Open Access Article
Open Access ArticleCyclic and acyclic acetals as safe, nonaqueous formaldehyde equivalents for the synthesis of pillararenes†
Babitha Machireddy and
Maggie He *
*
Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, AR 72701, USA. E-mail: maggiehe@uark.edu
First published on 20th August 2024
Abstract
Pillar[5]arene was synthesized using acyclic acetals diethoxymethane and dimethoxymethane, and cyclic acetals 1,3-dioxolane and 1,3,5-trioxane as an alternative to paraformaldehyde. Both Lewis and Brønsted acids were effective in catalyzing the hydrolysis of acetal and initiating the Friedel–Crafts reaction in pillararene synthesis.
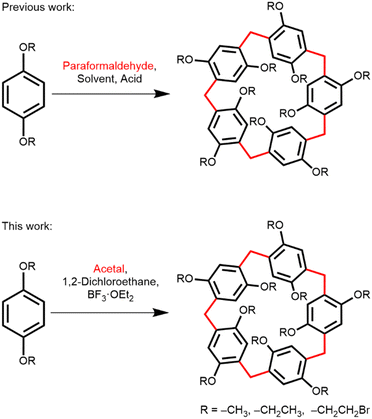
Pillar[n]arenes, a new class of aromatic macrocycles with a pillar shape, were first synthesized and reported by Ogoshi et al., in 2008.1 Pillar[n]arenes are composed of ‘n’ hydroquinone units connected to each other by para-methylene bridges (Scheme 1).2 Pillar[n]arenes are characterized by a partially rigid pillar shape (particularly when n = 5, 6), planar chirality and a very electron-rich aromatic cavity with a diameter of 5.1 Å for pillar[5]arene and 7.5 Å for pillar[6]arene.3 This electron-rich cavity facilitates a variety of host–guest interactions with cationic,4 neutral,5 and sometimes anionic6 guest molecules. Their ability to have host–guest interactions and their unique symmetrical pillar structures enabled pillararenes to contribute immensely to supramolecular chemistry by serving as building blocks for complex nanostructures such as 1D and 2D assemblies of pillararenes,7 rotaxanes,8,9 stimuli responsive supramolecules,10 drug delivery and vesicles,11 adaptive crystals,12,13 and small molecule recognition.14–16
Pillararenes can be synthesized by several methods. The simplest and commonly used approach involves the condensation of 1,4-dialkoxybenzene with excess paraformaldehyde in the presence of an appropriate Lewis acid or Brønsted acid catalyst, allowing for various alkoxy substituents1,2 to be attached to the arenes (Scheme 1). A wide variety of Lewis and Brønsted acids (BF3·OEt2,1 FeCl3,3 AlCl3,17 methanesulfonic acid,18 p-toluenesulfonic acid,19 triflic acid,20 etc.) are found to be applicable in different chlorinated solvents (dichloromethane, 1,2-dichloroethane, chloroform and chlorocyclohexane21) for this synthesis. Alternatively, pillararenes can be synthesized by condensation of 1,4-dialkoxy-2,5-bis(ethoxymethyl)benzene or 2,5-dialkoxy benzyl alcohol with a Lewis acid.19 However, these methods require multi-step synthesis to preinstall the methylene bridges in the arene starting material. Except for one report,22 paraformaldehyde was used exclusively to install the methylene bridges either in the arene starting material or in the final pillararene, regardless of the aforementioned methods.
Paraformaldehyde is a polymerized form of formaldehyde. It exists as a solid, typically available as powder or prill form, with a degree of polymerization ranging from 8 to 100 units. For chemical reactions, paraformaldehyde is commonly depolymerized in the presence of an acid, a base, or heat, to release formaldehyde. Paraformaldehyde is often used in the synthesis of pillararenes and similar macrocyclic hosts. However, due to the hazardous nature of the material, careful handling is important. Paraformaldehyde has a slight odor of formaldehyde due to the release of a low level of formaldehyde gas in air at room temperature.23,24 Formaldehyde itself is a colorless, flammable gas with a pungent smell. Both the International Agency for Research on Cancer (IARC) and the National Toxicology Program (NTP) have identified formaldehyde as a human carcinogen.25,26 Formaldehyde can also cause irritation to the skin, eyes, nose, and throat.27 Therefore, when handling paraformaldehyde, including opening its container and weighing the solid, it is recommended to work in a well-ventilated area, such as inside a fume hood. If a balance is not located inside a fume hood, extra care should be taken during weighing to minimize exposure to formaldehyde gas by adding paraformaldehyde inside a fume hood to a tared container and then covering the container before returning to the balance to weigh. For successful pillararene synthesis, fine paraformaldehyde powder is required (Fig. S1, ESI†), which presents additional handling considerations. The material can build up static electricity, causing the powder to cling or disperse unpredictably during weighing and transferring. With these considerations in mind, we began to explore alternative sources of formaldehyde that are easier and safer to handle.
Herein, we report that diethoxymethane (DEM) can serve as an alternative source of formaldehyde in pillararene synthesis (Scheme 1). DEM is a colorless liquid commonly used as an organic solvent and reagent for various organic transformations. DEM is relatively stable under basic, aqueous acidic, and oxidative conditions. However, DEM is unstable in homogeneous acidic conditions because of the liberation of formaldehyde in these conditions.28 The physical properties and stability of DEM present several advantages for safe handling of the material in pillararene synthesis. First, as a liquid, DEM can be easily measured volumetrically with a syringe or pipette inside a fume hood. Second, formaldehyde can be controllably released inside a reaction flask by adding a suitable Lewis or Brønsted acid, which also acts as a catalyst for commencing the Friedel–Crafts reaction in pillararene synthesis. Additionally, DEM is miscible in chlorinated solvents, forming a homogeneous solution. In contrast, paraformaldehyde forms a suspension in chlorinated solvents and requires depolymerization to dissolve. For effective pillararene synthesis, fine paraformaldehyde powder is necessary due its large surface area that allows sufficient depolymerization rate in chlorinated solvents. However, paraformaldehyde prills, which are easier to handle and dust-free, are ineffective for this synthesis (Table S1, ESI†).
The initial reaction of DEM with 1,4-diethoxybenzene and boron trifluoride diethyl etherate (BF3·OEt2) in dichloromethane successfully yields ethoxypillar[5]arene (EtO-PA[5]) in 61%, demonstrating that DEM releases formaldehyde necessary for the formation of the methylene bridges of pillararene. This reaction setup was relatively straightforward. It can be easily quenched, and the product can be readily purified by column chromatography. 1H NMR measurement also confirms the release of formaldehyde upon mixing DEM and BF3·OEt2 in CDCl3 as indicated by the appearance of a new peak at δ = 9.73 ppm (Fig. S2, ESI†).29,30 Other acid catalysts are also effective in combination with DEM for pillararene synthesis, albeit lower yields were obtained. All tested Lewis acids, BF3·OEt2, FeCl3, and Bi(OTf)3 effectively catalyze the hydrolysis of DEM acetal and initiate the Friedel–Crafts reaction. The low toxicity and low cost of bismuth compounds characterize it as a green element.31 Previously, Bi(OTf)3 was used to catalyze the Friedel–Crafts acylations32 and acetal deprotection.33 Although the yield was low, Bi(OTf)3 facilitated the formation of pillararene in the presence of DEM at room temperature. Brønsted acids such as methanesulfonic acid, triflic acid, and p-toluenesulfonic acid also successfully produced pillararenes. However, no pillararenes were observed with trifluoroacetic acid, concentrated HCl, or 2 M HCl in ether. 1H NMR measurements reveal that formaldehyde is in fact released in the presence of trifluoroacetic acid (Fig. S3, ESI†); however, the acid was inefficient at catalyzing the Friedel–Crafts reaction. These findings highlight the effectiveness of DEM as a formaldehyde source and the suitability of various acid catalysts in the pillararene synthesis.
Previous studies have shown that the choice of solvent significantly influences the size selectivity of pillararenes. Smaller solvent molecules favor the formation of pillar[5]arene and larger solvent molecules favor the formation of pillar[6]arene, due to the fitness of the size of the solvent molecule inside the macrocycle's cavity and its electronic interaction with the electron-rich macrocycle.18,21 In light of this, we examined several solvents for the reaction of 1,4-diethoxybenzene with DEM in the presence of BF3·OEt2. We found that when dichloromethane was the solvent, pillar[5]arene was exclusively the product, except in the cases of FeCl3 and p-toluenesulfonic acid, where 5% and 2% of pillar[6]arene were isolated, respectively (Table 1, entries 1, 2, and 6). 1,2-Dichloroethane proved to be the best solvent for the synthesis of pillar[5]arene, affording it in 71% yield (Table 2, entry 1). Larger solvent molecules increase the proportion of pillar[6]arene relative to pillar[5]arene. The highest amount of pillar[6]arene was obtained when chlorocyclohexane was used, resulting in a ratio of pillar[5]arene to pillar[6]arene of 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (Table 2, entry 5).
25 (Table 2, entry 5).
| Entry | Catalyst (equiv.) | Yield (%) | |
|---|---|---|---|
| EtO-PA[5] | EtO-PA[6] | ||
| Reaction conditions: 1,4-diethoxybenzene (1.00 equiv.), DEM (1.25 equiv.), acid catalyst, CH2Cl2 (0.05 M), 30 min.a Recovered 4% of 1,4-diethoxybenzene.b Recovered 63% of 1,4-diethoxybenzene after 48 h.c Recovered 16% of 1,4-diethoxybenzene after 9 h. | |||
| 1 | BF3·OEt2 (2.50) | 61 | 0 |
| 2 | FeCl3a (1.00) | 35 | 5 |
| 3 | Bi(OTf)3b (0.0500) | 9 | 0 |
| 4 | CH3SO3H (3.75) | 49 | 0 |
| 5 | CF3SO3H (1.00) | 37 | 0 |
| 6 | p-CH3C6H4SO3Hc (2.50) | 32 | 2 |
| 7 | CF3COOH (2.50) | 0 | 0 |
| 8 | Conc. HCl (2.00) | 0 | 0 |
| 9 | 2 M HCl in ether (2.00) | 0 | 0 |
| Entry | Solvent | Yield (%) | |
|---|---|---|---|
| EtO-PA[5] | EtO-PA[6] | ||
| Reaction conditions: 1,4-diethoxybenzene (1.00 equiv.), DEM (1.25 equiv.), BF3·OEt2 (2.50 equiv.), 30 min, 0.05 M.a Recovered 4% of 1,4-diethoxybenzene. | |||
| 1 | 1,2-Dichloroethane | 71 | 0 |
| 2 | Dichloromethane | 61 | 0 |
| 3 | Chloroform | 48 | 6 |
| 4 | 1,2-Dichlorobenzenea | 49 | 5 |
| 5 | Chlorocyclohexane | 43 | 25 |
During our solvent studies, we found that certain preservatives used to stabilize chlorinated solvents can affect the reactions. While chloroform stabilized with amylene smoothly afforded EtO-PA[5] in 48% yield, the same reaction carried out in chloroform stabilized with 1% ethanol resulted in only 14% EtO-PA[5] and mostly recovered unreacted 1,4-diethoxybenzene (Table S2, ESI†). Due to the high concentration of ethanol being used as a preservative, 1% v/v, we attribute that this is sufficient to deactivate the Lewis acid catalyst. The effect of ethanol preservative was further confirmed by the addition of 1% ethanol to the reaction with amylene stabilized chloroform. No reaction was observed in this case and only starting material was recovered.
We also explored the suitability of other acetals for pillararene synthesis, including dimethoxymethane and 1,3-dioxolane. The reactions of 1,4-diethoxybenzene and these acetals were conducted in 1,2-dichloroethane using BF3·OEt2 as the catalyst. Both acyclic and cyclic acetal were found to be equally effective in synthesizing pillar[5]arene (Table 3, entries 1–3). Additionally, varying the size of the alkoxy group on the acetal has little or no effect on the reaction yield (Table 3, entries 1 and 2). Interestingly, we did not observe the formaldehyde peak in the 1H NMR of a mixture of 1,3-dioxolane and BF3·OEt2 in CDCl3 over the course of 23 hours (Fig. S4, ESI†); however, pillararene was formed slowly in the presence of 1,4-diethoxybenzene in a mixture of 1,3-dioxolane and BF3·OEt2 in dichloromethane over 10 hours in 74% yield (Table 3, entry 3). We ascribe this observation to the equilibrium of hydrolysis lying largely toward the cyclic acetal under the conditions experimented, and the minute amount of formaldehyde produced in this equilibrium was quickly consumed by the Friedel–Crafts reaction. Lastly, we also found that the cyclic trimer of formaldehyde, 1,3,5-trioxane, under the same reaction conditions successfully produced 39% yield of EtO-PA[5].
| Entry | Acetal | Time (h) | Yield of EtO-PA[5] (%) |
|---|---|---|---|
| Reaction conditions: 1,4-diethoxybenzene (1.00 equiv.), acetal (1.25 equiv.), BF3·OEt2 (2.50 equiv.) in 1,2-dichloroethane, 0.05 M.a Recovered 20% of 1,4-diethoxybenzene. | |||
| 1 | Diethoxymethane | 0.5 | 71 |
| 2 | Dimethoxymethane | 1.5 | 69 |
| 3 | 1,3-Dioxolane | 10 | 74 |
| 4 | 1,3,5-Trioxanea | 1 | 39 |
We found that our method is suitable for the synthesis of pillararenes from different arene substrates. Both methoxy- and ethoxypillar[5]arene were obtained in high yields, 87% and 71%, in the presence of DEM, BF3·OEt2, and 1,2-dichloroethane, respectively (Table 4, entries 1 and 2). These yields of pillar[5]arenes were higher than those obtained from reported protocols.2,34 Our method is also efficient in synthesizing 2-bromoethoxypillar[5]arene from 1,4-bis(2-bromoethoxy)benzene in 60% yield. This decabrominated pillar[5]arene can be readily derivatized to access functionalized pillararenes for different targeted applications.35–38
| Entry | Substrate | Yield of pillar[5]arene (%) |
|---|---|---|
| Reaction conditions: arene (1.00 equiv.), diethoxymethane (1.25 equiv.), BF3·OEt2 (2.50 equiv.) in 1,2-dichloroethane, 0.05 M.a 0.23 M, 70 °C. | ||
| 1 | 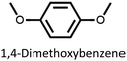 |
87 |
| 2 | 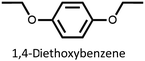 |
71 |
| 3 | 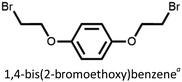 |
60 |
In summary, we have found that acetals serve as safe, nonaqueous formaldehyde equivalents for the synthesis of pillararenes. Acetals are easy to handle and release formaldehyde controllably only in the presence of an appropriate acid. Pillararenes can be synthesized in good yields from commercially available and inexpensive starting materials. Given the growing interest and development of pillararenes in functional material applications, employing a safer formaldehyde equivalent significantly benefits the health and safety of researchers.
The authors acknowledge the University of Arkansas for financial support and Rohana Liyanage for assistance with MALDI measurement.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Notes and references
- T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 Search PubMed.
- T. Ogoshi, K. Kitajima, T. Aoki, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Org. Chem., 2010, 75, 3268–3273 Search PubMed.
- M. Da Pian, C. A. Schalley, F. Fabris and A. Scarso, Org. Chem. Front., 2019, 6, 1044–1051 Search PubMed.
- Y. Ma, X. Chi, X. Yan, J. Liu, Y. Yao, W. Chen, F. Huang and J.-L. Hou, Org. Lett., 2012, 14, 1532–1535 Search PubMed.
- C. Li, Q. Xu, J. Li, Y. Feina and X. Jia, Org. Biomol. Chem., 2010, 8, 1568–1576 Search PubMed.
- Y. Ma, X. Ji, F. Xiang, X. Chi, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Chem. Commun., 2011, 47, 12340–12342 Search PubMed.
- S. Fa, T. Kakuta, T.-A. Yamagishi and T. Ogoshi, CCS Chem, 2019, 1, 50–63 Search PubMed.
- M. Rémy, I. Nierengarten, B. Park, M. Holler, U. Hahn and J.-F. Nierengarten, Chem. – Eur. J., 2021, 27, 8492–8499 Search PubMed.
- C. Ke, N. L. Strutt, H. Li, X. Hou, K. J. Hartlieb, P. R. McGonigal, Z. Ma, J. Iehl, C. L. Stern, C. Cheng, Z. Zhu, N. A. Vermeulen, T. J. Meade, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 17019–17030 Search PubMed.
- X. Li, M. Shen, J. Yang, L. Liu and Y.-W. Yang, Adv. Mater., 2024, 36, 2313317 Search PubMed.
- G. V. Zyryanov, D. S. Kopchuk, I. S. Kovalev, S. Santra, A. Majee and B. C. Ranu, Int. J. Mol. Sci., 2023, 24, 5167 Search PubMed.
- J.-R. Wu and Y.-W. Yang, Angew. Chem., Int. Ed., 2021, 60, 1690–1701 Search PubMed.
- Y. Wu, J. Zhou, E. Li, M. Wang, K. Jie, H. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 19722–19730 Search PubMed.
- B. Gómez-González, L. García-Río, N. Basílio, J. C. Mejuto and J. Simal-Gandara, Pharmaceutics, 2022, 14, 60 Search PubMed.
- J.-F. Chen, Q. Lin, Y.-M. Zhang, H. Yao and T.-B. Wei, Chem. Commun., 2017, 53, 13296–13311 Search PubMed.
- M. Ueno, T. Tomita, H. Arakawa, T. Kakuta, T.-A. Yamagishi, J. Terakawa, T. Daikoku, S.-I. Horike, S. Si, K. Kurayoshi, C. Ito, A. Kasahara, Y. Tadokoro, M. Kobayashi, T. Fukuwatari, I. Tamai, A. Hirao and T. Ogoshi, Commun. Chem., 2020, 3, 183 Search PubMed.
- Y. Ma, Z. Zhang, X. Ji, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Eur. J. Org. Chem., 2011, 5331–5335 Search PubMed.
- S. Mirzaei, D. Wang, S. V. Lindeman, C. M. Sem and R. Rathore, Org. Lett., 2018, 20, 6583–6586 Search PubMed.
- C. Han, F. Ma, Z. Zhang, B. Xia, Y. Yu and F. Huang, Org. Lett., 2010, 12, 4360–4363 Search PubMed.
- K. Wang, L.-L. Tan, D.-X. Chen, N. Song, G. Xi, S. X.-A. Zhang, C. Li and Y.-W. Yang, Org. Biomol. Chem., 2012, 10, 9405–9409 Search PubMed.
- T. Ogoshi, N. Ueshima, T. Akutsu, D. Yamafuji, T. Furuta, F. Sakakibara and T.-A. Yamagishi, Chem. Commun., 2014, 50, 5774–5777 Search PubMed.
- Z. Coady, J. N. Smith, K. A. Wilson and N. G. White, J. Org. Chem., 2024, 89, 1397–1406 Search PubMed.
- H. Hayashi, N. Kunugita, K. Arashidani, H. Fujimaki and M. Ichikawa, Brain Res., 2004, 1007, 192–197 Search PubMed.
- H. Hori and K. Arashidani, J. UOEH, 1997, 19, 123–131 Search PubMed.
- NTP (National Toxicology Program), Report on Carcinogens, Fifteenth Edition, Research Triangle Park, NC, U.S. Department of Health and Human Services, Public Health Service, 2021 DOI:10.22427/NTP-OTHER-1003.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 88: Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol, International Agency for Research on Cancer, 2006.
- Formaldehyde – OSHA Factsheet, https://www.osha.gov/sites/default/files/publications/formaldehyde-factsheet.pdf, accessed 2011.
- N. W. Boaz and B. Venepalli, Org. Process Res. Dev., 2001, 5, 127–131 Search PubMed.
- M. Rivlin, U. Eliav and G. Navon, J. Phys. Chem. B, 2015, 119, 4479–4487 Search PubMed.
- Y. Yu, X.-M. Zhang, J.-P. Ma, Q.-K. Liu, P. Wang and Y.-B. Dong, Chem. Commun., 2014, 50, 1444–1446 Search PubMed.
- R. Mohan, Nat. Chem., 2010, 2, 336 Search PubMed.
- J. R. Desmurs, M. Labrouillère, C. Le Roux, H. Gaspard, A. Laporterie and J. Dubac, Tetrahedron Lett., 1997, 38, 8871–8874 Search PubMed.
- M. D. Carrigan, D. Sarapa, R. C. Smith, L. C. Wieland and R. S. Mohan, J. Org. Chem., 2002, 67, 1027–1030 Search PubMed.
- T. Ogoshi, T. Aoki, K. Kitajima, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Org. Chem., 2011, 76, 328–331 Search PubMed.
- I. Nierengarten, S. Guerra, M. Holler, L. Karmazin-Brelot, J. Barberá, R. Deschenaux and J.-F. Nierengarten, Eur. J. Org. Chem., 2013, 3675–3684 Search PubMed.
- P. U. A. I. Fernando, Y. Shepelytskyi, P. T. Cesana, A. Wade, V. Grynko, A. M. Mendieta, L. E. Seveney, J. D. Brown, F. T. Hane, M. S. Albert and B. DeBoef, ACS Omega, 2020, 5, 27783–27788 Search PubMed.
- W. Zhao, J. Chu, F. Xie, Q. Duan, L. He and S. Zhang, J. Chromatogr. A, 2017, 1485, 44–51 Search PubMed.
- X.-Y. Hu, X. Liu, W. Zhang, S. Qin, C. Yao, Y. Li, D. Cao, L. Peng and L. Wang, Chem. Mater., 2016, 28, 3778–3788 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra for pillararenes, and deprotection of acetals in the presence of acids. See DOI: https://doi.org/10.1039/d4cc03306j |
| This journal is © The Royal Society of Chemistry 2024 |