 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The origin and evolution of molecular precursors for quantum dot synthesis
Mark
Green

Department of Physics, King's College London, The Strand, London, WC2R 2LS, UK. E-mail: mark.a.green@kcl.ac.uk
First published on 6th August 2024
Abstract
The 2023 Nobel Prize for Chemistry was awarded to Ekimov, Brus and Bawendi for the ‘discovery and synthesis of quantum dots.’ This was particularly gratifying for materials chemists as the award acknowledged the striking (and often overlooked) evolution of solution chemistry towards quantum-confined materials and solid-state materials in general. Traditionally, quantum dots have been highlighted for their remarkable optical properties (the ‘discovery’ part of the Nobel award), specifically the size-tuneable absorption and emission. In this review, we wish to highlight the origins and evolution of the synthetic chemistry, the development of which has ultimately led to the emergence of novel biological probes, solar cells and light emitting devices.
The now-routine availability of semiconductor quantum dots that exhibit size quantization effects is often taken for granted. These materials are now commercially available, and thousands of papers exist describing synthetic approaches to quantum dots, yet the advent of this field can be traced to a single report.1 This pioneering publication described a solution-based organometallic pathway to quantum dots of unparalleled quality, on a scale that allowed chemists, materials scientists, physicists and biologists – anyone who was used to handling lab samples – access to these hitherto unavailable nanomaterials. To the synthetic inorganic chemists with interests in the solid-state, the inception of solution-based precursors was of the utmost importance. It would, however, be a mistake to assume that the synthetic chemistry was a simple series of obvious reactions without deeper underlying studies. The 1993 publication was the culmination of two simultaneous, distinct and separate advances (the majority of which was developed at AT&T Bell labs under the guidance of Brus and Steigerwald), notably precursor and capping agent chemistries.
Capping agent chemistry
The development of the capping agents utilised in the Murray paper is relatively linear, the origins of which can be traced back to aqueous colloidal semiconductors developed for solar energy applications. In 1984, Henglein reported a water-based colloidal synthesis of CdS which utilised polyphosphates as surfactants and is considered a key step in quantum dot surface chemistry,2 as this allowed the particles to be isolated as a re-dispersible powder ‘without the loss of Q-state properties’ as opposed to the particles being held in a static matrix such as a glass, which is how size quantization effects were initially observed by Ekimov.3At AT&T Bell labs, interest in the development of an engineered surface species for colloidal semiconductors was extended to the use of inorganic monomers to passivate QDs grown in inverse micelles. A 1988 report by Steigerwald is significant as one of the first examples of the use of organometallic precursors in quantum dot synthesis, utilising a range of bis(silylated)chalcogen compounds in the preparation of capped CdSe, CdTe and HgSe rather than the then-typical and easily prepared CdS colloids.4 Specifically, metal salts and either bis(trimethylsilyl)selenium or bis(tert-butyldimethylsilyl)tellurium were added to preformed microemulsions immediately forming metal chalcogenide quantum dots in the micelle core. Importantly, the fully-formed particles were then further reacted with phenyl(trimethylsilyl)selenium, yielding phenyl-capped particles via a unique mix of organometallic and colloidal chemistry, allowing the particles to be isolated and redispersed in pyridine. The particles showed a blue shift in the absorption spectra relative to the bulk semiconductor band gap, consistent with quantum confinement, and were clearly crystalline as highlighted by powder X-ray diffraction and electron microscopy (Fig. 1). A significant extension of this work described the preparation of various core/shell structures, notably CdSe/ZnS complete with a terminal phenyl organic cap, using micelles and the same organometallic precursors.5 The precursors were added sequentially to form the core/shell morphologies, which were then annealed in 4-ethylpyridine. The bright emission from (CdSe)1(ZnS)4Ph – the nomenclature describing a CdSe core, and a ZnS shell of equal thickness to the core, due to 4 equivalents of shell precursor added, and a final terminal phenyl group – is the basis for the now universal CdSe/ZnS particles capped with organic groups. The resulting particles were again crystalline as determined by powder X-ray diffraction and electron microscopy, showed evidence of size quantization effects in the absorption spectra, and importantly, displayed band edge emission after passivation by the inorganic shell, as shown in Fig. 2.
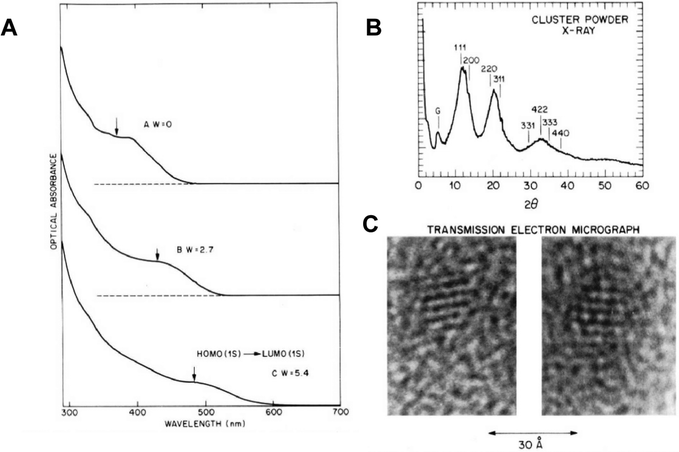 | ||
| Fig. 1 (A) Absorption spectra of CdSe nanocrystals prepared by varying synthesis conditions, showing differing blue shifted band edges and hence different sizes. (B) X-ray powder diffraction of CdSe nanoparticles. (C) Electron microscope image of CdSe nanoparticles, scale bar = 3 nm. All particles prepared by the micelle methods as described in ref. 4. Reprinted (adapted) with permission from M. L. Steigerwald, A. P. Alivisatos, J. M. Gibson, T. D. Harris, R. Kortan, A. J. Muller, A. M. Thayer, T. M. Duncan, D. C. Douglass, L. E. Brus, J. Am. Chem. Soc., 1988, 110, 3046–3050. Copyright 2024, American Chemical Society. | ||
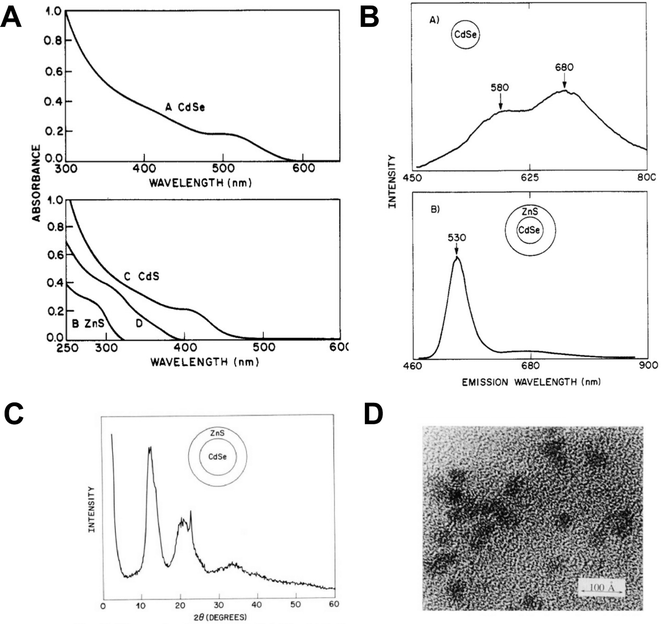 | ||
| Fig. 2 (A) Absorption spectra of A CdSe quantum dots showing excitonic shoulder. B ZnS particles made in a micelle. C CdS particles made in a similar fashion and D product of a ZnS particle reacted with a Cd ion, possibly producing an alloy. (B) Room temperature emission spectra of A CdSe particles with a phenyl surface and B (CdSe)1(ZnS)4Ph, which might be described as CdSe/ZnS core/shell quantum dots with a phenyl passivated surface. (C) X-ray powder diffraction pattern from (CdSe)1(ZnS)4Ph. (D) Electron microscope image of (ZnS)1(CdSe)4Ph quantum dots, which might now be described as an inverse core/shell with a phenyl passivated surface, scale bar = 10 nm. All particles prepared as described in ref. 5. Reprinted (adapted) with permission from A. R. Kortan, R. Hull, R. L. Opila, M. G. Bawendi, M. L. Steigerwald, P. J. Carroll, L. E. Brus, J. Am. Chem. Soc., 1990, 112, 1327–1332. Copyright 2024, American Chemical Society. | ||
The use of pyridine-based compounds was continued by Bawendi who utilised 4-ethylpyridine as a relatively high temperature Lewis base solvent to anneal the resulting QDs for powder X-ray diffraction studies.6 This study was extended to the use of other Lewis base species such as tributylphosphine, and tributylphosphine oxide, both possessing relatively high boiling points and hence providing a higher annealing temperature. It is reported that the use of the phosphine oxide was a serendipitous discovery, after Bawendi realised that annealing quantum dots in tributylphosphine from an old bottle was more effective than using the same solvent from a new bottle.7
Initial precursor development
Alongside the nanoparticle surface and micelle work at AT&T Bell Labs, solution-based precursor chemistry was also being simultaneously developed by Steigerwald and can be broken into three interweaving strands, emerging approximately over the same time period – notably single source precursors, phosphine chemistry, and binary organometallic precursors; all of which made significant contributions over the following three decades.Traditionally, precursors for compound semiconductors have been purely functional, with the simplest starting materials employed with little obvious thought given to molecular design, or their suitability for use in a standard inorganic or materials chemistry research lab. The genesis of precursors for the solution synthesis of nanosized semiconductors can be traced back to a report describing the use of ditellurides as replacements for the undesirably stable monotellurides in the OMVPE (organometallic vapour phase epitaxy) of CdTe thin films.8 The same group then examined the thermolysis of the compound [Hg(TePh)2]n as a comparative study, as this inorganic single source precursor was the product of a reaction between elemental mercury and diphenylditelluride.9 The synthesis of this compound was then replicated by the reaction of HgCl2 and two molar equivalents of PhTeLi, and thermolysis of the complex, under vacuum and at the surprisingly mild temperature of 120 °C (over 24 hours) yielded polycrystalline HgTe powder and Ph2Te. This reaction was then applied to solution synthesis, where the 4-methylphenyl analogue was thermolyzed in C6D6 and PEt3, generating HgTe and bis(4-methylphenyl)telluride. What is significant about the study, beyond being arguably one of the first solution synthetic pathways to compound semiconductors, is the unusual depth in which the group examined the reaction mechanisms, suggesting the reversable formation of the precursor from the ditelluride and metal, and the product of its ultimate thermolysis, as shown in reaction (1)
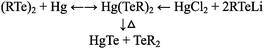 | (1) |
This approach was extended to the thermolysis of either Cd(SePh)2 or [Cd(SePh)2]2[Et2PCH2CH2PEt2] in vacuo which yielded bulk CdSe and Ph2Se.10 The precursors were prepared by the reaction between dimethylcadmium and benzeneselenol yielding the basic complex, whilst the diphosphine adduct was prepared by simple exposure of the complex to the phosphine compound. Refluxing the relatively insoluble Cd(SePh)2 in 4-ethylpyridine yielded CdSe quantum dots, with crystal lattice fringes observed by electron microscopy and, importantly, a tuneable absorption band edge showing a gradual red shift over 84 hours with a clear excitonic peak. A feature in the absorption spectra at 410 nm was assigned to what would become known as magic clusters, and although clusters were known by this point, having them observed during the synthesis of quantum dots through a molecular thermolysis process was remarkably far-sighted. Again, this publication is notable for the wealth of information provided for the quantum dots prepared by the thermolysis of molecular precursors, rather than materials prepared inside inverse micelles as described above.
