Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling coefficients of thermal expansion in thermoplastic materials: effects of zinc cyanide and ionic liquid†
Savannah
Egerton‡
a,
Claudia
Sim‡
a,
Heon E.
Park‡
 a,
Mark P.
Staiger
b,
Komal M.
Patil
a,
Mark P.
Staiger
b,
Komal M.
Patil
 c and
Matthew G.
Cowan
c and
Matthew G.
Cowan
 *ac
*ac
aDepartment of Chemical and Process Engineering, University of Canterbury, Christchurch, 8140, New Zealand. E-mail: matthew.cowan@canterbury.ac.nz
bDepartment of Mechanical Engineering, University of Canterbury, Christchurch, 8140, New Zealand
cMacDiarmid Institute for Advanced Materials and Nanotechnology, School of Physical and Chemical Sciences, University of Canterbury, Private Bag 4800, Christchurch, New Zealand
First published on 13th April 2022
Abstract
Tuning the coefficients of thermal expansion (CTE) of materials is necessary to control performance and extend the operational life of precision equipment. Herein, we report the strategy of creating 3-component composite materials from the thermoplastic poly(ethylene), zinc cyanide as a solid with a negative coefficient of thermal expansion (NTE), and ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM][TFSI]) as an interfacial agent that enhances the polymer/solid interface. Specifically, we produce thermoplastic composites with coefficients of thermal expansion reduced from 290 to 190 × 10−6 K−1, a significantly larger reduction than the ca. −15 × 10−6 K−1 reported for composites containing other NTE fillers. Additionally, the composite materials retain a Young's modulus comparable to low density polyethylene (80–110 MPa). Overall, this three-component design strategy shows promise for the development of thermoplastic materials with controlled coefficients of thermal expansion.
The coefficient of thermal expansion (CTE, α) describes a materials change in length in response to temperature. In electronic and mechanical devices, matching the CTE of components ensures the robustness, reliability and longevity of devices by reduces the likelihood of internal residual stresses developing due to temperature cycling.1–5 In contrast, mismatch of components CTE can compromise the strength and integrity of the device components,4,6,7 causing changes in temperature to render devices inoperable, particularly in electronics and high-precision applications.8–13 Strategies for designing materials with tailored CTE are therefore particularly advantageous for applications in electronic systems14–16 and optics,3,7,17,18 and seals (e.g. O-rings, gaskets).5,19
While most materials exhibit a positive CTE, some materials contract with increasing temperature and display a negative coefficient of thermal expansion (NTE). Well-known examples include highly orientated aromatic polyamides,20 graphite and graphene,21 metal oxides (e.g. PbTiO3,22 ZrW2O823), and metal–organic frameworks (e.g. zinc cyanide (Zn(CN)2)).24 Zn(CN)2 is known to have a relatively large NTE, ranging from −19.8 × 10−6 K−1 at <180 K to −14 × 10−6 K−1 at >400 K.3,8,9,25–27 The large NTE of Zn(CN)2 is due to the vibrational modes of the metal–ligand bonds that cause transverse vibrational displacement of the zinc ions, resulting in a decrease in the distance between adjacent Zn ions.8–10,28–31 The large NTE of Zn(CN)2 makes it an interesting material for forming composites with controlled CTE.
The CTE of materials can be manipulated through chemical treatments1,32–35 and compositional changes (e.g. fillers such as SiO2).36 To achieve a certain CTE, composite materials can incorporate a filler (or reinforcing) material with NTE.6,37 Examples include orientated aromatic polyamides or graphite fibers to create polymer matrix composites with a low (or near-zero) CTE.11,34 Recently, ZrW2O8 (NTE = −3 to −5 × 10−6 K−1) has been combined with epoxy,18 polyimide,23 and cement to reduce the CTE of polymer and bonding materials.3,7,19,37,38
The nature of the filler–matrix interface in these composite systems is poorly understood. However, as demonstrated by Shubin et al., the filler–matrix interfacial bonding (or incompatibility) will strongly influence both the overall CTE and mechanical properties of the resulting composite.19 This occurs via phase separation, lack of adhesion, and formation of void space defects at the filler–matrix interface, which reduces the toughness and strength of the composite by nucleating crack formation and initiating other failure mechanisms.5,19,39–41
Attempts to improve filler–matrix interfacial adhesion include the addition of linker molecules (aka compatibilizers) that permit chemical bonding between the filler and polymer matrix.39 Alternatively, surface modification of the ZrW2O8 is used to enhance interfacial bonding with the matrix, thereby reducing the presence of voids.38
Interfacial defects also play a key role in the field of mixed-matrix membrane materials. There, the incorporation of ionic liquids (ILs) such as 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide ([EMIM][TFSI]) has been reported to fill gas-transport defects at inorganic–organic interfaces improving selectivity and mechanical properties.42–45
Herein, we apply the strategy of ionic liquid and NTE filler incorporation to reduce the CTE of thermoplastic materials similar to those used in many electronic devices. Specifically, we demonstrate that [EMIM][TFSI] acts as an interfacial agent for eliminating interfacial defects in Zn(CN)2-filled low density polyethylene composites, allowing control over the CTE and mechanical properties.
The composite materials were prepared by melt-casting of the thermoplastic low density poly(ethylene) (LDPE), NTE filler Zn(CN)2, and ionic liquid [EMIM][TFSI]. Addition of Zn(CN)2 produced increasingly brittle composite materials, and composites containing > 40 wt. % Zn(CN)2 could not be removed from the mould without fracturing, which set an upper limit on the Zn(CN)2 content.
[EMIM][TFSI] was selected as the ionic liquid component due to its use studies on mixed-matrix membranes42–45 and because it contains both polar (ionic) and non-polar functionality ([TFSI]− anion), that make it a suitable interfacial agent for the Zn(CN)2 (polar) and LDPE (non-polar) interface. Interestingly, LDPE that contained [EMIM][TFSI], but no Zn(CN)2, produced phase-separated materials with separated ionic liquid pockets. Thus, the C-0-X (refers to: Composite (C) with 0 wt% Zn(CN)2 and x wt% [EMIM][TFSI]) samples were unsuitable for testing. In contrast, phase separation was not observed in composite formulations that contained both Zn(CN)2 and IL. This implies that [EMIM][TFSI] interacts with the polar Zn(CN)2 surface and therefore concentrates at the interface. The phase separation of [EMIM][TFSI] with neat LDPE suggests that future studies using ionic liquids more compatible with the LDPE matrix (e.g. longer aliphatic chains) may further enhance the interfacial adhesion at the polymer/NTE filler interface.
Scanning electron microscopy (SEM) images of the extreme cases of 40 wt% Zn(CN)2 and no [EMIM][TFSI] (C-40-0) or 10 wt% [EMIM][TFSI] (C-40-10) reveal that [EMIM][TFSI] plays a significant role in combining the polymer with the Zn(CN)2 (Fig. 1). All SEM images indicate that the composites are well-mixed with good dispersion of Zn(CN)2 and no stratification or settling of the solid material within the polymer matrix occurred during preparation. The defined edges of Zn(CN)2 in SEM images of (C-40-0) indicate that Zn(CN)2 is poorly adhered to the polymer matrix. In contrast, SEM images of (C-40-10) show that the polymer has fully and intimately coated the Zn(CN)2 particles. The rounded surfaces suggest good adherence has occurred at the interface.
Powder X-ray diffraction (PXRD) results (Fig. S2, ESI†) confirm that the crystal structure of Zn(CN)2 was maintained during preparation of the composites. Zinc and iron cyanide complexes are known to be insoluble in imidazolium ionic liquids.46
The moisture content and decomposition behaviour of the composites were measured using TGA to ensure that the materials were not hygroscopic and that exposure to ambient humidity causing variation in residual water content would not affect testing. No mass loss corresponding to water evaporation is observed (Fig. S1, ESI†) suggesting that the hydrophobic nature of the polymer and [EMIM][TFSI] are sufficient to prevent water absorption. The onset of decomposition (Td) of the composite occurs at ∼ 350 °C and the remaining residual mass corresponds to the amount of Zn(CN)2 (Td = 800 °C) within the composite.
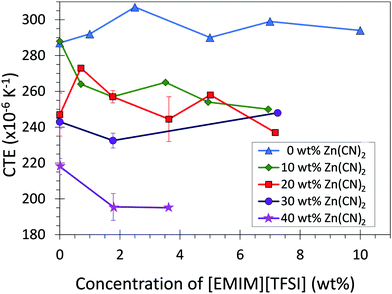
The CTE of the composite materials were measured using thermomechanical analysis (TMA) (Fig. 2; Table 1). Addition of Zn(CN)2 to the LDPE matrix decreased the coefficient of thermal expansion of the LDPE from 290 to 210 × 10−6 K−1 (The CTE values reported are average values for the range −40 °C to 80 °C). Incorporation of [EMIM][TFSI] content into the composites further decreased the CTE by ca. 20 × 10−6 K−1 (Fig. S4, ESI†). This result is in contrast to what is expected from a the law of mixtures (ESI,† Section 3), which predicts the addition of [EMIM][TFSI] (which has a positive CTE) to increase the overall CTE of the composites. This suggests that improving the LDPE/Zn(CN)2 interface enhances the ability of an NTE filler to reduce the overall CTE of a composite, significantly expanding the range of CTE values accessible to thermoplastics such as LDPE.
In absolute terms, the addition of Zn(CN)2 to LDPE decreased the CTE from 290 to 210 × 10−6 K−1 (Fig. 2; Table 1). The addition of 5 wt% [EMIM][TFSI] further decreases the CTE of the 40 wt% composite to 190 × 10−6 K−1 (ESI,† Section 3). The overall CTE decrease of 100 × 10−6 K−1 is an order of magnitude higher than the CTE reduction observed for polyimide composites containing 40 wt% ZrW2O8 (15 × 10−6 K−1) without the use of an interfacial agent.7,47
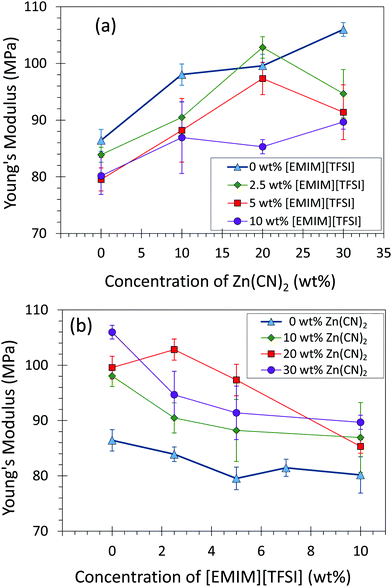
Addition of Zn(CN)2 enhanced the stiffness (Young's modulus) of the materials due to the solid nature of Zn(CN)2, while addition of [EMIM][TFSI] decreases the compliance due to the liquid nature of [EMIM][TFSI] (Fig. 3). Both toughness and tensile strength of the composites decreased with increasing amounts of Zn(CN)2 and [EMIM][TFSI] due to a decrease in elongation at break (Fig. S6–S11, ESI†). Although less ductile with increasing amounts of Zn(CN)2, the composite strength remains constant, especially encouraging for applications in devices where composites would not experience severe elongation.
In summary, we have developed a strategy for significantly reducing the CTE of thermoplastic composite materials and applied it to the production of LDPE composites with Zn(CN)2 fillers and ionic liquid interfacial agents. The reductions in CTE are an order of magnitude higher than reported from previous materials, allowing reduction of the neat polymer CTE by up to −80 × 10−6 K−1. Furthermore, inclusion of ionic liquid as an interfacial agent results in retention of tensile strength similar to neat LDPE. We anticipate the strategy of combining polymers with NTE fillers and ionic liquids will be useful for producing thermoplastic materials with controlled coefficients for use in precision electronic and mechanical devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the MacDiarmid Institute for supporting a Research Associate position for Savannah Egerton.References
- J. Chen, L. Hu, J. Deng and X. Xing, Chem. Soc. Rev., 2015, 44, 3522–3567 RSC
.
- R. C. Wetherhold and J. Wang, Compos. Sci. Technol., 1995, 53, 1–6 CrossRef
.
- J. Shi, Z. Pu, K. H. Wu and G. Larkins, MRS Online Proc. Libr., 1996, 445, 229–234 CrossRef
.
- E. Della Gaspera, R. Tucker, K. Star, E. H. Lan, Y. S. Ju and B. Dunn, ACS Appl. Mater. Interfaces, 2013, 5, 10966–10974 CrossRef CAS
.
- K. Takenaka, K. Kuzuoka and N. Sugimoto, J. Appl. Phys., 2015, 118, 084902 CrossRef
.
- R. Huang, Z. Chen, X. Chu, Z. Wu and L. Li, J. Compos. Mater., 2011, 45, 1675–1682 CrossRef CAS
.
- X. Shi, H. Lian, X. Yan, R. Qi, N. Yao and T. Li, Mater. Chem. Phys., 2016, 179, 72–79 CrossRef CAS
.
- A. L. Goodwin and C. J. Kepert, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 140301 CrossRef
.
- A. L. Goodwin, K. W. Chapman and C. J. Kepert, J. Am. Chem. Soc., 2005, 127, 17980–17981 CrossRef CAS
.
- A. E. Phillips, G. J. Halder, K. W. Chapman, A. L. Goodwin and C. J. Kepert, J. Am. Chem. Soc., 2010, 132, 10–11 CrossRef CAS
.
- K. Takenaka, T. Hamada, D. Kasugai and N. Sugimoto, J. Appl. Phys., 2012, 112, 083517 CrossRef
.
- K. Takenaka, Front. Chem., 2018, 6 Search PubMed
.
- L. Lei, L. Bolzoni and F. Yang, Adv. Compos. Hybrid Mater., 2021 DOI:10.1007/s42114-021-00248-7
.
- J. Chen, Y. Zhu, X. Chang, D. Pan, G. Song, Z. Guo and N. Naik, Adv. Funct. Mater., 2021, 31, 2104686 CrossRef CAS
.
- J. Chen, Y. Zhu, Z. Guo and A. G. Nasibulin, Eng. Sci., 2020, 12, 13–22 CAS
.
- N. Jiang, D. Hu, Y. Xu, J. Chen, X. Chang, Y. Zhu, Y. Li and Z. Guo, Adv. Compos. Hybrid Mater., 2021, 4, 574–583 CrossRef CAS
.
- R. Roy, D. K. Agrawal and H. A. McKinstry, Annu. Rev. Mater. Sci., 1989, 19, 59–81 CrossRef CAS
.
- P. Badrinarayanan, M. Rogalski, H. Wu, X. Wang, W. Yu and M. R. Kessler, Macromol. Mater. Eng., 2013, 298, 136–144 CrossRef CAS
.
- S. Shubin, A. Akulichev and A. Freidin, Mater. Phys. Mech., 2017, 32 Search PubMed
.
- S. Rojstaczer, D. Cohn and G. Marom, J. Mater. Sci. Lett., 1985, 4, 1233–1236 CrossRef CAS
.
- N. Mounet and N. Marzari, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 205214 CrossRef
.
- A. Chandra, W. H. Meyer, A. Best, A. Hanewald and G. Wegner, Macromol. Mater. Eng., 2007, 292, 295–301 CrossRef CAS
.
- J. Yang, Y. Yang, Q. Liu, G. Xu and X. Cheng, J. Mater. Sci. Technol., 2010, 26, 665–668 CrossRef CAS
.
- D. J. Williams, D. E. Partin, F. J. Lincoln, J. Kouvetakis and M. O'Keeffe, J. Solid State Chem., 1997, 134, 164–169 CrossRef CAS
.
- J. Zwanziger, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 052102 CrossRef
.
- T. Ravindran, A. Arora, S. Chandra, M. Valsakumar and N. C. Shekar, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 054302 CrossRef
.
- K. W. Chapman, P. J. Chupas and C. J. Kepert, J. Am. Chem. Soc., 2005, 127, 15630–15636 CrossRef CAS
.
- K. W. Chapman and P. J. Chupas, J. Am. Chem. Soc., 2007, 129, 10090–10091 CrossRef CAS
.
- J. O. Evans, J. Chem. Soc., Dalton Trans., 1999, 3317–3326 RSC
.
- V. E. Fairbank, A. L. Thompson, R. I. Cooper and A. L. Goodwin, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 104113 CrossRef
.
- J. Michael, W. Catherine and W. Mary, J. Therm. Anal. Calorim., 2009, 99, 165–172 Search PubMed
.
- J. Chen, X. Xing, R. Yu and G. Liu, J. Am. Ceram. Soc., 2005, 88, 1356–1358 CrossRef CAS
.
- C. Sun, Z. Cao, J. Chen, R. Yu, X. Sun, P. Hu, G. Liu and X. Xing, Phys. Status Solidi B, 2008, 245, 2520–2523 CrossRef CAS
.
- S. Wang, M. Tambraparni, J. Qiu, J. Tipton and D. Dean, Macromolecules, 2009, 42, 5251–5255 CrossRef CAS
.
- T.-C. Lim, Adv. Compos. Hybrid Mater., 2021, 4, 966–978 CrossRef
.
- X. Ye, B. Tian, Y. Guo, F. Fan and A. Cai, Beilstein J. Nanotechnol., 2020, 11, 671–677 CrossRef CAS
.
- C. Lind, Materials, 2012, 5, 1125–1154 CrossRef CAS
.
- C. Lind, M. R. Coleman, L. C. Kozy and G. R. Sharma, Phys. Status Solidi B, 2011, 248, 123–129 CrossRef CAS
.
- L. M. Sullivan and C. M. Lukehart, Chem. Mater., 2005, 17, 2136–2141 CrossRef CAS
.
- X. Chu, R. Huang, H. Yang, Z. Wu, J. Lu, Y. Zhou and L. Li, Mater. Sci. Eng., A, 2011, 528, 3367–3374 CrossRef
.
- A. George, Kirk-Othmer Encycl. Chem. Technol., 2000, 1–46 CAS
.
- Z. V. Singh, M. G. Cowan, W. M. McDanel, Y. Luo, R. Zhou, D. L. Gin and R. D. Noble, J. Membr. Sci., 2016, 509, 149–155 CrossRef CAS
.
- Y. C. Hudiono, T. K. Carlisle, J. E. Bara, Y. Zhang, D. L. Gin and R. D. Noble, J. Membr. Sci., 2010, 350, 117–123 CrossRef CAS
.
- Y. C. Hudiono, T. K. Carlisle, A. L. LaFrate, D. L. Gin and R. D. Noble, J. Membr. Sci., 2011, 370, 141–148 CrossRef CAS
.
- G. H. Teoh, P. C. Tan, A. L. Ahmad and S. C. Low, J. Membr. Sci. Res., 2021, 7, 29–37 CAS
.
- J. Shi, Z. Shi, H. Yan, X. Wang, X. Zhang, Q. Lin and L. Zhu, RSC Adv., 2018, 8, 6565–6571 RSC
.
- C. Yang, J. Li, D. Yang, S. Li, Y. Qin, S. Meng and S. Du, ACS Sustainable Chem. Eng., 2019, 7, 14747–14755 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00338d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |