Crystal engineering of porous coordination networks to enable separation of C2 hydrocarbons
Soumya
Mukherjee
 *ab,
Debobroto
Sensharma
*ab,
Debobroto
Sensharma
 a,
Kai-Jie
Chen
a,
Kai-Jie
Chen
 *c and
Michael J.
Zaworotko
*c and
Michael J.
Zaworotko
 *a
*a
aBernal Institute, Department of Chemical Sciences, University of Limerick, Limerick V94 T9PX, Republic of Ireland. E-mail: xtal@ul.ie
bCatalysis Research Center, Ernst-Otto-Fischer Straße 1 and Department of Chemistry, Technical University of Munich, Lichtenbergstraße 4, 85748 Garching bei München, Germany. E-mail: soumya.mukherjee@tum.de
cKey Laboratory of Special Functional and Smart Polymer Materials of Ministry of Industry and Information Technology, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China. E-mail: ckjiscon@nwpu.edu.cn
First published on 31st July 2020
Abstract
Crystal engineering, the field of chemistry that studies the design, properties, and applications of crystals, is exemplified by the emergence over the past thirty years of porous coordination networks (PCNs), including metal–organic frameworks (MOFs) and hybrid coordination networks (HCNs). PCNs have now come of age thanks to their amenability to design from first principles and how this in turn can result in new materials with task-specific features. Herein, we focus upon how control over the pore chemistry and pore size of PCNs has been leveraged to create a new generation of physisorbents for efficient purification of light hydrocarbons (LHs). The impetus for this research comes from the need to address LH purification processes based upon cryogenic separation, distillation, chemisorption or solvent extraction, each of which is energy intensive. Adsorptive separation by physisorbents (in general) and PCNs (in particular) can offer two advantages over these existing approaches: improved energy efficiency; lower plant size/cost. Unfortunately, most existing physisorbents suffer from low uptake and/or poor sorbate selectivity and are therefore unsuitable for trace separations of LHs including the high volume C2 LHs (C2Hx, x = 2, 4, 6). This situation is rapidly changing thanks to PCN sorbents that have set new performance benchmarks for several C2 separations. Herein, we review and analyse PCN sorbents with respect to the supramolecular chemistry of sorbent–sorbate binding and detail the crystal engineering approaches that have enabled the exquisite control over pore size and pore chemistry that affords highly selective binding sites. Whereas the structure–function relationships that have emerged offer important design principles, several development roadblocks remain to be overcome.
1. Introduction
The chemical industry has a turnover of $5.7 trillion per annum which represents ca. 7% of global GDP.1 Its energy footprint is even higher, with separation/purification of chemical commodities accounting for ca. 40% of industrial energy consumption. This underscores the societal need for greater energy efficiency and sustainability in the production of chemicals2 given that this energy footprint represents ca. 15% of global energy consumption.3 Further, there has been a forecast that suggests a threefold increase in demand for chemical commodities by 2050.2 The main reason for the energy footprint of commodity purification is reliance upon energy-intensive separation methods such as cryogenic separation, azeotropic and/or fractional distillation, chemisorption and solvent extraction.4Key to reducing the energy footprint of separations in today's ‘Age of Gas’2 are new technologies for gas and vapour purification. In this context, light hydrocarbon (LH) production is ever-increasing5 and chemists, material scientists and process engineers have been addressing the development of potentially disruptive energy-efficient LH separation processes that could be enabled by porous physisorbents.6 Herein, we address the rapid evolution of a new generation of physisorbents that have made significant progress with respect to addressing C2 LH purification, ethylene (C2H4), acetylene (C2H2) and ethane (C2H6).
Why C2 separations matter
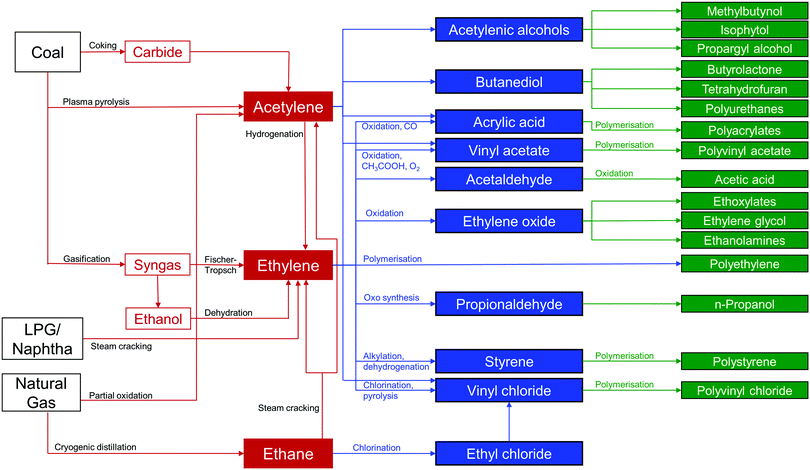
Millions of tonnes of C2 LHs are produced every year from coal, petroleum, and natural gas using a network of interrelated chemical processes and purification steps (Fig. 1). C2H4 is one of the highest volume products of the chemical industry and is the basic building block for a variety of polymers, solvents, detergents and coatings. The recent shale gas boom has reduced C2H4 costs by approximately half in Europe and North America in the past ten years, and this has consolidated its position as the “backbone of the global chemical industry.”2,7,8 The quantity of C2H4 produced annually was estimated to be ca. 143 Mt per year with a market value of US$254.6 billion in 2016. This is projected to reach US$475.8 billion in 2023 with an approximate growth rate of 5% per year.9,10 Although there is a wide variety of industrial uses for C2H4, over 80% of C2H4 production in the US, Europe and Japan is for the production of polyethylene, ethylene oxide and ethylene chlorides.11,12 Impurities in such processes can have substantial negative impacts on productivity.13 For example, if >5 ppm of C2H2 is present in C2H4 during polymerisation, the catalyst can become poisoned and its recovery is limited. Typically, polymer-grade specifications require C2H4 of >99.9% purity, with <2 ppm C2H2 and <200 ppm C2H6 and methane.12C2H4 is produced primarily by the steam cracking of C2H6 and light naphtha, with a small additional contribution from the hydrogenation of C2H2. During production from C2H6, C2H4 is typically the major product and C2H4/C2H6 separation is needed to remove C2H6 from incomplete conversion. Production by cracking of naphtha, affords C2H4 and propylene as the major products, but other C2–C6 olefins are present in significant quantities and a complex separation pathway is utilised.12 These processes require separation of C2 LHs from each other, a challenging proposition because of their similar boiling points, molecular sizes and properties (Fig. 2).14–16 Due in large part to these separation processes, the production of light olefins by steam cracking is the most energy-intensive process in the chemical industry, accounting for ca. 20% of its energy footprint and around 30% of its CO2 emission.7,17
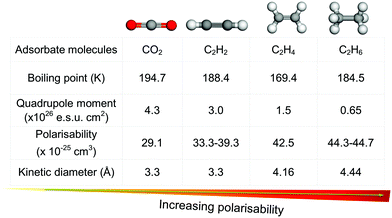 | ||
| Fig. 2 Comparison of key physicochemical properties of CO2 and C2 LHs reveals the similarities in properties for multiple industrially relevant gas pairs. | ||
C2H2 is also a major chemical building block. Production volumes have decreased from 10 Mt per year in 1960 to hundreds of kt per year at present, overtaken by cheaper, safer C2H4 as the C2 feedstock of choice after the shift from coal to a petroleum-based industrial economy.2,18–20 Nonetheless, C2H2 production is increasing again and the processes used for C2H2 all involve high temperatures; C2H2 is the most thermodynamically stable of the C2 LHs at temperatures above 1400 K.21,22 Partial oxidation of natural gas is an increasingly important route to C2H2 due to relatively low natural gas prices. C2H2 recovered by separation as a by-product of C2H4 production is also often commercially viable.18 C2H2 used as fuel in oxy-acetylene torches does not typically need to be highly pure (ca. 98%),22 however, for use as a chemical feedstock, high purity C2H2 is needed. For example, specifications for ‘Type A’ C2H2 in India require >99 volume% and <0.15% H2S, <0.1% NH3, <0.06% phosphine, <0.006% arsine when produced from the carbide process.23
C2H6 is the second most abundant component of natural gas (0.7–6.8%).24 Approximately 40% of C2H6 is recovered for chemical use, mainly as a feedstock in steam cracking. Purified C2H6 is used in small amounts in the synthesis of chloroethane.25 Purification of C2 LHs is therefore central to the chemical industry as a whole and represents a major portion of its energy usage and, in turn, global energy production. This means that, because of the production scale of C2 LHs and their derivatives, even minor improvements to purification processes could result in major economic and/or energy savings.
Why porous coordination networks, PCNs, promise to deliver on the challenge of C2 LH separations
That composition and structure profoundly impact the properties of crystalline solids has provided impetus for exponential growth in the field of crystal engineering over the past 30 years. Crystal engineering, the field of chemistry that studies the design, properties and applications of crystals, has evolved from focus upon structure (form) towards control over bulk properties (function).26 Crystal engineering now offers a paradigm shift from the more random, high-throughput methods that have traditionally been utilised in materials discovery and development. This situation is exemplified by porous physisorbents such as PCNs, a term coined by Ma and Zhou in the early 2000s.27 In essence, crystal engineering of PCNs has come of age thanks to their inherent modularity and two decades of ever-increasing activity from materials chemists who are now aiming to design the right material for the right application.28A subset of PCNs, metal–organic materials, MOMs,29 are particularly amenable to crystal engineering design principles that allow for “bottom-up” design approaches of a new generation of crystalline porous physisorbents suitable for application in commodity gas separations.4,15 The composition of PCNs makes them inherently amenable to design from first principles; they are typically comprised of metal cations or metal “node” clusters linked into 2D or 3D potentially porous networks by organic and/or inorganic “linker” ligands. This “node-and-linker” concept of designing specific structural motifs was introduced by Robson and Hoskins in 198930 and has subsequently afforded tens of thousands of CNs that can potentially exhibit permanent porosity.31 The potential utility of permanent porosity motivated Kitagawa and Yaghi to coin the terms PCPs, porous coordination polymers,32 and MOFs, metal–organic frameworks, respectively.33
1999 saw the seminal discoveries of the first two examples of extra-large surface-area PCNs: HKUST-134 [Cu3(1,3,5-benzenetricarboxylate)2]n, ca. 1900 m2 g−1; MOF-535 [Zn4O(1,4-benzenedicarboxylate)3]n, ca. 3800 m2 g−1. The quest for ultra-high surface area MOFs continues, with recent benchmarks set by DUT-60 (7839 m2 g−1) and NU-110 (7140 m2 g−1).36,37 Ironically, it is PCNs featuring much smaller pores i.e. ultramicropores (<0.7 nm), that are the focus herein. This is because ultramicropores tend to outperform other classes of physisorbents with respect to separation performance driven by selective binding of gases and optimal thermodynamics/kinetics. Ultramicropores function well in this context as they combine tight sorbent–sorbate binding with fine-tuned pore chemistry. Such selective binding is key to enabling separation of hard-to-separate gas molecules with similar size, shape and physical properties, as exemplified by hybrid ultramicroporous materials (HUMs).28 HUMs directly address a major weakness of most physisorbents, which bind sorbates too weakly to separate trace gas impurities from mixtures under ambient conditions. This is because HUMs offer energetic “sweet spots”, binding sites that are not too strong and not too weak, for a number of gas separations involving CO2,38–40 C2H2,41,42 and H2O.43,44 It has become apparent that ultramicroporous PCNs have emerged as the top-performing sorbents for gas separation and purification,45 as we detail herein with respect to C2 LHs. Notably, this means that interpenetration in HUMs, a phenomenon once considered detrimental to porosity,46 is key to controlling pore size and enabling tight C2 LH binding sites that result in exceptional sorption performance.41,42,47
2. The industrial state-of-the-art in C2 LH separations
Steam cracking accounts for a large share of the energy used by the chemical industry because of the high temperatures required for the pyrolysis of hydrocarbons. Nevertheless, 35–50% of the energy used in C2H4 production comes from the fractionation, compression and separation processes required to produce pure C2H4.7 In a typical process, C2H4 and other steam cracking products are separated by cryogenic distillation at conditions as extreme as 183–258 K and 7–28 bar compounded with >100 tray numbers and reflux ratios of 2.5–4 for C2H4/C2H6 separation to meet polymer-grade specifications.10C2H2 is also used as a feedstock but its explosive nature makes liquefaction hazardous and compression above 1.4 bar is avoided, discouraging cryogenic purification. Selective gas–liquid absorption processes are commonly used, employing solvents such as N-methyl pyrrolidone, N,N-dimethyl formamide, methanol, ammonia and acetone. A pre-scrubbing process is used to remove higher alkynes which tend to polymerise. Purified C2H2 is recovered by depressurising the solvent and elevating temperature. This process can yield C2H2 of >98.4% purity. Further treatment with aqueous H2SO4 and NaOH allows for recovery of 99.7% pure C2H2.18
Although gas–liquid absorption has some advantages over cryogenic distillation, it nonetheless operates at temperatures and pressures significantly above ambient, poses risks in terms of hazardous solvents and pressurised C2H2, and has a substantial energy cost. Further, the poor selectivity of solvents like N-methyl pyrrolidone for C2H2 over CO2 (present in high abundance in raw C2H2 streams, especially from partial oxidation) necessitates additional scrubbing steps using ammonia and NaOH.18,48,49
Gas–liquid absorption methods are also used for the recovery of C2H6 from natural gas streams. The heavier impurities, such as propane and butane, are absorbed into a “lean” absorption oil, while the light C2H6 fraction remains in the natural gas stream. Although this approach is less energy intensive than cryogenic distillation, it has much lower efficiency, and cryogenic techniques are generally preferred in industry.50 The cryogenic technique involves cooling natural gas to 188 K using an expansion turbine coupled with a fractionating column and liquefying the C2 and heavier fractions while methane, CH4, remains in the natural gas stream.25
In summary, the industrial state-of-the-art for purification of C2H2, C2H4 and C2H6 involves energy-intensive processes that are conducted at non-ambient conditions and industrial purification of chemical products accounts for ca. 15% of global energy production. It is therefore unsurprising that replacing such processes with sorbent-based separations that yield high purity C2 LHs and operate at near-ambient conditions was highlighted by Scholl and Lively as one of the seven “separations to change the world.”3,51 The processes outlined above purify C2 LHs from a variety of impurities including CH4, heavier hydrocarbons, and sulphur compounds, as well as purifying C2H4 and C2H2 from by-products. Herein, we address how and why PCNs have recently become the benchmark physisorbents for several C2 binary separations: CO2/C2H2,47 C2H2/CO2,52–54 C2H2/C2H4,52,55,56 C2H4/C2H6,57–59 and C2H6/C2H4.60,61
3. Chronology of key discoveries in the utility of PCNs as C2 sorbents
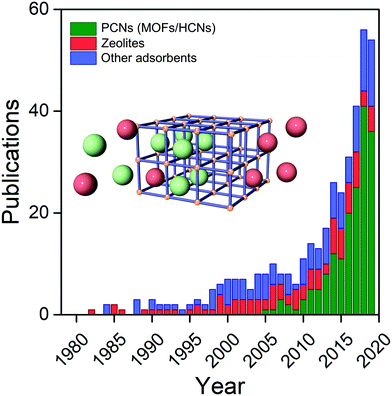
Interest in the utility of PCNs for C2 separations is a relatively recent phenomenon. As revealed by Fig. 3, the number of reported studies has grown exponentially over the past decade, especially since 2015. Prior to 2005, before PCNs were widely studied for gas separations, research tended to focus upon C2H4/C2H6, then considered the most important binary separation in industrial processes.3 In 2005, a 2D MOF, CPL-1, was reported by the Kitagawa group to possess excellent C2H2/CO2 selectivity and therefore offer potential for use in separations.62 To separate this pair of gas molecules, which exhibit identical kinetic diameters (Fig. 2), precise pore size/chemistry is needed, as subsequently demonstrated by several research groups (Fig. 4). For example, “Yin–Yang” separation of C2H2 and CO2 in two closely related HUMs (TIFSIX-2-Cu-i and SIFSIX-3-Ni) was realised in 2016 by the Zaworotko group thanks to the different pore structure of these two chemically related HUMs.47 In 2019, reverse C2H2/CO2 separation in two isostructural HUMs (SIFSIX-3-Ni and ZJUT-2) was achieved by B. Chen and Hu's groups.63 Most recently, two ultramicroporous PCNs (TCuCl and ZJU-74) were published by the Zaworotko and Qian groups, respectively.64 These materials were found to exhibit benchmark C2H2 capture performance from CO2 in terms of separation selectivity and uptake capacity, respectively.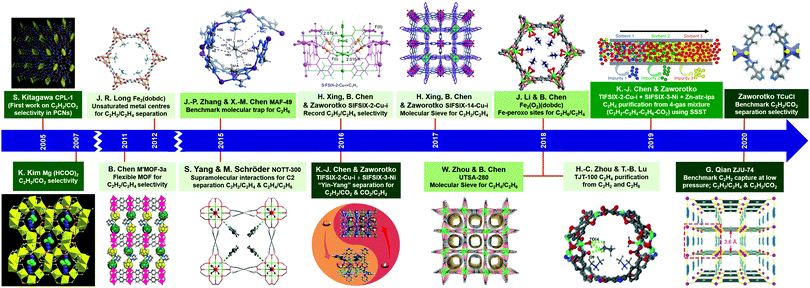 | ||
| Fig. 4 Chronology of the key developments in the design and separation/purification properties of PCNs for C2 LHs. (Reprinted with permissions from ref. 62, 69, 65, 66, 68, 67, 41, 47, 42, 57, 60, 70, 71, 53 and 64; copyright 2005, Springer Nature; copyright 2007, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2011, Springer Nature; copyright 2012, American Association for the Advancement of Science; copyright 2015, Springer Nature; copyright 2014, Springer Nature; copyright 2016, American Association for the Advancement of Science; copyright 2016, Elsevier Inc.; copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2018, Springer Nature; copyright 2018, American Association for the Advancement of Science; copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2019, American Association for the Advancement of Science; copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
For C2H2/C2H4 separation, high adsorption selectivity by a flexible PCN was reported in 2011 by B. Chen's group.65 In 2016, SIFSIX-1-Cu and SIFSIX-2-Cu-i were reported by the Xing, B. Chen and Zaworotko groups to deliver record-high C2H2 adsorption selectivity over C2H4.41 Another variant in this platform, SIFSIX-14-Cu-i (also known as UTSA-200a) was reported in 2017 to exhibit a sieving effect for C2H2 over C2H4.42 Regarding C2H4vs. C2H6, C2H4 selectivity in Fe-MOF-74 and NOTT-300 was reported by the Long and Schröder groups, respectively.66,67 These PCNs offer high C2H4 working capacities and moderate selectivity values. In 2018, the first, and thus far only, example of a C2H4 sieving PCN over C2H6, UTSA-280, was reported by B. Chen's group to exhibit ultra-high adsorption selectivity of >104.57 UTSA-280 also offers low production cost even when upscaled.
C2H6 selective adsorbents feature the advantage of incurring a minimal energy footprint during C2H4 production because a single-step adsorption process would purify C2H4 and replace the energy penalty for the regeneration process based upon C2H4 selective physisorbents. In this context, an azolate ultramicroporous material (AUM), MAF-49, first reported by Zhang and X.-M. Chen's group in 2015, was reported to exhibit record-high C2H6 adsorption energy and benchmark low-pressure uptake.68
In 2018, Fe-MOF-74 was post-synthetically modified with Fe–peroxo sites by B. Chen and Li's groups to afford Fe2(O2)(dobdc), which delivered inverse C2H6/C2H4 separation and continues to be the selectivity benchmark.60 To enable one-step C2H4 production, multiple impurities were removed in 2018 by an ionic PCN (TJT-100) via selective adsorption of C2H6 and C2H2 over C2H4. Zhou and Lu's findings on TJT-100 revealed co-adsorption of C2H6 and C2H2 to yield C2H4.70
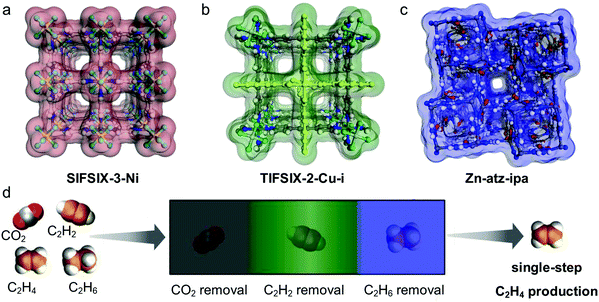
The discovery of sorbate-specific physisorbents that cover a range of sorbates and are selective enough for trace impurity removal suggests that it is now time to change focus from binary gas mixtures to multi-component gas mixtures. In principle, a single sorbent could be suitable for one-step separation of multiple minor impurities but would require high selectivity for several gases over the bulk component that is being purified. Alternatively, a series of custom sorbents, each one highly selective for one of the impurities in a gas mixture, would be expected to remove minor impurities in sequence. Such an approach, termed “synergistic sorbent separation technology” (SSST), was reported in 2019 through a collaboration between the groups of K. J. Chen and Zaworotko. Three ultramicroporous physisorbents (Zn-atz-ipa for C2H6 removal, SIFSIX-3-Ni for trace CO2 removal and TIFSIX-2-Cu-i for trace C2H2 removal) were packed in tandem in a single dynamic column breakthrough (DCB) setup and achieved one-step C2H4 production from a four-component gas mixture of C2H2/C2H4/C2H6/CO2. This report represents the prototypal example of SSST.71
Whereas Fig. 4 highlights the chronology of C2 separation-related discoveries, it is far from being an exhaustive account. The C2 separation literature continues to expand and is presented in more detail in Tables 1, 2, 3 and 4, which focus upon C2H2/C2H4, C2H4/C2H6, C2H6/C2H4, C2H2/CO2 and CO2/C2H2, respectively.
| Adsorbent, network dimensionality (nD) | S BET (m2 g−1) | Pore size (Å) | C2H2 uptake at 1 bar (mmol g−1) | C2H4 uptake at 1 bar (mmol g−1) | Q st(C2H2) at low loading (kJ mol−1) | S AE | Temperaturea (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
a Temperatures used in the determination of uptakes and SAE.
b IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (v/v) C2H2/C2H4.
c IAST selectivity at 1 bar for 1 99 (v/v) C2H2/C2H4.
c IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) C2H2/C2H4.
d IAST selectivity at lowest C2H2 loading for 1 1 (v/v) C2H2/C2H4.
d IAST selectivity at lowest C2H2 loading for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (v/v) C2H2/C2H4.
e Determined from CO2 isotherm recorded at 273 K.
f TCPE = tetrakis((4-carboxyphenyl)ethylene).
g Determined from Horvath–Kawazoe method or non-local density functional theory applied on N2 isotherm at 77 K.
h Pore size not defined due to post-synthetic metalation.
i IAST selectivities are qualitative, because of molecular sieving.
j Not applicable because of virial fits not conforming to stepped isotherms obtained at 298 and 273 K.
k Not mentioned.
l dps = 4,4′-dipyridylsulfide.
m Uptake ratio at C2H2/C2H4 (0.1/0.9). SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned. 99 (v/v) C2H2/C2H4.
e Determined from CO2 isotherm recorded at 273 K.
f TCPE = tetrakis((4-carboxyphenyl)ethylene).
g Determined from Horvath–Kawazoe method or non-local density functional theory applied on N2 isotherm at 77 K.
h Pore size not defined due to post-synthetic metalation.
i IAST selectivities are qualitative, because of molecular sieving.
j Not applicable because of virial fits not conforming to stepped isotherms obtained at 298 and 273 K.
k Not mentioned.
l dps = 4,4′-dipyridylsulfide.
m Uptake ratio at C2H2/C2H4 (0.1/0.9). SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned.
|
||||||||
| TIFSIX-14-Cu-i, 3D | 425 | 3.6 × 3.6 | 3.78 | 1.41 | 54 | 229b | 298 | 72 |
| GeFSIX-2-Cu-i, 3D | 467 | 4.5 × 4.5 | 3.9 | 2.2 | 42.6 | 67b | 298 | 73 |
| TIFSIX-2-Cu-i, 3D | 685 | 5.1 × 5.1 | 3.9 | 2.1 | 46 | 55b, 212.2c | 298 | 47 |
| SIFSIX-2-Cu-i, 3D | 503 | 5.2 × 5.2 | 4.02 | 2.19 | 52.9 | 44.54b, 41.01c | 298 | 41 |
| Ni-gallate, 3D | 424 | 3.5 × 4.9 | 3.59 | 1.97 | 46 | 43.7a | 298 | 74 |
| NbOFFIVE-2-Ni-i, 3D | 404 | 3.0 × 3.9 | 3.0 | 0.8 | 43 | 37.2b | 298 | 75 |
| NKMOF-1-Ni, 3D | 382 | 5.8 × 5.8 | 2.72 | 2.14 | 58 | 1272.6d, 30c | 298 | 76 |
| CPL-1, 3D | 414 | 4.0 × 6.0 | 2.07 | 0.31 | 40.2 | 26.75b | 298 | 77 |
| M′MOF-3a, 3D | 110 | 3.4 × 4.8 | 1.9 | 0.4 | 25 | 24.03b, 34.17c | 296 | 65 |
| Mg-gallate, 3D | 559 | 3.6 × 4.8 | 4.39 | 3.03 | 33 | 20.9a | 298 | 74 |
| UTSA-60a, 3D | 484 | 4.8 × 4.0 | 3.12 | 2.05 | 36 | 16b | 298 | 78 |
| Co-gallate, 3D | 475 | 3.7 × 5.0 | 3.88 | 3.37 | 47 | 15b | 298 | 74 |
| ELM-12, 2D | 706 | 4.3 × 3.9 | 2.56 | 1.0 | 25.4 | 14.8b | 298 | 79 |
| APPT-Cd-ClO4−, 3D | 205 | 11 × 11 | 1.75 | 0.44 | 28.6 | 14.71c | 298 | 80 |
| CPL-2, 3D | 495 | 9.0 × 6.0 | 3.13 | 1.86 | 30.8 | 12c | 298 | 77 |
| pacs-CoMOF-2a | 196 | 5.8g, 6.6g | 5.40 | 2.81 | 34.2 | 11.5b | 298 | 81 |
| UTSA-100a, 3D | 970 | 4.3 × 4.3 | 4.27 | 1.66 | 22 | 10.72b, 19.55c | 296 | 82 |
| SIFSIX-1-Cu, 3D | 1178 | 8.0 × 8.0 | 8.5 | 4.11 | 30/37 | 10.63b, 8.37c | 298 | 41 |
| UTSA-220, 3D | 577 | 4.5 × 4.1; 2.1 × 5.0 | 3.4 | 2.53 | 29 | 10b, 8.8c | 298 | 83 |
| SIFSIX-3-Zn, 3D | 250 | 4.2 × 4.2 | 3.64 | 2.24 | 21/31 | 8.82b, 13.72c | 298 | 41 |
| MUF-17, 3D | 247e | 3.1 × 3.5; 4.7 × 4.8 | 3.02 | 2.16 | 49.5 | 8.73c | 293 | 84 |
| JCM-1, 3D | 550 | 3.9 × 12.5 | 3.34 | 1.56 | 36.9 | 8.1c | 298 | 85 |
| Sr-TCPEf, 3D | NMk | 5.2 × 4.3; 5.9 × 5.2 | 1.52 | 0.9 | 29 | 8b | 298 | 86 |
| ZJU-198a, 3D | 343.1 | 3.6 × 4.1; 2.1 × 5.0 | 3.25 | 2.95 | 26.1 | 7.2c | 298 | 87 |
| UTSA-67a, 3D | 1136.7 | 3.3 × 3.3 | 5.13 | 2.81 | 32 | 6b | 298 | 88 |
| SIFSIX-2-Cu, 3D | 1881 | 10.5 × 10.5 | 5.38 | 2.02 | 26.3 | 6b, 4.95c | 298 | 41 |
| CPL-5, 3D | 523 | 11.0 × 6.0 | 3.01 | 1.84 | 31.3 | 6b | 298 | 77 |
| NBU-1, 3D | 368 | 3.8g | 3.64 | 2.07 | 38.3 | 5.9c | 298 | 89 |
| Ni-DCPTP, 3D | 857 | 6.7g, 10g | 6.54 | 4.48 | 38.9 | 5.5b | 298 | 90 |
| SIFSIX-3-Ni, 3D | 368 | 4.2 × 4.2 | 3.3 | 1.75 | 20.5 | 5.03b, 5.98c | 298 | 41 |
| HUST-6, 3D | 645.3 | NAh | 3.49 | 2.38 | 31.1 | 3.8c | 298 | 91 |
| Mg-MOF-74, 3D | 927 | 11 × 11 | 8.37 | 7.45 | 41 | 2.18b | 298 | 92 |
| NOTT-300, 3D | 1370 | 6.5 × 6.5 | 6.34 | 4.28 | 32 | 2.17b, 2.3c | 293 | 67 |
| Fe-MOF-74, 3D | 1350 | 11 × 11 | 6.8 | 6.1 | 46 | 2.08b, 2.1c | 318 | 66 |
| Co-MOF-74, 3D | 1018 | 11 × 11 | 8.17 | 7.02 | 45 | 1.7b | 298 | 92 |
| BUT-11, 3D | 1233 | 11g, 12.2g | 7.14 | 3.44 | 20 | NMi | 298 | 93 |
| Molecular sieves | ||||||||
| UTSA-300ai, 2D | 311 | 2.4 × 3.3 | 3.1 | 0.04 | 57.6 | ∼104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bj bj |
298 | 52 |
| NCU-100ai, 2D | 358 | 3.4 × 3.4 | 4.57 | 0.32 | 60.5 | 7291.3bj | 298 | 55 |
| bnn-1-Ca-H2Oi, 3D | 210 | 3.4 × 3.4 | 2.2 | 0.16 | NMk | 6966.4bj | 298 | 56 |
| SIFSIX-14-Cu-i , 3D | 612 | 3.4 × 3.4 | 1.8 | 0.6 | 40 | 6320bj | 298 | 42 |
| GeFSIX-14-Cu-ii, 3D | 424 | 3.0 × 3.0 | 4.1 | 0.76 | 43.6 | 1100bj | 298 | 73 |
| GeFSIX-dps-Cu,il 2D | 382 | 1.8 × 2.6; 2.5 × 4.4 | 4.28 | 0.16 | NMk | 19m | 298 | 94 |
| Adsorbent, network dimensionality (nD) | S BET (m2 g−1) | Pore size (Å) | C2H4 uptake at 1 bar (mmol g−1) | C2H6 uptake at 1 bar (mmol g−1) | Q st(C2H4) at low loading (kJ mol−1) | S C2H4/C2H6 | Temperaturea (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
a Temperatures used in the determination of uptakes and selectivities.
b IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) C2H4/C2H6.
c IAST selectivities are qualitative, because of molecular sieving.
d IAST selectivity at 0.01 bar for 1 1 (v/v) C2H4/C2H6.
c IAST selectivities are qualitative, because of molecular sieving.
d IAST selectivity at 0.01 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) C2H4/C2H6.
e Not mentioned.
f Determined from Horvath–Kawazoe method applied on N2 isotherm at 77 K.
g Ascribed to the combined effect of π-complexation and size-sieving.
h Atz = 3-amino-1,2,4-triazole.
i Equilibrium-kinetic combined selectivity.102
j Two consecutive reports on this sorbent document distinct values that are included using comma between them. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned. 1 (v/v) C2H4/C2H6.
e Not mentioned.
f Determined from Horvath–Kawazoe method applied on N2 isotherm at 77 K.
g Ascribed to the combined effect of π-complexation and size-sieving.
h Atz = 3-amino-1,2,4-triazole.
i Equilibrium-kinetic combined selectivity.102
j Two consecutive reports on this sorbent document distinct values that are included using comma between them. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned.
|
||||||||
| UTSA-280, 3D | 331 | 3.2 × 4.5; 3.8 × 3.8 | 2.5 | 0.098 | 34.1 | >104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bc bc |
298 | 57 |
| NUS-6(Hf)-Ag, 3D | 1027 | 10, 17 | 2.02 | 1.35 | 56.5 | 106.3d, 6b | 298 | 95 |
| ITQ-55, 3D | NMe | 2.07 × 5.86 | 1.28 | 0.76 | NMe | 90b | 303 | 96 |
| CuI@UiO-66-(COOH)2, 3D | 320 | 4.1f | 1.86 | 0.85 | 48.5 | 80.8bg | 298 | 58 |
| Co-gallate, 3D | 475 | 3.69 × 4.95 | 3.37 | 0.31 | 44 | 52b | 298 | 59 |
| NOTT-300, 3D | 1370 | 6.5 × 6.5 | 4.28 | 0.85 | 16 | 48.7b | 293 | 67 |
| Mg-gallate, 3D | 559 | 3.56 × 4.84 | 3.03 | 0.26 | 39 | 37.3b | 298 | 59 |
| PAF-1-SO3Ag, 3D | 783 | ∼8.0 | 4.06 | 2.23 | 106 | 27b | 296 | 97 |
| 10 wt% Ag/CPL-2, 3D | 12 | 7–11f | 0.9 | 0.15 | NMe | 26.1b | 298 | 98 |
| Fe2(m-dobdc), 3D | 1295 | 12 | 7.0 | 6.0 | 55 | 25b | 298 | 99 |
| Ni-gallate, 3D | 424 | 3.47 × 4.85 | 1.97 | 0.28 | 32 | 16.8b | 298 | 59 |
| NaETS-10, 3D | 289 | ∼8.0 | 1.7 | 1.3 | NMe | 14b | 298 | 100 |
| Fe-MOF-74, 3D | 1350 | 11 | 6.28 | 5.10 | 47.5 | 13.6b | 318 | 66 |
| ZnAtzPO4h,101 3D | 470 | 3.82 × 4.94 | 1.92 | 1.04 | 29.98 | 12.4i | 298 | 102 |
| (Cr)-MIL-101-SO3Agj, 3D | 1374, 1253 | NMe, 15–18f | 3.26, 4.32 | 1.47, 1.22 | 63, 120 | 9.7b, 16b | 296, 303 | 103 and 104 |
| 1.6AgM-DS, 3D | 846 | NMe | 3.37 | 0.94 | 59.2 | 9.5b | 298 | 105 |
| Co-MOF-74, 3D | 1341 | 11 | 6.21 | 5.25 | 43.6 | 5.82b | 318 | 106 |
| Mg-MOF-74, 3D | 927 | 11 | 7.4 | 6.4 | 42 | 5.6 | 296 | 92 |
| Zeolite 5A, 3D | 457–600 | ∼5.0 | 2.45 | 1.72 | 37 | 4.5b | 303 | 107 |
| NUS-36, 3D | 79.1 | NMe | 1.5 | 1.0 | 44 | 4.1b | 298 | 108 |
| HKUST-1, 3D | 1500–2100 | 10, 14 | 7.20 | 6.03 | 39 | 3.6b | 303 | 92 |
| UiO-66-ADC | 556 | 4.4 | 1.7 | 1.6 | 36 | 0.55b | 298 | 108 |
| Adsorbent, network dimensionality (nD) | S BET (m2 g−1) | Pore size (Å) | C2H6 uptake at 1 bar (mmol g−1) | C2H4 uptake at 1 bar (mmol g−1) | Q st(C2H6) at low loading (kJ mol−1) | S C2H6/C2H4 | Temperaturea (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
a Temperatures used in the determination of uptakes and selectivities.
b Pore size determined using published crystal structures.
c IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) C2H6/C2H4.
d Not mentioned.
e Pore limiting diameter.
f Largest pore opening.
g IAST selectivity at 1 bar for 1 1 (v/v) C2H6/C2H4.
d Not mentioned.
e Pore limiting diameter.
f Largest pore opening.
g IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v) C2H6/C2H4.
h IAST selectivity at 1 bar for 1 9 (v/v) C2H6/C2H4.
h IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (v/v) C2H6/C2H4. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned. 15 (v/v) C2H6/C2H4. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned.
