Breaking down phenylurea herbicides: advanced electrochemical approaches for environmental degradation and remediation
Ranil C. T.
Temgoua
 *,
Jan
Lisec
*,
Jan
Lisec
 and
Matthias
Koch
and
Matthias
Koch

Bundesanstalt für Materialforschung und -prüfung (BAM), Department of Analytical Chemistry and Reference Materials, Berlin, Germany. E-mail: raniltemgoua@yahoo.fr
First published on 17th November 2025
Abstract
Phenylurea herbicides (PUHs) represent one of the most extensively used herbicide families in agriculture worldwide. While effective for weed control, their environmental persistence, bioaccumulation potential, and formation of toxic metabolites raise significant environmental concerns. This review examines current electrochemical strategies for degrading phenylurea herbicides, with special emphasis on electrochemical oxidation (EC), photoelectrochemical processes (PEC), electro-Fenton (ECF) and photo-electro-Fenton (PECF), with particular attention to the various reactor configurations and their operational mechanisms. A critical innovation of this review lies in its systematic parameter assessment framework, which categorizes nine key operational parameters across all electrochemical degradation methods: electrode material, catalyst type, cell configuration, radiation source, operating conditions (pH, current density, temperature), removal efficiency, mineralization rate, degradation kinetics, identified intermediates, and energy consumption. For each technique, we highlight which parameters are essential, important, critical, or non-applicable, providing a structured framework to guide future experimental design. Selected case studies are presented to illustrate practical applications and performance outcomes. The review concludes with a critical analysis of current knowledge gaps and future research avenues that could enhance the sustainability, efficiency, and scalability of electrochemical remediation technologies. This work is intended as a comprehensive resource for environmental chemists, analytical scientists, and remediation engineers committed to addressing phenylurea herbicide contamination.
Water impactPhenylurea herbicides, among the most used in agriculture, persist in the environment and contaminate water with toxic residues, posing risks to both ecosystems and human health. This review highlights how advanced electrochemical processes, including electrochemical oxidation, photoelectrochemical, and electro-/photo-electro-Fenton systems, can effectively degrade these pollutants. By providing a structured framework to evaluate operational parameters, we point the way toward more sustainable, scalable, and practical solutions to safeguard clean water resources. |
1. Introduction
Phenylurea herbicides (PUHs) represent one of the most extensively utilized herbicide classes in both agricultural and non-agricultural applications, prized for their high efficacy at low application rates and their comparatively modest acute toxicity profiles. This chemical family encompasses widely implemented compounds including diuron, linuron, monuron, chlorotoluron, and isoproturon, which function as selective, non-systemic herbicides for controlling a broad spectrum of grasses and broadleaf weeds.1 Despite their agricultural value, these compounds pose considerable environmental concerns due to their limited biodegradability and high-water solubility, characteristics that facilitate their widespread distribution throughout aquatic ecosystems.1,2 The environmental persistence of phenylurea herbicides exhibits significant variation, with soil half-lives ranging from under 30 days to 166 days for compounds such as monuron.3 Their degradation pathways in soil and aquatic environments frequently generate metabolites including N-(3,4-dichlorophenyl)urea (DCPU), N-(3,4-dichlorophenyl)-N-methylurea (DCPMU), and 3,4-dichloroaniline (3,4-DCA), which often demonstrate enhanced toxicity compared to their parent compounds.4 These ecological implications have prompted increasing regulatory restrictions, with several phenylurea herbicides now prohibited in European nations while remaining in widespread use throughout other global regions.5The pervasive environmental presence of phenylurea herbicides necessitates the development of sophisticated analytical methodologies for their detection and monitoring, alongside efficient degradation techniques for environmental remediation. International regulatory frameworks have established stringent limitations for phenylurea herbicides across various water resources. For potable water, regulations mandate that the cumulative concentration of all pesticides must not surpass 0.5 μg L−1, with individual pesticide concentrations limited to a maximum of 0.1 μg L−1. The water framework directive (WFD) establishes environmental quality standards for pesticides in surface water to protect aquatic ecosystems and human health. Groundwater protection receives comparable attention through the groundwater directive, which implements a precautionary quality standard of 0.1 μg L−1 for individual pesticides, reflecting the priority to maintain minimal pesticide concentrations in these critical subsurface reserves.6,7 These comprehensive regulatory approaches acknowledge the interconnected nature of water resources and the potential for phenylurea herbicides to migrate between surface water, groundwater, and drinking water sources, necessitating consistent protective standards across all water compartments. Compliance with these regulatory thresholds requires analytical methods offering exceptional sensitivity and selectivity, complemented by robust remediation strategies for contaminated environments.
Standard degradation methods have been extensively employed historically and continue to be widely utilized in contemporary applications. These include photolysis and photocatalytic degradation (utilizing TiO2 catalysts as demonstrated by Lhomme et al.8 and Katsumata et al.9), biodegradation approaches (leveraging microbial consortia like those identified by Alba et al.10 that metabolize diuron, linuron, chlorotoluron and fluometuron, and Hussain et al.2), and advanced oxidation processes (AOPs) that generate highly reactive hydroxyl radicals. Additional techniques such as UV irradiation,11 persulfate,12 Fenton and photo-Fenton processes (which have shown >90% degradation efficiency for metobromuron as reported by Sellam et al.13), ozonation,14 and adsorption using activated carbon15 or biochar16 have demonstrated varying degrees of success in laboratory and field applications. While these methods have proven effective in many scenarios (due to high degradation efficiency, mineralization potential, and application at various environmental scales), they also entail significant limitations, including high energy consumption (UV, ozonation), formation of potentially toxic intermediates (e.g., Fenton's reagent), operational complexity, pH restrictions, and prohibitive costs for large-scale implementation. Photolysis, for example, may be relatively slow and have limited efficiency for certain herbicides, biodegradation can be highly specific and may require extended timeframes, AOPs may involve higher costs due to reagent requirements, and adsorption techniques primarily transfer pollutants from one phase to another, potentially requiring additional treatment of the adsorbent.17
Electrochemical-based methods have emerged as promising complementary and alternative approaches for phenylurea herbicide degradation and remediation, offering distinct advantages such as operational simplicity, minimal chemical input, ambient operation conditions, tunable reaction pathways, and cost-effectiveness, particularly when powered by renewable energy sources, making them especially valuable for point-source treatment applications and in resource-limited settings where conventional infrastructure may be unavailable.18 The electrochemical oxidation of phenylurea herbicides occurs through two primary mechanisms: direct electron transfer at the electrode surface, where organic molecules are oxidized directly upon contact with the anode, and indirect oxidation involving highly reactive hydroxyl radicals (·OH) and other oxidizing species generated during the process. These complementary pathways attack the herbicide molecules through different mechanisms, leading to their progressive degradation and mineralization. The mechanism typically involves two main pathways: aromatic ring hydroxylation and N-terminal group oxidation. The reactivity of phenylurea herbicides toward hydroxyl radicals depends on the number of chlorine substituents on the aromatic ring, with higher chlorine substitution leading to slower degradation rates.19,20 The degradation process often begins with the attack of hydroxyl radicals on the N-terminal group, followed by hydroxylation of the aromatic ring. This results in the formation of intermediate by-products, such as hydroxylated compounds, quinone imines, and dehalogenated species. These intermediates undergo further oxidation, leading to the mineralization of the herbicide into carbon dioxide, water, and inorganic ions.20,21
The efficiency of electrochemical oxidation processes for phenylurea herbicide degradation depends significantly on the electrode materials employed. Dimensionally stable anodes (DSA), such as Ti/Ru0.3Ti0.7O2, effectively degrade diuron following pseudo-first-order kinetics, with optimal performance at lower current densities.22,23 Lead dioxide (PbO2) electrodes, particularly carbon felt/β-PbO2, demonstrate high efficiency in degrading diuron, achieving up to 96.5% removal in three-dimensional electrode reactors under optimized conditions.24 Graphite-assisted alum sludge (GP/AS) electrodes effectively degrade diuron across a wide pH range (3–11) by generating surface-bonded free radicals and singlet oxygen.25 Boron-doped diamond (BDD) electrodes, known for their high oxidation power and durability, achieve mineralization rates exceeding 90% in electro-Fenton processes.26,27 The degradation pathways involve several key steps: hydroxylation, where hydroxyl radicals attack the aromatic ring forming hydroxylated intermediates;21,28 substitution reactions, where chlorine atoms are replaced by hydroxyl groups;19,20 dehalogenation, reducing environmental toxicity;27,29 and mineralization, where oxidative cleavage of the aromatic ring leads to carboxylic acids and ultimately CO2 and H2O.20,25
This review aims to provide a comprehensive overview of the most effective electrochemical methods for the degradation and remediation of phenylurea herbicides, with a focus on approaches that combine advanced oxidation processes with optimized electrode materials and operational conditions. Key electrochemical degradation methods to be discussed include anodic electrochemical oxidation (EC) and photoelectrochemical oxidation (PEC), electro-Fenton (ECF) and photo-electro-Fenton (PECF) processes. For each method, key considerations for optimization will be addressed, along with environmental applications and case studies. Structural analogs spanning the PUHs family (Fig. 1) will form the chemical basis for this systematic study. These include diuron (DIU) as primary target, complemented by linuron (LNR), isoproturon (ISO), monolinuron (MLN), fenuron (FNR), monuron (MNR), fluometuron (FTN), chlortoluron (CTR), chlorbromuron (CBR), and tebuthiuron (TBH).
 | ||
| Fig. 1 Structures of the investigated phenylurea herbicides. The blue skeleton is common to all herbicides of this class while red and purple groups are substituents (general structure of PUHs). | ||
Ultimately, by taking stock of the current state of this field, this review seeks to identify future perspectives and research needs to advance the development of electrochemical technologies for the remediation of phenylurea herbicides.
2. Advanced electrochemical degradation technologies for phenylurea herbicides
This section comprehensively examines the state-of-the-art electrochemical approaches for PUHs degradation and remediation, including EC, PEC, EF, and PEF processes. Additionally, we explore emerging bioelectrochemical approaches, specifically MERCs, as promising sustainable technologies for phenylurea degradation. For each degradation method, specific parameters are considered according to their relative importance in the process efficiency and performance. Table 1 provides an overview of the critical parameters influencing electrochemical degradation processes, highlighting how factors such as electrode materials, catalyst types, operating conditions, and energy consumption differ in significance across these technologies. These classifications are derived from a qualitative synthesis of findings from approximately 35 studies reviewed, reflecting the consensus or predominant influence reported in the literature for each technique. This comparative framework is intended to guide our systematic review and facilitate the identification of optimal conditions for effective phenylurea herbicide remediation.| Parameters | Electrochemical oxidation (EC) | Photoelectrochemical oxidation (PEC) | Electro-Fenton (EF) and photo-electro-Fenton (PEF) |
|---|---|---|---|
| Target herbicides | ✓ Essential | ✓ Essential | ✓ Essential |
| Electrode materials | ✓ Critical | ✓ Critical | ✓ Critical |
| Catalyst/photocatalyst | ✗ Not applicable | ✓ Critical | ✓ Critical |
| Cell configuration | ✓ Important | ✓ Important | ✓ Important |
| Radiation source | ✗ Not applicable | ✓ Critical | ✓ Critical for PEF (not for EF) |
| Operating conditions | ✓ Critical (current density, pH, electrolyte composition, temperature) | ✓ Critical (current density, pH, electrolyte composition, temperature, irradiation time) | ✓ Critical (current density, pH, H2O2 concentration, reaction time) |
| Removal efficiency | ✓ Essential | ✓ Essential | ✓ Essential |
| Mineralization rate | ✓ Important | ✓ Important | ✓ Important |
| Degradation kinetics | ✓ Important | ✓ Important | ✓ Important |
| Identified intermediates | ✓ Important | ✓ Important | ✓ Important |
| Energy consumption | ✓ Critical | ✓ Critical | ✓ Critical |
Critical (requires ±5% precision for reproducible results): parameters requiring precise control for optimal results; small variations significantly impact efficiency/mechanism. The parameter has a highly sensitive, often non-linear, impact on efficiency and mechanism. (e.g., electrode material, current density: above 50 mA cm−2 considered critical, pH in the electro-Fenton process: must be acidic).
Essential (>80% influence on degradation efficiency): parameters directly governing the degradation mechanisms; fundamental to the process, the process fundamentally cannot proceed without this parameter (e.g., an electrode in EC, target herbicide properties, removal efficiency).
Important (20–50% influence on performance): parameters that significantly influence performance but may have broader optimal ranges. The parameter has a significant and predictable influence on performance, but the process can still function if it is not perfectly optimized. (e.g., cell configuration, identified intermediates, mineralization rate).
Non-applicable: parameters not relevant to a specific technique (e.g., radiation source for pure EC, radiation source for electro-Fenton in the dark).
This classification system provides unprecedented guidance for researchers in parameter selection and optimization strategies.
Extensive literature exists describing the various mechanisms encountered during EC, PEC, EF, and PEF processes; however, these are not described here as they fall outside the scope of this review. To date, we have identified approximately sixty articles on the degradation/remediation of phenylurea herbicides using the aforementioned methods. Diuron dominates this field, accounting for more than half of these studies (approximately 35 articles), with about twenty focused specifically on EC. The remaining studies are distributed among the nine other herbicides in this family.
For each technique, we provide a brief overview and select representative herbicides for illustration, focusing primarily on the most recent and pertinent studies. Priority is given to works that can explain the underlying mechanisms and various parameters involved in these processes. These newer articles are assumed to have addressed the limitations of earlier studies.
2.1. Electrochemical oxidation (EC)
Electrochemical oxidation represents one of the most straightforward electrochemical approaches for herbicide degradation. This method utilizes electric current and appropriate electrodes to either directly oxidize organic pollutants at the electrode surface or generate powerful oxidizing species (such as hydroxyl radicals, ·OH) in solution that subsequently degrade the target compounds.18,19 The effectiveness of electrochemical oxidation is critically dependent on electrode materials, with various carbon-based electrodes (including boron-doped diamond, graphite, and carbon nanotubes) demonstrating promising results for phenylurea herbicide degradation. Recent investigations on the electrochemical behavior of phenylurea herbicides has employed various electrode configurations.22,24,30–37 These studies have revealed distinct electrochemical signatures for different phenylurea compounds, enabling both mechanistic understanding and quantitative analysis. Electrochemical oxidation offers several compelling advantages for herbicide degradation, including operational simplicity without complex reactor designs, the elimination of chemical additives, the potential for complete mineralization to CO2 and H2O, real-time process control, and excellent amenability to automation and scaling. However, electrode fouling, energy consumption, and the formation of chlorinated byproducts (particularly for chlorinated phenylureas) remain challenges for widespread application. A growing body of literature has explored electrochemical strategies for the degradation of Diuron, a persistent phenylurea herbicide, employing a range of materials, mechanisms, and system configurations. While all aim to enhance degradation efficiency and reduce environmental impact, the methodologies and mechanistic pathways vary significantly across studies.Fig. 2 illustrates the electrochemical degradation mechanism of diuron in a three-dimensional (3D) electro-peroxone system comprising a carbon felt/PbO2 anode, granular activated carbon (GAC) particle electrodes, and a cathode. The system operates through multiple synergistic oxidation pathways that significantly enhance diuron removal efficiency.
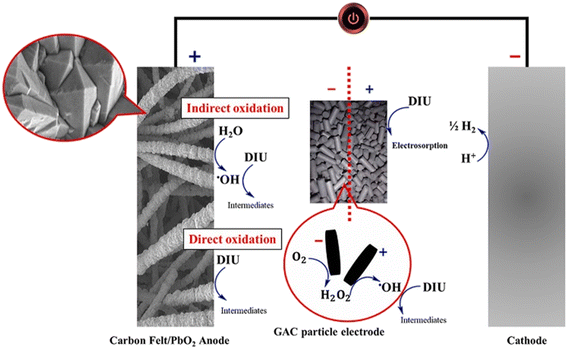 | ||
| Fig. 2 Schematic representation of Diuron degradation in a 3D electro-peroxone system using carbon felt/PbO2 anode and GAC particle electrodes. Reproduced from ref. 38 with permission from Elsevier, copyright 2021. | ||
Diuron is removed through both direct and indirect oxidation processes. At the anode, direct oxidation occurs via electron transfer, while indirect oxidation involves the generation of hydroxyl radicals (·OH) from water, which attack Diuron and form intermediates. In the middle zone, GAC particles act as 3D microelectrodes, enhancing the production of hydrogen peroxide (H2O2) from oxygen, which then reacts to form more ·OH, boosting the oxidative degradation. Meanwhile, the cathode facilitates the reduction of protons (H+ → 1/2 H2) and electrosorption of Diuron, bringing it closer to the reactive sites. The synergy between these mechanisms significantly increases Diuron removal efficiency, making this system highly effective for advanced water treatment.
Recent studies have demonstrated various electrochemical techniques for efficient Diuron degradation, each exploring different materials, mechanisms, and system configurations. Ghorban Asgari et al.36 and Irene Bavasso et al.35 focused on radical-based degradation. Asgari's use of TiO2-GAC in a 3D electro-peroxone system enhanced H2O2 production and ·OH-mediated oxidation. Similarly, Bavasso showed that combining ozonation with electrochemical treatment (especially with carbon-based cathodes) boosts ·OH generation, while metal cathodes favor direct ozone reduction. In contrast, Kai Zhu et al.34 used a Co3O4/graphite composite anode to achieve non-radical degradation. This system offered low energy demand and high electrochemical stability, showing promise for scale-up due to the use of low-cost materials. Alireza Rahmani et al.24 highlighted the importance of anode structure, demonstrating that a porous CF/b-PbO2 anode greatly improved Diuron removal rates and energy efficiency compared to a G/b-PbO2 anode. Lucas B. de Faria et al.22 provided insights into degradation kinetics and the need for economic optimization, while Lijing Zhu et al.37 emphasized energy savings with a Fe–Cu/HGF cathode in an E-PDS system and predicted the toxicity of degradation products using ECOSAR. These studies collectively underline that the efficiency of Diuron degradation depends on the degradation mechanism (radical vs. non-radical), electrode material, and system design. While direct quantitative comparison is challenging due to different experimental conditions, several trends emerge: hybrid systems (3D/E-peroxone, E-PDS) consistently show enhanced performance through synergistic effects, carbon-based electrodes generally favor hydrogen peroxide-mediated processes, and metal oxide composites offer promising alternatives with potentially lower energy requirements. The choice between radical and non-radical mechanisms depends on specific electrode materials and operational objectives, with radical-based systems typically showing higher mineralization rates but potentially higher energy consumption. In summary, energy efficiency, toxicity control, and scalability are key factors that should guide the development of future electrochemical remediation technologies.
As an illustration, Fig. 3 presents a simplified schematic showing (a) the preparation of PbO2 electrodes via anodic precipitation, (b) the 3D electrochemical reactor (3DER) setup for the degradation of the herbicide DIU in pesticide-contaminated wastewater, and (c and d) the proposed degradation pathways leading to DIU mineralization in two situations.24,38
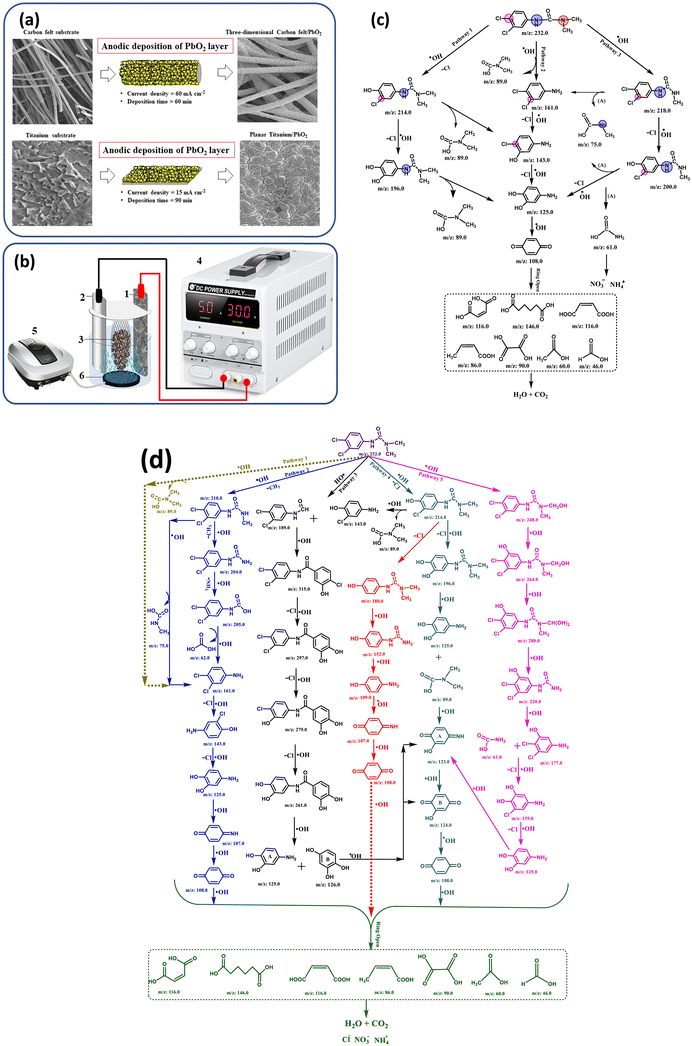 | ||
| Fig. 3 (a) Simple schematic of preparation of PbO2 electrodes by anodic precipitation method; (b) 3DER schematic for the degradation of DIU herbicide and pesticide wastewater (1. PbO2 anode, 2. stainless-steel cathode, 3. GAC particle pack, 4. DC power supply, 5. Air pump, and 6. air diffuser); (c and d) proposed degradation pathways for DIU mineralization. Reproduced from ref. 24, 38 with permission from Elsevier, copyright 2021, 2021. | ||
Fig. 3(a and b) illustrates (i) the anodic deposition process used to fabricate PbO2 electrodes on carbon felt and titanium substrates, and (ii) the 3DER setup integrating these electrodes. For carbon felt, the PbO2 layer is deposited at 60 mA cm−2 for 60 minutes, preserving its porous 3D structure, while for titanium, a compact PbO2 coating is achieved at 15 mA cm−2 for 90 minutes. The 3DER system includes a PbO2 anode, stainless-steel cathode, and a granular activated carbon (GAC) particle bed positioned between the electrodes to enhance conductivity and mass transfer. A DC power supply delivers the required current, while an air pump and diffuser ensure solution stirring. This integrated setup enables efficient electrocatalytic degradation of DIU via both direct and indirect oxidation mechanisms. Fig. 3c (first situation) illustrates the proposed mineralization pathways of the herbicide diuron (DIU) in a three-dimensional electrochemical reactor (3DER), emphasizing the role of hydroxyl radicals (HO·) in initiating degradation. Three main pathways are identified: (1) dechlorination and hydroxylation of the aromatic ring, leading to compounds such as 3-(3-chloro-4-hydroxyphenyl)-1,1-dimethylurea and eventually to low-mass by-products like methylcarbamic acid; (2) direct attack on the amide group, producing toxic intermediates such as 3,4-dichloroaniline; and (3) demethylation of the dimethyl urea moiety, forming intermediates like 1-(3,4-dichlorophenyl)-3-methylurea. All pathways converge toward the formation of common intermediates such as benzoquinone, which undergoes ring-opening to yield short-chain carboxylic acids (e.g., maleic, oxalic, and formic acids). These are ultimately mineralized into carbon dioxide and water, showcasing the effectiveness and complexity of 3DER systems for complete diuron degradation.
In the second situation, dealing with the degradation of diuron herbicide using a three-dimensional carbon felt/b-PbO2 anode as a highly porous electrode, the proposed degradation pathways reveal a complex sequence of hydroxyl radical (HO·) attacks that ultimately mineralize the pollutant into CO2, H2O, Cl−, and NH4+. Five main routes are identified: (i) direct attack on the amide group without initial dechlorination, producing intermediates such as 3,4-dichloroaniline, dimethylcarbamic acid, and later benzoquinone; (ii) hydroxylation of methyl groups leading to demethylated products that also degrade into 3,4-dichloroaniline and benzoquinone; (iii) recombination of organic radicals after successive HO· attacks, yielding hydroxylated aromatic intermediates like 1,2,4-benzenetriol; (iv) direct attack on the aromatic ring, involving dechlorination and hydroxylation, again producing benzoquinone; and (v) hydroxylation of the aliphatic portion, resulting in intermediates such as hydroxymethyl-methylurea derivatives that further break down into benzoquinone. In all cases, benzoquinone acts as a common intermediate, undergoing ring-opening reactions to form short-chain carboxylic acids before complete mineralization. This convergence highlights the central role of hydroxyl radicals in diuron degradation and underscores the efficiency of electrochemical advanced oxidation processes for pesticide remediation in complex matrices.
This detailed breakdown illustrates the complex series of reactions involved in the electrochemical degradation of diuron, emphasizing the role of HO· radicals and the formation of various intermediates before complete mineralization.
In summary, the study by Rahmani et al. demonstrated the superior electrocatalytic performance of the carbon felt/PbO2 anode compared to the titanium/PbO2 counterpart for the degradation of diuron (DIU) in a 3D electrochemical reactor (3DER). The carbon felt/PbO2 system achieved a 95% chemical oxygen demand (COD) removal efficiency after 300 minutes in real pesticide wastewater and reached complete (100%) total organic carbon (TOC) mineralization within just 50 minutes under optimal conditions. The degradation process was primarily driven by indirect oxidation mechanisms involving hydroxyl radicals (HO·). Additionally, the authors developed a predictive quadratic model to estimate the electrocatalytic efficiency of the system, and energy consumption was found to be 2.28 times lower with the carbon felt/PbO2 electrode compared to the titanium/PbO2, confirming both its effectiveness and energy efficiency.
2.2. Photoelectrochemical oxidation (PEC)
Photoelectrochemical oxidation (PEC) integrates electrochemical and photocatalytic processes to enhance the degradation efficiency of organic pollutants. This technique typically utilizes semiconductor materials (such as TiO2) as photoelectrodes that, when exposed to light, generate electron–hole pairs. The photogenerated holes actively oxidize target contaminants, while the electrons are directed through an external circuit, minimizing electron–hole recombination and boosting quantum efficiency compared to conventional photocatalysis. PEC systems have demonstrated strong potential for the removal of various organic pollutants, including phenylurea herbicides. The combined electrochemical and photochemical mechanisms not only accelerate degradation rates but also promote more complete mineralization. Furthermore, PEC often functions effectively under milder operational conditions and can offer lower energy demands than traditional electrochemical methods alone.39 Zhaozhi Zheng et al.40,41 present in their articles both a schematic diagram of a three-electrode photoelectrochemical system (Fig. 4a–c) and a real-life laboratory (Fig. 4d) setup used for herbicide removal from stormwater, as described in their studies on degradation mechanisms, operational conditions, and energy consumption in photoelectrochemical oxidation systems. | ||
| Fig. 4 (a–c) Simple schematic and (d) experimental setup of a three-electrode photoelectrochemical system for herbicide removal from stormwater. Reproduced from ref. 40, 41 with permission from Elsevier, copyright 2023, 2022. | ||
Recent research on this technique, specifically focusing on PUHs, includes fewer than ten reported studies in the literature.40–46 Zhaozhi Zheng et al. conducted a series of studies between 2021 and 2023 exploring the efficiency of PEC for degrading the herbicide diuron in stormwater. In the 2021 (ref. 42) study, they compared PEC with electrochemical oxidation (EC) and photocatalytic oxidation (PC), finding PEC to be the most effective, achieving over 90% diuron removal in 2 hours at 5 V, particularly when enhanced by solar thermal effects. The 2022 (ref. 41) study further confirmed PEC's superior performance, reporting a diuron decay rate of 3.978 h−1 for PEC versus 2.625 h−1 for EC, with minimal adsorption effects from the carbon fiber electrode, and identifying superoxide radicals as the dominant reactive species. In 2023,40 the team scaled the process to a flow PEC system, which successfully treated larger stormwater volumes (675 mL), maintaining >90% diuron removal over 6 hours at 2 V, even under high pollutant loads (240 μg L−1). Importantly, flow rate emerged as a critical factor, with 610 mL min−1 achieving optimal diuron degradation, while light intensity had only a minor effect on diuron, unlike atrazine. Overall, PEC consistently outperformed EC and PC in both batch and flow configurations for diuron removal, proving its strong potential for large-scale stormwater treatment applications.
Few studies have investigated the electrochemical and photoelectrochemical degradation of others phenylurea herbicides such as tebuthiuron, monuron, and fenuron, employing various advanced oxidation processes (AOPs). Isaac J. S. Montes et al.44 used a hybrid electrochemical–photochemical (HEP) system with DSA® anode and UVC light for the mineralization of tebuthiuron, achieving complete elimination of intermediates but noting high energy consumption, which could be optimized with higher NaCl concentrations. Similarly, Robson S. Souto et al.43 explored multiple EAOPs for tebuthiuron removal, highlighting the UVC/e-H2O2@e-ClO− system's superior performance (99.5% degradation in 60 min), with low energy demand (1.54 kWh g−1 TOC) and minimal ecotoxicity from by-products. For monuron, Brada M. et al.45 applied a photoelectrochemical process using WO3 photoanodes, achieving 86% degradation after 24 h but only 17% mineralization, pointing to significant intermediate formation and a lower Faradaic efficiency. In contrast, Karima Barbari et al.46 demonstrated that fenuron could be effectively degraded (86.3%) with nearly complete mineralization under neutral pH using photocatalytically-assisted electrooxidation and Ti/SnO2–Sb2O3/PbO2 electrodes at 30 mA cm−2. Overall, while all studies confirm the potential of AOPs for phenylurea herbicide degradation, degradation and mineralization rates, energy consumption, and efficiency depend heavily on the electrode material, current density, pH, and system configuration.
The figures presented by Zhaozhi Zheng et al. (2023)40 and Robson S. Souto et al. (2025)43 offer valuable insights into scalable, flow-based oxidation systems for pesticide degradation and are of great interest for future remediation technologies. Zheng et al. (Fig. 5a and b) illustrate a photoelectrochemical oxidation (PEC) flow system tailored for stormwater treatment, where a TiO2-coated carbon fiber photoanode and stainless-steel cathode are integrated into a compact, membrane-free reactor. The system operates under simulated solar light and a low applied voltage, with continuous water circulation ensured by a peristaltic pump. The design emphasizes light penetration (via a quartz plate), compactness, and energy efficiency, with a total system volume of 675 mL, ten times larger than in earlier batch studies, demonstrating scale-up potential. In comparison, Souto et al. (Fig. 5c and d) describe an electrochemical flow reactor (EFR) designed for the treatment of tebuthiuron-contaminated wastewater. The EFR features a gas diffusion cathode (for in situ H2O2 generation) and a DSA®-Cl2 anode, arranged in a membrane-less setup with a narrow inter-electrode gap to facilitate oxidant production and flow. This system also includes a UVC chamber to activate generated oxidants, a thermostated reservoir, and oxygen control components, operating at higher volumes (1.0 L) and flow rates (30 L h−1).
 | ||
| Fig. 5 (a and b) Schematic experiment setup diagram for flow reactor system (a- structure of reactor; b- setup of the system); (c) EFR configuration; (d) internal design of the flow reactor with gap between the electrodes; (e) electrochemical setup: 1-electrochemical flow reactor; 2-UVC chamber; 3-reservoir tank; 4-peristaltic pump; 5-power source; 6-gas flow meter; 7-oxygen cylinder. Reproduced from ref. 40, 43 with permission from Elsevier, copyright 2023, 2025. | ||
While both systems are continuous-flow and aim to reduce energy consumption while maximizing pollutant removal, the PEC system prioritizes solar-driven processes and compactness, whereas the EFR system focuses on multi-oxidant generation and hybrid activation strategies (electrochemical + photochemical). Together, these figures provide clear and complementary models for researchers aiming to transition from laboratory-scale to pilot-scale pesticide degradation systems.
2.3. Electro-Fenton (EF) and photo-electro-Fenton (PEF) processes
The electro-Fenton (EF) process represents an electrochemically assisted advanced oxidation process that overcomes key limitations of conventional Fenton reactions by generating H2O2in situ through cathodic oxygen reduction (O2 + 2H+ + 2e− → H2O2) while continuously regenerating Fe2+ from Fe3+, eliminating the need for continuous H2O2 addition and reducing iron sludge formation.47 The photo-electro-Fenton (PEF) process further enhances degradation efficiency through UV irradiation, which promotes photolysis of iron-hydroxo complexes and additional Fe2+ regeneration, often achieving complete mineralization of recalcitrant organic pollutants, including PUHs.48 While both processes offer high oxidation power and reduced chemical consumption, they require careful pH control and may face limitations from energy consumption and mass transfer constraints.For the electro-Fenton (EF) technique, we have identified approximately twenty publications in the literature,19,20,28,49–59 covering various phenylurea herbicides including chlorobromuron (CBR), chlorotoluron (CTR), diuron (DIU), fenuron (FNR), linuron (LNR), monuron (MNR), fluometuron (FTN) and tebuthiuron (TBH). This section provides a brief description of the most recent studies, offering a comparative analysis of these works. In contrast, the photo-electro-Fenton (PEF) technique has received limited attention for phenylurea herbicide degradation, with fewer than five studies reported, primarily focusing on DIU and TBH.60–62
Across multiple studies, the electro-Fenton (EF) process has been extensively investigated and proven effective for degrading various phenylurea herbicides and other persistent organic pollutants, each highlighting specific strategies, materials, and system designs to optimize degradation efficiency and mineralization. For instance, Susana Martínez et al. (2009)49 showed that using a stainless-steel anode in a simple two-electrode undivided cell (Fig. 6b) effectively removed low concentrations of CBR, achieving over 90% TOC removal with reduced costs, while Aida Abdessalem et al. (2008)50 achieved 98% mineralization of CTR under optimized conditions following pseudo-first order kinetics. Mehmet Oturan et al. (2010)19 explored the degradation of DIU, MNR, and FNR, demonstrating the dependence of degradation rate on chlorine substitution (DIU < MNR < FNR) and achieving over 90% COD removal within 3 hours. Pape Diaw et al. (2017, 2020)54,55 focused on FTN and MLN, respectively, finding EF more efficient than anodic oxidation, especially with BDD anodes, and identified comprehensive degradation pathways including demethylation and hydroxylation, leading to complete mineralization.
 | ||
| Fig. 6 (a and b) Experimental set-up (a-three-electrode divided cell and b-two-electrode undivided cell), (c) schematic diagram of the integrated bench-scale EF-FO (forward osmosis) system. Reproduced from ref. 49, 51 with permission from Elsevier, copyright 2009, 2023. | ||
Complementary innovations have further improved EF performance. Fabio Gozzi et al. (2017)62 highlighted the role of chelation and BDD electrodes in degrading TBH, while Haiqiang Qi et al. (2022)53 used hydrothermally modified graphite felt cathodes to enhance H2O2 production and Fe2+ regeneration, ensuring high degradation efficiency and electrode stability even after multiple cycles. Yulin Yang et al. (2024)52 introduced Sn-SbAS particle electrodes to increase H2O2 yield and target hydrophobic organic pollutants (HLOPs) effectively, supported by bonded free radical reactions. Meanwhile, Lei Zheng et al. (2023)51 integrated EF with forward osmosis (EF-FO) (Fig. 6c), demonstrating improved rejection and degradation of multiple trace organic contaminants like diuron, atrazine, and sulfamethoxazole, with membrane chemistry and pH significantly influencing degradation rates. Collectively, these studies confirm that EF, particularly when coupled with tailored electrode materials, integrated systems, or hybrid processes, not only achieves high mineralization rates and degradation efficiency but also adapts to different pollutants and operational contexts, making it a versatile and powerful tool for environmental remediation.
Fig. 6a–c illustrate complementary experimental strategies combining electro-Fenton (EF) degradation and membrane separation to remove persistent pollutants. Fig. 6a and b compare two EF setups for CBR removal: a three-electrode divided cell and a two-electrode undivided cell. The divided cell (Fig. 6a) separates anodic and cathodic compartments with a Nafion® membrane, enabling precise control of electrode potentials and preventing mixing of reactive species; it uses an RVC (reticulated vitreous carbon) cathode to generate H2O2 and a platinum anode, with Fe2+ added to catalyze the Fenton reaction. By contrast, the undivided cell (Fig. 6b) is simpler and more practical for scale-up: it employs a stainless-steel sacrificial anode that releases Fe2+ directly, combined with an RVC cathode to produce H2O2, allowing simultaneous generation of both reagents needed for pollutant degradation. This setup reduces costs and operational complexity, favoring the peroxi-coagulation mechanism where oxidation and coagulation act together.
Fig. 6c presents an integrated EF-forward osmosis (EF-FO) system that couples chemical oxidation and membrane separation to enhance removal of trace organic contaminants (TrOCs). In this design, the EF unit degrades pollutants in the feed solution via electrogenerated hydroxyl radicals, supported by oxygen purging to boost H2O2 production, while the FO unit extracts clean water across a semi-permeable membrane driven by osmotic pressure from a draw solution. Key advantages include the synergy between degradation and separation: FO concentrates residual contaminants, while reverse salt flux from the draw solution can generate additional reactive chlorine species, further aiding degradation. Together, these configurations highlight the versatility of EF approaches, from lab-scale divided cells to practical undivided reactors and integrated hybrid systems, each tailored to balance efficiency, cost, and operational simplicity for advanced wastewater treatment.
With regard to photoelectro-Fenton (PEF) process, it emerges as a highly promising advanced oxidation technique by coupling electrochemical generation of oxidants with UV irradiation, significantly boosting degradation rates and mineralization efficiency for various organic pollutants in wastewater. For instance, Aline da Costa et al. (2021)61 evaluated electrochemical processes for removing TBH from urban wastewater using a flow-by reactor and demonstrated that while the AO-H2O2UVC process achieved complete TBH removal and an impressive mineralization rate of 95%, simpler AO and AO-H2O2 setups only reached 22–28% efficiency, highlighting the critical role of UV irradiation and optimized current density in enhancing performance. Similarly, F. L. Sousa et al. (2019)60 investigated the coupling of conductive diamond electrochemical oxidation (CDEO) with electrogenerated H2O2, Fe2+ catalyst (EF), and UV irradiation (PEF) to treat a mixture of DIU and hexazinone herbicides, revealing that PEF achieved the fastest and most complete degradation, although some organic carbon persisted. Their results showed degradation rate trends of PEF > EF > CDEO/H2O2/UV > CDEO/H2O2 > CDEO > UV, demonstrating that hybrid systems produce more oxidants and yield synergistic effects (synergy coefficients > 1) that outperform single techniques. Together, these studies underscore that integrating photo-activation with electro-Fenton processes not only accelerates pollutant breakdown but also improves mineralization, making PEF especially advantageous for complex and persistent contaminants in real wastewater scenarios, despite possible byproduct formation that warrants further optimization.
3. Conclusions, perspectives, and research needs
Electrochemical oxidation has emerged as a highly effective and versatile strategy for degrading phenylurea herbicides, with process efficiency largely dependent on the choice of electrode material and operational parameters. Central to these processes is the generation of hydroxyl radicals, which initiate degradation through hydroxylation, dehalogenation, and subsequent mineralization of the parent compounds into less harmful or harmless by-products. Compared to conventional methods, electrochemical approaches offer notable advantages such as operational simplicity, lower chemical input, and adaptability to various environmental contexts. Additionally, electroanalytical tools and mass spectrometry techniques play a critical role in both tracking degradation progress and elucidating complex transformation pathways. Collectively, these advances demonstrate the strong potential of electrochemical methods to address the environmental persistence, toxicity, and mobility of PUHs.Looking ahead, further innovation is essential to translate laboratory successes into practical environmental applications. Priority areas include the development of advanced materials, not only novel electrode designs such as nanostructured carbons, metal oxide composites, and conducting polymers, but also catalytic supports, membranes, and hybrid materials that can enhance degradation rates, selectivity, and robustness in complex matrices. Equally important is the integration of renewable energy sources such as solar power to reduce operational costs and improve the sustainability of large-scale applications. Combining electrochemical processes with complementary methods, such as bio-electrochemical systems and in situ remediation techniques like bioelectroventing63 or microbial electroremediating cells (MECs),64 could further enhance pollutant removal and sustainability.
Additional research should focus on the comprehensive toxicity assessment of transformation products (TPs), employing both experimental bioassays and computational prediction tools such as ECOSAR, TEST, and ADMET. Particular attention should be given to the environmental implications of intermediates, as the formation of potentially toxic byproducts represents a critical consideration in evaluating the overall safety of electrochemical remediation. For instance, several studies have reported the generation of 3,4-dichloroaniline during the electrochemical degradation of diuron, a transformation product known for its persistence and toxicity. Future investigations should therefore not only identify and characterize such intermediates but also assess their environmental behavior, persistence, and toxicological profiles. Incorporating these insights into process design will help to mitigate risks associated with partial degradation, while the systematic optimization of operational parameters through design of experiments (DoE) can minimize the formation of hazardous byproducts and enhance process selectivity toward complete mineralization.
Importantly, while existing green metrics tools (e.g., Eco-Scale; Green Analytical Procedure Index, GAPI, Analytical GREEnness metric, AGREE)65–67 can be qualitatively adapted to electrochemical degradation processes, no standardized metric has yet been specifically developed for electrochemical oxidation, photoelectrochemical processes, electro-Fenton, or photo-electro-Fenton. Developing dedicated and quantitative green metrics, together with systematic life cycle assessments (LCA), represents a key research need to rigorously evaluate and compare the environmental sustainability of these advanced oxidation processes.
Beyond laboratory-scale demonstrations, future research must urgently address the integration of electrochemical processes into real environmental conditions and pilot-scale systems. While examples remain scarce for PUHs, lessons can be drawn from other recalcitrant pollutants, where batch configurations are progressively evolving toward continuous-flow reactors and modular systems.40,43 Critical challenges include the operational stability and long-term durability of electrodes such as BDD and DSA, which are often compromised by fouling, passivation, or corrosion in complex matrices containing natural organic matter and competing ions. Addressing these issues requires both robust electrode materials and operational strategies such as polarity reversal or novel coatings. Equally important, techno-economic assessments (TEA) and LCA remain largely unexplored for PUHs. Still, they are essential to quantify cost drivers (energy use, electrode replacement, chemical additives) and to benchmark performance using standardized metrics like the electrical energy per order (EEO). Ultimately, advancing this field will depend on pilot-scale studies that not only validate laboratory results but also confront the complexities of real wastewater environments, thereby paving the way for sustainable and scalable electrochemical remediation technologies.
In summary, future research on the electrochemical degradation of PUHs can be structured along three priority horizons. In the near term (1–3 years), efforts should focus on optimizing cost-effective materials and systematically exploring operational parameters using approaches such as design of experiments (DoE). In the mid-term (3–5 years), the emphasis should shift toward pilot-scale studies on real water matrices and the establishment of standardized protocols for assessing the toxicity of degradation intermediates. In the long term (>5 years), integration into hybrid treatment trains, field-scale demonstrations, and the development of robust green metrics and life cycle assessments (LCA) will be essential to support commercialization and sustainable deployment. This prioritization provides a clearer roadmap to guide the research community in advancing electrochemical remediation technologies from laboratory concepts to practical applications.
Author contributions
Ranil C. T. Temgoua: writing – original draft, investigation, methodology, conceptualization. Jan Lisec: writing – review and editing. Matthias Koch: writing – review and editing, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Glossary of key terms and abbreviations
Abbreviations
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| AOPs | Advanced oxidation processes |
| BDD | Boron-doped diamond |
| COD | Chemical oxygen demand |
| DSA | Dimensionally stable anode |
| EC | Electrochemical oxidation |
| ECOSAR | Ecological structure–activity relationship |
| EEO | Electrical energy per order |
| EF | Electro-Fenton |
| PEC | Photoelectrochemical oxidation |
| PEF | Photo-electro-Fenton |
| PUHs | Phenylurea herbicides |
| TEST | Toxicity estimation software tool |
| TOC | Total organic carbon |
Key Terms
| 3D Electrodes | Porous or foam-like electrode structures that increase surface area, mass transfer, and radical generation. |
| Current density | Electric current applied per unit electrode surface area; a key operational parameter influencing degradation kinetics. |
| Degradation kinetics | Mathematical description of pollutant removal rates often modeled using pseudo-first-order or Langmuir–Hinshelwood kinetics. |
| Degradation vs. remediation | Degradation refers to chemical breakdown of pollutants; remediation encompasses complete strategies to restore environmental quality. |
| Electro-Fenton | An electrochemical advanced oxidation process in which hydrogen peroxide (H2O2) is electrochemically generated in situ and reacts with ferrous ions (Fe2+) to produce hydroxyl radicals (·OH), leading to the degradation of organic pollutants. |
| Energy consumption | Total electrical energy required for treatment, expressed as kWh m−3 or EEO, a key techno-economic indicator. |
| Hydroxyl radical (·OH) | Highly reactive radical central to AOPs, with a near-diffusion-controlled reaction rate toward most organic pollutants. |
| Identified intermediates | Transformation products formed during degradation, usually detected by LC-MS, GC-MS, or HRMS, critical for assessing toxicity. |
| Mineralization rate | Rate at which pollutants are fully oxidized to CO2 and inorganic ions. |
| Mineralization | Complete conversion of organic pollutants into CO2, H2O, and inorganic ions. |
| Photo electrochemistry | Field combining electrochemistry and light irradiation to promote redox reactions for pollutant degradation. |
| Removal efficiency | Percentage reduction of pollutant concentration after treatment, often measured by HPLC, TOC, or COD. |
| Transformation products | Compounds formed during the degradation pathway, prior to complete mineralization; they may be more or less toxic than the parent pollutants. |
Data availability
No original experimental data were generated in this review. All information discussed is derived exclusively from published sources cited in the references.Acknowledgements
Ranil C. T. Temgoua acknowledges the Alexander von Humboldt Foundation (AvH) for the financial support through the Humboldt Research Fellowship for Postdoctoral Researchers. He also expresses his sincere gratitude to the BAM Federal Institute for Materials Research and Testing (Department of Analytical Chemistry and Reference Materials, Organic Trace and Food Analysis) for providing an excellent research environment and facilities that significantly contributed to the completion of this review article.References
- J. Liu, Chapter 80 - Phenylurea Herbicides, in Hayes' Handbook of Pesticide Toxicology, ed. R. Krieger, Academic Press, New York, 3rd edn, 2010, pp. 1725–1731, Available from: https://www.sciencedirect.com/science/article/pii/B978012374367100080X Search PubMed.
- S. Hussain, M. Arshad, D. Springael, S. R. SøRensen, G. D. Bending and M. Devers-Lamrani, et al., Abiotic and Biotic Processes Governing the Fate of Phenylurea Herbicides in Soils: A Review, Crit. Rev. Environ. Sci. Technol., 2015, 45(18), 1947–1998, DOI:10.1080/10643389.2014.1001141.
- W. Chu, W. K. Choy and C. Y. Kwan, Selection of Supported Cobalt Substrates in the Presence of Oxone for the Oxidation of Monuron, J. Agric. Food Chem., 2007, 55(14), 5708–5713, DOI:10.1021/jf063754r.
- C. Tixier, M. Sancelme, M. Sancelme, F. Bonnemoy, A. Cuer and H. Veschambre, Degradation products of a phenylurea herbicide, diuron: Synthesis, ecotoxicity, and biotransformation, Environ. Toxicol. Chem., 2001, 20(7), 1381–1389, DOI:10.1002/etc.5620200701.
- Pan Europe, Banned pesticides still in use in the EU, PAN Europe, 2023, Available from: https://www.pan-europe.info/resources/reports/2023/01/banned-pesticides-still-use-eu.
- Water Framework Directive, Pesticides in rivers, lakes and groundwater in Europe, 2024, Available from: https://www.eea.europa.eu/en/analysis/indicators/pesticides-in-rivers-lakes-and.
- W. Zhou, M. Li and V. Achal, A comprehensive review on environmental and human health impacts of chemical pesticide usage, Emerging Contam., 2025, 11(1), 100410 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S2405665024001112.
- L. Lhomme, S. Brosillon, D. Wolbert and J. Dussaud, Photocatalytic degradation of a phenylurea, chlortoluron, in water using an industrial titanium dioxide coated media, Appl. Catal., B, 2005, 61(3), 227–235 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S092633730500264X.
- H. Katsumata, M. Sada, Y. Nakaoka, S. Kaneco, T. Suzuki and K. Ohta, Photocatalytic degradation of diuron in aqueous solution by platinized TiO2, J. Hazard. Mater., 2009, 171(1), 1081–1087 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0304389409010462.
- L. M. Alba, M. Esmeralda and V. Jaime, Enhanced Biodegradation of Phenylurea Herbicides by Ochrobactrum anthrophi CD3 Assessment of Its Feasibility in Diuron-Contaminated Soils, Int. J. Environ. Res. Public Health, 2022, 19(3), 1365 CrossRef CAS PubMed , Available from: https://www.mdpi.com/1660-4601/19/3/1365.
- F. J. Benitez, F. J. Real, J. L. Acero and C. Garcia, Photochemical oxidation processes for the elimination of phenyl-urea herbicides in waters, J. Hazard. Mater., 2006, 138(2), 278–287 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0304389406005759.
- F. Lai, F. X. Tian, B. Xu, W. K. Ye, Y. Q. Gao and C. Chen, et al., A comparative study on the degradation of phenylurea herbicides by UV/persulfate process: Kinetics, mechanisms, energy demand and toxicity evaluation associated with DBPs, Chem. Eng. J., 2022, 428, 132088 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1385894721036676.
- B. Sellam, N. Seraghni, C. Bouaziz, S. Belaidi, N. Debbache and T. Sehili, Enhanced degradation of the herbicide metobromuron using calcium peroxide-based Fenton-like (Fe3+/CaO2) and photo-Fenton-like (Fe3+/CaO2/UVA) processes, J. Water Process Eng., 2025, 71, 107307 CrossRef , Available from: https://www.sciencedirect.com/science/article/pii/S2214714425003794.
- F. J. Benitez, F. J. Real, J. L. Acero and C. Garcia, Kinetics of the transformation of phenyl-urea herbicides during ozonation of natural waters: Rate constants and model predictions, Water Res., 2007, 41(18), 4073–4084 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0043135407003466.
- L. E. Z. de Moraes, F. A. O. Marcoti, M. A. N. Lucio, B. C. da S Rocha, L. B. Rocha and A. L. Romero, et al., Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans, Processes, 2024, 12(9), 1952 CrossRef , Available from: https://www.mdpi.com/2227-9717/12/9/1952.
- Y. Dan, M. Ji, S. Tao, G. Luo, Z. Shen and Y. Zhang, et al., Impact of rice straw biochar addition on the sorption and leaching of phenylurea herbicides in saturated sand column, Sci. Total Environ., 2021, 769, 144536 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0048969720380670.
- S. S. Mohanty, P. Singh, S. Nistala and K. Mohanty, Understanding the environmental fate and removal strategies of phenylurea herbicides: A comprehensive review, J. Hazard. Mater. Adv., 2024, 16, 100496 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S2772416624000974.
- C. Trellu, H. Olvera Vargas, E. Mousset, N. Oturan and M. A. Oturan, Electrochemical technologies for the treatment of pesticides, Curr. Opin. Electrochem., 2021, 26, 100677 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S2451910320302325.
- M. A. Oturan, M. C. Edelahi, N. Oturan, K. El kacemi and J. J. Aaron, Kinetics of oxidative degradation/mineralization pathways of the phenylurea herbicides diuron, monuron and fenuron in water during application of the electro-Fenton process, Appl. Catal., B, 2010, 97(1), 82–89 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0926337310001426.
- M. C. Edelahi, N. Oturan, M. A. Oturan, Y. Padellec, A. Bermond and K. El Kacemi, Degradation of diuron by the electro-Fenton process, Environ. Chem. Lett., 2003, 1(4), 233–236, DOI:10.1007/s10311-003-0052-5.
- R. C. T. Temgoua, U. Bussy, D. Alvarez-Dorta, N. Galland, E. Njanja and J. Hémez, et al., Electrochemistry-coupled to liquid chromatography-mass spectrometry-density functional theory as a new tool to mimic the environmental degradation of selected phenylurea herbicides, Environ. Sci.: Processes Impacts, 2021, 23(10), 1600–1611 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2021/em/d1em00351h.
- L. B. de Faria, G. F. Teixeira, A. C. F. Alves, J. J. Linares, S. B. Oliveira and A. J. Motheo, et al., Electrochemical Degradation of Diuron by Anodic Oxidation on a Commercial Ru0.3Ti0.7O2 Anode in a Sulfate Medium, ChemEngineering, 2023, 7(4), 73 CrossRef CAS , Available from: https://www.mdpi.com/2305-7084/7/4/73.
- L. B. De Faria, G. F. Teixeira, A. C. F. Alves, J. J. Linares, S. B. De Oliveira and A. J. Motheo, et al., Electrochemical Degradation of Diuron by anodic oxidation on DSA in sulfate medium, Environ. Earth Sci., 2023 Search PubMed , Available from: https://www.preprints.org/manuscript/202307.1614/v1.
- A. Rahmani, A. Seid-mohammadi, M. Leili, A. Shabanloo, A. Ansari and S. Alizadeh, et al., Electrocatalytic degradation of diuron herbicide using three-dimensional carbon felt/β-PbO2 anode as a highly porous electrode: Influencing factors and degradation mechanisms, Chemosphere, 2021, 276, 130141 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S004565352100610X.
- Y. Yang, J. Li, W. Qu, C. Ma, X. Feng and Y. Guo, et al., A novel particle electrode fabricated by graphite-assisted alum sludge for effective diuron degradation in wide pH ranges, Sep. Purif. Technol., 2024, 330, 125326 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1383586623022347.
- A. K. Abdessalem, M. A. Oturan, N. Oturan, N. Bellakhal and M. Dachraoui, Treatment of an aqueous pesticides mixture solution by direct and indirect electrochemical advanced oxidation processes, Int. J. Environ. Anal. Chem., 2010, 90(3–6), 468–477, DOI:10.1080/03067310902999132.
- K. Kovács, J. Farkas, G. Veréb, E. Arany, G. Simon and K. Schrantz, et al., Comparison of various advanced oxidation processes for the degradation of phenylurea herbicides, J. Environ. Sci. Health, Part B, 2016, 51(4), 205–214, DOI:10.1080/03601234.2015.1120597.
- I. Losito, A. Amorisco and F. Palmisano, Electro-Fenton and photocatalytic oxidation of phenyl-urea herbicides: An insight by liquid chromatography–electrospray ionization tandem mass spectrometry, Appl. Catal., B, 2008, 79(3), 224–236 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0926337307003724.
- T. Alapi, G. Simon, G. Veréb, K. Kovács, E. Arany and K. Schrantz, et al., Toxicology Aspects of the Decomposition of Diuron by Advanced Oxidation Processes, Hung. J. Ind. Chem., 2015, 25–32 CAS , Available from: https://hjic.mk.uni-pannon.hu/index.php/hjic/article/view/hjic-2015-0005.
- G. F. Pereira, B. F. Silva, R. V. Oliveira, D. A. C. Coledam, J. M. Aquino and R. C. Rocha-Filho, et al., Comparative electrochemical degradation of the herbicide tebuthiuron using a flow cell with a boron-doped diamond anode and identifying degradation intermediates, Electrochim. Acta, 2017, 247, 860–870 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0013468617314718.
- N. Abu Ghalwa, M. Hamada, H. M. Abu Shawish and O. Shubair, Electrochemical degradation of linuron in aqueous solution using Pb/PbO2 and C/PbO2 electrodes, Arabian J. Chem., 2016, 9, S821–S828 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S187853521100219X.
- G. Asgari, A. Seid-mohammadi, A. Rahmani, M. T. Samadi, S. Alizadeh and D. Nematollahi, et al., Carbon felt modified with N-doped rGO for an efficient electro-peroxone process in diuron degradation and biodegradability improvement of wastewater from a pesticide manufacture: Optimization of process parameters, electrical energy consumption and degradation pathway, Sep. Purif. Technol., 2021, 274, 118962 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1383586621006729.
- A. Rahmani, A. Ansari, A. Seid-mohammadi, M. Leili, D. Nematollahi and A. Shabanloo, Bismuth-doped 3D carbon felt/PbO2 electrocatalyst for degradation of diuron herbicide and improvement of pesticide wastewater biodegradability, J. Environ. Chem. Eng., 2023, 11(1), 109118 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S2213343722019911.
- K. Zhu, H. Qi, X. Sun and Z. Sun, Anodic oxidation of diuron using Co3O4/graphite composite electrode at low applied current, Electrochim. Acta, 2019, 299, 853–862 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0013468619300854.
- I. Bavasso, D. Montanaro, L. Di Palma and E. Petrucci, Electrochemically assisted decomposition of ozone for degradation and mineralization of Diuron, Electrochim. Acta, 2020, 331, 135423 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0013468619322959.
- G. Asgari, A. Seid-mohammadi, A. Rahmani, M. T. Samadi, M. Salari and S. Alizadeh, et al., Diuron degradation using three-dimensional electro-peroxone (3D/E-peroxone) process in the presence of TiO2/GAC: Application for real wastewater and optimization using RSM-CCD and ANN-GA approaches, Chemosphere, 2021, 266, 129179 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0045653520333762.
- L. Zhu, M. Li, H. Qi and Z. Sun, Using Fe–Cu/HGF composite cathodes for the degradation of Diuron by electro-activated peroxydisulfate, Chemosphere, 2022, 291, 132897 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0045653521033695.
- A. Rahmani, M. Leili, A. Seid-mohammadi, A. Shabanloo, A. Ansari and D. Nematollahi, et al., Improved degradation of diuron herbicide and pesticide wastewater treatment in a three-dimensional electrochemical reactor equipped with PbO2 anodes and granular activated carbon particle electrodes, J. Cleaner Prod., 2021, 322, 129094 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0959652621032832.
- T. Li, T. Kasahara, J. He, K. E. Dettelbach, G. M. Sammis and C. P. Berlinguette, Photoelectrochemical oxidation of organic substrates in organic media, Nat. Commun., 2017, 8(1), 390 CrossRef , Available from: https://www.nature.com/articles/s41467-017-00420-y.
- Z. Zheng, K. Zhang, C. Y. Toe, R. Amal and A. Deletic, Photo-electrochemical oxidation flow system for stormwater herbicides removal: Operational conditions and energy consumption analysis, Sci. Total Environ., 2023, 898, 166375 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0048969723050003.
- Z. Zheng, A. Deletic, C. Y. Toe, R. Amal, X. Zhang and R. Pickford, et al., Photo-electrochemical oxidation herbicides removal in stormwater: Degradation mechanism and pathway investigation, J. Hazard. Mater., 2022, 436, 129239 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0304389422010299.
- Z. Zheng, K. Zhang, C. Y. Toe, R. Amal, X. Zhang and D. T. McCarthy, et al., Stormwater herbicides removal with a solar-driven advanced oxidation process: A feasibility investigation, Water Res., 2021, 190, 116783 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0043135420313166.
- R. S. Souto, I. Sánchez-Montes, G. O. S. Santos, P. J. M. Cordeiro-Junior, R. Colombo and M. C. Santos, et al., Electrochemical degradation of tebuthiuron pesticide using a flow reactor: Insights into the co-generation of oxidants and ecotoxicological risks, J. Environ. Chem. Eng., 2025, 13(2), 115528 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S2213343725002234.
- I. J. S. Montes, B. F. Silva and J. M. Aquino, On the performance of a hybrid process to mineralize the herbicide tebuthiuron using a DSA® anode and UVC light: A mechanistic study, Appl. Catal., B, 2017, 200, 237–245 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0926337316305367.
- M. Brada, J. Rusek, T. Imrich, M. Neumann-Spallart and J. Krýsa, Photoelectrochemical degradation of selected organic pollutants on tungsten trioxide photoanodes, J. Photochem. Photobiol., A, 2024, 457, 115883 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1010603024004271.
- K. Barbari, R. Delimi, Z. Benredjem, S. Saaidia, A. Djemel and T. Chouchane, et al., Photocatalytically-assisted electrooxidation of herbicide fenuron using a new bifunctional electrode PbO2/SnO2-Sb2O3/Ti//Ti/TiO2, Chemosphere, 2018, 203, 1–10 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0045653518305435.
- H. Olvera-Vargas, C. Trellu, P. V. Nidheesh, E. Mousset, S. O. Ganiyu and C. A. Martínez-Huitle, et al., Challenges and opportunities for large-scale applications of the electro-Fenton process, Water Res., 2024, 266, 122430 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0043135424013290.
- H. H. Vigil-Castillo, E. J. Ruiz-Ruiz, K. López-Velázquez, L. Hinojosa-Reyes, O. Gaspar-Ramírez and J. L. Guzmán-Mar, Assessment of photo electro-Fenton and solar photo electro-Fenton processes for the efficient degradation of asulam herbicide, Chemosphere, 2023, 338, 139585 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0045653523018520.
- S. S. Martínez and C. L. Bahena, Chlorbromuron urea herbicide removal by electro-Fenton reaction in aqueous effluents, Water Res., 2009, 43(1), 33–40 CrossRef , Available from: https://www.sciencedirect.com/science/article/pii/S0043135408004442.
- A. K. Abdessalem, N. Oturan, N. Bellakhal, M. Dachraoui and M. A. Oturan, Experimental design methodology applied to electro-Fenton treatment for degradation of herbicide chlortoluron, Appl. Catal., B, 2008, 78(3), 334–341 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0926337307003049.
- L. Zheng, Y. Xiong, Y. Gao, F. Yin, M. Szczygiełda and M. Krajewska, et al., Tailoring the draw solution chemistry in the integrated electro-Fenton and forward osmosis for enhancing emerging contaminants removal: Performance, DFT calculation and degradation pathway, Sci. Total Environ., 2023, 872, 162155 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0048969723007714.
- Y. Yang, J. Li, W. Qu, W. Wang, C. Ma and H. Xue, et al., Sn/Sb–assisted alum sludge electrodes for eliminating hydrophilic organic pollutants in self-produced H2O2 electro-Fenton system: Insights into the co-oxidation mediated by 1O2 and ·OH(ads), J. Hazard. Mater., 2024, 471, 134457 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0304389424010367.
- H. Qi, W. Ren, X. Shi and Z. Sun, Hydrothermally modified graphite felt as the electro-Fenton cathode for effective degradation of diuron: The acceleration of Fe2+ regeneration and H2O2 production, Sep. Purif. Technol., 2022, 299, 121724 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1383586622012801.
- P. A. Diaw, N. Oturan, M. D. G. Seye, A. Coly, A. Tine and J. J. Aaron, et al., Oxidative degradation and mineralization of the phenylurea herbicide fluometuron in aqueous media by the electro-Fenton process, Sep. Purif. Technol., 2017, 186, 197–206 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S138358661730895X.
- P. A. Diaw, N. Oturan, M. D. Gaye Seye, O. M. A. Mbaye, M. Mbaye and A. Coly, et al., Removal of the herbicide monolinuron from waters by the electro-Fenton treatment, J. Electroanal. Chem., 2020, 864, 114087 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1572665720302708.
- F. Gozzi, I. Sirés, S. C. de Oliveira, A. Machulek and E. Brillas, Influence of chelation on the Fenton-based electrochemical degradation of herbicide tebuthiuron, Chemosphere, 2018, 199, 709–717 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0045653518302625.
- C. Lizama-Bahena, A. Álvarez-Gallegos, J. A. Hernandez and S. Silva-Martinez, Elimination of bio-refractory chlorinated herbicides like atrazine, alachlor, and chlorbromuron from aqueous effluents by Fenton, electro-Fenton, and peroxi-coagulation methods, Desalin. Water Treat., 2015, 55(13), 3683–3693 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S194439862405121X.
- M. A. Oturan, N. Oturan, M. C. Edelahi, F. I. Podvorica and K. El Kacemi, Oxidative degradation of herbicide diuron in aqueous medium by Fenton's reaction based advanced oxidation processes, Chem. Eng. J., 2011, 171(1), 127–135 CrossRef CAS.
- M. P. Rosa Barbosa, N. S. Lima, D. B. de Matos, R. J. Alves Felisardo, G. N. Santos and G. R. Salazar-Banda, et al., Degradation of pesticide mixture by electro-Fenton in filter-press reactor, J. Water Process Eng., 2018, 25, 222–235 CrossRef , Available from: https://www.sciencedirect.com/science/article/pii/S2214714418303453.
- F. L. Souza, R. S. Rocha, N. G. Ferreira, M. A. Rodrigo and M. R. V. Lanza, Effects of coupling hybrid processes on the treatment of wastewater containing a commercial mixture of diuron and hexazinone herbicides, Electrochim. Acta, 2019, 328, 135013 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0013468619318845.
- A. J. M. da Costa, M. S. Kronka, P. J. M. Cordeiro-Junior, G. V. Fortunato, A. J. dos Santos and M. R. V. Lanza, Treatment of Tebuthiuron in synthetic and real wastewater using electrochemical flow-by reactor, J. Electroanal. Chem., 2021, 882, 114978 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1572665721000047.
- F. Gozzi, I. Sirés, A. Thiam, S. C. de Oliveira, A. M. Junior and E. Brillas, Treatment of single and mixed pesticide formulations by solar photoelectro-Fenton using a flow plant, Chem. Eng. J., 2017, 310, 503–513 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1385894716301279.
- J. R. Quejigo, U. Dörfler, R. Schroll and A. Esteve-Núñez, Stimulating soil microorganisms for mineralizing the herbicide isoproturon by means of microbial electroremediating cells, Microb. Biotechnol., 2016, 9(3), 369–380, DOI:10.1111/1751-7915.12351.
- J. Rodrigo Quejigo, A. Domínguez-Garay, U. Dörfler, R. Schroll and A. Esteve-Núñez, Anodic shifting of the microbial community profile to enhance oxidative metabolism in soil, Soil Biol. Biochem., 2018, 116, 131–138 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0038071717302730.
- L. P. Kowtharapu, N. K. Katari, S. K. Muchakayala and V. M. Marisetti, Green metric tools for analytical methods assessment critical review, case studies and crucify, TrAC, Trends Anal. Chem, 2023, 166, 117196 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0165993623002832.
- M. Sajid and J. Płotka-Wasylka, Green analytical chemistry metrics: A review, Talanta, 2022, 238, 123046 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0039914021009681.
- S. I. Kaya, G. Ozcelikay-Akyildiz and S. A. Ozkan, Green metrics and green analytical applications: A comprehensive outlook from developing countries to advanced applications, Green Anal. Chem., 2024, 11, 100159 CrossRef , Available from: https://www.sciencedirect.com/science/article/pii/S2772577424000685.
| This journal is © The Royal Society of Chemistry 2026 |
