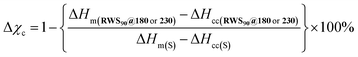Open Access Article
Open Access ArticleDrive-throughs to driveways: high-strength composites from multi-material post-consumer waste collected from fast food restaurants†
Katelyn M.
Derr
a,
Perla Y.
Sauceda-Oloño
 a,
Ashlyn D.
Smith
*a,
Andrew G.
Tennyson
a,
Ashlyn D.
Smith
*a,
Andrew G.
Tennyson
 *ab and
Rhett C.
Smith
*ab and
Rhett C.
Smith
 *a
*a
aDepartment of Chemistry, Clemson University, Clemson, SC 29634, USA. E-mail: rhett@clemson.edu
bDepartment of Materials Science and Engineering, Clemson University, Clemson, SC 29634, USA
First published on 30th May 2025
Abstract
Mixed post-consumer fast food waste poses a formidable challenge for chemical recycling due to its diverse composition of plastics, paper, and remnant foodstuffs. Herein is presented a strategy for kilogram-scale upcycling of such waste into high strength composites. This is accomplished by thiocracking fast food restaurant waste pulp (10 wt%) with sulfur (90 wt%) at 180 °C and 230 °C, giving composites RWS90@180 and RWS90@230, respectively. These composites display compressive strengths of 12.2–16.3 MPa and flexural strengths of 2.80–5.39 MPa, competitive with common construction materials such as standard building brick (C62 classification) and approaching values for Ordinary Portland Cement (OPC). Both materials exhibit favorable properties under acid challenge (retaining up to 91% of their compressive strength after immersion in 0.5 M H2SO4) and show exceptionally low water uptake (<0.5%). Composites RWS90@180 and RWS90@230 also showed low E factors, (0.1 and 0, respectively) and low global warming potentials (−0.0450 kg CO2e per kg and 0.0450 kg CO2e per kg, respectively). This work highlights an approach to upcycling otherwise problematic mixed-material restaurant waste into durable, corrosion-resistant composites with a low environmental impact.
1. Introduction
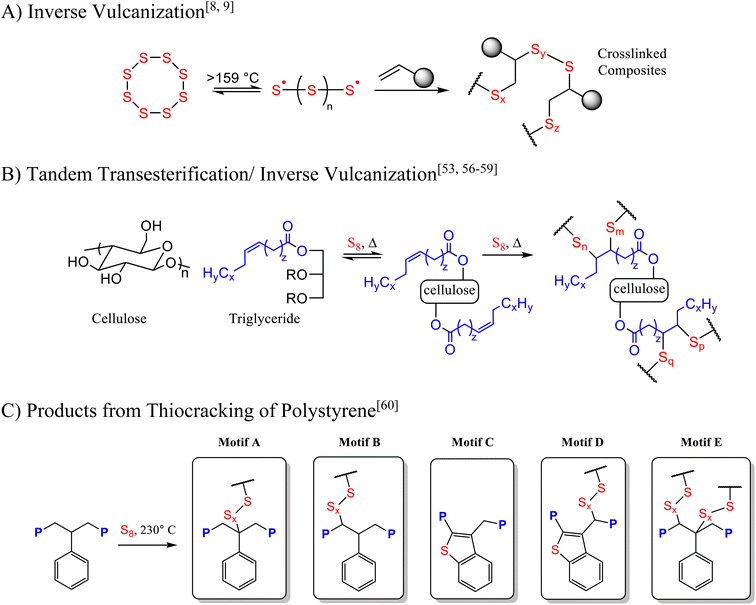
Single-use fast food packaging, often heavily soiled by food residues and comprising multiple types of materials (paper, plastic, adhesives, foil liners), remains a difficult target for conventional recycling. The scale of this challenge is enormous: in the United States alone, 11–16.5 million metric tons of food service waste is generated every year,1 with a substantial fraction (>15%) arising from fast food restaurants.2 Because this waste stream is a mixture of paper, polystyrene, polypropylene, coatings, adhesives, and food residue, achieving efficient chemical recycling of these comingled components is notoriously complicated.2 Plastic waste, in particular, degrades slowly and releases microplastics or other pollutants into natural ecosystems.3–5 As the global consumption of single-use items continues to rise, the need for processes that can upcycle these heterogeneous wastes in a single stage—without requiring extensive sorting—becomes ever more urgent.In parallel with the challenge of multi-material waste streams, the petrochemical industry generates vast amounts of elemental sulfur as a byproduct of crude oil refining, accruing an estimated 80 million metric tons yearly.6,7 Transforming this byproduct sulfur into value-added materials is also an important sustainability goal. One established method for sulfur-based polymeric materials is “inverse vulcanization” (Scheme 1A),8,9 in which thermally activated S–S radical chains react with unsaturated organic substrates to form crosslinked structures.10–44 Initial studies utilizing sulfur as a majority component to create high sulfur-content materials (HSMs) focused on using modified cellulose,45,46 plant oils,42,47–51 and biomass in the form of peanut shells.52–55 In the case of the reaction of sulfur with peanut shells – comprised of cellulose, lignin, and peanut oil – composite formation occurs via tandem transesterification/inverse vulcanization at 180 °C (Scheme 1B).
More recently, we demonstrated that additional S–C bond-forming mechanisms can be employed to address various single source waste plastics through thiocracking.56,57,61,62 Tandem transesterification/inverse vulcanization routes similar to that shown in Scheme 1B, for example, proved effective in recycling polyethylene terephthalate (PET) waste as well as more complex mixed-material packaging waste containing PET and triglycerides.59 Polystyrene (as post-consumer foam cups, lids, packaging cubes and plastic silverware) was also successfully converted into high strength composites by thiocracking.60 The mechanism of S–C bond formation during the thiocracking of polystyrene was investigated in some detail, and several microstructures were identified (Scheme 1C), all resulting from initial abstraction of benzylic hydrogen atoms from polystyrene by sulfur radicals.
The successful conversion of diverse waste materials to composites through S–C bond-forming chemistry led us to hypothesize that even complex mixed material waste containing plastic, paper, and food waste could be successfully converted to composites by heating with elemental sulfur. Herein we report the effectiveness of thiocracking to convert post-consumer fast food waste (polystyrene cups/lids, straws, wrappers, sandwich boxes, bags, napkins, and food remnants; Fig. 1) into composites in a single synthetic step.
Two reaction temperatures, 180 °C and 230 °C, were investigated to evaluate the extent to which temperature might influence crosslinking through accessible reaction pathways and consequently impact material properties. The resulting materials, RWS90@180 and RWS90@230, were characterized by mechanical strength testing, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), mechanical property retention after acid exposure, water uptake, and environmental impact metrics. Each composite exhibits compressive and flexural strengths comparable to or surpassing established construction materials such as C62 building brick and, in some respects, approaches Ordinary Portland Cement (OPC). Scalability of the process was demonstrated by preparing a ∼1 kg brick of RWS90@230 having compressional strength capable of supporting a residential vehicle. Not only are these composites prepared entirely from waste materials, but they also showed favorable environmental impact metrics. RWS90@180 has an E factor63 of 0.1 and a negative global warming potential of −0.0450 kg CO2e per kg, while RWS90@230 has an impressive E factor of 0 with a low global warming potential of 0.0450 kg CO2e per kg. The process's simplicity and excellent sustainability profile underscore the potential of this process for valorizing mixed-material food service waste while simultaneously finding practical uses for stockpiled elemental sulfur.
2. Results and discussion
2.1 Preliminary analysis of multi-material restaurant waste
Post-consumer fast food waste used in this study consisted of cups with lids and straws, sandwich containers (boxes and bags), napkins, straw wrappers, meal bags, and discarded French fries. The waste was collected directly from tables at source restaurants. Representative waste from one fast food meal was determined to include one cup (polystyrene) complete with the straw (polypropylene) and lid (polystyrene with <5 wt% added polybutadiene), a sandwich container (cardboard box or paper bag-like wrapper), one paper napkin, one paper straw wrapper, a paper meal bag, and 38.8 g of French fries. Less than 5 wt% of the representative restaurant waste was attributable to other sources. Waste from a total of five sample meals was utilized to serve as the organic component in this study. The relative contribution of various components to the mixture was determined (Table 1). The composition reported in Table 1 was determined by manually separating, drying, and weighing the components of five representative fast food meals. French fries used were from the same supply as those for which we previously reported the composition.64 Cellulose was the primary component of the meal bag, sandwich box/bag, napkin, and straw wrapper, comprising 26.5 wt% of the waste. These components also included minor contributors, such as dyes and adhesives, which were not further quantified. The polystyrene cup and lid comprised another 16.4 wt% of the sample, while the polypropylene straw accounted for 1.07 wt% of the sample. Discarded French fries comprised the largest proportion of the waste, at 56.0 wt%. We previously reported the composition of these fries as 41% water, 33% starch, 23% peanut oil, and 3% other materials such as vitamins and minerals.55 Preliminary analysis of the components comprising the sample meal was carried out using 1H NMR spectrometry, infrared spectroscopy, thermogravimetric analysis, and differential scanning calorimetry (Fig. S1–S10, S17–S25 and S28–S36†). These data are consistent with the known properties of the constituent components. For example, fries exhibit thermal mass loss below 150 °C due to evaporation of moisture and volatilization of oils, with a broad endothermic peak around 100 °C in the DSC trace.64 Polystyrene shows a sharp degradation onset near 400 °C in the TGA and a strong endothermic melting transition around 240 °C.60 Cellulose-based components, including napkins and sandwich wrappers, display a degradation onset at approximately 260 °C, with overlapping moisture loss and exothermic peaks attributed to cellulose depolymerization.46,53 These features help inform the thermal behavior of the composite precursors and explain some of the degradation patterns seen in the final composite materials (vide infra).| Component | Mass | Percent contribution |
|---|---|---|
| a Adhesives and dyes in meal bags and sandwich bags are included in the mass of cellulose-based materials. b Component contributions made by French fries. | ||
| Cellulose-based materialsa | 87.5 g | 25.6% |
| Polystyrene | 56.1 g | 16.4% |
| Polypropylene | 3.66 g | 1.07% |
| Starchb | 64.1 g | 18.8% |
| Peanut oilb | 44.7 g | 13.1% |
| Waterb | 79.6 g | 23.3% |
| Otherb | 5.80 g | 1.70% |
2.2 Preparation of composites
The fast food waste was processed in a blender to produce a fine aggregate restaurant waste pulp (RW, Fig. 1). The blended restaurant waste had a particle size range of approximately 0.5–2 mm based on sieve analysis. The bulk density of the dried pulp was determined to be 0.38 g cm−3 using volumetric displacement measurements. Processed RW (10 wt%) was combined with elemental sulfur (90 wt%) in a pressure flask under an atmosphere of dry nitrogen gas and then heated at 180 °C or 230 °C for 24 hours to give RWS90@180 or RWS90@230, respectively. Visually, RWS90@180 was a dark brown solid containing fine aggregates. When handled, this material was relatively brittle. In contrast, RWS90@230 was a black solid that was much more resistant to breakage when handled. The apparent difference in mechanical strength may be attributed to the higher degree of crosslinking anticipated in RWS90@230 compared to that in RWS90@180; prior studies indicated that minimal reaction of polystyrene (comprising 16.4 wt% of RW) occurs at 180 °C, whereas extensive reactivity (i.e., Scheme 1C) is observed at 230 °C.60 Further evidence for crosslink density dependence on temperature was also derived from thermal, morphological and mechanical tests (vide infra). Both composites were remeltable and could be readily poured into molds to prepare shapes for mechanical testing or applications (Fig. 1). The viability of the process for scaleup to the kilogram scale was further validated by preparing a brick (∼1 kg) of RWS90@230.FT-IR spectroscopy is often a useful technique providing evidence of S–C bond-formation following the reaction of organics with elemental sulfur. However, it is sometimes difficult to discern the already weak S–C stretch in spectra for HSMs comprising 90 wt% sulfur. For this reason, HSMs are often depolymerized using LiAlH4, which breaks S–S bonds and converts the S–C crosslink points to thiols.9 This significantly decreases the presence of sulfur in the material while retaining S–C bonds for analysis by IR spectroscopy with an improved signal-to-noise ratio. Depolymerization of RWS90@180 and RWS90@230 by a modification of the procedure reported by Pyun9 gave d-RWS90@180 and d-RWS90@230, respectively. Evidence of S–C bonds was observed as a broad stretch at 697 cm−1 in the depolymerized materials (Fig. 2).
 | ||
| Fig. 2 FT-IR spectra for RWS90@180 (black trace), d-RWS90@180 (gray trace), RWS90@230 (dark blue trace), and d-RWS90@230 (light blue trace) demonstrate the formation of S–C bonds evident by the band at 697 cm−1. ESI Fig. S11–S14† provide the full spectra for these materials. | ||
Elemental homogeneity was confirmed through scanning electron microscopy with elemental mapping by energy dispersive X-ray (SEM/EDX). These images showed little evidence of phase separation on the surface of the material on a 100 μm scale with an even distribution of carbon, oxygen, and sulfur throughout both RWS90@180 and RWS90@230 (Fig. S15 and S16†). Another measure of elemental distribution is the extent to which added sulfur is present as oligo- or poly-sulfur catenates covalently bound to organic comonomers versus being present as physically entrapped sulfur species known as “dark sulfur”.65 The amount of dark sulfur in RWS90@180 and RWS90@230 was quantified using a recently published method,66 wherein the dark sulfur in HSMs is extracted into ethyl acetate and quantified using UV-vis spectroscopy. RWS90@180 and RWS90@230 were determined to contain 64 wt% and 52 wt% dark sulfur, respectively. The greater proportion of dark sulfur in RWS90@180 is consistent with less crosslinking at the lower reaction temperature. The dark sulfur contents of RWS90@180 and RWS90@230 are also similar to those of HSMs prepared from thiocracking of 10 wt% polystyrene with 90 wt% sulfur at 230 °C, which contained 40–54 wt% dark sulfur.60
2.3 Thermal and morphological properties of composites
Thermogravimetric analysis (TGA) of RWS90@180 and RWS90@230 (Table 2 and Fig. S26, S27†) were performed to evaluate their thermal stability. The decomposition temperature (Td, defined as the temperature at which 5% mass loss has occurred) was determined to be 200 °C for RWS90@180 and 217 °C for RWS90@230, compared to 229 °C for elemental cyclo-S8. Mass loss in these materials is attributed to the sublimation of sulfur, both from dark sulfur and from sulfur formed during the thermal breakdown of polysulfur crosslinking chains. Smaller mass loss steps at higher temperatures are likely attributable to the decomposition of cellulose derivatives and organosulfur aryls formed from polystyrene-derived domains.| Material | T d °C | T m °C | T g °C | ΔHm J g−1 | ΔHcc J g−1 | Percent crystallinityd |
|---|---|---|---|---|---|---|
| a Temperature at which 5% mass loss is observed. b Temperature at the peak maximum of the endothermic melting from the third heating cycle. c Glass transition temperature. d Reduction of percent crystallinity of each sample calculated with respect to sulfur (normalized to 100%). | ||||||
| RWS90@180 | 200 | 118 | −38, −11 | 38 | −3, −5 | 72–77 |
| RWS90@230 | 217 | 117 | NA | 33 | −23 | 21 |
| S8 | 229 | 118 | NA | 44.8 | NA | 100 |
Differential scanning calorimetry (DSC) measurements (Table 2 and Fig. S37, S38†) further elucidated the thermomorphological properties of the composites. Both RWS90@180 and RWS90@230 exhibited a prominent melting event at 118 °C and 117 °C, respectively, consistent with the melting of orthorhombic sulfur. RWS90@230 exhibited a single cold crystallization peak at 23 °C and did not display an observable glass transition temperature (Tg). In contrast, RWS90@180 showed two cold crystallization events at −10 °C and 36 °C and a glass transition at −38 °C. The Tg in RWS90@180 is attributable to polymeric sulfur domains, confirming the presence of relatively long sulfur catenates in this material. Calculations based on melting and cold crystallization enthalpies indicate that RWS90@180 has a percent crystallinity of 72–77%, while RWS90@230 exhibits a significantly lower crystallinity of 21%. Both the absence of a Tg in the range typically observed for HSMs containing longer sulfur catenates and a lower observed crystallinity in RWS90@230 are consistent with the anticipated higher degree of crosslinking and concomitantly shorter sulfur catenates at the higher reaction temperature.
2.4 Mechanical properties and microstructures
Cylinders suitable for compressive strength analysis were prepared by melting each composite and pouring the molten materials into silicon molds. All compressive strength samples were allowed to sit for 96 h at room temperature before analysis to allow for post-reaction crosslinking, following standard conventions for other HSMs.45 The compressive strength of RWS90@180 was determined to be 12.2 ± 0.8 MPa, and RWS90@230 was found to have a compressive strength of 16.3 ± 0.6 MPa (Fig. S39 and S40†). These values are competitive with the minimum requirements for OPC in building foundations67 and 142% and 190% stronger than C62 brick, respectively. These composites made from multi-component restaurant waste have lower compressive strengths compared to other HSMs such as APS95 (made from allylated peanut shells and sulfur)52H0O10S90 (made from peanut oil, peanut shells, and sulfur) and WFFS90 (made from French fries and sulfur).55 However, the restaurant waste derived composites have comparable compressive strengths to those of HSMs made from polystyrene60 (more details on the composition of these composites are provided in the footnote to Table 3, and a visual synopsis of these data is provide in Fig. 3). A qualitative compressive strength test was also performed wherein a ∼1 kg brick of RWS90@230 was prepared. The brick's compressive strength was demonstrated by holding the weight of a 4-door sedan without any obvious physical deformation or damage to the brick. The improved mechanical properties of RWS90@230 (16.3 ± 0.6 MPa compressive, 5.39 ± 0.81 MPa flexural) compared to RWS90@180 are attributed to greater crosslink density, as evidenced by a 12% lower dark sulfur content and a moderately higher decomposition temperature (217 °C versus 200 °C). Significantly reduced crystallinity (21% versus 72–77%) of the sample made at 230 °C versus 180 °C also supports a denser, more amorphous network structure consistent with enhanced crosslinking. The denser crosslinking is also consistent with the report that polystyrene (comprising 16.4% of organics in restaurant waste) is not effectively crosslinked upon heating with sulfur at 180 °C, but is at 230 °C.60| Material | Compressive strength (MPa) | After acid (MPa) | Strength retained (%) | Flexural strength (MPa) |
|---|---|---|---|---|
| a Composite of allylated peanut shells (5 wt%) and sulfur (95 wt%).52 b Composite of peanut oil (10 wt%) and sulfur (90 wt%).55 c Composite of French fries (10 wt%) and sulfur (90 wt%).55 d Composite of a polystyrene cup (10 wt%) and sulfur (90 wt%).60 e Composite of a polystyrene lid (10 wt%) and sulfur (90 wt%).60 f Brick classification C62 for building brick with normal weathering.68 | ||||
| RWS90@180 | 12.2 ± 0.8 | 8.2 ± 1.0 | 67% | 2.80 ± 0.42 |
| RWS90@230 | 16.3 ± 0.6 | 14.9 ± 0.6 | 91% | 5.39 ± 0.81 |
| APS95 | 35.7 | ND | ND | 4.8 |
| H0O10S90 | 25.9 | ND | ND | ND |
| WFFS90 | 33.8 | ND | ND | ND |
| PSC90 | 7.7 | ND | ND | 2.15 |
| PSL90 | 12.8 | ND | ND | 1.92 |
| C62 brickf | 8.6 | ND | ND | ND |
| OPC | 17 | ND | ND | 3.7 |
 | ||
| Fig. 3 Compressive strengths of RWS90@180 and RWS90@230 compared to other high sulfur-content materials and commercial building materials. | ||
While mineral-based building materials like OPC are used in various construction applications, they exhibit some weaknesses, especially when exposed to acid or water. Sulfur's acid-resistance and hydrophobic properties can be imbued to the resulting HSMs. After submersion in 0.5 M H2SO4 for 24 h, RWS90@180 and RWS90@230 retained 67% and 91% of their strength, respectively. Under exposure to the same conditions, mineral-based legacy materials such as OPC dissolve. A main point of failure for OPC comes from the expansion and contraction of the material due to absorbed water freezing and thawing within the material, which can cause significant cracks. OPC can absorb up to 28% of its weight in water; conversely, following submersion in water for 24 h, RWS90@180 absorbs only 0.45% of its weight in water, and RWS90@230 exhibits a remarkably low 0.13% uptake of water.
Rectangular prisms for flexural strength determination by dynamic mechanical analysis (DMA, Table 3 and Fig. S41, S42†) were prepared by melting each material and pouring into molds. All samples were milled to a thickness of 1.5 mm and allowed to sit for 96 h at room temperature before testing. RWS90@180 was determined to have a flexural strength of 2.80 ± 0.42 MPa, similar to other HSMs prepared from polystyrene (PSC90 and PSL90 in Table 3). RWS90@230 was found to have a flexural strength of 5.39 ± 0.81 MPa which exceeds the average value of OPC's flexural strength of 3.7 MPa.
The successful incorporation of sulfur and organic components into an insoluble crosslinked composite, along with literature precedent for the reactivity of various components found in the fast food restaurant waste, allows us to propose the microstructural features of the restaurant waste-derived composites RWS90@T (Scheme 2). Prior studies establish S–C bond formation via inverse vulcanization (Scheme 1A) of plant oils such as the peanut oil in the restaurant waste,42,47–51 which takes place in tandem with transesterification in the presence of cellulose and starch (Scheme 1B).53,56–59 Polystyrene reacts with sulfur to form structural motifs A–E (Scheme 1C).60 All of these mechanisms are expected to occur when elemental sulfur reacts with the complex mixture of materials found in fast food restaurant waste to give structures such as those shown in Scheme 2.
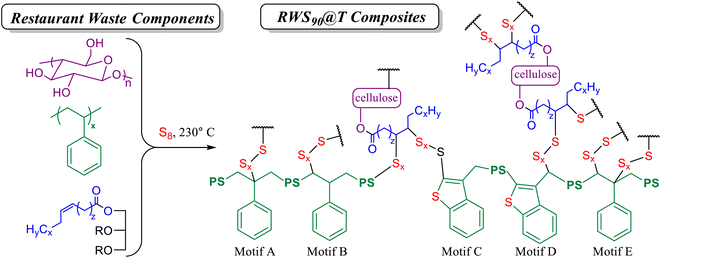 | ||
| Scheme 2 Representative structural features of composites RWS90@T, where PS indicated polystyrene chains, which will be further crosslinked at other positions along the chain. Polystyrene-derived motifs A–E are as based on our published study,60 as shown in Scheme 1C. | ||
2.5 Environmental impact
To fully assess the viability of RWS90@180 and RWS90@230 to serve as a replacement for mineral-based legacy materials such as OPC, it is essential to analyze the material's environmental impact. One metric that can help provide insight into the environmental impact is the E factor,63 which compares the amount of waste produced versus the amount of useful products created. A lower E factor correlates to less waste generated, whereas a higher E factor reflects a material that produces a greater amount of waste. For RWS90@180 and RWS90@230, the E factor was calculated based on the masses in Table 1; each part of the waste was included in the organic component in the preparation of the materials. Thus, the only waste comes from mass loss experienced during the reaction. The following calculations reflect the values had the total amount of waste collected been converted to HSMs:The composite RWS90@180 has an E factor of 0.1, whereas RWS90@230 has an impressive E factor of 0, as there was no waste in this process. These values are remarkably lower compared to many other commercial materials such as bulk commercial chemicals which can have E factors ranging from less than 1 to greater than 50,69,70 and OPC which has an E factor of 1.4.69
While the E factor provides insight into the environmental impact based on mass/atom balance, it does not account for energy consumption or emissions. To gain a more complete picture of the environmental impact of these materials, the global warming potential estimates the kilograms of carbon dioxide emitted per kilogram of useful material produced. For RWS90@180, the global warming potential was determined to be −0.00450 kg CO2e per kg whereas the global warming potential for RWS90@230 was determined to be +0.00450 kg CO2e per kg (the full scope and calculations can be found in the ESI†). Compared to the global warming potential of OPC (+1.0 kg CO2e per kg), whose carbon dioxide emissions account for roughly 8% of CO2 production, the values found for the RW–sulfur composites are far lower.71
3. Conclusions
This study demonstrates a noteworthy new approach for upcycling heterogeneous fast-food waste into high strength, sulfur-based composites via a one-pot thiocracking process. By employing a mixture of processed restaurant waste pulp (10 wt%) with elemental sulfur (90 wt%) at two reaction temperatures (180 °C and 230 °C), composites RWS90@180 and RWS90@230 were synthesized. These composites exhibit competitive mechanical properties relative to conventional construction materials. Notably, the composite prepared at 230 °C shows enhanced compressive (16.3 ± 0.6 MPa) and flexural strengths (5.39 ± 0.81 MPa), which can be attributed to a higher degree of crosslinking and shorter sulfur catenate lengths, as well as reduced dark sulfur content. These improvements are further reflected in the superior resistance of RWS90@230 to acid challenge and its remarkably low water uptake.72–87Importantly, the scalability of the process was validated by preparing a ∼1 kg brick of RWS90@230, underscoring the method's potential for industrial application. Additionally, the environmental impact assessments reveal exceptionally low E factors (0.1 for RWS90@180 and 0 for RWS90@230) and favorable global warming potential metrics, highlighting the minimal waste generation and reduced CO2 emissions associated with the process. This work not only provides a practical avenue for valorizing multi-material post-consumer waste but also offers a scalable route for transforming an abundant industrial byproduct, elemental sulfur, into value-added, durable construction materials.
Although the composites described here comprise only 10 wt% fast food waste, this method represents a scalable strategy for valorizing complex multi-material post-consumer waste streams and may contribute to reducing petroleum use in applications such as asphalt, for which sulfur-based alternatives have been previously explored.
Quantitative adhesion and wettability characterization (e.g., lap shear and contact angle measurements) are currently underway. Future studies will also aim at further optimizing reaction parameters and broadening the feedstock scope are underway to extend the applicability of this approach.
4. Experimental section
4.1 General considerations
A Blendtec Total blender was used to blend the restaurant waste used in this study.Proton NMR spectra were acquired on a Bruker NEO-300 MHz at room temperature, and data was processed with MestReNova x64-14.3-30573 software. All spectra reported were calibrated to the residual solvent peak from deuterated chloroform.
Fourier transform infrared spectra were obtained using a Shimadzu IR Affinity-1S instrument with an ATR attachment operating over 400–4000 cm−1 at ambient temperature.
UV-vis data was collected on an Agilent Technologies Cary 60 UV-Vis using Simple Reads software. The data was collected at 275 nm.
SEM and EDX were acquired on a Schottky Field Emission Scanning Electron Microscope SU5000 operating in variable pressure mode with an accelerating voltage of 15 keV.
Thermogravimetric analysis (TGA) data were recorded on a TA SDT Q600 instrument over the range 25 to 800 °C, with a heating rate of 10 °C min−1 under a flow of N2 (20 mL min−1).
DSC data were acquired (Mettler Toledo DSC 3 STARe System) over the range −60 to 140 °C with a heating rate of 10 °C min−1 under a flow of N2 (200 mL min−1). Each DSC measurement was carried out over three heat-cool cycles.
For percent crystallinity calculations, Tm, ΔHm and ΔHcc, the data was taken from the third heat/cool cycle. Melting enthalpies and cold crystallization enthalpies were calculated using DSC data. The reduction of the percent crystallinity of the composite RWS90@180 and RWS90@230 with respect to sulfur was calculated using the following equation.
 – melting enthalpy of composite materials (RWS90@180 or RWS90@230),
– melting enthalpy of composite materials (RWS90@180 or RWS90@230),  – cold crystallization enthalpy of composite materials, ΔHm(S) – melting enthalpy of sulfur, ΔHcc(S) – cold crystallization enthalpy of sulfur.
– cold crystallization enthalpy of composite materials, ΔHm(S) – melting enthalpy of sulfur, ΔHcc(S) – cold crystallization enthalpy of sulfur.
Compressional analysis was performed on a Mark-10 ES30 test stand equipped with a M3-200 force gauge (1 kN maximum force with ±1 N resolution) with an applied force rate of 3–4 N s−1. Compression cylinders were cast from silicone resin molds (Smooth-On Oomoo® 25 tin-cure) with diameters of approximately 6 mm and heights of approximately 10 mm. Samples were manually sanded to ensure uniform dimensions and measured with a digital caliper with ±0.01 mm resolution. Compressional analysis was performed in triplicate, and the results were averaged.
Flexural strength analysis was performed using a Mettler Toledo DMA 1 STARe System in single cantilever mode. The samples were cast from silicone resin molds (Smooth-On Oomoo® 30 tin-curve). The sample dimensions were approximately 1.5 × 10 × 23 mm. Flexural analysis was performed in triplicate and the results were averaged. The clamping force was 1 cN m.
4.2 Materials, methods, and safe handling warning
Sulfur powder (99.5%) was purchased from Alfa Aesar. Post-consumer restaurant waste (packaging and French fries) was collected from local chain restaurants on the day of disposal by customers. Post-consumer French fries were produced from Idaho potatoes produced in Idaho Falls, Idaho, USA, in the 2023 growing season. Composites were allowed to stand at room temperature for 4 days prior to mechanical testing.CAUTION: Heating elemental sulfur with organics can consequently result in H 2 S or other gas formation. Such gases can be corrosive, foul-smelling, and toxic. Temperature must be cautiously controlled in order to prevent thermal spikes, contributing to the potential for H 2 S or other gas evolution. Rapid stirring shortened heating times, and very slow addition of reagents can help avoid unforeseen temperature spikes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol until there was no more evolution of H2 gas. The mixture was filtered and washed with DI H2O (pH = 5) and the organic layer separated and the solvent removed to yield 7 mg of a brown crystalline solid.
ethanol until there was no more evolution of H2 gas. The mixture was filtered and washed with DI H2O (pH = 5) and the organic layer separated and the solvent removed to yield 7 mg of a brown crystalline solid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol until there was no more evolution of H2 gas. The mixture was filtered and washed with DI H2O (pH = 5) and the organic layer separated and the solvent removed to yield 7 mg of a tan crystalline solid.
ethanol until there was no more evolution of H2 gas. The mixture was filtered and washed with DI H2O (pH = 5) and the organic layer separated and the solvent removed to yield 7 mg of a tan crystalline solid.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by The National Science Foundation grant number CHE-2203669 to RCS and ACREC grant to RCS and ADS. The author primarily responsible for particular CRediT roles are provided here. K. M. D.: data curation, formal analysis, investigation, validation, writing – original draft. P. Y. S.-O.: supervision. A. D. S.: writing – review and editing, funding acquisition. A. G. T.: resources, supervision. R. C. S.: conceptualization, methodology, resources, supervision, funding acquisition, writing – original draft, review and editing.References
- Trash in America, Environment America Research & Policy Center, 2021, p. 2024 Search PubMed.
- Fast Food's Contribution to Food Waste, Skipshapiro Enterprises, 2024, p. 2024 Search PubMed.
- J. Jeong and J. Choi, Chemosphere, 2019, 231, 249 CrossRef CAS PubMed.
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Mar. Pollut. Bull., 2011, 62, 2588 CrossRef CAS PubMed.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Environ. Sci. Technol., 2012, 46, 3060 CrossRef CAS PubMed.
- US Geological Survey, Sulfur Production Worldwide in 2022, by Country, Statista, 2023, retrieved December 27, 2023, from https://www-statista-com.libproxy.clemson.edu/statistics/1031181/sulfur-production-globally-by-country/ Search PubMed.
- A. Tanimu and K. Alhooshani, Energy Fuels, 2019, 33, 2810 CrossRef CAS.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518 CrossRef CAS PubMed.
- J. Bao, K. P. Martin, E. Cho, K.-S. Kang, R. S. Glass, V. Coropceanu, J.-L. Bredas, W. O. N. Parker Jr, J. T. Njardarson and J. Pyun, J. Am. Chem. Soc., 2023, 145, 12386 CrossRef CAS PubMed.
- J. J. Griebel, R. S. Glass, K. Char and J. Pyun, Prog. Polym. Sci., 2016, 58, 90 CrossRef CAS.
- J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249 CrossRef CAS PubMed.
- J. J. Griebel, N. A. Nguyen, S. Namnabat, L. E. Anderson, R. S. Glass, R. A. Norwood, M. E. MacKay, K. Char and J. Pyun, ACS Macro Lett., 2015, 4, 862 CrossRef CAS PubMed.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, Á. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518 CrossRef CAS PubMed.
- J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, Top. Curr. Chem., 2019, 377, 16 CrossRef PubMed.
- A. Gupta, M. J. H. Worthington, H. D. Patel, M. R. Johnston, M. Puri and J. M. Chalker, ACS Sustain. Chem. Eng., 2022, 10, 9022 CrossRef CAS.
- N. A. Lundquist, A. D. Tikoalu, M. J. H. Worthington, R. Shapter, S. J. Tonkin, F. Stojcevski, M. Mann, C. T. Gibson, J. R. Gascooke, A. Karton, L. C. Henderson, L. J. Esdaile and J. M. Chalker, Chem.–Eur. J., 2020, 26, 10035 CrossRef CAS PubMed.
- P. Yan, W. Zhao, S. J. Tonkin, J. M. Chalker, T. L. Schiller and T. Hasell, Chem. Mater., 2022, 34, 1167 CrossRef CAS.
- I. Bu Najmah, N. A. Lundquist, M. K. Stanfield, F. Stojcevski, J. A. Campbell, L. J. Esdaile, C. T. Gibson, D. A. Lewis, L. C. Henderson, T. Hasell and J. M. Chalker, ChemSusChem, 2021, 14, 2352 CrossRef CAS PubMed.
- J. M. Chalker, M. Mann, M. J. H. Worthington and L. J. Esdaile, Org. Mater., 2021, 03, 362 CrossRef CAS.
- B. Zhang, H. Gao, P. Yan, S. Petcher and T. Hasell, Mater. Chem. Front., 2020, 4, 669 RSC.
- J. A. Smith, R. Mulhall, S. Goodman, G. Fleming, H. Allison, R. Raval and T. Hasell, ACS Omega, 2020, 5, 5229 CrossRef CAS PubMed.
- J. A. Smith, X. Wu, N. G. Berry and T. Hasell, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 1777 CrossRef CAS PubMed.
- D. J. Parker, S. T. Chong and T. Hasell, RSC Adv., 2018, 8, 27892 RSC.
- K. B. Sayer, V. L. Miller, Z. Merrill, A. E. Davis and C. L. Jenkins, Polym. Chem., 2023, 14, 3091 RSC.
- A. E. Davis, K. B. Sayer and C. L. Jenkins, Polym. Chem., 2022, 13, 4634 RSC.
- C. R. Westerman and C. L. Jenkins, Macromolecules, 2018, 51, 7233 CrossRef CAS.
- J. Dunn and C. L. Jenkins, Nat. Synth., 2022, 1, 835 CrossRef CAS.
- C. Herrera, K. J. Ysinga and C. L. Jenkins, ACS Appl. Mater. Interfaces, 2019, 11, 35312 CrossRef CAS PubMed.
- C. R. Westerman, P. M. Walker and C. L. Jenkins, J. Visualized Exp., 2019, e59620 Search PubMed.
- K. Orme, A. H. Fistrovich and C. L. Jenkins, Macromolecules, 2020, 53, 9353 CrossRef CAS.
- M. L. Eder, C. B. Call and C. L. Jenkins, ACS Appl. Polym. Mater., 2022, 4, 1110 CrossRef CAS.
- S. Diez, A. Hoefling, P. Theato and W. Pauer, Mechanical and Electrical Properties of Sulfur-Containing Polymeric Materials Prepared via Inverse Vulcanization, Polymers, 2017, 9, 59 CrossRef PubMed.
- H. Mutlu, E. B. Ceper, X. Li, J. Yang, W. Dong, M. M. Ozmen and P. Theato, Macromol. Rapid Commun., 2019, 40, 1800650 CrossRef PubMed.
- M. E. Duarte, B. Huber, P. Theato and H. Mutlu, Polym. Chem., 2020, 11, 241 RSC.
- I. Gomez, O. Leonet, J. A. Blazquez and D. Mecerreyes, ChemSusChem, 2016, 9, 3419 CrossRef CAS PubMed.
- I. Gomez, D. Mecerreyes, J. A. Blazquez, O. Leonet, H. Ben Youcef, C. Li, J. L. Gómez-Cámer, O. Bondarchuk and L. Rodriguez-Martinez, J. Power Sources, 2016, 329, 72 CrossRef CAS.
- I. Gomez, O. Leonet, J. Alberto Blazquez, H.-J. Grande and D. Mecerreyes, ACS Macro Lett., 2018, 7, 419 CrossRef CAS PubMed.
- I. Gomez, A. F. De Anastro, O. Leonet, J. A. Blazquez, H.-J. Grande, J. Pyun and D. Mecerreyes, Macromol. Rapid Commun., 2018, 39, 1800529 CrossRef PubMed.
- M. S. Karunarathna, M. K. Lauer, A. G. Tennyson and R. C. Smith, Polym. Chem., 2020, 11, 1621 RSC.
- C. V. Lopez, C. P. Maladeniya and R. C. Smith, Lithium-Sulfur Batteries: Advances and Trends, Electrochem, 2020, 1, 226 CrossRef.
- M. S. Karunarathna, M. K. Lauer and R. C. Smith, J. Mater. Chem. A, 2020, 8, 20318 RSC.
- C. V. Lopez, A. D. Smith and R. C. Smith, RSC Adv., 2022, 12, 1535 RSC.
- M. K. Lauer, A. G. Tennyson and R. C. Smith, Mater. Adv., 2021, 2, 2391 RSC.
- A. D. Smith, A. G. Tennyson and R. C. Smith, Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies, Sustainable Chem., 2020, 1, 209 CrossRef.
- M. K. Lauer, T. A. Estrada-Mendoza, C. D. McMillen, G. Chumanov, A. G. Tennyson and R. C. Smith, Adv. Sustainable Syst., 2019, 3, 1900062 CrossRef CAS.
- M. K. Lauer, A. G. Tennyson and R. C. Smith, ACS Appl. Polym. Mater., 2020, 2, 3761 CrossRef CAS.
- A. D. Smith, T. Thiounn, E. W. Lyles, E. K. Kibler, R. C. Smith and A. G. Tennyson, J. Polym. Sci., Part A:Polym. Chem., 2019, 57, 1704 CrossRef CAS.
- A. D. Smith, C. D. McMillin, R. C. Smith and A. G. Tennyson, J. Polym. Sci., 2020, 58, 438 CrossRef CAS.
- C. V. Lopez, M. S. Karunarathna, M. K. Lauer, C. P. Maladeniya, T. Thiounn, E. D. Ackley and R. C. Smith, J. Polym. Sci., 2020, 58, 2259 CrossRef CAS.
- C. P. Maladeniya, M. S. Karunarathna, M. K. Lauer, C. V. Lopez, T. Thiounn and R. C. Smith, Mater. Adv., 2020, 1, 1665 RSC.
- C. P. Maladeniya, N. L. K. Dona, A. D. Smith and R. C. Smith, Macromol, 2023, 3, 681 CAS.
- M. K. Lauer, M. S. Karunarathna, A. G. Tennyson and R. C. Smith, Materials Advances, 2020, 1, 590 RSC.
- M. K. Lauer, M. S. Karunarathna, A. G. Tennyson and R. C. Smith, Materials Advances, 2020, 1, 2271 RSC.
- M. K. Lauer, Z. E. Sanders, A. D. Smith and R. C. Smith, Mater. Adv., 2021, 2, 7413 RSC.
- B. G. S. Guinati, P. Y. Sauceda Oloño, N. L. Kapuge Dona, K. M. Derr, S. K. Wijeyatunga, A. G. Tennyson and R. C. Smith, RSC Sustainability, 2024, 2, 1819–1827 RSC.
- C. P. Maladeniya, A. G. Tennyson and R. C. Smith, J. Polym. Sci., 2023, 61, 787 CrossRef CAS.
- K. M. Derr, C. V. Lopez, C. P. Maladeniya, A. G. Tennyson and R. C. Smith, J. Polym. Sci., 2023, 61, 3075 CrossRef CAS.
- M. K. Lauer, M. S. Karunarathna, A. G. Tennyson and R. C. Smith, Materials Advances, 2020, 1, 590 RSC.
- K. M. Derr and R. C. Smith, Thiocracking of Multi-Materials: High-Strength Composites from Post-Consumer Food Packaging Jars, Sustainability, 2024, 16, 7023 CrossRef CAS.
- S. K. Wijeyatunga, A. G. Tennyson and R. C. Smith, ACS Sustainable Resour. Manage., 2024, 1, 2173 CrossRef CAS.
- S. K. Wijeyatunga, K. M. Derr, C. P. Maladeniya, P. Y. Sauceda-Oloño, A. G. Tennyson and R. C. Smith, J. Polym. Sci., 2024, 62, 554 CrossRef CAS.
- K. M. Derr and R. C. Smith, J. Polym. Sci., 2023, 62, 1115 CrossRef.
- R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC.
- B. G. S. Guinati, P. Y. Sauceda Oloño, N. L. Kapuge Dona, K. M. Derr, S. K. Wijeyatunga, A. G. Tennyson and R. C. Smith, RSC Sustainability, 2024, 2, 1819 RSC.
- J. J. Dale, S. Petcher and T. Hasell, ACS Appl. Polym. Mater., 2022, 4, 3169 CrossRef CAS.
- J. J. Dale, J. Stanley, R. A. Dop, G. Chronowska-Bojczuk, A. J. Fielding, D. R. Neill and T. Hasell, Eur. Polym. J., 2023, 195, 112198 CrossRef CAS.
- ACI Committee 332, Guide to Residential Concrete Construction, ACI 332.1R-06, American Concrete Institute, Farmington Hills, MI, 2006 Search PubMed.
- M. H. Riaz, A. Khitab, S. Ahmad, W. Anwar and M. T. Arshad, Asian Journal of Civil Engineering, 2020, 21, 243 CrossRef.
- T. V. T. Phan, C. Gallardo and J. Mane, Green Chem., 2015, 17, 2846 RSC.
- A. Dormer, D. P. Finn, P. Ward and J. Cullen, J. Cleaner Prod., 2013, 51, 133 CrossRef CAS.
- L. K. Turner and F. G. Collins, Constr. Build. Mater., 2013, 43, 125 CrossRef.
- K. E. Tomberlin, R. Venditti and Y. Yao, BioResources, 2020, 15, 3899 CAS.
- W. Russ and R. Meyer-Pittroff, Crit. Rev. Food Sci. Nutr., 2004, 44, 57 CrossRef PubMed.
- L. K. Brogaard, A. Damgaard, M. B. Jensen, M. Barlaz and T. H. Christensen, Resour., Conserv. Recycl., 2014, 87, 30 CrossRef.
- U. Arena and F. Ardolino, Resour., Conserv. Recycl., 2022, 183, 106379 CrossRef CAS.
- H. Jeswani, C. Krüger, M. Russ, M. Horlacher, F. Antony, S. Hann and A. Azapagic, Sci. Total Environ., 2021, 769, 144483 CrossRef CAS PubMed.
- F. Passarini, L. Ciacci, A. Santini, I. Vassura and L. Morselli, J. Cleaner Prod., 2012, 23, 28 CrossRef.
- W. Peukert, Int. J. Miner. Process., 2004, 74, S3 CrossRef CAS.
- G. Schubert and S. Bernotat, Int. J. Miner. Process., 2004, 74, S19 CrossRef CAS.
- D. Wüstenberg and J. Kasper, Int. J. Miner. Process., 2004, 74, S417 CrossRef.
- F. G. Müller, L. S. Lisboa and J. M. Chalker, Adv. Sustainable Syst., 2023, 7, 2300010 CrossRef.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748 RSC.
- T. Lee, P. T. Dirlam, J. T. Njardarson, R. S. Glass and J. Pyun, J. Am. Chem. Soc., 2022, 144, 5 CrossRef CAS PubMed.
- Y. Zhang, R. S. Glass, K. Char and J. Pyun, Polym. Chem., 2019, 10, 4078 RSC.
- J. Bao, K. P. Martin, E. Cho, K.-S. Kang, R. S. Glass, V. Coropceanu, J.-L. Bredas, W. O. N. Parker Jr, J. T. Njardarson and J. Pyun, J. Am. Chem. Soc., 2023, 145, 12386 CrossRef CAS PubMed.
- A. Amin, N. M. Mahmoud and Y. W. Z. Nisa, Green Mater., 2020, 8, 172 CrossRef.
- G. N. Lewis and M. Randall, J. Am. Chem. Soc., 1911, 33, 476 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta01674f |
| This journal is © The Royal Society of Chemistry 2025 |