Unveiling the structural and magnetic properties of RENaGeO4 (RE = Gd, Dy, and Ho) oxides and remarkable low-temperature magnetocaloric responses in GdNaGeO4 oxide
Yikun
Zhang
 *,
Yingzhe
Na
,
Yang
Xie
and
Xinyu
Zhao
*,
Yingzhe
Na
,
Yang
Xie
and
Xinyu
Zhao
School of Electronics and Information, Hangzhou Dianzi University, Hangzhou 310012, China. E-mail: ykzhang@hdu.edu.cn
First published on 15th May 2025
Abstract
Low-temperature magnetocaloric (MC) responses in various types of solid-state magnets have been extensively determined, with the aim of developing high-performing MC materials for magnetic refrigeration applications and deepening our understanding of their underlying intrinsic magneto-physical characteristics. Herein, we fabricated a family of single-phase rare-earth (RE)-dominated oxides, namely, RENaGeO4 (RE = Gd, Dy, and Ho), by applying the solid phase reaction method and unveiled their structural and magnetic properties, specifically to low-temperature MC responses through experimental determination and theoretical calculation. All RENaGeO4 oxides crystallize in an orthorhombic olivine-type structure with the space group Pnma (No. 62) and order magnetically at temperatures of 0.70, 2.28, and 2.15 K for GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides, respectively. The consistent elements in these RENaGeO4 oxides are distributed uniformly and present as RE3+, Na1+, Ge4+, and O2− valence states. The low-temperature MC responses in these RENaGeO4 oxides are identified by the MC parameters of maximum magnetic entropy change and relative cooling power. These MC parameters under magnetic field changes (Δμ0H) of 0–2/0–5 T are as follows: 34.98/47.30 J (kg K)−1 and 107.56/320.70 J kg−1 for GdNaGeO4, 11.23/14.82 J (kg K)−1 and 77.70/236.82 J kg−1 for DyNaGeO4, and 12.21/15.37 J (kg K)−1 and 81.18/239.47 J kg−1 for HoNaGeO4. Evidently, these determined MC parameters for GdNaGeO4 oxide, especially under relatively low Δμ0H, are much larger than those for the commercial MC material of Gd3Ga5O12 oxide and surpass those of most updated benchmarked low-temperature MC materials, making the GdNaGeO4 oxide an excellent candidate for low-temperature magnetic refrigeration application.
Introduction
Solid-state magnetic refrigeration,1–4 which leverages the magnetocaloric (MC) effect in solid-state magnets, is a distinct and prospective low-temperature cooling method3–8 owing to its notable economic and environmental benefits. The MC effect, recognized as an inherent magneto-physical property of all solid-state magnets,1–8 can be primarily accessed through the change in magnetic entropy (ΔSmaxM) under a fixed change in the magnetic field (Δμ0H). However, the development of practical magnetic refrigeration applications has been hindered by the lack of high-performing MC materials.3–8 Thus, various types of solid-state magnets4–16 have been fabricated and extensively determined regarding their MC responses over the last three decades, with the aim of exploring high-performing MC materials for practical refrigeration applications4–24 and better understanding their underlying intrinsic magneto-physical characters.6–18 Consequently, several high-performance MC materials capable of operating at various temperature ranges have been achieved,3–18 such as (Mn, Fe)2(P, X), Gd5(Si, Ge)4, La(Fe, Si)13Hy, Ni2MnX, Mn30(Fe, Cu)20Al50, and some rare earth (RE)-dominated solid-state magnets.Among the array of achieved high-performing low-temperature MC materials, rare-earth (RE)-dominated magnetic solids12–26 account for the highest proportions. For example, pure or partially doped RE(Co, Ni)2 Laves-phase compounds18–21 have recently been reported to possess remarkable MC responses, which are considerable for hydrogen liquification applications. A large low-temperature MC effect in A2GdSbO6 oxides was reported by Koskelo et al.,22 which correlates with their free-spin dominant magnetic behaviors.22 Remarkable low-temperature MC responses were very recently reported in Gd2Ti2O7 and SrGd2O4 oxides,23,24 which correlate with their geometrically frustrated magnetic structure.23,24 The Er1−xTmxGa compounds were fabricated by Wang et al.,25 which are reported to possess large MC responses around hydrogen liquification temperature. The low-temperature MC effects in RE(OH)3 compounds have recently been characterized, and Dy(OH)3 was observed to be a high-performing low-temperature MC material.26 Enhanced low-temperature MC responses were recently achieved in Gd2CuTiO6 and GdCoC compounds,27,28 which are governed by 4f-3d magnetic ground state interactions. The low-temperature MC effects of RE2CoTiO6 oxides29 were investigated, and remarkable MC responses were achieved in Gd2CoTiO6 oxide.29 These results collectively indicate that numerous unknown RE-dominated magnetic solids hold high potential as high-performing low-temperature MC materials, warranting our in-depth investigation.
Additionally, extensive investigations have been performed on RE-dominated magnetic oxides22–24,27–30 owing to their benefits of being easy to fabricate and good environmental stability. Thus, we herein shift our research interest to RENaGeO4 oxides,31–36 which have attracted research interest recently owing to their significant potential as luminescent phosphors.33–36 However, a detailed investigation of RENaGeO4 oxides on their magnetic properties and MC responses is still lacking. Therefore, building on our previous studies27–30 focused on developing high-performing low-temperature MC materials and deepening our understanding of the inherent magneto-properties of RENaGeO4 oxides, in this work, we fabricated three polycrystalline oxides of GdNaGeO4, DyNaGeO4, and HoNaGeO4 and unveiled their structural and magnetic properties, specifically their cryogenic MC responses experimentally and theoretically. Our studies indicate that GdNaGeO4 oxide possesses remarkably low-temperature MC responses, especially under relatively low Δμ0H, which is an exceptionally promising candidate for practical refrigeration applications.
Experimental and calculation details
Three polycrystalline samples of GdNaGeO4, DyNaGeO4 and HoNaGeO4 were fabricated by applying the method of solid phase reaction using raw materials of Gd2O3/Dy2O3/Ho2O3, Na2CO3 and GeO2 powders (purity all exceeding 99.9 wt.%). First, stoichiometric amounts of these oxide powders were weighed, hand-mixed and carefully ground. Second, the thoroughly mixed and ground powders were placed in a muffle furnace at 950 °C for 26 hours. Third, the resulting products were regrounded into fine powders and directly pelleted by cold pressing. Finally, the dense GdNaGeO4, DyNaGeO4 and HoNaGeO4 oxides were acquired by the final thermal treatment of these pellets at 1020 °C, 1120 °C and 1170 °C for 50 hours, respectively.The phase and structural properties of RENaGeO4 oxides are assessed by applying a Rigaku X-ray diffractor (XRD, SmartLab). The element distributions and microstructure of RENaGeO4 oxides were characterized by selective area electron diffraction (SAED), transmission electron microscopy (TEM, FEI-Talos-F200s) and attached energy dispersive spectroscopy (EDS). The valence states in RENaGeO4 oxides were accessed by Thermo Scientific X-ray photoelectron spectroscopy (XPS, K-Alpha) utilizing C-1s as the reference energy. The magnetization measurements of RENaGeO4 oxides were performed by applying a quantum design magnetic property measurement system (MPMS-Q3) with a magnetic field and temperature sweep rates of 30 mT s−1 and 3 K min−1, respectively. The magnetization results of GdNaGeO4 below 1.8 K were obtained using a dilute He-3 refrigerator with magnetic field and temperature sweep rates of 10 mT s−1 and 0.3 K min−1, respectively.
Atomic-level first-principles electronic and magnetic structure calculations of RENaGeO4 oxides were conducted within the framework of spin-polarized density functional theory (DFT).37–39 The calculations utilized the commercial Vienna ab initio Simulation Package (VASP) software, employing plane waves with a cutoff energy of 520 eV to reproduce the one-electron wave function for constructing the basis set and projected augmented waves (PAW) pseudopotentials.37–39 The valence electron states in PAW pseudopotentials of Gd [4f7 5s2 5p6 5d1 6s2], Dy [4f10 5s2 5p6 6s2], Ho [4f11 5s2 5p6 6s2], Na [2s2 2p6 3s1], Ge [3d10 4s2 4p2], and O [2s2 2p4] were considered in RENaGeO4 oxides explicitly during the calculations. The k-space integrations during calculation were set as 3 × 5 × 6 using the Monkhorst–Pack method.37–39 Structural optimizations of RENaGeO4 oxides were conducted using a conjugate gradient algorithm until each self-consistent electronic loop and the Hellman–Feynman forces converged to below 10−7 eV per atom and −0.01 eV Å−1, respectively.
Results and discussion
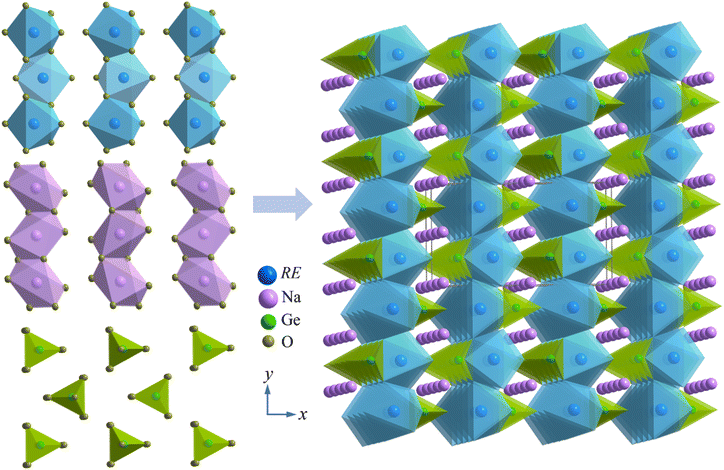
We first assessed the phase and structural characters of RENaGeO4 (RE = Gd, Dy, and Ho) oxides by XRD at room temperature (RT) and Rietveld structural refinement utilizing the Fullprof suite.40 The resulting experimental and refined XRD patterns of GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides are illustrated in Fig. 1(a)–(c), respectively. All the diffraction peaks of these RENaGeO4 oxides could be indexed and well-fitted to an orthorhombic Olivine-type structure (Pnma space group, No. 62). The values of fitted reliability parameters, including Rb, Rwp and Rexp, are as follows: 3.48%, 4.60% and 2.21% for GdNaGeO4; 3.63%, 5.01% and 1.98% for DyNaGeO4; and 4.05%, 6.89% and 1.67% for HoNaGeO4; respectively. These parameters are reasonably low, indicating the reliability of the present fitting and good matching with a structural model. Additionally, the refined lattice parameters a, b, c and V are 11.5487(15), 6.5334(9), 5.3012(7) Å and 399.984 Å3 for GdNaGeO4; 11.4400(17), 6.4839(9), 5.2764(8) Å and 391.378 Å3 for DyNaGeO4; and 11.4167(21), 6.4488(12), 5.2748(11) Å and 388.353 Å3 for HoNaGeO4, respectively, in which all decrease monotonically with increasing atom number of RE ions, as illustrated in Fig. 1(d), aligned well with the contraction principle of RE elemental. The schematic structural diagrams of RENaGeO4 oxides are illustrated in Fig. 2. Generally, the RE, Na, and Ge atoms in RENaGeO4 oxides are coordinated with six, six, and four O atoms and form irregular REO6 octahedra, NaO6 octahedra, and GeO4 tetrahedra, respectively. The REO6 and NaO6 octahedra exhibit similar behavior, sharing two O atoms with themselves and forming octahedral chains along the a-axis. Within the bc-plane, the NaO6 octahedra are corner shared with four equivalent REO6 octahedra and two equivalent GeO4 tetrahedra; it is also edge shared with two equivalent REO6 octahedra and GeO4 tetrahedron, resulting in a quasi-two-dimensional (2D) structural character of RENaGeO4 oxides. | ||
| Fig. 1 Experimental and calculated XRD patterns of (a) GdNaGeO4, (b) DyNaGeO4, and (c) HoNaGeO4 oxides. (d) Lattice parameter (a, b, c, and V) variations of RENaGeO4 oxides. | ||
The bright-field TEM images and corresponding fast Fourier transforms of GdNaGeO4, DyNaGeO4 and HoNaGeO4 oxides are illustrated in Fig. 3. The plane spacings of GdNaGeO4 oxide [Fig. 3(a2)] are deduced to be 2.631 and 3.859 Å, typically corresponding to its [410] and [111] diffraction planes, respectively. The plane spacings of DyNaGeO4 [Fig. 3(b2)] and HoNaGeO4 [Fig. 3(c2)] are deduced to be 3.137 and 2.970 Å, typically corresponding to their [301] and [400] diffraction planes, respectively. In addition, the observed clear ring shape in SAED patterns [Fig. 3(a3)–(c3)] of these RENaGeO4 oxides corresponding to the lattice planes are determined as follows: (524), (314), (102), (211) and (210) for GdNaGeO4 oxide [Fig. 3(a3)]; (724), (342), (141), (222), (301) and (111) for DyNaGeO4 oxide [Fig. 3(b3)]; and (024), (431), (331), (402) and (002) for HoNaGeO4 oxide [Fig. 3(c3)], respectively. The TEM-EDS mapping analysis of Gd/Dy/Ho [Fig. 3(a4)–(c4)], Na [Fig. 3(a5)–(c5)], Ge [Fig. 3(a6)–(c6)] and O [Fig. 3(a7)–(c7)] indicate the uniform distribution of all the constituent elements in RENaGeO4 oxides up to the nanoscale without notable segregations. Thus, these results reaffirm the single-phase character of these RENaGeO4 oxides with good homogeneity.
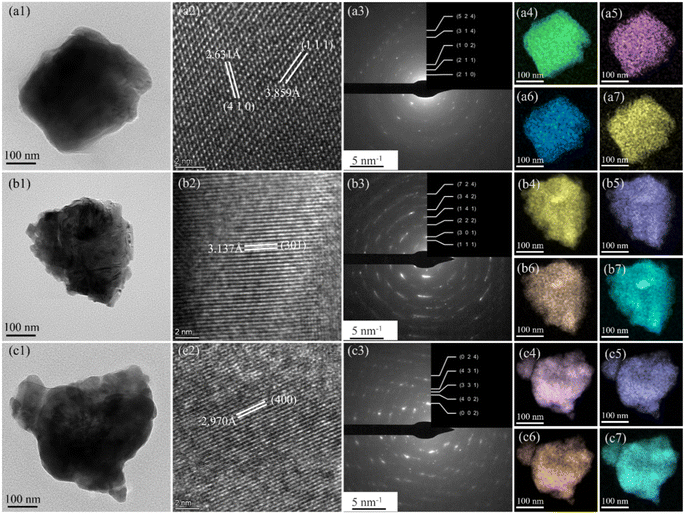 | ||
| Fig. 3 TEM, SAED, and EDS results of (a1–a7) GdNaGeO4, (b1–b7) Dy2NaGeO4, and (c1–c7) HoNaGeO4 oxides. | ||
The chemical valence states of RE (Gd, Dy, and Ho), Na, Ge and O in RENaGeO4 oxides were assessed using XPS at RT. The XPS spectra reveal distinct signals for C (reference), Gd/Dy/Ho, Na, Ge and O elements, which align well with those obtained from TEM-EDS analysis. The high-resolution core-level XPS spectra of Gd/Ho-4d, Dy-3d, Na-1s, Ge-3d, and O-1s in GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides and the corresponding peak-fitting results are illustrated in Fig. 4(a)–(c) respectively. The XPS core-level spectra of Gd-4d [Fig. 4(a1)], Dy-3d [Fig. 4(b1)] and Ho-4d [Fig. 4(c1)] all reveal two characteristic peaks located at 142.03 and 148.16 eV for GdNaGeO4 oxide, at 1334.75 and 1296.53 eV for DyNaGeO4 oxide, and at 163.28 and 161.12 eV for HoNaGeO4 oxide, respectively, indicating the presence of Gd3+, Dy3+ and Ho3+ valence states. The core-level XPS spectra of Na-1s [Fig. 4(a2), (b2), and (c2)] in RENaGeO4 oxides reveal a single character peak located around 1071.17 eV, indicating the presence of the Na1+ valence state. In the XPS spectra of Ge-2p core-level [Fig. 4(a3), (b3), and (c3)] in RENaGeO4 oxides, two characteristic peaks at around 32.03 and 31.23 eV corresponding to spin–orbit splitting of Ge-3d3/2 and Ge-3d5/2 can be noted, indicating the presence of Ge4+ valence state. Additionally, the XPS spectra of the O-1s core level [Fig. 4(a4), (b4), and (c4)] in RENaGeO4 oxides reveal two typical peaks at 529.98 and 531.43 eV, corresponding to the characteristic peak of O2− ions in the lattices and OH− on the surface, respectively.
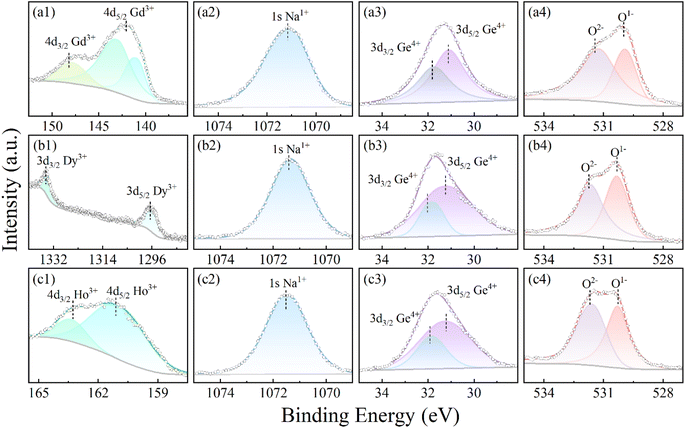 | ||
| Fig. 4 XPS core-level spectra of constituent elementals for (a) GdNaGeO4, (b) DyNaGeO4, and (c) HoNaGeO4 oxides. | ||
To assess the ground-state magnetic and electronic properties of these RENaGeO4 oxides, we performed spin-polarized first-principles DFT calculations37–39 using commercial VASP software with PAW pseudopotentials.37–39 We first evaluated the magnetic ground state of RENaGeO4 oxides by calculating the total energy (Etot) for various potential spin configurations. Parallel (FM) and three anti-parallel (AFM: AFM1, AFM2, and AFM3) states are considered, as illustrated in Fig. 5(a)–(d). The calculated Etot values of EFM, EAFM1, EAFM2 and EAFM3 are as follows: −226.7264, −226.7270, −226.7299 and −226.7297 eV per f.u. for GdNaGeO4; −210.3734, −210.3046, −210.3331 and −210.3344 eV per f.u. for DyNaGeO4; and −204.3413, −203.9209, −204.3958 and −204.2425 eV per f.u. for HoNaGeO4, respectively. Notably, the AFM2, FM and AFM2 states have the lowest Etot in GdNaGeO4, DyNaGeO4 and HoNaGeO4, respectively, illustrating that the AFM ground state is preferred for GdNaGeO4 and HoNaGeO4 oxides, whereas the FM ground state is preferred for DyNaGeO4 oxide. Furthermore, we calculated the spin-polarized total density of states (DOS) and projected-orbital partial DOS for Gd(4f), Dy(4f), Ho(4f), Na(3s), Ge(4p), and O(2p) orbitals to further assess their electronic and magnetic characters, as illustrated in Fig. 5(e)–(g) for GdNaGeO4, DyNaGeO4 and HoNaGeO4, respectively. Nearly identical states between spin-minority and spin-majority channels can be noted for GdNaGeO4 and HoNaGeO4 oxides with an evident band gap (BG) around the Fermi level, further proving their AFM ground state. However, the spin-minority and spin-majority channels in DyNaGeO4 are asymmetric, indicating the possibility of an FM ground state. Additionally, the projected-orbital RE(4f) partial DOS predominantly governs the total DOS and exhibits significant splitting behavior in these RENaGeO4 oxides, suggesting pronounced spontaneous polarization and large magnetic moments in RE3+ ions. The calculated average magnetic moment of GdNaGeO4 oxide is 6.88μB, which is close to the theoretical limitation of a free Gd3+ ion (7.0μB). However, the calculated average magnetic moments in DyNaGeO4 and HoNaGeO4 are 4.86 and 3.85μB, respectively, which are only around half of the theoretical limit of the corresponding free RE3+ ions. Such differences probably originate from the strong crystal field effects of Dy3+ and Ho3+ ions in RENaGeO4 oxides.
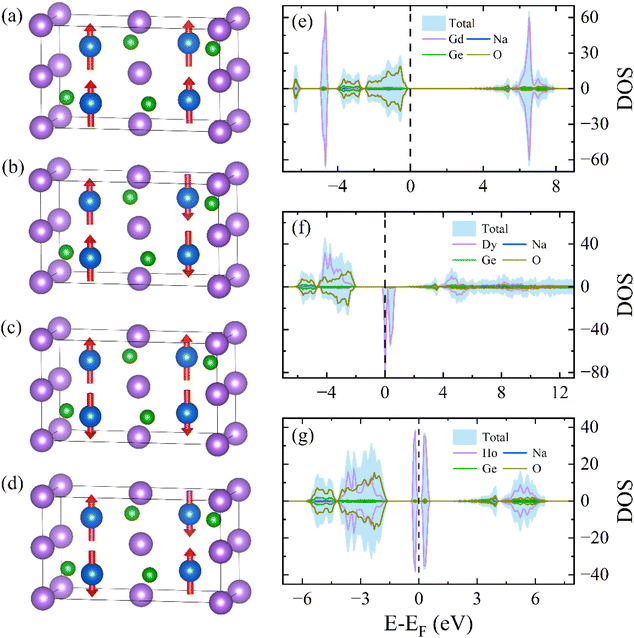 | ||
| Fig. 5 Potential magnetic coupling of (a) FM and (b–d) AFM in RENaGeO4 oxides. Spin-polarized total and partial DOS of (e) GdNaGeO4, (f) DyNaGeO4, and (g) HoNaGeO4 oxides. | ||
The magnetic properties of RENaGeO4 oxides were also experimentally determined with μ0H up to 7 T. The resulting M(T) and 1/χ(T) (χ = M/μ0H) curves of GdNaGeO4, DyNaGeO4 and HoNaGeO4 oxides under μ0H = 1 T are illustrated in Fig. 6(a)–(c), respectively. Despite slight differences in values, these thermomagnetic results in RENaGeO4 oxides exhibit similar behaviors: the values of M increase continuously as the temperature decreases, and the 1/χ(T) curves above 20 K of these RENaGeO4 oxides follow a Curie Weiss law, χ(T) = C/(T − θ) + χ0, in which θP and χ0 represent paramagnetic Curie temperature and temperature-independent part susceptibility, respectively. The Curie constant can be expressed asC = N(μBμeff)2/3κB, where μeff represents effective magnetic moment. The linear Curie Weiss fittings produce the θP values of −0.36, 0.23 and −4.71 K for GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides, respectively, indicating the possibility of ground state AFM interaction for GdNaGeO4 and HoNaGeO4 oxides, while FM interaction for DyNaGeO4, which aligns with those by DFT calculations. The corresponding deduced μeff values for RENaGeO4 oxides are 8.00, 10.58 and 10.62μB per f.u., which align well with their respective theoretical magnetic moment of free RE3+ ions, indicating the crucial roles of RE ions on magnetic properties in these RENaGeO4 oxides. Additionally, the M(T) curves for these RENaGeO4 oxides are illustrated in insets in Fig. 6(a)–(c) by FC and ZFC modes, which coincide within the experimental limitations, indicating the good thermal reversibility, which is demanded for magnetic refrigeration application. Moreover, the values of M in all RENaGeO4 oxides increase gradually as temperature decreases to 1.8 K and show a saturation tendency for DyNaGeO4 and HoNaGeO4 oxides around 2 K, resulting in magnetic phase transition (MPT) temperatures of ∼2.28 and 2.15 K, respectively, based on the infection point in their dM/dT(T) curves. However, the M value of GdNaGeO4 oxide increases continuously down to 1.8 K, indicating no distinct magnetic ordering. Thus, we further determined the thermomagnetic results of GdNaGeO4 oxide down to 0.4 K using a dilute He-3 refrigerator, as illustrated in Fig. 6(a). A clear peak in the M(T) curves of GdNaGeO4 oxide can be noted at around 0.7 K, indicating a typical MPT from the paramagnetic (PM) to AFM state.
 | ||
| Fig. 6 1/χ(T) and M(T) curves under 1 T for (a) GdNaGeO4, (b) DyNaGeO4, and (c) HoNaGeO4 oxides. Insets present corresponding FC, ZFC M(T) and dM/dT curves under 0.1 T. | ||
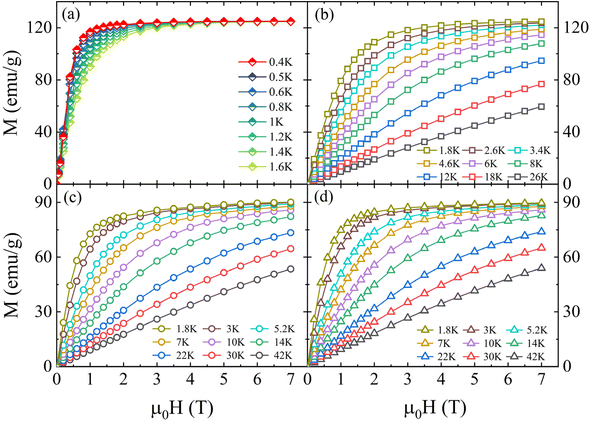
Moreover, a series of M(μ0H) curves of RENaGeO4 oxides from 1.8 to 42 K (down to 0.4 K for GdNaGeO4) with μ0H up to 7 T were determined to further assess their MC responses and MPT nature, as illustrated in Fig. 7(a)–(d). Except for some differences in values, the M(μ0H) results of these RENaGeO4 oxides exhibit similar performances. Generally, the M values increase abruptly in the low-field region, then become flat, and finally show saturation behavior in the high-field region. MC performance is known to be proportional to the saturation magnetic moment (Ms) of magnetic solids. The deduced Ms value for GdNaGeO4 reaches 7.07μB/Gd (0.4 K), which is close to the theoretical limitation of free Gd3+ ions; thus, notably low-temperature MC responses are expected. However, the Ms values are 5.20μB/Dy (1.8 K) and 5.21μB/Ho (1.8 K) for DyNaGeO4 and HoNaGeO4, respectively, which are obviously lower than the theoretical values of the corresponding free RE3+ ions; such differences mainly originate from the strong crystal field effect and single-ion anisotropy for Dy3+ and Ho3+ ions. Additionally, these values align well with those obtained by the DFT calculations, further proving the vital roles of RE ions in the magnetism of these RENaGeO4 oxides. Moreover, the MC responses in solid-state magnets are related to their corresponding MPT order types,27–30 which can be assessed by the slope signals in Arrott-plot (μ0H/M versus M2) curves, using the Banerjee criterion. The observation of a positive slope indicates the second-order type MPT,41 while the exhibition of a negative slope corresponds to first-order type MPT.41 Thus, to assess the order type of MPT in these RENaGeO4 oxides, the Arrott plot curves were transferred based on M(μ0H) results [Fig. 7(a)–(d)]. The resulting curves are correspondingly illustrated in Fig. 8(a)–(d), respectively. Clearly, negative slopes at low temperatures in GdNaGeO4 oxide [Fig. 8(a)] can be observed, illustrating the occurrence of first-order type MPT. However, only positive slopes are found for DyNaGeO4 and HoNaGeO4 oxides [Fig. 8(c)–(d)], indicating the second-order type MPT within the experimental limitations.
Subsequently, we first assessed the MC responses of these RENaGeO4 oxides using the temperature-dependent −ΔSM, evaluated using the equation 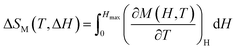 ,3–6 as illustrated in Fig. 9(a)–(c) for GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides, respectively, which reveal a conventional MC response: only positive −ΔSM(T) values can be observed within the experimental limitations, and the value of −ΔSM under a fixed Δμ0H increases continuously with decreases in T and reaches its maximum (−ΔSmaxM); after that, −ΔSM decreases gradually by further decreasing T. Under Δμ0H of 0–1, 0–2, 0–3, 0–5 and 0–7 T, the deduced −ΔSmaxM values are 22.22, 34.98, 40.90, 47.30 and 50.66 J (kg K)−1 for GdNaGeO4; 7.15, 11.23, 13.00, 14.82 and 15.83 J (kg K)−1 for DyNaGeO4; and 8.21, 12.21, 13.86, 15.37 and 16.22 J (kg K)−1 for HoNaGeO4, respectively. Evidently, the GdNaGeO4 possesses larger MC responses than those in DyNaGeO4 and HoNaGeO4 oxides, which is probably related to its low-field induced first order MPT and largest saturation magnetic moment with low crystal field effect. In addition, the values of refrigerant capacity
,3–6 as illustrated in Fig. 9(a)–(c) for GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides, respectively, which reveal a conventional MC response: only positive −ΔSM(T) values can be observed within the experimental limitations, and the value of −ΔSM under a fixed Δμ0H increases continuously with decreases in T and reaches its maximum (−ΔSmaxM); after that, −ΔSM decreases gradually by further decreasing T. Under Δμ0H of 0–1, 0–2, 0–3, 0–5 and 0–7 T, the deduced −ΔSmaxM values are 22.22, 34.98, 40.90, 47.30 and 50.66 J (kg K)−1 for GdNaGeO4; 7.15, 11.23, 13.00, 14.82 and 15.83 J (kg K)−1 for DyNaGeO4; and 8.21, 12.21, 13.86, 15.37 and 16.22 J (kg K)−1 for HoNaGeO4, respectively. Evidently, the GdNaGeO4 possesses larger MC responses than those in DyNaGeO4 and HoNaGeO4 oxides, which is probably related to its low-field induced first order MPT and largest saturation magnetic moment with low crystal field effect. In addition, the values of refrigerant capacity  and3–6 relative cooling3–6 power
and3–6 relative cooling3–6 power 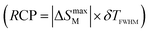 for RENaGeO4 oxides were deduced to further assess their MC responses. The Tcold and Thot represent the temperatures at
for RENaGeO4 oxides were deduced to further assess their MC responses. The Tcold and Thot represent the temperatures at 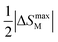 in ΔSM(T) curves at two sides, and the δTFWHM represents the absolute values of the difference between Thot and Tcold. Consequently, the deduced RC(RCP) values under Δμ0H of 0–1, 0–2, 0–3, 0–5 and 0–7 T are as follows: 30.35(38.64), 82.87(107.56), 137.76(178.87), 245.53(320.70) and 351.46(462.10) J kg−1 for GdNaGeO4; 21.23(28.73), 58.78(77.70), 100.02(130.92), 182.40(236.92) and 259.55(337.64) J kg−1 for DyNaGeO4; and 22.92(31.09), 61.66(81.18), 101.87(133.14), 183.25(239.47) and 262.50(345.20) J kg−1 for HoNaGeO4, respectively. Evidently, the GdNaGeO4 oxide shows the best MC responses among the RENaGeO4 oxides, aligning with its larger saturation magnetic moment. Finally, to further identify the MC responses of GdNaGeO4 oxide, we summarize its −ΔSmaxM values under Δμ0H of 0–2 and 0–5 T with the reported benchmarked low-temperature MC materials, as illustrated in Table 1. The MC responses of the present GdNaGeO4 oxide under low Δμ0H of 0–5 T are comparable to most of the reported materials. Interestingly, its MC performance under low Δμ0H of 0–2 T, which could be generated by RE-dominated permanent magnets, is much larger than that of the commercialized MC material of Gd3Ga5O12 oxide and surpasses most of the benchmarked MC materials. Although −ΔSmaxM value under Δμ0H of 0–2 T is lower than that of the divalent Eu-based EuB2O4 [44], the present GdNaGeO4 oxide has the benefit of being easy to fabricate by applying a simple solid-state reaction process with high environmental stability. These findings highlight GdNaGeO4 oxide as a top-level MC material that is attractive for practical low-temperature refrigeration applications.
in ΔSM(T) curves at two sides, and the δTFWHM represents the absolute values of the difference between Thot and Tcold. Consequently, the deduced RC(RCP) values under Δμ0H of 0–1, 0–2, 0–3, 0–5 and 0–7 T are as follows: 30.35(38.64), 82.87(107.56), 137.76(178.87), 245.53(320.70) and 351.46(462.10) J kg−1 for GdNaGeO4; 21.23(28.73), 58.78(77.70), 100.02(130.92), 182.40(236.92) and 259.55(337.64) J kg−1 for DyNaGeO4; and 22.92(31.09), 61.66(81.18), 101.87(133.14), 183.25(239.47) and 262.50(345.20) J kg−1 for HoNaGeO4, respectively. Evidently, the GdNaGeO4 oxide shows the best MC responses among the RENaGeO4 oxides, aligning with its larger saturation magnetic moment. Finally, to further identify the MC responses of GdNaGeO4 oxide, we summarize its −ΔSmaxM values under Δμ0H of 0–2 and 0–5 T with the reported benchmarked low-temperature MC materials, as illustrated in Table 1. The MC responses of the present GdNaGeO4 oxide under low Δμ0H of 0–5 T are comparable to most of the reported materials. Interestingly, its MC performance under low Δμ0H of 0–2 T, which could be generated by RE-dominated permanent magnets, is much larger than that of the commercialized MC material of Gd3Ga5O12 oxide and surpasses most of the benchmarked MC materials. Although −ΔSmaxM value under Δμ0H of 0–2 T is lower than that of the divalent Eu-based EuB2O4 [44], the present GdNaGeO4 oxide has the benefit of being easy to fabricate by applying a simple solid-state reaction process with high environmental stability. These findings highlight GdNaGeO4 oxide as a top-level MC material that is attractive for practical low-temperature refrigeration applications.
| Materials | T M (K) | −ΔSmaxM (0–2 T) J (kg K)−1 | −ΔSmaxM (0–5 T) J (kg K)−1 | Ref. |
|---|---|---|---|---|
| GdNaGeO4 | 0.7 | 34.98 | 47.30 | Present |
| Gd3Ga5O12 | 1.2 | 14.6 | 32.8 | 42 and 43 |
| EuB4O7 | 0.7 | 36.2 | 47.6 | 44 |
| Gd2CuTiO6 | 2.7 | 10.2 | 41.5 | 28 |
| NaGdS2 | 2.5 | 23.5 | 54 | 45 |
| Gd4.5K0.5Si3O13 | 0.7 | 31.9 | 58.2 | 46 |
| Gd3TeBO9 | 2.6 | 20.6 | 50.3 | 47 |
| Gd(OH)F2 | 1.31 | 32 | 65 | 48 |
| Gd9.33Si6O26 | 0.65 | 32.5 | 59.5 | 49 |
| Gd152Ni14@Cl24 | 2.5 | 20.5 | 46 | 50 |
Conclusions
In summary, three single-phased RENaGeO4 oxides were successfully fabricated and unveiled their structural and magnetic properties, specifically for low-temperature MC responses through experimental determination and theoretical calculation. All these RENaGeO4 oxides crystallize in an Olivine-type orthorhombic structure and order magnetically at low temperatures of 0.7, 2.28 and 2.15 K for GdNaGeO4, DyNaGeO4, and HoNaGeO4 oxides, respectively. The consistent elements in NaREGeO4 oxides are all distributed uniformly and present as Na1+, RE3+, Ge4+, and O2− valence states, respectively. Interestingly, the GdNaGeO4 oxide possesses remarkably low-temperature MC responses with the values of −ΔSmaxM reaching 22.22, 34.98, and 47.30 J (kg K)−1 under Δμ0H of 0–1, 0–2, and 0–5 T, respectively. These values, especially under low Δμ0H that can be generated by RE-dominated permanent magnets, are much larger than those of the commercial MC material of Gd3Ga5O12 oxide and surpass most of the updated benchmarked MC materials. These findings, in addition to their simple preparation process and high environmental stability, make the GdNaGeO4 oxide an excellent candidate for low-temperature magnetic refrigeration applications.Data availability
The data supporting the findings of this study are available from the corresponding author, Yikun Zhang, upon reasonable request.Author contributions
Y. Z.: conceptualisation, supervision, project administration, resources, writing original draft, and review and editing; Y. N., Y. X., and X. Z.: investigation, formal analysis, and data curation. All authors have reviewed and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ25E020002). The authors also acknowledge Dr Chao Zhang from the Instrumentation and Service Center for Physical Sciences at Westlake University for the magnetization measurements, and the Supercomputing Center of Hangzhou Dianzi University for providing computing resources.References
- M. H. Phan and S. C. Yu, J. Magn. Magn. Mater., 2007, 308, 325–340 CrossRef CAS.
- O. Gutfleisch, M. A. Willard, E. Brück, C. H. Chen, S. G. Sankar and J. P. Liu, Adv. Mater., 2011, 23, 821–842 CrossRef CAS PubMed.
- V. Franco, J. Blázqueza, J. Iplus, J. Law, L. Ramíreze and A. Comde, Prog. Mater. Sci., 2018, 93, 112–232 CrossRef.
- Y. Zhang, A. Li, W. Hao, H. F. Li and L. W. Li, Acta Mater., 2025, 292, 121033 Search PubMed.
- H. Zhong, Y. Song, F. Long, H. Lu, M. Ai, T. Li, Y. Yao, Y. Sakai, M. Ikeda, K. Takahashi, M. Azuma, F. Hu, X. Xing and J. Chen, Adv. Mater., 2024, 36, 2402046 CrossRef CAS PubMed.
- L. W. Li and M. Yan, J. Mater. Sci. Technol., 2023, 136, 1–12 CrossRef CAS.
- Y. Gong, X. Miao, F. Qian, F. Xu and L. Caron, J. Phys. Condens. Matter, 2024, 36, 503001 CrossRef CAS PubMed.
- F. Zhang, X. Miao, N. van Dijk, E. Brück and Y. Ren, Adv. Energy Mater., 2024, 14, 2400369 Search PubMed.
- Y. Xie, Y. Na, F. Chen and Y. Zhang, Inorg. Chem. Commun., 2025, 177, 114395 CrossRef CAS.
- J. Lin, P. Tong, K. Zhang, W. Lu, X. Wang, X. Zhang, W. Song and Y. Sun, Nat. Commun., 2022, 13, 596 Search PubMed.
- (a) Y. Zhang, W. Hao, C. L. Hu, X. Wang, X. Zhang and L. Li, Adv. Funct. Mater., 2023, 33, 2310047 Search PubMed; (b) W. Guo, X. Miao, J. Cui, S. Torii, F. Qian, Y. Bai, Z. Kou, J. Zha, Y. Shao, Y. Zhang, F. Xu and L. Caron, Acta Mater., 2024, 263, 119530 CrossRef CAS.
- F. Chen, Y. Na, Y. Xie and Y. K. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 52719–52726 CrossRef CAS PubMed.
- Y. Song, J. Zhang, H. Li, H. Zhong, F. Long, Z. Wang, Y. Xu, X. Zheng, H. Zhang, Q. Huang, Y. Zhang, X. Xing and J. Chen, Adv. Energy Mater., 2024, 14, 2402527 CrossRef CAS.
- J. Wang, R. Ji, J. Xu, Z. Li, Y. Zhang and L. Li, J. Magn. Magn. Mater., 2025, 619, 172897 CrossRef CAS.
- Z. Li, A. Arauzo, C. Roscini, J. G. Planas and E. Bartolomé, J. Mater. Chem. A, 2024, 12, 21971–21986 RSC.
- Y. Xie, L. F. Wang, Y. Z. Na and Y. Zhang, J. Non-Cryst. Solids, 2025, 658, 123526 CrossRef CAS.
- F. Yang, J. Wang, R. Ji and Y. Zhang, J. Electron. Mater., 2025, 54, 4642–4647 CrossRef CAS.
- X. Tang, H. Sepehri-Amin, N. Terada, A. Martin-Cid, I. Kurniawan, Y. Kotani, H. Takeya, J. Lai, Y. Matsushita, T. Ohkubo, Y. Miura, T. Nakamura and K. Hono, Nat. Commun., 2022, 13, 1817 CrossRef CAS PubMed.
- Y. Zhang, J. Ying, X. Gao, Z. Mo, J. Shen and L. W. Li, J. Mater. Sci. Technol., 2023, 159, 163–169 CrossRef CAS.
- J. Ćwik, Y. Koshkid’ko, P. Putyra, B. Weise, M. Małecka, D. Gajda, M. Babij and A. Czernuszewicz, Int. J. Hydrogen Energy, 2024, 87, 485 CrossRef.
- J. Ćwik, Y. Koshkid’ko, K. Shinde, J. Park, N. A. de Oliveira, M. Babija and A. Czernuszewicz, J. Mater. Chem. C, 2024, 12, 14421 RSC.
- E. A. C. Koskelo, N. D. Kelly, L. A. V. Nagle-Cocco, J. D. Bocarsly, P. Mukherjee, C. Liu, Q. Zhang and S. E. Dutton, Inorg. Chem., 2023, 62, 10317–10328 CrossRef CAS PubMed.
- Y. K. Zhang, W. X. Hao, J. L. Lin, H. F. Li and L. W. Li, Acta Mater., 2024, 272, 119946 CrossRef CAS.
- J. Lin, S. Wu, K. Sun, H. F. Li, W. Chen, Y. Zhang and L. Li, Ceram. Int., 2024, 50, 51269–51277 Search PubMed.
- D. Wang, X. Zheng, L. He, H. Wu, Y. Gao, G. Wang, H. Liu, T. Pan, A. Ma, L. Xi, J. Xu, S. Wang and B. Shen, Mater. Today Phys., 2025, 50, 101609 Search PubMed.
- P. W. Doheny, J. Chen, T. Gruner, F. M. Grosche and P. J. Saines, J. Mater. Chem. A, 2023, 11, 26474–26480 Search PubMed.
- Y. Zhang, W. Hao, J. Shen, Z. Mo, T. Gottschall and L. Li, Acta Mater., 2024, 276, 120128 Search PubMed.
- Y. K. Zhang, Y. Na, W. X. Hao, T. Gottschall and L. W. Li, Adv. Funct. Mater., 2024, 34, 2409061 Search PubMed.
- Y. K. Zhang, Y. Xie, J. J. Wei and W. X. Hao, J. Mater. Chem. A, 2024, 12, 32396–32407 Search PubMed.
- W. Hao, R. Ji, L. Zhu, S. Huang and Y. Zhang, Solid State Commun., 2025, 399, 115876 Search PubMed.
- M. Emirdag-Eanes, M. Krawiec and J. W. Kolis, J. Chem. Crystallogr., 2002, 31, 281–285 CrossRef.
- J. Yeon, J. B. Hardaway, A. S. Sefat, A. M. Latshaw and H. C. zur Loye, Solid State Sci., 2014, 34, 24–30 CrossRef CAS.
- X. Hou, T. Wan, D. Gao, X. Zhang, C. Jia, C. Du, R. Chai, Q. Pang, S. Yun and Y. Wang, Mater. Today Chem., 2024, 39, 102170 Search PubMed.
- Z. Liu, X. Yu, X. Yang, Q. Peng, X. Zhu, X. Xu and J. Qiu, Inorg. Chem., 2023, 62, 13362–13369 CrossRef CAS PubMed.
- X. Zhang, H. Song, R. Cui, K. Guo, M. Zhang and C. Deng, Mater. Res. Bull., 2023, 167, 112401 CrossRef CAS.
- L. Liu, K. Yu, L. Ming, Y. Sheng, S. Zheng, L. Song, J. Shi and Y. Zhang, J. Rare Earths, 2022, 40, 1424–1431 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 Search PubMed.
- G. Kresse and J. Furthmuller, Phys. Rev. B, 1996, 54, 11169 CrossRef CAS PubMed.
- R. A. Evarestov and V. P. Smirnov, Phys. Rev. B, 2004, 70, 233101 CrossRef.
- J. Rodriguez-Carvajal, FULLPROF: a Rietveld and Pattern Matching Analysis Program, Laboratoire Leon Brillouin CEA-CNRS, France, 2007 Search PubMed.
- B. Banerjee, Phys. Lett., 1964, 12, 16–17 Search PubMed.
- A. C. S. Hamilton, G. I. Lampronti, S. E. Rowley and S. E. Dutton, J. Phys.:Condens. Matter, 2014, 26, 116001 Search PubMed.
- M. Kleinhans, K. Eibensteiner, J. C. Leiner, C. Resch, L. Worch, M. A. Wilde, J. Spallek, A. Regnat and C. Pfleiderer, Phys. Rev. Appl., 2023, 19, 014038 CrossRef CAS.
- Y. Wang, J. Xiang, L. Zhang, J. Gong, W. Li, Z. Mo and J. Shen, J. Am. Chem. Soc., 2024, 146, 3315 Search PubMed.
- C. Delacotte, T. A. Pomelova, T. Stephant, T. Guizouarn, S. Cordier, N. G. Naumov and P. Lemoine, Chem. Mater., 2022, 34, 1829 Search PubMed.
- Y. Zhang, J. Y. Law, A. Li, W. Hao, V. Franco and L. Li, Small, 2025, 21, 2409981 Search PubMed.
- C. Q. Zhou and R. K. Li, Chem.–Eur. J., 2024, 30, 2303048 Search PubMed.
- Q. Xu, B. Liu, M. Ye, G. L. Zhuang, L. Long and L. Zheng, J. Am. Chem. Soc., 2022, 144, 13787 Search PubMed.
- Z. W. Yang, J. Zhang, B. Liu, X. Zhang, D. Lu, H. Zhao, M. Pi, H. Cui, Y. J. Zeng, Z. Pan, Y. Shen, S. Li and Y. W. Long, Adv. Sci., 2024, 11, 2306842 Search PubMed.
- N. Xu, W. Chen, Y. Ding and Z. Zheng, J. Am. Chem. Soc., 2024, 146, 9506–9511 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |