 Open Access Article
Open Access ArticleAn efficient route for the synthesis of phosphorus–selenium macro-heterocycles†
Guoxiong Hua,
Alexandra M. Z. Slawin,
Rebecca A. M. Randall,
David B. Cordes,
L. Ellis Crawford,
Michael Bühl and
J. Derek Woollins*
EaStChem School of Chemistry, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, UK. E-mail: jdw3@st-andrews.ac.uk; Fax: +44 (0)01334 470384; Tel: +44 (0)01334 463384
First published on 13th February 2013
Abstract
Four-membered ring [PhP(Se)(μ-Se)]2 (Woollins' reagent, WR) reacts with disodium alkenyl-diols followed by in situ ring-closure reaction with appropriate dibromoalkanes affording a series of unusual nine- to fifteen-membered organoselenophosphorus macrocycles bearing the O–P–Se–Cn–Se–P–O or O–P–Se–Cn–O–P–Se linkage.
The synthesis of macrocycles containing heavier elements is challenging and hetero-macrocycles are very rare1 thus organoselenium heterocycles containing more than eight-membered rings with at least two selenium atoms are limited.2–7 The known methods to prepare these heterocyclic compounds in general suffer from the use of highly toxic reagents with harsh reaction conditions, low yields or multi-steps. We, and others, have developed and exploited the chemistry of 2,4-bis(phenyl)-1,3-diselenadiphosphetane-2,4-diselenide (Woollins' reagent, WR) as a versatile selenation reagent in synthetic chemistry.8–19
Recently, we have successfully applied WR as an efficient building unit or block to synthesize a series of eight-, nine-, and ten-membered selenophosphorus heterocycles with the P–Se–Se–P linkage,20 and unique octaselenocyclododecane with four carbon atoms and eight selenium atoms in this fourteen-membered cycle.21 In this communication, we report a ‘one-pot’ approach via a small ring expansion by consecutively introducing two organic building blocks to construct a series of large organo-selenophosphorus heterocycles including two phosphorus atoms and two selenium atoms and two oxygen atoms in the ring. To the best of our knowledge, this is the first synthesis and X-ray structure of such organoselenophosphorus macrocycles consisting of the O–P–Se–Cn–Se–P–O linkage or the O–P–Se–Cn–O–P–Se linkage.
Cleavage of the four-membered ring P2S2 in WR by an equimolar amount of disodium ethane-1,2-bis(olate) or disodium propane-1,3-bis(olate) (derived from ethylene glycol or 1,3-propanediol and NaH) led to disodium bis(phenylphosphonodiselenoate)s 1 and 2, which were converted in situ into the corresponding nine- to fifteen-membered phosphorus–selenium macro-heterocycles 4–9 in moderate to good yields (36 to 56%) by further treatment with an equivalent amount of various dibromides (Br–E–Br, E = –(CH2)n– or –CH2C6H4CH2– or –CH2C6H4C6H4CH2–) at ambient temperature for 24 h (Scheme 1). In all the cases, insoluble (in water or normal organic solvent) side products which we were not able to identify were found resulting in the non-quantitative yields arising from the extensive formation of the possible polymers.
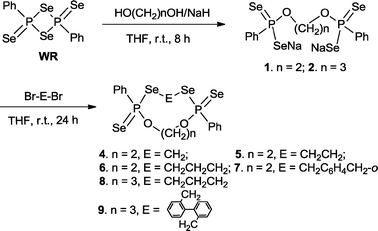 | ||
| Scheme 1 Synthesis of new macroheterocycles 4–9. | ||
However, analogous routes involving disodium butane-1,4-bis(olate) and 1,4-dibromobutanol gave rise to the surprising fourteen-membered macrocycle11 along with 10 (Scheme 2, obtained in 36.8% isolated yield). We speculate that 11 is formed by the reaction of 1,4-dibromobutane with an intermediate 3b, the latter being the intramolecular isomerization product of 3a. Monitoring the reaction of WR with NaO(CH2)4ONa by 31P NMR confirmed the presence of two isomers 3a and 3b in the reaction mixture (δP = 77.8 and 78.1 ppm).
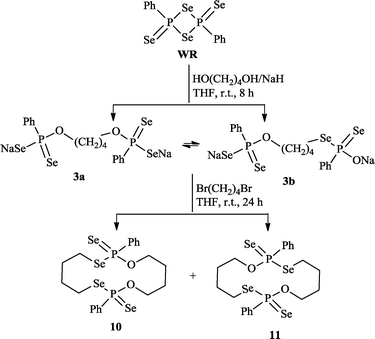 | ||
| Scheme 2 Synthesis of new 14-membered ring systems 10 and 11. | ||
Preliminary computations at the B3LYP/6-31G* level22,23 indicate selected stereoisomers of 11 to be slightly more stable in energy than their counterparts 10 (see Fig. S1 and Table S4 in the ESI†). We investigated increased reaction times and higher temperatures for the second stage of the reaction in Scheme 2 but this had no impact suggesting that although 11 is the more stable isomer there is no readily accessible pathway between 10 and 11.
Macrocycles 4–9 and 11 are soluble in common polar organic solvents and are stable to air and moisture at ambient temperature for several months without any sign of decomposition. All of the new compounds were characterized by standard analytical and spectroscopic techniques. The 31P NMR spectra of 4–9 and 11 exhibit sharp singlets in the range of δ = 67.6 to 82.4 ppm, which are accompanied by two sets of satellites for the endocyclic and exocyclic selenium atoms 1J(P,Seendo) and 1J(P,Seexo), thus indicating the presence of single and double P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se bonds in each compound. The results were further confirmed by the 77Se NMR spectra of compounds 4–9 and 11 that contain signals arising from exocyclic selenium in the range of δ = 341.3 to 490.1 ppm and endocyclic selenium atoms in the range of δ = −115.3 to −71.8 ppm with appropriate 1J(P,Seendo) and 1J(P,Seexo) coupling constants (ca. 450 and 830 Hz, respectively).
Se bonds in each compound. The results were further confirmed by the 77Se NMR spectra of compounds 4–9 and 11 that contain signals arising from exocyclic selenium in the range of δ = 341.3 to 490.1 ppm and endocyclic selenium atoms in the range of δ = −115.3 to −71.8 ppm with appropriate 1J(P,Seendo) and 1J(P,Seexo) coupling constants (ca. 450 and 830 Hz, respectively).
Macrocycles 5–7, 9 and 11 were crystallized from dichloromethane solutions with slow diffusion of hexane to give transparent, colorless, cubic crystals. The R,R/S,S and the R,S forms of 11 were crystallised independently. The X-ray structures of 5–7, 9 and 11 (Fig. 1–4) confirm the presence of nine- to fifteen-membered rings. Within 5–7, 9 and 11, the P–Se single bonds are in the range of 2.213–2.251 Å and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se double bonds are in the range of 2.078–2.098 Å similar to that in acyclic structures containing the P–Se–Se–P linkage.20,24 The macrocyclic frameworks in 5 and 9 are highly puckered with the two phenyl rings on phosphorus atoms on opposite sides of the macro-heterocyclic ring. Table 1 summarises the transannular Se⋯Se, P⋯P and O⋯O distances in the new macro-heterocycles and illustrates the ability to tune the cavity in this new class of macrocycles. The values are as anticipated significantly longer than those observed in the four-membered P2Se2 ring system (3.1 Å) and considerably longer than those in the six-membered P2Se4 ring system (4.3 Å).25 The geometry around P(1) and P(2) is distorted tetrahedral (Se(3)–P(1)–Se(1) and Se(4)–P(2)–Se(2): 116.5(6)° and 108.4(6)° for 5, 115.0(6)° and 116.2(6)° for 9). Macrocycles6, 7 and 11a (R,R/S,S isomer of 11) contain frameworks similar to 5 and 9, and are again highly puckered. The geometry around P(1) and P(2) is distorted tetrahedral (Se(3)–P(1)–Se(1) and Se(4)–P(2)–Se(2): 110.5(5)° and 113.7(5)° for 6, 115.0(6)° and 116.2(6)° for 7, and 106.7(2) [107.3(2)]° and 108.7(2) [107.5(2)]° for 11a).
Se double bonds are in the range of 2.078–2.098 Å similar to that in acyclic structures containing the P–Se–Se–P linkage.20,24 The macrocyclic frameworks in 5 and 9 are highly puckered with the two phenyl rings on phosphorus atoms on opposite sides of the macro-heterocyclic ring. Table 1 summarises the transannular Se⋯Se, P⋯P and O⋯O distances in the new macro-heterocycles and illustrates the ability to tune the cavity in this new class of macrocycles. The values are as anticipated significantly longer than those observed in the four-membered P2Se2 ring system (3.1 Å) and considerably longer than those in the six-membered P2Se4 ring system (4.3 Å).25 The geometry around P(1) and P(2) is distorted tetrahedral (Se(3)–P(1)–Se(1) and Se(4)–P(2)–Se(2): 116.5(6)° and 108.4(6)° for 5, 115.0(6)° and 116.2(6)° for 9). Macrocycles6, 7 and 11a (R,R/S,S isomer of 11) contain frameworks similar to 5 and 9, and are again highly puckered. The geometry around P(1) and P(2) is distorted tetrahedral (Se(3)–P(1)–Se(1) and Se(4)–P(2)–Se(2): 110.5(5)° and 113.7(5)° for 6, 115.0(6)° and 116.2(6)° for 7, and 106.7(2) [107.3(2)]° and 108.7(2) [107.5(2)]° for 11a).
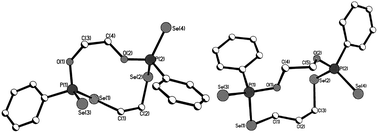 | ||
| Fig. 1 Molecular structures of 5 (left) and 6 (right) in the crystal. | ||
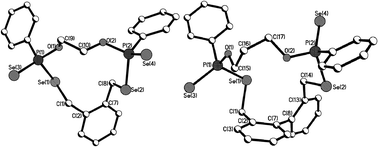 | ||
| Fig. 2 Molecular structures of 7 (left) and 9 (right) in the crystal. | ||
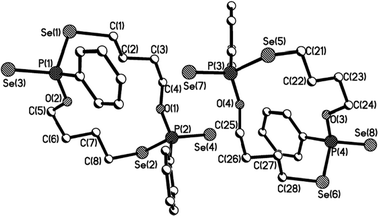 | ||
| Fig. 3 Molecular structure of 11a (R,R/S,S isomer of 11) in the crystal. | ||
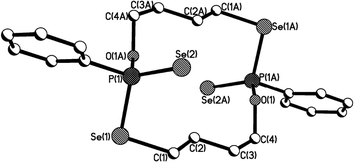 | ||
| Fig. 4 Crystal structure of 11b (R,S isomer of 11). | ||
| Macrocycle | Ring size & motif | O⋯O | P⋯P | Se⋯Se |
|---|---|---|---|---|
| R,S-5 | 10 P2Se2O2C4 | 2.850 | 5.278 | 5.119 |
| R,S-6 | 11 P2Se2O2C5 | 2.768 | 5.598 | 4.952 |
| R,R-7 | 12 P2Se2O2C6 | 3.140 | 4.793 | 3.596 |
| R,R-9 | 15 P2Se2O2C9 | 3.624 | 6.133 | 5.422 |
| R,R,S,S-11 | 14 P2Se2O2C8 | 4.656 [4.764] | 6.890 [6.968] | 7.575 [7.528] |
| R,S-11 | 14 P2Se2O2C8 | 5.162 | 6.504 | 7.561 |
The structure of 11b (R,S isomer of 11) exhibits a distinctly different pattern as shown in Fig. 4, compared with 11a (Fig. 3). The structure of 11b adopts a symmetric and puckered macrocyclic framework, with the two phenyl rings directly attached to phosphorus atoms being parallel to each other. The Se–P–Se bite angles are enlarged from ideal tetrahedral (Se(3)–P(1)–Se(1) and Se(4)–P(2)–Se(2): 115.9(8)°), and are considerably larger than those [106.7(2) [107.3(2)]° and 108.7(2) [107.5(2)]°] in 11a.
To probe the conformational preferences of the diastereomers of 11, some exploratory optimizations were performed at the B3LYP/6-31G* level of density functional theory. Starting from the relaxed coordinates of 11a and 11b with, respectively, (S,S) and (S,R) configurations at the stereogenic P centers, Se and Ph moieties were swapped at one P atom, affording the stereoisomers (S,R)-11a and (S,S)-11b. The relative energies of (S,S)-11a, (S,R)-11b, (S,S)-11b, and (S,R)-11a are 4.5, 0.0, 7.4 and 13.5 kJ mol−1, respectively. Thus, only the more stable conformer of each rac and meso isomer has been observed in the crystals.
Arguably, the flexible linkers will give rise to a plethora of possible conformers, and low kinetic barriers are expected for their interconversion. To confirm this expectation, a representative pathway was characterized leading from (S,S)-11a to (S,S)-11b (via one intermediate, see Fig. S1 in the ESI†). The highest barrier along this path is 23.6 kJ mol−1 at the B3LYP level (34.6 kJ mol−1 at B3LYP-D3). To freeze out such conformers would thus require very low temperatures, and would not seem possible with e.g. NMR under ambient conditions.
In summary, an efficient approach has been developed for the synthesis of a series of nine- to fifteen-membered phosphorus–selenium macro-heterocycles by consecutively inserting two difunctional organic units (alkyldiols and dibromoalkanes) into the four-membered ring in Woollins' reagent via one-pot reaction. The route is fast and efficient and simple. The demonstrated versatility of synthesis and X-ray structures provide a general and systemic approach to prepare such large phosphorus–selenium heterocyclic compounds. This method allows phosphorus–selenium macro-heterocycles to be easily available for further investigations into their chemical and biological properties.
The authors are grateful to the University of St Andrews, EaStCHEM and the Engineering and Physical Science Research Council (EPSRC, UK) for financial support and access to research computing facilities maintained by Dr H. Fruchtl, and the EPSRC National Mass Spectrometry Service Centre (Swansea) for mass spectral measurements.
References
- G. L. Sommen, ch. 16, pp. 863–899; A. M. Shestopalov and A. A. Shestopalov, ch. 17, pp. 900–943 in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katrizky and G. R. Newkome, Elsevier, Oxford, 2008, vol. 14 Search PubMed.
- S. Tomoda and M. Iwaoka, J. Chem. Soc., Chem. Commun., 1990, 231–233 RSC.
- J. L. Li, J. B. Meng, Y. M. Wang, J. T. Wang and T. Matsuura, J. Chem. Soc., Perkin Trans. 1, 2001, 1140–1146 RSC.
- A. Ishii, K. Furusawa, T. Omata and J. Nakyama, Heteroat. Chem., 2002, 13, 351–356 CrossRef CAS.
- A. U. R. Sankar, S. S. Reddy, G. C. S. Reddy, M. V. N. Reddy and C. N. Raju, Med. Chem. Res., 2011, 20, 962–967 CrossRef CAS.
- I. C. Reyes, E. VandenHoven, A. Mohammed and B. M. Pinto, Can. J. Chem., 1995, 73, 113–116 CrossRef.
- X. Zeng, X. Han, L. Chen, Q. Li, F. Xu, X. He and Z. Z. Zhang, Tetrahedron Lett., 2002, 43, 131–134 CrossRef CAS.
- For reviews see G. Hua and J. D. Woollins, Angew. Chem., Int. Ed., 2009, 48, 1368–1377 CrossRef CAS; J. D. Woollins, Synlett, 2012, 1154–1169 CrossRef.
- C. J. A. Gomez, R. M. Romano, H. Beckers, H. Willner and V. C. O. Della, Inorg. Chem., 2010, 49, 9972–9977 CrossRef.
- G. Hua, A. L. Fuller, Y. Li, A. M. Z. Slawin and J. D. Woollins, New J. Chem., 2010, 34, 1565–1571 RSC.
- M. Abdo, Y. Zhang and V. L. Schramm, Org. Lett., 2010, 12, 2982–2985 CrossRef CAS.
- Y. Huang, G. Jahreis, C. Luecke, D. Wildemann and G. Fischer, J. Am. Chem. Soc., 2010, 132, 7578–7579 CrossRef CAS.
- G. Hua, A. L. Fuller, A. M. Z. Slawin and J. D. Woollins, Eur. J. Org. Chem., 2010, 2607–2615 CrossRef CAS.
- G. Hua, J. B. Henry, Y. Li, A. R. Mount, A. M. Z. Slawin, J. D. Woollins and J. Derek, Org. Biomol. Chem., 2010, 8, 1655–1660 CAS.
- G. Hua, A. L. Fuller, A. M. Z. Slawin and J. D. Woollins, Polyhedron, 2011, 30, 805–808 CrossRef CAS.
- G. Hua, A. L. Fuller, A. M. Z. Slawin and J. D. Woollins, Eur. J. Org. Chem., 2011, 3067–3073 CrossRef CAS.
- G. Hua, D. B. Cordes, Y. Li, A. M. Z. Slawin and J. D. Woollins, Tetrahedron Lett., 2011, 52, 3311–3314 CrossRef CAS.
- R. C. S. Wong and M. L. Ooi, Inorg. Chim. Acta, 2011, 366, 350–356 CrossRef CAS.
- I. P. Gray, P. Bhattacharyya, A. M. Z. Slawin and J. D. Woollins, Chem.–Eur. J., 2005, 11, 6221–6227 CrossRef CAS.
- G. Hua, Y. Li, A. M. Z. Slawin and J. D. Woollins, Angew. Chem., Int. Ed., 2008, 47, 2857–2859 CrossRef CAS.
- G. Hua, J. M. Griffin, S. E. Ashbrook, A. M. Z. Slawin and J. D. Woollins, Angew. Chem., Int. Ed., 2011, 50, 4123–4126 CrossRef CAS.
- R. C. Binning and L. A. Curtiss, J. Comput. Chem., 1990, 11, 1206–1216 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104–154119 CrossRef.
- C. Q. Nguyen, A. Adeogun, M. Afzaal, M. A. Malik and P. O'Brien, Chem. Commun., 2006, 2179–2181 RSC.
- S. Parveen, P. Kilian, A. M. Z. Slawin and J. D. Woollins, Dalton Trans., 2006, 2586–2590 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 914877–914882. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc40515j |
| This journal is © The Royal Society of Chemistry 2013 |