|
||||||||
| Fe2(O2)(dobdc), 3D | 1073 | 7.6 × 7.6b | 3.3 | 2.6 | 66.8 | 4.4c | 298 | 60 |
| UTSA-30, 3D | 592 | 3.2 × 3.2b | 2.1 | 2.1 | 30 | 3.8c | 296 | 61 |
| Qc-5-Cu-sql-β, 2D | 240 | 3.3 × 3.3 | 1.8 | 0.8 | 37.6 | 3.4c | 298 | 109 |
| SBMOF-2, 3D | 195 | 3.6 × 3.6b | 2.8 | 2.7 | 32.3 | 3c | 298 | 110 |
| MAF-49, 3D | NMd | 3.3 × 3.0 | 1.7 | 1.7 | 60 | 2.7c | 316 | 68 |
| ZJU-30, 3D | 228 | 4.0 × 4.0; 5.6 × 5.6 | 2.1 | 2.0 | 29.7 | 2c | 298 | 111 |
| MUF-15, 3D | 1130 | 8.5 × 3.5; 7.0 × 3.8 | 1.7 | 1.7 | 29.2 | 1.95c | 298 | 112 |
| Y-BTC, 3D | 933 | 7.0 × 7.0 | 3.5 | 3.1 | 22 | 1.92c | 298 | 113 |
| PCN-250, 3D | 1470 | 5.5 × 5.5; 9.6 × 9.6 | 5.2 | 4.2 | 23 | 1.9c | 298 | 114 |
| C-PDA-3e, 3D | 3160 | NMd | 6.57 | 5.10 | 22 | 1.9c | 298 | 115 |
| Eu-BTC, 3D | 720 | 6.0 × 6.0 | 3.1 | 2.9 | 26 | 1.87c | 298 | 113 |
| IRMOF-8, 3D | 1360 | 11.0 × 11.0 | 4.1 | 2.9 | 54 | 1.8c | 298 | 116 |
| NUM-7a, 3D | 345 | 4.7 × 7.8 | 2.85 | 2.62 | 35.8 | 1.76c | 298 | 117 |
| CPM-733, 3D | 1328.5 | 7.3 × 7.3 | 7.1 | 6.4 | 23.4 | 1.75c | 298 | 118 |
| ZIF-8, 3D | 1844 | 3.5 × 3.5e; 11.6 × 11.6f | 2.5 | 1.5 | NMd | 1.7c | 293 | 119 |
| ZIF-4, 3D | 300 | 2.0 × 2.0e; 4.9 × 4.9f | 2.3 | 2.2 | NMd | 1.7c | 293 | 120 |
| SBMOF-1, 3D | 145 | 4.2 × 4.2 | 1.3 | 1.3 | 36.3 | 1.7c | 298 | 110 |
| Zn-atz-ipa, 3D | 650 | 2.8 × 2.8e; 5.5 × 5.5f | 1.8 | 1.8 | 45.8 | 1.7c | 298 | 71 |
| CPM-233, 3D | 1598 | 6.8 × 6.8 | 7.4 | 6.5 | 27.3 | 1.64c | 298 | 118 |
| JNU-2, 3D | 1219 | 3.7 × 3.7 | 4.1 | 3.6 | 29.4 | 1.6 | 298 | 121 |
| ZIF-7, 3D | 230 | 3.0 × 3.0e; 5.0 × 5.0f | 1.9 | 1.8 | NMd | 1.6c | 298 | 122 |
| UTSA-38, 3D | 1090 | 4.6 × 6.6 | 4.6 | 3.3 | 24.4 | 1.6c | 296 | 123 |
| [Ni(bdc)(ted)0.5], 3D | 1701 | 7.6 × 7.6; 5.1 × 3.7 | 5.0 | 3.4 | 21.5 | 1.6c | 298 | 124 |
| 1a-tz, 3D | 845 | 7.3 × 11.8 | 3.4 | 3.3 | 35 | 1.5c | 298 | 125 |
| MIL-142a, 3D | 1580 | 7.0 × 7.0 | 3.8 | 2.9 | 27.3 | 1.5c | 298 | 126 |
| Azole-Th-1, 3D | 983 | 10f | 4.5 | 3.6 | 28.6 | 1.46c | 298 | 127 |
| Zn-PNMI, 3D | 305 | 6.4 × 6.4b | 1.6 | 1.7 | 23.5 | 1.42g | 298 | 128 |
| In-soc-MOF-1, 3D | 1223 | 7.65 × 5.65; 10 × 10 | 4.0 | 3.7 | 28.4 | 1.4h | 298 | 129 |
| UTSA-33, 3D | 660 | 5.4 × 6.5; 4.8 × 5.8 | 2.8 | 2.7 | 32 | 1.4c | 296 | 130 |
| UTSA-35, 3D | 742 | 7.7 × 5.8 | 2.4 | 2.1 | 30 | 1.4c | 296 | 131 |
| Mn-PNMI, 3D | 818 | 8.0 × 8.0b | 2.8 | 2.0 | 24.5 | 1.38g | 298 | 128 |
| Cd-PNMI, 3D | 264 | 7.6 × 7.6b | 1.9 | 1.4 | 19.4 | 1.27g | 298 | 128 |
| TJT-100, 3D | 890 | 8.7 × 11.6 | 3.7 | 3.4 | 29 | 1.2c | 298 | 70 |
| Adsorbent, network dimensionality (nD) | S BET (m2 g−1) | Pore size (Å) | C2H2 uptake at 1 bar (mmol g−1) | CO2 uptake at 1 bar (mmol g−1) | Q st(C2H2) at low loading (kJ mol−1) | S AC | Temperaturea (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
a Temperatures used in the determination of uptakes and selectivities.
b IAST selectivity at 1 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) C2H2/CO2.
c Uptake ratio at 0.01 bar for 270 K measurements.
d Not mentioned.
e C2H2/CO2 uptake ratio at 0.5 bar.
f IAST selectivity at 0.15 bar for 1 1 (v/v) C2H2/CO2.
c Uptake ratio at 0.01 bar for 270 K measurements.
d Not mentioned.
e C2H2/CO2 uptake ratio at 0.5 bar.
f IAST selectivity at 0.15 bar for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) C2H2/CO2.
g Determined from Horvath–Kawazoe method applied on N2 isotherm at 77 K.
h Determined from CO2 isotherm at 195 K. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned.
i IAST selectivity at 1 bar for CO2/C2H2 (1 1 (v/v) C2H2/CO2.
g Determined from Horvath–Kawazoe method applied on N2 isotherm at 77 K.
h Determined from CO2 isotherm at 195 K. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned.
i IAST selectivity at 1 bar for CO2/C2H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture.
j Temperatures used in the determination of uptakes and SAE.
k IAST selectivity at 1 bar for CO2/C2H2 (1 1) mixture.
j Temperatures used in the determination of uptakes and SAE.
k IAST selectivity at 1 bar for CO2/C2H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture.
l Surface area calculated from CO2 195 K data.
m Desolvated phase pore size.
n MeOH solvated phase's pore size.
o Uptake ratio at 1 bar. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned. 2) mixture.
l Surface area calculated from CO2 195 K data.
m Desolvated phase pore size.
n MeOH solvated phase's pore size.
o Uptake ratio at 1 bar. SBET = Brunauer–Emmett–Teller (BET) theory based surface areas from N2 isotherm recorded at 77 K, unless otherwise mentioned.
|
||||||||
| (a) C2H2 selective adsorbents | ||||||||
| UTSA-300a, 2D | 311 | 2.4 × 3.3 | 3.3 | 0.2 | 57.6 | 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
298 | 52 |
| ZJU-74a, 3D | 694 | 3.6 × 3.8 | 3.83 | 3.08 | 44.5 | 36.5b | 298 | 53 |
| NKMOF-1-Ni, 3D | 382 | 5.8 × 5.8 | 2.7 | 2.3 | 60.3 | 30b | 298 | 76 |
| CPL-1, 2D | 571 | 4.0 × 6.0 | 1.9 | 0.07 | 42.5 | 26c | 270 | 62 |
| ZJU-196, 3D | NMd | 5.1 × 5.1 | 3.7 | 0.4 | 39.2 | 25e | 298 | 132 |
| FeNi-M′MOF, 3D | 383 | 4.15 × 4.27; 3.94 × 4.58 | 4.29 | 2.72 | 27 | 24b | 298 | 54 |
| [Ni3(HCOO)6]n, 3D | 289 | 4.3 × 4.3 | 2.4 | 1.6 | 40.9 | 22b | 298 | 133 |
| DICRO-4-Ni-i, 3D | 398 | 6.2 × 6.6 | 1.9 | 1.0 | 37.7 | 18.2e | 298 | 134 |
| TCuCl, 3D | 167 | 3.69 × 3.69 | 3.0 | 2.0 | 41 | 16.9b | 298 | 64 |
| pacs-CoMOF-2a | 196 | 5.8,g 6.6g | 5.40 | 2.81 | 34.2 | 13b | 298 | 81 |
| MIL-100(Fe), 3D | 2300 | 5.5 × 8.6 | 5.3 | 2.5 | 65 | 12.5e | 298 | 135 |
| ZJU-40a, 3D | 2858 | 10.2, 9.6 × 22.3 | 9.64 | 3.34 | 34.5 | 11.5b | 298 | 136 |
| Co-MOF, 3D | 973 | NMd | 6.47 | 2.68 | 33 | 11b | 298 | 137 |
| TIFSIX-2-Cu-i, 3D | 685 | 5.1 × 5.1 | 4.1 | 4.3 | 46 | 10e | 298 | 47 |
| JCM-1, 3D | 550 | 12.5 × 3.9 | 3.3 | 1.7 | 36.9 | 10b | 298 | 85 |
| ZJUT-2a, 3D | 350 | 3.2 × 3.2 | 3.4 | 2.2 | 41.5 | 10b | 298 | 63 |
| TCuBr, 3D | 173 | 3.59 × 3.59 | 2.8 | 2.0 | 36.6 | 9.5b | 298 | 64 |
| UTSA-74a, 3D | 830 | 8.0 × 8.0 | 4.8 | 3.2 | 31 | 9b | 298 | 138 |
| SNNU-150-Al, 3D | NMd | 8.5g | 4.33 | 1.98 | 29 | 7.27b | 298 | 139 |
| FJU-22a, 3D | 828 | 7.1 × 7.1 | 5.1 | 5.0 | 23 | 7.1f | 298 | 140 |
| ZJU-60a, 3D | 1627 | 4.4 × 5.4 | 6.7 | 3.3 | 17.6 | 6.7f | 298 | 141 |
| NTU-55, 3D | 2300 | 10.4g | 6.05 | 3.13 | 25 | 6.6f | 298 | 142 |
| UTSA-83a, 2D | 70h | 3.5 × 6.6 | 0.53 | 0.17 | 24.4 | 6.2b | 298 | 143 |
| MUF-17, 3D | 247 | 4.7 × 4.8 | 2.7 | 2.2 | 49.5 | 6b | 298 | 84 |
| CPM-107op, 3D | 319 | NMd | 4.35 | 1.55 | 37 | 5.7b | 298 | 144 |
| ZJNU-13, 3D | 1352 | 6.8g, 11.8g | 5.28 | 3.92 | 33.5 | 5.64b | 298 | 145 |
| PCP-33, 3D | 1248 | 11 × 20 | 5.4 | 2.6 | 27.5 | 5.6e | 298 | 146 |
| TCuI, 3D | 250 | 3.66 × 3.66 | 2.2 | 1.6 | 38.4 | 5.3b | 298 | 64 |
| UPC-110, 3D | 1384.3 | 6g | 3.27 | 1.08 | 24.6 | 5.1b | 298 | 147 |
| JXNU-5, 3D | 406 | 4.6g, 6.7g | 2.5 | 1.55 | 32.9 | 5b | 298 | 148 |
| Ag NP@Fe2O3@Zn-MOF-74, 3D | 936 | 7–10g | 6.7 | 5.13 | NMe | 4.73b | 293 | 149 |
| SNNU-45, 3D | 1006 | 4.5 | 5.98 | 4.33 | 40 | 4.5b | 298 | 150 |
| UTSA-220, 3D | 577 | 4.5–5.5; 3.1–4.8 | 3.40 | 3.38 | 29 | 4.4b | 298 | 83 |
| FJU-89a, 3D | 774 | 12 × 8 | 4.53 | 2.73 | 31 | 4.3b | 296 | 151 |
| FJU-90a, 3D | 1572 | 5.4 × 5.1 | 8.0 | 4.6 | 25.1 | 4.3b | 298 | 152 |
| Cu2(ade)2(PA)2, 3D | 401 | 2 × 6 | 2.19 | 1.5 | 26.8 | 4.2b | 298 | 153 |
| ZJU-199a, 3D | 987 | 5–7.5g | 5.71 | 2.78 | 38.5 | 4b | 296 | 154 |
| Hex-Zn-MOF 1a, 3D | 770.3 | 8.6g, 9.8g | 3.18 | 2.21 | 39 | 4b | 298 | 155 |
| mot-Cu(Br-BDC) MOF, 3D | 303 | 4.2 × 4.7; 12 × 24.1 | 1.53 | 1.08 | 26.1 | 3.9b | 298 | 156 |
| Cu-CPAH, 3D | 880 | 6–9g | 5.88 | 3.93 | 35.4 | 3.6b | 298 | 9 |
| NBU-3-Mn/Fe, 3D | 551 | NMd | 3.03 | 1.61 | 29 | 3.9b | 273 | 157 |
| UTSA-68a, 3D | 1954 | 6.5 × 6.5; 7.5 × 9.5 | 3.13 | 1.77 | 25.8 | 3.4b | 296 | 158 |
| UPC-200(Al)-F-BIM, 3D | 2212.8 | 7 × 11 | 6.2 | 2.5 | 20.5 | 3.15b | 298 | 159 |
| JNU-1, 3D | 818 | 16.3 × 6.6 | 2.7 | 2.2 | 13 | 3b | 298 | 160 |
| Cu-tztp MOF 1a, 3D | 798.9 | 5.4–8.6g | 5.02 | 3.35 | 38.3 | 2.7b | 298 | 161 |
| Zn-MOF-74, 3D | 1360 | 11 × 11 | 5.5 | 5.4 | 22.1 | 2b | 298 | 138 |
| ZJU-30a, 3D | 228 | 4.0 × 4.0; 5.6 × 5.6 | 2.31 | 1.87 | 31.3 | 1.7b | 296 | 158 |
| Adsorbent, network dimensionality (nD) | S BET (m2 g−1) | Pore size (Å) | CO2 uptake at 1 bar (mmol g−1) | C2H2 uptake at 1 bar (mmol g−1) | Q st(CO2) at low loading (kJ mol−1) | S CA | Temperaturej (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
| (b) CO2 selective adsorbents | ||||||||
| Tm(OH-bdc), 3D | 923 | 6.3 × 9.3; 6.3 × 10.6 | 5.8 | 2.0 | 45.2 | 17.5k | 298 | 162 |
| CD-MOF-2, 3D | 922 | 4.2 × 4.2, 7.8 × 7.8 (windows); 17 × 17 (cage) | 2.7 | 2.0 | 67.2 | 16.6k | 298 | 163 |
| Mn(bdc)(dpe), 3D | 535l | 3.3 × 3.5 | 2.1 | 0.3 | 29 | 8.8 | 273 | 164 |
| SIFSIX-3-Ni, 3D | 368 | 4.2 × 4.2 | 2.7 | 3.3 | 50.9 | 7.7 | 298 | 47 |
| CD-MOF-1, 3D | 1094 | 4.2 × 4.2, 7.8 × 7.8 (windows); 17 × 17 (cage) | 2.9 | 2.2 | 41.0 | 6.6k | 298 | 163 |
| K2[Cr3O(OOCH)6(4-ethylpyridine)3]2[α-SiW12O40], 0D | 75l | 2.6 × 2.6m; 3.5 × 3.5n | 2.4 | 0.5 | ca. 39 | 4.8o | 278 | 165 |
4. Separation of C2 gas mixtures by PCN sorbents
Whereas Section 3 details a chronology of the development of PCNs and highlights some key discoveries in the context of physisorbents with highly selective C2 binding sites, Section 4 presents an in-depth survey of the key structural and property parameters in the full range of PCNs that have been studied for C2 separations. PCN physisorbents and other classes of C2 sorbents are organised in tabular form according to parameters reported for the four most widely studied binary C2 separations: C2H2/C2H4 (Table 1); C2H4/C2H6 (Table 2); C2H6/C2H4 (Table 3); C2H2/CO2 and CO2/C2H2 (Table 4). Whereas no attempt is made to analyse the data in Sections 4 and 5 focuses upon analysis of the structural and chemical driving forces for selective molecular recognition with emphasis upon two aspects: the types of binding sites in PCNs that are key to strong C2 separation performance; how, once a binding site is recognised and understood, crystal engineering approaches can be exploited to fine-tune first generation sorbents in order to further enhance selectivity and separation performance in the second generation of sorbents.5. Crystal engineering of PCNs: in search of the optimal binding site
Section 4 tabulates some of the key structure and property parameters that are relevant to C2 LH separations (Tables 1–4). Now we address the various mechanisms that can drive selectivity (Sections 5.1–5.5) and present representative examples of binding sites (Section 5.6). That the availability of a new generation of highly selective PCN sorbents can enable C2 LH separation from multi-component gas mixtures is discussed in Section 5.7, in which the concept of SSST is explained.The modularity of PCNs is key to their enormous diversity of pore size, structure and chemistry and their amenability to crystal engineering strategies once a parent sorbent or “first generation” sorbent is identified. In essence, the modularity of PCNs enables platforms or families of closely related PCNs to be generated in a systematic manner. Structure–function relationships can then be extrapolated as fine tuning of pore size and pore chemistry is feasible in a manner that is infeasible for other classes of porous physisorbents such as zeolites. For example, first generation HUMs such as SIFSIX-3-Zn and SIFSIX-2-Cu-i offered more than an order of magnitude improvement for CO2/N238 and C2H2/C2H441 capture, respectively. The level of control that can be exerted over the pore environment in such HUMs has in a short time enabled the second generation of HUMs to exhibit a further order of magnitude improvement in selectivity towards CO2, C2 and C3 LHs.42,166–168 Two main factors contribute to the benchmark performance of HUMs: tight-fit binding pockets (pore diameter ≤0.7 nm, sometimes ≤0.4 nm); strong electrostatics from inorganic anions, e.g. MoO42−, SiF62−, TiF62− that serve as linkers/pillars.28 In essence, “lock-and-key” molecular recognition can occur in a manner that mimics selective substrate binding in enzymes. More generally, for hard-to-separate C2 LH pairs (Fig. 2), LHs are physisorbed in PCN pores and can preferentially interact with binding sites through strong electrostatics, weak van der Waals forces, sorbate-unsaturated metal centre (UMC) interactions, hydrogen bonding (H-bonding) interactions or a combination thereof.169 Binding site driven separations can be classified as equilibrium separations. Non-equilibrium separations are also possible with PCNs and would be driven by kinetics or molecular sieving.10 Overall, thermodynamics, kinetic effects and steric considerations have all been shown to contribute as driving forces for adsorptive C2 separations by physisorbents.
The rapid increase in the frequency of reports of C2 separation and the ever-improving performance benchmarks mean that there is now a body of understanding about structure–function with respect to which types of binding sites are selective to a particular C2 LH. There is also realisation that a high density of strong and, ideally, single binding sites can lead to commensurate packing of sorbate molecules. When these features are both in play, a PCN is primed to exhibit strong C2 LH separation performance.
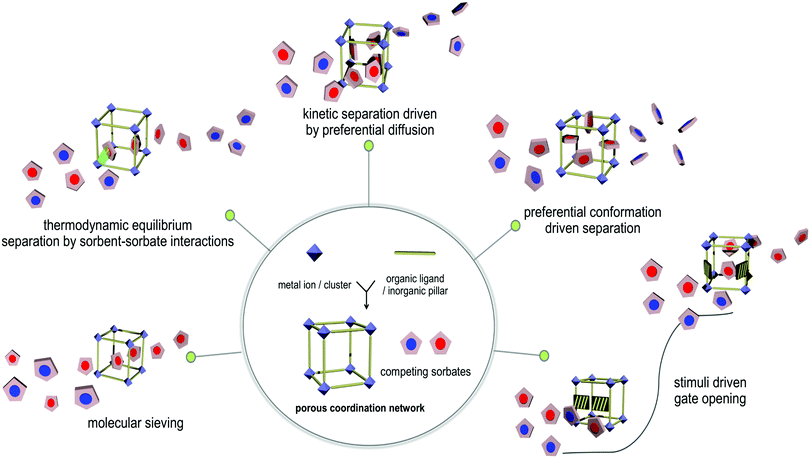
When one considers the full range of sorbents that have been studied, i.e. zeolites, activated carbons, mesoporous silica and PCNs (Fig. 3), preferred gas binding can be classified a being the consequence of one of five distinct mechanisms as follows: (a) size-exclusion guided molecular sieving; (b) thermodynamic equilibrium separation dictated by sorbent–sorbate binding; (c) differential diffusion to elicit kinetic i.e. non-equilibrium separation;170 (d) conformational preference for one of the C2 LHs; (e) stimulus-induced separation, often facilitated by structural flexibility in a breathing or switching PCN. We highlight these modes through prototypal examples below.
5.1. Unsaturated metal centre (UMC) driven binding of unsaturated LHs
That an olefin such as C2H4 possesses unsaturated carbon–carbon double bonds makes it behave differently versus the competing paraffin e.g. C2H6 in terms of binding to metal centres. This difference is driven by the diffuse π-orbitals of C2H4 that can result in selective binding interactions with metal centres that line the pore surfaces of some families of PCNs.PCNs can feature pore walls lined with coordinatively unsaturated metal centres (UMCs) and are therefore predisposed to preferentially bind to olefins over paraffins. Most typically, UMCs in as-synthesised PCNs are bonded to solvent molecules but activation results in removal of the solvent molecules and leads directly to the generation of UMCs that can interact with sorbates; interaction strength contingent on the relative electron densities of the UMCs.
Acetylene sorption studies on HKUST-1 conducted by B. Chen et al. resulted in structural determination of the C2H2 binding sites with Cu(II) UMCs (Fig. 6a).171 HKUST-1 was earlier identified as being C2H4/C2H6 selective.172 However, both C2 LHs are adsorbed by the Cu(II) UMCs in HKUST-1. The adsorption enthalpies (Qst) are relatively low at ca. 32 kJ mol−1 with [Qst(C2H4) − Qst(C2H6)] being <2 kJ mol−1. Modest selectivity was thereby observed.173 Nevertheless, the proof-of-principle established and a computational study174 led Long's group to explore the UMC rich PCN family M-MOF-74 (also known as CPO-27-M, M2(dhtp), or M2(dobdc); M = Mg, Mn, Fe, Co, Ni, Zn; dobdc4− = 2,5-dioxido-1,4-benzenedicarboxylate) for C2H2/C2H4 and C2H4/C2H6 separations.66,92 Fe-MOF-74 was found to exhibit the highest equimolar IAST selectivities of 2.08 and 13.6 for C2H2/C2H4 and C2H4/C2H6 respectively, in this family. The 1D hexagonal channels of ca. 11 Å are replete with a high density of UMCs that allow a limited degree of π-backbonding (Fig. 6b), despite the high-spin electronic configurations of transition metals in the respective M-MOF-74 analogues.175 Topological and structural analogues of M-MOF-74, M2(m-dobdc) MOFs (M = Mg, Mn, Fe, Co, Ni, Zn; m-dobdc4− = 4,6-dioxido-1,3-benzenedicarboxylate) were found to exhibit strong C2H4/C2H6 selectivity of ∼25 in Fe2(m-dobdc).99 Enhanced π-backbonding resulted in shorter M–Colefin distances and was cited as the key factor behind enhanced performance.176
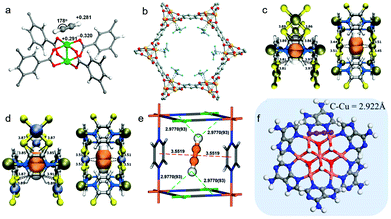 | ||
| Fig. 6 Examples of binding of unsaturated C2 LHs to unsaturated metal centres in PCNs: (a) C2H2 in HKUST-1 as determined by DFT calculations;171 (b) C2D4 in Fe-MOF-74 as determined by experimental NPD data;66 (c) C2H2 in NKMOF-1-Ni as determined by DFT calculations;76 (d) C2H2 in NKMOF-1-Cu as determined by DFT calculations;76 (e) C2D2 in FeNi-M′MOF as determined by experimental NPD data;54 (f) C2H2 in NBU-1 as determined by DFT-D calculations.89 The labelled distances are measured in Å. (Reprinted with permissions from ref. 171, 66, 76, 54 and 89; copyright 2009, American Chemical Society; copyright 2012, American Association for the Advancement of Science; copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2019, American Chemical Society.) | ||
Olefin-selective binding in PCN physisorbents by UMCs has been reported in subsequent studies (Tables 1–4), including NKMOF-1-Ni,76 NBU-189 and FeNi-M′MOF.54 Two ultramicroporous MOFs, NKMOF-1-M, Cu[M(pdt)]2 (M = Cu(II), Ni(II); pdt = pyrazine-2,3-dithiol) were introduced as C2 sorbents by Zhang's group in 2018. NKMOF-1-Ni was found to exhibit benchmark C2H2/C2H4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) selectivity of 1272.6 at low C2H2 coverage.76 A combination of ultramicropores (5.75 Å) and square planar Ni(II) UMC sites might have been expected to be responsible for C2H2-selective binding and the Qst(C2H2) value of ∼58 kJ mol−1. However, analysis by dispersion-corrected density functional theory (DFT-D) and Grand Canonical Monte Carlo (GCMC) modelling attributed the strong C2H2 binding to hydrogen bonding (HC
99) selectivity of 1272.6 at low C2H2 coverage.76 A combination of ultramicropores (5.75 Å) and square planar Ni(II) UMC sites might have been expected to be responsible for C2H2-selective binding and the Qst(C2H2) value of ∼58 kJ mol−1. However, analysis by dispersion-corrected density functional theory (DFT-D) and Grand Canonical Monte Carlo (GCMC) modelling attributed the strong C2H2 binding to hydrogen bonding (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH⋯S(MOF)) and π–π interactions between C2H2 and pyrazines from pdt ligands. Ni(II) or Cu(II) UMCs residing between the adjacent MS4 units were deemed responsible for a second but weaker binding site for selective binding to C2H2 (Fig. 6c and d).
CH⋯S(MOF)) and π–π interactions between C2H2 and pyrazines from pdt ligands. Ni(II) or Cu(II) UMCs residing between the adjacent MS4 units were deemed responsible for a second but weaker binding site for selective binding to C2H2 (Fig. 6c and d).
B. Chen and colleagues also exploited two distinct binding modes in a Hofmann-type PCN FeNi-M′MOF, ([Fe(pyz)Ni(CN)4], pyz = pyrazine) with Ni(II) UMCs and cyanide-linked ultramicropores of ∼4.0 Å diameter. High C2H2/CO2 IAST selectivity of ∼24 was calculated for ambient conditions.54 Uptake capacity of 4.54 mol L−1 during separation experiments from an equimolar C2H2/CO2 mixture at 298 K and 1 bar makes FeNi-M′MOF second behind the benchmark sorbent UTSA-74 (4.86 mol L−1).138 DFT-D modelled structures and high-resolution neutron powder diffraction (NPD) experiments indicated preferential distribution of C2D2 between the two pyz rings through π–π stacking with multiple intermolecular Dδ+⋯Nδ− and Cδ+⋯Nδ− interactions between C2D2 and FeNi-M′MOF (Fig. 6e).
UMC driven LH selectivity was also studied by H.-C. Zhou's group, who reported the highest kinetic separation efficiency for C2H2/C2H4 in the ultramicroporous sorbent NBU-1, (NH4){CuII3·[CuIICuI6(OH)6(Ad)6]2}·xH2O (Ad = adenine). The strong performance was attributed to its mixed-valence heptanuclear UMC-rich copper clusters and Lewis base adsorption sites. Spin-polarised DFT-D calculations revealed that, unlike the sorption mechanism shown by single Cu(II) UMCs, the C2H2 molecules in NBU-1 bind to the d-electron rich regions of two adjacent Cu(I) centres (Fig. 6f).89 Other notable examples of UMC-driven C2 separations in PCNs include UTSA-74a,138 ZJU-60a,141 PCP-33.146
5.2. Hydrogen bonded binding sites
The presence of functional groups, particularly Lewis base moieties such as amines and82,88,136 inorganic pillars such as SiF62−,41,42,52,73,76 on the Connolly surfaces of PCN sorbents has evolved as a paradigm to enhance C2 adsorption capacity and selectivity. As mentioned earlier, Kitagawa's group introduced the prototypal C2H2 selective sorbent in 2005, CPL-1 i.e. [Cu2(pzdc)2(pyz)] (pzdc = pyrazine-2,3-dicarboxylate). This low-surface area (ca. 571 m2 g−1) PCN exhibited uptakes consistent with strong C2H2/CO2 selectivity (uptake ratio ∼26 at 270 K).62 Maximum entropy method (MEM)/Rietveld analysis of CPL-1 revealed C2H2 molecules residing at periodic distances from one another sustained by H-bonding between two non-coordinated oxygen atoms of pzdc ligands and each of the two H-atoms of C2H2 (Fig. 7a). The C2H2-specific sorption of CPL-1 was attributed to a combination of electrostatic attractions and electron delocalization effects between C2H2(C–H) and O–C(sorbent), an example of a guest ‘confinement effect’ to elicit stoichiometric C2H2 trapping. O-donor based selective C2H2 binding has also been seen in a number of recent reports, including FJU-22a,140 TJT-10070 and JCM-1.85 In a related approach, amine introduction into ultramicropores in the prototypal AUM MAF-49, [Zn(batz)] (H2batz = bis(5-amino-1H-1,2,4-triazol-3-yl)methane), resulted in one of the first reports of C2H6 selective sorption from C2 LH mixtures.68 Strong C2H6 binding was manifested by high Qst(C2H6) ∼ 60 kJ mol−1 and the then benchmark C2H6/C2H4 selectivity was attributed to three strong C–H⋯N hydrogen bonds and three weak C–H⋯N electrostatic interactions (Fig. 7b).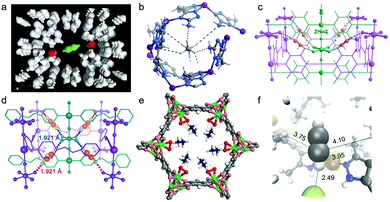 | ||
| Fig. 7 Illustrations of preferential H-bonded binding sites: (a) C2H2 in CPL-1 as determined by MEM/Rietveld analysis;62 (b) C2H6 in MAF-49 as determined by DFT calculations;68 (c) C2H2 in SIFSIX-2-Cu-i as determined by DFT-D calculations;41 (d) C2D2 in SIFSIX-14-Cu-i as determined by experimental NPD data;42 (e) C2D6 in Fe2(O2)(dobdc) as determined by experimental NPD data;60 (f) C2H2 in TCuCl as determined by simulated annealing.64 (Reprinted with permissions from ref. 62, 68, 41, 42, 60 and 64: copyright 2005, Springer Nature; copyright 2015, Springer Nature; copyright 2016, American Association for the Advancement of Science; copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2018, American Association for the Advancement of Science; copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
A key discovery concerning purification of C2H4 was realised by H. Xing, B. Chen and Zaworotko's collaborative studies on HUMs which included both non-interpenetrated and interpenetrated HUMs (i-HUMs). They reported a design and property breakthrough in terms of pore size and pore chemistry.41 From the sorbent design perspective, the HUMs studied each exhibit pores lined with hexafluorosilicate (SIFSIX) anions. From the property perspective, whereas the previous benchmark for C2H2/C2H4 selectivity exhibited an IAST selectivity of only 2.08 (Table 1),66 this family of HUMs, which comprises M(II)–Nheterocyclesql topology nets pillared by SIFSIX anions, resulted in more than an order of magnitude improvement in selectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 IAST selectivity at 1 bar, SAE ∼ 44.54) for the prototypal i-HUM, SIFSIX-2-Cu-i, a sorbent that exhibits 2-fold interpenetration. This exceptional selectivity was driven by exposed SIFSIX moieties that enable CH⋯F bonding to both sides of C2H2 molecules (Fig. 7c). More importantly, C2H2 binding was found to be markedly different in related materials such as SIFSIX-1-Cu, [Cu(SiF6)(bpy)2], which adsorbed 8.5 mmol g−1 of C2H2 at 298 K and 1 bar, ca. twice that of the larger-pore HUM SIFSIX-2-Cu [Cu(SiF6)(py2C2)2; py2C2 = 4,4′-dipyridylacetylene].41 However, the latter HUMs are just moderately C2H2 selective over C2H4 (SAE ∼ 10.6 and 6.0, respectively; Table 1) whereas SIFSIX-2-Cu-i binds C2H2 strongly with Qst(C2H2) = 52.9 kJ mol−1, a consequence of the aforementioned H-bonding interactions. Dynamic column breakthrough (DCB) experiments conducted upon SIFSIX-2-Cu-i yielded high-purity ethylene with C2H2 concentrations as low as 2 ppm. Substitution of linker 2 (py2C2) in SIFSIX-2-Cu-i with 4,4′-azopyridine (14) afforded the second generation HUM variant SIFSIX-14-Cu-i, which exhibits trace C2H2 capture from a 1
99 C2H2/C2H4 IAST selectivity at 1 bar, SAE ∼ 44.54) for the prototypal i-HUM, SIFSIX-2-Cu-i, a sorbent that exhibits 2-fold interpenetration. This exceptional selectivity was driven by exposed SIFSIX moieties that enable CH⋯F bonding to both sides of C2H2 molecules (Fig. 7c). More importantly, C2H2 binding was found to be markedly different in related materials such as SIFSIX-1-Cu, [Cu(SiF6)(bpy)2], which adsorbed 8.5 mmol g−1 of C2H2 at 298 K and 1 bar, ca. twice that of the larger-pore HUM SIFSIX-2-Cu [Cu(SiF6)(py2C2)2; py2C2 = 4,4′-dipyridylacetylene].41 However, the latter HUMs are just moderately C2H2 selective over C2H4 (SAE ∼ 10.6 and 6.0, respectively; Table 1) whereas SIFSIX-2-Cu-i binds C2H2 strongly with Qst(C2H2) = 52.9 kJ mol−1, a consequence of the aforementioned H-bonding interactions. Dynamic column breakthrough (DCB) experiments conducted upon SIFSIX-2-Cu-i yielded high-purity ethylene with C2H2 concentrations as low as 2 ppm. Substitution of linker 2 (py2C2) in SIFSIX-2-Cu-i with 4,4′-azopyridine (14) afforded the second generation HUM variant SIFSIX-14-Cu-i, which exhibits trace C2H2 capture from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2
99 C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4 mixture thanks to near-ideal molecular sieving.42 Typical of a molecular sieve, the record high IAST selectivity of 6320 at 1 bar (1
C2H4 mixture thanks to near-ideal molecular sieving.42 Typical of a molecular sieve, the record high IAST selectivity of 6320 at 1 bar (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4) and doubling of C2H4 production capacity compared to SIFSIX-2-Cu-i represented a significant breakthrough, more than an incremental improvement. Each adsorbed C2D2 interacts with two SiF62− anions from different interpenetrating nets through cooperative C–D⋯F H-bonds, the length of these bonds (1.921 Å) being smaller than those in SIFSIX-2-Cu-i (2.015 Å). These distances are reflective of stronger H-bonding interactions in the narrower-pore azopyridine HUM (Fig. 7d).
99 C2H2/C2H4) and doubling of C2H4 production capacity compared to SIFSIX-2-Cu-i represented a significant breakthrough, more than an incremental improvement. Each adsorbed C2D2 interacts with two SiF62− anions from different interpenetrating nets through cooperative C–D⋯F H-bonds, the length of these bonds (1.921 Å) being smaller than those in SIFSIX-2-Cu-i (2.015 Å). These distances are reflective of stronger H-bonding interactions in the narrower-pore azopyridine HUM (Fig. 7d).
The microporous MOF Fe2(O2)(dobdc) was recently reported by J. Li and B. Chen's group and binds ethane with a high Qst(C2H6) ∼ 67 kJ mol−1, leading to SC2H6/C2H4 of 4.4 for an equimolar mixture at 298 K and 1 bar. Breakthrough experiments using an equimolar mixture of C2H6 and C2H4 by a single DCB column of Fe2(O2)(dobdc) yielded polymer-grade C2H4 as effluent, with 99.99% purity. Prepared by addition of O2 to Fe2(dobdc), Fe2(O2)(dobdc) features η2-bound peroxo–Fe(II) sites, and NPD analysis recorded at 7 K indicated that these sites couple with electronegative surface oxygen distributions to engage in close contacts with –CH3 groups of the adsorbed ethane molecules (Fig. 7e). A downside of Fe2(dobdc) and Fe2(O2)(dobdc) is that they are air sensitive and must be handled in a moisture-free environment.
The benchmark C2H2 selectivity of i-HUMs such as SIFSIX-2-Cu-i,41TIFSIX-2-Cu-i,47 GeFSIX-2-Cu-i,73 NbOFFIVE-2-Ni-i,75SIFSIX-14-Cu-i,42 TIFSIX-14-Cu-i,72 GeFSIX-14-Cu-i73 is credited to cooperative C–H⋯F hydrogen bonding between acetylene and the inorganic pillars. Halide ligands bound to Cu(I) in an isostructural family of ultramicroporous MOFs, TCuX (X = Cl, Br, I), [Cu(TMBP)X] (TMBP = 3,3′,5,5′-tetramethyl-4,4′-bipyrazole) were also found to exhibit strong C2H2 binding driven by C–H⋯X H-bonds (Fig. 7f).64 A new benchmark for C2H2/CO2 separation selectivity was found for TCuCl with relative selectivities consistent with the H-bonding strength: C–H⋯Cl (2.49 Å) < C–H⋯Br (2.57 Å) < C–H⋯I (2.80 Å).
5.3. Olefin-π complexation to Ag(I) and Cu(I)
The first metal–olefin complex, platinum(II)–ethylene, Zeise's salt, can be traced back to 1827.177 Dewar, Chatt and Duncanson developed178 a π-back bonding model for such complexation (Fig. 8) which can be exploited to generate olefin-selective sorbents. Among transition metals that exhibit π-complexation with C2H4, Ag is the most widely used followed by Cu. Rather than physisorption, the binding here is regarded as reactive absorption via gas/liquid contact.179 Regardless of the generality of this approach, it was adjudged inefficient because of the weak contact between LH gases and liquid absorbents.180 In 2008, the nonporous compound, Ag2[Cr3O(OOCC2H5)6(H2O)3]2[α-SiW12O40], which is comprised of 2D layers of polyoxometalates and macrocations, exploited C2H4 complexation to exhibit strong C2H4/C2H6 sorption selectivity (uptake ratio >100 at 298 K and 1 bar).179 Silver-exchanged zeolite A (AgA) revealed size-selective molecular sieving of C2H6 and this “absolute” C2H4 selective sorbent was shown to be recyclable through vacuum and/or temperature swing experiments.181 Porous aromatic frameworks (PAFs) were also used to demonstrate this strategy in PAF-1-SO3Ag (SC2H4/C2H6 = 27). Sorption/selectivity experiments with PAF-1 (SC2H4/C2H6 = 0.7) and PAF-1-SO3H (SC2H4/C2H6 = 0.88) underscored the profound role of Ag-complexation behind the enhanced C2H4 selectivity.97 B. Chen and S. Ma's groups used this complexation strategy in mesoporous MIL-101, (Cr)-MIL-101-SO3Ag, leading to SC2H4/C2H6 = 16 versus the control variant, (Cr)-MIL-101-SO3H = 1.15.103,104 Zhao and co-workers further pursued this approach on a microporous Hf MOF, NUS-6(Hf)-Ag (SC2H4/C2H6 = 6) vs. that of NUS-6 (SC2H4/C2H6 = 0.9).95 Related reports include a study of CPL-2 (SC2H4/C2H6 = 1.4) modified to 10 wt% Ag/CPL-2 (SC2H4/C2H6 = 26.1)98 and 1.6AgM-DS.105 Qian's group recently extended this approach to the Cu(I) chelated physisorbent CuI@UiO-66-(COOH)2, which combines olefin complexation with controlled pore size to enable molecular sieving exclusion of C2H6 and SC2H4/C2H6 of 80.8.58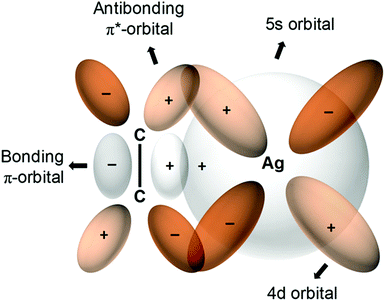 | ||
| Fig. 8 π-Complexation between an olefin such as C2H4 and Ag(I) ions182 results in enhanced C2H4/C2H6 selectivities in several PCNs. (Reprinted with permission from ref. 183: copyright 2018, American Chemical Society.) | ||
5.4. Flexible coordination networks
Several flexible PCNs with gated pores have been reported to achieve efficient separation of C2 LHs via gas-specific induced gate-opening. Unlike the canonical Langmuir model driven type I isotherms in rigid physisorbents, flexible PCNs are characterised by characteristic gating isotherms with five distinct isotherm types (F-I to F-V).184 A ‘step’ refers to a sudden increase in uptake at a threshold pressure that results from flexibility or a phase change of the adsorbent. Flexible PCNs that feature stepped type F-IV isotherms, which transform from non-porous to porous phases, can offer higher working capacity vs. rigid PCNs.185 The type F-IV C2 isotherms exhibited by ZIF-7 [Zn(bim)2, bim− = benzimidazolate] at ambient temperature feature lower gate-opening pressure for C2H6 than C2H4, making it an early example of an ethane-selective PCN (Table 3).122 Leveraging this C2H6 selectivity, C2H6/C2H4 separation performance was confirmed by equimolar binary DCB experiments. That C2H6 adsorption revealed a more exothermic profile versus C2H4 adsorption over the entire C2 sorption coverage can explain why gate opening occurred more readily for C2H6. With respect to sorbent–sorbate binding, C2H6 is thought to maximize van der Waals (vdW) interactions with the Connolly surface thanks to its 3-fold rotational symmetry matching that of ZIF-7 ultramicropores (pore limiting dimeter: 3.0 Å; largest pore opening: 5.0 Å) (Fig. 9a).122,186 Whereas H-bonding was identified as the key factor in realising C2H2 selectivity over CO2 in CPL-1 (Section 5.2),62 this sorbent exhibited an abrupt step increase in its C2H4 adsorption isotherm at 273 K and ∼2 bar. No step was noticed for C2H6 at 273 K, despite subjecting it to an elevated pressure of ∼10 bar.187 DCB experiments at 8 bar and 273 K demonstrated effective C2H4/C2H6 separation. Optimised geometries of C2H4 and C2H6 were consistent with the C2H2 binding modes earlier obtained via MEM/Rietveld analysis.62 An allosteric pore-opening mechanism for C2H4 selective sorption over C2H6 was observed in the dehydrated and guest-free, nonporous phase of the PCN [Co(vttf)]n {vttf2− = 2,2′-[1,2-bis(4-benzoate)-1,2-ethanediylidene]bis-1,3-benzodithiole}.188 The PCN structure is crosslinked by the coordination of tetrathiafulvalene sulphur atoms to the axial sites of Co2(COO)4 paddlewheels. Whereas [Co(vttf)]n is unresponsive to ethane, exposure to ethylene induces a cooperative transition driven by coordination to Co(II). This in turn displaces the tetrathiafulvalene linkers to afford an open architecture. Once open, [Co(vttf)]n co-adsorbs both C2 gases, resulting in only modest selectivity. Co-adsorption of multiple components represents an oft-encountered issue for flexible PCNs in separating C2 LH mixtures, especially when high purity in the sorbed phase is required.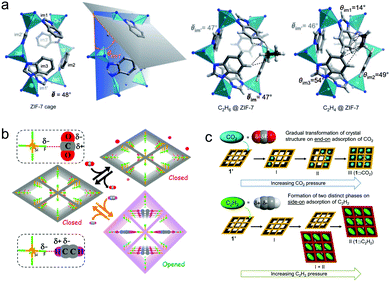 | ||
| Fig. 9 (a) Left: the optimised structure of the ZIF-7 cage entrance and a schematic illustration of the θim1 parameter (the angle between a plane accommodating Zn1, Zn2 and Zn3 atoms and a plane of the Im1 benzimidazole moiety), adsorption complexes of C2H6 and C2H4 in the window of ZIF-7 (average values of θ are presented when deviation between the individual values is minor).186 Schematic adsorption mechanisms showing distinct dynamic behaviour for CO2 and C2H2 adsorption in (b) UTSA-300a;52 (c) [Mn(bdc)(dpe)].164 Reprinted with permissions from ref. 186, 52 and 164: (reprinted with permissions from ref. 52, 164 and 186: copyright 2011 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2017, American Chemical Society; copyright 2016, American Chemical Society). | ||
Despite the prevalence of 3D HUMs for studies on C2 LHs, the 2D layered PCN [Zn(SiF6)(dps)2; dps = 4,4′-dipyridylsulfide], UTSA-300a, is the current benchmark for C2H2/CO2 and C2H2/C2H4 separation by a physisorbent thanks to its trace C2H2 capture performance.52 Interactions between pyridyl H atoms ortho to nitrogen and the SiF62− anions induce a tilting of the coordinated pyridyl rings. This blocks the pores of UTSA-300a from CO2 and/or C2H4 (Fig. 9b, top). However, C–H⋯F bonds drive cooperative gate opening upon exposure to C2H2 with pressures above ∼0.2 bar (at 298 K). C2H2 molecules bridge two diagonally opposite SiF62− (Fig. 9b, bottom). C2H2 selective flexibility driven by these binding modes was in agreement with the stepped gate opening isotherms observed exclusively for C2H2. Equimolar C2H2/C2H4 and C2H2/CO2 DCB experiments with UTSA-300a yielded C2H4 and CO2, respectively, with both effluents of purity >99.9%, a rarity among C2 purifying sorbents. Two recent follow-up studies were reported for NCU-100a55 and GeFSIX-dps-Cu.94 Both sorbents exhibited molecular sieving and C2H2 selective sorption to afford high-purity C2H4 as effluent from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 and equimolar (v/v) mixtures. Each sorbent exhibited stepped isotherms, suggesting that the combination of molecular sieving and C–H⋯F H-bonds might be of broad relevance for C2 LH separations.
99 and equimolar (v/v) mixtures. Each sorbent exhibited stepped isotherms, suggesting that the combination of molecular sieving and C–H⋯F H-bonds might be of broad relevance for C2 LH separations.
The 2-fold interpenetrated 3D PCN [Mn(bdc)(dpe)] (bdc = 1,4-benzenedicarboxylate, dpe = 1,2-di(4-pyridyl)ethylene) was observed to undergo sudden gate opening for CO2 and not for C2H2, implying CO2 sorption selectivity over C2H2, at 273 K. To examine the mechanism of this CO2 selective gated sorption (Fig. 9c), [2+2] photodimerization on Mn(bdc)(dpe) was conducted. The photodimerised variant, [Mn2(bdc)2(rctt-tpcb)] (rctt-tpcb = region-cis,trans,trans-tetrakis(4-pyridyl)cyclobutane), exhibited no CO2 selectivity. Other PCNs that rely upon flexibility as the primary mechanism for selective LH capture include M′MOF-3a65 and ELM-12.79 Both of these flexible PCNs are selective for C2H2 over C2H4 and offer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 selectivities >15 (Table 1).
99 C2H2/C2H4 selectivities >15 (Table 1).
5.5. Pore size control
Non-equilibrium physisorption from kinetic separation and molecular sieving170 relies upon the diffusivity difference of gas molecules. Relative pore sizes typically dictate separation performance. The profound impact that pore size/chemistry can exert on adsorption properties was exemplified by varying the pore size and degree of interpenetration in a series of pcu MFSIX HUMs (see Section 5.2 for details). In particular, thanks to near-ideal molecular sieving in SIFSIX-14-Cu-i, i.e. C2H2 trapped through cooperative C–H⋯F H-bonding (2.015 Å for C2H2, Fig. 10a), this HUM was reported as the benchmark sorbent for C2H2 capture (volumetric uptake, 58 cm3 cm−3) at 0.01 bar.42 Furthermore, SIFSIX-14-Cu-i recorded benchmark C2H4 productivity of 87.5 mmol g−1 per cycle, effluent C2H4 purity >99.99% and simultaneous production of high purity C2H2 (97%) via an energy-efficient desorption at 338 K. A follow-up study on the variants NCU-100a55 and GeFSIX-dps-Cu94 found record-high C2H4 purification performance by trace C2H2 capture which was also attributed to molecular sieving.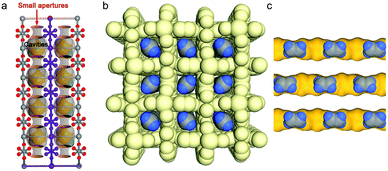 | ||
| Fig. 10 Schematic illustrations of pore size-controlled uptake of (a) C2H2 in SIFSIX-14-Cu-i;42 (b and c) C2H4 in UTSA-280.57 (Reprinted with permissions from ref. 42 and 57: copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2018, Springer Nature.) | ||
Another example of near-ideal molecular sieving was exemplified by UTSA-280, the easily scalable and low-cost MOF Ca(squarate).57 Unlike most of the MOFs that exhibit variable pore size owing to linker dynamics, UTSA-280 features 1D rigid pore channels (aperture sizes: 3.2 × 4.5; 3.8 × 3.8 in Å, Fig. 10b) and behaves as an ideal size-selective molecular sieve to exclude C2H6 from C2H4 even from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 trace gas mixtures. Ultramicropore windows in UTSA-280, with a cross-sectional area of ca. 14.4 Å2 (Fig. 10c), fit right between the minimum cross-sectional areas of the completing sorbates: C2H4 (13.7 Å2) and C2H6 (15.5 Å2), thus explaining the observed exclusion of C2H6.
99 trace gas mixtures. Ultramicropore windows in UTSA-280, with a cross-sectional area of ca. 14.4 Å2 (Fig. 10c), fit right between the minimum cross-sectional areas of the completing sorbates: C2H4 (13.7 Å2) and C2H6 (15.5 Å2), thus explaining the observed exclusion of C2H6.
5.6. Case studies for selective binding sites in C2 sorbents.
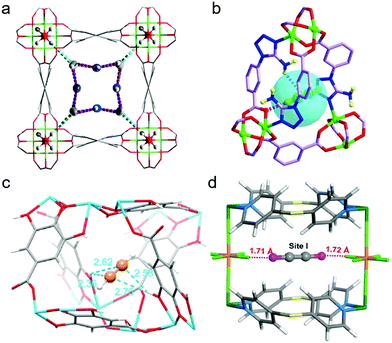 | ||
| Fig. 11 Preferential C2H2 binding sites in C2H2/C2H4 selective adsorbents: (a) NOTT-300, as determined by DFT-D modelling;67 (b) UTSA-100a, as determined by DFT-D calculations;82 (c) Mg-gallate, as determined by NPD experiments;74 (d) NCU-100a, as determined by Rietveld refinement.55 (Reprinted with permissions from ref. 67, 82, 55 and 74: copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2015, Springer Nature; copyright 2020, American Chemical Society; copyright 2014, Springer Nature.) | ||
The microporous MOF [Cu(ATBDC)] (ATBDC = 5-(5-amino-1H-tetrazol-1-yl)-1,3-benzenedicarboxylate), UTSA-100a, was reported by B. Chen's group to efficiently remove C2H2 from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 mixtures. C2H2 binding was studied by DFT-D calculations. One C2H2 molecule sits inside the small cage that links adjacent channels. This C2H2 binding mode, which resulted in an experimental Qst(C2H2) of ∼31.3 kJ mol−1, is an outcome of multiple supramolecular interactions of C2H2 with the pore wall of UTSA-100a (Fig. 11b). The weak basicity of aromatic –NH2 groups is complementary to weakly acidic C2H2 molecules (pKa = 25).190 Owing to its lower acidity, C2H4 (pKa = 44190) does not interact as strongly with the –NH2 moieties.
99 C2H2/C2H4 mixtures. C2H2 binding was studied by DFT-D calculations. One C2H2 molecule sits inside the small cage that links adjacent channels. This C2H2 binding mode, which resulted in an experimental Qst(C2H2) of ∼31.3 kJ mol−1, is an outcome of multiple supramolecular interactions of C2H2 with the pore wall of UTSA-100a (Fig. 11b). The weak basicity of aromatic –NH2 groups is complementary to weakly acidic C2H2 molecules (pKa = 25).190 Owing to its lower acidity, C2H4 (pKa = 44190) does not interact as strongly with the –NH2 moieties.
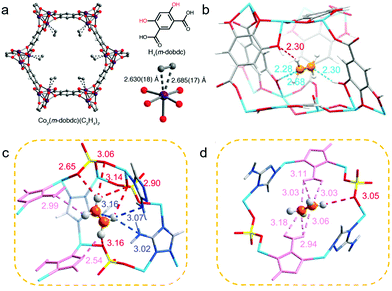
The aperture size of the 3D isostructural family of metal-gallate MOFs (M-gallates; M = Ni(II), Mg(II), Co(II)) ranged from 3.69 Å to 3.47 Å74 and SAE is highest for Ni-gallate. NPD studies of C2D2 and C2D4 loaded Mg-gallate phases revealed that C2D2 molecules locate at the centre of the Mg-gallate pore sustained by symmetrical Cdδ−⋯Hδ+O− interactions (C⋯H–O = 2.36–2.76 Å) from –OH groups of two neighbouring gallates (Fig. 11c). The strong C2H2 binding in Ni-gallate ranked it just after SIFSIX-14-Cu-i, resulting in ethylene productivity of 85.6 mol L−1 from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 mixture.
99 C2H2/C2H4 mixture.
Metal-node substitution of the current C2H2/C2H4 and C2H2/CO2 benchmark physisorbent, UTSA-300a (Section 5.4),52 afforded the isostructural variant NCU-100a, [Cu(SiF6)(dps)2].55 UTSA-300a possesses internal cages of 3.5 × 3.9 × 4.1 Å3 that are inaccessible to C2H2 molecules until dps linker rotation occurs at the C2H2 gate opening pressure of ∼0.2 bar at 298 K. On the contrary, elongated Cu–F bonds increase the pore cavities in NCU-100a thanks to Jahn–Teller distortion and result in expanded internal cages of 3.6 × 4.3 × 4.2 Å3. The cages can selectively accommodate C2H2 at low pressure. Rietveld refinement of the PXRD pattern recorded in situ for C2H2 saturated NCU-100a revealed C2H2 molecules trapped in cage-like pores with dual C–H⋯F hydrogen bonds between C2H2 terminal F atoms of different SiF62− units. C–H⋯F bond lengths of 1.71 and 1.72 Å were observed (Fig. 11d). C2H2-specific binding and molecular sieving enabled NCU-100a to achieve C2H2 uptake improvement (∼4.57 mmol g−1) vs. UTSA-300a (∼3.1 mmol g−1) and a high effluent C2H4 productivity of 14.9 mmol g−1. Remaining examples of C2H2/C2H4 selective physisorbents are listed by decreasing SAE in Table 1.
 | ||
| Fig. 12 Illustrations of preferential ethylene binding sites in C2H4/C2H6 selective adsorbents: (a) Co2(m-dobdc),99 as determined by in situ single-crystal X-ray diffraction under ∼0.3 bar of ethylene at 100 K; (b) Mg-gallate, as determined by NPD experiments (the C⋯H supramolecular interactions of C⋯H–O and C–D⋯O H-bonds are marked in cyan and red, respectively);59 (c and d) ZnatzPO4, as determined by DFT-D calculations.102 (Reprinted with permissions from ref. 57, 59 and 102: Copyright 2018, Springer Nature; copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright, 2020, the authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.) | ||
The M-gallates (M = Ni(II), Mg(II), Co(II)) detailed in Section 5.6.A (Fig. 11c) were also studied for C2H4/C2H6 selectivity and separation.59 The 3D interconnected zigzag channels of these ultramicroporous MOFs feature a narrow range of aperture sizes ∼3.47–3.69 Å, suitable for molecular sieving based upon selective entry of C2H4 (3.28 × 4.18 × 4.84 Å3) over C2H6 (3.81 × 4.08 × 4.82 Å3). Co-gallate, with SC2H4/C2H6 ∼ 52 and a C2H4 saturation uptake of 3.37 mmol g−1 at 298 K and 1 bar, performed well in equimolar (v/v) DCB experiments. NPD studies on Mg-gallate·0.485C2D4 at 200 K revealed C2D4 to be encircled by Mg(II) ions and two adjacent gallates. Cooperative interactions between C(δ−) of C2D4 and H(δ+) from –OH of the two parallel gallates (C⋯H–O = 2.28–2.68 Å) (Fig. 12b) play a key role in sorbent–sorbate binding. Furthermore, C–D⋯O interactions between C–D of C2D4 and gallate ligands further augments binding.
To lower the adsorption enthalpy of sorbent regeneration, the use of a phosphate anion in the pillared ultramicroporous MOF ZnAtzPO4101 (Atz = 3-amino-1,2,4-triazole) enabled C2H4/C2H6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) DCB separation performance with low Qst(C2H4) of ca. 30 kJ mol−1.102 That ZnAtzPO4 traps C2H4 and restricts the diffusion of C2H6 resulted in an equilibrium-kinetic combined selectivity of 32.4 as reported by H. Xing et al. The C2H4 binding mechanism was studied by first-principles DFT-D calculations, which revealed that ZnAtzPO4 provides two distinct “molecular trap” like pockets for C2H4 (Fig. 12c and d). At site-I (Fig. 12c), C2H4 molecules reside close to the pillaring PO43− anions and interact with neighbouring O (from PO43−) and N atoms (from Atz ligands) via weak H-bonds (2.54–3.16 Å) of two types: C–H⋯O and C–H⋯N, respectively. C2H4 binding site II (Fig. 12d) is centrally placed in the bottleneck-shaped scaffold that connects two adjacent pockets and features weak C–H⋯O interactions (3.05 Å) between C2H4 and the PO43− pillar. The authors credit the observed equilibrium-kinetic combined C2H4/C2H6 selectivity of ZnAtzPO4 to the absence of strong H-bonding interactions (C–H⋯O/N < 2.3 Å) in either of the two aforementioned binding sites. Other examples of C2H4 selective physisorbents versus C2H6 are given in Table 2 and are arranged by decreasing SC2H4/C2H6.
1, v/v) DCB separation performance with low Qst(C2H4) of ca. 30 kJ mol−1.102 That ZnAtzPO4 traps C2H4 and restricts the diffusion of C2H6 resulted in an equilibrium-kinetic combined selectivity of 32.4 as reported by H. Xing et al. The C2H4 binding mechanism was studied by first-principles DFT-D calculations, which revealed that ZnAtzPO4 provides two distinct “molecular trap” like pockets for C2H4 (Fig. 12c and d). At site-I (Fig. 12c), C2H4 molecules reside close to the pillaring PO43− anions and interact with neighbouring O (from PO43−) and N atoms (from Atz ligands) via weak H-bonds (2.54–3.16 Å) of two types: C–H⋯O and C–H⋯N, respectively. C2H4 binding site II (Fig. 12d) is centrally placed in the bottleneck-shaped scaffold that connects two adjacent pockets and features weak C–H⋯O interactions (3.05 Å) between C2H4 and the PO43− pillar. The authors credit the observed equilibrium-kinetic combined C2H4/C2H6 selectivity of ZnAtzPO4 to the absence of strong H-bonding interactions (C–H⋯O/N < 2.3 Å) in either of the two aforementioned binding sites. Other examples of C2H4 selective physisorbents versus C2H6 are given in Table 2 and are arranged by decreasing SC2H4/C2H6.
A 2D layered PCN studied by us for CO2 sieving,192 Qc-5-Cu-sql-β (Qc = quinoline-5-carboxylate), was also studied by B. Chen's group under the name Cu(Qc)2 to examine its SC2H6/C2H4vs. the isostructural isonicotinate variant Cu(ina)2.109 Cu(Qc)2 exhibits a narrow pore aperture size of 3.3 Å formed by aromatic rings and preferentially adsorbed C2H6 over C2H4 from calculated IAST selectivity and DCB experiments of an equimolar mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). NPD data indicates that C2H6 molecules are commensurately packed within the rhombic apertures of Cu(Qc)2 with multiple C–H⋯π interactions (marked in pink dashed bonds in Fig. 13a).
1, v/v). NPD data indicates that C2H6 molecules are commensurately packed within the rhombic apertures of Cu(Qc)2 with multiple C–H⋯π interactions (marked in pink dashed bonds in Fig. 13a).
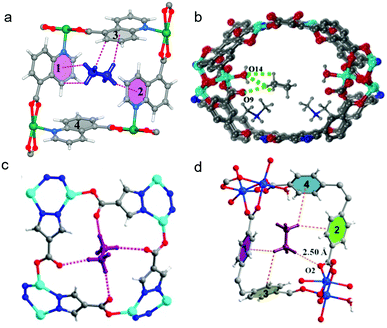 | ||
| Fig. 13 Preferential ethane binding sites in C2H6/C2H4 selective adsorbents: (a) Qc-5-Cu-sql-β as determined by NPD experiments;109 (b) TJT-100, as determined by GCMC simualtions;70 (c) JNU-2, as determined by DFT-D calculations;121 (d) NUM-7a, as determined by GCMC simulations.117 (Reprinted with permissions from ref. 109, 70, 121 and 117; copyright 2018, American Chemical Society; copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim; copyright 2019, American Chemical Society; copyright 2020, American Chemical Society.) | ||
(Me2NH2)[Co3(DCPN)2(μ3-OH)(H2O)]·11H2O (DCPN = 5-(3′,5′-dicarboxylphenyl)nicotinate), TJT-100, binds C2H2 and C2H6 over C2H4.70 Ambient temperature DCB experiments confirmed the potential use of TJT-100 for production of polymer-grade C2H4 from a ternary C2H2/C2H4/C2H6 (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, v/v/v) mixture. GCMC simulation results suggested that uncoordinated carboxylate oxygen atoms and coordinated water molecules on can trap C2H2 and C2H6 by formation of multiple C–H⋯O interactions (Fig. 13b), whereas the corresponding C2H4 interaction is much weaker.
0.5, v/v/v) mixture. GCMC simulation results suggested that uncoordinated carboxylate oxygen atoms and coordinated water molecules on can trap C2H2 and C2H6 by formation of multiple C–H⋯O interactions (Fig. 13b), whereas the corresponding C2H4 interaction is much weaker.
The Cu–Zn heterometallic MOF JNU-2 with xae topology features cage-like cavities interconnected through 3.7 Å ultramicroporous windows. Its C2H6 selectivity as determined by single-component gas sorption isotherms and DCB binary and ternary separation studies (10/90 C2H6/C2H4, v/v; 10/87/3 C2H6/C2H4/C2H2, v/v) was attributed by a molecular modelling study to multiple C–H⋯O hydrogen bonding interactions at the O-rich pore window. The limiting and cage-connecting pore apertures behaved like screening sites to promote C2H6 selectivity, whereas the internal cage porosity enabled high uptake at saturation pressure. C2H6 was calculated to form four weak H-bonds with JNU-2 (Fig. 13c) vs. only two H-bonds for C2H4. The DFT-D modelled observation on binding energy difference of 6.2 kJ mol−1 is consistent with that in electrostatic interactions (7.7 kJ mol−1) attributable to two weak H-bonds.
T.-L. Hu's group prepared the 3D ultramicroporous MOF NUM-7a by activating as-synthesised [Mn2(TCPE)(DMF)(H2O)]·DMF·CH3CN (TCPE = 4,4′,4′′,4′′′-(ethene-1,1,2,2-tetrayl)tetrabenzoate).117 The narrow pore aperture of 3.42 Å facilitated C–H⋯O and C–H⋯π interactions (Fig. 13d) upon adsorption of C2H6. NUM-7a is another PCN that exhibits a “best fit” for C2H6vs. the other C2 LHs. Planar configurations of adsorbed C2H2 and C2H4 restrict their weak interactions with the surrounding benzoate O-atoms and phenyl rings, as discussed therein.
Selectivity for CO2vs. C2H2 has only been reported for six physisorbents, five of them being PCNs (Table 4). Apart from [Mn(bdc)(dpe)]164 and the thulium(III) nitrate based material Tm(OH-bdc)162 (OH-bdc = 2,5-dihydroxyterephthalate), SIFSIX-3-Ni is the only example of a physisorbent that has been reported to exhibit CO2/C2H2 separation under DCB experimental conditions.47 GCMC simulations conducted upon SIFSIX-3-Ni suggested that, upon full saturation, C2H2 molecules align in a slipped parallel orientation to commensurately pack with two molecules per unit cell (Fig. 14a, left). Each C2H2 orients in a manner that allows C–H⋯C–H sorbate–sorbate interactions on both sides and a favourable C–H⋯F interaction on one side. In contrast, the single binding site for CO2 in SIFSIX-3-Ni was calculated and experimentally validated in an earlier in situ study.193 CO2 molecules are proximate to the four electro-negative F atoms from four independent SiF62− pillars with Cδ+⋯Fδ− contacts of ∼2.75 Å (Fig. 14a, right). A 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 C2H2
85 C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2
CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He DCB experiment validated CO2/C2H2 binary separation that produces high-purity C2H2 effluent in a one-step adsorption process that does not need an energy-intensive regeneration step.
He DCB experiment validated CO2/C2H2 binary separation that produces high-purity C2H2 effluent in a one-step adsorption process that does not need an energy-intensive regeneration step.
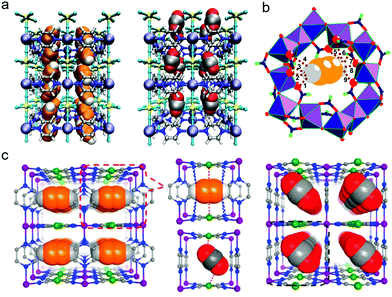 | ||
| Fig. 14 Illustrations of preferential binding sites for (a) C2H2 (left) and CO2 (right) in SIFSIX-3-Ni as determined by GCMC simulations;47 (b) C2H2 in [Ni3(HCOO)6], as determined by GCMC simulations;133 (c) C2H2 and CO2 in ZJU-74a as determined by GCMC simulations.53 (Reprinted with permissions from ref. 47, 133 and 53; copyright 2016, Elsevier Inc.; copyright 2019, American Chemical Society; copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.) | ||
Early reports with metal formates69 prompted B. Chen and Qian's groups to explore the moisture and H2S-stable MOF [Ni3(HCOO)6]n for C2H2/CO2 equimolar (v/v) separation.133 The ultramicroporous aperture of 4.3 Å and O donor sites from formate ligands on the pore walls enable moderate selectivity for C2H2 as validated by GCMC simulations which revealed that each unit cell binds one C2H2 molecule through such H-bonding (Fig. 14b).
Ultramicroporous pillared Hofmann clathrate sorbents are a promising but understudied PCN platform for adsorptive separation studies. Recent reports suggested their possible utility for selective C2H2 adsorption.53,54 In ZJU-74a, reported by Qian and coworkers in 2020, a “sandwich-type” binding site is created by the exposed square planar Ni(II) centres located 3.6 Å apart at diametrically opposite positions in a cuboidal pore. GCMC simulations revealed that the Ni(II) centres interact strongly with the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond of acetylene, while eight C
C bond of acetylene, while eight C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N N atoms from two different [Ni(CN)4]2− groups are H-bonded to the H atoms of C2H2, creating a tight, specific binding site (Fig. 14c). The effect of this cooperative “sandwich-type” binding site can be seen in the very high IAST selectivity of ZJU-74a for C2H2/CO2 separation (36.5), which in turn results in excellent DCB separation performance with dry and wet equimolar C2H2/CO2 mixtures. A high selectivity for C2H2 over C2H4 was also reported and 1
N N atoms from two different [Ni(CN)4]2− groups are H-bonded to the H atoms of C2H2, creating a tight, specific binding site (Fig. 14c). The effect of this cooperative “sandwich-type” binding site can be seen in the very high IAST selectivity of ZJU-74a for C2H2/CO2 separation (36.5), which in turn results in excellent DCB separation performance with dry and wet equimolar C2H2/CO2 mixtures. A high selectivity for C2H2 over C2H4 was also reported and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 C2H2/C2H4 DCB experiments demonstrated trace acetylene removal. The chemical stability of ZJU-74a is an advantage for development at higher technological readiness levels (TRLs).53
99 C2H2/C2H4 DCB experiments demonstrated trace acetylene removal. The chemical stability of ZJU-74a is an advantage for development at higher technological readiness levels (TRLs).53
5.7. Separation of multi-component gas mixtures by SSST
Whereas we and others have tended to focus upon binary separations, the most relevant industrial gas mixtures (e.g. biogas, syngas, air, natural gas, C2 gases, C3 gases) are multicomponent gas mixtures of varying composition. As detailed herein, advances in the past five years have provided families of physisorbents that exhibit new selectivity benchmarks for each of the trace impurities present in the most relevant gas mixtures.38,40–42,64,68,76 To address purification of the largest volume chemical building block chemical, C2H4, we recently introduced the use of multiple bespoke sorbents to enable “synergistic sorbent separation technology”, SSST, for the one-step production of polymer-grade (>99.9% purity) C2H4 from ternary (C2H2/C2H6/C2H4) or quaternary (CO2/C2H2/C2H6/C2H4) gas mixtures. SSST was demonstrated with a column packed with a series of three ultramicroporous PCNs, SIFSIX-3-Ni,194TIFSIX-2-Cu-i47 and Zn-atz-ipa,195 in a packed-bed geometry (Fig. 15).71 SSST exploited the three bespoke physisorbents, one for each trace impurity, to enable single-step removal of multiple impurities. This approach enabled one-step purification of multicomponent gas mixtures that mimic real-world gas mixtures. That SSST was effective under two different quaternary mixture concentrations: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and 1
33 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, implies that the choice of task-specific ultraselective sorbents in tandem-packed sorbent beds of the type used here is unlikely to be limited to the three sorbents or gas mixtures that we investigated. Further, performance could be enhanced by substitution of second generation sorbents with higher selectivity, higher uptake capacity, or both, to optimize overall performance. The strong performance of SSST with respect to the purification of C2 gas mixtures and the availability of an ever-increasing number of ultraselective physisorbents suggests that the scope of SSST will be broad enough to address the high energy footprint of other industrial commodity purifications.
1, implies that the choice of task-specific ultraselective sorbents in tandem-packed sorbent beds of the type used here is unlikely to be limited to the three sorbents or gas mixtures that we investigated. Further, performance could be enhanced by substitution of second generation sorbents with higher selectivity, higher uptake capacity, or both, to optimize overall performance. The strong performance of SSST with respect to the purification of C2 gas mixtures and the availability of an ever-increasing number of ultraselective physisorbents suggests that the scope of SSST will be broad enough to address the high energy footprint of other industrial commodity purifications.
6. Critical analysis and future outlook
Herein, we have detailed the emergence and rapid development of PCNs as physisorbents for the challenging and industrially important separation of C2 LHs. We have also delineated structure–function relationships in terms of pore structure, size and chemistry and how they impact sorbent–sorbate interactions at the molecular level. PCNs have thereby emerged as the leading adsorbent class for C2 separations to the extent that they now represent a greater share of research output in this area than all other classes of sorbents combined (Fig. 3). We attribute this upsurge of interest to the exceptional tunability of pore size and pore chemistry offered by PCNs that has enabled unmatched selectivities for C2 separations through careful control of pore dimensions (to exclude larger adsorbates) or the incorporation of bespoke functionalities to enhance sorbate binding. Crystal engineering of PCN adsorbents has thereby enabled the design of new generations of sorbents with favourable thermodynamics for selective binding, energy-efficient regeneration (Qst ∼ 35–50 kJ mol−1) and fast sorption kinetics.6,45 These characteristics are perhaps best exemplified by ultramicroporous (<0.7 nm) PCNs as pioneered by several groups, including ours. The combination of strongly interacting functional groups (e.g. inorganic anions) and narrow channels results in tight fitting binding sites that offer highly specific interactions for key adsorbates. This is borne out by a comparison of the leading physisorbents for the binary C2 separations detailed herein. Plots of IAST selectivity versus uptake (Fig. 16) reveal that several ultramicroporous PCNs are the best performing class of materials, sometimes orders of magnitude ahead of their larger-pore counterparts. Indeed, the top performing materials for C2H2/C2H4, C2H4/C2H6 and C2H2/CO2 selectivity are all ultramicroporous PCNs.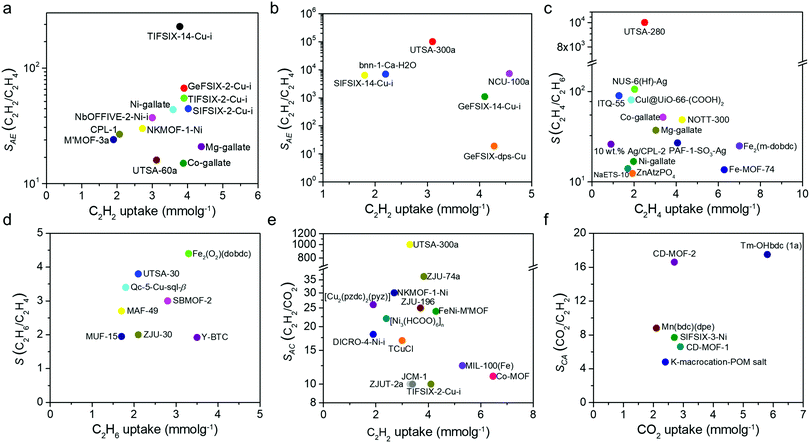 | ||
Fig. 16 Selectivity versus uptake plots for (a) C2H2/C2H4 selective adsorbents with a threshold C2H2 selectivity, SAE > 15 (calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 mixtures of C2H2 99 mixtures of C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4 unless otherwise stated in Table 1); (b) C2H2/C2H4 selective adsorbents that exhibit molecular sieving (calculated for 1 C2H4 unless otherwise stated in Table 1); (b) C2H2/C2H4 selective adsorbents that exhibit molecular sieving (calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 mixtures of C2H2 99 mixtures of C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4 unless otherwise stated in Table 1). The IAST derived selectivities are therefore qualitative; (c) C2H4/C2H6 selective adsorbents with a threshold C2H4 selectivity, SC2H4/C2H6 > 10 (calculated for 1 C2H4 unless otherwise stated in Table 1). The IAST derived selectivities are therefore qualitative; (c) C2H4/C2H6 selective adsorbents with a threshold C2H4 selectivity, SC2H4/C2H6 > 10 (calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of C2H4 1 mixtures of C2H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H6,unless otherwise stated in Table 2); (d) C2H6/C2H4 selective adsorbents with a threshold C2H6 selectivity, SC2H6/C2H4 > 1.9 (calculated for 1 C2H6,unless otherwise stated in Table 2); (d) C2H6/C2H4 selective adsorbents with a threshold C2H6 selectivity, SC2H6/C2H4 > 1.9 (calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of C2H6 1 mixtures of C2H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4 unless otherwise stated in Table 3); (e) C2H2/CO2 selective adsorbents with a threshold C2H2 selectivity, SAC > 10 (calculated for 1 C2H4 unless otherwise stated in Table 3); (e) C2H2/CO2 selective adsorbents with a threshold C2H2 selectivity, SAC > 10 (calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of C2H2 1 mixtures of C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 unless otherwise stated in Table 4); (f) CO2/C2H2 selective adsorbents (calculated for 1 CO2 unless otherwise stated in Table 4); (f) CO2/C2H2 selective adsorbents (calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2 1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of CO2 1 mixtures of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H2 as stated in Table 4). Uptakes and selectivities are considered at 1 bar, at the temperatures specified in Tables 1–4. C2H2 as stated in Table 4). Uptakes and selectivities are considered at 1 bar, at the temperatures specified in Tables 1–4. | ||
We also note that the ultramicroporous sorbents with tight binding sites have resulted in examples of ‘reverse selectivity’ such as C2H6/C2H4 and CO2/C2H2 selective sorbents. These sorbents are not outliers. Rather, they are powerful illustrations of how pore structure, chemistry and shape can lead to profound property effects and task-specific binding sites. Whereas crystal engineering of binding sites with just the right charge distributions to harness the slight differences in hard-to-separate sorbate pairs remains challenging, growing insight into the mechanisms underlying this type of ‘reverse’ selectivity, have been aided by computational chemistry and in situ structural studies. Even when adsorbates are of the same kinetic diameter (or indeed, the larger one is selectively adsorbed), ultramicroporous PCNs feature among the top performing adsorbents and demonstrate their versatility as tunable sorbent platforms.28
The body of research on C2 LHs has established that crystal engineering can take first generation PCNs with benchmark properties and quickly iterate families of second generation PCNs with even better C2 separation performance. Nevertheless, in order for PCNs to replace existing separation technologies, some obstacles must be overcome. Future research must address the full “spectrum of performance parameters” that is relevant to commercial applications (Fig. 17). Since the eventual goal of the development of sorbents is industrial utility, factors such as cost, stability, scale-up and multi-cycle regenerability must also be considered, beginning at the lab scale.
 | ||
| Fig. 17 The spectrum of performance parameters that must be exhibited by a sorbent with respect to gas separation/purification technologies. | ||
In addition, the study of highly selective flexible adsorbents is in its infancy and is still looking at first generation materials for which the thermodynamics and kinetics of phase transformations remain poorly understood. Nevertheless, the high working capacities that can arise from type F-IV isotherms could lead to benchmark separation performance. In this context, whether selectivity is retained in the ‘open’ phase also remains understudied. Advanced in situ techniques196 that provide clues to the processes underlying stimulus-responsive adsorption197 are needed for further development of flexible C2-selective adsorbents.
Several other aspects of PCN sorbent performance remain understudied. For example, adsorption/desorption kinetics and co-adsorption are areas that must be addressed. In addition, multicomponent dynamic column breakthrough experiments can provide vital insight into the performance of sorbents under industrially relevant conditions with more complex gas mixtures than those typically studied at the lab scale. The stability of candidate PCNs to H2, CO and sulphur-containing compounds, as well as the retention of their performance is also an important factor in determining the viability of sorbents at higher TRLs.198,199 The further development of ‘reverse’ selectivity in, for example, C2H6/C2H4 and CO2/C2H2 separations, is also an area for that needs more study and insight. Reverse selectivity can be advantageous for removal of common trace impurities from feedstock gases during the adsorption cycle of fixed-bed processes. Synergistic sorbent separation technology, as put forward by our group, is a recent highlight in this context.71 The use of combinations of two or more sorbents with specific properties offers an simple but effective approach to the challenge of multicomponent “real-world” gas mixtures of varying composition.
In summary, crystal engineering of PCN platforms has enabled fine tuning of families of ultramicroporous PCNs that offer new benchmarks for separation performances of C2 LHs, but in many ways we are only at the end of the beginning. Moving forward, the next steps will involve the design and discovery of third generation sorbents that offer strong separation performances addressing other properties that collectively enable further development of PCN sorbents at higher TRLs.
Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
The authors acknowledge the generous support of Science Foundation Ireland (13/RP/B2549 and 16/IA/4624). S. M. thanks the Alexander von Humboldt Foundation for awarding a postdoctoral research fellowship and Prof. Dr Roland A. Fischer (Chair of Inorganic and Metal–Organic Chemistry, TU Munich) for hosting his research tenure.Notes and references
- https://www.icca-chem.org/wp-content/uploads/2019/03/ICCA_EconomicAnalysis_Report_030819.pdf, Report for International Council of Chemical Associations (ICCA), accessed 04/07/2020.
- S. Kitagawa, Angew. Chem., Int. Ed., 2015, 54, 10686–10687 CrossRef CAS.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef.
- Z. Bao, G. Chang, H. Xing, R. Krishna, Q. Ren and B. Chen, Energy Environ. Sci., 2016, 9, 3612–3641 RSC.
- D. G. Madden, D. O’Nolan, K.-J. Chen, C. Hua, A. Kumar, T. Pham, K. A. Forrest, B. Space, J. J. Perry and M. Khraisheh, Chem. Commun., 2019, 55, 3219–3222 RSC.
- W.-G. Cui, T.-L. Hu and X.-H. Bu, Adv. Mater., 2020, 32, 1806445 CrossRef CAS.
- T. Ren, M. K. Patel and K. Blok, Energy, 2008, 33, 817–833 CAS.
- https://cefic.org/app/uploads/2019/01/The-European-Chemical-Industry-Facts-And-Figures-2020.pdf, Conseil Européen des Fédérations de l'Industrie Chimique, accessed 04/07/2020.
- L. Meng, L. Yang, C. Chen, X. Dong, S. Ren, G. Li, Y. Li, Y. Han, Z. Shi and S. Feng, ACS Appl. Mater. Interfaces, 2020, 12, 5999–6006 CrossRef CAS.
- Y. Wang, S. B. Peh and D. Zhao, Small, 2019, 15, 1900058 CrossRef.
- L. Kniel, O. Winter and K. Stork, Ethylene: Keystone to the petrochemical industry, Dekker, 1980 Search PubMed.
- J. J. McKetta Jr, Encyclopedia of Chemical Processing and Design: Volume 20 - Ethanol as Fuel, CRC Press, 1984 Search PubMed.
- J. H. Kang, E. W. Shin, W. J. Kim, J. D. Park and S. H. Moon, Catal. Today, 2000, 63, 183–188 CrossRef CAS.
- S. Sircar, Ind. Eng. Chem. Res., 2006, 45, 5435–5448 CrossRef CAS.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- D. R. Lide, CRC handbook of chemistry and physics, CRC Press, 2004 Search PubMed.
- M. Neelis, M. Patel, K. Blok, W. Haije and P. Bach, Energy, 2007, 32, 1104–1123 CrossRef.
- P. Pässler, W. Hefner, K. Bucki, H. Meinass, A. Meiswinkel, H. Wernicke, G. Ebersberg, R. Muller, J. Bässler and H. Behringer, Acetylene, Ullmann's Encyclopedia of Industrial Chemistry, 2011 DOI:10.1002/14356007.a01_097.pub4.
- K. Weissermel, H.-J. Arpe, C. R. Lindley and S. Hawkins, Industrial organic chemistry, Wiley Online Library, 1997 Search PubMed.
- H. Schobert, Chem. Rev., 2014, 114, 1743–1760 CrossRef CAS.
- S. H. Pine, J. Hendrickson, D. Cram and G. Hammond, Organic Chemistry, McGraw-Hill Book Company, New York City, 1987 Search PubMed.
- R. E. Gannon, V. J. Krukonis and T. Schoenberg, Product R&D, 1970, 9, 343–347 Search PubMed.
- Ascent Supply Chain Consultants Pvt. Ltd Calcium Carbide, https://www.ascconline.com/img/services/project_report/Calcium_Carbide_Project_Report_Sample.pdf, (accessed 04/07/2020).
- N. Magnowski, A. Avila, C. Lin, M. Shi and S. Kuznicki, Chem. Eng. Sci., 2011, 66, 1697–1701 CrossRef CAS.
- S. Matar and L. F. Hatch, Chemistry of petrochemical processes, Elsevier, 2001 Search PubMed.
- S. Bhattacharya, K. S. Peraka and M. J. Zaworotko, Co-crystals: Preparation, Characterization and Applications, The Royal Society of Chemistry, 2018, pp. 33–79 10.1039/9781788012874-00033.
- S. Ma and H.-C. Zhou, J. Am. Chem. Soc., 2006, 128, 11734–11735 CrossRef CAS.
- S. Mukherjee and M. J. Zaworotko, Trends Chem., 2020, 2, 506–518 CrossRef.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M. Paik Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715 CAS.
- M. Kondo, T. Yoshitomi, H. Matsuzaka, S. Kitagawa and K. Seki, Angew. Chem., Int. Ed. Engl., 1997, 36, 1725–1727 CrossRef CAS.
- H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8571–8572 CrossRef CAS.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- I. M. Hönicke, I. Senkovska, V. Bon, I. A. Baburin, N. Bönisch, S. Raschke, J. D. Evans and S. Kaskel, Angew. Chem., Int. Ed., 2018, 57, 13780–13783 CrossRef.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016–15021 CrossRef CAS.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS.
- P. M. Bhatt, Y. Belmabkhout, A. Cadiau, K. Adil, O. Shekhah, A. Shkurenko, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2016, 138, 9301–9307 CrossRef CAS.
- S. Mukherjee, N. Sikdar, D. O’Nolan, D. M. Franz, V. Gascón, A. Kumar, N. Kumar, H. S. Scott, D. G. Madden and P. E. Kruger, Sci. Adv., 2019, 5, eaax9171 CrossRef CAS.
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Science, 2016, 353, 141–144 CrossRef CAS.
- B. Li, X. Cui, D. O'Nolan, H.-M. Wen, M. Jiang, R. Krishna, H. Wu, R.-B. Lin, Y.-S. Chen, D. Yuan, H. Xing, W. Zhou, Q. Ren, G. Qian, M. J. Zaworotko and B. Chen, Adv. Mater., 2017, 29, 1704210 CrossRef.
- D. O’Nolan, A. Kumar and M. J. Zaworotko, J. Am. Chem. Soc., 2017, 139, 8508–8513 CrossRef.
- X. Liu, X. Wang and F. Kapteijn, Chem. Rev., 2020 DOI:10.1021/acs.chemrev.9b00746.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- B. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Science, 2001, 291, 1021–1023 CrossRef CAS.
- K.-J. Chen, H. S. Scott, D. G. Madden, T. Pham, A. Kumar, A. Bajpai, M. Lusi, K. A. Forrest, B. Space, J. J. Perry and M. J. Zaworotko, Chem, 2016, 1, 753–765 CAS.
- H. Nonnenmacher et al., Production of acetylene by thermal cracking of liquid hydrocarbons, US Pat., US3242223A, 1966 Search PubMed.
- S. Mukherjee, Y. He, D. Franz, S.-Q. Wang, W.-R. Xian, A. Bezrukov, B. Space, Z. Xu, J. He and M. Zaworotko, Chem. – Eur. J., 2020, 26, 4923–4929 CrossRef CAS.
- R. Chebbi, N. Al-Amoodi, N. A. Jabbar, G. Husseini and K. Al Mazroui, Chem. Eng. Res. Des., 2010, 88, 779–787 CrossRef CAS.
- S. Brueske, C. Kramer and A. Fisher, Bandwidth Study on Energy Use and Potential Energy Saving Opportunities in US Chemical Manufacturing, Energetics, 2015 CAS.
- R.-B. Lin, L. Li, H. Wu, H. Arman, B. Li, R.-G. Lin, W. Zhou and B. Chen, J. Am. Chem. Soc., 2017, 139, 8022–8028 CrossRef CAS.
- J. Pei, K. Shao, J.-X. Wang, H.-M. Wen, Y. Yang, Y. Cui, R. Krishna, B. Li and G. Qian, Adv. Mater., 2020, 32, 1908275 CrossRef CAS.
- J. Gao, X. Qian, R.-B. Lin, R. Krishna, H. Wu, W. Zhou and B. Chen, Angew. Chem., Int. Ed., 2020, 59, 4396–4400 CrossRef CAS.
- J. Wang, Y. Zhang, P. Zhang, J. Hu, R.-B. Lin, Q. Deng, Z. Zeng, H. Xing, S. Deng and B. Chen, J. Am. Chem. Soc., 2020, 142, 9744–9751 CAS.
- S. Mukherjee, S. Chen, A. Bezrukov, M. Mostrom, V. Terskikh, D. Franz, S.-Q. Wang, A. Kumar, M. Chen, B. Space, Y. Huang and M. J. Zaworotko, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202006414.
- R.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS.
- L. Zhang, L. Li, E. Hu, L. Yang, K. Shao, L. Yao, K. Jiang, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, Adv. Sci., 2020, 7, 1901918 CrossRef CAS.
- Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen and Q. Ren, Angew. Chem., Int. Ed., 2018, 57, 16020–16025 CrossRef CAS.
- L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS.
- Y. He, S. Xiang, Z. Zhang, S. Xiong, F. R. Fronczek, R. Krishna, M. O'Keeffe and B. Chen, Chem. Commun., 2012, 48, 10856–10858 RSC.
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Nature, 2005, 436, 238–241 CrossRef CAS.
- H.-M. Wen, C. Liao, L. Li, L. Yang, J. Wang, L. Huang, B. Li, B. Chen and J. Hu, Chem. Commun., 2019, 55, 11354–11357 RSC.
- S. Mukherjee, Y. He, D. Franz, S.-Q. Wang, W.-R. Xian, A. A. Bezrukov, B. Space, Z. Xu, J. He and M. J. Zaworotko, Chem. – Eur. J., 2020, 26, 4923–4929 CrossRef CAS.
- S.-C. Xiang, Z. Zhang, C.-G. Zhao, K. Hong, X. Zhao, D.-R. Ding, M.-H. Xie, C.-D. Wu, M. C. Das, R. Gill, K. M. Thomas and B. Chen, Nat. Commun., 2011, 2, 204 CrossRef.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS.
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef.
- D. G. Samsonenko, H. Kim, Y. Sun, G.-H. Kim, H.-S. Lee and K. Kim, Chem. – Asian J., 2007, 2, 484–488 CrossRef CAS.
- H.-G. Hao, Y.-F. Zhao, D.-M. Chen, J.-M. Yu, K. Tan, S. Ma, Y. Chabal, Z.-M. Zhang, J.-M. Dou, Z.-H. Xiao, G. Day, H.-C. Zhou and T.-B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS.
- K.-J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS.
- D. O’Nolan, A. Kumar, K.-J. Chen, S. Mukherjee, D. G. Madden and M. J. Zaworotko, ACS Appl. Nano Mater., 2018, 1, 6000–6004 CrossRef.
- Z. Zhang, X. Cui, L. Yang, J. Cui, Z. Bao, Q. Yang and H. Xing, Ind. Eng. Chem. Res., 2018, 57, 7266–7274 CrossRef CAS.
- J. Wang, L. Li, L. Guo, Y. Zhao, D. Xie, Z. Zhang, Q. Yang, Y. Yang, Z. Bao and Q. Ren, Chem. – Eur. J., 2019, 25, 15516–15524 CrossRef CAS.
- L. Yang, A. Jin, L. Ge, X. Cui and H. Xing, Chem. Commun., 2019, 55, 5001–5004 RSC.
- Y. L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K. J. Chen, K. A. Forrest, B. Space, P. Cheng and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS.
- F. Zheng, L. Guo, B. Gao, L. Li, Z. Zhang, Q. Yang, Y. Yang, B. Su, Q. Ren and Z. Bao, ACS Appl. Mater. Interfaces, 2019, 11, 28197–28204 CAS.
- H.-M. Wen, B. Li, H. Wang, C. Wu, K. Alfooty, R. Krishna and B. Chen, Chem. Commun., 2015, 51, 5610–5613 RSC.
- L. Li, R.-B. Lin, R. Krishna, X. Wang, B. Li, H. Wu, J. Li, W. Zhou and B. Chen, J. Mater. Chem. A, 2017, 5, 18984–18988 RSC.
- G.-X. Jin, X. Niu, J. Wang, J.-P. Ma, T.-L. Hu and Y.-B. Dong, Chem. Mater., 2018, 30, 7433–7437 CrossRef CAS.
- D.-M. Chen, C.-X. Sun, N.-N. Zhang, H.-H. Si, C.-S. Liu and M. Du, Inorg. Chem., 2018, 57, 2883–2889 CrossRef CAS.
- T.-L. Hu, H. Wang, B. Li, R. Krishna, H. Wu, W. Zhou, Y. Zhao, Y. Han, X. Wang, W. Zhu, Z. Yao, S. Xiang and B. Chen, Nat. Commun., 2015, 6, 7328 CrossRef CAS.
- H. Li, L. Li, R.-B. Lin, G. Ramirez, W. Zhou, R. Krishna, Z. Zhang, S. Xiang and B. Chen, ACS Sustainable Chem. Eng., 2019, 7, 4897–4902 CrossRef CAS.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Chem. Mater., 2019, 31, 4919–4926 CrossRef CAS.
- J. Lee, C. Y. Chuah, J. Kim, Y. Kim, N. Ko, Y. Seo, K. Kim, T. H. Bae and E. Lee, Angew. Chem., Int. Ed., 2018, 57, 7869–7873 CrossRef CAS.
- J. Xiong, A. Li, Y. Fan, Z. Xu, H. Feng, Q. Gao, Q. Fan, Y. He, Z. Gao and F. Luo, J. Solid State Chem., 2020, 288, 121337 CrossRef CAS.
- L. Zhang, X. Cui, H. Xing, Y. Yang, Y. Cui, B. Chen and G. Qian, RSC Adv., 2017, 7, 20795–20800 RSC.
- H.-M. Wen, B. Li, H. Wang, R. Krishna and B. Chen, Chem. Commun., 2016, 52, 1166–1169 RSC.
- J. Li, L. Jiang, S. Chen, A. Kirchon, B. Li, Y. Li and H.-C. Zhou, J. Am. Chem. Soc., 2019, 141, 3807–3811 CrossRef CAS.
- H.-H. Wang, Q.-Y. Liu, L. Li, R. Krishna, Y.-L. Wang, X.-W. Peng, C.-T. He, R.-B. Lin and B. Chen, Inorg. Chem., 2018, 57, 9489–9494 CrossRef CAS.
- F. Yu, B.-Q. Hu, X.-N. Wang, Y.-M. Zhao, J.-L. Li, B. Li and H.-C. Zhou, J. Mater. Chem. A, 2020, 8, 2083–2089 RSC.
- Y. He, R. Krishna and B. Chen, Energy Environ. Sci., 2012, 5, 9107–9120 RSC.
- X. Wang, L. Li, Y. Wang, J.-R. Li and J. Li, CrystEngComm, 2017, 19, 1729–1737 RSC.
- T. Ke, Q. Wang, J. Shen, J. Zhou, Z. Bao, Q. Yang and Q. Ren, Angew. Chem., Int. Ed., 2020, 59, 12725–12730 CrossRef CAS.
- Y. Wang, Z. Hu, Y. Cheng and D. Zhao, Ind. Eng. Chem. Res., 2017, 56, 4508–4516 CrossRef CAS.
- P. J. Bereciartua, Á. Cantín, A. Corma, J. L. Jordá, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop and G. L. Casty, Science, 2017, 358, 1068–1071 CrossRef CAS.
- B. Li, Y. Zhang, R. Krishna, K. Yao, Y. Han, Z. Wu, D. Ma, Z. Shi, T. Pham, B. Space, J. Liu, P. K. Thallapally, J. Liu, M. Chrzanowski and S. Ma, J. Am. Chem. Soc., 2014, 136, 8654–8660 CrossRef CAS.
- H. Xiang, A. Ameen, J. Shang, Y. Jiao, P. Gorgojo, F. R. Siperstein and X. Fan, Microporous Mesoporous Mater., 2020, 293, 109784 CrossRef CAS.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS.
- A. Anson, Y. Wang, C. C. H. Lin, T. M. Kuznicki and S. M. Kuznicki, Chem. Eng. Sci., 2008, 63, 4171–4175 CrossRef CAS.
- R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Angew. Chem., Int. Ed., 2012, 51, 1826–1829 CrossRef CAS.
- Q. Ding, Z. Zhang, C. Yu, P. Zhang, J. Wang, X. Cui, C.-H. He, S. Deng and H. Xing, Sci. Adv., 2020, 6, eaaz4322 CrossRef.
- Y. Zhang, B. Li, R. Krishna, Z. Wu, D. Ma, Z. Shi, T. Pham, K. Forrest, B. Space and S. Ma, Chem. Commun., 2015, 51, 2714–2717 RSC.
- G. Chang, M. Huang, Y. Su, H. Xing, B. Su, Z. Zhang, Q. Yang, Y. Yang, Q. Ren, Z. Bao and B. Chen, Chem. Commun., 2015, 51, 2859–2862 RSC.
- Y. Yin, Z. Zhang, C. Xu, H. Wu, L. Shi, S. Wang, X. Xu, A. Yuan, S. Wang and H. Sun, ACS Sustainable Chem. Eng., 2020, 8, 823–830 CrossRef CAS.
- S. J. Geier, J. A. Mason, E. D. Bloch, W. L. Queen, M. R. Hudson, C. M. Brown and J. R. Long, Chem. Sci., 2013, 4, 2054–2061 RSC.
- M. Mofarahi and S. M. Salehi, Adsorption, 2013, 19, 101–110 CrossRef CAS.
- Y. Wang, S. Yuan, Z. Hu, T. Kundu, J. Zhang, S. B. Peh, Y. Cheng, J. Dong, D. Yuan, H.-C. Zhou and D. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 7118–7126 CrossRef CAS.
- R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS.
- A. M. Plonka, X. Chen, H. Wang, R. Krishna, X. Dong, D. Banerjee, W. R. Woerner, Y. Han, J. Li and J. B. Parise, Chem. Mater., 2016, 28, 1636–1646 CrossRef CAS.
- J. Cai, J. Yu, H. Xu, Y. He, X. Duan, Y. Cui, C. Wu, B. Chen and G. Qian, Cryst. Growth Des., 2013, 13, 2094–2097 CrossRef CAS.
- O. T. Qazvini, R. Babarao, Z.-L. Shi, Y.-B. Zhang and S. G. Telfer, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS.
- C. He, Y. Wang, Y. Chen, X. Wang, J. Yang, L. Li and J. Li, Ind. Eng. Chem. Res., 2020, 59, 6123–6129 CrossRef CAS.
- Y. Chen, Z. Qiao, H. Wu, D. Lv, R. Shi, Q. Xia, J. Zhou and Z. Li, Chem. Eng. Sci., 2018, 175, 110–117 CrossRef CAS.
- X. Wang, Y. Wu, X. Zhou, J. Xiao, Q. Xia, H. Wang and Z. Li, Chem. Eng. Sci., 2016, 155, 338–347 CrossRef CAS.
- J. Pires, M. L. Pinto and V. K. Saini, ACS Appl. Mater. Interfaces, 2014, 6, 12093–12099 CrossRef CAS.
- F.-Z. Sun, S.-Q. Yang, R. Krishna, Y.-H. Zhang, Y.-P. Xia and T.-L. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 6105–6111 CrossRef CAS.
- H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS.
- U. Böhme, B. Barth, C. Paula, A. Kuhnt, W. Schwieger, A. Mundstock, J. Caro and M. Hartmann, Langmuir, 2013, 29, 8592–8600 CrossRef.
- M. Hartmann, U. Böhme, M. Hovestadt and C. Paula, Langmuir, 2015, 31, 12382–12389 CrossRef CAS.
- H. Zeng, X.-J. Xie, M. Xie, Y.-L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef.
- M. C. Das, H. Xu, Z. Wang, G. Srinivas, W. Zhou, Y.-F. Yue, V. N. Nesterov, G. Qian and B. Chen, Chem. Commun., 2011, 47, 11715–11717 RSC.
- W. Liang, F. Xu, X. Zhou, J. Xiao, Q. Xia, Y. Li and Z. Li, Chem. Eng. Sci., 2016, 148, 275–281 CrossRef CAS.
- X.-Y. Li, Z.-J. Li, Y.-Z. Li, L. Hou, Z. Zhu and Y.-Y. Wang, Inorg. Chem., 2018, 57, 12417–12423 CrossRef CAS.
- Y. Chen, H. Wu, D. Lv, R. Shi, Y. Chen, Q. Xia and Z. Li, Ind. Eng. Chem. Res., 2018, 57, 4063–4069 CrossRef CAS.
- Z. Xu, X. Xiong, J. Xiong, R. Krishna, L. Li, Y. Fan, F. Luo and B. Chen, Nat. Commun., 2020, 11, 3163 CrossRef CAS.
- L. Yang, Y. Wang, Y. Chen, J. Yang, X. Wang, L. Li and J. Li, Chem. Eng. J., 2020, 387, 124137 CrossRef CAS.
- H. Wu, Y. Chen, D. Lv, R. Shi, Y. Chen, Z. Li and Q. Xia, Sep. Purif. Technol., 2019, 212, 51–56 CrossRef CAS.
- Y. He, Z. Zhang, S. Xiang, F. R. Fronczek, R. Krishna and B. Chen, Chem. – Eur. J., 2012, 18, 613–619 CrossRef CAS.
- Y. He, Z. Zhang, S. Xiang, F. R. Fronczek, R. Krishna and B. Chen, Chem. Commun., 2012, 48, 6493–6495 RSC.
- L. Zhang, K. Jiang, L. Li, Y.-P. Xia, T.-L. Hu, Y. Yang, Y. Cui, B. Li, B. Chen and G. Qian, Chem. Commun., 2018, 54, 4846–4849 RSC.
- L. Zhang, K. Jiang, J. Zhang, J. Pei, K. Shao, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, ACS Sustainable Chem. Eng., 2019, 7, 1667–1672 CrossRef CAS.
- H. S. Scott, M. Shivanna, A. Bajpai, D. G. Madden, K.-J. Chen, T. Pham, K. A. Forrest, A. Hogan, B. Space, J. J. Perry Iv and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2017, 9, 33395–33400 CrossRef CAS.
- J. W. Yoon, J. S. Lee, S. Lee, K. H. Cho, Y. K. Hwang, M. Daturi, C.-H. Jun, R. Krishna and J.-S. Chang, Chem. – Eur. J., 2015, 21, 18431–18438 CrossRef CAS.
- H.-M. Wen, H. Wang, B. Li, Y. Cui, H. Wang, G. Qian and B. Chen, Inorg. Chem., 2016, 55, 7214–7218 CrossRef CAS.
- D.-M. Chen, X.-H. Liu, J.-Y. Tian, J.-H. Zhang, C.-S. Liu and M. Du, Inorg. Chem., 2017, 56, 14767–14770 CrossRef CAS.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O’Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS.
- H.-J. Lv, Y.-P. Li, Y.-Y. Xue, Y.-C. Jiang, S.-N. Li, M.-C. Hu and Q.-G. Zhai, Inorg. Chem., 2020, 59, 4825–4834 CrossRef CAS.
- Z. Yao, Z. Zhang, L. Liu, Z. Li, W. Zhou, Y. Zhao, Y. Han, B. Chen, R. Krishna and S. Xiang, Chem. – Eur. J., 2016, 22, 5676–5683 CrossRef CAS.
- X. Duan, Q. Zhang, J. Cai, Y. Yang, Y. Cui, Y. He, C. Wu, R. Krishna, B. Chen and G. Qian, J. Mater. Chem. A, 2014, 2, 2628–2633 RSC.
- Q. Dong, Y. Guo, H. Cao, S. Wang, R. Matsuda and J. Duan, ACS Appl. Mater. Interfaces, 2020, 12, 3764–3772 CrossRef CAS.
- H. Cui, S. Chen, H. Arman, Y. Ye, A. Alsalme, R.-B. Lin and B. Chen, Inorg. Chim. Acta, 2019, 495, 118938 CrossRef CAS.
- H. Yang, T. X. Trieu, X. Zhao, Y. Wang, Y. Wang, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2019, 58, 11757–11762 CrossRef CAS.
- T. Xu, Z. Jiang, P. Liu, H. Chen, X. Lan, D. Chen, L. Li and Y. He, ACS Appl. Nano Mater., 2020, 3, 2911–2919 CrossRef CAS.
- J. Duan, W. Jin and R. Krishna, Inorg. Chem., 2015, 54, 4279–4284 CrossRef CAS.
- W. Fan, X. Wang, X. Liu, B. Xu, X. Zhang, W. Wang, X. Wang, Y. Wang, F. Dai, D. Yuan and D. Sun, ACS Sustainable Chem. Eng., 2019, 7, 2134–2140 CrossRef CAS.
- R. Liu, Q.-Y. Liu, R. Krishna, W. Wang, C.-T. He and Y.-L. Wang, Inorg. Chem., 2019, 58, 5089–5095 CrossRef CAS.
- H. Q. Wu, C. S. Yan, F. Luo and R. Krishna, Inorg. Chem., 2018, 57, 3679–3682 CrossRef CAS.
- Y.-P. Li, Y. Wang, Y.-Y. Xue, H.-P. Li, Q.-G. Zhai, S.-N. Li, Y.-C. Jiang, M.-C. Hu and X. Bu, Angew. Chem., Int. Ed., 2019, 58, 13590–13595 CrossRef CAS.
- Y. Ye, S. Chen, L. Chen, J. Huang, Z. Ma, Z. Li, Z. Yao, J. Zhang, Z. Zhang and S. Xiang, ACS Appl. Mater. Interfaces, 2018, 10, 30912–30918 CrossRef CAS.
- Y. Ye, Z. Ma, R.-B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS.
- H. Li, H. Bonduris, X. Zhang, Y. Ye, A. Alsalme, R.-B. Lin, Z. Zhang, S. Xiang and B. Chen, J. Solid State Chem., 2020, 284, 121209 CrossRef CAS.
- L. Zhang, C. Zou, M. Zhao, K. Jiang, R. Lin, Y. He, C.-D. Wu, Y. Cui, B. Chen and G. Qian, Cryst. Growth Des., 2016, 16, 7194–7197 CrossRef CAS.
- P. Yan, J. Yang, X. Hao, Z. Chen, G. Shen, Y. Zhao, D. Ma and J. Zhu, CrystEngComm, 2020, 22, 275–282 RSC.
- H. Cui, Y. Ye, H. Arman, Z. Li, A. Alsalme, R.-B. Lin and B. Chen, Cryst. Growth Des., 2019, 19, 5829–5835 CrossRef CAS.
- L. Jiang, N. Wu, Q. Li, J. Li, D. Wu and Y. Li, Inorg. Chem., 2019, 58, 4080–4084 CrossRef CAS.
- G. Chang, B. Li, H. Wang, T. Hu, Z. Bao and B. Chen, Chem. Commun., 2016, 52, 3494–3496 RSC.
- W. Fan, S. Yuan, W. Wang, L. Feng, X. Liu, X. Zhang, X. Wang, Z. Kang, F. Dai, D. Yuan, D. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 8728–8737 CrossRef.
- H. Zeng, M. Xie, Y.-L. Huang, Y. Zhao, X.-J. Xie, J.-P. Bai, M.-Y. Wan, R. Krishna, W. Lu and D. Li, Angew. Chem., Int. Ed., 2019, 58, 8515–8519 CrossRef CAS.
- X.-Y. Li, Y.-Z. Li, L.-N. Ma, L. Hou, C.-Z. He, Y.-Y. Wang and Z. Zhu, J. Mater. Chem. A, 2020, 8, 5227–5233 RSC.
- D.-Y. Ma, z li, J. Zhu, Y. Zhou, L. Chen, X. Mai, M. Liufu, Y. Wu and Y. Li, J. Mater. Chem. A, 2020, 8, 11933–11937 RSC.
- L. Li, J. Wang, Z. Zhang, Q. Yang, Y. Yang, B. Su, Z. Bao and Q. Ren, ACS Appl. Mater. Interfaces, 2019, 11, 2543–2550 CrossRef CAS.
- M. L. Foo, R. Matsuda, Y. Hijikata, R. Krishna, H. Sato, S. Horike, A. Hori, J. Duan, Y. Sato, Y. Kubota, M. Takata and S. Kitagawa, J. Am. Chem. Soc., 2016, 138, 3022–3030 CrossRef CAS.
- R. Eguchi, S. Uchida and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 1635–1639 CrossRef CAS.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS.
- L. Yang, X. Cui, Z. Zhang, Q. Yang, Z. Bao, Q. Ren and H. Xing, Angew. Chem., Int. Ed., 2018, 57, 13145–13149 CrossRef CAS.
- H.-M. Wen, L. Li, R.-B. Lin, B. Li, B. Hu, W. Zhou, J. Hu and B. Chen, J. Mater. Chem. A, 2018, 6, 6931–6937 RSC.
- B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS.
- Y. Wang and D. Zhao, Cryst. Growth Des., 2017, 17, 2291–2308 CrossRef CAS.
- S. Xiang, W. Zhou, J. M. Gallegos, Y. Liu and B. Chen, J. Am. Chem. Soc., 2009, 131, 12415–12419 CrossRef CAS.
- Q. Min Wang, D. Shen, M. Bülow, M. Ling Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef.
- T. M. Nicholson and S. K. Bhatia, J. Phys. Chem. B, 2006, 110, 24834–24836 CrossRef CAS.
- Z. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. Ren, X. Lu and S. Deng, Langmuir, 2011, 27, 13554–13562 CrossRef CAS.
- K. Lee, W. C. Isley, A. L. Dzubak, P. Verma, S. J. Stoneburner, L.-C. Lin, J. D. Howe, E. D. Bloch, D. A. Reed, M. R. Hudson, C. M. Brown, J. R. Long, J. B. Neaton, B. Smit, C. J. Cramer, D. G. Truhlar and L. Gagliardi, J. Am. Chem. Soc., 2014, 136, 698–704 CrossRef CAS.
- J. E. Bachman, D. A. Reed, M. T. Kapelewski, G. Chachra, D. Jonnavittula, G. Radaelli and J. R. Long, Energy Environ. Sci., 2018, 11, 2423–2431 RSC.
- W. C. Zeise, Ann. Phys., 1831, 97, 497–541 CrossRef.
- J. Chatt and L. A. Duncanson, J. Chem. Soc. (Resumed), 1953, 2939–2947, 10.1039/JR9530002939.
- S. Uchida, R. Kawamoto, H. Tagami, Y. Nakagawa and N. Mizuno, J. Am. Chem. Soc., 2008, 130, 12370–12376 CrossRef CAS.
- D. J. Safarik and R. B. Eldridge, Ind. Eng. Chem. Res., 1998, 37, 2571–2581 CrossRef CAS.
- S. Aguado, G. Bergeret, C. Daniel and D. Farrusseng, J. Am. Chem. Soc., 2012, 134, 14635–14637 CrossRef CAS.
- R. B. Eldridge, Ind. Eng. Chem. Res., 1993, 32, 2208–2212 CrossRef.
- A. C. C. Campos, R. A. dos Reis, A. Ortiz, D. Gorri and I. Ortiz, Ind. Eng. Chem. Res., 2018, 57, 10071–10085 CrossRef CAS.
- Q.-Y. Yang, P. Lama, S. Sen, M. Lusi, K.-J. Chen, W.-Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry Iv, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- J. van den Bergh, C. Gücüyener, E. A. Pidko, E. J. M. Hensen, J. Gascon and F. Kapteijn, Chem. – Eur. J., 2011, 17, 8832–8840 CrossRef CAS.
- K. Kishida, Y. Watanabe, S. Horike, Y. Watanabe, Y. Okumura, Y. Hijikata, S. Sakaki and S. Kitagawa, Eur. J. Inorg. Chem., 2014, 2747–2752 CrossRef CAS.
- S. Sen, N. Hosono, J.-J. Zheng, S. Kusaka, R. Matsuda, S. Sakaki and S. Kitagawa, J. Am. Chem. Soc., 2017, 139, 18313–18321 CrossRef CAS.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS.
- J. M. Michael and B. Smith, March's Advanced Organic Chemistry, Wiley, 6th edn, 2007, pp. 363–364 Search PubMed.
- W. S. W. Ho, G. Doyle, D. W. Savage and R. L. Pruett, Ind. Eng. Chem. Res., 1988, 27, 334–337 CrossRef CAS.
- K.-J. Chen, D. G. Madden, T. Pham, K. A. Forrest, A. Kumar, Q.-Y. Yang, W. Xue, B. Space, J. J. Perry Iv, J.-P. Zhang, X.-M. Chen and M. J. Zaworotko, Angew. Chem., Int. Ed., 2016, 55, 10268–10272 CrossRef CAS.
- S. K. Elsaidi, M. H. Mohamed, H. T. Schaef, A. Kumar, M. Lusi, T. Pham, K. A. Forrest, B. Space, W. Xu, G. J. Halder, J. Liu, M. J. Zaworotko and P. K. Thallapally, Chem. Commun., 2015, 51, 15530–15533 RSC.
- A. Kumar, D. G. Madden, M. Lusi, K.-J. Chen, E. A. Daniels, T. Curtin, J. J. Perry Iv and M. J. Zaworotko, Angew. Chem., Int. Ed., 2015, 54, 14372–14377 CrossRef CAS.
- K.-J. Chen, R.-B. Lin, P.-Q. Liao, C.-T. He, J.-B. Lin, W. Xue, Y.-B. Zhang, J.-P. Zhang and X.-M. Chen, Cryst. Growth Des., 2013, 13, 2118–2123 CrossRef CAS.
- V. Bon, E. Brunner, A. Pöppl and S. Kaskel, Adv. Funct. Mater., 2020 DOI:10.1002/adfm.201907847.
- N. Hosono, A. Terashima, S. Kusaka, R. Matsuda and S. Kitagawa, Nat. Chem., 2019, 11, 109–116 CrossRef CAS.
- S. Mukherjee, A. V. Desai and S. K. Ghosh, Coord. Chem. Rev., 2018, 367, 82–126 CrossRef CAS.
- X. Han, S. Yang and M. Schröder, Nat. Rev. Chem., 2019, 3, 108–118 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |