Emergence of electrical conductivity in a flexible coordination polymer by using chemical reduction†
Kentaro
Fuku
a,
Momoka
Miyata
a,
Shinya
Takaishi
 a,
Takefumi
Yoshida
a,
Takefumi
Yoshida
 ab,
Masahiro
Yamashita
abc,
Norihisa
Hoshino
ab,
Masahiro
Yamashita
abc,
Norihisa
Hoshino
 d,
Tomoyuki
Akutagawa
d,
Tomoyuki
Akutagawa
 d,
Hiroyoshi
Ohtsu
d,
Hiroyoshi
Ohtsu
 e,
Masaki
Kawano
e,
Masaki
Kawano
 e and
Hiroaki
Iguchi
e and
Hiroaki
Iguchi
 *a
*a
aDepartment of Chemistry, Graduate School of Science, Tohoku University, 6-3 Aza-Aoba, Aramaki, Sendai 980-8578, Japan. E-mail: h-iguchi@tohoku.ac.jp
bAdvanced Institute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
cSchool of Materials Science and Engineering, Nankai University, Tianjin 300350, China
dInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
eDepartment of Chemistry, School of Science, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan
First published on 22nd June 2020
Abstract
A flexible coordination polymer with a naphthalenediimide core exhibited reversible desorption–adsorption of solvent molecules and an enhancement of electrical conductivity (∼10−7 S cm−1) upon chemical reduction using hydrazine.
Electrically conductive coordination polymers (CPs) have been one of the most attractive targets in solid-state coordination chemistry from the viewpoint of both fundamental and applied sciences.1 For instance, Magnus-type salts,2 and MX-3 and MMX-type4 chain complexes are classic but still actively investigated one-dimensional (1D) CPs owing to their conductivity,2b,3d,e,4a,c optical properties3a and potential application in nanotechnology.4b Recently, studies on electrically conductive CPs have been extended to 2D CPs5 and porous CPs like so-called metal–organic frameworks (MOFs),6 which are promising as sensors,7 electrode materials8 and other electronic devices.9 In typical electrically conductive CPs, the covalent bonding character existing between metals and ligands enables carrier transport along the framework (“through-bond” conduction). On the other hand, conductive CPs with the “through-space” conduction pathway such as infinite π-stacked columns are rare.10 Although many CPs and MOFs with large π-conjugated ligands have been reported, the ligands are mostly isolated from one another11 or the π-stack assembly stops at the oligomer level.12 This is because the π-stacking interaction is generally not strong enough for compatibility with the rigid frameworks. To enhance the π-stacking interaction and to rationally design a π-stacked column among CPs, introduction of π-radicals has recently been proposed.13,14
In this work, we chose another strategy, that is, using flexible CPs, because flexible ligands occasionally achieve the co-existence of coordination networks and π-stacked columns.15 A naphthalenediimide (NDI) core was selected as the π-conjugated moiety, since it has been known as a stable electron acceptor16 and as the main component in several conductive crystals.17 We herein report the synthesis of a new flexible CP with the through-space conduction pathway. It showed a reversible structural change upon desorption–adsorption of chloroform molecules and an enhancement of electrical conductivity by postsynthetic carrier doping.
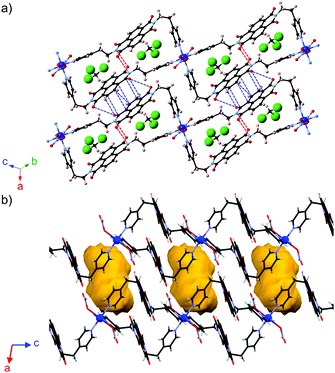
N,N′-Di(2-(pyridin-4yl)ethyl)-1,4,5,8-naphthalenetetracarb-oxdiimide (NDI-enpy)18 was synthesized as the ligand, which contains flexible ethylene spacers between the NDI core and pyridyl groups. The slow diffusion of NDI-enpy in chloroform and Cu(NO3)2·3H2O in N,N-dimethylformamide (DMF) yielded purple crystals of [Cu(NDI-enpy)2(NO3)2]·2CHCl3 (1). Single crystal X-ray structure analysis indicates that 1 is a 1D CP consisting of a periodic array of rectangular units, which is constructed from two NDI-enpy molecules and two Cu2+ ions as metal nodes (Fig. 1a). Since 1 contains a loop structure, it can be regarded as a 1D coordination network.19 The NDI core of the ligand exhibits π–π interaction with the other NDI core in the same rectangular unit (intrachain π-stacking; red dashed lines in Fig. 1a). Although only two atom pairs are overlapped, the interatomic distance is quite short (3.175(5) Å). Moreover, the π–π interaction between NDI cores in adjacent chains (interchain π-stacking; blue dashed lines in Fig. 1a) is also formed. More atoms are involved in the interchain π–π interaction though the interatomic distances are in the moderate range (3.228–3.477 Å). Consequently, the slipped π-stacked columnar structure is realized along the a-axis. As shown in Fig. 1b, the closed inner space surrounded by the NDI cores, pyridyl planes and NO3− ions (axial ligands) confines two chloroform molecules.
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) exhibited a 17.9% endothermic weight loss from 170 to 200 °C (Fig. 2a), consistent with the weight of the two chloroform molecules (17.3%). This result indicates that the elimination of chloroform occurred at an abnormally high temperature compared with the boiling point of chloroform (61 °C). This high thermal stability is probably derived from the strong confinement of the chloroform molecules in 1. In fact, the Cl⋯O distances between chloroform and NDI-enpy molecules (2.842(4) Å and 2.886(5) Å) are 13.1% and 11.7% shorter than the sum of the van der Waals radii,20 respectively. This indicates the formation of halogen bonds,21 which should enhance the durability. The exothermic weight loss over 250 °C may indicate the decomposition of the framework. The powder X-ray diffraction (PXRD) pattern of the desolvated complex, [Cu(NDI-enpy)2(NO3)2] (1d), was different from that of 1, reflecting a large structural change of the flexible framework (Fig. 2b). The PXRD pattern, however, returned to the initial one after soaking 1d in chloroform for a day. Thus, it is concluded that 1 is flexible enough to achieve a reversible structural change via desorption–adsorption of chloroform molecules.
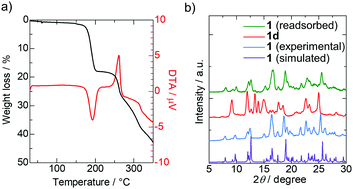 | ||
| Fig. 2 (a) TGA and DTA trace of 1. (b) PXRD patterns of 1d (red) and 1 (simulated from the crystal structure: purple, experimental: blue, after soaking 1d in chloroform for a day: green). | ||
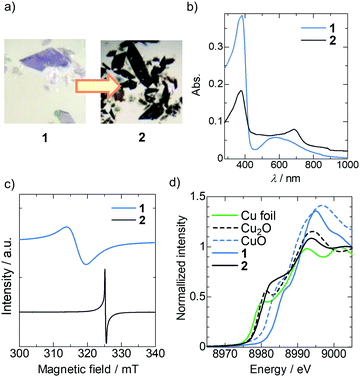
Since 1 showed porosity and an infinite π-stacking structure, we considered postsynthetic carrier doping. To date, some NDI-based electron conductors have been reported.17 However, the enhancement of electrical conductivity of NDI-based materials via electron doping has been limited to solids with dopant-accessible pores such as polymer films22 and porous 3D MOFs,23 to the best of our knowledge. Hence, the applicability of doping to densely packed crystalline solids has remained unclear. According to previous reports,24 hydrazine was selected as the reductant to generate the NDI anion radical (NDI˙−). In addition, it is applicable in aqueous media, where the dissolution of 1 does not occur. The postsynthetic reduction of 1 was therefore carried out by soaking 1 in an aqueous solution of hydrazine monohydrate, yielding black solid 2, as shown in Fig. 3a. The colour change proceeded from the surface to the inside with the evolution of bubbles (see video in the ESI†), suggesting the reaction of hydrazine (N2H4 → N2 + 4H+ + 4e−). On the basis of PXRD measurement (Fig. S1, ESI†), 2 is a crystalline solid, and its crystal structure is different from that of either 1 or 1d. The reduction of 1d using the same method also gave a similar PXRD pattern, and thus the structure of the reduced state 2 is not affected by the existence of chloroform molecules.
In order to study the electronic state of 2, UV-vis-NIR diffuse reflectance spectra of 1 and 2 were acquired. As shown in Fig. 3b, the absorption band derived from the π–π* transition in the NDI-enpy moiety was observed below 400 nm in both 1 and 2. A relatively weak Cu2+ d–d absorption band was observed around 550 nm in 1. However, this d–d band was unclear and a new peak appeared around 690 nm in the case of 2. This new absorption band was assigned to the intramolecular transition of the NDI˙− core according to previous reports.25 The ESR spectra exhibited a more distinct difference between 1 and 2. As shown in Fig. 3c, a broad signal (g = 2.0594) attributed to the unpaired d electron of Cu2+ was observed in the spectrum of 1. On the other hand, a sharp signal (g = 2.0031) appeared in the spectrum of 2. Because a sharp signal is typically observed for NDI˙− species, the ESR spectra indicate that both NDI cores and Cu2+ ions were reduced to NDI˙− and nonmagnetic Cu+ ions, respectively. In order to further confirm the oxidation state of the Cu atom, X-ray absorption near edge structure (XANES) spectra of the Cu K-edge were acquired at room temperature. In general, the onset of the absorption edge (Eedge) of a metal ion decreases with the decrease of the oxidation number. As shown in Fig. 3d, the Eedge of 2 was smaller than that of 1, indicating a lower oxidation number of the Cu ion in 2. Moreover, compared with the reference samples (Cu0 foil, Cu+2O and Cu2+O), the Eedge and the shape of the XANES spectra of 1 and 2 were similar to those of CuO and Cu2O, respectively. Therefore, we conclude that the oxidation state of the Cu atom in 2 is +1.
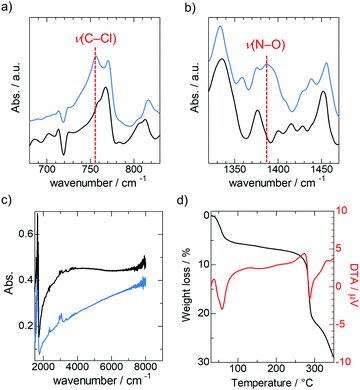
IR spectroscopy, TGA, and elemental analysis were performed to determine the chemical formula of 2. In the IR spectra, the signals of the C–Cl stretching vibration mode (756 cm−1)26 and N–O stretching vibration mode (1388 cm−1)27 disappeared after the reduction (Fig. 4a and b). These results suggest that elimination of nitrate and chloroform occurred during the reduction. Therefore, we assumed the chemical formula of the framework in 2 to be [Cu(NDI-enpy)2]. From the thermogravimetric analysis of 2, an 8.0% weight loss was observed from 30 to 260 °C (Fig. 4d). This corresponds to the elimination of five water molecules (8.1%), consistent with the results of the elemental analysis (see the ESI†). Accordingly, the chemical formula of 2 was determined as [Cu(NDI-enpy)2]·5H2O. Because the charge of the Cu ion is +1, the average charge of the NDI core is presumed to be −0.5.
The electrical conductivity (σ) of 1 and 2 was measured in pressed pellet form by using a two-probe method. The σ of 1 was below the lower detection limit of the source meter (σ < 10−9 S cm−1), which agrees with the lack of carriers in the π-stacked column. In contrast, 2 showed semiconducting behaviour and σ reached over 10−7 S cm−1 at room temperature, as shown in Fig. 5, reflecting the carrier doping in the π-stacked column achieved by the reduction. The activation energy (Ea) was calculated to be 0.267 eV from the Arrhenius model (σ = σ0exp(−Ea/kBT)), where kB is the Boltzmann constant and T is the temperature. Although the crystal structure of 2 has not been identified, the emergence of σ strongly suggests the retention of the π-stacked columnar structure. This is also supported by the manifestation of the broad band from 3000 to 6000 cm−1 (Fig. 4c), which is typically attributed to intermolecular charge transfer between NDI cores in the π-stacked column.14
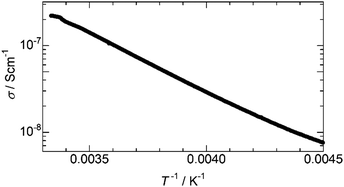 | ||
| Fig. 5 Temperature dependence of the electrical conductivity (σ) of 2 measured by using a two-probe method on a pellet sample. | ||
In summary, we synthesized a flexible 1D coordination network, [Cu(NDI-enpy)2(NO3)2]·2CHCl3 (1), which shows a reversible structural change upon desorption–adsorption of internally accommodated chloroform molecules. The flexible ethylene moiety in 1 enables the co-existence of the CP framework and the infinite π-stacked column as a through-space conduction pathway. 1 demonstrated electrical conductivity upon hydrazine reduction. To the best of our knowledge, 1 is the first densely packed CP that undergoes postsynthetic carrier doping with a structural change, enabled by its flexible feature. This work offers a new basis for designing electrically conductive CPs as conductive soft crystals.28
This work was partly supported by JSPS KAKENHI Grant Numbers JP18H04498 (H. I.), JP18K14233 (H. I.), and JP19H05631 (H. I., S. T., and M. Y.); by the CASIO Science Promotion Foundation (H. I.); by the Ogasawara Foundation for the Promotion of Science and Engineering (H. I.); by the Program for Interdisciplinary Research in Tohoku University Frontier Research Institute for Interdisciplinary Sciences (H. I.); and by the Toyota Riken Scholar Program (H. I.). This work was performed under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2019G117, beamline 12C). M. Y. acknowledges the support from the 111 project (B18030) from China.
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- G. Givaja, P. Amo-Ochoa, C. J. Gómez-García and F. Zamora, Chem. Soc. Rev., 2012, 41, 115 RSC
.
-
(a) E.-G. Kim, K. Schmidt, W. R. Caseri, T. Kreouzis, N. Stingelin-Stutzmann and J.-L. Brédas, Adv. Mater., 2006, 18, 2039 CrossRef CAS
; (b) C. H. Hendon, A. Walsh, N. Akiyama, Y. Konno, T. Kajiwara, T. Ito, H. Kitagawa and K. Sakai, Nat. Commun., 2016, 7, 11950 CrossRef CAS PubMed
.
-
(a) H. Kishida, H. Matsuzaki, H. Okamoto, T. Manabe, M. Yamashita, Y. Taguchi and Y. Tokura, Nature, 2000, 405, 929 CrossRef CAS PubMed
; (b) M. Yamashita and S. Takaishi, Chem. Commun., 2010, 46, 4438 RSC
; (c) S. Kumagai, S. Takaishi, H. Iguchi and M. Yamashita, Dalton Trans., 2015, 44, 8590 RSC
; (d) M. R. Mian, H. Iguchi, S. Takaishi, H. Murasugi, T. Miyamoto, H. Okamoto, H. Tanaka, S. Kuroda, B. K. Breedlove and M. Yamashita, J. Am. Chem. Soc., 2017, 139, 6562 CrossRef CAS PubMed
; (e) M. R. Mian, U. Afrin, H. Iguchi, S. Takaishi, T. Yoshida, T. Miyamoto, H. Okamoto, H. Tanaka, S. Kuroda and M. Yamashita, CrystEngComm, 2020, 22, 3999 RSC
.
-
(a) M. Mitsumi, K. Kitamura, A. Morinaga, Y. Ozawa, M. Kobayashi, K. Toriumi, Y. Iso, H. Kitagawa and T. Mitani, Angew. Chem., Int. Ed., 2002, 41, 2767 CrossRef CAS
; (b) L. Welte, A. Calzolari, R. D. Felice, F. Zamora and J. Gómez-Herrero, Nat. Nanotechnol., 2010, 5, 110 CrossRef CAS PubMed
; (c) H. Iguchi, S. Takaishi and M. Yamashita, Chem. Lett., 2014, 43, 69 CrossRef CAS
.
-
(a) H. Miyasaka, N. Motokawa, S. Matsunaga, M. Yamashita, K. Sugimoto, T. Mori, N. Toyota and K. R. Dunbar, J. Am. Chem. Soc., 2010, 132, 1532 CrossRef CAS PubMed
; (b) X. Huang, P. Sheng, Z. Tu, F. Zhang, J. Wang, H. Geng, Y. Zou, C.-A. Di, Y. Yi, Y. Sun, W. Xu and D. Zhu, Nat. Commun., 2015, 6, 7408 CrossRef CAS PubMed
.
-
(a) S. Takaishi, M. Hosoda, T. Kajiwara, H. Miyasaka, M. Yamashita, Y. Nakanishi, Y. Kitagawa, K. Yamaguchi, A. Kobayashi and H. Kitagawa, Inorg. Chem., 2009, 48, 9048 CrossRef CAS PubMed
; (b) C. F. Leong, P. M. Usov and D. M. D'Alessandro, MRS Bull., 2016, 41, 858 CrossRef
; (c) W.-H. Li, W.-H. Deng, G.-E. Wang and G. Xu, EnergyChem, 2020, 2, 100029 CrossRef
; (d) L. S. Xie, G. Skorupskii and M. Dinca, Chem. Rev., 2020 DOI:10.1021/acs.chemrev.9b00766
.
-
(a) A. Chidambaram and K. C. Stylianou, Inorg. Chem. Front., 2018, 5, 979 RSC
; (b) V. Rubio-Giménez, N. Almora-Barrios, G. Escorcia-Ariza, M. Galbiati, M. Sessolo, S. Tatay and C. Martí-Gastaldo, Angew. Chem., Int. Ed., 2018, 57, 15086 CrossRef PubMed
; (c) M. L. Aubrey, M. T. Kapelewski, J. F. Melville, J. Oktawiec, D. Presti, L. Gagliardi and J. R. Long, J. Am. Chem. Soc., 2019, 141, 5005 CrossRef CAS PubMed
; (d) J. Chen, Y. Sekine, A. Okazawa, H. Sato, W. Kosaka and H. Miyasaka, Chem. Sci., 2020, 11, 3610 RSC
.
-
(a) L. Wang, Y. Han, X. Feng, J. Zhou, P. Qi and B. Wang, Coord. Chem. Rev., 2016, 307, 361 CrossRef CAS
; (b) D. Sheberla, J. C. Bachman, J. S. Elias, C.-J. Sun, Y. Shao-Horn and M. Dincă, Nat. Mater., 2017, 16, 220 CrossRef CAS PubMed
; (c) M. E. Ziebel, C. A. Gaggioli, A. B. Turkiewicz, W. Ryu, L. Gagliardi and J. R. Long, J. Am. Chem. Soc., 2020, 142, 2653 CrossRef CAS PubMed
.
-
(a) D. M. D’Alessandro, Chem. Commun., 2016, 52, 8957 RSC
; (b) I. Stassen, N. Burtch, A. Talin, P. Falcaro, M. Allendorf and R. Ameloot, Chem. Soc. Rev., 2017, 46, 3185 RSC
.
-
(a) S. S. Park, E. R. Hontz, L. Sun, C. H. Hendon, A. Walsh, T. Van Voorhis and M. Dincă, J. Am. Chem. Soc., 2015, 137, 1774 CrossRef CAS PubMed
; (b) D. Chen, H. Xing, Z. Su and C. Wang, Chem. Commun., 2016, 52, 2019 RSC
; (c) X. Kuang, S. Chen, L. Meng, J. Chen, X. Wu, G. Zhang, G. Zhong, T. Hu, Y. Li and C.-Z. Lu, Chem. Commun., 2019, 55, 1643 RSC
.
-
(a) M. Pan, X. M. Lin, G. B. Li and C. Y. Su, Coord. Chem. Rev., 2011, 255, 1921 CrossRef CAS
; (b) A. Mallick, B. Garai, M. A. Addicoat, P. S. Petkov, T. Heine and R. Banerjee, Chem. Sci., 2015, 6, 1420 RSC
; (c) S. Suginome, H. Sato, A. Hori, A. Mishima, Y. Harada, S. Kusaka, R. Matsuda, J. Pirillo, Y. Hijikata and T. Aida, J. Am. Chem. Soc., 2019, 141, 15649 CrossRef CAS PubMed
.
-
(a) S. S. Park, C. H. Hendon, A. J. Fielding, A. Walsh, M. O’Keeffe and M. Dincă, J. Am. Chem. Soc., 2017, 139, 3619 CrossRef CAS PubMed
; (b) C. Hua, P. W. Doheny, B. Ding, B. Chan, M. Yu, C. J. Kepert and D. M. D'Alessandro, J. Am. Chem. Soc., 2018, 140, 6622 CrossRef CAS PubMed
.
-
(a) J. Y. Koo, Y. Yakiyama, G. R. Lee, J. Lee, H. C. Choi, Y. Morita and M. Kawano, J. Am. Chem. Soc., 2016, 138, 1776 CrossRef CAS PubMed
; (b) J. Y. Ha, J. Y. Koo, H. Ohtsu, Y. Yakiyama, K. Kim, D. Hashizume and M. Kawano, Angew. Chem., Int. Ed., 2018, 57, 4717 CrossRef CAS PubMed
.
- L. Qu, H. Iguchi, S. Takaishi, F. Habib, C. F. Leong, D. M. D'Alessandro, T. Yoshida, H. Abe, E. Nishibori and M. Yamashita, J. Am. Chem. Soc., 2019, 141, 6802 CrossRef CAS PubMed
.
-
(a) C.-L. Chen, C.-Y. Su, Y.-P. Cai, H.-X. Zhang, A.-W. Xu, B.-S. Kang and H.-C. zur Loye, Inorg. Chem., 2003, 42, 3738 CrossRef CAS PubMed
; (b) C. Jia, Q. Lin and W. Yuan, CrystEngComm, 2014, 16, 2508 RSC
.
- M. Al Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685 CrossRef PubMed
.
-
(a) G. Heywang, L. Born, H.-G. Fitzky, T. Hassel, J. Hocker, H.-K. Müller, B. Pittel and S. Roth, Angew. Chem., Int. Ed. Engl., 1989, 28, 483 CrossRef
; (b) A. Mizuno, Y. Shuku, R. Suizu, M. M. Matsushita, M. Tsuchiizu, D. R. Manñeru, F. Illas, V. Robert and K. Awaga, J. Am. Chem. Soc., 2015, 137, 7612 CrossRef CAS PubMed
; (c) M. T. Nguyen, M. D. Krzyaniak, M. Owczarek, D. P. Ferris, M. R. Wasielewski and J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 5795 CrossRef CAS PubMed
.
- Y. Tsukada, K. Hirao and J. Mizuguchi, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o3872 CrossRef CAS
.
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715 CAS
.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS
.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478 CrossRef CAS PubMed
.
-
(a) H. Luo, Z. Liu, Z. Cai, L. Wu, G. Zhang, C. Liu and D. Zhang, Chin. J. Chem., 2012, 30, 1453 CrossRef CAS
; (b) Y. Han, Z. Fei, Y.-H. Lin, J. Martin, F. Tuna, T. D. Anthopoulos and M. Heeney, npj Flexible Electron., 2018, 2, 11 CrossRef
; (c) Y. M. Gross, D. Trefz, C. Dingler, D. Bauer, V. Vijayakumar, V. Untilova, L. Biniek, M. Brinkmann and S. Ludwigs, Chem. Mater., 2019, 31, 3542 CrossRef CAS
.
-
(a) Z. Guo, D. K. Panda, K. Maity, D. Lindsey, T. G. Parker, T. E. Albrecht-Schmitt, J. L. Barreda-Esparza, P. Xiong, W. Zhou and S. Saha, J. Mater. Chem. C, 2016, 4, 894 RSC
; (b) H. Wentz, G. Skorupskii, A. B. Bonfim, J. L. Mancuso, C. H. Hendon, E. H. Oriel, G. T. Sazama and M. G. Campbell, Chem. Sci., 2020, 11, 1342 RSC
.
- D. Zhou, Y. Wang, J. Jia, W. Yu, B. Qu, X. Li and X. Sun, Chem. Commun., 2015, 51, 10656 RSC
.
-
(a) D. Gosztola, M. P. Niemczyk, W. Svec, A. S. Lukas and M. R. Wasielewski, J. Phys. Chem. A, 2000, 104, 6545 CrossRef CAS
; (b) Y. Matsunaga, K. Goto, K. Kubono, K. Sako and T. Shinmyozu, Chem. – Eur. J., 2014, 20, 7309 CrossRef CAS PubMed
.
- DBS Web: https://sdbs.db.aist.go.jp National Institute of Advanced Industrial Science and Technology, accessed April 2020.
- F. A. Miller and C. H. Wilkins, Anal. Chem., 1952, 24, 1253 CrossRef CAS
.
- M. Kato, H. Ito, M. Hasegawa and K. Ishii, Chem. – Eur. J., 2019, 25, 5105 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, elemental analysis, PXRD data and a video. CCDC 1999098 (1). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc03062g |
| This journal is © The Royal Society of Chemistry 2020 |