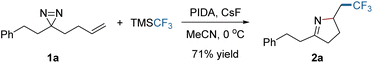Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Access to pyrrolines and fused diaziridines by selective radical addition to homoallylic diazirines†‡
Zhigang
Ma§
a,
Xinxin
Wu§
 a,
Haotian
Li
a,
Zhu
Cao
b and
Chen
Zhu
a,
Haotian
Li
a,
Zhu
Cao
b and
Chen
Zhu
 *ab
*ab
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu 215123, China. E-mail: chzhu@suda.edu.cn
bFrontiers Science Center for Transformative Molecules, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China. E-mail: chzhu@sjtu.edu.cn
First published on 29th December 2023
Abstract
Pyrroline derivatives are common in bioactive natural products and therapeutic agents. We report here a synthesis of pyrrolines and fused diaziridines by divergent radical cyclization of homoallylic diazirines, which can serve as an internal radical trap and a nitrogen source. This reaction proceeds by selective radical addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or N
C or N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds followed by intramolecular cyclization. Frontier molecular orbital analysis provides a deep insight into the origin of the selectivity. The reaction demonstrates a new cyclization mode, broad functional group compatibility and high product diversity, and reveals a much broader chemical space for diazirine studies.
N bonds followed by intramolecular cyclization. Frontier molecular orbital analysis provides a deep insight into the origin of the selectivity. The reaction demonstrates a new cyclization mode, broad functional group compatibility and high product diversity, and reveals a much broader chemical space for diazirine studies.
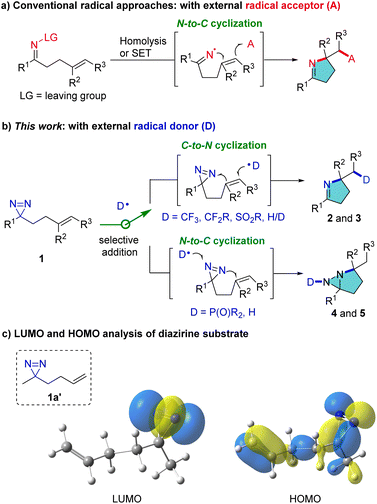
Pyrroline and pyrrolidine structural motifs are widely encountered in bioactive natural products in plants, insects such as myrmicine ants and animals, including poison frogs.1,2 Consequently, new strategies to access pyrrolines and analogues address a long-standing interest of chemists. Notwithstanding the interest in transition-metal catalyzed synthesis of pyrroline derivatives over the past few decades,3 radical approaches have also received remarkable attention owing to their innately attractive features which include mild reaction conditions and good tolerance of susceptible functional groups.4 The conventional radical approaches proceed through the generation of an iminyl radical by homolysis or single-electron transfer (SET) in labile precursors and the subsequent intramolecular N → C cyclization of the iminyl radical with C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. External radical acceptors are sought which can terminate the cascade reaction and furnish pyrroline products (Fig. 1a).5 We have conceived a reverse route which can access pyrrolines by C → N cyclization using external radical donors rather than acceptors (Fig. 1b). This process is intended to enrich product diversity and lead to distinct pyrrolines, such as CF3-substituted pyrrolines, which are difficult to produce by conventional methods. This is because there are no SOMOphiles that lead to the introduction of, for example, CF3 groups.
C bonds. External radical acceptors are sought which can terminate the cascade reaction and furnish pyrroline products (Fig. 1a).5 We have conceived a reverse route which can access pyrrolines by C → N cyclization using external radical donors rather than acceptors (Fig. 1b). This process is intended to enrich product diversity and lead to distinct pyrrolines, such as CF3-substituted pyrrolines, which are difficult to produce by conventional methods. This is because there are no SOMOphiles that lead to the introduction of, for example, CF3 groups.
Diazirines, which consist of one carbon and two nitrogen atoms in an unsaturated three-membered ring, are often exploited in chemical biology as carbene precursors.6 They have been reported only rarely in radical reactions. The use of specific perfluoroalkyl-substituted diazirines to trap carbon-centered radicals was pioneered by Barton,7 and recently extended by Lopchuk et al.8 and Liao et al.9 Despite this elegant research, the use of diazirines as a nitrogen source for construction of valuable N-heterocycles remains underexplored. Herein, we report novel, divergent radical cyclization reactions of homoallylic diazirines (1) for the synthesis of pyrrolines and fused diaziridines (Fig. 1b). The selectivity of addition of an external radical to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or N
C or N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond is dictated by the inherent polarity of the radical. The transformation of 1 can proceed through serial radical addition to C
N bond is dictated by the inherent polarity of the radical. The transformation of 1 can proceed through serial radical addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N
C and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, leading to diverse multi-functionalized pyrrolines (2 and 3). Alternatively, the reaction can proceed by first adding the external radical to the N
N bonds, leading to diverse multi-functionalized pyrrolines (2 and 3). Alternatively, the reaction can proceed by first adding the external radical to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, followed by cyclization with the C
N bond, followed by cyclization with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, resulting in unusual fused diaziridines (4 and 5). The products may define a much broader chemical space for diazirine studies, and in particular they can probe the reactivity and synthetic potential of diazirines.
C bond, resulting in unusual fused diaziridines (4 and 5). The products may define a much broader chemical space for diazirine studies, and in particular they can probe the reactivity and synthetic potential of diazirines.
At the outset, frontier molecular orbital (FMO) analysis of the simplified model (1a′) was conducted. The aim was to gain an insight into the discrepancy in the energy levels of alkenes and diazirines in the same molecule and to examine the feasibility of the hypothesis from a theoretical point of view. The results of the analysis demonstrated that the lowest unoccupied molecular orbital (LUMO) of 1a′ is dominated by the LUMO of the diazirine moiety (Fig. 1c, left), while the highest occupied molecular orbital (HOMO) of 1a′ is located more in the alkenyl moiety than in the diazirine (Fig. 1c, right). It was postulated that selective radical addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or N
C or N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond might be controlled with an appropriate external radical. Experimental investigations began with the addition of a CF3 radical to the dialiphatic diazirine (1a). The reactivity in radical reactions of dialiphatic diazirines without aryl or perfluoroalkyl substituents has not been explored. The CF3 radical was generated from the Ruppert–Prakash reagent (TMSCF3) which was oxidized by (diacetoxyiodo)benzene (PIDA) at 0 °C (for details, see the ESI‡). The transformation was initiated by adding a CF3 radical to the C
N bond might be controlled with an appropriate external radical. Experimental investigations began with the addition of a CF3 radical to the dialiphatic diazirine (1a). The reactivity in radical reactions of dialiphatic diazirines without aryl or perfluoroalkyl substituents has not been explored. The CF3 radical was generated from the Ruppert–Prakash reagent (TMSCF3) which was oxidized by (diacetoxyiodo)benzene (PIDA) at 0 °C (for details, see the ESI‡). The transformation was initiated by adding a CF3 radical to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 1a and ended with N
C bond of 1a and ended with N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N interception, leading as expected, to the pyrroline (2a) (Scheme 1). This product is unlikely to be formed by the previously described N → C cyclization in Fig. 1b, due to the lack of a radical acceptor that can transfer the CF3 group. The use of CsF as an additive is crucial to the reaction outcome, and other bases such as Cs2CO3 or KF were ineffective in this reaction.
N interception, leading as expected, to the pyrroline (2a) (Scheme 1). This product is unlikely to be formed by the previously described N → C cyclization in Fig. 1b, due to the lack of a radical acceptor that can transfer the CF3 group. The use of CsF as an additive is crucial to the reaction outcome, and other bases such as Cs2CO3 or KF were ineffective in this reaction.
With the optimized reaction conditions established, the scope of aliphatic diazirines was explored (Fig. 2). An array of diazirines bearing diverse skeletons were tested and easily converted into the corresponding pyrrolines in synthetically useful yields. The benzyl-substituted diazirine (1h) gave rise to a mixture of a product (2h) with an over-oxidized product (2h′), resulting from the spontaneous oxidation of the methylene between the phenyl and the pyrroline in air during the work-up procedure.3f,10 Diazirines bearing 1,1-disubstituted alkenes tended to construct pyrrolines containing quaternary centers regardless of steric hindrance. The substituents could contain alkyl, alkenyl, aryl, or heteroaryl groups (2i–2t). The electronic properties of aryl substituents had only a modest impact on the reaction outcomes (2m–2r). In those cases, the undesired oxidation of the tertiary benzylic radical intermediates to cations, which can occur under oxidative conditions, was not observed, indicating the priority of the intramolecular trapping of the benzylic radical by the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. The reaction of a diazirine (1u) bearing an internal alkene also proceeded smoothly, leading to an aza-spiro product (2u) with exclusive diastereoselectivity (dr > 20
N bond. The reaction of a diazirine (1u) bearing an internal alkene also proceeded smoothly, leading to an aza-spiro product (2u) with exclusive diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Aryl-substituted diazirines (2v) are also suitable substrates for the reaction. In addition to pyrrolines, tetrahydropyridine as a six-membered analogue could also be produced under the same conditions (2w, 2x and 2y), enriching the product library. Construction of other six-membered N-containing heterocycles could also be anticipated by placing various heteroatoms on the aliphatic chain. Attempts to construct seven- or eight-membered or even larger N-containing heterocycles by extending the aliphatic chain were unsuccessful. Remarkably, it was found however that this protocol can be applied to the dearomative 2,3-difunctionalization of indoles, providing spiro-indoline products (2z–2ab) with excellent stereo-selectivity. These three-dimensional indolines are widely distributed in various bioactive molecules.11
1). Aryl-substituted diazirines (2v) are also suitable substrates for the reaction. In addition to pyrrolines, tetrahydropyridine as a six-membered analogue could also be produced under the same conditions (2w, 2x and 2y), enriching the product library. Construction of other six-membered N-containing heterocycles could also be anticipated by placing various heteroatoms on the aliphatic chain. Attempts to construct seven- or eight-membered or even larger N-containing heterocycles by extending the aliphatic chain were unsuccessful. Remarkably, it was found however that this protocol can be applied to the dearomative 2,3-difunctionalization of indoles, providing spiro-indoline products (2z–2ab) with excellent stereo-selectivity. These three-dimensional indolines are widely distributed in various bioactive molecules.11
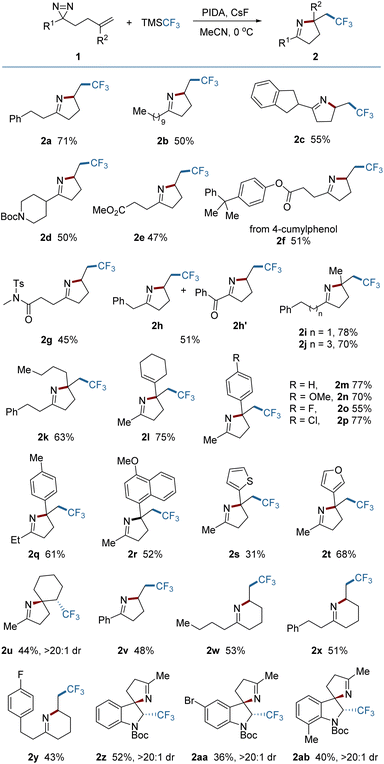 | ||
| Fig. 2 Scope of diazirines. Reaction conditions: 1 (0.2 mmol), TMSCF3 (0.6 mmol), PIDA (0.3 mmol), and CsF (0.3 mmol) in 2.0 mL MeCN under Ar at 0 °C for 1 h. Yields of isolated products are given. | ||
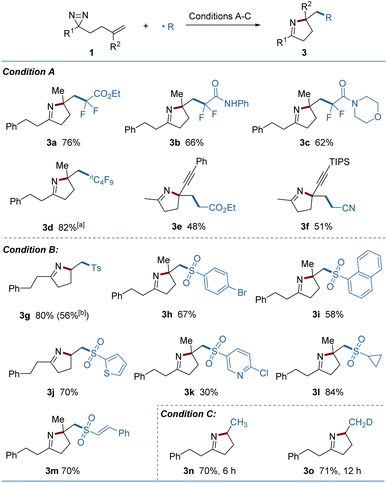
Using electrophilic radicals other than the CF3 radical further enriched the diversity of products from the reaction. As shown in Fig. 3, a portfolio of external radicals, such as di- or perfluoroalkyl radicals, electron-deficient alkyl radicals and sulfonyl radicals proved to be amenable to photoredox catalytic conditions, leading to the corresponding pyrrolines (3a–3m) in acceptable yields. The formation of 3e and 3f from the enyne-substituted diazirine precursors, is noteworthy, as the nascent propargyl radical was retained and engaged in the cyclization without conversion to the tautomeric allenyl radical.12 A set of (hetero)aryl, alkyl and styryl substituted sulfonyl radicals were added readily to the substrates, but the strong electron-deficient radicals delivered a lower yield (3k). A sulfonyl radical could also be generated under thermal conditions using copper(II) acetate as the oxidant and sodium p-toluenesulfinate as the radical source (3g). Combining this process with a metal-hydride hydrogen atom transfer (MHAT) led to the products containing incorporated hydrogen or deuterium (3n, 3o) in good yields, demonstrating the breadth of the method.
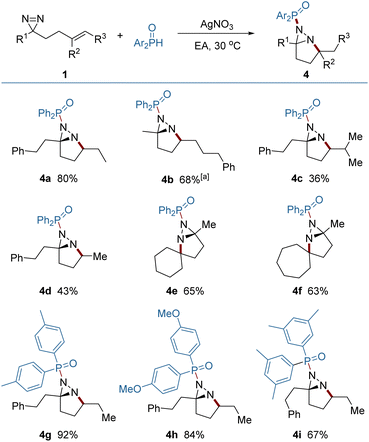
It was found that the addition of P-centered radicals to diazirine (1) proceeded through a distinct pathway and resulted in fused diaziridine products (Fig. 4). Diarylphosphinoyl radicals were generated from diarylphosphine oxides in the presence of silver nitrate as a catalyst, and were selectively added to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N rather than the C
N rather than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. The N → C cyclization generated an alkyl radical reaction which was terminated by HAT from diarylphosphine oxide. Meanwhile, the diarylphosphinoyl radical was regenerated, perpetuating the radical chain process. Compared to the diazirines (4a, 4b) which bear a 1,2-dialkyl alkene, the diazirine bearing a trisubstituted alkene delivered a lower yield (4c), probably because of the weak H-atom transfer (HAT) ability of the tertiary alkyl radical and the steric congestion that impeded the HAT. The engagement of the highly reactive primary alkyl radicals from HAT was less controllable, and also led to a decreased yield (4d). Skeletal complex polycyclic diaziridines (4e, 4f) are otherwise difficult to prepare, but were furnished in useful yields by this method. A quaternary carbon center can be readily constructed in this reaction.
C bond. The N → C cyclization generated an alkyl radical reaction which was terminated by HAT from diarylphosphine oxide. Meanwhile, the diarylphosphinoyl radical was regenerated, perpetuating the radical chain process. Compared to the diazirines (4a, 4b) which bear a 1,2-dialkyl alkene, the diazirine bearing a trisubstituted alkene delivered a lower yield (4c), probably because of the weak H-atom transfer (HAT) ability of the tertiary alkyl radical and the steric congestion that impeded the HAT. The engagement of the highly reactive primary alkyl radicals from HAT was less controllable, and also led to a decreased yield (4d). Skeletal complex polycyclic diaziridines (4e, 4f) are otherwise difficult to prepare, but were furnished in useful yields by this method. A quaternary carbon center can be readily constructed in this reaction.
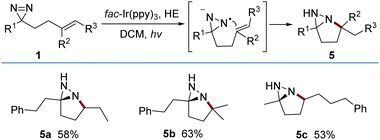
The reductive cyclization of 1 was accomplished to generate a new type of fused diaziridine (5) under photochemical conditions using a Hantzsch ester (HE) as a reducing agent (Fig. 5). These fused diaziridines might serve as versatile precursors for various N-heterocycles according to published approaches.13 The reaction leading to fused diaziridines was initiated by the reductive quenching of excited fac-Ir(ppy)3 with the Hantzsch ester to form an Ir(II) species. Its strong reduction potential (EIII/II1/2 = −2.19 V vs. SCE) enables single-electron reduction of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of diazirine (ERE1/2 = −2.06 V vs. SCE) to an N-centered radical anion (for details, see the ESI‡), and this is followed by a sequence of intramolecular cyclization, HAT and protonation. It should be noted that excited fac-Ir(ppy)3
N bond of diazirine (ERE1/2 = −2.06 V vs. SCE) to an N-centered radical anion (for details, see the ESI‡), and this is followed by a sequence of intramolecular cyclization, HAT and protonation. It should be noted that excited fac-Ir(ppy)3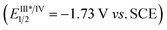 is not sufficient to reduce diazirines to trigger the reaction.
is not sufficient to reduce diazirines to trigger the reaction.
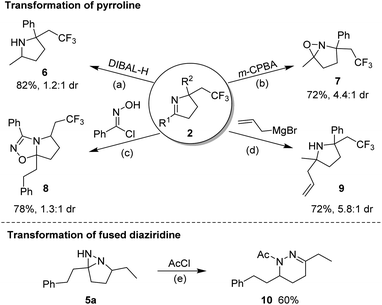
A scaled-up preparation of pyrroline (2m) from 2 mmol of 1m was achieved with 82% isolated yield, illustrating the practicality of the protocol. The products could be further converted to other valuable molecules (Fig. 6). For example, in the presence of diisobutyl aluminum-hydride (DIBAL-H), 2m was readily reduced to a pyrrolidine (6). The treatment of 2m with m-CPBA gave rise to a fused oxaziridine (7). The [3 + 2] cycloaddition of 2a with N-hydroxybenzimidoyl chloride generated the biologically relevant, CF3-substituted 1,2,4-oxadiazoline (8).14 Addition of an allyl Grignard reagent to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of 2m in the presence of BF3·Et2O afforded 2,2,5,5-tetrasubstituted pyrrolidine (9). Simple treatment of 5a with acetyl chloride resulted in N-acetyl-tetrahydropyridazine (10) by a ring-opening process.
N bond of 2m in the presence of BF3·Et2O afforded 2,2,5,5-tetrasubstituted pyrrolidine (9). Simple treatment of 5a with acetyl chloride resulted in N-acetyl-tetrahydropyridazine (10) by a ring-opening process.
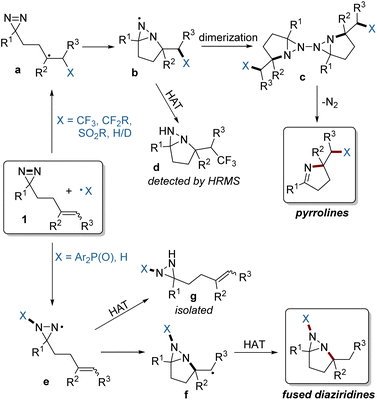
Based on the above experimental and computational results, a plausible mechanism is depicted in Fig. 7. For electrophilic radicals such as the fluoroalkyl and sulfonyl radicals, the selective addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 1, followed by intramolecular trapping of a radical (a) by the N
C bond of 1, followed by intramolecular trapping of a radical (a) by the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond gives rise to an N-centered radical (b), which can form a dimer (c). Intermediate c is unstable, and simultaneously undergoes N2 extrusion to afford two molecules of the pyrroline product.7,15 The formation of radical b was confirmed by the detection by HRMS of the fused diaziridine (d) which is gained from competitive HAT. In contrast, the relatively nucleophilic phosphinoyl radical prefers addition to the N
N bond gives rise to an N-centered radical (b), which can form a dimer (c). Intermediate c is unstable, and simultaneously undergoes N2 extrusion to afford two molecules of the pyrroline product.7,15 The formation of radical b was confirmed by the detection by HRMS of the fused diaziridine (d) which is gained from competitive HAT. In contrast, the relatively nucleophilic phosphinoyl radical prefers addition to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of 1, resulting in radical e. The subsequent intramolecular cyclization to the C
N bond of 1, resulting in radical e. The subsequent intramolecular cyclization to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and HAT affords the fused diaziridine products. The formation of intermediate e was confirmed by the isolation of byproduct g derived from HAT.
C bond and HAT affords the fused diaziridine products. The formation of intermediate e was confirmed by the isolation of byproduct g derived from HAT.
Conclusions
In summary, a novel radical-mediated divergent synthesis of pyrrolines and fused diaziridines from rationally designed alkene-substituted diazirines is described. The key to the success of the reaction is the selective addition of an external radical to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or N
C or N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, which is controlled by the inherent electronic characteristics of the external radical. FMO analysis sheds light on the underlying reason for the chemoselectivity. The protocol features good functional group tolerance and high product diversity, and creates a much broader chemical space for diazirine studies.
N bond, which is controlled by the inherent electronic characteristics of the external radical. FMO analysis sheds light on the underlying reason for the chemoselectivity. The protocol features good functional group tolerance and high product diversity, and creates a much broader chemical space for diazirine studies.
Data availability
All relevant experimental and computational data and characterization details are provided in the ESI.‡Author contributions
C. Z. conceived the idea and designed the experiments, Z. M. performed most of the experiments, X. W. performed the DFT calculations, H. L. synthesized some of the starting materials, Z. C. helped with analysis of the data, and C. Z. supervised the research and co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22171201, 22001185, and 21971173), the Fundamental Research Funds for the Central Universities (22X010201631), the Program of Shanghai Academic/Technology Research Leader (23XD1421900), the Natural Science Foundation of Jiangsu Province (BK20200852), the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201905), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- (a) J. W. Daly, H. M. Garraffo and T. F. Spande, in Alkaloids Chem. Biol. Perspect., ed. S. W. Pelletier, Elsevier, Oxford, 1999, pp. 1–161 Search PubMed; (b) T. Aniszewski, in Alkaloids – Secrets Life, Elsevier, 2007, pp. 61–139 Search PubMed; (c) T. H. Jones, V. E. Zottig, H. G. Robertson and R. R. Snelling, J. Chem. Ecol., 2003, 29, 2721–2727 CrossRef CAS PubMed; (d) T. H. Jones, A. N. Andersen and J. C. Kenny, Chem. Biodiversity, 2009, 6, 1034–1041 CrossRef CAS PubMed; (e) J. Chen, C. L. Cantrell, D. Oi and M. J. Grodowitz, Toxicon, 2016, 122, 127–132 CrossRef CAS PubMed.
- (a) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (c) A. Kondo, N. Morita, T. Ishikawa, M. Yoshida, A. Moriyama and I. Wanajo, US Pat., US10995084B2, 2018 Search PubMed; (d) A. Kondo, N. Morita, T. Ishikawa, M. Yoshida, A. Moriyama and I. Wanajo, EP3459941A1, 2019; (e) A. Kondo, N. Morita, T. Ishikawa, M. Yoshida, A. Moriyama and I. Wanajo, US Pat., US2020/0299273A1, 2020 Search PubMed; (f) E. Martinborough, Y. Shen, A. van Oeveren, Y. O. Long, T. L. S. Lau, K. B. Marschke, W. Y. Chang, F. J. López, E. G. Vajda, P. J. Rix, O. H. Viveros, A. Negro-Vilar and L. Zhi, J. Med. Chem., 2007, 50, 5049–5052 CrossRef CAS PubMed; (g) H. Ü. Kaniskan, M. S. Eram, K. Zhao, M. M. Szewczyk, X. Yang, K. Schmidt, X. Luo, S. Xiao, M. Dai, F. He, I. Zang, Y. Lin, F. Li, E. Dobrovetsky, D. Smil, S. J. Min, J. Lin-Jones, M. Schapira, P. Atadja, E. Li, D. Barsyte-Lovejoy, C. H. Arrowsmith, P. J. Brown, F. Liu, Z. Yu, M. Vedadi and J. Jin, J. Med. Chem., 2018, 61, 1204–1217 CrossRef CAS PubMed.
- For selected examples, see: (a) A. Faulkner, J. S. Scott and J. F. Bower, Chem. Commun., 2013, 49, 1521–1523 RSC; (b) A. Faulkner, N. J. Race, J. S. Scott and J. F. Bower, Chem. Sci., 2014, 5, 2416–2421 RSC; (c) A. Faulkner, J. S. Scott and J. F. Bower, J. Am. Chem. Soc., 2015, 137, 7224–7230 CrossRef CAS PubMed; (d) C. Chen, L. Hou, M. Cheng, J. Su and X. Tong, Angew. Chem., Int. Ed., 2015, 54, 3092–3096 CrossRef CAS PubMed; (e) N. J. Race, A. Faulkner, G. Fumagalli, T. Yamauchi, J. S. Scott, M. R. Landergren, H. A. Sparkes and J. F. Bower, Chem. Sci., 2017, 8, 1981–1985 RSC; (f) H.-B. Yang, S. R. Pathipati and N. Selander, ACS Catal., 2017, 7, 8441–8445 CrossRef CAS; (g) L. Wang and C. Wang, J. Org. Chem., 2019, 84, 6547–6556 CrossRef CAS PubMed; (h) Y. Zhang, H.-J. Ai and X.-F. Wu, Org. Chem. Front., 2020, 7, 2986–2990 RSC; (i) X. Zhang, D. Qi, C. Jiao, Z. Zhang, X. Liu and G. Zhang, Org. Chem. Front., 2021, 8, 6522–6529 RSC.
- For selected reviews, see: (a) M. M. Jackman, Y. Cai and S. L. Castle, Synthesis, 2017, 49, 1785–1795 CrossRef CAS; (b) J. Fu, G. Zanoni, E. A. Anderson and X. Bi, Chem. Soc. Rev., 2017, 46, 7208–7228 RSC; (c) P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 12–22 CrossRef PubMed; (d) X.-Y. Yu, Q.-Q. Zhao, J. Chen, W.-J. Xiao and J.-R. Chen, Acc. Chem. Res., 2020, 53, 1066–1083 CrossRef CAS PubMed; (e) J. Boivin, E. Fouquet and S. Z. Zard, Tetrahedron Lett., 1991, 32, 4299–4302 CrossRef CAS; (f) X. Sun and S. Yu, Chem. Commun., 2016, 52, 10898–10901 RSC; (g) X.-Y. Yu, J.-R. Chen, P.-Z. Wang, M.-N. Yang, D. Liang and W.-J. Xiao, Angew. Chem., Int. Ed., 2018, 57, 738–743 CrossRef CAS PubMed; (h) E. M. Dauncey, S. U. Dighe, J. J. Douglas and D. A. Leonori, Chem. Sci., 2019, 10, 7728–7733 RSC; (i) J. Singh, G. A. Nickel, Y. Cai, D. D. Jones, T. J. Nelson, J. E. Small and S. L. Castle, Org. Lett., 2021, 23, 3970–3974 CrossRef CAS PubMed.
- (a) J. Davies, S. G. Booth, S. Essafi, R. A. W. Dryfe and D. Leonori, Angew. Chem., Int. Ed., 2015, 54, 14017–14021 CrossRef CAS PubMed; (b) J. Davies, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2017, 56, 13361–13365 CrossRef CAS PubMed; (c) H. Jiang and A. Studer, Angew. Chem., Int. Ed., 2017, 56, 12273–12276 CrossRef CAS PubMed; (d) S.-H. Cai, J.-H. Xie, S. Song, L. Ye, C. Feng and T.-P. Loh, ACS Catal., 2016, 6, 5571–5574 CrossRef CAS; (e) S.-H. Cai, D.-X. Wang, L. Ye, Z.-Y. Liu, C. Feng and T.-P. Loh, Adv. Synth. Catal., 2018, 360, 1262–1266 CrossRef CAS; (f) X. Shen, C. Huang, X.-A. Yuan and S. Yu, Angew. Chem., Int. Ed., 2021, 60, 9672–9679 CrossRef CAS PubMed.
- (a) R. A. Moss, Acc. Chem. Res., 2006, 39, 267–272 CrossRef CAS PubMed; (b) J. Das, Chem. Rev., 2011, 111, 4405–4417 CrossRef CAS PubMed.
- D. H. R. Barton, J. C. Jaszberenyi, E. A. Theodorakis and J. H. Reibenspies, J. Am. Chem. Soc., 1993, 115, 8050–8059 CrossRef CAS.
- (a) P. P. Chandrachud, L. Wojtas and J. M. Lopchuk, J. Am. Chem. Soc., 2020, 142, 21743–21750 CrossRef CAS PubMed; (b) V. Maharaj, P. P. Chandrachud, W. Che, L. Wojtas and J. M. Lopchuk, Org. Lett., 2021, 23, 8838–8842 CrossRef CAS PubMed.
- X. Shu, R. Xu and S. Liao, Sci. China: Chem., 2021, 64, 1756–1762 CrossRef CAS.
- (a) M. Lebœuf, A. Ranaivo, A. Cavé and H. Moskowitz, J. Nat. Prod., 1989, 52, 516–521 CrossRef; (b) S. Kim, Y. M. Lee, J. Lee, T. Lee, Y. Fu, Y. Song, J. Cho and D. Kim, J. Org. Chem., 2007, 72, 4886–4891 CrossRef CAS PubMed.
- (a) Y. J. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed; (b) N. Ye, H. Y. Chen, E. A. Wold, P. Y. Shi and J. Zhou, ACS Infect. Dis., 2016, 2, 382–392 CrossRef CAS PubMed; (c) Y. T. Yang, J. F. Zhu, G. C. Liao, H. J. Xu and B. Yu, Curr. Med. Chem., 2018, 25, 2233–2244 CrossRef CAS PubMed.
- (a) F. Wang, D. Wang, Y. Zhou, L. Liang, R. Lu, P. Chen, Z. Lin and G. Liu, Angew. Chem., Int. Ed., 2018, 57, 7140–7145 CrossRef CAS PubMed; (b) K.-F. Zhang, K.-J. Bian, C. Li, J. Sheng, Y. Li and X.-S. Wang, Angew. Chem., Int. Ed., 2019, 58, 5069–5074 CrossRef CAS PubMed; (c) X. Zhu, W. Deng, M.-F. Chiou, C. Ye, W. Jian, Y. Zeng, Y. Jiao, L. Ge, Y. Li, X. Zhang and H. Bao, J. Am. Chem. Soc., 2019, 141, 548–559 CrossRef CAS PubMed.
- (a) Y. B. Koptelov, Russ. J. Org. Chem., 2006, 42, 1510–1515 CrossRef CAS; (b) J. M. Allen and T. H. Lambert, Tetrahedron, 2014, 70, 4111–4117 CrossRef CAS; (c) A. O. Chagarovskiy, V. S. Vasin, V. V. Kuznetsov, O. A. Ivanova, V. B. Rybakov, A. N. Shumsky, N. N. Makhova and I. V. Trushkov, Angew. Chem., Int. Ed., 2018, 57, 10338–10342 CrossRef CAS PubMed; (d) H. Zheng and M. P. Doyle, Angew. Chem., Int. Ed., 2019, 58, 12502–12506 CrossRef CAS; (e) H. Hu, J. Xu, F. Wang, S. Dong, X. Liu and X. Feng, Org. Lett., 2020, 22, 93–97 CrossRef CAS PubMed; (f) T. Sarkar, K. Talukdar, S. Roy and T. Punniyamurthy, Chem. Commun., 2020, 56, 3381–3384 RSC; (g) A. A. Penney, M. M. Efremova, A. P. Molchanov, M. A. Kryukova, A. Y. Kudinov, A. S. Bunev, V. M. Keresten and M. A. Kuznetsov, ChemistrySelect, 2022, 7, e202202627 CrossRef CAS.
- G. Dannhardt, K. K. Mayer and I. Sommer, Sci. Pharm., 1984, 52, 280–290 CAS.
- (a) D. M. Gale, W. J. Middleton and C. G. Krespan, J. Am. Chem. Soc., 1966, 88, 3617–3623 CrossRef; (b) D. H. R. Barton, N. Ozbalik and B. Vacher, Tetrahedron, 1988, 44, 7385–7392 CrossRef CAS.
Footnotes |
| † Dedicated to the 70th birthday of Professor Dennis Curran. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc04886a |
| § These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |