Thermal treatment options for biosolids management: a critical review
Savankumar
Patel
 ab,
Ibrahim Gbolahan
Hakeem
ab,
Ibrahim Gbolahan
Hakeem
 ab,
Mojtaba Hedayati
Marzbali
ab,
Pobitra
Halder
bc,
Arun K.
Vuppaladadiyam
ad,
Lalit
Kumar
a,
Aravind
Surapaneni
ab,
Mojtaba Hedayati
Marzbali
ab,
Pobitra
Halder
bc,
Arun K.
Vuppaladadiyam
ad,
Lalit
Kumar
a,
Aravind
Surapaneni
 bf,
Abhishek
Sharma
bg,
Damien J.
Batstone
be and
Kalpit
Shah
bf,
Abhishek
Sharma
bg,
Damien J.
Batstone
be and
Kalpit
Shah
 *ab
*ab
aChemical and Environmental Engineering, School of Engineering, RMIT University, VIC 3000, Australia. E-mail: kalpit.shah@rmit.edu.au
bARC Training Centre for the Transformation of Australia's Biosolids Resource, RMIT University, Bundoora, VIC 3083, Australia
cSchool of Engineering, Deakin University, Geelong, VIC 3216, Australia
dSchool of Civil and Mechanical Engineering, Curtin University, Perth, WA 6102, Australia
eAustralian Centre for Water and Environmental Biotechnology, The University of Queensland, St Lucia, Brisbane, QLD 4072, Australia
fSouth East Water, Frankston, VIC 3199, Australia
gDepartment of Biotechnology and Chemical Engineering, Manipal University Jaipur, Rajasthan 303007, India
First published on 10th November 2025
Abstract
Thermal treatment of biosolids is receiving significant attention in the water industry as an alternative management option to land application. Traditional thermal treatment processes for biosolids management include drying and incineration, whereas emerging thermal technologies comprise dry thermal processes, such as pyrolysis and gasification, and wet thermal processes, such as hydrothermal carbonisation/liquefaction and supercritical water gasification. Thermal treatment is considered an efficient approach for the volume reduction of biosolids, contaminant destruction, and valuable product generation. However, there is a clear gap in the literature in benchmarking the range of available technologies, considering their techno-economic viability, emission potential, resource (energy and nutrient) recovery, and contaminant reduction. This knowledge is crucial for understanding the techno-commercial readiness, integration flexibility, and potential adoption of the thermal treatment technologies for biosolids management in wastewater treatment facilities. This critical review provides a comprehensive comparison of the various thermal treatment processes based on the parameters such as fate of nutrients and emerging contaminants, emissions, energy requirement, capital and operating expenditures, and scale-up maturity. It was found that dry thermal processes have substantial benefits over traditional incineration technologies, with pyrolysis and gasification being more energy-efficient and providing opportunities to generate valuable products (biochar and bioenergy). Hydrothermal liquefaction offers further benefits with high bio-oil and nutrient recovery and strong synergies with the existing water treatment infrastructures. Gasification and pyrolysis have high technology- and commercial-readiness level for biosolids treatment, making them suitable for the wastewater treatment industry. However, to ensure efficient and sustainable management of biosolids through thermal processes, there are some techno-commercial challenges, which are highlighted as future research perspectives.
Water impactThermal treatment offers a sustainable alternative for biosolids (stabilised sewage sludge) management, enabling volume reduction, contaminant destruction, and resource recovery. This critical review benchmarks key thermal technologies across different technical and commercial indicators. The findings highlight pyrolysis, gasification, and hydrothermal processes as promising solutions for wastewater treatment facilities, seeking energy-efficient, low-emission, and commercially viable strategies for biosolids valorisation and circular resource integration. |
1. Introduction
Biosolids are stabilised sewage sludge produced in wastewater treatment plants (WWTPs) from wastewater treatment. The growth in global population and urbanisation has led to the rapid expansion of WWTPs, leading to increased production of biosolids. Globally, biosolids production is around 100 million dry tonnes, which is projected to reach 175 million dry tonnes by 2050,1 with a daily per capita generation of 35–85 g dry matter.2,3 In Australia, 372![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dry tonnes of biosolids were generated in 2023 and New Zealand produced 75
000 dry tonnes of biosolids were generated in 2023 and New Zealand produced 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of dry biosolids in 2023.4 In 2024, over 2300 WWTPs in the US generated around 4 million dry tonnes of biosolids,5 while 15 million tonnes of dry biosolids were generated in EU-28 in 2021.6 In a typical WWTP, the management of biosolids accounts for more than 50% of the operating cost and about 40% of greenhouse gas emissions.7 The increasing production volume and stricter regulations pose challenges for the sustainable management of biosolids.
000 tonnes of dry biosolids in 2023.4 In 2024, over 2300 WWTPs in the US generated around 4 million dry tonnes of biosolids,5 while 15 million tonnes of dry biosolids were generated in EU-28 in 2021.6 In a typical WWTP, the management of biosolids accounts for more than 50% of the operating cost and about 40% of greenhouse gas emissions.7 The increasing production volume and stricter regulations pose challenges for the sustainable management of biosolids.
Biosolids consist of organic matter (polysaccharides, proteins, lipids, and non-degradable organics such as humic substances, lignin, and synthetic organics), inorganic materials (such as clay, sediments and minerals), macronutrients (such as potassium, calcium, magnesium, nitrogen, phosphorus, and sulfur) and micronutrients (such as zinc, iron, copper, boron, manganese and molybdenum).8 The composition of biosolids, particularly their macro- and micro-nutrient constituents, makes them suitable materials for land application in agricultural soils. Land application is one of the major traditional routes for biosolids management. For example, the US land applied about 60% of its biosolids produced in 2024.4 In Australia, 80% of biosolids were used for land application in agricultural soils in 2023.9 However, growing concerns regarding the presence of contaminants such as microplastics, per- and poly-fluoroalkyl substances (PFAS), pharmaceuticals, and pesticides, in biosolids and their associated impacts on soil and groundwater may restrain their direct land application in the future.10 In addition, heavy metals and microbial pathogens are present in biosolids, often at levels that raise concerns. The uptake of these contaminants by crops may result in their entry into the food chain and pose severe risks to the human health if not managed effectively. While pathogens can be effectively controlled by biological treatment or eliminated (by moderate thermal treatment), heavy metals are difficult to remove by existing treatment approaches and should be source controlled. Emerging contaminants such as microplastics and PFASs are potentially subject to regulations as they present a risk to the agricultural use of biosolids.11
Other conventional disposal methods of biosolids, such as landfilling and stockpiling, are not considered environmentally sustainable. Globally, about 30% of biosolids produced yearly are stockpiled or landfilled. In Australia, the US, and China, about 15%, 28%, and 44% of biosolids are either stockpiled or landfilled, respectively.12 While stockpiling and landfilling have been conventional biosolids management options, they offer little environmental benefits and face several issues including health risks, greenhouse gas emissions, groundwater contamination, and increasingly stringent legislations and negative public perceptions.13 Composting is another popular alternative option for biosolids management. However, it is a labour-intensive and costly approach, which effectively increases the biosolids volume, dilutes rather than eliminates contaminants, and requires a market for the composted biosolids.14 Therefore, the increasing biosolids production and stringent environmental regulations are pushing the wastewater industries to look for alternatives that could massively reduce biosolids volume, destruct/eliminate contaminants, and recover resources.
Thermal treatment of biosolids, having undergone numerous technological iterations in the last 100 years, has emerged as an attractive approach focusing on emission management and energy efficiency. Established technologies include thermal drying, thermal hydrolysis, and incineration. Emerging technologies include dry thermal processes (torrefaction, pyrolysis and gasification), and wet thermal processes (hydrothermal carbonisation, hydrothermal liquefaction, and hydrothermal gasification). The traditional thermal treatment methods, such as drying and incineration, can destroy organic contaminants and pathogens and facilitate energy recovery, but have a few shortcomings.15 For instance, incineration produces CO2, NOx, dioxins, and particulate matter, which must be controlled. The emerging thermal techniques have substantial benefits over conventional ones in generating valuable products, such as char and bio-oil and non-condensable gas for usable heat and power generation.16
Recently, there has been significant interest by water utilities in adopting thermal technologies for biosolids management. Plausible benefits include reducing biosolids volume by up to 70%, greatly reducing or eliminating odour, pathogens and persistent organic contaminants, and recovery of valuable resources (carbon, energy, and nutrients).15 However, a lack of understanding about the environmental performance and suitability of the diverse thermal treatment technologies in recovering valuable products limits their adoption at a commercial scale in the water industries. Therefore, benchmarking thermal and hydrothermal technologies is essential to evaluating their suitability for water industries, which is missing in the current literature.
Numerous reviews are available in the literature and have focused on the operating principles of different thermal/hydrothermal technologies and effects of process conditions on product distribution and properties.17–22 For example, Gao et al. and Sharma et al. reviewed the effects of critical process conditions on product yields during thermochemical treatment of biosolids and evaluated the energy recovery potential of the various processes.3,18 Patel et al. reviewed the relationships of pyrolysis conditions with char, oil and syngas product fractions and overviewed the numerous environmental and energy applications of the biochar product.23 Zhang et al. reported heavy metals and nutrient migration behaviour during the hydrothermal treatment of biosolids as a function of process conditions.22 The integration of thermal drying process with pyrolysis and gasification for energy-efficient biosolids conversion and contaminant reduction was reviewed by Nylen and Sheeban.24 In recent studies, Marzbali et al. demonstrated a range of emerging industrial applications of char product from the thermal treatment of biosolids.25 Vuppaladadiyam et al. provided a review of application of biosolids-derived biochar and hydrochar for H2S removal from biogas with a demonstration of how WWTPs can benefit from this circular model of utilising biosolids char for the upgrading of biogas generated from the anaerobic digestion of their sewage sludge.26
In all the existing reviews, there has been limited focus on the technological challenges, energy and nutrient recovery potential, emission characteristics, and techno-commercial feasibility of the different thermal treatment processes ranging from the traditional thermal process (drying and incineration) to emerging thermal process (pyrolysis, gasification and hydrothermal). The understanding of these various aspects of the diverse thermal treatment technologies is essential for fair comparison and benchmarking of the available technologies. The knowledge will guide the WWTPs on the identification and adoption of the most efficient, cost-effective, and commercially matured thermal technologies for their biosolids treatment.
Therefore, the primary aim of this review is to critically discuss the technological level assessment of different thermal and hydrothermal processes, considering the fate of nutrients and contaminants, emission characteristics, range of generated product streams, scale-up plants and technology maturity level, as well as costs and energy requirements. As such, we compare the conventional and emerging thermal technologies by considering their energy requirements, transformation and fate of nutrients and contaminants during treatment, and economic analysis of each process. Furthermore, this study benchmarked the thermal treatment technology and assessed its integration prospects with the existing sludge treatment process, particularly anaerobic digestion. Lastly, the opportunities and barriers to implementing the thermal treatment technologies in the water industry were highlighted.
2. Thermal technologies for biosolids transformation
Thermal technologies for biosolids transformation can be grouped into two main categories: (i) wet processes and (ii) dry processes. Each of the processes can further be classified as oxidative and non-oxidative processes depending on whether oxygen or air is added for the process operation, as summarised in Fig. 1, where the added oxygen concentration is at or above the stoichiometric oxygen requirement for the complete conversion of the organic fuels to CO2 and H2O, and such process is referred to as combustion. In this case, incineration is an example of an oxidative process where the added oxygen is at or above the stoichiometric concentration. However, where the added oxygen concentration is below the stoichiometric requirement for the complete combustion of the hydrocarbon fuel, such oxidative process is considered partial/incomplete combustion. In this case, gasification process is a typical example. However, non-oxidative processes operate in an inert environment with no external oxygen or air added, thus preventing organic matter oxidation (as in pyrolysis). Therefore, incineration is a dry and oxidative thermal process, while hydrothermal oxidation and supercritical wet air oxidation are wet oxidative thermal processes. Similarly, pyrolysis is a dry and non-oxidative thermal process, while hydrothermal carbonisation/liquefaction is a wet and non-oxidative thermal process.Thermal processes for biosolids transformation operate under a spectrum of temperature and oxygen concentration with different energy requirements, conversion efficiencies, and product streams. In addition, each of the thermal processes highlighted in Fig. 1 has different resource (energy and nutrient) recovery potentials, emission profile, product distributions, contaminant destruction efficiency, and product quality. Furthermore, some of the thermal treatment techniques such as drying, thermal hydrolysis, and incineration are well established and already adopted by the water industry for treating sludge and biosolids. While other treatment techniques such as pyrolysis, gasification, and hydrothermal processes are yet to be widely adopted and are considered rapidly emerging treatment technologies. In this section, we discuss a range of thermal treatment technologies for biosolids transformation across different metrics, such as energy requirements, fate of nutrients, fate of organic and metal contaminants, capital and operating costs and scale-up plants.
2.1 Thermal drying
Drying is employed as a pre-treatment step prior to incineration or other thermal treatment processes, as the low feedstock moisture content needed for these processes cannot be achieved by mechanical dewatering means alone. Drying helps to reduce volume and serves as a stabilisation method for biosolids use in agricultural soils. For instance, thermally stabilised (dried) biosolids in the US received substantial brand recognition and the final product has a higher sale price than the raw nutrient value (e.g., milorganite, bloom, and “bay state fertilizer”).The thermal drying of biosolids usually begins with a constant drying phase, during which unbound moisture or free water is removed from the surface. This is followed by two falling rate phases, where bound moisture is gradually eliminated.8 Biosolids are dried using different types of dryer technologies, which can be operated in direct or indirect mode. Direct dryers operate by direct contact of biosolids with a hot stream (generally air, steam, or combustion flue gas). Indirect dryers operate by heat transfer from an internal or an external heating element (normally by steam, hot air, or hot oil).17,27 A comparison of the different types of dryers is shown in Table 1. Direct dryers are generally simpler and may have a smaller footprint due to higher operating temperatures and heat transfer rates. However, they generate contaminated off-gases (even when the contact gas is recycled). Indirect dryers are more complex and larger but allow better heat usage efficiency, as heat is not lost with the exhaust air.28 Since most of the water vapour generated is condensed, it enhances energy efficiency. Both dryer types can be configured to produce a pelletised product, which is formed through agglomeration of particles (particle–particle contact within the dryer). Indirect dryers have better control over pellet size and can produce large pellets. Both approaches allow for the classification of dried biosolids products allowing for the recycling of undersized particle sizes and fines.29 Pellets are generally preferred when the agricultural use of the dried product is desired or when the dried sludge is to be incinerated. Alternative non-pelletising drying processes such as flash, fluidised bed (and spray) are used with off-gases fed with entrained sludge to the incinerator.29 While both types of dryers can be designed to operate without solids recycling, direct dryers are generally dependent on recycling undersize particles due to classification issues, while indirect dryers benefit from recycling to enhance heat transfer.30 Recycling avoids the “sticky zone” at <55–70% solids and allows for the recycling of undersized particles.30,31
| Direct dryer | Indirect dryer |
|---|---|
| • Types: rotary drum, belt, flash, fluidized bed, spray, toroidal | • Types: tray (including multistage and rotating tray), paddle, disc, hollow flight |
| • Faster heat transfer (smaller dryer) | • More energy efficient (no convective losses, may allow condensate energy recovery) |
| • Higher thermal energy consumption (3.5–4 MJ kg−1) | • Higher electrical energy consumption (50 Wh kg−1) |
| • Contact gases must be treated (may be reused depending on configuration) | • Reduced off-gases (mainly evaporate, CO2etc.) |
| • Simpler, available at smaller scale | • Only at larger scale (>10 tpd) |
| • More market penetration (in USA) | • Mature tech, well established in Europe |
| • Generally requires product recycle | • Products recycle reduced |
| • Lower CAPEX | • Lower OPEX |
| • Can operate at higher contact temperature | • Higher temperature reduces energy efficiency, dependent on HX |
| • Generates dust in off-gas | • Generates dustier product |
| • Process explosive risk due to off-gas/dust | • Product explosive risk due to dust |
| • Smaller pellets (less controllable, configured by design) | • Larger pellets (more controllable) |
 | ||
| Fig. 2 Thermal energy required by the dryer to dry biosolids cake to 95% solids using 3 GJ per tonne water. Energy costs $10 per GJ. | ||
The energy requirements of biosolids drying technologies vary with the dryer type.27 For instance, the thermal energy requirement for a drum-type dryer is estimated between 0.85 and 1.07 kWhth kg−1 water, and for fluidised bed-type and disc-type dryers. it is 0.8–1.1 kWhth kg−1 water. The belt and paddle dryer requires 0.95 kWhth kg−1 water while the flash and rotary dryer consumes around 1.05 kWhth kg−1 water removed. The solar greenhouse drying technology exhibits a significantly lower energy consumption than that of conventional thermal drying. For instance, the energy consumption of solar greenhouse drying to dry flocculated and unflocculated sludge is estimated as 0.22 and 0.28 kWhth kg−1 water, respectively, which can be further reduced using open airflow greenhouses than the climate-controlled ones.32,35 It is worth mentioning that Germany and Poland treat around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 9000 tonnes of wet biosolids per year, respectively, using a solar greenhouse drying technology.32 Although solar dryers do not require any additional thermal energy, they need prolonged exposure time and rely on weather conditions.17,18
000 and 9000 tonnes of wet biosolids per year, respectively, using a solar greenhouse drying technology.32 Although solar dryers do not require any additional thermal energy, they need prolonged exposure time and rely on weather conditions.17,18
Arjona and Cisneros performed a feasibility assessment of commercially available dryers in different scenarios in a typical WWTP.34 The authors observed that the net energy from drying anaerobically digested sludge was negative, suggesting that conventional drying is not feasible to produce dried digested sludge for energy applications. However, drying undigested sludge results in a net positive energy of about 1185.98 kWhth per tonne dry sludge. Đurđević et al. also studied the drying energy balance of sludge, which is the difference between combustion energy recovery and energy required for the drying process.33 Considering the specific energy consumption as 0.9 kWhth kg−1 water, energy recovery efficiency as 0.8, and initial sludge solid content as 30%, the thermal energy balance (QEB) in kWhth kg−1 DM can be expressed using eqn (1):
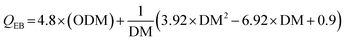 | (1) |
Đurđević et al. reported a negative energy balance for sludge with ODM < 0.44, irrespective of the value of the DM, indicating the case of drying digested sludge.33 On the contrary, the energy balance was positive for ODM > 0.66 over the entire range of DM tested, indicating the case of drying non-digested sludge. The positive thermal energy balance in the case of drying undigested sludge is most likely due to the high calorific value associated with the high organic matter in the undigested sludge. Fig. 3 shows the thermal energy balance profile as a function of sludge ODM and DM.
 | ||
| Fig. 3 Energy balance of sludge drying with respect to the ODM and DM content (adapted with permission33). | ||
2.2 Incineration
Incineration is a high-temperature (800–1200 °C) thermal process that completely combusts biosolids in the presence of air (with or without other fuels), converting them to ash, heat, and gases. This process significantly reduces the volume of biosolids by over 90%, produces recoverable heat energy, and can also destroy harmful contaminants and pathogens.51 Incineration is widely employed in Asia, North America, and Europe for managing biosolids. The number of incinerators in Japan was around 1173 in 2013, whereas this number in Korea was close to 476 in 2014.52 In the USA, 16% of biosolids are disposed of via incineration in about 340 incinerators,53 and in Europe, approximately 28% of biosolids are disposed of by incineration.54 Incineration has received serious disapproval in the recent past primarily due to the introduction of EPA emission regulations in 2011 and enforced from 2016.55 In the USA, nearly 30 incinerators (mainly multiple hearth furnaces) have been decommissioned in the last 10 years.Incineration is effectively a two-stage process consisting of drying wet biosolids followed by combustion. Initial drying is required to achieve the necessary flame temperatures required for emission control. Drying can be done initially in the incinerator but with lower efficiency than a dedicated dryer unit, which is economically justified in plant sizes above 20 ML d−1.56 The most common incinerator types in the US are multiple hearth furnace (MHF) and fluidised bed furnace (FBF).53 A MHF consists of 5–12 hearths, and the solids are fed at >20% dry solids. MHFs are relatively cheaper (low capital cost), and reasonably energy efficient. However, they are difficult to operate, mechanically complex, and produce high-emission loads (due to variable combustion zones). Fluidised bed furnaces utilise an inert bed (normally sand) as a heat reservoir and fluidizing agent. Sand is fluidized by the added air, which ensures uniform drying and combustion and hence a consistent combustion temperature. Employing fluidising media in FBF furnaces facilitate easy operational control but reduce energy efficiency (as the inert bed material must be preheated to 490 °C). While an FBF is more easily controlled than an MHF, it is more difficult than a standard combustion process, since both air–fuel mixture and combustion temperature must be controlled to minimize emissions. To address this, even when sludge is sufficiently dry to maintain combustion auto-ignition, gaseous fuel must be continuously fed, which reduces energy efficiency.
Thermal energy balance is also impacted by the gross calorific value (GCV) or higher heating value (HHV) of the biosolids feed material. GCV or HHV is the maximum recoverable thermal energy including the latent heat recovery of the generated water vapour. On the other hand, net calorific value (NCV) or lower heating value (LHV) is the recoverable thermal energy excluding the latent heat recovery of the generated water vapour. Calorific value is a measure of energy content and mineral content and can vary substantially depending on the redox of the inputs. HHV impacts many other design and operational parameters including combustion flame temperature, optimal air mix, and net thermal efficiency. The LHV or NCV can be calculated from the HHV using eqn (2) (the second term represents latent heat of vaporisation of the evaporated water):58
| LHV (MJ kg−1) = HHV − 0.02454(M + 9H) | (2) |
HHV is ideally measured in a bomb calorimeter, though there are a large number of correlations that use proximate compositions (ash, volatile matter, and fixed carbon) or ultimate compositions (CHONS), or both.61,62 These have mean absolute errors of the order of 4–7% on plant biomass.62 In general, proximate analysis correlations are biomass specific, while ultimate analysis correlations consider variation in biomass compositions more thoroughly. Ideally, correlations which include a minimum of CHON should be utilised (since these vary in biosolids). Assessing the correlations reported in previous studies,61,62 which are general for biomass, eqn (3) from a previous study62 (source ref. 63) fulfils the above-mentioned criteria (and also includes an ash correction), and predicts HHV of both reference materials (CH4, ethanol) and sludges effectively:59,60
| HHV (MJ kg−1) = 0.3491C + 1.1783H + 0.1005S − 0.1034O − 0.0151N − 0.0211Ash | (3) |
HHV is fundamentally linked to chemical oxygen demand (COD) of sludge and 1 kg COD is correlated to an HHV of 13.875 MJ kg−1 (corrected from ref. 64). However, accurately measuring sludge COD can be difficult as the chemical COD test does not oxidise all materials in the sludge matrix. This means that HHV calculations using COD correlations can show substantial variation. In a recent study, Schaum et al. reported a HHV coefficient of 14.98 MJ kgCOD−1.64
 | ||
| Fig. 5 Nitrogen transformation during sewage sludge combustion (adapted with permissions65). | ||
CAPEX = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797 × P0.82 797 × P0.82 | (4) |
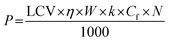 | (5) |
The CAPEX and OPEX, in million USD per year, of the incineration plant can also be estimated as a function of plant capacity (C) in 1000 tonnes per year using eqn (6) and (7), respectively.77
| CAPEX = 2.3507 × C0.7753 | (6) |
| OPEX = 0.0744 × C0.8594 | (7) |
2.3 Dry thermal processes (torrefaction, pyrolysis, and gasification)
Torrefaction, pyrolysis, and gasification are thermal conversion processes designed to operate under atmospheric conditions and elevated temperatures (300–800 °C) under different gas environments, such as inert and reactive. The three processes operate similarly over a spectrum of temperature and time scale. Pyrolysis is generally conducted in the absence of air or oxygen (inert environment), while gasification may be with oxygen or air (reactive environment) or without (thermal cracking). The basic difference between pyrolysis and torrefaction is that torrefaction employs lower temperatures (200–300 °C) in comparison to pyrolysis at 400–700 °C, and are both conducted at low heating rates in an inert atmosphere.80,81Operating below the biochar temperature/timeline produces bio-coal and highly volatile organics (as in the torrefaction process). Operating above the biochar temperature/timeline results in biochar, either via slow pyrolysis or via fast pyrolysis. Gasification is conducted at elevated temperatures and results in minimal residual carbon with the main product referred to as syngas. The residual carbon can be partly oxidised with the addition of steam or other oxidising agents, resulting in the production of activated carbon.80,81
Pyrolysis and gasification have been extensively demonstrated for biochar and bioenergy production from biosolids. However, these works are mostly based on laboratory-scale batch operation rather than continuous pilot or full-scale demonstrations. It is worth mentioning that though the literature in the area is informative, key technical challenges around reactor design and scale-up, technology variation on energy requirement, product quality, and emission profile as well as product application limitations are often underreported. For example, the most important environmental impact of using biochar on land is for carbon sequestration as well as renewable bioenergy and greenhouse gas emission reductions from utilising pyrolysis- and gasification-derived products as opposed to fossil-based fuels. Nevertheless, pyrolysis/gasification could have negative impacts on the environment including direct and indirect greenhouse gas emissions, energy requirements for pre-processing of feedstock and flue gas cleaning, and the production of pollutants with human or environmental toxicity.
Nevertheless, pyrolysis and gasification processes generate product streams whose energy content exceeds that of the parent feedstock helping to generate net positive thermal energy.82 It has been reported that recycling the excess heat for drying incoming feedstock and/or for supplying the process energy can significantly improve the overall energy performance of these technologies. Using the bioenergy recovered from the syngas or bio-oil product instead of fossil-derived energy can significantly reduce greenhouse gas emissions and lower the carbon footprint of pyrolysis or gasification processes. The heat released during biosolids pyrolysis or gasification can also be captured and used to dry feedstock, thus lowering the need for external energy.81 Furthermore, integrating pyrolysis or gasification systems with existing sewage treatment units such as anaerobic digestion and dewatering/drying could be more efficient as it allows for the effective reuse of excess heat, biosolids feedstock, and products within the treatment system.81
Volatile organic nitrogen in biosolids is mostly converted to ammonia, but the relative fraction changes from 50% conversion of feed nitrogen to 80% conversion as the process temperature increases from 400 to 800 °C. As the temperature increases, other volatile nitrogen compounds progress from volatile organic nitrogen compounds to HCN, ammonia, and finally, nitrogen gas.86 Elevated levels of HCN require post pyrolysis volatile combustion or reforming of mixed pyrolysis gases. Ammonia recovery is possible in high-temperature pyrolysis or gasification but is more complex due to the availability of toxic compounds such as cyanide and sulfides.
Phosphorus and potassium are other critical nutrient elements in biosolids. During pyrolysis/gasification, the majority of P and K are retained in the resulting char product with generally more than 80% recovery reported. In addition, the concentration of these elements in the char typically increases by at least 1.5-fold compared to the feed, depending on the process temperature.87 The increase in the concentration of P and K in the char residues during thermal treatment of biosolids is mainly due to volume reduction and the relative thermal stability of these elements. However, the retention of P and K is accompanied by some transformation induced by temperature, chemical speciation of the elements, and mineral composition of the biosolids.88 For example, labile P and K in biosolids are transformed to reducible and oxidisable fractions in the biochar, forming complex minerals with major inorganic elements in the char such as Ca, Si, Al, and Fe.89 Notably, high-temperature pyrolysis and gasification (>700 °C) can cause K volatilisation to the gas phase, thereby reducing the K content in the biochar product. It was reported that K would volatilise at 700–800 °C from the carbon matrix having a C/K ratio of 6.2–9.5 wt%.90 It is generally reported that the bioavailability of the N, P, and K content of biochar is generally lower compared to that of the biosolids feed attributed to the thermally induced stabilisation of the elements in the char matrix.91 It is important to optimise the thermal treatment process for maximum retention of nutrient elements in the biochar.
Microplastics and PFASs are substantially decomposed under pyrolysis/gasification environment as low as 500 °C.48,97 PFAS levels in biochar are generally observed to be below the detection limit following biosolids pyrolysis and gasification at ≥500 °C. For example, more than 90% removal of PFOA and PFOS from biosolids having an input concentration of 0.2–87 ng g−1 for PFOA and 13–30 ng g−1 for PFOS was observed under pyrolysis conditions operating at 500–700 °C. Residual PFOS/PFOA concentration was <0.2 ng g−1 or in most cases below detectable levels in the biochar.98–101 Similarly, organic micropollutants such as pesticides, pharmaceuticals, and surfactants are also sufficiently eliminated during biosolids pyrolysis.95,102 Polyaromatic hydrocarbons (PAHs) are formed during biosolids pyrolysis between 450 and 650 °C, reaching a peak value around 550 °C.103 Most 5-ring or lower PAH compounds are expected to get volatilised at these temperatures and potentially destroyed in the gas-phase combustion process. Long-chain PAH compounds (above 5-ring) are largely retained in the biochar with naphthalene being the most prominent PAH compound detected in biosolids biochar.104 Generally, the char product has a low level of POP contamination due to the thermal decomposition of the organic material in a reducing environment, relatively high thermal volatility of the compounds, and longer solid retention times in pyrolysis environment. However, gas phase contamination can be an issue due to shorter reactive retention time and the volatility of POPs into the gas phase, with most POPs being condensable with the tar. Notably, the quality of bio-oil from biosolids is relatively low, containing high-molecular weight aromatic nitrogenated and oxygenated compounds including PAHs.105 The Diels–Alder reactions result in PAH synthesis, particularly at >500 °C, and PAHs would have a high fraction of C5–C6 nitrogenous compounds, including nitriles, pyridines, amides, amines and polyaromatic nitrogenated compounds.106 Sulfur is largely volatilised as hydrogen sulfide but also forms carbonyl sulfides, methyl sulfides and other organosulfides, imparting a strong odour to pyrolysis tar.107
Overall, the utilisation of biosolids tar product can be problematic. For this reason, volatiles are either converted post-pyrolysis in a reformer or combusted for energy in a thermal oxidiser. The gas phase emissions from the combustion of volatile products from biosolids can be carefully managed by following the best available practices for thermal plants using appropriate gas cleaning units.95 Beyond the environmental aspects of pyrolysis and gasification, it is critical to examine the economic impacts to determine their cost-effectiveness.
EnerSludge™ set up a commercial plant in Perth, Australia, to transform 45% of the energy in the sludge feed into bio-oil. However, the plant was shut down after 16 months of operation because it was not cost-effective and raised concerns over the bio-oil quality.113 In 1986, Battelle Memorial Institute in the United States proposed a pilot plant process to produce bio-oil from sewage sludge and patented it as the sludge-to-oil-recovery system (STORS). ThermoEnergy in 2007 commercialised STORS with the following operational conditions: 275–315 °C, 11.4–14.8 MPa, and 1–3 h. The process converted sludge (20% dry solids) into a liquid fuel having 90% of diesel's HHV and solid char similar to coal.114 The engine could use the bio-oil to make electricity and heat. Molton et al. provided a detailed cost estimate of STORS technology for small WWTPs processing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population equivalent dry tonnes per year sludge, medium WWTPs processing 100
000 population equivalent dry tonnes per year sludge, medium WWTPs processing 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population equivalent dry tonnes per year sludge, and large WWTP processing 1 million population equivalent dry tonnes per year sludge.115 The estimated energy used in the process were 1410, 1394 and 1394 kWh per dry MT, and the electricity produced from oil for the small- (1898 kWh per dry MT), medium- (1480 kWh per dry MT) and large-sized population (1480 kWh per dry MT). However, no full-scale installation is currently in operation.
000 population equivalent dry tonnes per year sludge, and large WWTP processing 1 million population equivalent dry tonnes per year sludge.115 The estimated energy used in the process were 1410, 1394 and 1394 kWh per dry MT, and the electricity produced from oil for the small- (1898 kWh per dry MT), medium- (1480 kWh per dry MT) and large-sized population (1480 kWh per dry MT). However, no full-scale installation is currently in operation.
Goldhöfe in Germany has a biosolids pyrolysis demonstration plant that uses a pyrolysis drum as the reactor.118 The heat from natural gas combustion is used to pyrolyze biosolids in the reactor. Pyrolytic gas is used to provide heat for gas purification and recycling.
The Silicon Valley Clean Water Authority in California commissioned the BioForceTech Corporation BioDryer integrated with PYREG Pyrolysis technology in 2018. BioForceTech's “biosolids to energy plant” consists of 6 BioDryers coupled with a P-FIVE pyrolysis system. The system has a rated capacity of 14 wet tonnes per day (wtpd) of dewatered, digested biosolids with 25% total solids. The BioDryers consume only 220 kWh of energy to remove moisture from 1 tonne biosolids. The process can produce up to 320 MJ h−1 heat energy used to generate hot water for the drying purposes. The pyrolysis reactor temperature ranges between 450 and 750 °C. The reported mass output of the system is 1.1 tpd biochar at 90% solid content.81
In 2015, Anaergeia's Technologies commissioned a pyrolysis system (PyroSys™) at the Encina Wastewater Authority's Water Pollution Control Facility in California. The system has a rated capacity of 12 wtpd and is designed to process digested biosolids.
RMIT University in collaboration with South East Water, Greater Western Water, and Intelligent Water Networks has developed an innovative efficient fluidised bed heat exchanger reactor (PYROCO™) with optimised heat and mass transfer for biosolids pyrolysis, which is the first of its kind in Australia. The PYROCO™ pyrolysis system was trialled on a semi-pilot scale with a capacity of 0.25 kg h−1 in 2019.119 The pilot-scale with a capacity of 1 tonne wet biosolids per day in 2021 and 3 tonne per day wet biosolids in 2023 were trialled at a Greater Western Water treatment site in Melbourne, Australia.82 The fluidised bed pyrolysis reaction in a PYROCO™ reactor was carried out at 500–600 °C using oxygen lean syngas generated in the preceding gas producer unit for fluidisation and heat carrier media. The pilot-scale PYROCO™ plant demonstrated no detectable PFAS and other organic contaminants in the biochar product from biosolids pyrolysis.95 The commercial-scale PYROCO™ demonstration plant is expected to be ready by the end of 2026.
Low-temperature circulating fluidised bed has gained popularity as a fuel-flexible gasification technology. A classic example is a 6 MW demonstration project developed by the Technical University of Denmark at DONG Energy's Asnaes Powerplant in Kalundborg, Denmark.123 High ash content in biosolids feedstock necessitates ash removal, and a high flow rate for fluidisation degrades syngas quality during fluidised bed gasification. Recovery of energy-rich syngas via a commercial EBARA-fluidised bed gasification technology was demonstrated in Japan. Six TwinRec process lines were functioning in September 2002 and 14 more were planne.124 A recent report has demonstrated that only three commercial sludge gasification plants are in operation in Europe, all located in Germany. The smaller one, located in Balingen, has a capacity of 1955 dry solids per year, the medium size, located in Koblenz, has a capacity of 4000 dry solids per year and the largest, located in Mannheim, has a capacity of nearly 5000 dry solids per year.125,126 All plants are based on a two-stage gasification process. The KOPF gasification technology is an example of a two-stage process and includes a post-combustion chamber and a solar drying unit.127 The solar unit dries the wet-digested sludge to a solid content of 70–85%. The fluidized bed operates at 900 °C for 30 minutes. The syngas product is combusted at 850 °C and the gas engine generates around 70 kW electricity. A post-combustion chamber is used to flare unused gas.
Logan City Council in Queensland, Australia, conducted a biosolids gasification demonstration project using a multiple hearth gasifier with a bed temperature ranging from 400 °C to 700 °C, and a 100 s retention time.128 With a dried-biosolids feed rate of 480 kg h−1 (74% of maximum capacity at 650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kg h−1), soot and tar buildup in the air manifolds reduced system throughput during longer runs. However, these deposits were easily burned off during feed pauses, and an automated burn-off sequence is planned for future operation. Modifications were made to the spray absorber scrubber and barrier filter due to overwhelming non-sticky carbon and dust carryover from the pyrolysis off-gas. A venturi device was installed, and a wet electrostatic precipitator is planned for improved dust control.
kg h−1), soot and tar buildup in the air manifolds reduced system throughput during longer runs. However, these deposits were easily burned off during feed pauses, and an automated burn-off sequence is planned for future operation. Modifications were made to the spray absorber scrubber and barrier filter due to overwhelming non-sticky carbon and dust carryover from the pyrolysis off-gas. A venturi device was installed, and a wet electrostatic precipitator is planned for improved dust control.
At the Morrisville Municipal Authority in Pennsylvania, the Ecoremedy Fluid Lift Gasification technology, commissioned in 2019, has a rated capacity of 32 wtpd (with 27% total solids).129 It produces a maximum energy of 2640 MJ h−1 in the form of heat for thermal drying. The reported mass output of the system is 2.4 wtpd.
The City of Lebanon, Tennessee commissioned an Aries Clean Technologies Downdraft Gasification system in 2016.130 The system has a rated capacity of 29 wet tons per day and processes a blend of waste wood, scrap tires, and dewatered, digested biosolids. It produces a maximum energy output of 420 kW electricity using a flue gas-driven organic Rankine cycle generator. The reported mass output is 1.5 wtpd.130,131
2.4 Wet thermal processes
Raw biosolids has about 95–99% water, which can be reduced to 70–85% through mechanical dewatering.132 The cost of sludge management in WWTPs is nearly 50–60% of the total operating costs, and moisture removal is one of the main contributors, given the huge amount of energy required to evaporate water.133 Incineration and other dry thermal treatment processes (torrefaction, pyrolysis, and gasification) require a feedstock with a moisture content below 20% to achieve net positive thermal energy.134 Consequently, the dry thermal processes require prohibitive energy for biosolids drying, which might be avoided in wet thermal processes. This is because, in wet thermal processes, the inherent water content in biosolids is simultaneously used as the reaction medium, solvent and catalyst to decompose the organic fraction into a hydrophobic solid char product (hydrochar).134Wet thermal processes cover hydrothermal carbonisation (HTC), hydrothermal liquefaction (HTL), hydrothermal oxidation (HTO), and supercritical water gasification (SCWG), which vary over a spectrum of temperature and oxygen inputs (Fig. 1). Hydrothermal techniques are ideally suitable for processing highly wet biosolids or even raw sewage sludge.135 Autogenous pressure builds up during the process, helping to keep the water in its liquid state and avoiding evaporation. Treatment in such a water-rich medium not only alleviates the loss of carbon caused due to coke formation but also takes advantage of hydrogen donated by water to enhance the quality of products. Hydrochar, bio-oil, aqueous phase and gas products are the main products of hydrothermal treatment; however, their distribution depends on the process conditions.135 The water-soluble intermediates are formed relatively rapidly and are then converted to bio-oil and hydrochar. The liquefaction reactions can occur under mild conditions; for instance, the hydrolysis of hemicellulose and cellulose occurs at 140 and 220 °C, respectively.136 Hydrolysis can contribute to liquid-phase products or be followed by isomerization, dehydration, decarboxylation and condensation/repolymerization to mainly produce secondary char at low temperatures (<250 °C).137 Increasing the temperature up to the critical point of water, i.e., 374 °C, may decrease the chances of re-polymerization and accelerate the decomposition of protein, lignin, and lipid in the sludge.137 Under HTL conditions, more liquefied products, including bio-oil or aqueous phases, are generated. Bio-oil yield depends on the extraction solvent and can substantially vary depending on the solvent used. Bio-oil formation is favoured with microbial, protein, and lipid-rich feedstock such as sewage sludge over lignocellulosic biomass feedstock.138 The aqueous phase contains largely polar and water-soluble organic acids, alcohols, ammonia, sulfides, and other compounds, which cannot be extracted using a solvent. The aqueous phase is organic-rich with chemical oxygen demand (COD) exceeding 30 g L−1, which may be anaerobically digested to produce biogas or further processed to recover energy and nutrients.139 Furthermore, increasing the temperature above water supercritical temperature (>375 °C) generates mainly H2, CO2, CH4 and CO as the products of SCWG. Fig. 6 summarises the hydrothermal processes based on their conditions and potential products. In general, for HTC (<250 °C), hydrochar is the dominant product, with 50–70% yield. For the HTL, the process occurs in the range of 250–370 °C and bio-oil is the main product with 30–60% yield. In SCWG (>370 °C), gas is the predominant product.140 Inherent catalytic hydrothermal conversion occurs in sewage sludge due to the presence of high concentrations of alkali and alkaline earth metals.141 Additionally, hydrothermal treatment of sewage sludge or biosolids offers many advantages including reduction in waste volume and complete sterilization and eradication of microbial pathogens for biosolids stabilisation.142
Biosolids are also rich in micronutrients. For example, Australian biosolids comprise Mn, Cu, Fe, Zn, Ni, at a concentration of 50–500, 92–1996, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 824–18
824–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026, 210–3060, 20–250 mg kg−1, respectively.147 Given the main application of biosolids as soil amendment in Australia, the concentration of micronutrients must be within the environmental loading limits and helps plant growth.148 Hydrothermal conversion of sewage sludge at 230 °C could slightly increase the concentration of micronutrients such as Mn, Cu, Zn and Ni. One plausible reason could be the subsequent precipitation of initially solubilized micronutrients with other chemical species.149 Similar observations were reported by Wang et al.150 Although some elements such as Zn and Cu are beneficial plant micronutrients, at higher concentrations, they are toxic to plants and/or animals.
026, 210–3060, 20–250 mg kg−1, respectively.147 Given the main application of biosolids as soil amendment in Australia, the concentration of micronutrients must be within the environmental loading limits and helps plant growth.148 Hydrothermal conversion of sewage sludge at 230 °C could slightly increase the concentration of micronutrients such as Mn, Cu, Zn and Ni. One plausible reason could be the subsequent precipitation of initially solubilized micronutrients with other chemical species.149 Similar observations were reported by Wang et al.150 Although some elements such as Zn and Cu are beneficial plant micronutrients, at higher concentrations, they are toxic to plants and/or animals.
Hydrothermal treatment of sewage sludge concentrates and immobilises heavy metals in the hydrochar. However, despite the increased heavy metal concentration in hydrochar, the direct bioavailability of the metals was exceedingly low.150 Under severe processing conditions, as shown in HTL and SCWG, more heavy metals may be released to the liquid phase and the hydrochar may be lean in heavy metals.158 The heavy metals during hydrothermal treatment transform from weakly bounded fractions to stable fractions with reduced toxicity and bioavailability, possibly due to the formation of a complex with inert minerals and immobilisation in the crystal lattices of the hydrochar.150 A detailed review of the fate of heavy metals during the hydrothermal conversion of sewage sludge was conducted by Leghari et al.159 Co-hydrothermal treatment of sewage sludge with other feedstock like food and garden waste, alum sludge, or lignocellulosic biomass can help to reduce the total concentration of heavy metals by dilution effect as well as enhance metals immobilisation, reduce bioavailability and generate stable precipitates via beneficial synergistic interactions of feed materials.160,161
 | ||
| Fig. 7 Process diagram for the HTL of sewage sludge (adapted with permission162). | ||
Starting with the pump, several vendors reported that the pumps could effectively transfer feedstock having up to 15 wt% solid content.163 A higher solid content means that less water must be heated and pressurized, offering a more compact plant. However, the resulting high viscous feed slurry may create problems for pumps and the low heat transfer coefficients may significantly increase the operating costs. Furthermore, pumps will likely be subjected to downtime and regular maintenance to preserve efficiency. The viscosity of the feed and transport rheology can be improved by pre-heating or thermal hydrolysis before pumping. A hydrothermal reactor is operated at high pressures. Therefore, to avoid installing excessively thick walls requiring larger diameter pipes in a single pipe train, it is suggested to install four process trains, which not only reduce the pipe diameter but also provide process redundancy.164 The holding time represented by liquid hourly space velocity (LHSV) can significantly impact the reactor size and the overall capital investment. According to a report from Pacific Northwest National Laboratory (PNNL) published by the US Department of Energy, a reactor system comprising a pump, heat exchanger and plug flow reactor, handling a sludge flow rate of 139 tons h−1 at 4 LHSV costs around $18.7 million.165 The price of a pre-heater, usually referred to as a hot-oil system, is reported as $4.7 million and is installed to heat the feed mixture to the processing temperature. Downstream separation and material processing are fundamentally different. While phase separation can be achieved by depressurisation and flashing, the material can also be selectively separated through variation in the dielectric constant of water and using the pressure differential (for example, to extract water and gases) through membranes. However mineral and organic fouling can be a substantial issue. A phase separator, including a solid filter and an oil/water separator, costs around $4 MM for a flow rate of 553 tons kg−1 solids. Ideally, the electrical work required to pressurise 1 ton of water to 220 bar is 5.56 kWh (or 40 kWh per ton-dry solids). Modelled energy consumption of HTL plants is estimated at 100 kWh per ton dry solids.162
Aalborg University in Denmark collaborated with Steeper Energy to design and construct a continuous hydrothermal liquefaction (HTL) research facility.167 This facility was utilised to convert non-digested sewage sludge into HTL biocrude, water, and gas under supercritical conditions, reaching a temperature of around 400 °C and 320 bar pressure. The HTL process effectively transformed the sewage sludge into valuable biocrude, along with the generation of water and gas as byproducts.
The Altaca Energy facility, located in Gönen, Turkey, is a demonstration plant that was constructed and completed in 2016.168 It utilizes the catalytic hydrothermal liquefaction technology known as CatLiq®, developed by SCF Technologies based in Denmark. This innovative plant is designed to convert diverse biomass sources, such as biogas plant digestate, forest waste, sewage sludge, agricultural waste, food plant waste, and organic household waste, into HTL bio-crude.
Licella Pty Ltd, an Australian company, has developed advanced catalytic hydrothermal liquefaction technology (Cat-HTR™).169 Licella commissioned their first Cat-HTR™ pilot-scale plant in 2007. Two commercial Cat-HTR™ demonstration plants are in operation in Somersby, Australia since 2008 and 2015 which are producing 350 tonnes per year bio-oil from lignocellulosic biomass and 1 tonne per year bio-oil from plastics, respectively. Mura Technology is constructing the first commercial HTL plant for waste plastic in the UK using the Cat-HTR™ technology. Mura also aims to build a Cat-HTR™ technology-based advanced recycling plant in Japan in collaboration with Mitsubishi Chemicals. Licella is developing a Cat-HTR™ technology-based facility in Altona, Australia, for converting 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year plastics into liquid and gaseous hydrocarbon products, which has received approval from the Environmental Protection Agency Victoria.
000 tonnes per year plastics into liquid and gaseous hydrocarbon products, which has received approval from the Environmental Protection Agency Victoria.
Generally, hydrothermal processing has a low technology readiness level (TRL) of 4–6.5 when compared with a TRL of 6–9 for other thermochemical processes across the fossil and biofuels industries due to the need for higher capital investment to handle high amounts of water for processing.169–171 Hydrothermal process is a complex treatment generating multiple product phases and has substantial safety and operational challenges due to the high pressures employed in the process.
3. Integration potential of thermal technologies with anaerobic digestion
Anaerobic digestion (AD) is a well-established biochemical process in WWTPs for treating sewage sludge by degrading volatile organic solids and converting them into biogas leaving bio-recalcitrant residues as digestate. The integration of AD being a critical sludge treatment unit with thermal technologies such as pyrolysis, gasification, and hydrothermal processes can offer promising solutions for sludge volume reduction, bioenergy recovery, contaminant destruction, and valuable product generation.172 This integrated approach combines the strengths of biological and thermal treatments to achieve more efficient sludge management and resource recovery outcomes. Coupling AD upstream of thermal process offers a promising cascaded approach, converting the residual organic matter in the AD digestate into syngas, bio-oil, and biochar via thermal processes.173 The syngas can be used for energy production, and the organic phase of bio-oil can be used for electricity and heat, or upgraded to petroleum products or hydrogen fuel. Studies have demonstrated that integrating AD with the thermal process increases biogas and electricity production and reduces greenhouse gas emissions.174,175 There are several potential process configurations for integrating thermal processes downstream of AD for intensive treatment of wastewater sludge and their by-products. The different cases have been grouped into AD-gasification, AD-pyrolysis, AD-HTC, and AD-HTL, as well as their process variant as summarised in Fig. 8. The practical feasibility of the proposed integration will depend on the choice of thermal treatment technology, operating requirements, quality of the sewage sludge feed (physicochemical and biochemical properties), quality of side product streams, auxiliary processing requirements, and overall conversion efficiency.1763.1 Description of scenarios
3.2 Considerations for process integration
To determine the most efficient process integration from energy point of view, we modelled the various configurations shown in Fig. 8 (scenarios 1–8) focusing on mass and energy balance. The investigations considered the processing of 1000 kg (1 tonne) of mixed sludge (combination of primary sludge and waste activated sludge), which is the typical sludge stream being used in WWTPs for biogas production. The mixed sludge has 4.9% total solid content containing 80% volatile solids and 20% ash content. The study considered several combinations of AD with pyrolysis, low- and high-temperature gasification, HTC, and HTL. The volatiles generated during pyrolysis and gasification are combusted in the thermal oxidiser for energy recovery utilised in digestate drying, endothermic energy required for pyrolysis/gasification. Char was considered as the primary product of the thermal treatment process, and the biogas product from AD has not been combusted for energy recovery. The study also evaluated the possibility of recycling bio-oil and aqueous phase from pyrolysis and hydrothermal units for biogas production in the AD unit. The calculation only accounts for thermal energy and does not consider electricity consumption in the dewatering unit.The AD process in this study has several key parameters and considerations. First, at a temperature of 35 °C, 400 mL of biogas is produced per gram of volatile solids (mL g-VS−1) added. The biogas composition is 59 wt% CH4 and 41 wt% CO2, with a higher heating value (HHV) of 21.55 MJ Nm−3 and a density of 1.159 g L−1. Additionally, the study assumed that AD converts 75% of the pyrolysis oil and 75% of the HTL oil to biogas.183 The aqueous phase produced from HTC at 200 °C generates 400 mL of biogas per gram of COD when subjected to AD. However, the aqueous phase produced from HTL at 339 °C generates 120 mL of biogas per gram COD due to the accumulation of refractory compounds at higher temperatures, resulting in a lower biomethane potential than that of HTC.184 The hydrochar produced from the HTC can be anaerobically digested to produce 400 mL of biogas per g-VS added, while that from the liquefaction process was not digested.
The study also examined the dewatering process, which increases the total solids to 27% for pyrolysis/gasification and 12% for HTC/HTL. The reason for maintaining this level of water content is because hydrothermal treatment uses water as the medium of reaction, and having enough water content is necessary to ensure a favourable conversion.
As for pyrolysis, the study assumed that at a temperature of 500 °C, the product yield for biochar, bio-oil, and gas is 40%, 35%, and 25%, respectively. Additionally, it is assumed that the heat of pyrolysis would be around 1.8 MJ kg−1 which is in the range for similar biomass feedstock.185 In the case of gasification, the process is assumed to operate in energy-neutral mode (autothermal), which means that no external heat energy is required. At a low temperature of 600 °C, it is assumed that 35% of the feedstock will be converted to biochar, while 65% will be converted to syngas. Tar production is considered to be negligible at this temperature. At a higher temperature of 800 °C, it is assumed that the yield of biochar will decrease to 27%, while the yield of syngas will increase to 73%. Tar production is still assumed to be negligible.
The assumptions for the dryer are that all the water will be evaporated, and the specific heat capacity of water and sludge solids is 4.18 and 1.6 kJ kgK−1, respectively.186 Additionally, the latent heat of evaporation of water is assumed to be 2257 kJ kg−1.187 In the thermal oxidiser, the heating value of the mixed volatiles from pyrolysis is 20 MJ kg−1, while that of the non-condensable gases is 12 MJ kg−1.188 The syngas produced from low- and high-temperature gasification has a heating value of 6 and 8 MJ Nm−3, respectively,189 and the HTL gas product has a heating value of 2.84 MJ kg−1.
HTC at 200 °C for 30 min results in a yield of 67% hydrochar, 20% aqueous phase, 10% bio-oil, and 3% gas.190 The aqueous phase has a COD concentration of 21.5 g L−1,184 while the gas phase is mainly composed of CO2.191 The enthalpy of water was obtained from the literature189 and verified by the STEAMNBS method in Aspen Plus. In the HTL reaction, operating at 340 °C for 30 min results in an ash-free yield of 3.6% hydrochar, 40.2% oil, 34.6% aqueous phase, and 21.6% gas.192 The aqueous phase has a COD content of 41.2 g L−1 (ref. 184) and the gas phase is assumed to consist of 30 mol% H2 and 70 mol% CO2.193 The heat exchanger is also used to pre-heat the feedstock using the thermal energy of the product stream from the hydrothermal reactor. Exiting product stream temperature decreases to 100 °C and 170 °C after heat recovery in HTC and HTL scenarios, respectively.
3.3 Thermal energy performance of the integrated processes
Integrating different thermal treatment techniques with AD for primary sludge processing had different thermal energy efficiencies and environmental impacts. On the one hand, pyrolysis and gasification require a pre-dried feedstock, which adds to the energy requirements, while hydrothermal treatment does not need feedstock pre-drying. On the other hand, the requirement for high pressures in hydrothermal treatment poses a major disadvantage in terms of safety precautions and design complexity. The energy requirements across each unit operation and the overall thermal energy balance in all the scenarios investigated are summarised in Table 2. Notably, all scenarios investigated in this study resulted in a net positive energy balance ranging from 92.1 to 405.9 MJ per tonne of mixed sludge processed. It should be noted that the energy data are obtained from theoretical calculations based on certain assumptions, regarding the product yield and biomethane production potential of different organic substrates. This range of energy balance data need to be validated through experimental investigations involving the integration of various thermal processes and AD, as there are limited pilot- and industrial-scale studies reported. Nevertheless, based on the net energy balance (MJ), the energy integration efficiency of AD with thermal techniques can be ranked as high temperature gasification (405.9) > low temperature gasification (316.3) > pyrolysis (268.9) > HTL (150) > HTC (92.1). Gasification downstream of AD (scenario 3 and 4) gave the highest net positive energy due to their autothermal operation and the generation of high-volume and high-quality syngas for energy recovery, albeit at the expense of quality char product. In contrast, AD upstream of HTC/HTL (scenarios 7 and 8) gave the least net positive energy due to the low organic matter conversion, yielding low volumes of energy-carrying volatiles and gas streams. Pyrolysis generates a net positive energy value (192.4–268.9 MJ), which is in between that of gasification (316.3–405.9 MJ) and hydrothermal (228.3–92.1 MJ)-integrated processes (Table 2). This was attributed to the moderate conversion and generation of volatiles, which are either combusted for energy recovery (as in scenario 1) or condensed as bio-oil and partly recycled as organic substrates to the AD unit (as in scenario 2).| Scenarios | AD | Dryer | HTC | HTL | Pyrolysis | Gasification | Oxidiser | Balance | |
|---|---|---|---|---|---|---|---|---|---|
| a All values are expressed in MJ for processing 1000 kg (1 tonne) of mixed sludge at 4.9% TS. | |||||||||
| 1 | AD-pyrolysis | +232.7 | −108.0 | — | — | −390.9 | — | +183.2 | +268.9 |
| 2 | AD-pyrolysis (recycled bio-oil) | +286.0 | −108.0 | — | — | −390.9 | — | +534.5 | +192.4 |
| 3 | AD-low temp. Gasification | +232.7 | −108.0 | — | — | — | 0 | +191.6 | +316.3 |
| 4 | AD-high temp. gasification | +232.7 | −108.0 | — | — | — | 0 | +281.2 | +405.9 |
| 5 | AD-HTL-(recycled bio-oil) | +303.9 | — | — | −826.6 | — | — | +698.6 | +228.3 |
| 6 | AD-HTC-(recycled aqueous phase) | +249.4 | — | −434.4 | — | — | — | — | +206.0 |
| 7 | HTL-AD | +244.6 | — | — | −253.8 | — | — | +242.3 | +150.1 |
| 8 | HTC-AD | +216.9 | — | −124.8 | — | — | — | — | +92.1 |
Gasification gave the highest net positive energy value available for use in non-process heating or for grid integration. The process produced low yield and low-quality char compared to the char product from pyrolysis due to the partial oxidation of the biochar carbon. Pyrolysis sequesters carbon in biochar with a higher calorific value, suggesting that the combustion of biochar will add to the overall net energy value as in scenarios 1 and 2. In addition, the economic attractiveness of biochar from pyrolysis is higher than that from gasification considering the widely demonstrated applications of biochar, thanks to their physicochemical and structural properties.194 The recycling of pyrolysis bio-oil to anaerobic digestion could help convert inherent nitrogenated compounds into biogas, a cleaner form of energy compared to bio-oil. However, the recycling of bio-oil resulted in a decrease in the total energy balance since 75% of bio-oil is converted to a biogas stream containing 41% CO2 (as in scenario 2). Meanwhile, in scenario 1 without bio-oil recycle, the use of thermal oxidiser for bio-oil combustion may result in the production of nitrogen oxides (NOx) in the flue gas. This is an important consideration in terms of environmental impact, as NOx can contribute to air emissions. Therefore, downstream gas treatment in the scrubbing unit may be necessary to reduce NOx contents in the flue gas released into the environment.
