A single-step multi-level supramolecular system for cancer sonotheranostics†
Huirong
Lin‡
a,
Shuang
Li‡
a,
Junqing
Wang‡
 a,
Chengchao
Chu
a,
Yang
Zhang
a,
Xin
Pang
a,
Peng
Lv
a,
Xiaoyong
Wang
a,
Qingliang
Zhao
a,
Junjie
Chen
b,
Hongmin
Chen
a,
Chengchao
Chu
a,
Yang
Zhang
a,
Xin
Pang
a,
Peng
Lv
a,
Xiaoyong
Wang
a,
Qingliang
Zhao
a,
Junjie
Chen
b,
Hongmin
Chen
 a,
Wen
Liu
b,
Xiaoyuan
Chen
a,
Wen
Liu
b,
Xiaoyuan
Chen
 c and
Gang
Liu
c and
Gang
Liu
 *a
*a
aState Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, 361102, China. E-mail: gangliu.cmitm@xmu.edu.cn
bSchool of Pharmaceutical Science, Xiamen University, Xiamen 361102, China
cNational Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, MD 20892, USA
First published on 1st October 2018
Abstract
A multi-level supramolecular system produced by single-step Fe3+-mediated ionic crosslinking self-assembly can overcome the critical issues of current sonodynamic therapy (SDT) and address the need to monitor therapeutic effects in vivo with a non-invasive approach. This rational design of organic sonosensitizer-based formulation shows great potential for clinical SDT against deep-seated cancer.
Conceptual insightsDeveloping an effective sonodynamic therapy (SDT) for solid malignancies toward clinical use is challenging. The clinical applications of sonosensitizers have largely been hindered by the poor quantum yield of reactive oxygen species (ROS), potential biosafety issues, and the difficulty of scaled-up production. Herein, a facile route is presented for the fabrication of a multi-level self-assembling supramolecular system that overcomes the critical issues of current SDT and addresses the need to noninvasively monitor the therapeutic effects in vivo. Indocyanine green, the only FDA-approved theranostic dye, is introduced to form a self-organized coacervate-microbubble formulation via Fe3+-mediated ionic crosslinking using a single-step multi-level approach. The sonotheranostic value of this design was verified in orthotopic mouse models of hepatocellular carcinoma (HCC) (i.e., human LM-3 HCC and murine Hep1-6 HCC). Intravenous administration of this formulation increased the generation of ROS by enhancing the transfer of sound energy in non-covalent contacts. Furthermore, upon sono-activation, the selective accumulation at the target site was enhanced by converting the microbubbles into nanoparticles in situ, leading to significant HCC suppression. The results of this study could potentially be important for the design of organic sonosensitizer-based formulations for clinical SDT against deep-seated cancers. |
Sonodynamic therapy (SDT) uses therapeutic ultrasound instead of light to activate a sensitizer, which could potentially enable cost-effective and safe approaches for both diagnostic and therapeutic purposes. In the application of SDT, the sensitizers can be activated by sonoluminescence during the implosion of cavitation bubbles, which function in a similar manner to photodynamic therapy. The most commonly reported organic sonosensitizers are porphyrins, phthalocyanines, and their derivatives,1 followed by other photosensitizers,2 fluorescent dyes,3,4 and chemotherapeutic agents.5,6 However, light-sensitive agents are often phototoxic to the skin,7 they exhibit poor water solubility, and they promote hydrophobic aggregation, which further reduces the solubility and the generation of ROS.8 This results in unsatisfactory pharmacokinetic profiles and limited exposure to pathological sites in clinical practice. Additionally, the side effects are a concern for the long-term use of sonosensitizers derived from chemotherapeutic agents, such as doxorubicin5 and levofloxacin.6 Very recently, titanium dioxide (TiO2),9 gold/silver,10 carbon fluoroxide,11 porous silicon,12 and metal–organic-framework nanoparticles (MOF NPs)13 have also been explored as inorganic-based nanosensitizers. However, they are largely hindered by potential biosafety issues in human patients and are still far from ideal for clinical application.
We demonstrate a single-step, multi-level, metal-coordination-assisted self-assembly of theranostic dye-bearing microbubbles (MBs), which can overcome the critical issues of current SDT and address the need to monitor therapeutic effects in vivo and in real time. To identify potential theranostic dyes that are safe and close to clinical application, we screened three candidates that have been approved for clinical intravenous use by the United States Food and Drug Administration (FDA): sodium fluorescein (SF), methylthioninium chloride (MC), and indocyanine green (ICG). A sonodynamic effect has been described for cancer therapy using these compounds.4 However, SF and MC can cause serious intolerance reactions and serotonin toxicity.14 In contrast, ICG has a lower incidence of side effects,15 and allows for deep-tissue diagnosis through NIR fluorescence and photoacoustic imaging. However, the use of ICG as a sonosensitizer is limited by various characteristics, particularly instability, rapid clearance, and a lack of targeting. Incorporating ICG into NP systems is a promising way to overcome these barriers. Using a similar approach to our recent research,16 we envision that the limitations could be overcome and that the ROS generation could be increased by complexation mediated by ICG–metal ion binding through ionic crosslinking between anionic sulfonic groups and Fe3+ ions.17 Moreover, a lipid MB system is introduced to encapsulate Fe3+/ICG through multi-level self-assembly, which consists of growing Fe3+/ICG clusters with simultaneous MB formation (Fig. 1a). The MBs enable site-specific release of the Fe3+/ICG, improve their accumulation at the target site, and prevent spontaneous precipitation. We demonstrate that the Fe3+/ICG@MB system can be optimized to achieve greater generation of ROS than free ICGs, as well as sufficient optical and acoustic signal intensities that allow imaging-guided SDT against orthotopic hepatocellular carcinoma (HCC).
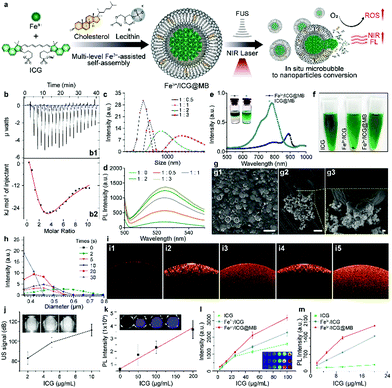
To establish the supramolecular self-assembly system, the exact binding affinity of Fe3+ for ICG was first characterized by isothermal titration calorimetry (ITC). The binding isotherm obtained by the interpretation of the raw data (Fig. 1b1) showed a biphasic process that includes an exothermic interaction at 20 °C (Fig. 1b2), which responds to increasing ICG concentrations. The resulting isotherm is best fitted to a two-site model to determine the dissociation constant (KD). The binding affinity at the first stage was approximately six times stronger than that of the second stage, as evidenced by the KD values of 21 μM for the first stage and 131 μM for the second stage. This indicates that an equal amount of Fe3+ was able to bind to ICG. The lowest KD was expected to occur first since ICG was the titrant and there was excessive Fe3+ in early injections. Subsequently, an increased amount of ICG molecules would attach by weak interactions and finally form assembled systems of –ICG–Fe3+–ICG–. This ion effect is called the Hofmeister phenomenon.18 Notably, increasing the [Fe3+]/[ICG] ratio results in greater size of the Fe3+/ICG assembly (Fig. 1c). To investigate the effect of the aggregate size on the ROS generation ability, a dichloro-dihydro-fluorescein diacetate (DCFH-DA) ROS detection kit was employed to monitor the sonoactivity by fluorometric detection. Interestingly, as the amount of Fe3+ increased, an aberrant gain in the fluorescence intensity of Fe3+/ICG was observed upon sono-activation (Fig. 1d). This may be attributed to the larger assembly of metal–organic sonosensitizers increasing the transfer of sound energy in comparison to a single-molecule.16 To enable the targeted delivery and on-demand release of Fe3+/ICG, a multi-level hetero-assembly of Fe3+/ICG and cholesterol-incorporated MB was investigated. Similar titrations were carried out with lecithin and Fe3+/ICG, and the ITC profiles in Fig. S1a (ESI†) show that injecting 1 mM of lecithin into a suspension of Fe3+/ICG results in both endothermic and exothermic components with uncounted KD. This process suggests that some lecithin molecules may also take part in Fe3+/ICG binding and are likely to replace one of the ICG molecules in the –ICG–Fe3+–ICG– systems. In contrast, no interaction was found when cholesterol was introduced due to the high KD value (1791 μM) (Fig. S1b, ESI†).
UV-vis-NIR absorption spectra were then analysed to evaluate the changes in optical properties among the free ICG, Fe3+/ICG, ICG/lecithin, Fe3+/ICG/lecithin and Fe3+/ICG@MB (Fig. S1c and d, ESI†). The free ICG has absorption peaks at around 710 nm and 780 nm. No distinct peak change was observed after chelating Fe3+ or adding lecithin. Notably, a new red-shift shoulder at around 890 nm was formed upon adding lecithin to Fe3+/ICG, indicating the aggregation of Fe3+ and ICG into large assemblies. Furthermore, this shoulder absorption transformed into a new peak that occupied a leading position at 890 nm with Fe3+/ICG@MB, suggesting further self-organization. These results likely resulted from cation–π interactions.19 Fe3+ induces assembly between ICG and lecithin molecules in MBs, which leads to donor–π–acceptor push–pull character of the hybrids and shifts the absorption to a greater wavelength (Fig. 1e). The desired Fe3+/ICG@MB was obtained by sonication with perfluorocarbon (C3F8) gas, based on an optimized [ICG]/[Fe3+] ratio of 1/0.5. As shown in the inset of Fig. 1e, Fe3+/ICG@MB (left) exhibited less solubility in methanol than ICG@MB (right), indicating that Fe3+/ICG enabled more stable MBs with less inclusion leakage. The multi-level Fe3+/ICG@MB formulation also exhibited better dispersity in phosphate buffered saline (PBS) (Fig. 1f), while ICG alone and Fe3+/ICG assemblies exhibited poor dispersity and precipitated rapidly after centrifugation at 5000 rpm for 5 min. Thus, the direct interaction between ICG and HSA could be avoided, which would prolong bioavailability after administration. The scanning electron microscopy (SEM) results in Fig. 1g1 show that the size of most Fe3+/ICG@MB is around 3 μm. The release of integrated Fe3+/ICG assemblies at the moment when bubbles burst was clearly observed in the magnified SEM image (Fig. 1g2 and g3). In addition, the size distribution of Fe3+/ICG@MB was progressively reduced after low frequency (1 MHz) was applied for 30 s (Fig. 1h). Optical coherence tomography (OCT) was performed to examine the detection capability among free ICG, ICG@MB, and Fe3+/ICG@MB with and without sono-activation at 1 MHz (Fig. 1i). The results show that the ultrasound treatment can significantly enhance the depth of signal penetration in both assemblies, in which the arch cavities can be clearly seen (Fig. 1i3 and i5). The signal coverage from Fe3+/ICG@MB is slightly higher, which may be attributable to the red-shift of ICG. A positive correlation was observed between the signal intensities of sonography/fluorescence imaging and increases in the concentration of Fe3+/ICG@MB (Fig. 1j and k). Furthermore, Fe3+/ICG@MB seems to outperform free ICG and Fe3+/ICG in both photoacoustic imaging (Fig. 1l) and ROS generation ability (Fig. 1m), especially at higher concentrations. This demonstrates the improved functionality of ICG as a sonotheranostic agent in the proposed scheme.
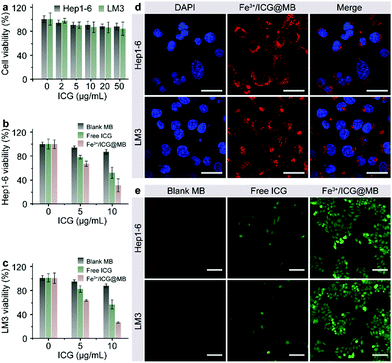
The cytotoxicity, cellular internalization, and sonodynamic effects of Fe3+/ICG@MB were analyzed using Hep1-6 and LM3 cells. First, a standard MTT assay was performed to determine the cell viability after incubation with Fe3+/ICG@MB at increasing concentrations for 24 h. As shown in Fig. 2a, negligible toxicity was observed in both cell lines even at the highest concentration of ICG applied. Conversely, upon sono-activation, the cell viability of Hep1-6 (Fig. 2b) and LM3 (Fig. 2c) decreased by approximately 3-fold when treated with 10 μg mL−1 of Fe3+/ICG@MB. Treatment with blank MB and free ICG only exhibited negligible and much lower cytotoxicity at equal concentrations. These results preliminarily demonstrate that Fe3+/ICG@MB has a desirable safety profile and pronounced sonotoxicity at the cellular level. Confocal laser scanning microscopy (CLSM) was carried out to quantitatively determine the uptake of Fe3+/ICG@MB into cells. As shown in Fig. 2d, the Hep1-6 and LM3 cells displayed strong fluorescence signals (red) in the cytoplasm after incubation with Fe3+/ICG@MB for 6 h. This observation could be attributed to the lecithin-based MBs, which enhance the interactions with bio-amphiphilic molecules such as cellular membrane lipids and allow the subsequent release of Fe3+/ICG within the cytoplasm. To verify the ROS generation ability of the Fe3+/ICG@MB in vitro, Hep1-6 and LM3 cell lines were stained with DCFH-DA after being treated with PBS, blank MB, free ICG, ICG@MB and Fe3+/ICG@MB upon sono-activation. As shown in Fig. 2e, cells treated with Fe3+/ICG@MB showed the brightest green fluorescence, revealing the highest amount of ROS generated from the Fe3+/ICG@MB. The cells treated with PBS, blank MB, free ICG, and ICG@MB (Fig. 2e and Fig. S3, ESI†) still maintained physiological activity and a weak fluorescence signal. Collectively, these results imply that Fe3+/ICG@MB enabled high-efficiency ultrasonic energy conversion due to the Fe3+-mediated supramolecular self-organization in the multi-level system.
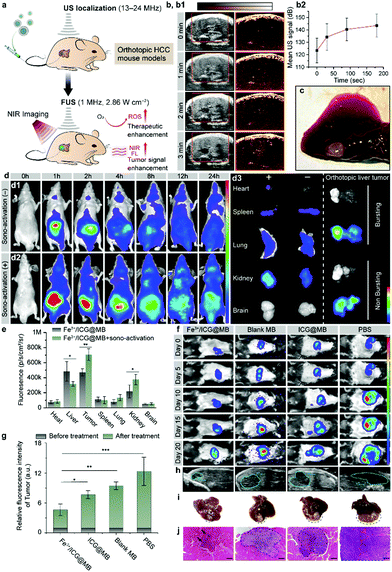
Precise localization and remote-controlled therapeutic activation are extremely important for achieving successful SDT against deep-seated solid tumors (Fig. 3a).20 Hence, imaging-guided SDT combined with sonographic imaging21 and NIR fluorescence imaging are highly desirable for both pre-treatment tumor localization and the spatiotemporal evaluation of therapeutic gain throughout the course of treatment. By taking advantage of NIR fluorescence of ICG and the ultrasound contrast-enhancement of MB, in vivo SDT assessment was carried out through a non-invasive multimodal imaging approach. BALB/c nude mice were implanted with LM3 cells orthotopically in the liver tissue to establish a tumor model of HCC. One pulse sono-activation was done at 30 s after the intravenous (i.v.) injection of Fe3+/ICG@MB. We then used sonography to observe the pathological site accumulation of Fe3+/ICG@MB. The sonographic images displayed a steady rise of signal and reached maximum enhancement at 3 min after injection (Fig. 3b1). This was also confirmed by quantifying the average ultrasound signal intensity at different time points (Fig. 3b2). The results indicate that Fe3+/ICG@MB could be selectively deposited at the tumor site (Fig. 3c). For further verification, we applied equal ultrasound pulses in a tube filled with Fe3+/ICG@MB and detected the change in forward scatter (FSC-A) via flow cytometry. Based on the results and Fig. 1h, we found that there was an obvious increase of smaller MBs after sono-activation was applied (Fig. S2a and b, ESI†). Therefore, we hypothesize that the well-formed Fe3+/ICG@MB could possibly allow for the in situ conversion of MBs to NPs when receiving sono-activation.
Next, the time-dependent biodistribution of Fe3+/ICG@MB with and without sono-activation was monitored by an in vivo optical imaging system. As illustrated in Fig. 3d, after the injection of equal quantities of Fe3+/ICG@MB, mice treated with sono-activation gained strong PL intensity at the tumor site at 1 h post-injection, and the tumor site was highly enriched 4 h later (Fig. 3d1). The control mice exhibited much lower PL intensity, which quickly diminished in 2 h (Fig. 3d2). This was also confirmed by the semiquantitative (Fig. 3d3) and quantitative ROI (Fig. 3e) data based on ex vivo fluorescence images of resected organs and tumors. Fe3+/ICG@MB combined with sono-activation demonstrated 1.3 times higher deposition in the tumor tissue, 0.3 times lower non-specific accumulation in the liver, and 0.4 times higher accumulation in the kidneys compared with Fe3+/ICG@MB alone (Fig. 3e). The higher accumulation of Fe3+/ICG@MB at the target site may be attributed to MB bursting and transient pores forming, which allow drugs to exit from leaky tumor vessels under sono-activation.
We next investigated the accumulation behavior of Fe3+/ICG@MB in a different model of tumor heterogeneity. C57BL/6 mice bearing Hep1-6 orthotopic HCC were intravenously injected with Fe3+/ICG@MB. A consistent accumulation pattern was observed (Fig. S3, ESI†). The sonography (Fig. S3a, ESI†) and ultrasound signal intensities (Fig. S3b, ESI†) show that the signal contrast reached 10% at 3 min at the tumor site after one pulse of sono-activation. The PL signals in the sono-activation group exhibited higher sono-material enrichment and were retained for a longer time (Fig. S3d, ESI†). The ex vivo fluorescence images of the resected organs and tumors were also recorded (Fig. S3e, ESI†). The sono-activation group had 1.33 times higher accumulation of sono-material (Fig. S3f, ESI†). This indicates that our assumption that size conversion mediated selective targeting has been confirmed for this model. We next provide further insight into examining the anticancer activities of Fe3+/ICG@MB in vivo with the firefly luciferase (fLuc) reporter gene. C57BL/6 mice bearing fLuc-Hep1-6 orthotopic HCC were intravenously injected with PBS, blank MB, ICG@MB, and Fe3+/ICG@MB, followed by 5 min sonication for bubble bursting and a second sonication (5 min) was applied 6 h later. As shown in Fig. 3f, mice in the PBS group had a rapid growth of tumor volume, and the group treated with blank MB showed only a slight delay compared with the PBS group. Notably, the ICG@MB treated group exhibited a tumor-suppression effect, while the Fe3+/ICG@MB treated group exhibited almost complete tumor remission. The quantitative bioluminescence measurements of the tumors among different groups showed a significant difference after treatment (Fig. 3g). The tumor luminescence intensity of the Fe3+/ICG@MB treated group was 3 times less than that of the PBS group and 39% less than that of the ICG@MB treated group. This revealed that Fe3+/ICG@MB enhanced the SDT effect due to the sonication-induced passive targeting and increased ROS generation.
The tumor morphology was also monitored by post-treatment diagnostic sonography at day 20 (Fig. 3h) and the tumor size in the Fe3+/ICG@MB-treated group was much smaller than that in the other groups. This is clearly demonstrated in the photographs of resected orthotopic HCC tumors (Fig. 3i). The orthotopic tumor tissues were then stained with hematoxylin and eosin (H&E), and the histological slides showed that there was less tumor tissue surrounded by normal tissue in the Fe3+/ICG@MB-treated group (Fig. 3j and Fig. S4, ESI†). In contrast, the other groups showed obvious tumor invasion in the liver and different degrees of liver function impairment (Fig. S5a, ESI†). During 20 days of therapy, the body weights of the mice showed no obvious changes among all groups (Fig. S5b, ESI†), suggesting no severe adverse effects from this therapy. These results imply that Fe3+/ICG@MB can abrogate tumor growth when combined with SDT and sonication-induced conversion of MBs to NPs in situ.
Conclusions
In summary, this study highlighted the importance of Fe3+-mediated supermolecular assembly of ICG for cancer sonotheranostics. The assembly increased ROS generation by enhancing the transfer of sound energy in non-covalent contacts during SDT, and the conversion of MBs to NPs promotes the selective accumulation of Fe3+/ICG@MB at the target site. The Fe3+/ICG@MB also exhibit biocompatibility and high loading efficiency, and they could be used for non-invasive multimodal imaging. Therefore, the results of this study are a step forward toward the clinical use of ICG-mediated SDT against deep-seated cancer.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFA0205201 and 2018YFA0107301), the National Natural Science Foundation of China (81422023, 81371596, U1705281, and U1505221), the Fundamental Research Funds for the Central Universities (20720160065 and 20720150141), and the Program for New Century Excellent Talents in University, China (NCET-13-0502). All animal experiments were approved by the Animal Management and Ethics Committee of Xiamen University.References
- Y. Nagahiko, H. Qing-Song, K. Ikuko and U. Shin-ichiro, Cancer Sci., 2008, 99, 166 Search PubMed; S. Guo, X. Sun, J. Cheng, H. Xu, J. Dan, J. Shen, Q. Zhou, Y. Zhang, L. Meng, W. Cao and Y. Tian, Int. J. Nanomed., 2013, 8, 2239 Search PubMed; S. Endo, N. Kudo, S. Yamaguchi, K. Sumiyoshi, H. Motegi, H. Kobayashi, S. Terasaka and K. Houkin, Ultrasound Med. Biol., 2015, 41, 2458 CrossRef PubMed.
- S. Umemura, N. Yumita, R. Nishigaki and K. Umemura, Jpn. J. Cancer Res., 1990, 81, 962 CrossRef CAS PubMed; H. Xu, H. Chen, B. Zheng, Y. Zheng, M. Ke and J. Huang, Ultrason. Sonochem., 2015, 22, 125 CrossRef PubMed.
- N. Nomikou, C. Sterrett, C. Arthur, B. McCaughan, J. F. Callan and A. P. McHale, ChemMedChem, 2012, 7, 1465 CrossRef CAS PubMed; Y. Li, Q. Zhou, Z. Deng, M. Pan, X. Liu, J. Wu, F. Yan and H. Zheng, Sci. Rep., 2016, 6, 25968 CrossRef PubMed.
- J. Xiang, X. Xia, Y. Jiang, A. W. Leung, X. Wang, J. Xu, P. Wang, H. Yu, D. Bai and C. Xu, Ultrasonics, 2011, 51, 390 CrossRef CAS PubMed; M. Zou, L. Zhang, J. Wang, Q. Wang, J. Gao and P. Fan, Spectrochim. Acta, Part A, 2013, 110, 364 CrossRef PubMed; Q. Tang, S. Chang, Z. Tian, J. Sun, L. Hao, Z. Wang and S. Zhu, Ultrasound Med. Biol., 2017, 43, 2690 CrossRef PubMed; H. Chen, X. Zhou, Y. Gao, B. Zheng, F. Tang and J. Huang, Drug Discovery Today, 2014, 19, 502 CrossRef PubMed.
- L. Liang, S. Xie, L. Jiang, H. Jin, S. Li and J. Liu, Ultrasound Med. Biol., 2013, 39, 146 CrossRef PubMed.
- B. Liu, J. Wang, X. Wang, B.-M. Liu, Y.-M. Kong, D. Wang and S.-K. Xu, J. Fluoresc., 2010, 20, 985 CrossRef CAS PubMed.
- T. J. Dougherty, M. T. Cooper and T. S. Mang, Lasers Surg. Med., 1990, 10, 485 CrossRef CAS PubMed.
- Y. N. Konan, R. Gurny and E. Allemann, J. Photochem. Photobiol., B, 2002, 66, 89 CrossRef CAS.
- Y. Harada, K. Ogawa, Y. Irie, H. Endo, L. B. Feril, T. Uemura and K. Tachibana, J. Controlled Release, 2011, 149, 190 CrossRef CAS PubMed; S. Yamaguchi, H. Kobayashi, T. Narita, K. Kanehira, S. Sonezaki, N. Kudo, Y. Kubota, S. Terasaka and K. Houkin, Ultrason. Sonochem., 2011, 18, 1197 CrossRef PubMed.
- A. Sazgarnia, A. Shanei, A. R. Taheri, N. T. Meibodi, H. Eshghi, N. Attaran and M. M. Shanei, J. Ultrasound Med., 2013, 32, 475 CrossRef PubMed; V. Bernard, V. Mornstein, J. Jaroš, M. Sedláčková and J. Škorpíková, J. Appl. Biomed., 2014, 12, 137 CrossRef.
- A. Kharin, O. Syshchyk, A. Geloen, S. Alekseev, A. Rogov, V. Lysenko and V. Timoshenko, Sci. Technol. Adv. Mater., 2015, 16, 044601 CrossRef PubMed.
- L. A. Osminkina, A. L. Nikolaev, A. P. Sviridov, N. V. Andronova, K. P. Tamarov, M. B. Gongalsky, A. A. Kudryavtsev, H. M. Treshalina and V. Y. Timoshenko, Microporous Mesoporous Mater., 2015, 210, 169 CrossRef CAS.
- X. Pan, L. Bai, H. Wang, Q. Wu, H. Wang, S. Liu, B. Xu, X. Shi and H. Liu, Adv. Mater., 2018, 30, 1800180 CrossRef PubMed.
- M. P. Lopez-Saez, E. Ordoqui, P. Tornero, A. Baeza, T. Sainza, J. M. Zubeldia, M. L. Baeza and M. L. Baeza, Ann. Allergy, Asthma, Immunol., 1998, 81, 428 CrossRef CAS; P. K. Gillman, Anaesthesia, 2006, 61, 1013 CrossRef PubMed.
- J. R. Paugh, in Clinical Ocular Pharmacology, ed. J. D. Bartlett, S. D. Jaanus, R. G. Fiscella, N. R. Holdeman and C. L. Prokopich, Butterworth-Heinemann, Saint Louis, 5th edn, 2008, p. 283 Search PubMed.
- C. Chu, H. Lin, H. Liu, X. Wang, J. Wang, P. Zhang, H. Gao, C. Huang, Y. Zeng, Y. Tan, G. Liu and X. Chen, Adv. Mater., 2017, 29, 1605928 CrossRef PubMed.
- J. Wang, A. Z. Wang, P. Lv, W. Tao and G. Liu, Adv. Sci., 2018, 1800564 CrossRef PubMed.
- A. W. Omta, M. Kropman, S. Woutersen and H. Bakker, Science, 2003, 301, 347 CrossRef CAS PubMed; P. Lo Nostro and B. W. Ninham, Chem. Rev., 2012, 112, 2286 CrossRef PubMed.
- A. S. Mahadevi and G. N. Sastry, Chem. Rev., 2013, 113, 2100 CrossRef CAS PubMed.
- S. N. Goldberg, C. J. Grassi, J. F. Cardella, J. W. Charboneau, I. Gerald, D. Dodd, D. E. Dupuy, D. Gervais, A. R. Gillams, R. A. Kane, J. Fred, T. Lee, T. Livraghi, J. McGahan, D. A. Phillips, H. Rhim and S. G. Silverman, Radiology, 2005, 235, 728 CrossRef PubMed; J. Wang, W. Tao, X. Chen, O. C. Farokhzad and G. Liu, Theranostics, 2017, 7, 3915 CrossRef PubMed.
- K. Ferrara, R. Pollard and M. Borden, Annu. Rev. Biomed. Eng., 2007, 9, 415 CrossRef CAS PubMed; N. Nomikou, C. Fowley, N. M. Byrne, B. McCaughan, A. P. McHale and J. F. Callan, Chem. Commun., 2012, 48, 8332 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nh00276b |
| ‡ H. L., S. L., and J. W. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |