Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Perylene bisimide hydrogels and lyotropic liquid crystals with temperature-responsive color change†
Daniel
Görl
,
Bartolome
Soberats
,
Stefanie
Herbst
,
Vladimir
Stepanenko
and
Frank
Würthner
*
Institut für Organische Chemie & Center for Nanosystems Chemistry, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: wuerthner@chemie.uni-wuerzburg.de
First published on 22nd July 2016
Abstract
The self-assembly of perylene bisimide (PBI) dyes bearing oligo ethylene glycol (OEG) units in water affords responsive functional nanostructures characterized by their lower critical solution temperature (LCST). Tuning of the LCST is realized by a supramolecular approach that relies on two structurally closely related PBI–OEG molecules. The two PBIs socially co-assemble in water and the resulting nanostructures exhibit a single LCST in between the transition temperatures of the aggregates formed by single components. This permits to precisely tune the transition from a hydrogel to a lyotropic liquid crystal state at temperatures between 26 and 51 °C by adjusting the molar fraction of the two PBIs. Owing to concomitant changes in PBI–PBI interactions this phase transition affords a pronounced color change with “fluorescence-on” response that can be utilized as a smart temperature sensory system.
Introduction
Molecular self-assembly has attracted a great deal of attention as an elegant and inexpensive approach for fabricating nanostructured functional materials.1 In particular, self-assembly of π-conjugated dye molecules has intensively been studied,2 because π–π-interactions between dyes lead to peculiar changes of electronic and optical properties which holds great promise for applications in organic electronics,3 photonics4 and sensing.5 Materials composed of dye aggregates have recently also gained much interest in an aqueous environment because the intermolecular interactions between the π-conjugated chromophores are remarkably enhanced due to the hydrophobic effect.6 The majority of the so far investigated π-amphiphilic molecules rely on oligoethylene glycol (OEG) as a water solubility providing element. Interestingly, this structural motif can provide additional features to the material such as formation of hydrogels at higher concentration and lower critical solution temperature (LCST) behaviour.7 LCST is a phenomenon based on the entropy-driven phase transition of an at least binary mixture at a specific temperature due to changes in the noncovalent interactions with surrounding solvent molecules. However, the relevance of LCST behaviour for self-assembled discrete molecules has only recently come into focus.8–10 It is of special interest in π-conjugated systems because the structural changes accompanying the phase transition can be used to trigger an optical or electronic response, as has been shown for oligo(p-phenylene) derivatives9 and photo-switchable diarylethenes.10 In this context, the use of perylene bisimide (PBI) dyes is very attractive due to their outstanding optical and self-assembly properties.2f,11 In our recent work on PBI 2 self-assembly in water we noted an unexpected high solubility at lower temperatures,12a but precipitation at higher temperatures, i.e. LCST behaviour.12b Our subsequent research was accordingly devoted to reveal the origin of this effect which led to several interesting discoveries in the field of amphiphilic dyes.Herein we report on hydrogel and lyotropic liquid crystal mesophase formation and a novel supramolecular approach to tune the LCST properties in self-assembled aggregates of bolaamphiphilic PBI dyes. Fluorescent PBI 1 and PBI 2 differ only in a methylene bridge in the OEG termini (Fig. 1a) but their aqueous aggregates exhibit remarkably different LCSTs (Fig. 1b). This unique feature allows the creation of PBI 1/PBI 2 co-aggregates with a single LCST phase transition that is precisely tuned between 26 and 51 °C by adjusting the molar fraction of the two PBI components (Fig. 1b). This unprecedented strategy is further applied for the development of tunable temperature responsive PBI 1/PBI 2 hydrogels that afford a remarkable color change during LCST phase transition, overall highlighting the impact of implementing small structural changes at the molecular periphery for fine-tuning of the LCST.
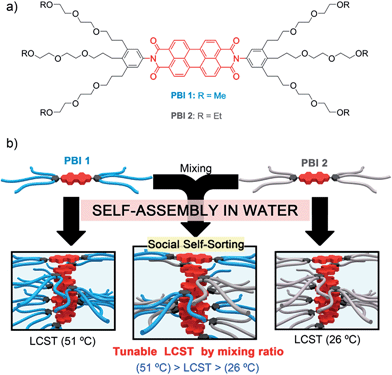 | ||
| Fig. 1 (a) Chemical structures of PBI 1 and PBI 2. (b) Schematic illustration of the self-assembly features and LCST properties of PBI 1, PBI 2 and PBI 1/PBI 2 co-aggregates. | ||
Results and discussion
In the present work, we synthesized the new PBI–OEG derivative PBI 1 that differs from the previously reported PBI 2 (ref. 12) by one methylene unit at the termini of the OEG side chains (Fig. 1a) (for synthetic details, see the ESI†). According to their structural similarity, PBI 1 and PBI 2 exhibit identical optical and aggregation properties, but surprisingly show a strikingly distinct LCST behaviour in water, which has been of great relevance for the LCST tuning in the mixtures described below.We have previously shown that PBI 2 self-organizes hierarchically into columnar and lamellar superstructures in water, which are soluble at room temperature but precipitate at increased temperatures, indicative of LCST behaviour.12 For PBI 1, we found a similar aggregation behaviour like for PBI 2 (Fig. S1†). For example, the UV-vis absorption spectra of both compounds in THF show the vibronic fine structure commonly observed for monomerically dissolved PBI dyes, whereas the UV-vis spectra in water reveal a hypsochromically shifted absorption maximum concomitant with a loss of the vibronic fine structure, indicating the self-assembly of the PBIs into well-defined H-aggregates. Moreover, both compounds exhibit almost identical extinction coefficients and fluorescence quantum yields in both THF (Φfl(PBI 1) = 42%, Φfl(PBI 2) = 43%) and in pure water (Fig. S1†). These results confirm that PBI 1 and PBI 2 self-assemble in water with the same chromophore arrangement, forming long aggregates even at nanomolar concentrations.12
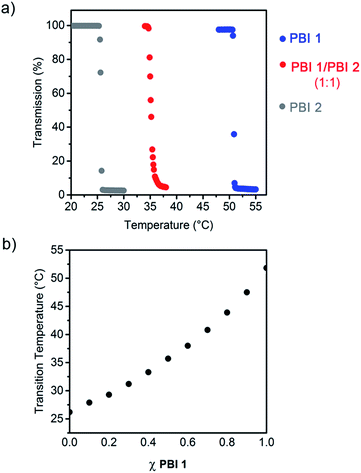
The temperature response of PBI 1 and PBI 2 aggregates was examined by turbidity measurements in water (c = 2.5 × 10−4 M) (Fig. 2a and S2†). These experiments revealed abrupt drops in the transmission at 800 nm after exceeding a certain temperature (Fig. 2a). This behaviour is attributed to the temperature dependent dehydration of the OEG chains which provoke the phase separation of the aggregates and water, which is known as critical solution temperature phenomenon.7 In the present work, the onset temperature of this phase transition (Fig. S2†) will be referred as LCST. Interestingly, it was found that PBI 1 exhibits a phase transition at 51 °C, while we previously reported that PBI 2 exhibits a LCST transition at 26 °C (Fig. 2a and S2†). Hence, small differences in the molecular structure such as the replacement of ethyl by methyl end groups (Fig. 1a) have remarkable impact on the LCST behaviour, which has barely been addressed in literature so far.13 The heating curves are highly reproducible, which suggests that both dyes form thermodynamically stable aggregates in water.
Inspired by previous studies on random copolymers, which showed that the LCST behavior can be tuned by the control of the ratio of two different polymer building blocks,7 we hypothesized that PBI 1 and PBI 2 would socially self-assemble to form co-aggregated structures14 exhibiting a LCST behaviour dependent of the PBIs mixing ratio. Accordingly, different aqueous solutions of PBI 1/PBI 2 mixtures were prepared and their transmission at 800 nm was monitored as a function of temperature (Fig. 2 and S2†). For example, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of PBI 1 and PBI 2 exhibits a single transition at 35 °C (Fig. 2a), which is a strong indication that both dyes perfectly mix with each other even without an orthogonal binding motif, thus forming socially self-sorted heteroaggregates.14 Pleasingly, the LCSTs of the PBI 1/PBI 2 system is consequently adjusted in between the temperature interval ranging from 26 °C (LCST of PBI 2 aggregate) to 51 °C (LCST of PBI 1 aggregate) depending on the molar fraction of both dyes (Fig. 2b). Such control of the LCST properties in co-aggregated structures of discrete molecules is indeed unprecedented but constitutes a facile and efficient strategy to fine-tune temperature responsive systems. Therefore the LCST seems to be a powerful tool for the investigation of supramolecular co-assemblies.
1 solution of PBI 1 and PBI 2 exhibits a single transition at 35 °C (Fig. 2a), which is a strong indication that both dyes perfectly mix with each other even without an orthogonal binding motif, thus forming socially self-sorted heteroaggregates.14 Pleasingly, the LCSTs of the PBI 1/PBI 2 system is consequently adjusted in between the temperature interval ranging from 26 °C (LCST of PBI 2 aggregate) to 51 °C (LCST of PBI 1 aggregate) depending on the molar fraction of both dyes (Fig. 2b). Such control of the LCST properties in co-aggregated structures of discrete molecules is indeed unprecedented but constitutes a facile and efficient strategy to fine-tune temperature responsive systems. Therefore the LCST seems to be a powerful tool for the investigation of supramolecular co-assemblies.
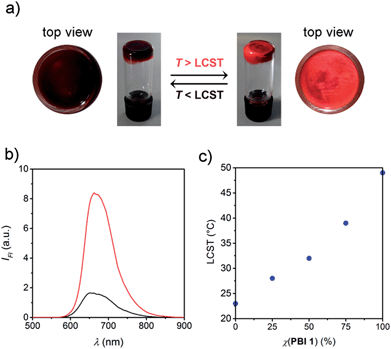
In the aim to demonstrate that this strategy can be applied for the development of stimuli responsive materials, we focused on highly concentrated aqueous materials based on PBI 1 and PBI 2. Both PBIs exhibit thermotropic columnar liquid crystalline phases under anhydrous conditions (Fig. S3 and S4†), whereas they form lyotropic columnar hexagonal phases upon addition of 20 wt% of water (Fig. S5 and S6†).15 After further addition of water up to 80 wt% water content, the dye molecules form hydrogels at room temperature. Most interestingly, these hydrogels are subject to LCST behaviour at temperatures of 51 (for PBI 1) and 26 °C (for PBI 2) which are accompanied by a significant color change from dark red to bright orange-red that can easily be followed by the naked eye (Fig. 3a). It is noteworthy that this process is reversible and proceeds in quasisolid state without any appreciable volume change.
In order to unravel the origin of the color change we next conducted temperature-dependent fluorescence measurements of hydrogel films by fluorescence microscopy (ESI†). Indeed, the fluorescence emitted at the surface of the hydrogels is enhanced remarkably upon LCST phase transition (Fig. 3b and S7†) and resembles the excimer-type emission known for PBI 2 aggregates,12 which is consistent with the formation of face-to-face stacked PBI assemblies (Fig. 1b). The fluorescence intensity is increased by a factor of ∼7, which we attribute to a rigidification effect,16i.e. decrease in the rotational and translational motion of the stacked PBI molecules. These motions, which are known as fluorescence quenching pathways in photoexcited PBI aggregates,17 are less restricted below the LCST, in which the OEG chains are solvated. Also for less concentrated samples (c = 8 × 10−5 M) an increase in fluorescence intensity has been observed upon LCST phase transition (Fig. S8a and c†). Corresponding absorption measurements revealed insignificant changes of the absorption profile, while scattering effects become pronounced due to the turbidity of the sample (Fig. S8b and d†). Accordingly, the thermally induced color change in PBI hydrogels is mainly attributed to an increase of the excimer-type fluorescence intensity (Fig. 3b, S7 and S8†) as well as other factors such as changes in light scattering and diffraction indices induced by the phase separation (vide infra).
In order to prove the tunability of the LCSTs of PBI 1/PBI 2 mixtures in the hydrogel state, we prepared mixtures of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratios of PBI 1/PBI 2 and we examined their temperature behaviour by differential scanning calorimetry (DSC) measurements (Fig. S9†). These experiments revealed indeed only one phase transition for each mixture, which is accompanied by a single, fast and reversible color change that can be easily followed by naked eye. Like in case of the diluted samples, the LCST phase transition in the PBI 1/PBI 2 hydrogels occurs at a temperature in between 26 (LCST of PBI 2) and 51 °C (LCST of PBI 1) (Fig. 3c). Accordingly, the LCST process in PBI 1 and PBI 2 is not influenced by the concentration but highly dependent on the mixing ratio of the PBIs. It is noteworthy that the optical response is identical for PBI 1, PBI 2 and for their corresponding mixtures, because the two PBIs differ in the OEG-chains which does not influence the electronic coupling between the photoactive part of the self-assembled structure (PBI-cores).18 Therefore, the small structural changes in the molecular periphery allow to affect LCST transition temperatures while keeping the optoelectronic and self-assembly properties of the PBI material unchanged.
3 ratios of PBI 1/PBI 2 and we examined their temperature behaviour by differential scanning calorimetry (DSC) measurements (Fig. S9†). These experiments revealed indeed only one phase transition for each mixture, which is accompanied by a single, fast and reversible color change that can be easily followed by naked eye. Like in case of the diluted samples, the LCST phase transition in the PBI 1/PBI 2 hydrogels occurs at a temperature in between 26 (LCST of PBI 2) and 51 °C (LCST of PBI 1) (Fig. 3c). Accordingly, the LCST process in PBI 1 and PBI 2 is not influenced by the concentration but highly dependent on the mixing ratio of the PBIs. It is noteworthy that the optical response is identical for PBI 1, PBI 2 and for their corresponding mixtures, because the two PBIs differ in the OEG-chains which does not influence the electronic coupling between the photoactive part of the self-assembled structure (PBI-cores).18 Therefore, the small structural changes in the molecular periphery allow to affect LCST transition temperatures while keeping the optoelectronic and self-assembly properties of the PBI material unchanged.
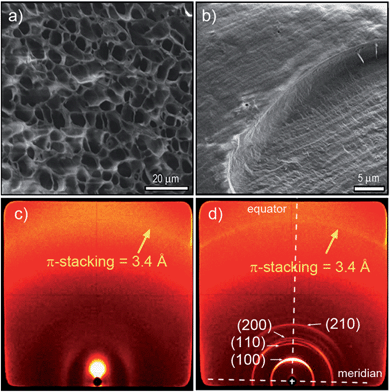
To gain insight into the origin of this LCST phase transition in these hydrogels, we have examined the structural features of PBI 1 and PBI 2 with 80 wt% water content below and above the LCST by means of scanning electron microscopy (SEM), X-ray diffraction (XRD) and optical microscopy (OM) (Fig. 4, 5 and S10–S15†). Fig. 4a and b show the cryo-SEM images of PBI 1 hydrogel below and above LCST, respectively, which reveal a significant morphology change upon LCST phase transition. A porous network with rather random branching has been obtained below LCST (Fig. 4a, S10a, b and S11a, b†), which is common for gel materials.19 In contrast, above the critical temperature a compact and non-porous structure consisting of bundled aggregate strands with a diameter of up to 400 nm (Fig. 4b, S10c, d and S11c, d†) is observed. These results indicate that PBI 1 forms a higher ordered state after LCST phase transition.
This observation was further corroborated by X-ray scattering below LCST and above LCST (Fig. 4c and d and S12†). The 2D X-ray patterns of PBI 1 with 80 wt% water content at 35 °C (below LCST) show no reflection in the small angle region (Fig. 4c), which indicates the absence of a well-ordered nanostructure. In contrast, the X-ray pattern of the same sample at 70 °C exhibits one intense and three weak reflections along the equator corresponding to the (100), (110), (200) and (210) lattices of a columnar hexagonal phase (Fig. 4d).
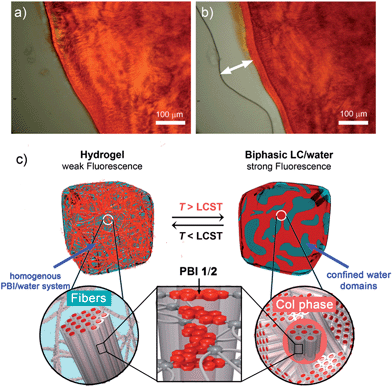
Corresponding OM images of PBI 1 hydrogel revealed a homogenous phase below the LCST (Fig. 5a), but after heating above the LCST a biphasic mixture consisting of a red phase and a transparent liquid (water) is observed (Fig. 5b). These microscope observations show that the phase transition is accompanied by the expulsion of water from the gel matrix, which likely triggers the temperature-induced transition between the hydrogel state and a biphasic state consisting of pure water and a nanostructured columnar PBI phase (Fig. 5c).
This LCST transition behaviour was found in hydrogels of PBI 1, PBI 2 as well as in PBI 1/PBI 2 mixtures (Fig. S13–S15†) and is always accompanied by the remarkable color change. It should be noted that the biphasic state observed above LCST exhibits a similar X-ray pattern to that observed for the lyotropic columnar hexagonal phase (20 wt% water content) of the same compound (Fig. S5†). For the latter, temperature-induced water expulsion has not been observed. Accordingly, a rather high concentration of water is required for the formation of PBI 1 and PBI 2 self-assemblies that are subject to LCST phase transition.
Conclusion
In summary, we have developed a new supramolecular strategy to tune LCST properties in self-assembled aggregates of OEG-substituted dyes. PBI 1 and PBI 2 differ only in one methylene group at the peripheral ends of the OEG side chains, but their aggregated structures in water exhibit remarkably different LCST properties. This unique feature allows for the development of mixed hydrogel materials with only one phase transition due to the social co-assembly of PBI 1 and PBI 2 into supramolecular co-aggregates. The phase transition of these hydrogels is accompanied by a remarkable color change from dark red to bright orange-red that can accordingly be adjusted to any temperature between 51 and 26 °C by controlling the molar ratio of PBI 1 and PBI 2. Taking into account that the LCSTs of the present hydrogels cover environmentally and physiologically relevant temperatures, we foresee smart sensory systems based on these novel stimuli responsive materials. Applications in (bio-)medical sensing20 and health monitoring21 should be possible as well if our concept can be extended to other self-assemblies of π-conjugated molecules.Acknowledgements
We thank the DFG (grant no. Wu 317/11) and the Bavarian State Ministry of Science, Research, and the Arts (establishment of the Key Laboratory for Supramolecular Polymers at the Universität Würzburg) for financial support.Notes and references
- (a) J.-M. Lehn, Angew. Chem., Int. Ed., 2013, 52, 2836–2850 CrossRef CAS PubMed; (b) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed; (c) T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38–68 CrossRef CAS PubMed; (d) C. F. J. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS; (e) H.-J. Sun, S. Zhang and V. Percec, Chem. Soc. Rev., 2015, 44, 3900–3923 RSC; (f) C. F. J. Faul, Acc. Chem. Res., 2014, 47, 3428–3438 CrossRef CAS PubMed; (g) L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089–6102 RSC; (h) E. Elacqua, D. S. Lye and M. Weck, Acc. Chem. Res., 2014, 47, 2405–2416 CrossRef CAS PubMed; (i) E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 116, 2414–2477 CrossRef CAS PubMed.
- (a) H. J. Kim, T. Kim and M. Lee, Acc. Chem. Res., 2011, 44, 72–82 CrossRef CAS PubMed; (b) L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196–7239 CrossRef CAS PubMed; (c) Y. Sagara, S. Yamane, M. Mitani, C. Weder and T. Kato, Adv. Mater., 2016, 28, 1073–1095 CrossRef CAS PubMed; (d) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed; (e) D. González-Rodríguez and A. P. H. J. Schenning, Chem. Mater., 2011, 23, 310–325 CrossRef; (f) S. Yagai, Bull. Chem. Soc. Jpn., 2015, 88, 28–58 CrossRef; (g) H. Frauenrath and E. Jahnke, Chem.–Eur. J., 2008, 14, 2942–2955 CrossRef CAS PubMed; (h) A. Das and S. Ghosh, Chem. Commun., 2016, 52, 6860–6872 RSC.
- (a) A. Jain and S. J. George, Mater. Today, 2015, 18, 206–214 CrossRef CAS; (b) Z. B. Henson, K. Müllen and G. C. Bazan, Nat. Chem., 2012, 4, 699–704 CrossRef CAS PubMed; (c) A. N. Sokolov, B. C. K. Tee, C. J. Bettinger, J. B. H. Tok and Z. Bao, Acc. Chem. Res., 2012, 45, 361–371 CrossRef CAS PubMed.
- (a) H. G. Li, J. Choi and T. Nakanishi, Langmuir, 2013, 18, 5318–5406 Search PubMed; (b) M. O'Neill and S. M. Kelly, Adv. Mater., 2011, 23, 566–584 CrossRef PubMed; (c) F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef PubMed.
- S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 4, 1339–1386 CrossRef PubMed.
- (a) M. R. Molla and S. Ghosh, Phys. Chem. Chem. Phys., 2014, 16, 26672–26683 RSC; (b) C. F. J. Faul, Acc. Chem. Res., 2014, 47, 3428–3438 CrossRef CAS PubMed; (c) C. Rest, M. J. Mayoral and G. Fernández, Int. J. Mol. Sci., 2013, 14, 1541–1565 CrossRef CAS PubMed; (d) D. Görl, X. Zhang and F. Würthner, Angew. Chem., Int. Ed., 2012, 51, 6328–6348 CrossRef PubMed; (e) B. Rybtchinski, ACS Nano, 2011, 5, 6791–6818 CrossRef CAS PubMed; (f) C. V. Hoven, A. Garcia, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2008, 20, 3793–3810 CrossRef CAS.
- (a) C. Li and S. Liu, Chem. Commun., 2012, 48, 3262–3278 RSC; (b) C. Weber, R. Hoogenboom and U. S. Schubert, Prog. Polym. Sci., 2012, 37, 686–714 CrossRef CAS.
- (a) P. Wei, T. R. Cook, X. Yan, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2014, 136, 15497–15500 CrossRef CAS PubMed; (b) T. Ogoshi, K. Kida and T.-a. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed; (c) L. Li, Y. Che, D. E. Gross, H. Huang, J. S. Moore and L. Zang, ACS Macro Lett., 2012, 1, 1335–1338 CrossRef CAS; (d) X. Yao, X. Wang, T. Jiang, X. Ma and H. Tian, Langmuir, 2015, 31, 13647–13654 CrossRef CAS PubMed; (e) K. R. Raghupathi, U. Sridhar, K. Byrne, K. Raghupathi and S. Thayumanavan, J. Am. Chem. Soc., 2015, 137, 5308–5311 CrossRef CAS PubMed.
- (a) Y. Kim, W. Li, S. Shin and M. Lee, Acc. Chem. Res., 2013, 46, 2888–2897 CrossRef CAS PubMed; (b) Z. Huang, H. Lee, E. Lee, S.-K. Kang, J.-M. Nam and M. Lee, Nat. Commun., 2011, 2, 459 CrossRef PubMed; (c) E. Lee, Y.-H. Jeong, J.-K. Kim and M. Lee, Macromolecules, 2007, 40, 8355–8360 CrossRef CAS; (d) K.-S. Moon, H.-J. Kim, E. Lee and M. Lee, Angew. Chem., Int. Ed., 2007, 46, 6807–6810 CrossRef CAS PubMed.
- (a) T. Hirose, K. Matsuda and M. Irie, J. Org. Chem., 2006, 71, 7499–7508 CrossRef CAS PubMed; (b) T. Hirose, M. Irie and K. Matsuda, Adv. Mater., 2008, 20, 2137–2141 CrossRef CAS.
- (a) F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed; (b) T. Weil, T. Vosch, J. Hofkens, K. Peneva and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 9068–9093 CrossRef CAS PubMed.
- (a) X. Zhang, D. Görl, V. Stepanenko and F. Würthner, Angew. Chem., Int. Ed., 2014, 53, 1270–1274 CrossRef CAS PubMed; (b) D. Görl, X. Zhang, V. Stepanenko and F. Würthner, Nat. Commun., 2015, 6, 7009 CrossRef PubMed.
- (a) T. Ishizone, A. Seki, M. Hagiwara and S. Han, Macromolecules, 2008, 41, 2963–2967 CrossRef CAS; (b) W. Li, A. Zhang and A. D. Schlüter, Chem. Commun., 2008, 5523–5525 RSC; (c) S. Aoshima, H. Oda and E. Kobayashi, J. Polym. Sci., Part A: Polym. Chem., 1992, 30, 2407–2413 CrossRef CAS.
- M. M. Safont-Sempere, G. Fernández and F. Würthner, Chem. Rev., 2011, 111, 5784–5814 CrossRef CAS PubMed.
- (a) F. Rodler, B. Schade, C. M. Jäger, S. Backes, F. Hampel, C. Böttcher, T. Clark and A. Hirsch, J. Am. Chem. Soc., 2015, 137, 3308–3317 CrossRef CAS PubMed; (b) S. Yagai, M. Usui, T. Seki, H. Murayama, Y. Kikkawa, S. Uemura, T. Karatsu, A. Kitamura, A. Asano and S. Seki, J. Am. Chem. Soc., 2012, 134, 7983–7994 CrossRef CAS PubMed; (c) A. Wicklein, A. Lang, M. Muth and M. Thelakkat, J. Am. Chem. Soc., 2009, 131, 14442–14453 CrossRef CAS PubMed; (d) Y. Zakrevskyy, C. F. J. Faul, Y. Guan and J. Stumpe, Adv. Funct. Mater., 2004, 14, 835–841 CrossRef CAS; (e) V. Percec, M. Peterca, T. Tadjiev, X. Zeng, G. Ungar, P. Leowanawat, E. Aqad, M. R. Imam, B. M. Rosen, U. Akbey, R. Graf, S. Sekharan, D. Sebastiani, H. W. Spiess, P. A. Heiney and S. D. Hudson, J. Am. Chem. Soc., 2011, 133, 12197–12219 CrossRef CAS PubMed.
- B.-K. An, S.-K. Kwon, S.-D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410–14415 CrossRef CAS PubMed.
- J. Sung, P. Kim, B. Fimmel, F. Würthner and D. Kim, Nat. Commun., 2015, 6, 8646 CrossRef CAS PubMed.
- F. Würthner, Chem. Commun., 2004, 1564–1579 RSC.
- (a) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766–1776 CrossRef CAS PubMed; (b) J. H. van Esch and B. L. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263–2266 CrossRef CAS.
- M. Sun, K. Müllen and M. Yin, Chem. Soc. Rev., 2016, 45, 1513–1528 RSC.
- (a) A. Chortos and Z. Bao, Mater. Today, 2014, 17, 321–331 CrossRef CAS; (b) T. Someya, T. Sekitani, S. Iba, Y. Kato, H. Kawaguchi and T. Sakurai, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9966–9970 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures and results for all reported experiments, along with synthetic details for PBI 1. See DOI: 10.1039/c6sc02249a |
| This journal is © The Royal Society of Chemistry 2016 |