Open Access Article
Open Access ArticleFacile synthesis of fluorescent hetero[8]circulene analogues with tunable solubilities and optical properties†
Yusuke
Matsuo
,
Fengkun
Chen
,
Koki
Kise
,
Takayuki
Tanaka
 * and
Atsuhiro
Osuka
* and
Atsuhiro
Osuka

Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan. E-mail: taka@kuchem.kyoto-u.ac.jp
First published on 30th October 2019
Abstract
Hetero[8]circulenes are an interesting class of polycyclic heteroaromatic molecules having rigid and planar structures, which are promising in light of their potential applications for OLEDs, OFETs and so forth. Although their synthetic methods have been developed in some specific cases, a facile synthetic protocol of novel hetero[8]circulenes with tunable properties is highly desirable. We herein report the unexpected formation of methoxy-substituted quasi-aza[8]circulene and its conversion into unprecedented triazaoxa[8]circulene. The structures and optical properties were comparatively studied. Remarkably, triazaoxa[8]circulene is highly soluble in THF, acetone and DMSO mainly because of effective hydrogen-bonding of the NH moieties to these solvents. Their highly soluble nature in various solvents enabled us to study the solvent effects of these molecules. In particular, triazaoxa[8]circulene displays a high fluorescence quantum yield of 0.72 in DMSO. Furthermore, enantiomeric separation of highly distorted quasi-aza[8]circulene was successfully achieved by chiral HPLC. Thus, these novel hetero[8]circulene derivatives are practically useful fluorescent nanographene-like molecules with intriguing optical properties.
Introduction
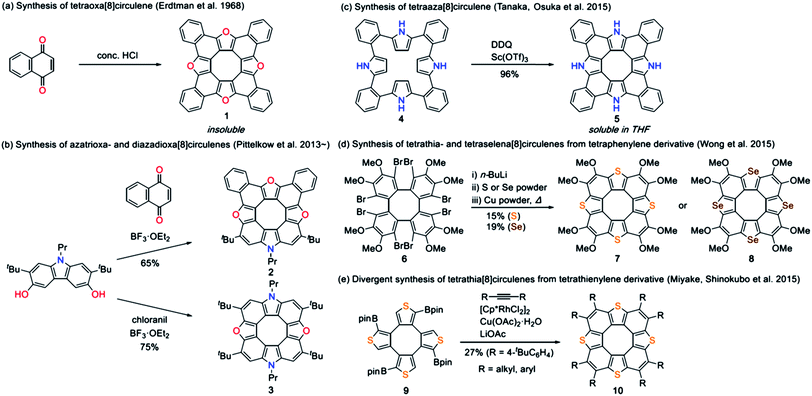
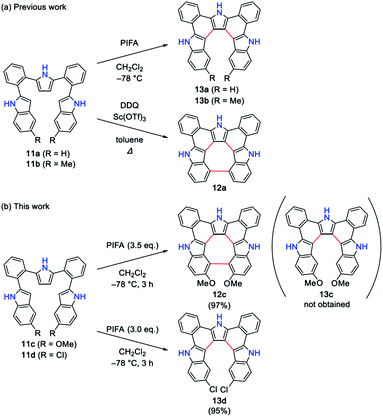
Hetero[8]circulenes belong to a class of polycyclic heteroaromatic molecules (PHAs) possessing eight alternately arranged condensed arenes and/or heteroles that surround a central eight-membered ring.1–3 Unlike the parent all-benzene analogue (i.e. [8]circulene) adopting a saddle-shaped conformation,4 most of the hetero[8]circulenes take planar structures and have attracted considerable attention as promising materials for organic electronics.5–10 One of the old examples is tetraoxa[8]circulene 1 reported by Erdtman et al. in 1968,5 which was synthesized by acid-catalyzed condensation of α-naphthoquinone although its formation was only identified by mass analysis due to its extremely poor solubility (Chart 1). Several tetraoxa[8]circulene derivatives were reported later on,6 but it was not until 2013 that hetero[8]circulenes including units other than furan units were prepared. Azatrioxa[8]circulene 2 and diazadioxa[8]circulene 3 were synthesized by Pittelkow et al. by using hydroxy-substituted carbazole precursors under acidic conditions.7 Recently, we reported the first synthesis of tetraaza[8]circulene 5 by a fold-in type oxidative fusion reaction of ortho-phenylene-bridged cyclic tetrapyrrole 4.8a From octabromotetraphenylene 6, Wong et al. synthesized tetrathia- and tetraselena[8]circulenes 7 and 8 by Li-mediated nucleophilic reactions followed by thermal annealing.9a Miyake and Shinokubo utilized tetraboryltetrathienylene 9 as a precursor of tetrathia[8]circulenes 10.10 Interestingly, multiply alkylated tetrathia[8]circulenes form aggregates in CDCl3 depending on the number of peripheral substituents.10b Despite this progress, all these reactions required specific synthetic precursors or harsh reaction conditions, precluding the fine property tunability of [8]circulenes. A facile synthetic approach toward unprecedented hetero[8]circulenes is thus highly desirable.Recently, we reported the synthesis of triaza-quasi-[8]circulene 12a,11 which lacks one heterole moiety from the hetero[8]circulene structure.12 Due to the structural constraint, 12a takes on a non-planar geometry. Interestingly, under milder conditions, triaza[7]helicene 13a was exclusively obtained from the identical precursor 11a. Dimethyl-substituted triaza[7]helicene 13b was then synthesized to estimate a helicene racemization activation free energy of 23.8 kcal mol−1 (at 298.15 K). On the other hand, the racemization rate of 12a was computed to be too fast for experimental determination.
Herein, we report an unexpected formation of dimethoxy-substituted triaza-quasi-[8]circulene 12c and its conversion to the hitherto unknown triazaoxa[8]circulene 14. The planar and non-substituted triazaoxa[8]circulene 14 was surprisingly well soluble in polar solvents such as THF and acetone. The solvent-dependent optical properties will be discussed in conjunction with further N-substituted derivatives.
Results and discussion
Synthesis
The synthesis of triaza-quasi-[8]circulene is straightforward; the coupling reaction of indole derivatives with 2,5-bis(2-bromophenyl)pyrrole followed by oxidation under appropriate conditions.12 This time, we synthesized a dimethoxy-substituted precursor 11c as a potential cyclization substrate leading to dimethoxy-substituted triaza[7]helicene 13c. However, even under milder oxidative reaction conditions (PIFA at −78 °C), quasi-[8]circulene 12c was produced in 97% yield and 13c was not detected (Scheme 1). This result has been ascribed to the higher spin density at the 4-position than at the 3-position in the cation radical of 11c (see Fig. S5-1, ESI†). Therefore, intramolecular 4,4′-oxidative coupling in 11c proceeded preferably in spite of the steric repulsion imposed by the two methoxy groups. In contrast, the spin density distribution is reversed in the case of the dichloro-substituted precursor 11d, which preferred oxidative coupling between the central pyrrole and indole 3-position to give triaza[7]helicene 13d in 95% yield. The structures of 12c and 13d were unambiguously revealed by X-ray diffraction analysis (Fig. 1). Both compounds displayed highly twisted structures, the degree of which can be quantified by mean-plane deviation (MPD) values to be 0.45 Å for 12c and 0.55 Å for 13d (35 atoms). The value of 12c is distinctly larger than that of 12a (0.31 Å) due to the two methoxy groups. Because of the twisted structures, intermolecular π–π stacking is not favorable. In the crystal lattice, two or three THF molecules are encapsulated and form hydrogen bonds with pyrrolic NH sites.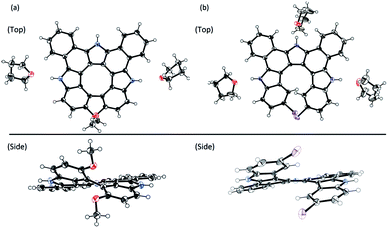 | ||
| Fig. 1 X-ray crystal structures of (a) 12c and (b) 13d. The thermal ellipsoids were scaled to 50% probability level. Solvent molecules were omitted in the side views. | ||
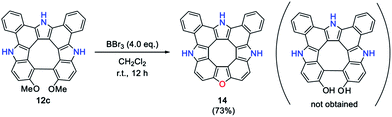
It is well known that biphenylene-2,2′-diol derivatives undergo thermal dehydration reaction to afford the corresponding dibenzofuran derivatives.13 We attempted demethylation of 12c with BBr3 in CH2Cl2 at room temperature, which gave triazaoxa[8]circulene 14 as a yellow solid in 73% yield (Scheme 2). Matrix-assisted-laser-desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) revealed the molecular ion peak of 14 at m/z = 457.1218 (calcd for C32H15N3O = 457.1210 [M]+). The 1H NMR spectrum in DMSO-d6 showed two NH peaks at 12.93 and 12.64 ppm and six peaks at 9.09, 8.82, 7.98, 7.96, 7.83 and 7.77 ppm. Even at low temperature, the corresponding diol intermediate was not isolated, presumably because of the fast dehydration reaction in the diol intermediate driven by strain release. It is noteworthy that this is the first example of hetero[8]circulene consisting of three pyrrole units and one furan unit. All the reaction steps are high yielding and the reaction products are easily separable, thus allowing us to obtain 14 in more than 300 mg quantity. In spite of the lack of solubilizing substituents, 14 is quite soluble in acetone and THF but less soluble in CH2Cl2. The single crystal structure of 14 is shown in Fig. 2. The molecule is almost planar with a MPD value of 0.014 Å (35 atoms). Three acetone molecules adopt hydrogen bonding with the three NH sites, and the layer of three acetone molecules prevents the planar core molecule from intermolecular π–π contact. The contribution of the central formal 8π cyclooctatetraene moiety is another point to discuss. The constituent eight C–C bond lengths are in the range of 1.404(2)–1.431(2) Å (see Fig. S6-5, ESI†). The NICS(0) and NICS(1)zz values at the center of the eight-membered ring were calculated by the GIAO method to be +7.00 and +18.05 ppm, respectively (Fig. S5-12 and S5-13, ESI†). This situation is in analogy to other hetero[8]circulenes.7,8 Although it may have decent antiaromatic contributions, we would say that the aromatic contributions of each benzene, pyrrole and furan moiety are predominant as indicated by HOMA and NICS(0) values at the centre points of each aromatic ring (see Fig. S5-14, ESI†).
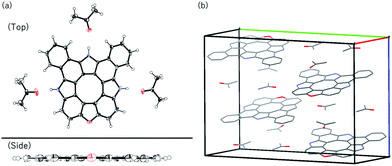 | ||
| Fig. 2 (a) X-ray crystal structures of 14. The thermal ellipsoids were scaled to 50% probability level. Solvent molecules in the side view were omitted for clarity. (b) Packing diagram. | ||
N-substitution reactions
An advantage of the new synthetic scheme for 14 against other [8]circulene syntheses may be the presence of the intact NH moieties which can be further decorated. Previously, we reported that sequential N-butylation of tetraaza[8]circulene 5 slightly altered its optical properties.14 Along with this strategy, N-alkylation reactions were tested for 12a, 12c and 14. N-Butylation was easily achieved for 12a and 14 by utilizing the reported method (excess NaH, 1-iodobutane in THF) to give tributylated products 12a-Bu and 14-Bu in 43% and 78% yields, respectively.14,15 On the other hand, the same reaction of 12c resulted in a very low yield of N-butylated products. It is likely that the highly twisted structure of 12c causes degradation under these conditions. Then, the reaction conditions were screened, and we found that the treatment of 12c with 200 equivalents of 1-iodobutane in the presence of potassium tert-butoxide at room temperature for 24 hours afforded 12c-Bu in 46% yield. All the solid-state structures have been revealed by X-ray diffraction analysis as shown in Scheme 3. The planarity and bond-length alternation do not change so much for 14-Bu, while the degree of non-planarity of 12a-Bu and 12c-Bu increased compared with 12a and 12c.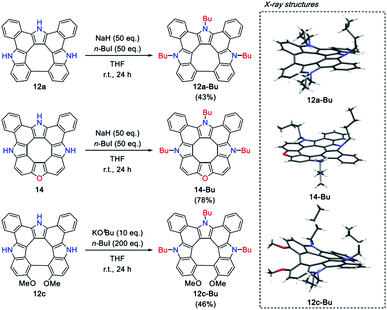 | ||
| Scheme 3 N-substitution reactions and the X-ray crystal structures of 12a-Bu, 12c-Bu and 14-Bu. Solvent molecules were omitted for clarity. | ||
With these in hand, we next investigated their aggregation behaviours in solution. Basically, all these hetero[8]circulene analogues are quite soluble in THF and the absorption spectra obey the Beer–Lambert law in the UV/vis concentration (ca. 10−5–10−6 M) (see Fig. S7-1 and S7-2, ESI†). The tri-N-butylated products were more soluble in less polar solvents as compared with non-substituted ones. For instance, the solubilities of 14 and 14-Bu were roughly estimated by a simple filtration experiment to be 0.15 mg mL−1 in CH2Cl2 for 14 and 26 mg mL−1 in CH2Cl2 for 14-Bu at room temperature, while those in acetone were comparable (4.6 mg mL−1 for 14 and 5.1 mg mL−1 for 14-Bu) (see Table S9-1, ESI†). In addition, 12a-Bu, 12c-Bu and 14-Bu are even soluble in n-hexane. On the other hand, 12a, 12c and 14 bearing intact NH sites are especially soluble in hydrogen-bond accepting solvents such as THF, acetone and DMSO. They are also soluble in a mixed solvent, up to 10% THF in n-hexane, without the observation of aggregation-induced fluorescence quenching (Fig. S7-9†). In the solid state, the emission spectra were completely quenched.
Nanographenes are, in many cases, only soluble in halogenated solvents, and the low solubility often hampers further functionalization and applications.16 The high solubility can be a unique feature of hetero[8]circulenes possessing intact NH sites, and is useful for solution-processed electronics applications and is potentially applicable for biochemistry.17
Optical properties
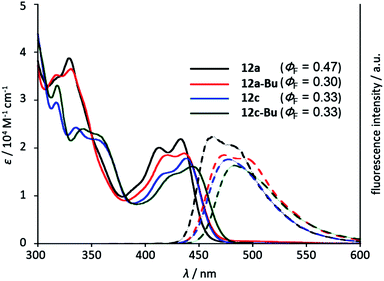
Hetero[8]circulenes are highly luminescent molecules upon photoirradiation because of their rigid structures that prevent thermal energy loss due to structural change in the excited state. In fact, tetraaza[8]circulene 5 showed a very small Stokes shift (ca. 180 cm−1) in THF with a high fluorescence quantum yield (ΦF = 0.59). The absorption and emission spectra of quasi-aza[8]circulene 12a are red-shifted with a relatively larger Stokes shift (1450 cm−1) probably due to their non-planar conformations. The absorption spectrum of 12c is further shifted up to 474 nm compared with that of 12a (433 nm) (Fig. 3 and Table 1). DFT calculations revealed that the larger non-planarity of 12c raised its HOMO energy level, giving rise to the decreased HOMO–LUMO gap (see Fig. S5-8, ESI†). Instead, the emission quantum yield was decreased to ΦF = 0.33. Upon N-butylation, the absorption and emission spectra were red-shifted. While 12a-Bu and 14-Bu showed decreased ΦF values from non-butylated ones, 12c and 12c-Bu displayed the same ΦF values in THF.| Compound | Solvent | λ abs (nm) | λ flu (nm) | Stokes shift (cm−1) | Φ F | τ F (ns) | k r (×108 s−1) | k nr (×108 s−1) |
|---|---|---|---|---|---|---|---|---|
| a See ref. 8a. | ||||||||
| 5 | THF | 413 | 416 | 180 | 0.59 | 3.8 | 1.6 | 1.1 |
| 12a | THF | 433 | 462 | 1450 | 0.47 | 5.6 | 0.83 | 0.94 |
| 12a | DMSO | 436 | 490 | 2530 | 0.58 | 8.2 | 0.71 | 0.51 |
| 12a-Bu | THF | 436 | 473 | 1790 | 0.30 | 5.1 | 0.59 | 1.4 |
| 12a-Bu | DMSO | 435 | 496 | 2830 | 0.42 | 6.7 | 0.62 | 0.87 |
| 12c | THF | 426 | 476 | 1680 | 0.33 | 3.6 | 0.92 | 1.8 |
| 12c | DMSO | 444 | 485 | 1900 | 0.49 | 4.6 | 1.1 | 1.1 |
| 12c-Bu | THF | 443 | 491 | 1870 | 0.33 | 4.0 | 0.82 | 1.7 |
| 12c-Bu | DMSO | 447 | 494 | 2130 | 0.42 | 5.0 | 0.84 | 1.2 |
| 14 | THF | 426 | 437 | 590 | 0.52 | 4.5 | 1.2 | 1.1 |
| 14 | DMSO | 429 | 449 | 1040 | 0.72 | 5.7 | 1.3 | 0.50 |
| 14-Bu | THF | 440 | 451 | 550 | 0.40 | 4.1 | 0.97 | 1.5 |
| 14-Bu | DMSO | 442 | 460 | 890 | 0.70 | 4.8 | 1.5 | 0.62 |
Triazaoxa[8]circulene 14 has a rigid and planar structure like 5, but exhibited red-shifted absorption and emission bands (Fig. 4). The decrease of the molar extinction coefficient is ascribed to its lower symmetry, while the red-shift may be due to the lack of two benzo-fused segments. Azatrioxa[8]circulene 2 also showed a similar shift compared with a non-benzo-fused analogue.7a This tendency is consistent with theoretical calculations (see Fig. S5-10, ESI†).18
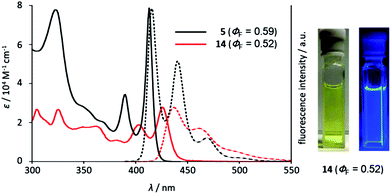 | ||
| Fig. 4 UV/vis absorption and emission spectra of 5 and 14 in THF. The right figures show the photos (under room light and under UV light). | ||
As described above, 14-Bu is quite soluble both in polar and non-polar solvents without any aggregation effects. Thus, the absorption and emission spectra were recorded in various solvents as shown in Fig. 5. The absorption spectra are almost unchanged in various solvents with clear vibronic structures, while the emission spectra are slightly but gradually red-shifted as the polarity of the solvent increases. Interestingly, the emission is largely enhanced up to ΦF = 0.70 in DMSO. This tendency is also observed for 14 (Table 1 and Fig. S7-5†). The fluorescence lifetimes (τF) were measured using the time-correlated single photon counting (TCSPC) technique. The longest lifetimes were observed in DMSO in all cases, but mostly the τF values are in the range of 3.0–7.0 ns. From these optical parameters, the radiative rate constant (kr) and non-radiative rate constant (knr) were estimated. In 14 and 14-Bu, larger kr and smaller knr both contributed to higher emission in DMSO.19 For others, the larger ΦF values in polar solvents were mainly attributed to smaller knr values. Thus, deactivation processes in the excited state can be somehow suppressed in polar solvents.
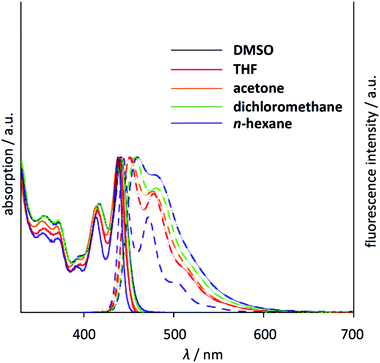 | ||
| Fig. 5 Normalized UV/vis absorption (solid lines) and emission (dashed lines) spectra of 14-Bu in n-hexane (purple), THF (red), CH2Cl2 (green), acetone (orange) and DMSO (blue). | ||
Chiral separation
The highly twisted structure of quasi-aza[8]circulene 12c prompted us to examine its enantiomeric separation. Although the racemization activation barrier of 12a was computed to be about 6.0 kcal mol−1,12 that of 12c was estimated to be more than 40 kcal mol−1 (see Fig. S10-2, ESI†). Through screening of the chiral stationary phase and eluents, we found that 12c-Bu was successfully separated by HPLC equipped with a CHIRALPAK IA-3 (φ = 4.6 mm, Daicel Co.) with CH2Cl2 as an eluent. The enantiomers were completely separated and the circular dichroism (CD) spectra showed a mirror-image pattern (Fig. 6). The first fraction which showed a negative Cotton effect at around 450 nm was assigned as the (M) twisted form by comparing with the calculated CD spectrum (see Fig. S10-3, ESI†). This CD spectral feature resembles that of quasi-[7]circulene derivatives,20 but is reversed when compared with azahelicene derivatives.12,21 The enantiomers were quite stable and no racemization took place under heating in toluene. Since 12c-Bu is soluble in various solvents, this may be useful in chiral recognition in suitable media. While Wong et al. reported the chiral properties of tetraphenylene-based molecules,22 enantiomeric separation of polycondensed nanographene-like molecules other than helicenes is quite rare.23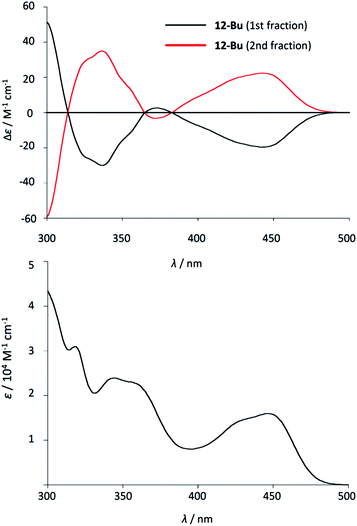 | ||
| Fig. 6 (Top) CD and (bottom) UV/vis absorption spectra of the first fraction (black) and the second fraction (red) of 12c-Bu in CH2Cl2. | ||
Conclusions
In summary, non-planar quasi-aza[8]circulene derivatives and planar triazaoxa[8]circulene were prepared in good yields via a facile synthetic protocol. These hetero[8]circulenes are highly soluble in organic solvents without solubilizing substituents due to the hydrogen bonding interaction between the outer-pointing NH site and hydrogen-bond accepting solvent. Furthermore, N-alkylation reactions facilitated tuning of the solubility and optical properties. Taking advantage of the non-aggregation behaviours in various solvents, we studied the solvent effects on the absorption and emission spectra as well as emission lifetime. As an important finding, the fluorescence quantum yields were enhanced in DMSO mostly due to the suppression of non-radiative decay from the excited state. Among them, triazaoxa[8]circulenes 14 and 14-Bu showed high fluorescence quantum yields of 0.72 and 0.70 in DMSO, respectively. Enantiomeric separation of 12c-Bu was also successfully achieved using the chiral HPLC technique as the first chiral quasi-[8]circulene derivative. Further exploration of the synthesis and characterization of novel hetero[8]circulenes is in progress in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by JSPS KAKENHI Grant Numbers (JP18K14199, JP16H00909 and JP18H03910). K. K. acknowledges a JSPS Fellowship for Young Scientists. We appreciate Prof. Tsuyoshi Kawai, Dr Mihoko Yamada and Mr Yuto Yoshida (NAIST) for circular dichroism measurements.Notes and references
- (a) Carbon-Rich Compounds, ed. M. M. Haley and R. R. Tykwinski, Wiley-VCH, Weinheim, 2006 Search PubMed; (b) Functional Organic Materials, ed. T. J. J. Müller and U. H. F. Bunz, Wiley-VCH, Weinheim, 2007 Search PubMed; (c) Polycyclic Arenes and Heteroarenes, ed. Q. Miao, Wiley-VCH, New York, 2015 Search PubMed.
- (a) A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616 RSC; (b) M. Stępień, E. Gońka, M. yła and N. Sprutta, Chem. Rev., 2017, 117, 3479 CrossRef PubMed; (c) M. A. Majewski and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 86 CrossRef CAS PubMed; (d) M. Grzybowski, B. Sadowski, H. Butenschön and D. T. Gryko, Angew. Chem., Int. Ed., 2019, 58 DOI:10.1002/anie.201904934.
- (a) M. Stępień, Synlett, 2013, 24, 1316 CrossRef; (b) C.-N. Feng, Y.-C. Hsieh and Y.-T. Wu, Chem. Rec., 2015, 15, 266 CrossRef CAS PubMed; (c) T. Hensel, N. N. Andersen, M. Plesner and M. Pittelkow, Synlett, 2016, 27, 498 CAS.
- (a) C.-N. Feng, M.-Y. Kuo and Y.-T. Wu, Angew. Chem., Int. Ed., 2013, 52, 7791 CrossRef CAS PubMed; (b) Y. Sakamoto and T. Suzuki, J. Am. Chem. Soc., 2013, 135, 14074 CrossRef CAS PubMed; (c) R. W. Miller, A. K. Duncan, S. T. Schneebeli, D. L. Gray and A. C. Whalley, Chem.–Eur. J., 2014, 20, 3705 CrossRef CAS PubMed; (d) C.-N. Feng, W.-C. Hsu, J.-Y. Li, M.-Y. Kuo and Y.-T. Wu, Chem.–Eur. J., 2016, 22, 9198 CrossRef CAS PubMed.
- H. Erdtman and H.-E. Högberg, The first synthetic attempt can be further traced back to 1881 when Knapp and Schultz reported that α-naphthoquinone was reacted with concentrated hydrochloric acid to give insoluble material. Erdtman also synthesized the same material in 1933. However, the product had been mis-assigned and it was finally proved to be 1 in 1968, Chem. Commun., 1968, 773 RSC.
- (a) H. Erdtman and H.-E. Högberg, Tetrahedron Lett., 1970, 11, 3389 CrossRef; (b) J.-E. Berg, H. Erdtman, H.-E. Högberg, B. Karlsson, A.-M. Pilotti and A.-C. Söderholm, Tetrahedron Lett., 1977, 18, 1831 CrossRef; (c) H. Erdtman and H.-E. Högberg, Tetrahedron, 1979, 35, 535 CrossRef CAS; (d) J. Eskildsen, T. Reenberg and J. B. Christensen, Eur. J. Org. Chem., 2000, 1637 CrossRef CAS; (e) R. Rathore and S. H. Abdelwahed, Tetrahedron Lett., 2004, 45, 5267 CrossRef CAS; (f) C. B. Nielsen, T. Brock-Nannestad, T. K. Reenberg, P. Hammershøj, J. B. Christensen, J. W. Stouwdam and M. Pittelkow, Chem.–Eur. J., 2010, 16, 13030 CrossRef CAS PubMed; (g) T. Brock-Nannestad, C. B. Nielsen, M. Schau-Magnussen, P. Hammershøj, T. K. Reenberg, A. B. Petersen, D. Trpcevski and M. Pittelkow, Eur. J. Org. Chem., 2011, 6320 CrossRef CAS; (h) S. L. Mejlsøe and J. B. Christensen, J. Heterocycl. Chem., 2014, 51, 1051 CrossRef.
- (a) C. B. Nielsen, T. Brock-Nannestad, P. Hammershøj, T. K. Reenberg, M. Schau-Magnussen, D. Trpcevski, T. Hensel, R. Salcedo, G. V. Baryshnikov, B. F. Minaev and M. Pittelkow, Chem.–Eur. J., 2013, 19, 3898 CrossRef CAS PubMed; (b) T. Hensel, D. Trpcevski, C. Lind, R. Grosjean, P. Hammershøj, C. B. Nielsen, T. Brock-Nannestad, B. E. Nielsen, M. Schau-Magnussen, B. Minaev, G. V. Baryshnikov and M. Pittelkow, Chem.–Eur. J., 2013, 19, 17097 CrossRef CAS PubMed; (c) M. Plesner, T. Hensel, B. E. Nielsen, F. S. Kamounah, T. Brock-Nannestad, C. B. Nielsen, C. G. Tortzen, O. Hammerich and M. Pittelkow, Org. Biomol. Chem., 2015, 13, 5937 RSC.
- (a) F. Chen, Y. S. Hong, S. Shimizu, D. Kim, T. Tanaka and A. Osuka, Angew. Chem., Int. Ed., 2015, 54, 10639 CrossRef CAS PubMed; (b) Y. Nagata, S. Kato, Y. Miyake and H. Shinokubo, Org. Lett., 2017, 19, 2718 CrossRef CAS PubMed.
- (a) X. Xiong, C.-L. Deng, B. F. Minaev, G. V. Baryshnikov, X.-S. Peng and H. N. C. Wong, Chem.–Asian J., 2015, 10, 969 CrossRef CAS PubMed; (b) X. Xiong, C.-L. Deng, Z. Li, X.-S. Peng and H. N. C. Wong, Org. Chem. Front., 2017, 4, 682 RSC.
- (a) S. Kato, Y. Serizawa, D. Sakamaki, S. Seki, Y. Miyake and H. Shinokubo, Chem. Commun., 2015, 51, 16944 RSC; (b) S. Kato, S. Akahori, Y. Serizawa, X. Lin, M. Yamauchi, S. Yagai, T. Sakurai, W. Matsuda, S. Seki, H. Shinokubo and Y. Miyake, J. Org. Chem., 2019 DOI:10.1021/acs.joc.9b01655.
- A. Rajca, M. Miyasaka, S. Xiao, P. J. Boratyński, M. Pink and S. Rajca, Another expression of 13a is dibenzohepta[8]circulene as previously reported,12,19 but here we use the term, quasi-[8]circulene to emphasize the resemblance as referred to the Rajca's report, J. Org. Chem., 2009, 74, 9105 CrossRef CAS PubMed.
- F. Chen, T. Tanaka, T. Mori and A. Osuka, Chem.–Eur. J., 2018, 24, 7489 CrossRef CAS PubMed.
- (a) K. Nakanishi, D. Fukatsu, K. Takaishi, T. Tsuji, K. Uenaka, K. Kuramochi, T. Kawabata and K. Tsubaki, J. Am. Chem. Soc., 2014, 136, 7101 CrossRef CAS PubMed; (b) M. S. Sundar and A. V. Bedekar, Org. Lett., 2015, 17, 5808 CrossRef PubMed; (c) C. Mitsui, M. Yamagishi, R. Shikata, H. Ishii, T. Matsushita, K. Nakahara, M. Yano, H. Sato, A. Yamano, J. Takeya and T. Okamoto, Bull. Chem. Soc. Jpn., 2017, 90, 931 CrossRef CAS; (d) T. Okamoto, H. Dosei, M. Mitani, Y. Murata, H. Ishii, K.-i. Nakamura, M. Yamagishi, M. Yano and J. Takeya, Asian J. Org. Chem., 2018, 7, 2309 CrossRef CAS.
- (a) F. Chen, Y. S. Hong, D. Kim, T. Tanaka and A. Osuka, ChemPlusChem, 2017, 82, 1048 CrossRef CAS; (b) G. V. Baryshnikov, R. R. Valiev, B. F. Minaev and H. Ågren, New J. Chem., 2017, 41, 7621 RSC.
- (a) F. Chen, T. Tanaka, Y. S. Hong, W. Kim, D. Kim and A. Osuka, Chem.–Eur. J., 2016, 22, 10597 CrossRef CAS PubMed; (b) F. Chen, T. Tanaka and A. Osuka, Chem. Commun., 2017, 53, 2705 RSC.
- J. C. Fetzer, Large Polycyclic Aromatic Molecules – Chemistry and Analysis, Wiley Interscience, New York, 2000 Search PubMed.
- Water-soluble nanographenes and their biological applications: (a) H. A. Lin, Y. Sato, Y. Segawa, T. Nishihara, N. Sugimoto, L. T. Scott, T. Higashiyama and K. Itami, Angew. Chem., Int. Ed., 2018, 57, 2874 CrossRef CAS PubMed; (b) C. Liu, S. Zhang, J. Li, J. Wei, K. Müllen and M. A. Yin, Angew. Chem., Int. Ed., 2019, 58, 1638 CrossRef CAS PubMed.
- N. N. Karaush, G. V. Baryshnikov, V. A. Minaeva, H. Ågren and B. F. Minaev, Mol. Phys., 2017, 115, 2218 CrossRef CAS.
- From the Strickler–Berg relationship, larger kr values can be attributed to a larger absorption coefficient at the lowest-energy band. Indeed, 12 and 12-Bu displayed absorption bands at 429 nm (ε = 38000 M−1 cm−1) and 441 nm (ε = 39000 M−1 cm−1) in DMSO, which are larger than those in THF (Fig. S7-11, ESI†).
- K. Yamamoto, T. Harada, Y. Okamoto, H. Chikamatsu, M. Nakazaki, Y. Kai, T. Nakao, M. Tanaka, S. Harada and N. Kasai, J. Am. Chem. Soc., 1988, 110, 3579 Search PubMed.
- I. Pischel, S. Grimme, S. Kotila, M. Nieger and F. Vögtle, Tetrahedron: Asymmetry, 1996, 7, 109 CrossRef CAS.
- (a) J.-W. Han, X. Li and H. N. C. Wong, Chem. Rec., 2015, 15, 107 CrossRef CAS PubMed; (b) P. Rashidi-Ranjbar, Y. M. Man, J. Sandstroem and H. N. C. Wong, J. Org. Chem., 1989, 54, 4888 CrossRef CAS; (c) H.-Y. Peng, C.-K. Lam, T. C. W. Mak, Z. Cai, W.-T. Ma, Y.-X. Li and H. N. C. Wong, J. Am. Chem. Soc., 2005, 127, 9603 CrossRef CAS PubMed.
- Selected reviews of chiral nanographenes: (a) E. E. Maroto, M. Izquierdo, S. Reboredo, J. Marco-Martínez, S. Filippone and N. Martin, Acc. Chem. Res., 2014, 47, 2660 CrossRef CAS PubMed; (b) I. R. Márquez, S. Castro-Fernández, A. Millán and A. G. Campaña, Chem. Commun., 2018, 54, 6705 RSC; (c) M. Rickhaus, M. Mayor and M. Juríĉek, Chem. Soc. Rev., 2017, 46, 1643 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational details and X-ray crystallographic data for 12c, 13d, 14, 12a-Bu, 12c-Bu and 14-Bu are available. CCDC 1953592–1953597. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc05087f |
| This journal is © The Royal Society of Chemistry 2019 |