The HTL/HTC downstream AD with recycling of bio-oil/aqueous phase (scenarios 5 and 6) shows a slightly lower energy output than that of pyrolysis (Table 2). However, the partial return of one of the liquid product streams to the upstream AD unit increases the biogas production volume, thereby generating a higher net surplus energy than in scenarios 7 and 8. This integrated process is expected to have a less emission load, particularly SOx and NOx in the gas stream due to the milder treatment conditions producing negligible volume of bio-oil for combustion. Moreover, hydrochar produced by this method has a better quality and economic value than pyrolysis and gasification char due to the preservation of surface functional groups and energy densification at low to medium temperatures. The last two scenarios involving hydrothermal processes before AD showed the lowest net surplus energy (92.1–150.1 MJ), attributed to the energy-intensive nature of processing a large volume of wet feedstock containing 88 wt% water to elevated temperatures and pressure. However, all the generated product streams, namely hydrochar, aqueous phase, bio-oil, and gas, are available for use in their raw or refined form, which can increase the economic prospects of these integrated configurations (HTC/HTL-AD).
In selecting an optimum process configuration for treating wet sewage sludge via integrated AD and thermal techniques, it is important to consider various factors, such as surplus energy available, environmental footprint, and the quality of end products (economic potential). Careful consideration of these factors holistically is crucial to achieve an effective, efficient, and sustainable treatment of wet sewage sludge. Combining AD and thermal processes presents a promising pathway for maximizing energy recovery from sewage sludge while generating value-added products such as biochar, syngas, nutrient-enriched aqueous phase, bio-oil, and platform chemicals. Other potential synergies of coupling AD upstream of thermal units include:
1. The utilisation of dewatered and dried digestates as thermal/hydrothermal feedstock can eliminate microbial and organic contaminants without the need for traditional sludge stabilisation methods.
2. Increased overall plant capacity by reducing the solid residence time in the digester by directly feeding biosolids to the downstream thermal unit (gasification/pyrolysis/hydrothermal).
3. Efficient utilisation of process heat from thermal units in other processes like anaerobic digestion, biosolids drying, and gas upgrading.
4. Pre-separation of soluble plant nutrients, such as potassium and sulfur, into the liquid fraction, mitigating corrosion and deposition issues in thermal gasification/pyrolysis.
5. Application of the thermal process char product (biochar/ash/hydrochar), which contains macro- and micro-nutrients (N, P, and K), as fertiliser and soil amendment.
6. Diversion of condensation tar and hydrothermal process water to the digester, enhancing the overall gas yield.
7. Elimination of direct gaseous emissions from biosolids stockpiling and land application, contributing to environmental sustainability.
8. The integrated approach enhances waste-to-energy conversion, improves resource efficiency, and promotes a circular economy model for the water industry.
4. Perspectives for future works
1. Development of technology assessment tool
While qualitative comparisons can be established from the current study and many other similar literature reviews, a detailed benchmarking, which is crucial for the technology/process selection, must be developed. The optimum technology/process configuration may be site specific. Therefore, developing a technology assessment tool covering technology and site-specific parameters is crucial.2. Advances in the technologies
Commercial thermal technologies suffer from a range of bottlenecks such as poor heat and mass transfer, limited scalability and higher capital and operating costs. Future research efforts should be carried out to improve the reactor design to address the above-mentioned bottlenecks.3. Pilot-scale and industrial-scale studies
Limited data are available in the open literature on large-scale trials including pilot plants, demonstration production plants and industrial-scale studies of biosolids thermal treatment. The availably of data from large-scale field trials is essential to understand the range of technical, environmental, and techno-commercial challenges associated with biosolids thermal treatment. This will help improving the fundamental understanding and adoption of these technologies for biosolids management in water industries.4. Advances in energy and process modelling
Energy and resource recovery estimates published in the literature vary significantly. One of the reasons may be the empirical nature of the published models. Efforts should be made to derive process models based on the first principles to accurately predict product distribution, emission profile, mass and energy balance, and heat recovery potential.5. Fundamental understanding of contaminant destruction/removal
Though the volume of work published on fate of contaminants during the thermal treatment of biosolids is on the rapid increase, data on the fate and transport of multiple contaminants (as inherent in biosolids) across all effluent streams are still sparsely available. Siloxane has been identified as an emerging contaminant of concern during biosolids' thermal treatment and requires deeper investigations. The studies in the literature have mainly focused on the fate of contaminants in the solid stream, mostly biochar. Therefore, extensive research is required to understand the fate and distribution of contaminants in other product streams including liquid (scrubber effluent) and gaseous streams (flue gas and stack gas), during the thermal process, and evaluate their concentrations in line with relevant environmental regulation guidelines. Thermodynamic equilibrium calculations of contaminants using computational software can provide a good understanding, though the calculations are not based on real-time conditions. This will help to take appropriate measures to overcome the environmental and other practical challenges during the design and operation of scale-up facilities.6. Life cycle analysis
Many studies have focused on the life cycle assessment (LCA) of thermal technologies for energy recovery from different wastes. However, only a handful of studies considered different sludge compositions in LCA. It is worth noting that biosolids composition can significantly affect the technical and environmental performance of the thermal technology. Therefore, future research should focus on the comprehensive LCA of biosolids thermal processing, considering the goals of water industries including volume reduction, contaminant reduction, biochar production, and bioelectricity generation, for biosolids transformation, considering spatial and temporal variation in wastewater treatment process and biosolids production.5. Conclusions
The following key conclusions have been reached from this critical review.1. Drying is a critical thermal treatment process for biosolids management either as a standalone biosolids stabilisation method or as a pre-treatment step for high-temperature thermal processes. Dryers are necessary for drying and/or pelletising biosolids (if necessary) before feeding into incineration, pyrolysis and gasification reactors. The viability of indirect dryers for the thermal drying of biosolids is enhanced by energy efficiency improvements, exhaust management, and the ability to capture and utilise evaporated moisture. Thermally dried and pelletised biosolids products are potentially saleable, and a market has been established particularly in the US. However, the thermal drying of biosolids alone without high thermal treatment via pyrolysis or gasification is not sufficient to remove persistent organic contaminants in biosolids.
2. Although incineration is a well-established thermal treatment technique for biosolids management, it has been and will continue to be challenged by emission control. In addition, incineration does not allow for beneficial carbon sequestration from biosolids in the form of biochar. The solid-phase combustion process and the extensive nature of gas cleaning and emission capture equipment increase the capital and operational costs and reduce the thermal efficiency. Incinerators need external energy for startup and require sludge cake solids of 28–32% fed into a dryer to avoid any external input of thermal energy for the process.
3. Thermal treatment, particularly pyrolysis, should be regarded as an advanced, thermally efficient technique for organic pollutant destruction methods rather than a resource recovery platform. Biosolids pyrolysis at 500 °C integrated with the combustion of the pyrolysis volatile products can make pyrolysis plants achieve thermal breakeven (autothermal operations) with 22–23% cake solids. In addition, emissions can be more readily and economically controlled since combustion occurs in the gas phase at high temperatures.
4. Hydrothermal liquefaction (HTL) produces a hydro-oil that is usable than recovered pyrolysis oil but requires high temperatures and pressures (though in a small footprint due to the liquid nature of the process and less rigorous back-end gas cleaning units). The main barrier to HTL is the low technological readiness level and high capital and operating cost due to the need for specialised construction material for the reactor suitable to handle the process autogenous pressure.
5. Biochar and hydrochar are usable products, mainly for agriculture. Pyrolysis char has stable carbon structures, while hydrochar will break down, making it unsuitable for carbon fixation in soils. Both biochar and hydrochar from biosolids thermal treatment have several non-agricultural applications.
6. The integration of AD with thermal treatment processes offers beneficial synergy in organic matter conversion, energy recovery, and valuable product generation (biogas, biochar, and bioenergy). The most advanced integration of AD with thermal processes from thermal energy and contaminant destruction perspectives is AD-gasification and AD-pyrolysis. However, the integration of AD with hydrothermal treatment (HTL/HTC) can be highly favourable for sewage sludge treatment given the milder processing conditions. However, this type of configuration might require extensive downstream secondary treatment of the products.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work is supported by the Australian Research Council (ARC) Training Centre for the Transformation of Australia's Biosolids Resource, RMIT University, Australia, under the ARC Industrial Transformation Training Centre scheme (grant number IC190100033).References
- J. L. Cárdenas-Talero, J. A. Silva-Leal, A. Pérez-Vidal and P. Torres-Lozada, The Influence of Municipal Wastewater Treatment Technologies on the Biological Stabilization of Sewage Sludge: A Systematic Review, Sustainability, 2022, 14, 5910 CrossRef.
- S. Werle and S. Sobek, Gasification of sewage sludge within a circular economy perspective: a Polish case study, Environ. Sci. Pollut. Res., 2019, 26, 35422–35432 CrossRef CAS.
- T. Sharma, I. G. Hakeem, A. B. Gupta, J. Joshi, K. Shah, A. K. Vuppaladadiyam and A. Sharma, Parametric influence of process conditions on thermochemical techniques for biochar production: A state-of-the-art review, J. Energy Inst., 2024, 113, 101559 CrossRef CAS.
- ANZBP, Biosolids Production and End Use Survey – Australia 2022/23, 2024 Search PubMed.
- USEPA, Sewage Sludge Laws and Regulations, 2025 Search PubMed.
- D. Koumoulidis, I. Varvaris, Z. Pittaki and D. Hadjimitsis, Sewage Sludge in Agricultural Lands: The Legislative Framework in EU-28, Sustainability, 2024, 16, 10946 CrossRef CAS.
- S. Marchuk, S. Tait, P. Sinha, P. Harris, D. L. Antille and B. K. McCabe, Biosolids-derived fertilisers: A review of challenges and opportunities, Sci. Total Environ., 2023, 875, 162555 CrossRef CAS PubMed.
- A. M. Elgarahy, M. Eloffy, A. Priya, V. Yogeshwaran, Z. Yang, K. Z. Elwakeel and E. A. Lopez-Maldonado, Biosolids management and utilizations: A review, J. Cleaner Prod., 2024, 451, 141974 CrossRef CAS.
- ANZBP, Australian Biosolids Statistics, 2023 Search PubMed.
- W. Shu, G. Price, K. Khosravi and C. Metcalfe, Presence and accumulation of pharmaceutical compounds in agricultural soils receiving six years of biosolids applications, Environ. Pollut., 2025, 126736 CrossRef CAS.
- E. A. Pozzebon and L. Seifert, Emerging environmental health risks associated with the land application of biosolids: a scoping review, Environ. Health, 2023, 22, 57 CrossRef PubMed.
- J. Xue, W. Verstraete, B.-J. Ni, J. P. Giesy, G. Kaur, D. Jiang, E. McBean, Z. Li, H.-M. Shin, F. Xiao, Y. Liu, J. Liu, L. Chibwe, K. T. Wai Ng and Y. Uchida, Rethink biosolids: Risks and opportunities in the circular economy, Chem. Eng. J., 2025, 510, 161749 CrossRef CAS.
- J. Xue, W. Verstraete, B.-J. Ni, J. P. Giesy, G. Kaur, D. Jiang, E. McBean, Z. Li, H.-M. Shin and F. Xiao, Rethink biosolids: Risks and opportunities in the circular economy, Chem. Eng. J., 2025, 510, 161749 CrossRef CAS.
- E. E. Manea, C. Bumbac, L. R. Dinu, M. Bumbac and C. M. Nicolescu, Composting as a sustainable solution for organic solid waste management: Current practices and potential improvements, Sustainability, 2024, 16, 6329 CrossRef CAS.
- A. G. Capodaglio and A. Callegari, Energy and resources recovery from excess sewage sludge: A holistic analysis of opportunities and strategies, Resour. Conserv. Recycl. Adv., 2023, 19, 200184 Search PubMed.
- S. Patel, S. Kundu, P. Halder, N. Ratnnayake, M. H. Marzbali, S. Aktar, E. Selezneva, J. Paz-Ferreiro, A. Surapaneni and C. C. de Figueiredo, A critical literature review on biosolids to biochar: an alternative biosolids management option, Rev. Environ. Sci. Bio/Technol., 2020, 1–35 Search PubMed.
- S. S. A. Syed-Hassan, Y. Wang, S. Hu, S. Su and J. Xiang, Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations, Renewable Sustainable Energy Rev., 2017, 80, 888–913 CrossRef CAS.
- N. Gao, K. Kamran, C. Quan and P. T. Williams, Thermochemical conversion of sewage sludge: A critical review, Prog. Energy Combust. Sci., 2020, 79, 100843 CrossRef.
- M. Hu, Z. Ye, H. Zhang, B. Chen, Z. Pan and J. Wang, Thermochemical conversion of sewage sludge for energy and resource recovery: technical challenges and prospects, Environ. Pollut. Bioavailability, 2021, 33, 145–163 CrossRef CAS.
- S. Nanda and F. Berruti, A technical review of bioenergy and resource recovery from municipal solid waste, J. Hazard. Mater., 2021, 403, 123970 CrossRef CAS PubMed.
- L. Wang, Y. Chang and A. Li, Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review, Renewable Sustainable Energy Rev., 2019, 108, 423–440 CrossRef CAS.
- X. Zhang, X. Li, R. Li and Y. Wu, Hydrothermal Carbonization and Liquefaction of Sludge for Harmless and Resource Purposes: A Review, Energy Fuels, 2020, 34, 13268–13290 CrossRef CAS.
- S. Patel, S. Kundu, P. Halder, N. Ratnnayake, M. H. Marzbali, S. Aktar, E. Selezneva, J. Paz-Ferreiro, A. Surapaneni and C. C. de Figueiredo, A critical literature review on biosolids to biochar: an alternative biosolids management option, Rev. Environ. Sci. Bio/Technol., 2020, 19, 807–841 CrossRef.
- J. Nylen and M. Sheehan, Review of the integration of drying and thermal treatment processes for energy efficient reduction of contaminants and beneficial reuse of wastewater treatment plant biosolids, Energies, 2023, 16, 1964 CrossRef CAS.
- M. H. Marzbali, I. G. Hakeem, T. Ngo, R. Balu, M. K. Jena, A. Vuppaladadiyam, A. Sharma, N. R. Choudhury, D. J. Batstone and K. Shah, A critical review on emerging industrial applications of chars from thermal treatment of biosolids, J. Environ. Manage., 2024, 369, 122341 CrossRef.
- A. K. Vuppaladadiyam, M. K. Jena, I. G. Hakeem, S. Patel, G. Veluswamy, A. V. Thulasiraman, A. Surapaneni and K. Shah, A critical review of biochar versus hydrochar and their application for H2S removal from biogas, Rev. Environ. Sci. Bio/Technol., 2024, 23, 699–737 CrossRef CAS.
- M. Schnell, T. Horst and P. Quicker, Thermal treatment of sewage sludge in Germany: A review, J. Environ. Manage., 2020, 263, 110367 CrossRef CAS PubMed.
- I. Zbicinski, K. Ciesielski and B. Ge, Mechanism of particle agglomeration for single and multi-nozzle atomization in spray drying: A review, Processes, 2022, 10, 727 CrossRef.
- V. Mislej and B. Novosel, Specification and Classification of Pelletised Dried Sewage Sludge: Identifying Its Key Properties as a Renewable Material for Enabling Environmentally Non-Harmful Energy Utilisation, Sustainability, 2022, 14, 14836 CrossRef CAS.
- USEPA, Biosolids Technology Fact Sheet: Heat Drying, United States Environmental Protection Agency, 2006, pp. 1–13, EPA 832-F-06-029 Search PubMed.
- G. Chen, P. Lock Yue and A. S. Mujumdar, Sludge dewatering and drying, Drying Technol., 2002, 20, 883–916 CrossRef CAS.
- J. Boguniewicz-Zablocka, I. Klosok-Bazan and A. G. Capodaglio, Sustainable management of biological solids in small treatment plants: overview of strategies and reuse options for a solar drying facility in Poland, Environ. Sci. Pollut. Res., 2021, 28, 24680–24693 CrossRef PubMed.
- D. Đurđević, P. Blecich and Ž. Jurić, Energy recovery from sewage sludge: The case study of Croatia, Energies, 2019, 12, 1927 CrossRef.
- B. Treviño Arjona and J. R. Cisneros, presented in part at the Third LACCEI International Latin American and Caribbean Conference for Engineering and Technology (LACCET’2005), 8 june 2005, 2005 Search PubMed.
- M. O. Belloulid, H. Hamdi, L. Mandi and N. Ouazzani, Solar drying of wastewater sludge: a case study in Marrakesh, Morocco, Environ. Technol., 2019, 40, 1316–1322 CrossRef CAS PubMed.
- S. B. Tiwari, W. P. Chan, A. Veksha, G. Lisak and T.-T. Lim, Advancing insights into phosphorus transformation during thermochemical treatment of sewage sludge, J. Environ. Manage., 2025, 391, 126505 CrossRef CAS PubMed.
- I. Deviatkin, L. Lyu, S. Chen, J. Havukainen, F. Wang, M. Horttanainen and M. Mänttäri, Technical implications and global warming potential of recovering nitrogen released during continuous thermal drying of sewage sludge, Waste Manage., 2019, 90, 132–140 CrossRef CAS.
- D. Szypulska, Ł. Kokurewicz, B. Zięba, S. Miodoński, M. Muszyński-Huhajło, A. Jurga and K. Janiak, Impact of the thermal drying of sludge on the nitrogen mass balance of a WWTP, and GHG emissions with classical and novel treatment approach-A full-scale case study, J. Environ. Manage., 2021, 294, 113049 CrossRef CAS.
- S. O'Shaughnessy, I. Song, J. Artiola and C. Choi, Nitrogen loss during solar drying of biosolids, Environ. Technol., 2008, 29, 55–65 CrossRef.
- M. H. Romdhana, D. Lecomte, B. Ladevie and C. Sablayrolles, Monitoring of pathogenic microorganisms contamination during heat drying process of sewage sludge, Process Saf. Environ. Prot., 2009, 87, 377–386 CrossRef CAS.
- Q. Le and G. Price, A review of the influence of heat drying, alkaline treatment, and composting on biosolids characteristics and their impacts on nitrogen dynamics in biosolids-amended soils, Waste Manage., 2024, 176, 85–104 CrossRef CAS.
- M. Gomez-Rico, A. Fullana and R. Font, Volatile organic compounds released from thermal drying of sewage sludge, WIT Trans. Ecol. Environ., 2008, 111, 425–433 CAS.
- X. Zhu, X. Yang, W. Gao, R. Jiao, S. Zhao, J. Yu and D. Wang, Effect of low-temperature thermal drying on malodorous volatile organic compounds (MVOCs) emission of wastewater sludge: The relationship with microbial communities, Environ. Pollut., 2022, 306, 119423 CrossRef CAS PubMed.
- E. F. Shanahan, A. Roiko, N. W. Tindale, M. P. Thomas, R. Walpole and D. İ. Kurtböke, Evaluation of pathogen removal in a solar sludge drying facility using microbial indicators, Int. J. Environ. Res. Public Health, 2010, 7, 565–582 CrossRef CAS PubMed.
- W. Yang, C. Cai, Y. Guo, H. Wu, Y. Guo and X. Dai, Diversity and fate of human pathogenic bacteria, fungi, protozoa, and viruses in full-scale sludge treatment plants, J. Cleaner Prod., 2022, 380, 134990 CrossRef CAS.
- T. Dziok, M. Bury and P. Burmistrz, Mercury release from municipal solid waste in the thermal treatment process, Fuel, 2022, 329, 125528 CrossRef CAS.
- P. J. McNamara, J. Calteux, E. Redman, T. McKnight, L. Moss, W. Hoener, S. Carr and Z. Liu, Drying reduces the total PFAS concentration in biosolids and alters the PFAS profile, Environ. Sci.: Water Res. Technol., 2025, 11, 1007–1015 RSC.
- I. G. Hakeem, P. Halder, S. Patel, E. Selezneva, N. Rathnayake, M. H. Marzbali, G. Veluswamy, A. Sharma, S. Kundu and A. Surapaneni, Current understanding on the transformation and fate of per-and polyfluoroalkyl substances before, during, and after thermal treatment of biosolids, Chem. Eng. J., 2024, 493, 152537 CrossRef CAS.
- A. Rahman, S. Grieco, B. Bahman, A. Friedenthal, A. White and T. Williams, Understanding dynamics of PFAS in biosolids processed through composting, thermal drying and high temperature pyrolysis, J. Water Process Eng., 2025, 72, 107508 CrossRef.
- USEPA, Biosolids Technology Fact Sheet, 2006 Search PubMed.
- P. Peltola, L. Ruottu, M. Larkimo, A. Laasonen and K. Myöhänen, A novel dual circulating fluidized bed technology for thermal treatment of municipal sewage sludge with recovery of nutrients and energy, Waste Manage., 2023, 155, 329–337 CrossRef CAS PubMed.
- J. Kim and S. Jeong, Economic and environmental cost analysis of incineration and recovery alternatives for flammable industrial waste: The case of South Korea, Sustainability, 2017, 9, 1638 CrossRef.
- USEPA, Biosolids Technology Fact Sheet: Use of Incineration for Biosolids Management, United States Environmental Protection Agency, 2003, pp. 1–14, EPA 832-F-03-013 Search PubMed.
- M. C. Collivignarelli, A. Abbà, A. Frattarola, M. Carnevale Miino, S. Padovani, I. Katsoyiannis and V. Torretta, Legislation for the reuse of biosolids on agricultural land in Europe: Overview, Sustainability, 2019, 11, 6015 CrossRef CAS.
- NEBRA, Incineration/Thermal Conversion, North East Biosolids and Residual Association, 2020 Search PubMed.
- S. Schwarz, T. J. Overcamp and T. M. Keinath, An Economic Evaluation of Indirect Sludge Drying Prior to Incineration Processes, Res. J. Water Pollut. Control Fed., 1990, 62, 275–281 CAS.
- S. Donatello and C. R. Cheeseman, Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review, Waste Manage., 2013, 33, 2328–2340 CrossRef CAS PubMed.
- USEPA, Methodology for Thermal Efficiency and Energy Input Calculations and Analysis of Biomass Cogeneration Unit Characteristics, USEPA, Washington, DC, 2007 Search PubMed.
- W. Barber, Observing the Effects of Digestion and Chemical Dosing on the Calorific Value of Sewage Sludge, 2007 Search PubMed.
- V. Singh, H. C. Phuleria and M. K. Chandel, Estimation of energy recovery potential of sewage sludge in India: Waste to watt approach, J. Cleaner Prod., 2020, 276, 122538 CrossRef.
- M. Hasan, Y. Haseli and E. Karadogan, Correlations to Predict Elemental Compositions and Heating Value of Torrefied Biomass, Energies, 2018, 11, 2443 CrossRef.
- C.-Y. Yin, Prediction of higher heating values of biomass from proximate and ultimate analyses, Fuel, 2011, 90, 1128–1132 CrossRef CAS.
- S. A. Channiwala and P. P. Parikh, A unified correlation for estimating HHV of solid, liquid and gaseous fuels, Fuel, 2002, 81, 1051–1063 CrossRef CAS.
- C. Schaum, D. Lensch and P. Cornel, Evaluation of the energetic potential of sewage sludge by characterization of its organic composition, Water Sci. Technol., 2016, 73, 3072–3079 CrossRef CAS PubMed.
- H. Kobayashi, A. Hayakawa, K. K. A. Somarathne and E. C. Okafor, Science and technology of ammonia combustion, Proc. Combust. Inst., 2019, 37, 109–133 CrossRef CAS.
- M. Anderson, Encouraging prospects for recycling incinerated sewage sludge ash (ISSA) into clay-based building products, J. Chem. Technol. Biotechnol., 2002, 77, 352–360 CrossRef CAS.
- N. R. Council, in Waste incineration & public health, National Academies Press (US), 2000 Search PubMed.
- J. M. Park, S. B. Lee, J. P. Kim, M. J. Kim, O. S. Kwon and D. I. Jung, Behavior of PAHs from sewage sludge incinerators in Korea, Waste Manage., 2009, 29, 690–695 CrossRef CAS.
- L. Lundin and S. Jansson, A desktop study on destruction of persistent organic compounds in combustion systems, Swedish Environmental Protection Agency, 2017 Search PubMed.
- J. A. Conesa, Sewage sludge as inhibitor of the formation of persistent organic pollutants during incineration, Sustainability, 2021, 13, 10935 CrossRef CAS.
- D. Wenyi, Y. Jianhua, L. Xiaodong, W. Fei, C. Yong and L. Shengyong, Emission characteristics of dioxins, furans and polycyclic aromatic hydrocarbons during fluidized-bed combustion of sewage sludge, J. Environ. Sci., 2009, 21, 1747–1752 CrossRef.
- D. Vamvuka, S. Alexandrakis and M. Galetakis, Combustion performance of sludge from a wastewater treatment plant in fluidized bed, Factorial modeling and optimization of emissions, Front. Energy Res., 2019, 7, 43 CrossRef.
- S. Reuna and A. Väisänen, To incinerate or not?–Effects of incineration on the concentrations of heavy metals and leaching efficiency of post-precipitated sewage sludge (RAVITA™), Waste Manage., 2020, 118, 241–246 CrossRef CAS.
- D. Pudasainee, Y.-C. Seo, J.-H. Kim and H.-N. Jang, Fate and behavior of selected heavy metals with mercury mass distribution in a fluidized bed sewage sludge incinerator, J. Mater. Cycles Waste Manage., 2013, 15, 202–209 CrossRef CAS.
- I. Gulyurtlu, M. H. Lopes, P. Abelha, I. Cabrita and J. S. Oliveira, The study of partitioning of heavy metals during fluidized bed combustion of sewage sludge and coal, J. Energy Resour. Technol., 2006, 128(2), 104–110 CrossRef CAS.
- P. E. Escamilla-García, R. H. Camarillo-López, R. Carrasco-Hernández, E. Fernández-Rodríguez and J. M. Legal-Hernández, Technical and economic analysis of energy generation from waste incineration in Mexico, Energy Strat. Rev., 2020, 31, 100542 CrossRef.
- E. H. Farzad Bazdidi Tehrani, presented in part at the The First Sustainable Development of Engineering Systems in Energy, Water, and Environment. A, 21/05/2015, 2015 Search PubMed.
- S. Kaza and P. Bhada-Tata, Decision maker's guides for solid waste management technologies, 2018 Search PubMed.
- A. Kumar and S. R. Samadder, A review on technological options of waste to energy for effective management of municipal solid waste, Waste Manage., 2017, 69, 407–422 CrossRef CAS PubMed.
- F. Mercl, Z. Košnář, P. Maršík, M. Vojtíšek, J. Dušek, J. Száková and P. Tlustoš, Pyrolysis of biosolids as an effective tool to reduce the uptake of pharmaceuticals by plants, J. Hazard. Mater., 2021, 405, 124278 CrossRef CAS.
- L. J. Winchell, J. J. Ross, D. A. Brose, T. B. Pluth, X. Fonoll, J. W. Norton Jr and K. Y. Bell, Pyrolysis and gasification at water resource recovery facilities: Status of the industry, Water Environ. Res., 2022, 94, e10701 CrossRef PubMed.
- N. Rathnayake, S. Patel, I. G. Hakeem, G. Veluswamy, I. Al-Waili, S. Agnihotri, A. K. Vuppaladadiyam, A. Surapaneni, D. Bergmann and K. Shah, The pyrolysis of biosolids in a novel closed coupled pyrolysis and gasification technology: pilot plant trials, Aspen plus modelling, and a techno-economic analysis, Water, 2024, 16, 3399 CrossRef CAS.
- Y. Sun, Y. Zuo, Y. Shao, L. Wang, L.-M. Jiang, J. Hu, C. Zhou, X. Lu, S. Huang and Z. Zhou, Carbon footprint analysis of wastewater treatment processes coupled with sludge in situ reduction, Water Res.: X, 2024, 24, 100243 CAS.
- M. Hu, J. Ma, Z. Jiang, J. Wang, Z. Pan, Z.-T. Hu, S. Tang, R. Beims and C. Xu, New insights into nitrogen control strategies in sewage sludge pyrolysis toward environmental and economic sustainability, Sci. Total Environ., 2023, 882, 163326 CrossRef CAS PubMed.
- J.-P. Cao, X.-Y. Zhao, K. Morishita, X.-Y. Wei and T. Takarada, Fractionation and identification of organic nitrogen species from bio-oil produced by fast pyrolysis of sewage sludge, Bioresour. Technol., 2010, 101, 7648–7652 CrossRef CAS.
- L. Wei, L. Wen, T. Yang and N. Zhang, Nitrogen Transformation during Sewage Sludge Pyrolysis, Energy Fuels, 2015, 29, 5088–5094 CrossRef CAS.
- I. G. Hakeem, P. Halder, S. Patel, A. Sharma, R. Gupta, A. Surapaneni, J. Paz-Ferreiro and K. Shah, Enhancing the pyrolytic conversion of biosolids to value-added products via mild acid pre-treatment, J. Anal. Appl. Pyrolysis, 2023, 173, 106087 CrossRef CAS.
- J. Kooij, P.-T. Yang, S. Bruun, J. Magid, U. G. Nielsen, L. T. Kuhn and D. Müller-Stöver, Phosphorus speciation in different sewage sludges and their biochars and its implications for movement of labile phosphate in two soils, J. Environ. Manage., 2024, 370, 122565 CrossRef CAS PubMed.
- Y. Xiao, R. Zhao, Z. Xiong, X. Li, J. Chen and H. Yao, Transformation of phosphorous during incineration of sewage sludge: Influence of steam and mineral, Fuel, 2021, 303, 121307 CrossRef CAS.
- X. Wang, Q. Chen, B. Wei, G. Yu, H. Li, X. Chen, H. Liu and F. Wang, Volatile potassium transfer behavior in carbon matrix and its effect on char gasification, Fuel, 2023, 333, 126302 CrossRef CAS.
- M. Novotný, M. Marković, J. Raček, M. Šipka, T. Chorazy, I. Tošić and P. Hlavínek, The use of biochar made from biomass and biosolids as a substrate for green infrastructure: A review, Sustainable Chem. Pharm., 2023, 32, 100999 CrossRef.
- S. Aktar, M. A. Hossain, N. Rathnayake, S. Patel, G. Gasco, A. Mendez, C. de Figueiredo, A. Surapaneni, K. Shah and J. Paz-Ferreiro, Effects of temperature and carrier gas on physico-chemical properties of biochar derived from biosolids, J. Anal. Appl. Pyrolysis, 2022, 164, 105542 CrossRef CAS.
- I. G. Hakeem, P. Halder, C. C. Dike, K. Chiang, A. Sharma, J. Paz-Ferreiro and K. Shah, Advances in biosolids pyrolysis: Roles of pre-treatments, catalysts, and co-feeding on products distribution and high-value chemical production, J. Anal. Appl. Pyrolysis, 2022, 166, 105608 CrossRef CAS.
- J. Moško, M. Pohořelý, T. Cajthaml, M. Jeremiáš, A. A. Robles-Aguilar, S. Skoblia, Z. Beňo, P. Innemanová, L. Linhartová and K. Michalíková, Effect of pyrolysis temperature on removal of organic pollutants present in anaerobically stabilized sewage sludge, Chemosphere, 2021, 265, 129082 CrossRef PubMed.
- I. G. Hakeem, S. Agnihotri, S. Patel, G. Veluswamy, N. Rathnayake, A. K. Vuppaladadiyam, I. Al-Waili, S. Pabba, A. V. Thulasiraman and A. Surapaneni, The pyrolysis of biosolids in a novel fluidized bed heat exchanger reactor: pilot plant trials, biochar properties, gas emissions testing, and fate of PFAS, J. Anal. Appl. Pyrolysis, 2025, 107343 Search PubMed.
- M. J. Bentel, Y. Yu, L. Xu, Z. Li, B. M. Wong, Y. Men and J. Liu, Defluorination of Per- and Polyfluoroalkyl Substances (PFASs) with Hydrated Electrons: Structural Dependence and Implications to PFAS Remediation and Management, Environ. Sci. Technol., 2019, 53, 3718–3728 CrossRef CAS.
- B.-J. Ni, Z.-R. Zhu, W.-H. Li, X. Yan, W. Wei, Q. Xu, Z. Xia, X. Dai and J. Sun, Microplastics mitigation in sewage sludge through pyrolysis: the role of pyrolysis temperature, Environ. Sci. Technol. Lett., 2020, 7, 961–967 CrossRef CAS.
- H. Bamdad, S. Papari, E. Moreside and F. Berruti, High-temperature pyrolysis for elimination of per-and polyfluoroalkyl substances (PFAS) from biosolids, Processes, 2022, 10, 2187 CrossRef CAS.
- E. D. Thoma, R. S. Wright, I. George, M. Krause, D. Presezzi, V. Villa, W. Preston, P. Deshmukh, P. Kauppi and P. G. Zemek, Pyrolysis processing of PFAS-impacted biosolids, a pilot study, J. Air Waste Manage. Assoc., 2022, 72, 309–318 CrossRef CAS PubMed.
- P. McNamara, M. S. Samuel, S. Sathyamoorthy, L. Moss, D. Valtierra, H. C. Lopez, N. Nigro, S. Somerville and Z. Liu, Pyrolysis transports, and transforms, PFAS from biosolids to py-liquid, Environ. Sci.: Water Res. Technol., 2023, 9, 386–395 RSC.
- E. Sørmo, G. Castro, M. Hubert, V. Licul-Kucera, M. Quintanilla, A. G. Asimakopoulos, G. Cornelissen and H. P. H. Arp, The decomposition and emission factors of a wide range of PFAS in diverse, contaminated organic waste fractions undergoing dry pyrolysis, J. Hazard. Mater., 2023, 454, 131447 CrossRef.
- J. Ross, D. Zitomer, T. Miller, C. Weirich and P. J. McNamara, Emerging investigators series: Pyrolysis removes common microconstituents triclocarban, triclosan, and nonylphenol from biosolids, Environ. Sci.: Water Res. Technol., 2016, 2, 282–289 RSC.
- A. Mohseni-Bandpei, M. Majlesi, M. Rafiee, S. Nojavan, P. Nowrouz and H. Zolfagharpour, Polycyclic aromatic hydrocarbons (PAHs) formation during the fast pyrolysis of hazardous health-care waste, Chemosphere, 2019, 227, 277–288 CrossRef CAS.
- A. Krzyszczak, M. P. Dybowski and B. Czech, Formation of polycyclic aromatic hydrocarbons and their derivatives in biochars: The effect of feedstock and pyrolysis conditions, J. Anal. Appl. Pyrolysis, 2021, 160, 105339 CrossRef CAS.
- M. E. Sánchez, J. A. Menéndez, A. Domínguez, J. J. Pis, O. Martínez, L. F. Calvo and P. L. Bernad, Effect of pyrolysis temperature on the composition of the oils obtained from sewage sludge, Biomass Bioenergy, 2009, 33, 933–940 CrossRef.
- P. Manara and A. Zabaniotou, Towards sewage sludge based biofuels via thermochemical conversion - A review, Renewable Sustainable Energy Rev., 2012, 16, 2566–2582 CrossRef CAS.
- S. Liu, M. Wei, Y. Qiao, Z. Yang, B. Gui, Y. Yu and M. Xu, Release of organic sulfur as sulfur-containing gases during low temperature pyrolysis of sewage sludge, Proc. Combust. Inst., 2015, 35, 2767–2775 CrossRef CAS.
- G. Lourinho, O. Alves, B. Garcia, B. Rijo, P. Brito and C. Nobre, Costs of gasification technologies for energy and fuel production: overview, analysis, and numerical estimation, Recycling, 2023, 8, 49 CrossRef.
- N. Mills, P. Pearce, J. Farrow, R. Thorpe and N. Kirkby, Environmental & economic life cycle assessment of current & future sewage sludge to energy technologies, Waste Manage., 2014, 34, 185–195 CrossRef CAS.
- Q. Yang, H. Zhou, X. Zhang, C. P. Nielsen, J. Li, X. Lu, H. Yanga and H. Chen, Hybrid life-cycle assessment for energy consumption and greenhouse gas emissions of a typical biomass gasification power plant in China, J. Cleaner Prod., 2018, 205, 661–671 CrossRef.
- S. G. Nkuna, T. O. Olwal, S. D. Chowdhury and J. M. Ndambuki, A review of wastewater sludge-to-energy generation focused on thermochemical technologies: An improved technological, economical and socio-environmental aspect, Clean. Waste Syst., 2024, 7, 100130 CrossRef.
- K. M. Holmgren, T. Berntsson and T. Lönnqvist, Profitability and greenhouse gas emissions of gasification-based biofuel production-Analysis of sector specific policy instruments and comparison to conventional biomass conversion technologies, Energy, 2018, 165, 997–1007 CrossRef CAS.
- T. Bridle, L. Molinari, S. Dr Skrypski-Mantele, P. Dr Ye and J. Mills, Start-up of the Subiaco Enersludge™ plant, Water Sci. Technol., 2000, 41, 31–36 CrossRef CAS.
- V. K. Tyagi and S.-L. Lo, Sludge: a waste or renewable source for energy and resources recovery?, Renewable Sustainable Energy Rev., 2013, 25, 708–728 CrossRef CAS.
- P. Molton, A. Fassbender and M. D. Brown, STORS: The sludge-to-oil reactor system, Pacific Northwest Lab., Richland, WA (USA), 1986 Search PubMed.
- T. Oda, Making fuel charcoal from sewage sludge for thermal power generation plant-First in Japan, Proceedings of the Water Environment Federation, 2007, vol. 2007, pp. 2943–2951 Search PubMed.
- KORE, Unlock carbon-negative energy in waste, https://koreinfrastructure.com/, 2025).
- G. Jiang, D. Xu, B. Hao, L. Liu, S. Wang and Z. Wu, Thermochemical methods for the treatment of municipal sludge, J. Cleaner Prod., 2021, 311, 127811 CrossRef CAS.
- S. Kundu, S. Patel, P. Halder, T. Patel, M. H. Marzbali, B. K. Pramanik, J. Paz-Ferreiro, C. C. de Figueiredo, D. Bergmann and A. Surapaneni, Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water, Environ. Sci.: Water Res. Technol., 2021, 7, 638–649 RSC.
- A. Murray, A. Horvath and K. L. Nelson, Hybrid life-cycle environmental and cost inventory of sewage sludge treatment and end-use scenarios: a case study from China, Environ. Sci. Technol., 2008, 42(9), 3163–3169 CrossRef CAS.
- M. Campoy, A. Gómez-Barea, P. Ollero and S. Nilsson, Gasification of wastes in a pilot fluidized bed gasifier, Fuel Process. Technol., 2014, 121, 63–69 CrossRef CAS.
- K.-Y. Chiang, C.-H. Lu, C.-K. Liao and R. H.-R. Ger, Characteristics of hydrogen energy yield by co-gasified of sewage sludge and paper-mill sludge in a commercial scale plant, Int. J. Hydrogen Energy, 2016, 41, 21641–21648 CrossRef CAS.
- T. P. Thomsen, H. Hauggaard-Nielsen, B. Gøbel, P. Stoholm, J. Ahrenfeldt, U. B. Henriksen and D. S. Müller-Stöver, Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 2: Evaluation of ash materials as phosphorus fertilizer, Waste Manage., 2017, 66, 145–154 CrossRef CAS PubMed.
- C. Steiner, EBARA's fluidized bed gasification: atmospheric 2× 225t/d for shredding residues recycling and two-stage pressurized 30t/d for ammonia synthesis from waste plastics, 2002 Search PubMed.
- J. W. Judex, M. Gaiffi and H. C. Burgbacher, Gasification of dried sewage sludge: Status of the demonstration and the pilot plant, Waste Manage., 2012, 32, 719–723 CrossRef CAS.
- J. D. Bień and B. Bień, Sludge thermal utilization, and the circular economy, Civ. Environ. Eng. Rep., 2019, 29, 157–175 Search PubMed.
- Y. Kalogo, H. Monteith and P. Eng, State of science report: energy and resource recovery from sludge, WERF, Water Environment Research Foundation, 2008 Search PubMed.
- L. C. Council, Technical Report Loganholme Wastewater Treatment Plant: Biosolids Gasification Demonstration Project (PBE-075), 2021 Search PubMed.
- Ecoremedy, Due diligence report: Biosolids gasification and drying system (ECR-642), Morrisville Municipal Authority, 2021 Search PubMed.
- TPO, Fort wayne wurns biosolids and water plant lime residuals into beneficial products, 2018 Search PubMed.
- M. Environmental, A new way of solving manure issues, 2025 Search PubMed.
- B. Bień and J. D. Bień, Conditioning of sewage sludge with physical, chemical and dual methods to improve sewage sludge dewatering, Energies, 2021, 14, 5079 CrossRef.
- S. Borzooei, G. Campo, A. Cerutti, L. Meucci, D. Panepinto, M. Ravina, V. Riggio, B. Ruffino, G. Scibilia and M. Zanetti, Optimization of the wastewater treatment plant: From energy saving to environmental impact mitigation, Sci. Total Environ., 2019, 691, 1182–1189 CrossRef CAS.
- S. Patel, S. Kundu, J. Paz-Ferreiro, A. Surapaneni, L. Fouche, P. Halder, A. Setiawan and K. Shah, Transformation of biosolids to biochar: a case study, Environ. Prog. Sustainable Energy, 2019, 38, 13113 CrossRef.
- K. Nahar, M. H. Marzbali, I. G. Hakeem, A. Sharma, K. Chiang, A. Surapaneni, R. Gupta, A. Ball and K. Shah, Hydrothermal processing of primary, waste-activated, and digested sewage sludge: Products characterisation, fate of heavy metals and nutrients, and process integration, J. Ind. Eng. Chem., 2025, 145, 519–533 CrossRef CAS.
- Y. Fan, U. Hornung and N. Dahmen, Hydrothermal liquefaction of sewage sludge for biofuel application: A review on fundamentals, current challenges and strategies, Biomass Bioenergy, 2022, 165, 106570 CrossRef CAS.
- K. Czerwińska, M. Śliz and M. Wilk, Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge, A review, Renewable Sustainable Energy Rev., 2022, 154, 111873 CrossRef.
- W.-T. Chen, M. A. Haque, T. Lu, A. Aierzhati and G. Reimonn, A perspective on hydrothermal processing of sewage sludge, Curr. Opin. Environ. Sci. Health., 2020, 14, 63–73 CrossRef PubMed.
- A. R. K. Gollakota, N. Kishore and S. Gu, A review on hydrothermal liquefaction of biomass, Renewable Sustainable Energy Rev., 2018, 81, 1378–1392 CrossRef.
- M. H. Marzbali, S. Kundu, P. Halder, S. Patel, I. G. Hakeem, J. Paz-Ferreiro, S. Madapusi, A. Surapaneni and K. Shah, Wet organic waste treatment via hydrothermal processing: A critical review, Chemosphere, 2021, 279, 130557 CrossRef CAS.
- A. Matayeva, F. Basile, F. Cavani, D. Bianchi and S. Chiaberge, in Studies in Surface Science and Catalysis, ed. S. Albonetti, S. Perathoner and E. A. Quadrelli, Elsevier, 2019, vol. 178, pp. 231–256 Search PubMed.
- K. Nahar, A. V. Thulasiraman, A. K. Vuppaladadiyam, I. G. Hakeem and K. Shah, Current understanding on the fate of contaminants during hydrothermal treatment of sewage sludge, Curr. Opin. Green Sustainable Chem., 2024, 49, 100960 CrossRef CAS.
- S. S. Amorim Junior, V. S. Hwa Mazucato, B. d. S. Machado, D. de Oliveira Guilherme, R. Brito da Costa and F. J. Correa Magalhães Filho, Agronomic potential of biosolids for a sustainable sanitation management in Brazil: Nutrient recycling, pathogens and micropollutants, J. Cleaner Prod., 2021, 289, 125708 CrossRef CAS.
- H. Wang, S. L. Brown, G. N. Magesan, A. H. Slade, M. Quintern, P. W. Clinton and T. W. Payn, Technological options for the management of biosolids, Environ. Sci. Pollut. Res., 2008, 15, 308–317 CrossRef.
- K. McGaughy and M. T. Reza, Recovery of macro and micro-nutrients by hydrothermal carbonization of septage, J. Agric. Food Chem., 2018, 66, 1854–1862 CrossRef CAS PubMed.
- J. D. Marin-Batista, A. F. Mohedano, J. J. Rodríguez and M. A. de la Rubia, Energy and phosphorous recovery through hydrothermal carbonization of digested sewage sludge, Waste Manage., 2020, 105, 566–574 CrossRef CAS.
- B. Sharma, A. Sarkar, P. Singh and R. P. Singh, Agricultural utilization of biosolids: A review on potential effects on soil and plant grown, Waste Manage., 2017, 64, 117–132 CrossRef CAS PubMed.
- T. Yuan, Y. Cheng, W. Huang, Z. Zhang, Z. Lei, K. Shimizu and M. Utsumi, Fertilizer potential of liquid product from hydrothermal treatment of swine manure, Waste Manage., 2018, 77, 166–171 CrossRef CAS.
- X. Lu, X. Ma, Z. Qin, C. Ke, L. Chen and X. Chen, Co-Hydrothermal Carbonization of Sewage Sludge with Wood Chip: Fuel Properties and Heavy Metal Transformation Behavior of Hydrochars, Energy Fuels, 2021, 35, 15790–15801 CrossRef CAS.
- L. Wang, Y. Chang and Q. Liu, Fate and distribution of nutrients and heavy metals during hydrothermal carbonization of sewage sludge with implication to land application, J. Cleaner Prod., 2019, 225, 972–983 CrossRef CAS.
- A. Czechowska-Kosacka, Y. Cao and A. Pawłowski, Criteria for sustainable disposal of sewage sludge, Rocznik Ochrona Srodowiska, 2015, vol. 17, pp. 337–350 Search PubMed.
- L. Zhan, L. Jiang, Y. Zhang, B. Gao and Z. Xu, Reduction, detoxification and recycling of solid waste by hydrothermal technology: a review, Chem. Eng. J., 2020, 390, 124651 CrossRef CAS.
- B. Weiner, I. Baskyr, J. Poerschmann and F.-D. Kopinke, Potential of the hydrothermal carbonization process for the degradation of organic pollutants, Chemosphere, 2013, 92, 674–680 CrossRef CAS PubMed.
- K. Nahar, A. V. Thulasiraman, A. K. Vuppaladadiyam, I. G. Hakeem and K. Shah, Current understanding on the fate of contaminants during hydrothermal treatment of sewage sludge, Curr. Opin. Green Sustainable Chem., 2024, 100960 CrossRef CAS.
- T. F. Ducey, J. C. Collins, K. S. Ro, B. L. Woodbury and D. D. Griffin, Hydrothermal carbonization of livestock mortality for the reduction of pathogens and microbially-derived DNA, Front. Environ. Sci. Eng., 2017, 11, 1–8 CAS.
- J. Yu, A. Nickerson, Y. Li, Y. Fang and T. Strathmann, Fate of Per- and Polyfluoroalkyl Substances (PFAS) during Hydrothermal Liquefaction of Municipal Wastewater Treatment Sludge, Environ. Sci.: Water Res. Technol., 2020, 6, 1388–1399 RSC.
- S. Hao, Y.-J. Choi, B. Wu, C. P. Higgins, R. Deeb and T. J. Strathmann, Hydrothermal Alkaline Treatment for Destruction of Per-and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam, Environ. Sci. Technol., 2021, 55, 3283–3295 CrossRef CAS PubMed.
- W. Shi, C. Liu, D. Ding, Z. Lei, Y. Yang, C. Feng and Z. Zhang, Immobilization of heavy metals in sewage sludge by using subcritical water technology, Bioresour. Technol., 2013, 137, 18–24 CrossRef CAS PubMed.
- A. Leghari, Y. Xiao, L. Ding, A. Raheem, A. Ryzhkov and G. Yu, Research advancements in nutrients and heavy metals, its speciation and behavior during hydrothermal carbonization of sludge–A critical review, Fuel, 2023, 352, 129082 CrossRef CAS.
- X. Lu, X. Ma and X. Chen, Co-hydrothermal carbonization of sewage sludge and lignocellulosic biomass: Fuel properties and heavy metal transformation behaviour of hydrochars, Energy, 2021, 221, 119896 CrossRef CAS.
- K. Nahar, I. G. Hakeem, A. Surapaneni, A. S. Ball and K. Shah, Co-processing of sewage sludge with alum sludge and food and garden organics: Towards boehmite generation and enhanced product properties, J. Environ. Chem. Eng., 2025, 13, 115792 CrossRef CAS.
- L. J. Snowden-Swan, R. T. Hallen, Y. Zhu, J. M. Billing, S. B. Jones, T. R. Hart, D. C. Elliott, S. P. Fox, A. J. Schmidt and G. D. Maupin, Hydrothermal Liquefaction and Upgrading of Municipal Wastewater Treatment Plant Sludge: A Preliminary Techno-Economic Analysis, Washington, DC, 2016 Search PubMed.
- E. Medina-Martos, I.-R. Istrate, J. A. Villamil, J.-L. Gálvez-Martos, J. Dufour and Á. F. Mohedano, Techno-economic and life cycle assessment of an integrated hydrothermal carbonization system for sewage sludge, J. Cleaner Prod., 2020, 277, 122930 CrossRef CAS.
- D. Knorr, J. Lukas and P. Schoen, Production of Advanced Biofuels via Liquefaction-Hydrothermal Liquefaction Reactor Design: April 5, 2013, National Renewable Energy Lab.(NREL), Golden, CO (United States), 2013 Search PubMed.
- S. B. Jones, Y. Zhu, D. B. Anderson, R. T. Hallen, D. C. Elliott, A. J. Schmidt, K. O. Albrecht, T. R. Hart, M. G. Butcher and C. Drennan, Process design and economics for the conversion of algal biomass to hydrocarbons: whole algae hydrothermal liquefaction and upgrading, Pacific Northwest National Lab (PNNL), Richland, WA (United States), 2014 Search PubMed.
- M. Hedayati Marzbali, S. Kundu, S. Patel, P. Halder, J. Paz-Ferreiro, S. Madapusi and K. Shah, Hydrothermal carbonisation of raw and dewatered paunch waste: Experimental observations, process modelling and techno-economic analysis, Energy Convers. Manage., 2021, 245, 114631 CrossRef CAS.
- E. Heracleous, M. Vassou, A. A. Lappas, J. K. Rodriguez, S. Chiaberge and D. Bianchi, Understanding the Upgrading of Sewage Sludge-Derived Hydrothermal Liquefaction Biocrude via Advanced Characterization, Energy Fuels, 2022, 36, 12010–12020 CrossRef CAS.
- R. d. S. Deuber, D. S. Fernandes, J. M. Bressanin, J. Watson, M. F. Chagas, A. Bonomi, L. V. Fregolente and M. D. B. Watanabe, Techno-economic assessment of HTL integration to the Brazilian sugarcane industry: An evaluation of different scenarios, Ind. Crops Prod., 2021, 173, 114139 CrossRef CAS.
- Licella, Enabiling Suistainable Transformation, https://www.licella.com/, (accessed 14/10/2025).
- D. Reißmann, D. Thrän and A. Bezama, How to identify suitable ways for the hydrothermal treatment of wet bio-waste? A critical review and methods proposal, Waste Manage. Res., 2018, 36, 912–923 CrossRef PubMed.
- P. Biller and A. Roth, in Biokerosene, Springer, 2018, pp. 607–635 Search PubMed.
- M. Pecchi and M. Baratieri, Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: a review, Renewable Sustainable Energy Rev., 2019, 105, 462–475 CrossRef CAS.
- J. Yang, S. Tang, B. Song, Y. Jiang, W. Zhu, W. Zhou and G. Yang, Optimization of integrated anaerobic digestion and pyrolysis for biogas, biochar and bio-oil production from the perspective of energy flow, Sci. Total Environ., 2023, 872, 162154 CrossRef CAS PubMed.
- D. Fabbri and C. Torri, Linking pyrolysis and anaerobic digestion (Py-AD) for the conversion of lignocellulosic biomass, Curr. Opin. Biotechnol., 2016, 38, 167–173 CrossRef CAS.
- D. Andreides, K. O. Fliegerova, D. Pokorna and J. Zabranska, Biological conversion of carbon monoxide and hydrogen by anaerobic culture: Prospect of anaerobic digestion and thermochemical processes combination, Biotechnol. Adv., 2022, 58, 107886 CrossRef CAS PubMed.
- V. S. Sikarwar, M. Pohořelý, E. Meers, S. Skoblia, J. Moško and M. Jeremiáš, Potential of coupling anaerobic digestion with thermochemical technologies for waste valorization, Fuel, 2021, 294, 120533 CrossRef CAS.
- S. Tayibi, F. Monlau, F. Marias, N. Thevenin, R. Jimenez, A. Oukarroum, A. Alboulkas, Y. Zeroual and A. Barakat, Industrial symbiosis of anaerobic digestion and pyrolysis: Performances and agricultural interest of coupling biochar and liquid digestate, Sci. Total Environ., 2021, 793, 148461 CrossRef CAS PubMed.
- J. González-Arias, M. V. Gil, R. Á. Fernández, E. J. Martínez, C. Fernández, G. Papaharalabos and X. Gómez, Integrating anaerobic digestion and pyrolysis for treating digestates derived from sewage sludge and fat wastes, Environ. Sci. Pollut. Res., 2020, 27, 32603–32614 CrossRef.
- S. Tayibi, F. Monlau, A. Bargaz, R. Jimenez and A. Barakat, Synergy of anaerobic digestion and pyrolysis processes for sustainable waste management: A critical review and future perspectives, Renewable Sustainable Energy Rev., 2021, 152, 111603 CrossRef CAS.
- S. Sanaye, P. Alizadeh and M. Yazdani, Thermo-economic analysis of syngas production from wet digested sewage sludge by gasification process, Renewable Energy, 2022, 190, 524–539 CrossRef CAS.
- X. Guo, Y. Zhang, Q. Guo, R. Zhang, C. Wang, B. Yan, F. Lin, G. Chen and L. a. Hou, Evaluation on energetic and economic benefits of the coupling anaerobic digestion and gasification from agricultural wastes, Renewable Energy, 2021, 176, 494–503 CrossRef CAS.
- H. K. Tatla, P. Niknejad, S. Ismail, M. A. Khan, R. Gupta and B. R. Dhar, A comprehensive assessment of Integrating anaerobic digestion and hydrothermal liquefaction Processes: Harnessing energy from sewage sludge, Energy Convers. Manage., 2024, 322, 119187 CrossRef.
- Y. S. Trevor Shackelford, Green Waste to Renewable Natural Gas by Pyrobiomethane - Anaerobic Codigestion of Pyrolysis Oil and Green Waste Sludge, Anaergia Services, 2021 Search PubMed.
- R. Z. Gaur, O. Khoury, M. Zohar, E. Poverenov, R. Darzi, Y. Laor and R. Posmanik, Hydrothermal carbonization of sewage sludge coupled with anaerobic digestion: Integrated approach for sludge management and energy recycling, Energy Convers. Manage., 2020, 224, 113353 CrossRef CAS.
- L. Reyes, L. Abdelouahed, C. Mohabeer, J.-C. Buvat and B. Taouk, Energetic and exergetic study of the pyrolysis of lignocellulosic biomasses, cellulose, hemicellulose and lignin, Energy Convers. Manage., 2021, 244, 114459 CrossRef CAS.
- G. Müller, The atmospheric steam engine as energy converter for low and medium temperature thermal energy, Renewable Energy, 2013, 53, 94–100 CrossRef.
- B. Reinhardt, Thermal Energy Storage, technology, 2010 Search PubMed.
- J. Oladejo, K. Shi, X. Luo, G. Yang and T. Wu, A review of sludge-to-energy recovery methods, Energies, 2018, 12, 60 CrossRef.
- J. Lappalainen, D. Baudouin, U. Hornung, J. Schuler, K. Melin, S. Bjelić, F. Vogel, J. Konttinen and T. Joronen, Sub-and supercritical water liquefaction of kraft lignin and black liquor derived lignin, Energies, 2020, 13, 3309 CrossRef CAS.
- E. Danso-Boateng, G. Shama, A. D. Wheatley, S. J. Martin and R. G. Holdich, Hydrothermal carbonisation of sewage sludge: effect of process conditions on product characteristics and methane production, Bioresour. Technol., 2015, 177, 318–327 CrossRef CAS PubMed.
- M. Wilk, K. Czerwińska, M. Śliz and M. Imbierowicz, Hydrothermal carbonization of sewage sludge: Hydrochar properties and processing water treatment by distillation and wet oxidation, Energy Rep., 2023, 9, 39–58 CrossRef.
- L. J. Snowden-Swan, Y. Zhu, S. B. Jones, D. C. Elliott, A. J. Schmidt, R. T. Hallen, J. M. Billing, T. R. Hart, S. P. Fox and G. D. Maupin, Hydrothermal liquefaction and upgrading of municipal wastewater treatment plant sludge: a preliminary techno-economic analysis, rev. 1, Pacific Northwest National Lab (PNNL), Richland, WA (United States), 2016 Search PubMed.
- T. M. Brown, P. Duan and P. E. Savage, Hydrothermal liquefaction and gasification of Nannochloropsis sp, Energy Fuels, 2010, 24, 3639–3646 CrossRef CAS.
- A. K. Sakhiya, A. Anand and P. Kaushal, Production, activation, and applications of biochar in recent times, Biochar, 2020, 2, 253–285 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |