Scalable co-cured polyimide/poly(p-phenylene benzobisoxazole) all-organic composites enabling improved energy storage density, low leakage current and long-term cycling stability†
Peiyuan
Zuo
,
Jinpeng
Li
,
Donglin
Chen
,
Lingzhi
Nie
,
Liang
Gao
 ,
Jingyu
Lin
and
Qixin
Zhuang
,
Jingyu
Lin
and
Qixin
Zhuang
 *
*
The Key Laboratory of Advanced Polymer Materials of Shanghai, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: qxzhuang@ecust.edu.cn
First published on 8th November 2023
Abstract
The all-organic high-temperature polymer dielectrics with promising scale-up potential have witnessed much progress in the energy storage area, etc. However, the electron suppression trap mechanisms behind many all-organic dielectrics are still unclear, especially for high temperature resistant poly(p-phenylene benzobisoxazole) (PBO) polymers. To resolve this tough issue, we herein innovatively prepared PBO-based all-organic thin films containing sulfone-based polyimide (P(DSDA-ODA)) functioning as an electron trap phase using a facile and scalable co-curing method. The great linear dielectric properties of the prepared P(DSDA-ODA)/PBO films hold high dielectric thermal stability over the temperature range from 25 °C to 200 °C. The 60 wt% P(DSDA-ODA) systems yield the lowest leakage current (3.8 × 10−8 A cm−2). The tight structure and reduced leakage current enable an enhanced breakdown strength of 60 wt% P(DSDA-ODA)/PBO (470 kV mm−1), which is 1.7 times that of pure PBO. Meanwhile, it can reach 4.16 J cm−3 of energy density, which is 257% higher than that for pure PBO thin films while concurrently maintaining a long stable charge–discharge cycle (at least 5000 times) and high charge–discharge efficiency at 85.10%. Moreover, P(DSDA-ODA)/PBO still exhibits excellent energy storage performance at high temperature compared to PBO. This innovative strategy is further verified by replacing P(DSDA-ODA) with P(6FDA-ODA), and therefore lays a solid foundation for more investigation on scalable all-organic dielectrics.
New conceptsThe commercial biaxially-oriented-polypropylene (BOPP) dielectric capacitors face many tough issues such as poor energy storage properties and high leakage current at high temperature. To resolve these problems, we innovatively used a facile and scalable co-curing method to prepare polyimide/poly(p-phenylene benzobisoxazole) all-organic dielectrics. The introduced dipole groups from polyimide construct electron traps within the blending material system, therefore forming scattering centers to suppress the movement of free electrons. To the best of our knowledge, this is the first experimental and theoretical verification derived from polyimide/poly(p-phenylene benzobisoxazole) all-organic composites, which exhibit concurrently improved energy storage density, low leakage current and long-term cycling stability. |
Introduction
Recently, dielectric polymers are receiving ever-increasing attention due to their high breakdown strength (Eb), high temperature tolerance and excellent energy density storage (Ue) characteristics for advanced power and electrical equipment,1–4 and electronic devices such as polymer-based film capacitors and gate dielectrics.5–8 Currently, the main method is focused on adding ceramic nanoparticles with a giant dielectric constant into dielectric polymers to form inorganic–organic nanocomposites and substantially enhance the comprehensive dielectric properties.9,10 However, compared to all-organic composites, the inorganic–organic nanocomposite dielectric materials usually result in obvious dielectric loss.11,12 Moreover, the interfacial area of the inorganic–organic composition frequently exhibits an internal electric field concentration due to heterogenous phases, hence easily leading to premature breakdown due to the overhigh local electric field.9,13Different from typical high-k inorganic nanofillers, boron nitride nanosheets (BNNs) with relatively low dielectric constant but large band gap have been preferred to enhance the breakdown strength and energy storage capacity in recent years.14 Despite this, the exfoliation of BNNs is time-consuming and low-efficiency, so it is not realizable to have mass production in short times. Another important strategy is to construct electron traps to effectively suppress the leakage current and dielectric loss, thus realizing an improvement in the dielectric energy storage properties.15–18 Zha et al. intensively investigated the imidization reaction kinetics of polyimide films at elevated temperature and found that the introduced –COOH/–CN–OH– polar groups realized an increased dielectric constant by enhancing dipole polarization.19 They reported that the –COOH/–CN–OH– functional groups can act as deep traps to reduce the mobility of the carriers, thus increasing the Eb and Ue. A recent report also firmly clarified that the electron traps can be effectively introduced in a polymer matrix using small molecular semiconductors featuring high electron affinity.20–22 Compared to other inorganic doping, the organic traps enable less voids and pores although the molecular semiconductors are still expensive and scarce. As such, how to build all-organic composites with commercially acceptable polymers while endowing some blending phase with a trap function is an interesting topic to be further investigated.
Noticeably, although the conventional dielectric polymers such as traditional biaxially oriented polypropylene (BOPP) have been successfully commercialized and widely applied, there still exist many problems such as poor high temperature resistance due to low glass transition temperature (Tg). Once the temperature approaches Tg, the BOPP polymers will lose their dimensional and dielectric stability, thus seriously displaying low dielectric constant and high dielectric loss.23 To circumvent these problems, high temperature resistant polymers such as polyimide (PI),17,24–26 polyetherimide (PEI)27,28 and poly(p-phenylene benzobisoxazole) (PBO)29–32 have been intensively exploited due to their superior thermal stability. In particular, PBO with much less water absorption and low dielectric loss characteristics is becoming a promising matrix for high-temperature dielectric materials. Despite that, for pure PBO, it is difficult to introduce a controllable number of polar groups into the PBO molecular chain through complex chemical grafting.
Based on this above consideration, we herein innovatively prepared high-temperature resistant polymer dielectric materials based on PBO using an all-organic material strategy. The excellent polyimide polymer containing a sulfone group is introduced into PBO to regulate the number of polar groups and thus improve the polarization strength of the PBO-based film. The mixed PI and PBO precursors are further subjected to a co-curing process to reduce the free volume in the all-organic system, therefore enhancing the comprehensive dielectric properties. At the same time, the breakdown and mechanical performance of PBO is also improved by the mixing of the PBO and PI polymer molecular chain. Also, the introduction of sulfone groups can construct electron traps within the blending material system, therefore forming scattering centers to suppress the movement of free electrons. This can effectively reduce the generation of internal leakage current in the system under high temperature conditions and improve the high-temperature energy storage performance of blending dielectrics. More noticeably, the small size of sulfonyl groups enables less hysteresis during dipole switching. In addition, to explore whether the introduction of PI containing functional groups into PBO can be widely applied, we also synthesized PI polymers containing trifluoromethyl groups and the corresponding PBO films and tested the dielectric and energy storage properties of the films. The results showed that the introduction of polyimide containing trifluoromethyl groups into PBO polymers can also significantly improve the energy storage performance of PBO-based films. Accordingly, we therefore believe the simple PI and PBO blending system with a co-curing process would be a facile and scalable direction to support the wide dielectric applications.
Experimental and characterizations
Materials
4,6-Diaminoresorcinol dihydrochloride (AR) was provided by Shanghai Lingfeng Chemicals. tert-Butyldimethylsilyl chloride (97%), triethylamine (GC), p-phthaloyl chloride (99%), 3,3′,4,4′-diphenylsulfonetetracarboxylicdianhydride (DSDA) (HPLC) and 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (98%) were purchased from Aladdin (Shanghai, China). 4,4′-Oxydianiline (98%) was supplied by Macklin (Shanghai, China). N-Methylpyrrolidone (NMP), N,N-dimethylacetamide (DMAc) and N,N-dimethylformamide (DMF) were provided by J&K (Shanghai, China). All chemicals are used as received without any further treatment.Synthesis of polyimide and PI-PBO films
Characterization
The cross-sectional images of thin film samples were collected using an S-4800 high-power field emission scanning electron microscope (SEM, Hitachi). The thermal weight loss (TG) curves of the samples were collected by the ATA409PC thermal analyzer, and the thermal weight loss test was conducted under nitrogen fractionation. The dielectric properties of the thin film samples at room temperature were tested using the Concept 40 broadband dielectric spectrometer (Novocontrol Technologies). Before testing, a gold electrode with a diameter of 3 mm was deposited on the surface of the thin film using a gold spray instrument. The variation of the dielectric properties for the thin films with frequency at different temperatures was measured by a 4980A impedance analyzer produced by Agilent (Palo Alto, CA). The breakdown strength of the thin films was tested using the CS2674AX withstand voltage tester manufactured by Nanjing Changsheng. The D–E electric field curve and leakage current of the all-organic dielectrics at room and high temperatures were determined by a Radiant Precision Premier II ferroelectric analyzer (Radiant Technologies, Inc., Albuquerque, NM). Mechanical properties were conducted by the universal testing machine (E42.503) with a strain rate of 2 mm min−1. Each result was obtained based on repeating at least five specimens. The thermal transition temperatures of PBO and its blending composites were studied by differential scanning calorimetry (DSC) under a N2 atmosphere at a rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
°C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min−1.
min−1.
Results and discussion
Fig. 1(a) shows the X-ray diffraction spectra of PBO and P(DSDA-ODA)/PBO films with different P(DSDA-ODA) contents after co-curing. The diffraction peaks are observed in PBO and all-organic films near 16.0° and 26.1°, indicating the successful preparation of PBO-based films. The added P(DSDA-ODA) enables increased diffraction angle intensity of the molecular chain at a 16.0° diffraction angle, suggesting that the introduction of P(DSDA-ODA) is beneficial for increased order degree in PBO. Fig. 1(b) shows the infrared spectra of PBO and P(DSDA-ODA)/PBO films after heat treatment. With the completion of co-curing, the characteristic peak of PrePBO disappears while the appearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N characteristic peak at 1630 cm−1 suggests the successful formation of PBO. In addition, no characteristic peak of CO–NH– is observed at 1550 cm−1 in the infrared spectrum of the P(DSDA-ODA)/PBO film, implying that DSDA-ODA-PAA completes the ring closure reaction and successfully transforms into P(DSDA-ODA) during the co-curing process of PBO. The co-curing process enables the Pre-PBO to form an oxazole ring. This process always transforms the soluble Pre-PBO to insoluble and dark PBO polymers. The film exhibits characteristic peaks of C
N characteristic peak at 1630 cm−1 suggests the successful formation of PBO. In addition, no characteristic peak of CO–NH– is observed at 1550 cm−1 in the infrared spectrum of the P(DSDA-ODA)/PBO film, implying that DSDA-ODA-PAA completes the ring closure reaction and successfully transforms into P(DSDA-ODA) during the co-curing process of PBO. The co-curing process enables the Pre-PBO to form an oxazole ring. This process always transforms the soluble Pre-PBO to insoluble and dark PBO polymers. The film exhibits characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric and symmetric vibrations at 1782 cm−1 and 1728 cm−1, and a sulfone-based characteristic peak at 1176 cm−1, respectively. The characteristic peaks of the imide ring can also be observed at 742 cm−1, and the relative intensity of these characteristic peaks of P(DSDA-ODA) increases with the increase of the P(DSDA-ODA) content, showing that organic films with different ratios of P(DSDA-ODA)/PBO have been successfully prepared through a solution blending and co-curing method. Fig. 1(c) shows the thermal weight loss curves of P(DSDA-ODA)/PBO at different concentrations. One can see that the Td5 of P(DSDA-ODA)/PBO is greater than 400 °C, demonstrating excellent heat resistance. Besides the high decomposition temperature, it can also be observed that the increased content of P(DSDA-ODA) also brings additional weight loss from 20 wt% to 80 wt%. This is mainly due to the excellent thermal-resistance of PBO itself derived from the existence of an oxazole ring. The higher P(DSDA-ODA) content that replaces the oxazole ring will therefore slightly weaken the high-temperature thermal resistance. Fig. S1 (ESI†) also provides more morphological details for P(DSDA-ODA)/PBO films before/after co-curing, and the films’ colour turns from light to deep yellow. After undergoing high-temperature co-curing, it tends to deepen while accompanying metallic luster (Fig. 1(d)) and good bendability features (Fig. 1(c) inserted photo and Fig. S1(c) and (d), ESI†).
O asymmetric and symmetric vibrations at 1782 cm−1 and 1728 cm−1, and a sulfone-based characteristic peak at 1176 cm−1, respectively. The characteristic peaks of the imide ring can also be observed at 742 cm−1, and the relative intensity of these characteristic peaks of P(DSDA-ODA) increases with the increase of the P(DSDA-ODA) content, showing that organic films with different ratios of P(DSDA-ODA)/PBO have been successfully prepared through a solution blending and co-curing method. Fig. 1(c) shows the thermal weight loss curves of P(DSDA-ODA)/PBO at different concentrations. One can see that the Td5 of P(DSDA-ODA)/PBO is greater than 400 °C, demonstrating excellent heat resistance. Besides the high decomposition temperature, it can also be observed that the increased content of P(DSDA-ODA) also brings additional weight loss from 20 wt% to 80 wt%. This is mainly due to the excellent thermal-resistance of PBO itself derived from the existence of an oxazole ring. The higher P(DSDA-ODA) content that replaces the oxazole ring will therefore slightly weaken the high-temperature thermal resistance. Fig. S1 (ESI†) also provides more morphological details for P(DSDA-ODA)/PBO films before/after co-curing, and the films’ colour turns from light to deep yellow. After undergoing high-temperature co-curing, it tends to deepen while accompanying metallic luster (Fig. 1(d)) and good bendability features (Fig. 1(c) inserted photo and Fig. S1(c) and (d), ESI†).
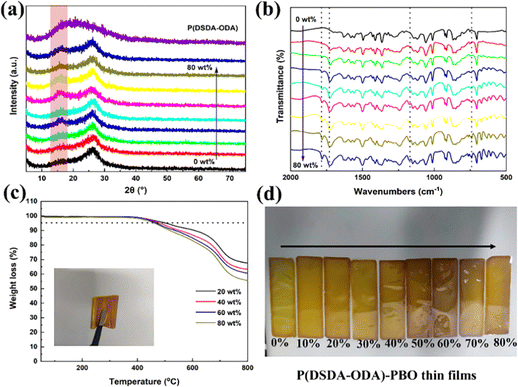 | ||
| Fig. 1 (a)–(c) XRD, FTIR spectrum and TGA curve of P(DSDA-ODA)/PBO films with 0 to 80 wt% P(DSDA-ODA) and (d) digital photos of P(DSDA-ODA)/PBO films. | ||
Fig. 2(a)–(i) provide cross-sectional SEM images of pure PBO and 10–80 wt% P(DSDA-ODA)/PBO thin films, respectively. All P(DSDA-ODA)/PBO thin films exhibit good continuity at the cross-section without significant phase separation, therefore suggesting that the prepared P(DSDA-ODA) has good compatibility with PBO. We have conducted a DSC test and found that P(DSDA-ODA) presents a glass transition temperature at 311 °C while the 60% P(DSDA-ODA)/PBO shows unobvious Tg around 318 °C. In comparison, the pure PBO does not exhibit Tg below 350 °C. As such, we conclude that the P(DSDA-ODA)/PBO would therefore exhibit high thermal resistance and dielectric thermal stability.
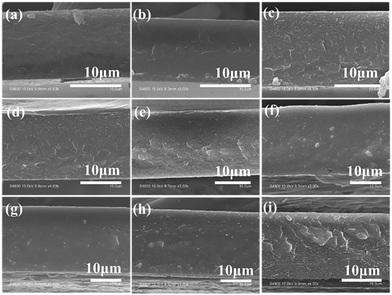 | ||
| Fig. 2 ((a)–(i)) Cross-sectional SEM images of the P(DSDA-ODA)/PBO films with the P(DSDA-ODA) weight fractions from 0 to 80 wt%. | ||
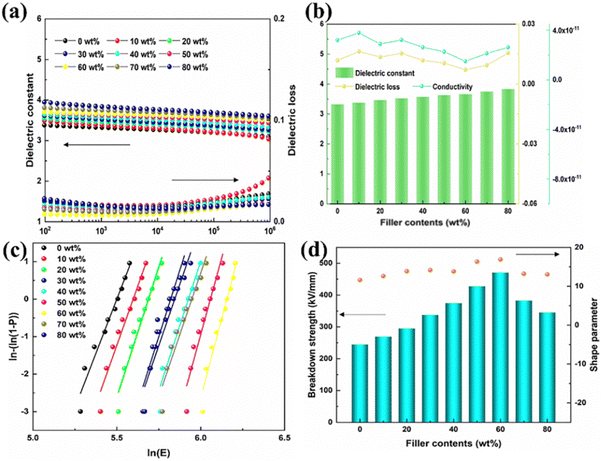
Fig. 3(a) shows the dielectric constant/loss of P(DSDA-ODA)/PBO all-organic thin films in the range of 102–106 Hz. Due to the linear dielectric properties and excellent compatibility of PBO and P(DSDA-ODA), the dielectric constant of P(DSDA-ODA)/PBO exhibits a low frequency dependence. As the amount of P(DSDA-ODA) addition increases, the high dipole moment-SO2 (4.5 D) in the P(DSDA-ODA)/PBO film gives rise to a dielectric constant of the films. Unlike inorganic–organic films, all-organic composites can greatly alleviate the dielectric loss derived from interface polarization occurring at the heterogeneous interface.34 Therefore, the dielectric loss of P(DSDA-ODA)/PBO is maintained at a low value (<0.05). Even if the addition of P(DSDA-ODA) reaches 80 wt%, the dielectric loss of the films is still similar to that of pure PBO (Fig. 3(b)), which is obviously beneficial to improve the dielectric energy storage efficiency and the service life of the capacitor. When the amount of P(DSDA-ODA) reaches 60 wt%, the dielectric loss of the material reaches its lowest in the low-frequency region. When the content of P(DSDA-ODA) further increases, the dielectric loss of the P(DSDA-ODA)/PBO film begins to increase. Similarly, the conductivity of the film material is also maintained in a very low range, indicating the excellent insulation performance of the film (Fig. S3, ESI†). When the addition amount of P(DSDA-ODA) is 60 wt%, the conductivity of the film material in the low-frequency range also reaches its lowest level. Noticeably, the dielectric constant/loss for PBO and P(DSDA-ODA)/PBO exhibit high thermal stability from RT to 200 °C over the whole testing frequency range (Fig. S4, ESI†). As shown in Fig. S4 (ESI†), due to the strong and stable molecular structure, the dielectric constant/loss of PBO and 60 wt% P(DSDA-ODA) are almost insensitive to the temperature. This is mainly due to the high thermal resistance of the PBO and PI polymers, which ensures the dielectric thermal stability over a wide range. Fig. 3(c) shows the Weibull distribution of the breakdown strength for all polymer films with different P(DSDA-ODA) contents. The addition of P(DSDA-ODA) has to some extent improved the breakdown strength of PBO. The addition of P(DSDA-ODA) can reduce the defects in PBO, thereby delaying the occurrence of the breakdown phenomenon. One can also see the changes in the shape factor of the Weibull distribution for P(DSDA-ODA)/PBO and PBO thin films. Fig. 3(d) shows the characteristic breakdown strength of PBO based on all organic films with different levels of P(DSDA-ODA) addition. When the addition amount is 60 wt%, the highest characteristic breakdown strength of P(DSDA-ODA)/PBO reaches 470 kV mm−1, which is 1.7 times higher than the original PBO film (246 kV mm−1). When the content of P(DSDA-ODA) further increases, it will cause an overlap of energy traps, followed by reducing the energy level of energy traps, therefore leading to a decrease in the breakdown strength of the films when the content of P(DSDA-ODA) is too high. Compared to the original PBO thin film, the addition of P(DSDA-ODA) significantly increases the material's shape factor. There are two reasons that can be concluded as follows: first, besides phonon electron scattering occurring within the polymer, the Coulomb interaction between sulfone dipoles and carriers can cause additional electron scattering, hence stabilizing the high-energy charges and limiting the formation of secondary electrons due to collisions during the movement of carriers. Second, the dipoles can form localized states in the bandgap as energy traps, therefore leading to the formation of localized states.35 The P(DSDA-ODA)/PBO film has a certain degree of improvement compared to PBO, as it can capture charges under the action of an external electric field and limit the movement of charge carriers.
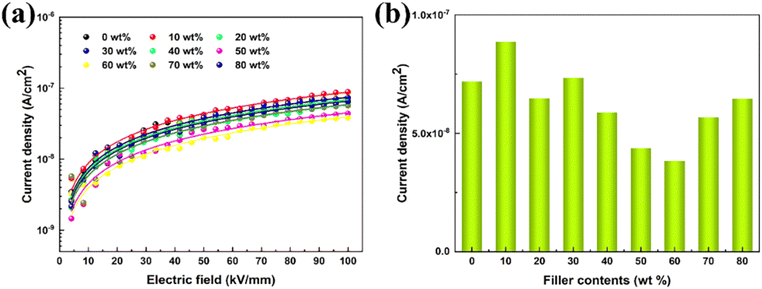
To further clarify the mechanism behind in film materials with increased breakdown strength, the variation of the leakage current density of P(DSDA-ODA)/PBO with an applied electric field at room temperature is presented (Fig. 4(a)). By nonlinear fitting the leakage current density (J) of the dielectric thin film with the applied electric field (E), it is shown that the variation pattern follows the hopping conduction mechanism; that is, the relationship between J and E follows formula (1). By integrating the constants in formula (1), the relationship between J and E can be simplified as formula (2):
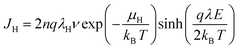 | (1) |
| J = A × sinh(BE) | (2) |
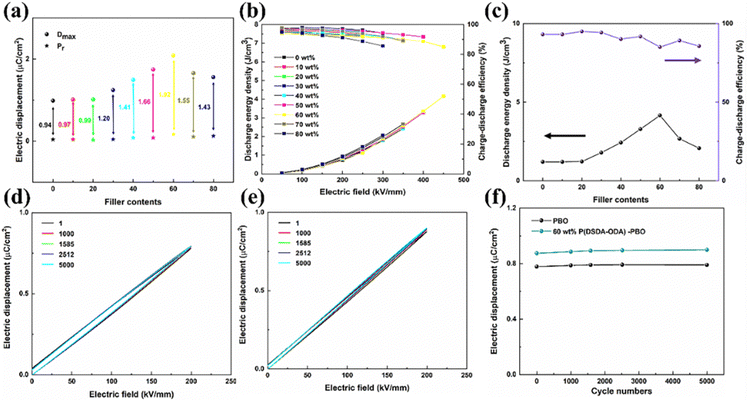
Fig. 5(a) shows the maximum applied electric field potential shift (Dmax), the residual polarization (Pr), and their differences for thin films with different levels of P(DSDA-ODA) addition. One can see that the Dmax of pure PBO is only 0.99 μC cm−2, when the addition amount of P(DSDA-ODA) is 60 wt%, Dmax reaches the maximum value of 2.09 μC cm−2, increasing by 110% when compared to pure PBO. The difference between Dmax and Pr of P(DSDA-ODA)/PBO thin films shows a trend of first increasing and then decreasing, but both values are higher than those of pure PBO thin films, therefore indicating that the introduction of P(DSDA-ODA) is beneficial for improving the effective polarization of the material. By calculating the hysteresis loop areas of polymer films with different contents, their energy storage density and charge discharge efficiency can be obtained (Fig. 5(b)). With the addition of P(DSDA-ODA), the energy storage density of the dielectric shows an increasing trend. On the one hand, the introduction of –SO2 strengthens the dielectric constant of the all-organic system while the addition of P(DSDA-ODA) also improves the breakdown strength of PBO. When the addition amount of P(DSDA-ODA) is 60 wt%, P(DSDA-ODA)/PBO can withstand higher external electric fields and possesses the highest energy storage density. At 450 kV mm−1, the discharge energy storage density reaches 4.16 J cm−3, which is 257% higher than that for pure PBO film (1.16 J cm−3), while maintaining a charge discharge efficiency at 85.10%, thus indicating less Joule heat generated in the charge–discharge process, which is beneficial to thermal runway resistance.36 As the content of P(DSDA-ODA) further increases, the density of dipoles in the all-organic system continuously changes, causing the energy traps constructed by charge carriers to pile up with each other, therefore resulting in a decrease in their energy levels and a weakened ability to limit charge carriers and hence leading to a declination in voltage resistance performance. Although the electric displacement is enhanced under the same electric field, the decreased breakdown strength limits the further improvement of the energy storage performance of the thin film. Fig. 5(c) shows the energy storage density and charge discharge efficiency of thin films versus different P(DSDA-ODA) contents under the maximum applied electric field. The evolution of the energy storage density of P(DSDA-ODA)/PBO thin films is consistent with the relationship between the Dmax–Pr values. In addition, the energy storage density of all P(DSDA-ODA)/PBO thin films is higher than that of pure PBO thin films while maintaining a high charge discharge efficiency. Although the 60% P(DSDA-ODA)/PBO films show relatively low charge–discharge efficiency, it still yields an 85% charge–discharge efficiency. It is understood that at low P(DSDA-ODA) content, the energy storage density is low with high efficiency. In comparison, the high P(DSDA-ODA) content usually results in more dielectric loss and hence relatively lower efficiency. Nevertheless, the general charge–discharge efficiency is satisfying.
To further investigate the electrical fatigue resistance of the prepared all-organic thin films, the PBO-based thin films were subjected to 5000 electrical cycling tests under an applied electric field of 200 kV mm−1. Fig. 5(d) and (e) show the D–E curves of PBO and 60 wt% P(DSDA-ODA)/PBO under different cycle numbers, respectively. It is obvious to observe that the D–E loops of PBO and 60 wt% P(DSDA-ODA)/PBO present different shapes. The PBO shows relatively fat shapes while the 60 wt% P(DSDA-ODA)/PBO exhibits an extremely slim one. This indicates that the PBO films possess high dielectric loss while the addition of P(DSDA-ODA) can effectively alleviate this serious dielectric loss, which also therefore leads to better dielectric performance based on 60 wt% P(DSDA-ODA)/PBO thin film. As the number of cycles increases, it can still hold stable performance until at least 5000 cycles, therefore suggesting the excellent charge–discharge fatigue resistance of the prepared PBO-based all-organic film (Fig. 5(f)).
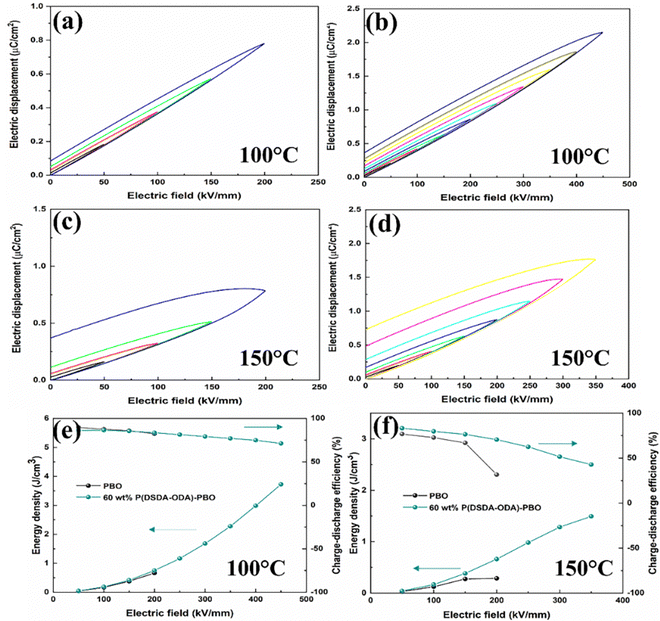
To further investigate the energy storage performance of the P(DSDA-ODA)/PBO film at different temperatures, Fig. 6 shows the D–E curves of PBO and 60 wt% P(DSDA-ODA)/PBO at 100 °C and 150 °C, respectively. As the temperature increases, the heat accumulation inside the dielectric is more severe than at room temperature.4 Compared with dielectric materials used at room temperature, the dielectric system not only needs to withstand the electrical breakdown caused by carrier movement but also the impact of thermal breakdown. Therefore, as the testing temperature increases, the external electric field that the films can withstand shows a decreasing trend. Compared with pure PBO films, P(DSDA-ODA)/PBO films still exhibit excellent energy storage properties at 100 °C and 150 °C. At 100 °C, 60 wt% P(DSDA-ODA)/PBO can still withstand an external electric field of 450 kV mm−1, while the external electric field that 60 wt% P(DSDA-ODA)/PBO can withstand decreases to 350 kV mm−1 when the temperature rises to 150 °C. The leakage current is the reflection of conduction loss. With the increase of the temperature, the more active electrons usually result in higher leakage current, that is higher conduction loss, therefore easily leading to low breakdown strength. In addition, with the increase of the testing temperature, the hysteresis loops of PBO and 60 wt% P(DSDA-ODA)/PBO thin films show a widening phenomenon, indicating that the increment of the testing temperature leads to a certain degree of decrease in the charge–discharge efficiency.
To further clarify the addition effect of P(DSDA-ODA) on the energy storage performance of PBO films under different test temperature conditions, Fig. 6 respectively shows the discharge energy storage density and charge discharge efficiency of PBO and 60 wt% P(DSDA-ODA)/PBO films under different external electric fields at 100 °C and 150 °C. As shown in Fig. 6(a), when the test temperature is 100 °C, the maximum discharge density achieved by 60 wt% P(DSDA-ODA)/PBO reaches 3.72 J cm−3 while the charge–discharge efficiency remains at 71.25%. However, the pure PBO film possesses a storage density of only 0.66 J cm−3 at 100 °C. When the testing temperature is further raised to 150 °C, the number of charges in the dielectric system increases sharply due to the influence of the Poole–Frenkel effect (as shown in eqn (3))
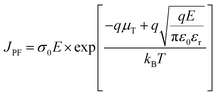 | (3) |
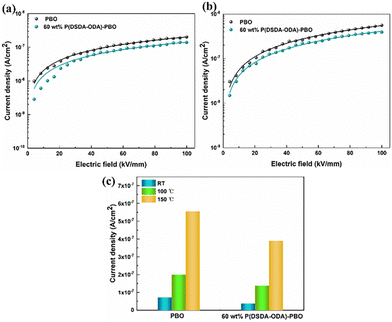
It is significant to accurately figure out the high-temperature conduction loss since it accounts for energy storage density, charge–discharge efficiency, and operating temperature. Fig. 7 shows the variation of leakage current with electric field for PBO and 60 wt% P(DSDA-ODA)/PBO at 100 °C and 150 °C, respectively, which can illustrate the phenomenon presented in terms of high-temperature energy storage performance. One can observe that the electronic conduction of the two specimens still follows the hopping conduction mechanism model at high temperatures. In addition, by comparing the leakage current at 100 kV mm−1 electric field versus different temperatures (Fig. 7(c)), we speculate that the addition of an appropriate amount of P(DSDA-ODA) is beneficial to reduce the leakage current density of the system, which also enables 60 wt% P(DSDA-ODA)/PBO to withstand higher external electric fields at high temperatures, thus yielding excellent energy storage capacity. At high temperature, the comparison of the hopping distances for pure PBO (0.96 nm at 100 °C and 1.03 nm at 150 °C) and 60% P(DSDA-ODA) (0.87 nm at 100 °C and 1.0 nm at 150 °C) illustrates that the P(DSDA-ODA) can better function as deep traps, therefore limiting the electron hopping phenomenon.
To further explore the generalizability of various functional groups in PAA precursors, we herein replace DSDA by using 6FDA containing trifluoromethyl groups to prepare P(6FDA-ODA)/PBO films. The corresponding morphological images in Fig. S5 (ESI†) show that there is no significant phase separation phenomenon on the cross sections of the films with an addition amount of 10–80 wt% P(6FDA-ODA), thus demonstrating excellent compatibility between the two polymers. The following D–E curve and energy storage performance was calculated based on the D–E curve of P(6FDA-ODA)/PBO at a content of 10 wt% to 80 wt% P(6FDA-ODA) (Fig. S6, ESI†). When the addition amount of P(6FDA-ODA) is 50 wt%, the energy storage performance of the entire polymer is the highest, reaching 3.33 J cm−3 under an electric field of 450 kV mm−1 while maintaining a high energy efficiency (86.24%). The overall dielectric constant of the films shows a decreasing trend (Fig. S7, ESI†). This is due to the strong electronegativity of fluorine atoms, which can better fix electrons, reduce the electron and ion polarizability of polymer molecules, and thus reduce the polarization rate of polymer molecules. Similarly, the conductivity of P(6FDA-ODA)/PBO thin films remains at a relatively low level, and there is no significant increase compared to pure PBO (Fig. S8, ESI†).
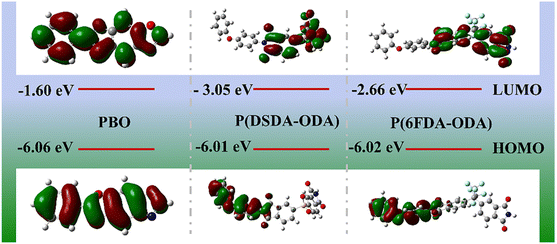
On the other hand, the more detailed information of energy band structure, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is further obtained by conducting density functional theory (DFT) calculations.27,37,38 It is commonly accepted that the valence band is below the HOMO level while the conduction band is above the LUMO level. According to the HOMO levels and LUMO levels, the band gap can therefore be calculated. The HOMO energy level represents the capacity to capture holes or lose electrons, while the LUMO level stands for the ability to capture electrons. In Fig. 8, the LUMO levels for PBO, P(DSDA-ODA) and P(6FDA-ODA) are −1.6 eV, −3.05 eV and −2.66 eV, respectively. The lowest LUMO level for P(DSDA-ODA) leads to its highest dielectric energy storage properties due to the strong electron capture ability due to the SO2 group. Also, the P(6FDA-ODA) also has lower LUMO level than pure PBO, thus resulting in its better dielectric properties. In general, the scalable blending of PI and PBO is further confirmed from the LUMO–HOMO level information.
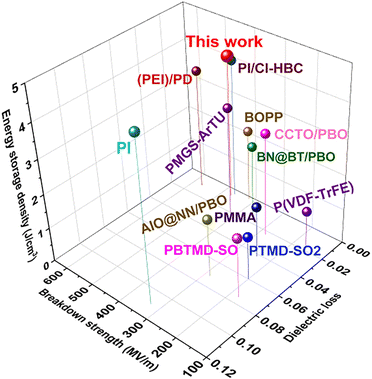
Fig. 9 summarizes the relevant outputs of comprehensive properties including energy storage density, breakdown strength and dielectric loss for all-organic or PBO-based dielectrics in the past decade.32–34,39–46 These outputs can be categorized roughly as three types. The first type includes PI, PMMA, PBTMD-SO2 and PTMD-SO2 as well as Al2O3@NaNbO3/PBO, all of which exhibit high dielectric loss (>0.06) while the breakdown strength is also below 400 kV mm−1; the second type is those that have reduced dielectric loss (<0.03) although the breakdown is still not very high (<400 kV mm−1). These material systems consist of CCTO/PBO, P(VDF-TrFE), and BNNs@BT/PBO. In comparison, the third type of dielectric materials yield concurrently an improved breakdown strength (>400 kV mm−1) while maintaining very low dielectric loss (<0.02). These typical materials are 60 wt% P(DSDA-ODA)/PBO, PMGS-ArTU, (PEI)/PD, PI/Cl-HBC, BOPP, etc. Among these representative materials, our prepared 60 wt% P(DSDA-ODA)/PBO still outperforms especially in terms of energy storage density using scalable blending process methodology. The additional tensile test results in Fig. S9 (ESI†) also confirm that the blending is beneficial to the obvious improvement in comprehensive mechanical properties of PBO-based all-organic films. When the addition amount of P(DSDA-ODA) is 60 wt%, the leakage current of P(DSDA-ODA)/PBO is the lowest. The tight structure and reduced leakage current enable the addition of P(DSDA-ODA) to enhance the breakdown strength of PBO. The breakdown strength of 60 wt% P(DSDA-ODA)/PBO can reach 470 kV mm−1, which is 1.7 times that of pure PBO. The low dielectric loss and excellent breakdown performance enable the maximum energy storage density of 60 wt% P(DSDA-ODA)/PBO to reach 4.16 J cm−3, which is 257% higher than that of pure PBO thin films while maintaining a charge discharge efficiency of 85.10% with a long charge–discharge cycle (at least 5000 times). In addition, in high-temperature environments, P(DSDA-ODA)/PBO still exhibits excellent energy storage performance compared to PBO. Finally, the introduction of PI polymers containing functional groups into PBO has scalability by replacing P(DSDA-ODA) with P(6FDA-ODA), thereby achieving the goal to similarly improve the energy storage performance of PBO films.
Conclusions
This work successfully and innovatively prepares PBO based all-organic thin films containing sulfone-based polyimide P(DSDA-ODA) using precursor solution blending and thermal co-curing methods. The good compatibility between P(DSDA-ODA) and PBO enables no obvious phase separation phenomenon on the cross-section of the prepared P(DSDA-ODA)/PBO film. The introduced molecular chains containing dipole groups effectively increase the dielectric constant while remaining within a relatively low dielectric loss range.Thanks to the excellent heat resistance of P(DSDA-ODA) and PBO, the linear dielectric properties of the prepared materials are almost independent of temperature till 200 °C. The mutually interacted system helps to fill the defects and thus suppress the movement of charge carriers. The SO2 serves as the charge center in the localized state not only playing a scattering role on high-energy carriers but also serving as an energy trap to limit the movement of carriers. When the addition amount of P(DSDA-ODA) is 60 wt%, the leakage current of P(DSDA-ODA)/PBO is the lowest. The tight structure and reduced leakage current enable the addition of P(DSDA-ODA) to enhance the breakdown strength of PBO. The breakdown strength of 60 wt% P(DSDA-ODA)/PBO can reach 470 kV mm−1, which is 1.7 times that of pure PBO. The low dielectric loss and excellent breakdown performance enable the maximum energy storage density of 60 wt% P(DSDA-ODA)/PBO to reach 4.16 J cm−3, which is 257% higher than that of pure PBO thin films while maintaining a charge discharge efficiency at 85.10% with a long charge–discharge cycle (at least 5000 times). In addition, in high-temperature environments, P(DSDA-ODA)/PBO still exhibits excellent energy storage performance compared to PBO. Finally, the introduction of PI polymers containing functional groups into PBO has scalability by replacing P(DSDA-ODA) with P(6FDA-ODA), thereby achieving the goal to similarly improve the energy storage performance of PBO films.
Author contributions
P. Z.: writing – original draft, conceptualization, methodology, investigation. J. L.: investigation. D. C.: validation. L. N.: software. L. G.: software. J. L.: validation. Q. Z.: supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All authors are grateful for financial support from the National Natural Science Foundation of China (52073091, 52303083, 52373073, 2171086), Shanghai Pujiang Program (22PJ1402500), Shanghai Rising-Star Program (21QA1403900) and the Key Laboratory of Advanced Polymer Materials of Shanghai.Notes and references
- X. Wu, X. Chen, Q. M. Zhang and D. Q. Tan, Advanced dielectric polymers for energy storage, Energy Storage Mater., 2022, 44, 29–47 CrossRef
.
- L. Ren, H. Li, Z. Xie, D. Ai, Y. Zhou, Y. Liu, S. Zhang, L. Yang, X. Zhao and Z. Peng, High-Temperature High-Energy-Density Dielectric Polymer Nanocomposites Utilizing Inorganic Core–Shell Nanostructured Nanofillers, Adv. Energy Mater., 2021, 11(28), 2101297 CrossRef CAS
.
- V. Sharma, C. Wang, R. G. Lorenzini, R. Ma, Q. Zhu, D. W. Sinkovits, G. Pilania, A. R. Oganov, S. Kumar, G. A. Sotzing, S. A. Boggs and R. Ramprasad, Rational design of all organic polymer dielectrics, Nat. Commun., 2014, 5(1), 4845 CrossRef CAS PubMed
.
- Y. Zhang, Z. Liu, L. Zhu, J. Liu, Y. Zhang and Z. Jiang, Enhanced discharged efficiency and high energy density at elevated temperature in polymer dielectric via manipulating relaxation behavior, CCS Chem., 2020, 2(5), 1169–1177 CrossRef CAS
.
- M. Jang, S. Y. Park, S. K. Kim, D. Jung, W. Song, S. Myung, S. S. Lee, D. H. Yoon and K. S. An, Strategic Customization of Polymeric Nanocomposites Modified by 2D Titanium Oxide Nanosheet for High-k and Flexible Gate Dielectrics, Small, 2021, 17(17), 2007213 CrossRef CAS
.
- C. Bin, X. Hou, Y. Xie, J. Zhang, H. Yang, L. Xu, H. Wei and J. Wang, Ultrahigh Energy Storage Performance of Flexible BMT-Based Thin Film Capacitors, Small, 2022, 18(4), 2106209 CrossRef CAS PubMed
.
- J. Hu, S. Zhang and B. Tang, 2D filler-reinforced polymer nanocomposite dielectrics for high-k dielectric and energy storage applications, Energy Storage Mater., 2021, 34, 260–281 CrossRef
.
- J. Dong, R. Hu, Y. Niu, L. Sun, L. Li, S. Li, D. Pan, X. Xu, R. Gong and J. Cheng, Enhancing high-temperature capacitor performance of polymer nanocomposites by adjusting the energy level structure in the micro-/meso-scopic interface region, Nano Energy, 2022, 99, 107314 CrossRef CAS
.
- S. Wang, Z. Luo, J. Liang, J. Hu, N. Jiang, J. He and Q. Li, Polymer Nanocomposite Dielectrics: Understanding the Matrix/Particle Interface, ACS Nano, 2022, 16(9), 13612–13656 CrossRef CAS PubMed
.
- L. Li, J. Cheng, Y. Cheng, T. Han, Y. Liu, Y. Zhou, G. Zhao, Y. Zhao, C. Xiong and L. Dong, Significant improvements in dielectric constant and energy density of ferroelectric polymer nanocomposites enabled by ultralow contents of nanofillers, Adv. Mater., 2021, 33(35), 2102392 CrossRef CAS
.
- Z. Pan, L. Yao, J. Zhai, X. Yao and H. Chen, Interfacial coupling effect in organic/inorganic nanocomposites with high energy density, Adv. Mater., 2018, 30(17), 1705662 CrossRef
.
- J. Liu, Z. Shen, W. Xu, Y. Zhang, X. Qian, Z. Jiang and Y. Zhang, Interface-strengthened polymer nanocomposites with reduced dielectric relaxation exhibit high energy density at elevated temperatures utilizing a facile dual crosslinked network, Small, 2020, 16(22), 2000714 CrossRef CAS
.
- Y. Huan, X. Wang, Y. Zheng, X. Wang, T. Wei, J. Ouyang and X. Wang, Achieving excellent energy storage reliability and endurance via mechanical performance optimization strategy in engineered ceramics with core-shell grain structure, J. Materiomics, 2022, 8(3), 601–610 CrossRef
.
- J. Chen, Z. Shen, Q. Kang, X. Qian, S. Li, P. Jiang and X. Huang, Chemical adsorption on 2D dielectric nanosheets for matrix free nanocomposites with ultrahigh electrical energy storage, Sci. Bull., 2022, 67(6), 609–618 CrossRef CAS
.
- R. Wang, H. Xu, S. Cheng, J. Liang, B. Gou, J. Zhou, J. Fu, C. Xie, J. He and Q. Li, Ultrahigh-energy-density dielectric materials from ferroelectric polymer/glucose all-organic composites with a cross-linking network of hydrogen bonds, Energy Storage Mater., 2022, 49, 339–347 CrossRef
.
- X. Wu, Y. Ivry, J. Zheng, P. Zhang, Z. Zheng and D. Q. Tan, Dielectric loss reduction and validation of P (VDF-HFP) films via sandwiching film fabrication for capacitive energy storage, Mater. Today Energy, 2023, 31, 101209 CrossRef CAS
.
- J.-W. Zha, Y. Tian, M.-S. Zheng, B. Wan, X. Yang and G. Chen, High-temperature energy storage polyimide dielectric materials: polymer multiple-structure design, Mater. Today Energy, 2022, 101217 Search PubMed
.
- G. He, H. Luo, C. Yan, Y. Wan, D. Wu, H. Luo, Y. Liu and S. Chen, Achieving Synergistic Improvement in Dielectric and Energy Storage Properties of All-Organic Poly(Methyl Methacrylate)-Based Copolymers Via Establishing Charge Traps, Energy Environ. Mater., 2023, e12577 CrossRef
.
- X.-J. Liu, M.-S. Zheng, G. Wang, Y.-Y. Zhang, Z.-M. Dang, G. Chen and J.-W. Zha, High energy density of polyimide films employing an imidization reaction kinetics strategy at elevated temperature, J. Mater. Chem. A, 2022, 10(20), 10950–10959 RSC
.
- M. Feng, Y. Feng, C. Zhang, T. Zhang, Q. Chen and Q. Chi, Ultrahigh energy storage performance of all-organic dielectrics at high-temperature by tuning the density and location of traps, Mater. Horiz., 2022, 9(12), 3002–3012 RSC
.
- B. Zhang, X. m Chen, Z. Pan, P. Liu, M. Mao, K. Song, Z. Mao, R. Sun, D. Wang and S. Zhang, Superior High-Temperature Energy Density in Molecular Semiconductor/Polymer All-Organic Composites, Adv. Funct. Mater., 2023, 33(5), 2210050 CrossRef CAS
.
- C. Yuan, Y. Zhou, Y. Zhu, J. Liang, S. Wang, S. Peng, Y. Li, S. Cheng, M. Yang and J. Hu, Polymer/molecular semiconductor all-organic composites for high-temperature dielectric energy storage, Nat. Commun., 2020, 11(1), 3919 CrossRef CAS
.
- A. Mannodi-Kanakkithodi, A. Chandrasekaran, C. Kim, T. D. Huan, G. Pilania, V. Botu and R. Ramprasad, Scoping the polymer genome: A roadmap for rational polymer dielectrics design and beyond, Mater. Today, 2018, 21(7), 785–796 CrossRef CAS
.
- D. Ji, T. Li, W. Hu and H. Fuchs, Recent progress in aromatic polyimide dielectrics for organic electronic devices and circuits, Adv. Mater., 2019, 31(15), 1806070 CrossRef
.
- R. Wang, Y. Zhu, J. Fu, M. Yang, Z. Ran, J. Li, M. Li, J. Hu, J. He and Q. Li, Designing tailored combinations of structural units in polymer dielectrics for high-temperature capacitive energy storage, Nat. Commun., 2023, 14(1), 2406 CrossRef CAS
.
- Z. Ran, R. Wang, J. Fu, M. Yang, M. Li, J. Hu, J. He and Q. Li, Spiral-structured dielectric polymers exhibiting ultra-high energy density and charge-discharge efficiency at high Temperatures, Adv. Mater., 2023, 2303849 CrossRef CAS PubMed
.
- P. Zuo, J. Jiang, D. Chen, J. Lin, Z. Zhao, B. Sun and Q. Zhuang, Enhanced Interfacial and Dielectric Performance for Polyetherimide Nanocomposites through Tailoring Shell Polarities, ACS Appl. Mater. Interfaces, 2023, 15(19), 23792–23803 CrossRef CAS PubMed
.
- J. Jiang, J. Li, Y. Zhang, Y. Yuan, X. Liu, P. Zuo, J. Qian and Q. Zhuang, Tuning the interfacial insulating shell characteristics in CaCu3Ti4O12 nanowires/polyetherimide nanocomposites for high-temperature capacitive energy storage, J. Mater. Chem. C, 2022, 10(20), 7962–7969 RSC
.
- Q. Liu, Z. Cheng, J. Qian, X. Chen, Y. Zhang and Q. Zhuang, A core@ double shell-structured PBO composite with excellent dielectric properties and high heat resistance, J. Mater. Chem. A, 2019, 7(18), 11195–11204 RSC
.
- H. Feng, W. Ma, Z.-K. Cui, X. Liu, J. Gu, S. Lin and Q. Zhuang, Core/shell-structured hyperbranched aromatic polyamide functionalized graphene nanosheets-poly(p-phenylene benzobisoxazole) nanocomposite films with improved dielectric properties and thermostability, J. Mater. Chem. A, 2017, 5(18), 8705–8713 RSC
.
- Y. Chen, S. Zhang, X. Liu, Q. Pei, J. Qian, Q. Zhuang and Z. Han, Preparation of solution-processable reduced graphene oxide/polybenzoxazole nanocomposites with improved dielectric properties, Macromolecules, 2015, 48(2), 365–372 CrossRef CAS
.
- X. Wang, Y. Yuan, Y. Zhang, J. Qian, P. Zuo, X. Liu and Q. Zhuang, Covalently modified and hierarchically structured corn-like BNNs@ BT/benzoxazole composites with enhanced dielectric properties over an ultra-wide temperature range, Composites, Part A, 2022, 160, 107027 CrossRef CAS
.
- J. Li, J. Jiang, Q. Cheng, Z. Cui, X. Liu, P. Zuo and Q. Zhuang, Construction of a flexible 1D core–shell Al2O3@NaNbO3 nanowire/poly(p-phenylene benzobisoxazole) nanocomposite with stable and enhanced dielectric properties in an ultra-wide temperature range, J. Mater. Chem. C, 2022, 10(2), 716–725 RSC
.
- J. Ding, W. Xu, X. Zhu, Z. Liu, Y. Zhang and Z. Jiang, All-organic nanocomposite dielectrics contained with polymer dots for high-temperature capacitive energy storage, Nano Res., 2023, 1–8 Search PubMed
.
- Z. Zhang, D. H. Wang, M. H. Litt, L. S. Tan and L. Zhu, High-temperature and high-energy-density dipolar glass polymers based on sulfonylated poly(2,6-dimethyl-1,4-phenylene oxide), Angew. Chem., Int. Ed., 2018, 130(6), 1544–1547 CrossRef
.
- Z. Pan, L. Li, L. Wang, G. Luo, X. Xu, F. Jin, J. Dong, Y. Niu, L. Sun and C. Guo, Tailoring poly(styrene-co-maleic anhydride) networks for all-polymer dielectrics exhibiting ultrahigh energy density and charge–discharge efficiency at elevated temperatures, Adv. Mater., 2023, 35(1), 2207580 CrossRef CAS
.
- C. Yuan, Y. Zhou, Y. Zhu, S. Hu, J. Liang, Z. Luo, B. Gao, T. Zeng, Y. Zhang, J. Li, S. Huang, Z. Han, X. Yang, Y. Yang, P. Meng, J. Hu, J. He, H. Yuan and Q. Li, Improved High-Temperature Electrical Properties of Polymeric Material by Grafting Modification, ACS Sustainable Chem. Eng., 2022, 10(27), 8685–8693 CrossRef CAS
.
- Y. Liu, Z. Tai, Q. Zhang, H. Wang, W. K. Pang, H. K. Liu, K. Konstantinov, Z. Guo and A. New, Energy Storage System: Rechargeable Potassium-Selenium Battery, Nano Energy, 2017, 35, 36 CrossRef CAS
.
- H. Xu, G. He, S. Chen, S. Chen, R. Qiao, H. Luo and D. Zhang, All-organic polymer dielectrics containing sulfonyl dipolar groups and π–π stacking interaction in side-chain architectures, Macromolecules, 2021, 54(17), 8195–8206 CrossRef CAS
.
- J. Jiang, J. Li, J. Qian, X. Liu, P. Zuo, Y. Yuan and Q. Zhuang, Benzoxazole-polymer@ CCTO hybrid nanoparticles prepared via RAFT polymerization: toward poly(p-phenylene benzobisoxazole) nanocomposites with enhanced high-temperature dielectric properties, J. Mater. Chem. A, 2021, 9(46), 26010–26018 RSC
.
- S. Gross, D. Camozzo, V. Di Noto, L. Armelao and E. Tondello, PMMA: A key macromolecular component for dielectric low-κ hybrid inorganic–organic polymer films, Eur. Polym. J., 2007, 43(3), 673–696 CrossRef CAS
.
- P. Hu, Y. Song, H. Liu, Y. Shen, Y. Lin and C.-W. Nan, Largely enhanced energy density in flexible P (VDF-TrFE) nanocomposites by surface-modified electrospun BaSrTiO3 fibers, J. Mater. Chem. A, 2013, 1(5), 1688–1693 RSC
.
- Y. Liu, J. Chen, X. Jiang, P. Jiang and X. Huang, All-organic cross-linked polysiloxane-aromatic thiourea dielectric films for electrical energy storage application, ACS Appl. Energy Mater., 2020, 3(6), 5198–5207 CrossRef CAS
.
- L.-j Zhang, J. Liu, L.-b Luo, X.-y Liu and X. Wang, All-organic polyimide/Cl-HBC composite film with high breakdown strength and ultra-low dielectric loss, Polymer, 2022, 245, 124702 CrossRef CAS
.
- A. Ahmad, G. Liu, S. Cao, X. Liu, J. Luo, L. Han, H. Tong and J. Xu, Significantly Improved Dielectric Performance of All-Organic Parylene/Polyimide/Parylene Composite Films with Sandwich Structure, Macromol. Rapid Commun., 2023, 44(2), 2200568 CrossRef CAS
.
- X. Zhang, Y. Zhao, Y. Wu and Z. Zhang, Poly(tetrafluoroethylene-hexafluoropropylene) films with high energy density and low loss for high-temperature pulse capacitors, Polymer, 2017, 114, 311–318 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01479g |
| This journal is © The Royal Society of Chemistry 2024 |