Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reactive oxygen species in iridium-based OER catalysts†
Verena
Pfeifer
ab,
Travis E.
Jones
*a,
Sabine
Wrabetz
a,
Cyriac
Massué
ac,
Juan J.
Velasco Vélez
ac,
Rosa
Arrigo
 d,
Michael
Scherzer
ac,
Simone
Piccinin
e,
Michael
Hävecker
ac,
Axel
Knop-Gericke
a and
Robert
Schlögl
ac
d,
Michael
Scherzer
ac,
Simone
Piccinin
e,
Michael
Hävecker
ac,
Axel
Knop-Gericke
a and
Robert
Schlögl
ac
aDepartment of Inorganic Chemistry, Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, Berlin, 14195, Germany. E-mail: trjones@fhi-berlin.mpg.de
bCatalysis for Energy, Group EM-GKAT, Helmholtz-Zentrum Berlin für Materialien und Energie GmbH, Elektronenspeicherring BESSY II, Albert-Einstein-Str. 15, Berlin, 12489, Germany
cDepartment of Heterogeneous Reactions, Max-Planck-Institut für Chemische Energiekonversion, Stiftstr. 34-36, Mülheim a. d. Ruhr, 45470, Germany
dDiamond Light Source Ltd., Harwell Science & Innovation Campus, Didcot, Oxfordshire OX 11 0DE, UK
eConsiglio Nazionale delle Ricerche – Istituto Officina dei Materiali, c/o SISSA, Via Bonomea 265, Trieste, 34136, Italy
First published on 19th July 2016
Abstract
Tremendous effort has been devoted towards elucidating the fundamental reasons for the higher activity of hydrated amorphous IrIII/IV oxyhydroxides (IrOx) in the oxygen evolution reaction (OER) in comparison with their crystalline counterpart, rutile-type IrO2, by focusing on the metal oxidation state. Here we demonstrate that, through an analogy to photosystem II, the nature of this reactive species is not solely a property of the metal but is intimately tied to the electronic structure of oxygen. We use a combination of synchrotron-based X-ray photoemission and absorption spectroscopies, ab initio calculations, and microcalorimetry to show that holes in the O 2p states in amorphous IrOx give rise to a weakly bound oxygen that is extremely susceptible to nucleophilic attack, reacting stoichiometrically with CO already at room temperature. As such, we expect this species to play the critical role of the electrophilic oxygen involved in O–O bond formation in the electrocatalytic OER on IrOx. We propose that the dynamic nature of the Ir framework in amorphous IrOx imparts the flexibility in Ir oxidation state required for the formation of this active electrophilic oxygen.
Ir-based materials are promising candidates to catalyze the oxygen evolution reaction (OER) in acidic media. Iridium oxides, both anhydrous rutile-type IrO2 and hydrated X-ray amorphous forms, have been widely considered as possible OER catalysts.1–8 Of these, the amorphous and nanostructured Ir-based catalysts have been shown to exhibit higher OER activities than rutile-type IrO2.9,10 Although numerous studies exist on such amorphous structures, and activity descriptors like mixed iridium oxidation states11,12 and the surface OH-concentration13 have been proposed, a convincing explanation for the enhanced activity of amorphous structures over crystalline ones is lacking. While many studies concentrated on investigating active metal centers and the iridium oxidation state,11,12,14 by analogy with the well-studied biological water splitting, an investigation of preactivated oxygen species contained in OER catalysts seems appropriate.
Oxygen preactivated for O–O bond formation is essential in photocatalytic water splitting over the tetranuclear Mn water oxidation complex (WOC) of photosystem II (PS II).15,16 A proposed mechanism of O–O bond formation in PS II is the nucleophilic attack of oxygen adsorbed in the WOC (O*) by a (preadsorbed) water molecule/hydroxide:17,18
| WOC–O* + H2O(ads) → WOC–O–O–H + H+ + e−. | (1) |
In this mechanism water acts as the nucleophile, donating electrons to the reactive oxygen, which can alternatively be formulated WOC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O+ to emphasize its susceptibility to nucleophilic attack.15 Within this mechanism, the ability of Mn to accommodate different oxidation states enables the preparation of the electrophilic oxygen.
O+ to emphasize its susceptibility to nucleophilic attack.15 Within this mechanism, the ability of Mn to accommodate different oxidation states enables the preparation of the electrophilic oxygen.
Nucleophilic attack of adsorbed oxygen by water/hydroxide has also been proposed to describe the mechanism of O–O bond formation on transition metal oxides during the OER.4,19 For iridium oxide, the importance of reactive oxygen is evident from isotope labeling experiments,5 which suggest the outer layers of the catalyst are involved directly in the electrocatalytic OER. Thus, O–O bond formation can be written as:
| IrOxO(ads) + H2O → IrOx–O–O–H + H+ + e−, | (2) |
A powerful means of uncovering the chemistry of the OI− is through CO oxidation, a reaction that has proven essential in developing a fundamental understanding of heterogeneous processes.20 Herein we use this probe to test if the OI− in X-ray amorphous IrOx can act as a strong electrophile. If this species is sufficiently electrophilic, we expect it to readily react with CO by accommodating the electron density of the partially negatively charged carbon atom of CO.20,21
The sample investigated is a commercially available, hydrated, X-ray amorphous IrIII/IV oxyhydroxide (IrOx, Premion, AlfaAesar). In a control experiment, a less active, crystalline rutile-type IrO2 (Sigma Aldrich) was examined. The properties of both materials are described in ref. 10. Therein, we showed that OI− is identifiable by an excitation energy resonance at 529 eV in the near-edge X-ray absorption fine structure (NEXAFS) of the O K-edge that is not present in pristine rutile-type IrO2. Herein, the spectroscopic features corresponding to these OI− species were monitored by quasi in situ X-ray photoemission spectroscopy (XPS) and NEXAFS before and after CO exposure to observe whether OI− reacts with CO during titration of the IrOx surface. This approach ensures high quality artifact free oxygen spectra by preventing alterations of the sample during air exposure between measurements and CO exposure and eliminating gas phase contributions of CO and CO2 to the oxygen absorption spectra. The results are compared to theoretical calculations and a microcalorimetric analysis.
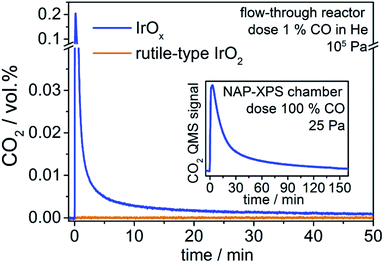
In a preliminary experiment, we tested the general activity of the two iridium oxide powders towards a stoichiometric oxidation of CO to CO2 at room temperature and atmospheric pressure in a standard flow-through reactor (details in ESI†). To ensure reaction of CO was exclusively with active oxygen from the iridium oxide samples, we did not add O2 to the gas feed and had oxygen filters attached to the gas dosing lines.
We observe that introducing CO into the reactor filled with rutile-type IrO2 leads to no change in the CO2 signal (Fig. 1). In contrast, when introducing CO into the reactor loaded with IrOx, the CO2 signal shows a sharp increase followed by a decline towards the baseline (Fig. 1). Such full CO conversion at ≈300 K is extremely unusual, having only been observed in a few novel systems, such as oxide-supported gold catalysts,22 and reveals the high reactivity of the IrOx. As there is no external oxygen source available in the present experiment, the effluent CO2 must be due to the stoichiometric reaction of CO with an oxygen species present in IrOx and nearly absent from the rutile-type powder. Because only IrOx has an abundance of OI−, this type of oxygen is a likely candidate for that active in CO oxidation. To test this hypothesis, we employ XPS/NEXAFS and monitor the regions characteristic for OI− (≈529 eV).
In a quasi in situ experiment, we first measured the powder in vacuum as received (base pressure 10−6 Pa). We then exposed the IrOx to pure CO in the NAP-XPS chamber (25 Pa, 2 mL min−1) for 3 h and observed a CO2 profile similar to that seen in the standard reactor (see inset Fig. 1). Finally, we evacuated the chamber and without air contact measured the sample after CO (details see ESI†).
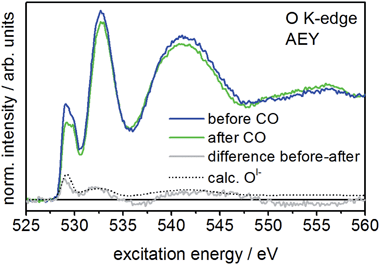
When comparing the XPS core lines recorded before and after CO exposure, the Ir 4f spectra show no obvious changes (Fig. S3†). In contrast, the intensity at low binding energies (529–530 eV) in the O 1s spectrum is slightly reduced after CO exposure (Fig. S3†), indicating the potential loss of OI−. The corresponding O K-edge NEXAFS collected in the surface sensitive Auger electron yield mode (AEY) corroborate this finding. Here we see CO exposure leads to a loss in intensity at excitation energies around 529 eV (Fig. 2). A comparison of the difference spectrum of the O K-edges before and after CO exposure with that calculated for OI− (see Fig. 2) supports this conclusion, suggesting OI− is consumed from IrOx by oxidizing CO to CO2 at room temperature.
Interestingly, more bulk sensitive total electron yield (TEY) measurements of the O K-edge also show reduced 529 eV intensity after CO exposure (see Fig. S5†). Hence, subsurface OI− species migrating to the surface also appear to be involved in the reaction with CO. This observation alludes to the dynamic mobility of OI− in the porous structure10 of IrOx. Nevertheless, since the CO2 signal in the standard reactor and the NAP-XPS chamber declines immediately after the initial increase, the reservoir of mobile OI− species seems to be rapidly exhausted. However, since the 529 eV component does not vanish completely in either AEY and TEY, part of the OI− seem unavailable for reaction with CO over the time scale of our experiment.
Though comparison of our theoretical and experimental O K-edge spectra supports the conclusion that OI− participates in stoichiometric room temperature CO oxidation, we have not demonstrated such a reaction is feasible. To do so, we begin by considering the thermodynamics of the reaction of gas phase CO with oxygen, in oxidation state n (On−), in an IrOx matrix (IrOxOn−):
| IrOxOn− + CO(g) → IrOx + CO2(g) + ne−. | (3) |
The liberated ne− may reduce adjacent Ir or O. We have assumed the product desorbs from the surface after forming, an assumption we will show to be valid. It is then straightforward to compute the heat of reaction for this process for different types of oxygen species by way of DFT (see ESI† for details), as the energies of the gas phase reactant and product are constants and only the adsorption energy of the oxygen species involved in the reaction changes the heat evolved (using the positive sign convention of microcalorimetry). And while we do not know the structure of the amorphous IrOx, we can use model systems to gain useful insights into the relationships between the electronic structure of oxygen and its adsorption energy.
To investigate the reaction of CO(g) with surfaces species, we employed IrO2 (110) and IrO2 (113) surfaces as model structures, using both oxygen-terminated and partially reduced (110) surfaces along with a partially reduced (113) surface (see ESI† for full details). Both types of surface have under coordinated O atoms whose simulated O K-edge spectra agree with that measured for OI− (see ESI†). The reduced surfaces also have under coordinated Ir atoms that can adsorb CO with adsorption energies of ≈180–210 kJ mol−1 (see ESI†).
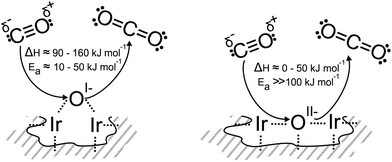
As a first consideration, we computed the minimum energy path for the reaction of CO(g) with an oxygen-terminated (110) surface. This surface contains three unique types of oxygen, an OII− like species, an OI− like species, and oxygen on the coordinatively unsaturated site (CUS), the last of which was not observed experimentally (Fig. S14†). The OI− has a barrier for reaction (3), ≈10 kJ mol−1, that is slightly larger than kBT at room temperature, and the reaction is ≈90 kJ mol−1 exothermic. In contrast, the activation energy associated with the reaction of CO(g) directly with OII− is significantly larger than the energy available at room temperature, 170 kJ mol−1, and is exothermic by ≈50 kJ mol−1.
To test the reaction of adsorbed CO with an OI− like species, we computed minimum energy paths on the partially reduced (113) surface (see ESI† for details). In this case, CO oxidation by an OI− has a slightly higher barrier, 50 kJ mol−1, than was observed on the fully oxidized (110) surface. Also, the initial product on the reduced surface is a strongly adsorbed CO2, not CO2(g). Overall reaction (3) is ≈160 kJ mol−1 exothermic on the reduced (113) surface, which is in agreement with the 165 kJ mol−1 computed for the reaction of CO(g) with OI− on a partially reduced (110) surface with an Ir vacancy.
These results suggest that, if present, surface OI− species are highly electrophilic, with the fully oxidized surface perhaps showing higher reactivity. These electrophilic anionic defects are, in fact, predicted to be so reactive that they should not persist except under ultra-high vacuum, which may explain the their absence in rutile-type IrO2 loaded from air10 and the lack of CO2 formation when rutile-type IrO2 is exposed to CO (see Fig. 1). However, we know from the TEY measurements that, unlike the rutile-type oxide, the amorphous oxides contain a significant concentration of potentially mobile OI− in the subsurface/bulk region. These subsurface species are depleted during CO oxidation (see Fig. S5†). If we assume that once on the surface any OI− will behave in a manner analogous to the aforementioned surface species, we can focus on the bulk as a thermodynamic source of oxygen and compute its associated heat of reaction with CO(g).
We find that reaction with the bulk OII− of rutile-type IrO2 is thermoneutral, whereas reaction with bulk OI− species10 is 110–120 kJ mol−1 exothermic, depending on the atomic structure around the anionic defect. As with the surfaces, we see that bulk OI− species are more weakly bound, hence more electrophilic, than the bulk OII−. These results are summarized schematically in Fig. 3.
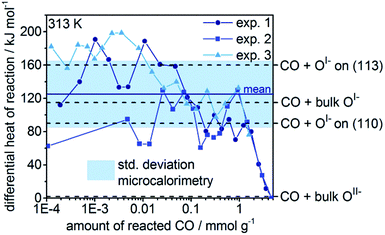
When the heats of reaction for all the oxygen species studied in this work are considered, we find that the OI− type generally gives rise to higher heats of reaction than the formally OII−. Remarkably, in the absence of major structural relaxations of the surface after reaction, there is a rough linear relationship between the position of the white line in the calculated O K-edge spectrum of an oxygen species and its corresponding heat of reaction with CO (see Fig. S15†). Thus, the O K-edge spectra appear to offer a means of probing the electrophilic character of the oxygen. From this measure, we would predict the heat of reaction of the dominant OI− species present in our experiments (O K ≈ 529 eV) is 100 kJ mol−1.
To validate the calculated heats of reaction for room temperature CO oxidation on IrOx, we titrated IrOx with CO at 313 K using in situ microcalorimetry.23 A gas phase analysis after the experiment confirmed the predominant presence of CO2(g), supporting our assumption in eqn (3) and alluding to complete oxidation of the dosed CO (see Fig. S11†). Hence, in the determination of the differential heat of reaction, we took into account the entire dosed CO. Doing so, and averaging over three measurements, we obtain an exothermic heat of reaction of 125 kJ mol−1 ± 40 kJ mol−1 for the 2 mmol g−1 of reacted CO (see Fig. 4). These measured differential heats of reaction agree with those calculated for CO(g) oxidation by OI− species (see Fig. 4), whereas they are much larger than the thermoneutral reaction of CO(g) with OII−. In fact, the mean measured value (125 kJ mol−1) is similar to the heats calculated for reaction with bulk OI−, while the upper and lower ends of the standard deviation window agree with the calculated reaction enthalpies for CO(g) with OI− on the partially reduced (113) and fully oxidized (110) surfaces, respectively. In addition, we observe a broadening of the thermosignal time profiles with increasing amount of reacted CO (see Fig. S8–S10†), which could be an indication for the involvement of subsurface OI− liberated by the reaction heat evolved in the surface region.
Hence, the calculations corroborate our assumption that during the exposure of IrOx to CO, OI− reacts with CO at room temperature. Combining our NEXAFS and microcalorimetric measurements with our theoretical calculations therefore suggests that the OI− is a stoichiometric oxidant of CO to CO2, and therefore strongly electrophilic.
Now that we have demonstrated the reactivity of the OI− species, it seems appropriate to consider their regeneration since for the OER cycle to be catalytic, reactive oxygen must be replenished. To test whether OI− can be replenished on IrOx we performed additional quasi in situ XPS and NEXAFS experiments. Following the CO titration, we exposed the sample successively to different gas atmospheres (25 Pa O2, 25 Pa H2O, and 25 Pa O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O3 (99
O3 (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) in the NAP-XPS chamber for 20 min each to discern whether any of these gases can replenish the reactive oxygen sites. After each gas treatment, we evacuated the chamber and measured XPS and NEXAFS in UHV without allowing the samples to contact air.
1)) in the NAP-XPS chamber for 20 min each to discern whether any of these gases can replenish the reactive oxygen sites. After each gas treatment, we evacuated the chamber and measured XPS and NEXAFS in UHV without allowing the samples to contact air.
We observed distinct changes in the O K-edge only after the exposure of the sample to the ozone-containing gas mixture. A comparison of the O K-edges after CO and after subsequent ozone exposure indicates a replenishment of OI− by a more intense 529 eV feature after ozone exposure, see Fig. S6.† However, we found that the formally OI− species replenished by ozone were not stable, as indicated by a reduction in the 529 eV resonance after 30 min in vacuum. This observation suggests that parts of the matrix stabilizing these OI− species are lost during the oxidation of CO and cannot be restructured under such low pressure at room temperature. Nevertheless, ozone, as a source of atomic oxygen, seems to be capable of at least temporarily refilling the OI− vacancies formed during reaction with CO. Hence, we have demonstrated that the replenishment of these reactive oxygen species is feasible, which makes them relevant for the catalytic OER cycle.
The local structure of the OI− species considered in our work can be characterized as a µ2-oxo bridge, which can be seen to arise naturally when defects are introduced into the cationic framework of the parent rutile-type structure, thereby transforming µ3-oxo into µ2-oxo bridges. This process gives rise to holes in the O 2p states, due to the removal of IrIV and 4e−, making the µ2-oxo bridges charge deficient.10,24 Such µ2-oxo bridged atomic structures have been identified as key motifs in amorphous IrOx thin films25 and as active centers in liquid-phase iridium oxidation catalysts26 by extended X-ray absorption fine structure (EXAFS) measurements, which are able to resolve the local atomic structure of amorphous materials. It is worth noting, however, that the electrophilic OI− species seen in XPS and NEXAFS need not exclusively be due to such µ2-oxo bridges. Instead, they may also emerge when substituting an IrIV with a lower-valent element, such as NiII,13 as the holes formed by replacing IrIV and 4e− with NiII and 2e− can localize on neighboring oxygen atoms. Thus, both types of environment, the under-coordinated and the lower-valent, can result in the emergence of an electron deficiency on oxygen. And while other potentially electron deficient oxygen species, in particular Ir![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, can also be imagined, we find no evidence for them in the O K-edge spectra of the rutile-type IrO2 or amorphous IrOx samples, see Fig. S14.†
O, can also be imagined, we find no evidence for them in the O K-edge spectra of the rutile-type IrO2 or amorphous IrOx samples, see Fig. S14.†
In conclusion, we showed that the OI− species adsorbed on the surface and in the subsurface of OER-active IrIII/IV oxyhydroxides are extremely electrophilic. By combining advanced experimental and theoretical techniques we found that this electrophilic oxygen can even oxidize CO to CO2 at room temperature, with a portion of the reacting oxygen coming from the subsurface region of the amorphous catalyst. The exceptional reactivity of the formally OI− species is suspected to play a critical role in the OER by reacting with OH/H2O to form the OOH intermediate, which, when considering that weakly OER-active crystalline rutile-type IrO2 is nearly devoid of this reactive oxygen, explains the exceptional reactivity of amorphous IrIII/IV oxyhydroxides. Like in biological water splitting, the electrophilic oxygen in IrIII/IV oxyhydroxides is an optimal precursor site for the nucleophilic attack of (preadsorbed) water during the O–O bond formation. The creation of electrophilic oxygen in PS II is enabled by the easy accommodation of different oxidation states of the coordinated Mn ions. Likewise flexibility in the oxidation state for iridium is needed for electrophilic oxygen formation in iridium, which is apparently found in the IrIII/IV oxyhydroxides.
Acknowledgements
The authors gratefully acknowledge BESSY II/HZB for granting beam time under the proposal #15101925 and Höchst-Leistungs-Rechenzentrum Stuttgart (HLRS) for computational facilities. We also thank Pierre Kube for conducting the post gas phase analysis of the microcalorimetry experiment. T. E. J. acknowledges the Alexander-von-Humboldt foundation for financial support. This work was further supported by the Ministry of Education and Science of the Russian Federation (RFMEFI61614X0007) and the Bundesministerium für Bildung und Forschung (05K14EWA) through the joint Russian-German research project “SYnchrotron and NEutron STudies for Energy Storage” (SYNESTESia).References
- D. N. Buckley and L. D. Burke, J. Chem. Soc., Faraday Trans. 1, 1976, 72, 2431–2440 RSC
.
- R. Kötz, H. Neff and S. Stucki, J. Electrochem. Soc., 1984, 131, 72–77 CrossRef
.
- M. Vuković, J. Appl. Electrochem., 1987, 17, 737–745 CrossRef
.
- J. Rossmeisl, Z.-W. Qu, H. Zhu, G.-J. Kroes and J. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS
.
- S. Fierro, T. Nagel, H. Baltruschat and C. Comninellis, Electrochem. Commun., 2007, 9, 1969–1974 CrossRef CAS
.
- T. Reier, M. Oezaslan and P. Strasser, ACS Catal., 2012, 2, 1765–1772 CrossRef CAS
.
- H. N. Nong, L. Gan, E. Willinger, D. Teschner and P. Strasser, Chem. Sci., 2014, 5, 2955–2963 RSC
.
- M. Bernicke, E. Ortel, T. Reier, A. Bergmann, J. Ferreira de Araujo, P. Strasser and R. Kraehnert, ChemSusChem, 2015, 8, 1908–1915 CrossRef CAS PubMed
.
- T. Reier, D. Teschner, T. Lunkenbein, A. Bergmann, S. Selve, R. Kraehnert, R. Schlögl and P. Strasser, J. Electrochem. Soc., 2014, 161, 876–882 CrossRef
.
- V. Pfeifer, T. E. Jones, J. J. Velasco Vélez, C. Massué, M. T. Greiner, R. Arrigo, D. Teschner, F. Girgsdies, M. Scherzer, J. Allan, M. Hashagen, G. Weinberg, S. Piccinin, M. Hävecker, A. Knop-Gericke and R. Schlögl, Phys. Chem. Chem. Phys., 2016, 18, 2292–2296 RSC
.
- A. Minguzzi, C. Locatelli, O. Lugaresi, E. Achilli, G. Cappelletti, M. Scavini, M. Coduri, P. Masala, B. Sacchi, A. Vertova, P. Ghigna and S. Rondinini, ACS Catal., 2015, 5, 5104–5115 CrossRef CAS
.
- A. Minguzzi, O. Lugaresi, E. Achilli, C. Locatelli, A. Vertova, P. Ghigna and S. Rondinini, Chem. Sci., 2014, 5, 3591–3597 RSC
.
- T. Reier, Z. Pawolek, S. Cherevko, M. Bruns, T. Jones, D. Teschner, S. Selve, A. Bergmann, H. N. Nong, R. Schlögl, K. J. J. Mayrhofer and P. Strasser, J. Am. Chem. Soc., 2015, 137, 13031–13040 CrossRef CAS PubMed
.
- H. G. Sanchez Casalongue, M. L. Ng, S. Kaya, D. Friebel, H. Ogasawara and A. Nilsson, Angew. Chem., Int. Ed., 2014, 53, 7169–7172 CrossRef CAS PubMed
.
- W. Lubitz, E. J. Reijerse and J. Messinger, Energy Environ. Sci., 2008, 1, 15–31 CAS
.
- J. P. McEvoy and G. W. Brudvig, Chem. Rev., 2006, 106, 4455–4483 CrossRef CAS PubMed
.
- J. Messinger, Phys. Chem. Chem. Phys., 2004, 6, 4764–4771 RSC
.
- Y. Gao, T. Åkermark, J. Liu, L. Sun and B. Åkermark, J. Am. Chem. Soc., 2009, 131, 8726–8727 CrossRef CAS PubMed
.
- M. Zhang, M. de Respinis and H. Frei, Nat. Chem., 2014, 6, 362–367 CrossRef CAS PubMed
.
- H.-J. Freund, G. Meijer, M. Scheffler, R. Schlögl and M. Wolf, Angew. Chem., Int. Ed., 2011, 50, 10064–10094 CrossRef CAS PubMed
.
- B. Rosenblum, A. H. Nethercot and C. H. Townes, Phys. Rev., 1958, 109, 400–412 CrossRef CAS
.
- A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed
.
- L. C. Jozefowicz, H. G. Karge and E. N. Coker, J. Phys. Chem., 1994, 98, 8053–8060 CrossRef CAS
.
- V. Pfeifer, T. E. Jones, J. J. Velasco Vélez, C. Massué, R. Arrigo, D. Teschner, F. Girgsdies, M. Scherzer, M. T. Greiner, J. Allan, M. Hashagen, G. Weinberg, S. Piccinin, M. Hävecker, A. Knop-Gericke and R. Schlögl, Surf. Interface Anal., 2016, 48, 261–273 CrossRef CAS
.
- J. Huang, J. D. Blakemore, D. Fazi, O. Kokhan, N. D. Schley, R. H. Crabtree, G. W. Brudvig and D. M. Tiede, Phys. Chem. Chem. Phys., 2014, 16, 1814–1819 RSC
.
- K. R. Yang, A. J. Matula, G. Kwon, J. Hong, S. W. Sheehan, J. M. Thomsen, G. W. Brudvig, R. H. Crabtree, D. M. Tiede, L. X. Chen and V. S. Batista, J. Am. Chem. Soc., 2016, 138, 5511–5514 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc01860b |
| This journal is © The Royal Society of Chemistry 2016 |