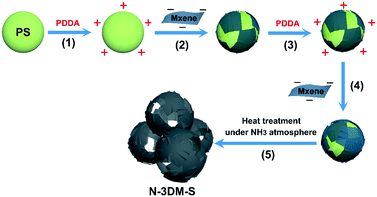
Interconnected N-doped MXene spherical shells for highly efficient capacitive deionization†
Gujia
Zhang
a,
Luhua
Wang
a,
Rongjian
Sa
 *b,
Chao
Xu
*b,
Chao
Xu
 *a,
Zhaohui
Li
a and
Lianzhou
Wang
*a,
Zhaohui
Li
a and
Lianzhou
Wang
 *c
*c
aState Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou 350108, P. R. China. E-mail: cxu@fzu.edu.cn
bFujian Key Laboratory of Functional Marine Sensing Materials, Institute of Oceanography, Minjiang University, Fuzhou, 350108, P. R. China
cSchool of Chemical Engineering and Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, QLD. 4072, Australia. E-mail: l.wang@uq.edu.au
First published on 17th November 2021
Abstract
MXenes have been considered as promising electrode materials for capacitive deionization (CDI). However, the availability of space for trapping and storing ions is usually inhibited in Mxene-based assemblies due to their special lamellar structure, which affects their desalination performance. Herein, a kind of N-doped Ti3C2Tx electrode with a unique interconnected porous structure (N-3DM-S) is designed to improve the active site accessibility of the electrodes. The Ti3C2Tx shell layers with flaws are prepared on spherical sacrificial templates, which then lead to the formation of an interconnected N-doped porous architecture upon the heat treatment under an NH3 atmosphere. The interconnected porous feature improves the availability of electrode materials, while the doping of nitrogen further enhances the accessibility of the electrode to electrolyte in addition to the chemical and electrochemical properties. As a result, the as-obtained N-3DM-S samples with more valid surface active sites exhibit excellent CDI performance compared to the control samples with isolated pore structure and the ones without N-doping. In particular, the optimal CDI capacities of N-3DM-S electrodes can reach up to 53 ± 2 mg g−1 in aqueous NaCl of 5000 mg L−1 at 1.6 V. Moreover, such N-3DM-S electrodes exhibit good regeneration stability as well, indicating their potential applications in the field of desalination.
Environmental significanceCapacitive deionization (CDI) technology has shown potential application in the field of water treatment in recent years due to its simple operating conditions, low energy consumption, environmental friendliness, etc. Since the CDI performance is strongly dependent on the properties of electrodes, construction of novel materials with a unique micro-nano structure is critical for the development of high-performance CDI electrodes. MXenes have been considered as promising electrode materials for capacitive deionization (CDI) due to their excellent physicochemical and electrical properties. Unfortunately, MXene nanosheets are prone to restack into a compact lamellar structure caused by the van der Waals force, which hinders the effective transport/adsorption of substances in the corresponding assemblies, thereby affecting their desalination capacity. Therefore, it is necessary to design a unique MXene-based architecture with more available and accessible surface area/pores to improve the desalination performance of such electrode materials, promoting the application of these MXenes in the field of water desalination. |
1. Introduction
The energy-efficient removal of ions from brackish media is crucial for sustainable supply of clean water. As a promising desalting technology, capacitive deionization (CDI) has recently attracted great attention in water treatment areas, mainly due to its low energy consumption, environmental friendliness, and easy operation.1 Conventional CDI is usually based on electrosorption of ions by forming electric double layers (EDLs) on the surface of carbonaceous porous electrodes. However, the applications of carbon-based electrodes in traditional CDI systems are seriously impeded by the co-ion expulsion effect, the limited available surface areas, the low charge-storage capacity and so on.2–4 In contrast, faradaic materials, which store ions via intercalation or conversion reactions, can address these issues and demonstrate much stronger charge-storage capacities at both low and high ion strengths than conventional electrodes, becoming promising alternative electrodes.5–13MXene, a two-dimensional (2D) metal carbide/nitride, has shown great promise in film separation, high-rate pseudo-capacitive energy storage applications, and ion batteries due to its excellent physicochemical and electrical properties.14,15 In particular, the typical MXene material Ti3C2Tx, has recently been found as an intercalation-type pseudocapacitive electrode for CDI, setting a new start for the research of such unique materials in the field of electrochemical desalination.16–19 Usually, MXene nanosheets are prone to restack into a compact lamellar structure caused by the van der Waals force, which hinders the effective transport/adsorption of substances in the corresponding assemblies, thereby affecting their desalination capacity. Several strategies have been carried out to overcome these issues,20–29 for instance adopting small-sized Ti3C2Tx sheets to shorten the lamellar channels and increase the ion diffusion pathways, using alkali-induced Ti3C2Tx floccules as intercalators to increase the interlayer distance and so on.26,27 Despite some progress, the sinuous and narrow 2D channels in these lamellar stacked assemblies still hinder the efficient diffusion and adsorption of ions to a certain extent, which makes the active sites for capturing ions in the electrode underutilized.
Assembling 2D sheets into three-dimensional (3D) porous networks has been considered as a promising strategy to improve the CDI performance of fabricated electrodes by reducing the aggregation of nanosheets and improving the permeability of the assemblies.30–41 For example, Wang and co-authors had prepared porous Ti3C2Tx aerogel through a freeze-drying method, which demonstrated higher desalination capacity almost twice that of traditional re-stacked samples.20 However, it is worth noting that these 2D sheets that consist of pore walls usually possess a certain barrier feature, which reduces the interconnectivity between pores in the monolithic structure. As a result, the pores/surface areas cannot be fully accessed, which would also affect the effective improvement of the desalination performance of these MXene-based porous electrodes. As demonstrated by our recent work,42 the open interconnectivity between pores can be enhanced efficiently by designing graphene-based pore walls with certain flaws, which greatly increases the availability of pores/surface areas, improving the CDI performance of the as-obtained porous electrodes. Conceivably, it is of great possibility that MXene-based porous electrodes with higher desalination ability can be obtained by improving the interconnectivity of pores in MXene electrodes.
In addition to structural regulation, the surface affinity properties of electrode materials also contribute to the improvement of the availability of active sites, especially inside the electrodes.43–48 As is known, heteroatom-doping plays a significant role in modifying the structure, composition, and especially the electrochemical performance of MXene materials.49–51 For example, Amiri and co-authors had indicated that the doping of nitrogen increased the conductivity of Ti3C2Tx electrodes, which would lead to the improvement of their corresponding CDI capacity (43.5 mg g−1). Meanwhile, our groups also showed that the introduction of nitrogen elements subtlety adjusted the Ti3C2Tx lamellar structure, which greatly benefited the intercalation adsorption of ions between the stacked layers. Actually, element-doping can improve the hydrophilic properties of as-formed materials as well, which is good for the quick diffusion of the aqueous solution inside the electrodes, increasing the effective contact between the electrode and electrolyte. Therefore, heteroatom-doping may further promote the availability of MXene materials in the desalination process.
Herein, we demonstrate a special 3D porous N-doped Ti3C2Tx electrode with excellent CDI performance by effectively improving the accessibility of pores and active sites. Incomplete Ti3C2Tx shells are constructed firstly on sacrificial sphere templates by adopting relatively small sized Ti3C2Tx sheets. After the removal of the template under an NH3 atmosphere, reserved N-doped spherical shells make up the target electrode architecture (N-3DM-S) with good interconnectivity and accessibility. The results of desalination indicate that such an N-3DM-S electrode exhibits superior CDI performance compared to the control samples with isolated pores (N-3DM-L) and those without N-doping (3DM-S). In particular, the CDI capacity of N-3DM-S electrodes can reach up to 53 ± 2 mg g−1 under optimized test conditions (5000 mg L−1 NaCl, 1.6 V). Moreover, such N-3DM-S electrodes also exhibit good deionization stability and regeneration performance over ongoing cycling, suggesting their potential application in the CDI field.
2. Experimental
2.1 Preparation and modification of polystyrene spheres
Polystyrene (PS) spheres with a diameter of about 500 nm were prepared by emulsifier-free emulsion polymerization according to previous work.52 Then, poly(diallyldimethylammonium chloride) (PDDA) was utilized to modify these spheres. PS spheres were dispersed in water (5 wt%), in which PDDA (1 wt%) was added. After stirring for 2 hours at room temperature, the modified PS sphere templates were washed thoroughly, and re-dispersed in water (5 wt%).2.2 Synthesis of Ti3C2Tx nanosheets with different sizes
MXene Ti3C2Tx was synthesized by etching commercial Ti3AlC2 powder according to the reported methods.14,15 The as-obtained Ti3C2Tx suspension was centrifuged at 3500 rpm for 1 hour firstly to remove the un-exfoliated sheets. Then, the collected supernatant was further treated using an ultrasound probe (135 W) for 6 minutes in an Ar atmosphere, in which small Ti3C2Tx sheets with a size of about 400 nm were obtained after centrifugation. The untreated Ti3C2Tx sheets with an average size of about 2 μm were used as control samples.2.3 Preparation of N-3DM-S and control samples
Typically, a Ti3C2Tx suspension (25 ml, 2 mg L−1) was added into a solution containing the modified PS microspheres (100 ml, 5 wt%) with an injection pump (1 mL min−1). After stirring for 2 hours, the composite was collected by centrifugation to remove the excess Ti3C2Tx. Subsequently, the as-obtained PS@Ti3C2Tx composites were modified with PDDA according to the aforesaid method, which were then re-dispersed in water (100 mL). After that, another Ti3C2Tx suspension (25 ml, 2 mg L−1) was mixed with the PDDA modified PS@Ti3C2Tx composites under the same conditions. The resulting composites were collected and dried at 60 °C, and then heat treated at 600 °C for 4 h under an NH3 atmosphere, resulting in the formation of N-3DM-S samples. Similarly, N-3DM-L and 3DM-S control samples were prepared using the same process by adopting large Ti3C2Tx sheets and heat treatment under a N2 atmosphere, respectively.2.4 Characterization
The size of the nanosheets was analyzed by atomic force microscopy (AFM, Dimension Icon Bruker). A Malvern Zetasizer Nano-ZS90 particle analyzer was used to determine the zeta potential of the samples. Thermogravimetric (TG) analyses were performed on a thermogravimetric analyzer (Mettler Toledo) in a nitrogen flow. A transmission electron microscope equipped with an energy dispersive spectrometer (TEM, Tecnai G2 F20 S-TWIN) and a field emission scanning electron microscope (Hitachi SU8010) were utilized to analyze the micromorphology and the element composition of the samples. The crystal form of the samples was confirmed by X-ray diffraction (XRD) with Cu Kα radiation (Bruker D8 Advance). X-ray photoelectron spectroscopy (XPS) was carried out on a Thermo Scientific Escalab 250 X-ray photoelectron spectrometer using Mg Kα radiation (hν = 1253.6 eV) as an excitation source. Nitrogen adsorption and desorption isotherms were measured using an ASAP3020M. A mercury intrusion analyzer (AutoPore IV 9500) was used to analyze the macroporous feature of the samples. The contact angle tests were carried out on an OCA20 (Dataphysics Co.).2.5 Electrochemical measurement
The MXene electrodes for electrochemical measurements were obtained from the following procedure. MXene, acetylene black, and polyvinylidene difluoride (PVDF) were firstly mixed with a mass ratio of 90%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%
5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% in water. Then, the obtained slurry was brushed on graphite paper, and dried naturally. Analysis of the traditional electrochemical properties of the samples including constant voltage (CV), galvanostatic charging–discharging (GCD), and electrochemical impedance spectroscopy (EIS) tests were performed on a three-electrode electrochemical workstation (Autolab M204) with Pt as the counter electrode, Ag/AgCl as the reference electrode, and 1 M NaCl as the electrolyte.
5% in water. Then, the obtained slurry was brushed on graphite paper, and dried naturally. Analysis of the traditional electrochemical properties of the samples including constant voltage (CV), galvanostatic charging–discharging (GCD), and electrochemical impedance spectroscopy (EIS) tests were performed on a three-electrode electrochemical workstation (Autolab M204) with Pt as the counter electrode, Ag/AgCl as the reference electrode, and 1 M NaCl as the electrolyte.
The contribution of the capacitive and diffusion (intercalation pseudocapacitive) effects was calculated according to eqn (1).20,53–55
| i(V) = k1v + k2v1/2 | (1) |
The diffusion coefficients of ions in electrodes were calculated according to eqn (2):
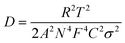 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1), C is the ion concentration (1 mol L−1) and σ is the Warburg coefficient.
500 C mol−1), C is the ion concentration (1 mol L−1) and σ is the Warburg coefficient.
2.6 CDI measurement
The capacitance performance of Ti3C2Tx-based electrodes was determined on a home-made asymmetric CDI device, which consists of an activated carbon (AC) anode and an MXene cathode. These CDI electrodes were fabricated using the same method as for electrochemical tests. Ti3C2Tx (or AC), acetylene black and PVDF were mixed in water. Subsequently, the as-obtained mixed slurry containing about 40 mg of Ti3C2Tx (or AC) was cast onto graphite paper current collectors to cover an area of about 12.5 cm2.The CDI experiments were performed at constant voltage. A NaCl solution (50 mL) was recirculated through the HCDI cell with a flow rate of 14 mL min−1 using a peristaltic pump. The conductivity of the solution was monitored by a conductivity meter (Leici, DDSJ-308F). The salt electrosorption capacities (SEC) and salt electrosorption rate (SER) of the electrodes were calculated using the following eqn (3) and (4), respectively:
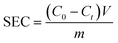 | (3) |
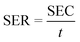 | (4) |
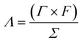 | (5) |
3. Results and discussion
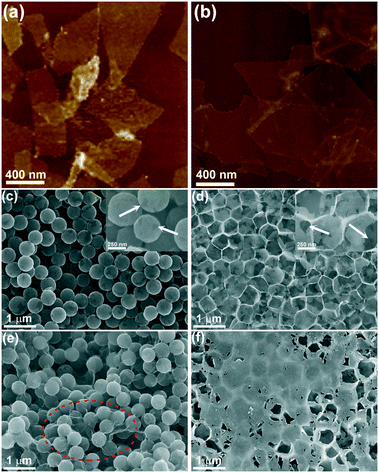
The N-doped porous Ti3C2Tx architecture is constructed using a template-mediated self-assembly method,56,57 as the schematic shows in Fig. 1. First, small Ti3C2Tx sheets with an average lateral size of about 400 nm (Fig. 2(a)) are collected by ultrasonication and centrifugation according to a previous study,58 and the relatively large ones (∼2.0 μm, Fig. 2(b)) are utilized as control samples in this work. Typically, negatively charged Ti3C2Tx sheets with a small size (−41 mV, zeta-potential values of the samples are listed in Fig. S1(a)†) are pasted conformably on the surface of the PS template (+44 mV, 450 nm) by the electrostatic adsorption effect, achieving single layer saturated adsorption (about 10 wt%, TG curves shown in Fig. S1 (b)†). Consistent with our previous work, the single Ti3C2Tx shell layer is discontinuous even in the case of saturated adsorption, so single-layer spherical shells are prone to collapse after the removal of the support templates (Fig. S2†). To reinforce these fragile spherical shells, a second Ti3C2Tx layer (∼10 wt%) is attached again on such composite templates that were modified with PDDA (+24 mV), forming the target core–shell building blocks. After heat treatment under an NH3 atmosphere, these reserved Ti3C2Tx spherical shells will take shape into an interconnected porous architecture.Fig. 2(c) and (d) show the typical FESEM images of the N-3DM-S sample before and after heat treatment. From Fig. 2(c), it is found that the core–shell composite units with a uniform structure can be prepared by enwrapping small Ti3C2Tx nanosheets on the PS templates. Because of the difference in geometry between the sheets and the spherical templates, the enwrapped shell layers in most core–shell units are incomplete, which is similar to the structure that we used to wrap templates with graphene.42 After the in situ removal of templates, a 3D porous network architecture is constructed successfully by the assembly of these reserved Ti3C2Tx spherical shells (Fig. 2(d)).59 Owing to the incompleteness of the shells, these large voids induced by templates are not totally isolated by the pore walls, resulting in the formation of an open and interconnected porous structure, as the arrows indicate in Fig. 2(d). By comparison, the large Ti3C2Tx sheets are inclined to cover several templates simultaneously (as the circle shows in Fig. 2(e), which makes the as-formed voids be covered after template removal as well (Fig. 2(f)). As a result, the connectedness between these pores and the outside surroundings in the control N-3DM-L sample will be hindered.
In addition, we have utilized small sheets to prepare control samples with different shell layers, for example a single layer and three layers of spherical shells (Fig. S2†). It is found that the single-layer Ti3C2Tx shell hardly maintains the spherical porous structure after the removal of the support templates, because these discontinuous ultrathin shells assembled by single-step wrapping are prone to collapse (Fig. S2(a)†). While a relatively sealed spherical shell structure is inclined to form when three layers of Ti3C2Tx sheets are enwrapped on PS spheres, which results in large amounts of unavailable pores (Fig. S2(b)†). Accordingly, it is more appropriate to adopt a double-layer structure, which can both effectively maintain the structural feature of the spherical shell, and ensure the good interconnectivity between these spherical shells at the same time.
In this work, we heat treat our core–shell samples under an NH3 atmosphere in order to remove the core templates and integrate the N element into the shells (pore walls) simultaneously. From the XRD patterns (Fig. 3(a) and S3(a)†), it is found that the typical lamellar diffraction peak of Ti3C2Tx sheets still exists after heat treatment, which is caused by the restacking of these as-formed sphere shells. However, probably due to the introduction of the macroporous structure and the nitrogen element doping, the orderly lamellar structure of Ti3C2Tx is destroyed, giving rise to a certain shift and broadening of the diffraction patterns. Meanwhile, there are no other obvious diffraction signals in the XRD patterns, indicating that such 3D porous materials still mainly consist of Ti3C2Tx species.60Fig. 3(b) presents the typical XPS survey spectra of N-3DM-S and original Ti3C2Tx sheets. It has been demonstrated that ammonia treatment at higher temperatures could lead to the integration of N atoms into Ti3C2Tx (∼4 wt% content of N element) and the loss of the F element simultaneously, which is clearly shown in Fig. 3(b). In particular, the appearance of the Ti–N peak in the N1s spectrum (around 396.5 eV in Fig. 3(c)) and the decrease of the Ti–C peak intensity in the C1s spectrum (around 282 eV in Fig. 3(d)) provide the confirmation of the substitution of partial C atoms with N atoms in the Ti3C2Tx structure,61–63 which is also consistent with our previous DFT simulation calculations.30 In addition, the Ti–N band also appears in the Ti2p spectrum (Fig. S3(b)†), suggesting the effective formation of N-doped samples.
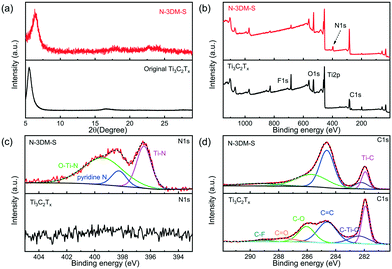 | ||
| Fig. 3 (a) XRD patterns and (b–d) XPS curves of N-3DM-S and original Ti3C2Tx sheets. (c) and (d) Deconvolution of high resolution XPS spectra for N1s and C1s in samples, respectively. | ||
Furthermore, such a N-doped porous architecture can also be confirmed by TEM images and the corresponding elemental mappings. From Fig. 4(a), a network framework structure is clearly found, which is similar to that shown in the FESEM images (Fig. 3(b)). The high-resolution TEM (HRTEM) image indicates the existence of orderly lamellar features in N-3DM-S samples, which is mainly ascribed to the stacking of Ti3C2Tx sheets and the adjacent spherical shells. Fig. 4(c) and (d) show the elemental mappings in the selected area of the N-3DM-S sample. Obviously, the even distribution of the N and Ti elements further proves that the doping of the nitrogen element in N-3DM-S is relatively uniform.
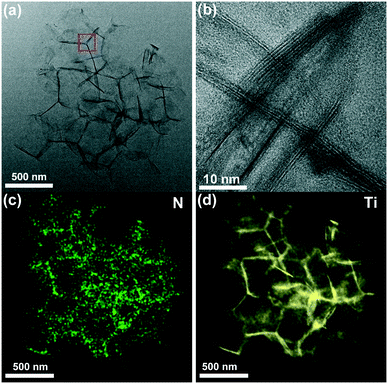 | ||
| Fig. 4 (a) TEM and (b) HRTEM images of N-3DM-S. Elemental mapping of the N-3DM-S sample: (c) N and (d) Ti. | ||
Generally, assembling 2D sheets into 3D porous networks will decrease the aggregation of nanosheets, and increase their surface areas simultaneously. According to the nitrogen adsorption–desorption isotherms (Fig. S4(a) and (b)†), the specific surface area of N-3DM-S samples is improved greatly over that of traditional restacked ones, which is increased from ∼17 to 183 m2 g−1. However, the introduction of pores doesn't mean that they are effectively used, for example forming closed pores. Fortunately, as indicated by morphological characterization (Fig. 2), these N-3DM-S samples constructed from small sheets have a more open and interconnected porous architecture, which enables more surfaces and pores to be detected as compared with that of N-3DM-L derived from large sheets. As indicated in Table 1, the specific surface area of N-3DM-S is indeed much larger than that of N-3DM-L (about 94 m2 g−1), although the preparation method is almost the same (The N element contents are similar to each other, ∼4 wt%, Fig. S3(c)†). Moreover, we also use Hg penetration tests to analyze the template-induced macroporous features of both N-3DM-S and N-3DM-L samples (Fig. S4(c) and (d)†). As expected, the typical total intrusion volume and porosity of N-3DM-S are higher than that of N-3DM-L samples (as the data listed Table 1), further suggesting that such N-3DM-S samples possess more open porous features. Accordingly, it is assumed that more available surface areas and pores could increase the contact probability of substances on the inner and outer surfaces of the electrodes, which will be beneficial for the adsorption and diffusion of ions by electrodes during the CDI process.
| Sample | N2 physisorption | Hg penetration | ||
|---|---|---|---|---|
| Specific surface area (m2 g−1) | Mesopore volume (cm3 g−1) | Total intrusion volume (cm3 g−1) | Porosity (%) | |
| Ti3C2Tx | 16.6 | 0.03 | 2.6 | 83.6 |
| N-3DM-S | 182.6 | 0.23 | 12.2 | 95.0 |
| N-3DM-L | 93.9 | 0.11 | 9.5 | 84.9 |
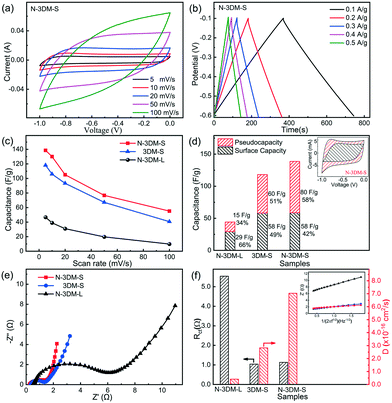
The electrochemical properties of N-3DM-S and reference samples (N-3DM-L and 3DM-S) are investigated firstly using a three-electrode system before CDI measurements. Fig. 5(a) shows the typical cyclic voltammetry (CV) curves of N-3DM-S at scan rates of 5 to 100 mV s−1, in which no obvious redox peaks (faradaic reaction) are observed. Moreover, the charge and discharge curves at different current densities (from 0.1 to 0.5 A g−1) have a nearly perfect triangular shape, indicating the good coulombic efficiency of the N-3DM-S electrode (Fig. 5(b)). According to these CV curves, it can be calculated that the specific capacitances of N-3DM-S electrodes are higher than those of N-3DM-L and 3DM-S ones at different scan rates (Fig. 5(c)). For instance, the capacitance value of N-3DM-S is 138 F g−1 at a scan rate of 5 mV s−1, while that of N-3DM-L is only about 44 F g−1, which could be due to the more available surface areas in the N-3DM-S sample (Table 1). Besides, the discharge curves of the N-3DM-S sample are visibly longer than those of the control ones (Fig. 5(b) and S5†), which is another indication that N-3DM-S has a considerably higher specific capacitance at all current densities.
It has been demonstrated that the capacitive feature of porous MXene electrodes usually includes both EDL capacitance and intercalation pseudocapacitance.20,64 As the inset shows in Fig. 5(d), the CV curves of N-3DM-S electrodes are not typical rectangles like those of capacitive materials, but distorted rectangles at low scan rates, indicating the existence of pseudocapacitance in addition to EDL capacitance. Following previous studies,20,53–55,65 we calculated the contribution of outer charge (capacitance) and diffusion control (faradaic) processes to illustrate the capacitive and intercalation behaviour of these Ti3C2Tx electrodes. As the result shows in Fig. 5(d), the calculated capacitance and faradaic contribution of N-3DM-S are about 58 F g−1 (42.0%) and 80 F g−1 (58.0%), respectively, suggesting the dual-mode ion storage in this electrode (the detailed calculation steps are shown in the ESI†). Due to its relatively isolated porous feature, both the EDL capacitance and intercalation pseudocapacitance of N-3DM-L are lower than those of N-3DM-S and 3DM-S electrodes. On the other hand, some work including ours had indicated that the doping of nitrogen could improve the electron concentration of N-doped Ti3C2Tx and its conductivity, which effectively promote the intercalation and/or adsorption behaviours of ions between Ti3C2Tx layers. Accordingly, the specific capacitance, especially the pseudocapacitive portion of N-3DM-S, is higher than that of the control 3DM-S without doping.49,61,66,67
The electrochemical impedance spectroscopy (EIS) results of these porous electrodes in 1.0 M NaCl aqueous solution are presented in Fig. 5(e), which can provide more detailed information on the charge transport and ion diffusion inside electrodes. Previous studies demonstrated42,57 that network porous architectures built with small nanosheets are inclined to form more electron transfer pathways in the monolithic frameworks, which effectively decrease the charge transfer resistance of such assembled electrodes. Thus, in the high-frequency region, both N-3DM-S and 3DM-S possess smaller semicircle diameters compared with that of N-3DM-L, indicating their better conductivity properties. However, probably due to the facts that the electrodes contain polymer binder (PVDF) and the electrolyte is not a traditional proton electrolyte, the charge transfer resistance of these electrodes is relatively low compared with that of some reports. The linear part in the low-frequency region of EIS reflects the diffusion resistance encountered by ions migrating through the electrode (Warburg impedance). Usually, the higher the slope of the line, the better diffusion performance of ions into the porous electrodes. Obviously, N-3DM-S displays a more vertical line than N-3DM-L and 3DM-S, indicating the faster and easier diffusion of ions into this electrode. This can be attributed to the fact that the interconnectivity between pores in the porous structure that were assembled from small sheets is much better than that derived from larger ones, which lead to the formation of more abundant diffusion pathways in the monolithic samples. Meanwhile, the enhanced affinity of the samples towards the electrolyte caused by N-doping is also conducive to the penetration of solutions and ions inside the electrodes. For example, the N-3DM-S samples possessed good hydrophilicity, and their water contact angle decreased quickly from 68° to 0° in 30 seconds. By comparison, the contact angle of the un-doped 3DM-S samples only decreased from 79° to 38° at the same time interval (Fig. S6†). According to eqn (2),68 we calculated the Warburg and ion diffusion coefficients of these electrodes, which fully proves the superiority of N-3DM-S in terms of ion migration (Fig. 5(f)). Therefore, it is reasonable to speculate that the N-3DM-S electrode with a unique porous structure and superior electrochemical properties would exhibit excellent CDI performances.
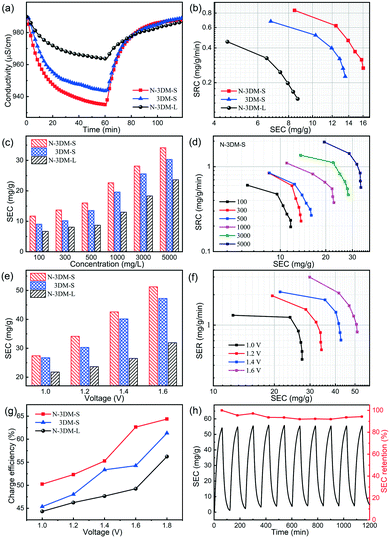
Generally, MXene electrodes are prone to oxidation at anodic potentials when operated in a symmetric cell configuration, eventually influencing their stability. So in this work, we assembled HCDI cells using commonly used activated carbon (AC) as the positive electrode to evaluate the CDI performances of these newly prepared electrodes at constant voltage (Fig. 6).69Fig. 7(a) shows the typical CDI performance of 3DM electrodes in 500 mg L−1 NaCl solution at a voltage of 1.2 V. It is found that the conductivities of the saline solutions decrease at the applied voltage and then recover after the removal of voltage, indicating that these porous electrodes have good CDI properties in this system. In the meantime, the solution conductivity using the N-3DM-S electrode is much lower than that using the control ones, suggesting the better deionization performance of the N-3DM-S electrode, which is also confirmed by the Ragone curves (Fig. 7(b)). It is known that the accessibility of the active sites is a key factor affecting the desalination performance of CDI electrodes, either by the EDL or intercalation adsorption effects. Fortunately, the formation of a N-doped interconnected porous structure obviously increases the accessibility of the N-3DM-S electrode compared with that of original Ti3C2Tx sheets and N-3DM-L with isolated pores (Table 1). Moreover, the enhanced hydrophilicity of the electrodes caused by N doping further improve the affinity of the monolithic electrodes towards the saline solution (Fig. 5(f) and S6†). Accordingly, the N-3DM-S samples can capture more ions through both EDL storage and ion intercalation (Fig. 5(d)), achieving relatively high CDI capacities (Fig. 6).
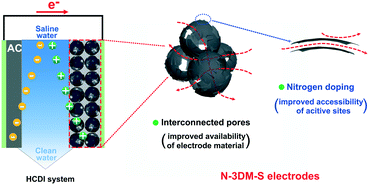 | ||
| Fig. 6 Schematic illustration of the working HCDI unit cell and the potential advantages of such interconnected N-doped Mxene spherical shells as desalination electrodes. | ||
It is well known that traditional carbonaceous electrodes are always subjected to the co-ion expulsion effect, so their CDI tests could be only carried out in solution with low salt concentration (≤500 mg L−1). By contrast, these MXene electrodes that possess special pseudocapacitance features can tolerate higher salt concentration, which enables their CDI performance to be evaluated over a relatively wide concentration range. Fig. 7(c) shows the CDI capacities of these Ti3C2Tx porous electrodes in the NaCl concentration range from 100 to 5000 mg L−1 at 1.2 V. The results show that the desalination capacities of the N-3DM-S electrode increase gradually along with the NaCl concentration, which are always better than those of the control samples. Moreover, the Ragone curves shifting to the upper right also indicates that the high salt concentration is beneficial for increasing the salt adsorption capacity and adsorption rate of the N-3DM-S electrode (Fig. 7(d)).
On the other hand, we also test the CDI performances of these electrodes at different operating voltages at a fixed salt concentration as shown in Fig. 7(e). Consistent with previous reports, the desalination capacities of the electrodes are improved at higher operating voltages due to the increased coulombic interaction. For example, the CDI capacities of N-3DM-S electrodes increase obviously from ∼18 to ∼53 mg with an increase in the operating voltage from 1.0 to 1.6 V at a NaCl concentration of 5000 mg L−1. Meanwhile, the desalination efficiency and charge efficiency of the electrodes also increase along with the voltage, as shown in Fig. 7(f) and (g). Probably due to the polarization effect, no bubbles were generated on the surface of these electrodes during the whole desalination process (the electrolysis of water does not occur), suggesting that such electrodes operate normally at high voltage. To further investigate the regeneration properties of such CDI electrodes, repeated charge–discharge experiments are also carried out under optimized conditions (Fig. 7(g)). Typically, the desalination capacities of the N-3DM-S sample can be maintained at 95% after 10 cycles in NaCl solution of 5000 mg L−1 at 1.6 V, indicating its good deionization stability and regeneration performance during the test process. Therefore, it can be determined that such N-3DM-S samples work well under both high voltage and/or high salt concentration, showing their potential applications in relatively harsh environments.
To benchmark the CDI performance of our electrodes, we have also summarized the desalination performance of our samples and most recently reported MXene-based electrodes especially those without ion exchange membranes (Table 2). It is found that the as-obtained N-3DM-S samples in this work also exhibit excellent desalination capacity, which is probably due to their interconnected porous structure and the good accessibility of active adsorption sites in our electrodes (Fig. 6). Accordingly, there's a reason to believe that the constitution of MXene-based electrodes with more available spaces and accessible active sites is beneficial for further improving the desalination performance of MXenes, expanding the vast use of such materials in the field of CDI.1
| Entry | CDI architecture | Applied voltage (V) | Concentration (mg L−1, NaCl) | SEC (mg g−1) | Ref. |
|---|---|---|---|---|---|
| a Ion exchange membranes are not utilized in these studies. | |||||
| 1 | CDI | 1.2 V | 292 | 13 | 16 |
| 2 | CDI | 1.2 V | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
45 | 20 |
| 3 | CDI | 0.8 V | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 064 064 |
15 | 21 |
| 4 | CDI | 1.4 V | 500 | 26.8 | 22 |
| 5 | CDI | 1.6 V | 498 | 12.1 | 23 |
| 6 | CDI | 1.2 V | 5000 | 43.5 | 24 |
| 7 | HCDI | 1.2 V | 500 | 16 | 25 |
| 8 | HCDI | 1.6 V | 5000 | 53 | This work |
4. Conclusions
In summary, a new kind of N-doped Ti3C2Tx-based porous electrode (N-3DM-S) with excellent CDI performance has been successfully prepared in this work. We intentionally adopt relatively small Ti3C2Tx sheets to construct incomplete shell layers on the sacrificial templates, which enable the reserved spherical shells after heat treatment to assemble into a 3D architecture with integrally interconnected porous features. Combined with the contributions of N-doping to conductivity, and hydrophilicity, etc., the as-obtained N-3DM-S samples exhibit excellent physical–chemical properties including the specific surface area and ion diffusion ability, which greatly improve the availability of the active sites for capturing ions. As a result, these N-3DM-S electrodes exhibit superior CDI performance compared with N-3DM-L and 3DM-S under different test conditions. In particular, the optimal CDI capacities of N-3DM-S electrodes reach up to 53 mg g−1 in NaCl aqueous solution. Furthermore, the N-3DM-S electrodes also show good cyclic desalination performance, indicating their potential applications in the CDI field.This work demonstrated that construction of a more interconnected and accessible porous architecture is an effective means to increase the desalination capacity of Ti3C2Tx-based electrodes, which provides a potential strategy for the design of MXene porous electrodes with a novel structure and excellent performance, better promoting the application of such electrode materials in the field of CDI.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the National Key Research and Development Program of China (2018YFE0208500), National Natural Science Foundation of China (52172292) and Natural Science Foundation of Fujian Province, China (2020J01439).Notes and references
- P. Srimuk, X. Su, J. Yoon, D. Aurbach and V. Presser, Nat. Rev. Mater., 2020, 5, 517–538 CrossRef CAS
.
- S. Porada, L. Borchardt, M. Oschatz, M. Bryjak, J. S. Atchison, K. J. Keesman, S. Kaskel, P. M. Biesheuvel and V. Presser, Energy Environ. Sci., 2013, 6, 3700–3712 RSC
.
- C. Tsouris, R. Mayes, J. Kiggans, K. Sharma, S. Yiacoumi, D. DePaoli and S. Dai, Environ. Sci. Technol., 2011, 45, 10243–10249 CrossRef CAS PubMed
.
- P. Liu, T. Yan, L. Shi, H. S. Park, X. Chen, Z. Zhao and D. Zhang, J. Mater. Chem. A, 2017, 5, 13907–13943 RSC
.
- C. Zhang, D. He, J. Ma, W. Tang and T. D. Waite, Water Res., 2018, 128, 314–330 CrossRef CAS PubMed
.
- D. He, C. E. Wong, W. Tang, P. Kovalsky and T. D. Waite, Environ. Sci. Technol. Lett., 2016, 3, 222–226 CrossRef CAS
.
- Y.-H. Liu, H.-C. Hsi, K.-C. Li and C.-H. Hou, ACS Sustainable Chem. Eng., 2016, 4, 4762–4770 CrossRef CAS
.
- N. Kim, S. P. Hong, J. Lee, C. Kim and J. Yoon, ACS Sustainable Chem. Eng., 2019, 7, 16182–16189 CrossRef CAS
.
- Z. Zhang and H. Li, Environ. Sci.: Nano, 2021, 8, 1886–1895 RSC
.
- W. Si, Z. Liu, Z. Zhang, W. Ji and H. Li, Environ. Sci.: Nano, 2021, 8, 657–665 RSC
.
- Y. Zhao, B. Liang, M. Zong, M. Duan, K. Li and C. Lv, Environ. Sci.: Nano, 2019, 6, 3091–3101 RSC
.
- J. Adorna Jr, M. Borines, D. Van Dien and R.-A. Doong, Desalination, 2020, 492, 114602 CrossRef
.
- X. Xu, C. Li, C. Wang, L. Ji, Y. V. Kaneti, H. Huang, T. Yang, K. C. W. Wu and Y. Yamauchi, ACS Sustainable Chem. Eng., 2019, 7, 13949–13954 CrossRef CAS
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS
.
- Y.-Y. Peng, B. Akuzum, N. Kurra, M.-Q. Zhao, M. Alhabe, B. Anasori, E. C. Kumbur, H. N. Alshareef, M.-D. Ger and Y. Gogotsi, Energy Environ. Sci., 2016, 9, 2847–2854 RSC
.
- P. Srimuk, F. Kaasik, B. Kruener, A. Tolosa, S. Fleischmann, N. Jaeckel, M. C. Tekeli, M. Aslan, M. E. Suss and V. Presser, J. Mater. Chem. A, 2016, 4, 18265–18271 RSC
.
- J. Ma, Y. Cheng, L. Wang, X. Dai and F. Yu, Chem. Eng. J., 2020, 384, 123329 CrossRef CAS
.
- J. Ai, J. Li, K. Li, F. Yu and J. Ma, Chem. Eng. J., 2021, 408, 127256 CrossRef CAS
.
- Z. Chen, X. Xu, Z. Ding, K. Wang, X. Sun, T. Lu, M. Konarova, M. Eguchi, J. G. Shapter, L. Pan and Y. Yamauchi, Chem. Eng. J., 2021, 407, 127148 CrossRef CAS
.
- W. Bao, X. Tang, X. Guo, S. Choi, C. Wang, Y. Gogotsi and G. Wang, Joule, 2018, 2, 778–787 CrossRef CAS
.
- P. Srimuk, J. Halim, J. Lee, Q. Tao, J. Rosen and V. Presser, ACS Sustainable Chem. Eng., 2018, 6, 3739–3747 CrossRef CAS
.
- L. Guo, X. Wang, Z. Y. Leong, R. Mo, L. Sun and H. Y. Yang, Flatchem, 2018, 8, 17–24 CrossRef CAS
.
- A. Feng, Y. Yu, L. Mi, Y. Yu and L. Song, Ionics, 2019, 25, 727–735 CrossRef CAS
.
- A. Amiri, Y. Chen, C. B. Teng and M. Naraghi, Energy Storage Mater., 2020, 25, 731–739 CrossRef
.
- B. Chen, A. Feng, R. Deng, K. Liu, Y. Yu and L. Song, ACS Appl. Mater. Interfaces, 2020, 12, 13750–13758 CrossRef CAS
.
- S. Buczek, M. L. Barsoum, S. Uzun, N. Kurra, R. Andris, E. Pomerantseva, K. A. Mahmoud and Y. Gogotsi, Energy Environ. Mater., 2020, 3, 398–404 CrossRef CAS
.
- X. Shen, Y. Xiong, R. Hai, F. Yu and J. Ma, Environ. Sci. Technol., 2020, 54, 4554–4563 CrossRef CAS
.
- L. Agartan, K. Hantanasirisakul, S. Buczek, B. Akuzum, K. A. Mahmoud, B. Anasori, Y. Gogotsi and E. C. Kumbur, Desalination, 2020, 477, 114267 CrossRef CAS
.
- G. S. Gund, J. H. Park, R. Harpalsinh, M. Kota, J. H. Shin, T.-i. Kim, Y. Gogotsi and H. S. Park, Joule, 2019, 3, 164–176 CrossRef CAS
.
- Z. Li, B. Song, Z. Wu, Z. Lin, Y. Yao, K.-S. Moon and C. P. Wong, Nano Energy, 2015, 11, 711–718 CrossRef CAS
.
- Y. Wu, G. Jiang, G. Liu, G. Lui, Z. P. Cano, Q. Li, Z. Zhang, A. Yu, Z. Zhang and Z. Chen, J. Mater. Chem. A, 2019, 7, 15633–15639 RSC
.
- P. Liu, H. Wang, T. Yan, J. Zhang, L. Shi and D. Zhang, J. Mater. Chem. A, 2016, 4, 5303–5313 RSC
.
- Z. U. Khan, T. Yan, L. Shi and D. Zhang, Environ. Sci.: Nano, 2018, 5, 980–991 RSC
.
- W. Dianbudiyanto and S.-H. Liu, Desalination, 2019, 468, 114069 CrossRef CAS
.
- Y. Li, Y. Liu, M. Wang, X. Xu, T. Lu, C. Q. Sun and L. Pan, Carbon, 2018, 130, 377–383 CrossRef CAS
.
- J. Li, B. Ji, R. Jiang, P. Zhang, N. Chen, G. Zhang and L. Qu, Carbon, 2018, 129, 95–103 CrossRef CAS
.
- A. G. El-Deen, R. M. Boom, H. Y. Kim, H. Duan, M. B. Chan-Park and J.-H. Choi, ACS Appl. Mater. Interfaces, 2016, 8, 25313–25325 CrossRef CAS PubMed
.
- Y. Zhao, X. Li, X. Mo and K. Li, Environ. Sci.: Nano, 2020, 7, 3575–3586 RSC
.
- X. Wei, X. Li, C. Lv, X. Mo and K. Li, Electrochim. Acta, 2020, 354, 136590 CrossRef CAS
.
- J. Kim, Y. Yi, D.-H. Peck, S.-H. Yoon, D.-H. Jung and H. S. Park, Environ. Sci.: Nano, 2019, 6, 916–924 RSC
.
- X. Xu, H. Tan, Z. Wang, C. Wang, L. Pan, Y. V. Kaneti, T. Yang and Y. Yamauchi, Environ. Sci.: Nano, 2019, 6, 981–989 RSC
.
- Y. Zhu, G. Zhang, C. Xu and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 29706–29716 CAS
.
- Z. U. Khan, T. Yan, J. Han, L. Shi and D. Zhang, Environ. Sci.: Nano, 2019, 6, 3442–3453 RSC
.
- Y. Huang, J. Yang, L. Hu, D. Xia, Q. Zhang, Y. Liao, H. Li, W. Yang, C. He and D. Shu, Environ. Sci.: Nano, 2019, 6, 1430–1442 RSC
.
- A. B. Ganganboina and R.-A. Doong, Environ. Sci.: Nano, 2020, 7, 228–237 RSC
.
- Q. Zhang, Y. Huang, D. Xia, L. Hu, P. Li, L. Tan, Y. Wang, C. He, D. Shu and X. Xie, Environ. Sci.: Nano, 2019, 6, 3359–3373 RSC
.
- D.-C. Han, C.-M. Zhang, J. Guan, L.-H. Gai, R.-Y. Yue, L.-N. Liu, M. Z. Afzal, C. Song, S.-G. Wang and X.-F. Sun, Electrochim. Acta, 2020, 336, 135639 CrossRef CAS
.
- X. Xu, J. Tang, Y. V. Kaneti, H. Tan, T. Chen, L. Pan, T. Yang, Y. Bando and Y. Yamauchi, Mater. Horiz., 2020, 7, 1404–1412 RSC
.
- Y. Wen, T. E. Rufford, X. Chen, N. Li, M. Lyu, L. Dai and L. Wang, Nano Energy, 2017, 38, 368–376 CrossRef CAS
.
- C. Yang, Y. Tang, Y. Tian, Y. Luo, X. Yin and W. Que, ACS Appl. Mater. Interfaces, 2020, 3, 586–596 CAS
.
- Y. Li, J. Qi, J. Li, J. Shen, Y. Liu, X. Sun, J. Shen, W. Han and L. Wang, ACS Sustainable Chem. Eng., 2017, 5, 6635–6644 CrossRef CAS
.
- C. C. Ho, A. Keller, J. A. Odell and R. H. Ottewill, Colloid Polym. Sci., 1993, 271, 469–479 CrossRef CAS
.
- A. VahidMohammadi, J. Moncada, H. Chen, E. Kayali, J. Orangi, C. A. Carrero and M. Beidaghi, J. Mater. Chem. A, 2018, 6, 22123–22133 RSC
.
- V. Augustyn, J. Come, M. A. Lowe, J. W. Kim, P. L. Taberna, S. H. Tolbert, H. D. Abruna, P. Simon and B. Dunn, Nat. Mater., 2013, 12, 518–522 CrossRef CAS
.
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. Barsoum, P. Simon and Y. Gogotsi, Nat. Energy, 2017, 2, 17105 CrossRef CAS
.
- M.-Q. Zhao, X. Xie, C. E. Ren, T. Makaryan, B. Anasori, G. Wang and Y. Gogotsi, Adv. Mater., 2017, 29, 1702410 CrossRef
.
- R. Sun, H.-B. Zhang, J. Liu, X. Xie, R. Yang, Y. Li, S. Hong and Z.-Z. Yu, Adv. Funct. Mater., 2017, 27, 1702807 CrossRef
.
- E. Kayali, A. VahidMohammadi, J. Orangi and M. Beidaghi, ACS Appl. Mater. Interfaces, 2018, 10, 25949–25954 CrossRef CAS PubMed
.
- X. Song, D. Fang, S. Huo and K. Li, Environ. Sci.: Nano, 2021, 8, 2191–2203 RSC
.
- Z. Fan, C. Wei, L. Yu, Z. Xia, J. Cai, Z. Tian, G. Zou, S. X. Dou and J. Sun, ACS Nano, 2020, 14, 867–876 CrossRef PubMed
.
- W. Bao, L. Liu, C. Wang, S. Choi, D. Wang and G. Wang, Adv. Energy Mater., 2018, 8, 1702485 CrossRef
.
- C. Lu, L. Yang, B. Yan, L. Sun, P. Zhang, W. Zhang and Z. Sun, Adv. Funct. Mater., 2020, 30, 2000852 CrossRef CAS
.
- H. Liu, Z. Hu, Q. Liu, P. Sun, Y. Wang, S. Chou, Z. Hu and Z. Zhang, J. Mater. Chem. A, 2020, 8, 24710–24717 RSC
.
- X. Shen, R. Hai, X. Wang, Y. Li, Y. Wang, F. Yu and J. Ma, J. Mater. Chem. A, 2020, 8, 19309–19318 RSC
.
- S. Ardizzone, G. Fregonara and S. Trasatti, Electrochim. Acta, 1990, 35, 263–267 CrossRef CAS
.
- L. Yu, Z. Fan, Y. Shao, Z. Tian, J. Sun and Z. Liu, Adv. Energy Mater., 2019, 9, 1901839 CrossRef
.
- J. Wang, G. Wang, T. Wu, D. Wang, Y. Yuan, J. Wang, T. Liu, L. Wang and J. Qiu, ACS Sustainable Chem. Eng., 2018, 6, 17204–17210 CrossRef CAS
.
- O. Noonan, Y. Liu, X. Huang and C. Yu, J. Mater. Chem. A, 2018, 6, 14272–14280 RSC
.
- J. Zhao, Z. Zhao, Y. Sun, X. Ma, M. Ye and X. Wen, Environ. Sci.: Nano, 2021, 8, 2059–2068 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Zeta-potential, FESEM images, XRD patterns, XPS spectra, nitrogen adsorption–desorption isotherms, mercury penetration curves, galvanic charging curves and contact angle tests of the samples. See DOI: 10.1039/d1en00821h |
| This journal is © The Royal Society of Chemistry 2022 |