These two initial studies were further developed to address a range of materials, using the precursor M(ER)2 (M = Zn, Cd, Hg; E = S, Se, Te; R = organic group), synthesised using a range of approaches, incorporating metal alkyls, selenols, thiols and other inorganic compounds.11 It was observed that again, the parent compound was usually insoluble until complexed to a diphosphine species, after which, single crystals could be isolated. The majority of these species were thermolyed in sealed tubes, to mimic the vapour deposition processes, except for the solution thermolysis of the Cd(TePh)2 precursor in 4-ethylpyridine giving CdTe quantum dots, with a shifted optical band edge and electron micrograph and diffraction patterns consistent with a wurtzite crystalline nanomaterial. The solution-based thermolysis of Hg(TePh)2 reportedly yielded nanostructured HgTe as described above, yet the particles rapidly proceeded through the nanoscale to the bulk phase. This was remedied by replacing the phenyl groups in the compound with n-butyl groups and exposing the resulting Hg(Te-n-Bu)2 complex, in a diphosphine/pyridine solution, to room light whereupon the structure photolytically decomposed yielding nanostructured HgTe quantum dots. Interestingly, the authors stated the reasons for choosing CdSe and CdTe for the studies into nanoparticles were the convenient decomposition temperatures of the precursors, and the spectral range of the nanomaterials prepared, which would shift from the near IR of the bulk materials, across the visible spectra to the UV region, thus making them simple to analyse with standard spectroscopy, unlike, for example, the zinc chalcogenides.
The initial advantages of such a single source approach are obvious; the use of just one, often air-stable, crystalline solid to deliver all elements required in the target semiconductor presents an elegant alternative to the use of multiple volatile compounds. However, barriers to their routine use exist. The inability to alter elemental ratios in the precursor limits some studies, and the atom efficiency of most single source precursors is low – which presents practical difficulties in adding large amounts of precursors to reactions, resulting in a significant quantity of unknown or undesired side products. The compounds can also be relatively inaccessible – single source compounds such as cadmium diselenocarbamates, (the use of which was reported just after the seminal quantum dot paper of Murray) required the use of starting materials such as CSe2, which is commercially unavailable, difficult to prepare and noxious.12 In such cases, the use of a binary precursor approach is easier and often preferable. Where the single source precursor is readily available (such as zinc diethyldithiocarbamate) and easier to use than, for example, the volatile silylated thiols, then the use of such complexes has been readily adopted, notably in the deposition of a ZnS shell on ‘naked’ quantum dots.13 Another notable single source precursor based on cluster compounds, (X)4[M10Se4(SPh)16] (X = Li+, (CH3)3NH+; M = Cd, Zn) has been utilised in the preparation of metal selenide quantum dots, with the clear advantage of using elemental selenium during the cluster synthesis rather than, for example, CSe2.14 Again, these compounds are based on much earlier work in the 1980s, on, for example, the preparation of fragments of a solid-state lattice15 and might be therefore thought of as ideal precursors, as they have already adopted a specific crystalline core structure.16 Indeed, the idea that the geometry of a precursor can influence the crystalline structure of the proceeding material has been explored by Barron, when using [(t-Bu)GaS]4 as a single source precursor towards GaS thin films by CVD.17 There are numerous examples of clusters that can potentially be used as precursors, or bridge the gap between molecules and quantum sized nanomaterials.18,19 Recently, magic sized clusters such as In37P20(O2CR)51 have been explored as effective single source precursors to InP quantum dots.20
Phosphine chalcogenides
Whilst the use of phosphines and phosphine oxides was described earlier as a capping agent development,6 their use is also intimately linked to precursor chemistry. Murray initiated the routine use of trialkylphosphine chalcogenides in his pioneering publication,1 and this could be argued as the most significant precursor development in quantum dot chemistry. Whilst the use of purely organometallic precursors for chalcogen delivery is both elegant and effective, allowing access to a range of previously unexploited solution-based solid-state materials, significant synthetic skills are required to prepare and handle the compounds. The use of, for example, trioctylphosphine selenide (TOPSe) is simpler, efficacious and provides a convenient route to a group VI element. Whilst the use of TOPSe is now ubiquitous in quantum dot synthesis and widely attributed to Murray et al., its initial utilisation was a much earlier development.Phosphine chalcogenides have been known since at least the 1960s, the preparation of which has changed little to this day – the simple dissolution of elemental chalcogens in the liquid phosphine. A brief report in 1963 on the reaction of a solution of tributylphosphine and elemental tellurium described the isolation of tributylphosphine telluride as a solid, which deposited elemental tellurium on surfaces upon standing, highlighting its potential as a group VI precursor.21 Similarly, a larger study with numerous other phosphines described the preparation and isolation of a range of solid phosphine tellurides that exhibited remarkable stability until exposed to oxygen or heated. Gentle heating of the compounds in saturated hydrocarbons resulted in the formation of ‘very even, shiny, tellurium mirrors on the surface of the glass’, thus confirming their suitability as thermal precursors for tellurium.22
It may be argued that the first (albeit inadvertent) synthesis of a nanocrystalline compound semiconductor using a phosphine telluride was reported in 1985 by Darkowski and Cocivera, who were exploring alternative, low valency and organically-soluble tellurium precursors for electro-deposited thin films, after being made aware of the above compounds by R. T. Oakley.23 The choice of tributylphosphine telluride as an intentionally low valency starting material (as compared to tellurium dioxide) was a surprisingly prescient example of molecular precursor design, as they reported the use of the higher valency compound yielded an alloy rather than the binary compound semiconductor.24
In this electrochemical solution deposition method,23,24 tri(n-butylphosphine)telluride was used with cadmium perchlorate in propylene carbonate, yielding amorphous CdTe thin films, which transformed to a polycrystalline material when annealed at 400 °C under an inert atmosphere. Upon heating, the films converted from p type to n type and the powder X-ray diffraction patterns suggested CdTe particles (or domains) as small as 50 nm, too large for quantum size effects, but notable for their observed nanoscale dimensions a year before the term ‘quantum dot’ was first used.25 During the investigation, anomalies observed in current density were attributed to a cadmium phosphine telluride intermediate, which was insightful as such transitional compounds were not generally suggested until specific studies into precursor chemistry highlighted complexes which could be thought of as in situ single source precursors.26
In 1988, Steigerwald, inspired by the earlier reports by Zingaro, reported an in-depth investigation into the use of phosphine tellurides as tellurium transport agents for the solution synthesis of HgTe.27 It was highlighted that there were few effective telluride precursors, and standard vapour deposition precursors such as dimethyl- and diethyl-telluride had, as previously mentioned, been problematic due to their inherent stability. This study is significant as it attempts to uncover the molecular basis of precursor reactions and suggested an equilibrium between the compound and free elemental chalcogenide and phosphine, and that tellurium was transported by the volatile phosphine as shown in eqn (2).
| R3P + Te ⇔ R3PTe | (2) |
This is important as semiconductors deposited by chemical vapour deposition (CVD) were usually the product of a molecular decomposition reaction, rather than elemental transport. Steigerwald reported a solution-based experiment to explore the (usual) vapour phase reactions with elemental mercury and Et3PTe, giving HgTe in quantitative yields, hypothesising that the telluride precursor was a coordinate complex of Te(0). As, at this point in time, alkylmercury compounds were the usual mercury precursors, the experiment was repeated using either Et2Hg or Ph2Hg, giving the same product, but in the case of Ph2Hg, Ph2Te and Ph2Te2 were observed as side products. Whilst referring to their previous work on the preparation of HgTe via the single source precursor Hg(TePh)2, a related mechanism was suggested;
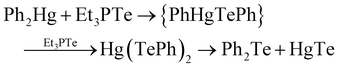 | (3) |
This report is therefore noteworthy as it is the first report of a simple (non-electrochemical) solution route to a compound semiconductor using phosphine tellurides, and the reaction explained by molecular inorganic chemistry; specifically, the insertion of Te atoms in the metal (Hg)–carbon bond of the metal alkyl, in a similar manner to other well-defined pathways such as Grignard reactions. Furthermore, as shown in the eqn (3) and discussed in more depth in the published report, the role of phosphine tellurides in materials synthesis is clearly more nuanced, with the product dependant on the ratio of precursors. A prime example of this would be reported much later – where one reaction between HgBr2 and tributylphosphine telluride gave the ring compound [Hg3(TePR3)3Br5]2[Hg2Br6].28
This use of trialkylphosphine telluride was then extended to the synthesis of numerous solid-state materials via intermediate organometallic complexes and clusters,29 again often described as fragments of the bulk lattice which effectively functioned as single source precursors; MnTe (via [(Et3P)2(CO)3MnTe]),30 NiTe (via Ni20Te18(PEt3)12),31 and Cr3Te4 (via Cr6Te8(PEt3)6).32 Likewise Ni3Se2 (via Ni23Se12(PEt3)13) was prepared from the selenium analogue,33 whilst an extension of the technique to the reaction between Co2(CO)8 and EPnBu3 (E = S, Se, Te) yielded Co6E8(PnBu3)6 (and Co4Se2(CO)6(PnBu3)4) which yielded β-CoTe, CoS and γ-CoSe when thermolyzed.34 This chemical route, combining zero valent metal complexes and the phosphine chalcogenide was described as a ‘masked atom approach’ as the individual precursors yielded the solid-state material of interest whilst liberating the ligands. The phosphine–tellurium interaction was described as a simple phosphorous to tellurium donor acceptor bond, which was simply broken by gentle heating as described in 1965 by Zingaro et al.,22 and the overall reaction was described thus:
| LnM(0) + R3PTe(0) → MxTey + nL + R3P | (4) |
Steigerwald's use of phosphine chalcogenides with other zero valent complexes, which he described as a ‘combination of atoms’ approach not only highlighted a generic route to II–VI materials in solution, but also uncovered the underlying inorganic chemistry suggesting that complexes that could be isolated and utilised as single source precursors. Whilst these types of clusters were developed in the 1980s, they are still of interest today, not as precursors, but as constituent parts of binary assemblies that mimic magnetic atomic solid-state structures, for example.35
Binary inorganic precursors
Discreet from the phosphine chemistry, phosphine-derived single source precursors and clusters, Steigerwald simultaneously developed solution-based binary precursor routes to II–VI semiconductors using metal alkyls and silylated chalcogenide compounds.36 These routes benefit from being non-ionic, and therefore could proceed in organic solvents which were not restricted to the limited boiling point of water and are routinely provided anhydrously. The silylated precursors utilised, whilst still toxic, are liquid and could be handled easier than gaseous reagents and presented a significant advantage to synthetic chemists.The initial reaction, between dimethylcadmium and bis(trimethylsilyl)selenide carried out in either toluene, heptane, THF or dichloromethane, gave an amorphous (or nanocrystalline) powder that required annealing at 400 °C for 4 hours to yield a polycrystalline material that exhibited a diffraction pattern identical to commercially available CdSe. This dealkylsilylation reaction scheme was extended to other II–VI semiconductor targets as shown in eqn (5)–(7)
| CdMe2 + Se(SiMe3)2 → CdSe + 2SiMe4 | (5) |
| ZnEt2 + Se(SiMe3)2 → ZnSe + 2SiEtMe3 | (6) |
| CdMe2 + Te(SiiPrMe2)2 → CdTe + 2SiiPrMe3 | (7) |
It was also observed that the single source precursor Cd(EPh2) (E = S, Se, Te), as described earlier, could also be prepared as shown in eqn (8).
| CdMe2 + 2PhESiMe3 → Cd(EPh)2 (E = S, Se, Te) | (8) |
Combining precursors and capping agent chemistry
Murray, Norris and Bawendi then combined the phosphine and phosphine oxide capping agents with the organometallic and phosphine chalcogenide precursors described above.1 Two methods were reported; the first and widely adopted method involved the use of Me2Cd and TOPE (E = Se, Te) and the second involving the use of Me2Cd and either Se(SiMe3)2, S(SiMe3)2 or Te(SiBuMe2)2. Both routes involved the thermolysis at elevated temperatures via injection of a tri-n-octylphosphine solution of the precursors under an inert atmosphere into hot (tri-n-octylphosphine oxide) as a direct solution synthesis of phosphine/phosphine oxide passivated quantum dots, negating the need for microemulsions. Deeper, intricate details of capping agent chemistry, now itself a major research discipline, have been described elsewhere,37 with the essential role of impurities38 and the ‘magic bottle’ effect39 being of particular note. Other elegant aspects of this reaction include the separation of the nucleation and growth steps using the sudden introduction of the room temperature (and hence cooler) precursor solution into the hot solvent system (termed hot injection), and the ability to controllably precipitate out different particle sizes using solvent/non-solvent interaction, termed size-selective precipitation. The extremely high quality of the quantum dots produced, in terms of both optical properties and structural purity, allowed detailed chemical, materials and physical analysis to be undertaken and new solid-state phenomena to be uncovered. Notably, the highly crystalline materials showed clear evidence of quantum confinement, not only as a blue shift in the band gap but also by the emergence of discrete energy levels in the absorption spectra and band edge emission without the needs for a further inorganic shell (Fig. 3). The exquisite control over particle size, coupled with the ease of manipulation and superior optical properties led to an immediate explosion of related quantum dot research ultimately resulting in the award of the Nobel prize, in part, for the synthetic chemistry.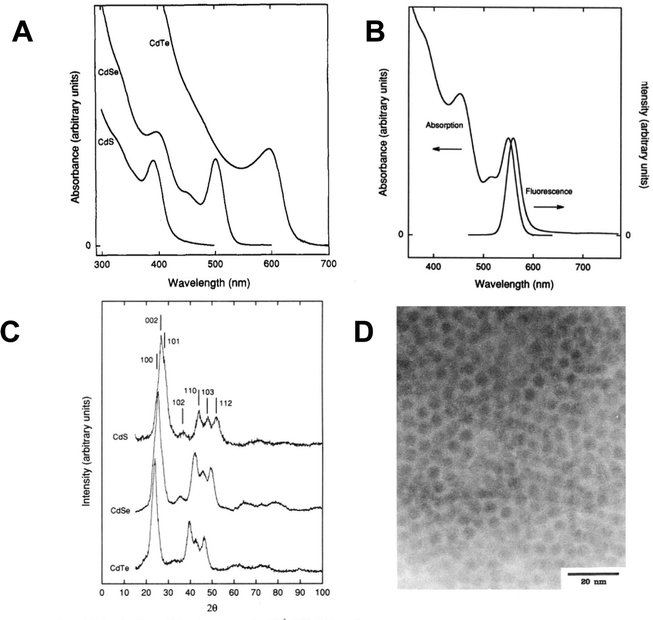 | ||
| Fig. 3 (A) Absorption spectra of CdS, CdSe, and CdTe quantum dots produced by the thermolysis of inorganic and organometallic precursors in tri-n-octylphosphine oxide. (B) Absorption and emission spectra of CdSe quantum dots, displaying a blue shifted band edge with discrete energy levels and near band edge emission. (C) X-ray diffraction patterns from a selection of CdS, CdSe and CdTe quantum dots. (D) Electron micrograph image of CdSe quantum dots, scale bar = 20 nm. All particles prepared as described in ref. 1. Reprinted (adapted) with permission from C. B. Murray, D. J. Norris, M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715. Copyright 2024, American Chemical Society. | ||
Going forwards
The advent of this pioneering reaction scheme, sometimes referred to as the ‘TOPO’ route (after the use of tri-n-octylphosphine oxide as the solvent and capping agent) provided a well-defined pathway to solid-state nanomaterial science for the synthetic chemist, and developments were quickly reported. Perhaps the most important advance in quantum dot structures was the synthesis of core/shell particles using the TOPO route, making the quantum dots robust enough to withstand purification and hence capable of being incorporated into to real life applications.40 In the seminal report, Me2Zn and S(SiMe3)2 were added to preformed CdSe particles at a Cd/Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn/S ratio of 1
Zn/S ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (as used in the earlier core/shell study5), and thermolyzed, resulting in core/shell materials with enhanced emission quantum yields and prolonged stability in ambient conditions.
4 (as used in the earlier core/shell study5), and thermolyzed, resulting in core/shell materials with enhanced emission quantum yields and prolonged stability in ambient conditions.
One issue with the organometallic reactions, as described above, was the use of metal alkyls as precursors. Whilst effective, the compounds are highly toxic and volatile, which makes routine handling difficult. Peng proposed the use of CdO41 and Cd(COOCH3)242 as safer alternatives in the preparation of CdSe, the inherent stability allowing greater control of the reaction. It was observed that the metal precursors reacted with phosphinic acid impurities in the phosphine oxide capping agent, and that by altering the chain length of intentionally added ‘impurities’, particle growth could be controlled, highlighting the intricate link between reagents and capping agents. As this route is much simpler than the use of metal alkyls, it has been readily embraced and is very much the standard precursor system, in the synthesis of core and core/shell particles alike. An excellent overview of the synthesis has been reported by García-Rodríguez et al.,43 which describes numerous key parameters in some depth. The review notably covers the structure and intermediates of the metal precursors and clusters, the equilibria and cleavage mechanism of phosphine tellurides, the role of secondary phosphine chalcogenides and the actual identity of many of the reactive precursor species.
Another key development was the introduction of non-coordinating solvents which could be used at elevated temperatures in conjunction with a separate capping agent. The separation of capping agent and solvent allowed a high degree of control of the reaction conditions and particle size, and has now become a standard technique.44,45 Likewise, the nomenclature of coordinating ligands is now better defined and routinely assigned whilst referring to Green's classification.46–48 X-type ligands are one electron donors, Z-type ligands are neutral electron accepter (Lewis acids), whilst L-type ligands are neutral electron donors (Lewis bases).
Similarly, the application of single-source precursor to quantum dot synthesis was independently revisited by O’Brien, applying expertise from vapour deposition chemistry.12,49 As a result, readily available and easily manipulated compounds such as zinc diethyldithiocarbamate have become a standard precursor for the deposition of a ZnS shell to quantum dots.13
Other systems
Despite the advances in the preparation of the II–VI family of quantum dots (such as CdSe/ZnS), the utilisation of heavy metal-containing materials is unlikely to be universally adopted in everyday applications due to disposal and (potentially ill-perceived) toxicity issues. As such, the III–V family of semiconducting materials, notably InP, have emerged as alternatives with InP/ZnS based quantum dots having equally impressive optical properties.50 The emergence of the synthetic solution-based chemistry behind III–V materials is comparable to the evolution of II–VI materials. In this case, two research groups who were independently developing chemical routes to semiconductors reported key papers in 1989 which described the dehalosilylation reaction. Wells et al.51 reported a straight forwards reaction as shown in eqn (9);| (Me3Si)3As + MX3 → MAs + 3Me3SiX | (9) |
Whilst Healy et al.52 suggested a slightly different reaction as shown in eqn (10) (although the intermediates are likely to be similar in both reactions);
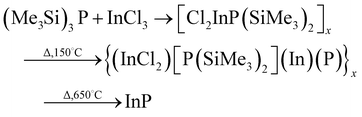 | (10) |
Initial work towards applying this chemistry to quantum dot synthesis was reported by Olshavsky in 1990.53 In this synthesis, GaCl3 was reacted with (Me3Si)3As in quinoline, a nitrogen-containing heterocyclic compound, similar to 4 ethylpyridine but with a much higher boiling point. This reaction is notable as the nanosized III–V materials are often seen as a much later development when compared to the nanosized II–VI materials, where in fact this paper reported a solution route to quantum confined semiconductors prior to the emergence of TOPO-capped CdSe described by Murray et al.1 Likewise in 1991, Uchida essentially repeated the same experiment but explored the optical properties in more depth, highlighting issues with molecular species masking the emissive properties of the quantum dots.54 Such interference from solvents plagued the early reports of the III–V quantum dots and was a major hindrance of applying the materials to applications, along with the problem associated with preparing monodispersed samples.55 Similarly, Uchida extended this work in 1993 to the preparation of InAs quantum dots, using indium acetylacetonate and (Me3Si)3As in triglyme,56 although it is unclear if the materials still exhibited interference from the solvent.57 Several groups then adapted the TOPO reaction to accommodate the dehalosilylation reaction, notably Alivisatos58 and Mićić.59 In these examples, similar reagents were used whilst trioctylphosphine and trioctylphosphine oxide were utilised as high temperature solvents and capping agents, as described by Murray.1 This route afforded higher temperature reaction conditions yielding higher quality particles. The reaction has been refined over the last two decades, notably by the inclusion of zinc,60 surface etching using HF,61 and the addition of multiple shells62 leading to materials that could be argued as the most efficient colloidal quantum dot materials available to date.62 Regardless, the synthetic chemistry remains essentially unchanged, and despite minor advances, the dehalosilylation reaction is still preferred and widely used.
The TOPO route was not restricted to semiconductor quantum dots and opened solution pathways to other material. The high temperatures afforded by the solvents systems (up to 350 °C) and the inert atmosphere employed meant that organometallic and inorganic precursors routinely used for CVD could be used in the solution preparation of other solid-state nanostructures. Murray extended his route to magnetic nanoparticles of cobalt, by reducing CoCl2 with LiBEt3H at 200 °C (under an inert atmosphere) in the presence of high boiling point solvents and capping agents such as oleic acid and trialkylphosphines.63 Of particular interest is the extremely high monodispersity of the particles, the inherent self-assembly into ordered superlattices, and the relatively rare crystalline phase of cobalt obtained, termed ε-Co. Dinega and Bawendi also reported the synthesis of ε-Co via the thermolysis of Co2CO8 in TOPO, further highlighting the capability of the reaction technique to access previously unobtainable materials.64 Murray then reported a synthesis of FePt particles,65 using a route that drew on all engineered aspects of the above-described chemistry. The capping agents and solvents were chosen with reference to previous results, notably the use of long chain amines and carboxylates as passivating agents, and the inclusion of a long chain diol to reduce the Pt precursor, Pt acetylacetonate. The high temperatures afforded by such long chain species allowed the simultaneous high temperature thermolysis of Fe(CO)5, yielding size controllable particles of magnetic FePt. Again, the particles self-assembled into highly organised lattices. Indeed, numerous, highly controlled and engineered lattices have been reported by Murray using the chemistry in the papers we have described, and these are arguably unrivalled in the quantum dots/nanoparticle space and the epitome of this particular branch of nanotechnology.66
Other materials of note prepared by this type of chemistry include lead chalcogenides,67 mercury chalcogenides68 and more recently still, perovskites,69 all of which exhibit exceptional optical and structural properties afforded by solution chemistry derived from the above-mentioned reactions. This is not to ignore other chemical routes – unusually, aqueous routes to CdTe70 and HgTe71 provide equally impressive materials, although these are to be considered the exception rather than the rule.
The number of significant publications born from this chemistry are too numerous to mention in one report, and we simply wish to highlight the overlooked genesis of this unique branch of materials chemistry. We note that going forwards, design criteria for precursor chemistry should approach the mechanistic sophistication of other branches of synthetic chemistry. Factors such as the thermodynamics and kinetics of precursor decomposition, atom efficiency, the elucidation of mechanistic pathways, etc. will be considered alongside simple benchmarks such as commercial availability. Whilst biological applications such as cellular labelling were, perhaps, a surprising candidate for the first commercial implementation of quantum dots, made possible by the superior optical properties inherent in the nanoparticles afforded by the chemistry described above and the associated developments in surface engineering, applications such as displays, and solar energy harvesting were more predictably close to follow. Now that monodispersed, size-controlled particles can be prepared on a regular basis, the underlying novel and unexpected optoelectronics and physics are beginning to be uncovered. The stringent requirements for, for example, quantum computing, dictate that optical properties such as on-demand, pure single photon generation are realised, and colloidal quantum dots prepared as described in this article appear to be ideal candidates.72 This eventuality could not have been predicted in the 1980s when the solution chemistry for semiconductors synthesis was being developed, and the best application of colloidal quantum dots may as-yet be unknown. The 2023 Nobel prize for chemistry is unlikely to be the only award with its basis in the described work.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MG acknowledges the Engineering and Physical Sciences Research Council (EPSRC) for funding (EP/X014495/1).References
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- A. Fojtik, H. Weller, U. Koch and A. Henglein, Ber. Bunsenges. Phys. Chem., 1984, 88, 969–977 CrossRef CAS.
- A. I. Ekimov and A. A. Onushchenko, J. Exp. Theor. Phys. Lett., 1981, 34, 345–349 Search PubMed.
- M. L. Steigerwald, A. P. Alivisatos, J. M. Gibson, T. D. Harris, R. Kortan, A. J. Muller, A. M. Thayer, T. M. Duncan, D. C. Douglass and L. E. Brus, J. Am. Chem. Soc., 1988, 110, 3046–3050 CrossRef CAS.
- A. R. Kortan, R. Hull, R. L. Opila, M. G. Bawendi, M. L. Steigerwald, P. J. Carroll and L. E. Brus, J. Am. Chem. Soc., 1990, 112, 1327–1332 CrossRef CAS.
- M. G. Bawendi, A. R. Kortan, M. L. Steigerwald and L. E. Brus, J. Chem. Phys., 1989, 91, 7282–7290 CrossRef CAS.
- K. Yu and K. S. Schanze, ACS Cent. Sci., 2023, 9, 1989–1992 CrossRef CAS PubMed.
- D. W. Kisker, M. L. Steigerwald, T. Y. Kometani and K. S. Jeffers, Appl. Phys. Lett., 1987, 50, 1681–1683 CrossRef CAS.
- M. L. Steigerwald and C. R. Sprinkle, J. Am. Chem. Soc., 1987, 109, 7200–7201 CrossRef CAS.
- J. G. Brennan, T. Siegrist, P. J. Carroll, S. M. Stuczynski, L. E. Brus and M. L. Steigerwald, J. Am. Chem. Soc., 1989, 111, 4141–4143 CrossRef CAS.
- J. G. Brennan, T. Siegrist, P. J. Carroll, S. M. Stuczynski, P. Reynders, L. E. Brus and M. L. Steigerwald, Chem. Mater., 1990, 2, 403–409 CrossRef CAS.
- T. Trindade and P. O’Brien, Adv. Mater., 1996, 8, 161–163 CrossRef CAS.
- J. R. Dethlefsen and A. Døssing, Nano Lett., 2011, 11, 1964–1969 CrossRef CAS PubMed.
- S. L. Cumberland, K. M. Hanif, A. Javier, G. A. Khitrov, G. F. Strouse, S. M. Woessner and C. S. Yun, Chem. Mater., 2002, 14, 1576–1584 CrossRef CAS.
- I. G. Dance, A. Choy and M. L. Scudder, J. Am. Chem. Soc., 1984, 106, 6285–6295 CrossRef CAS.
- N. Herron, J. C. Calabresse, W. E. Farnath and Y. Wang, Science, 1993, 259, 1426–1428 CrossRef CAS PubMed.
- A. N. MacInnes, M. B. Power and A. R. Barron, Chem. Mater., 1992, 4, 11–14 CrossRef CAS.
- O. Fuhr, S. Dehnen and D. Fenske, Chem. Soc. Rev., 2013, 42, 1871–1906 RSC.
- J. F. Corrigan, O. Fuhr and D. Fenske, Adv. Mater., 2009, 21, 1867–1871 CrossRef CAS.
- M. R. Friedfeld, D. A. Johnson and B. M. Cossairt, Inorg. Chem., 2019, 58, 803–810 CrossRef CAS PubMed.
- R. A. Zingaro, J. Organomet. Chem., 1963, 1, 200 CrossRef CAS.
- R. A. Zingaro, B. H. Steeves and K. Irgolic, J. Organomet. Chem., 1965, 4, 320–323 CrossRef CAS.
- A. Darkowski and M. Cocivera, J. Electrochem. Soc., 1985, 132, 2768–2771 CrossRef CAS.
- A. Darkowski and M. Cocivera, J. Electrochem. Soc., 1987, 134, 226–229 CrossRef CAS.
- M. A. Reed, R. T. Bate, K. Bradshaw, W. M. Duncan, W. R. Frensley, J. W. Lee and H. D. Shih, J. Vac. Sci. Technol., B, 1986, 4, 358–360 CrossRef CAS.
- R. L. Wells, M. F. Self, A. T. McPhail and S. R. Aubuchon, Organometallics, 1993, 12, 2832–2834 CrossRef CAS.
- M. L. Steigerwald and C. R. Sprinkle, Organometallics, 1988, 7, 245–246 CrossRef CAS.
- L. Huang, R. A. Zingaro, E. A. Meyers and J. H. Reibenspies, Heteroat. Chem., 1996, 7, 57–65 CrossRef CAS.
- M. L. Steigerwald, Polyhedron, 1994, 13, 1245–1252 CrossRef CAS.
- M. L. Steigerwald and C. E. Rice, J. Am. Chem. Soc., 1988, 110, 4228–4231 CrossRef CAS.
- J. G. Brennan, T. Siegrist, S. M. Stuczynski and M. L. Steigerwald, J. Am. Chem. Soc., 1989, 111, 9240–9241 CrossRef CAS.
- B. Hessen, T. Siegrist, T. Palstra, S. M. Tanzler and M. L. Stiegerwald, Inorg. Chem., 1993, 32, 5165–5169 CrossRef CAS.
- J. G. Brennan, T. Siegrist, Y.-U. Kwon, S. M. Stuczynski and M. L. Steigerwald, J. Am. Chem. Soc., 1992, 114, 10334–10338 CrossRef CAS.
- S. M. Stuczynski, Y.-U. Kwon and M. L. Steigerwald, J. Organomet. Chem., 1993, 449, 167–172 CrossRef CAS.
- X. Roy, C.-H. Lee, A. C. Crowther, C. L. Schenck, T. Besara, R. A. Lalancette, T. Siegrist, P. W. Stephens, L. E. Brus, P. Kim, M. L. Steigerwald and C. Nuckolls, Science, 2013, 341, 157–160 CrossRef CAS PubMed.
- S. M. Stuczynski, J. G. Brennan and M. L. Steigerwald, Inorg. Chem., 1989, 28, 4431–4432 CrossRef CAS.
- J. J. Calvin, A. S. Brewer and A. P. Alivisatos, Nat. Synth., 2022, 1, 127–137 CrossRef.
- F. Wang, R. Tang and W. E. Buhro, Nano Lett., 2008, 8, 3521–3524 CrossRef CAS PubMed.
- C. M. Tyrakowski and P. T. Snee, Phys. Chem. Chem. Phys., 2014, 16, 837–855 RSC.
- M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468–471 CrossRef CAS.
- Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 183–184 CrossRef CAS PubMed.
- L. Qu, Z. A. Peng and X. Peng, Nano Lett., 2001, 1, 333–337 CrossRef CAS.
- R. García-Rodríguez, M. P. Hendricks, B. M. Cossairt, H. Liu and J. S. Owen, Chem. Mater., 2013, 25, 1233–1249 CrossRef.
- W. W. Yu and X. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368–2371 CrossRef CAS PubMed.
- C. Bullen, J. van Embden, J. Jasieniak, J. E. Cosgriff, R. J. Mulder, E. Rizzardo, M. Gu and C. L. Raston, Chem. Mater., 2010, 22, 4135–4143 CrossRef CAS.
- M. L. H. Green, J. Organomet. Chem., 1995, 500, 127–148 CrossRef CAS.
- J. De Roo, K. De Keukeleere, Z. Hens and I. Van Driessche, Dalton Trans., 2016, 45, 13277–13283 RSC.
- J. Owen, Science, 2015, 347, 615–616 CrossRef CAS PubMed.
- T. Trindade, P. O’Brien and X.-M. Zhang, Chem. Mater., 1997, 9, 523–530 CrossRef CAS.
- S. Tamang, C. Lincheneau, Y. Hermans, S. Jeong and P. Reiss, Chem. Mater., 2016, 28, 2491–2506 CrossRef CAS.
- R. L. Wells, C. G. Pitt, A. T. McPhail, A. P. Purdy, S. Shafieezad and R. B. Hallock, Chem. Mater., 1989, 1, 4–6 CrossRef CAS.
- M. D. Healy, P. E. Laibinis, P. D. Stupik and A. R. Barron, J. Chem. Soc., Chem. Commun., 1989, 359–360 RSC.
- M. A. Olshavsky, A. N. Goldstein and A. P. Alivisatos, J. Am. Chem. Soc., 1990, 112, 9438–9439 CrossRef CAS.
- H. Uchida, C. J. Curtis and A. J. Nozik, J. Phys. Chem., 1991, 95, 5382–5384 CrossRef CAS.
- J. R. Heath and J. J. Shiang, Chem. Soc. Rev., 1998, 27, 65–71 RSC.
- H. Uchida, T. Matsunaga, H. Yoneyama, T. Sakata, H. Mori and T. Sasaki, Chem. Mater., 1993, 5, 716–719 CrossRef CAS.
- M. Furis, Y. Sahoo, D. J. MacRae, F. S. Manciu, A. N. Cartwright and P. N. Prasad, J. Phys. Chem. B, 2003, 107, 11622–11625 CrossRef CAS.
- A. A. Guzelian, J. E. B. Katari, A. V. Kadavanich, U. Banin, K. Hamad, E. Juban, A. P. Alivisatos, R. H. Wolters, C. C. Arnold and J. R. Heath, J. Phys. Chem., 1996, 100, 7212–7219 CrossRef CAS.
- O. I. Mićić, C. J. Curtis, K. M. Jones, J. R. Sprague and A. J. Nozik, J. Phys. Chem., 1994, 98, 4966–4969 CrossRef.
- S. Xu, J. Ziegler and T. Nann, J. Mater. Chem., 2008, 18, 2653–2656 RSC.
- O. I. Mićić, J. Sprague, Z. Lu and A. J. Nozik, Appl. Phys. Lett., 1996, 68, 3150–3152 CrossRef.
- Y.-H. Won, O. Cho, T. Kim, D.-Y. Chung, T. Kim, H. Chung, H. Jang, J. Lee, D. Kim and E. Jang, Nature, 2019, 575, 634–671 CrossRef CAS PubMed.
- S. Sun and C. B. Murray, J. Appl. Phys., 1999, 85, 4325–4330 CrossRef CAS.
- D. P. Dinega and M. G. Bawendi, Angew. Chem., Int. Ed., 1999, 38, 1788–1791 CrossRef CAS PubMed.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1992 CrossRef CAS PubMed.
- E. V. Shevchenko, D. V. Talapin, N. A. Kotov, S. O’Brien and C. B. Murray, Nature, 2006, 439, 55–59 CrossRef CAS PubMed.
- C. B. Murray, S. Sun, W. Gaschler, H. Doyle, T. A. Betley and C. R. Kagan, IBM J. Res. Dev., 2001, 45, 47–56 CAS.
- C. Gréboval, A. Chu, N. Goubet, C. Livache, S. Ithurria and E. Lhuillier, Chem. Rev., 2021, 121, 3627–3700 CrossRef PubMed.
- Q. A. Akkerman, G. Rainò, M. V. Kovalenko and L. Manna, Nat. Mater., 2018, 17, 394–405 CrossRef CAS PubMed.
- N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmüller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177–7185 CrossRef CAS.
- A. Rogach, S. Kershaw, M. Burt, M. Harrison, A. Kornowski, A. Eychmüller and H. Weller, Adv. Mater., 1999, 11, 552–555 CrossRef CAS.
- A. H. Proppe, D. B. Berkinsky, H. Zhu, T. Sverko, A. E. K. Kaplan, J. R. Horowitz, T. Kim, H. Chung, S. Jun and M. G. Bawendi, Nat. Nanotechnol., 2023, 18, 993–999 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
