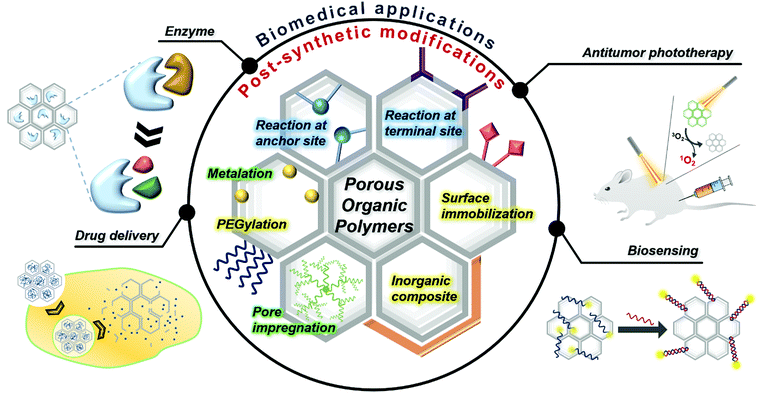
Post-synthetic modifications in porous organic polymers for biomedical and related applications
Ji Hyeon Kim†
 ,
Dong Won Kang†
,
Dong Won Kang† ,
Hongyeol Yun†,
Minjung Kang,
Nem Singh
,
Hongyeol Yun†,
Minjung Kang,
Nem Singh *,
Jong Seung Kim
*,
Jong Seung Kim * and
Chang Seop Hong
* and
Chang Seop Hong *
*
Department of Chemistry, Korea University, Seoul 02841, Korea. E-mail: nsingh@korea.ac.kr; jongskim@korea.ac.kr; cshong@korea.ac.kr
First published on 3rd December 2021
Abstract
Porous organic polymers (POPs) are prepared by crosslinked polymerization of multidimensional rigid aromatic building blocks. Generally, POPs can be classified into crystalline covalent organic frameworks (COFs) and other poorly crystalline or amorphous porous polymers. Due to their remarkable intrinsic properties, such as high porosity, stability, tunability, and presence of numerous building blocks, several new POPs are being developed for application across various scientific fields. The essential sensitive functional groups needed for specific applications are not sustained under harsh POP preparation conditions. The recently developed post-synthetic modification (PSM) strategies for POPs have enabled their advanced applications that are otherwise restricted. Owing to the advanced PSM strategies POPs have experienced a blossoming resurgence with diverse functions, particularly in biomedical applications, such as bioimaging tools, drugs, enzymes, gene or protein delivery systems, phototherapy, and cancer therapy. This tutorial review focuses on the recently developed PSM strategies for POPs, especially for biomedical applications, and their future perspectives as promising bioapplicable materials.
Key learning points(A) PSM strategies of POPs for biological applications, such as cancer therapy, biosensing, and biocatalysts.(B) Diverse POPs in the field of bioapplications that have endured PSM. (C) Differences in functionality before and after undergoing PSM. (D) Current challenges and perspectives in the PSM of POPs toward biological applications. (E) Future potential and challenges in the clinical trials of POPs. |
1. Introduction
The recent few years have witnessed a rapid upsurge in the development of new porous organic polymers (POPs) owing to their widespread applications across science.1,2 The potential of POPs is attributed to their beneficial properties, such as intrinsic porosity, high stability, high modularity, easy access to building blocks, etc.3 POPs include crystalline porous organic frameworks, popularly known as covalent organic frameworks (COFs), and other poorly crystalline or non-crystalline porous organic polymers.4–6 Based on preparation methods and building blocks used, poorly crystalline or amorphous POPs are known as conjugated porous polymers (CPPs), conjugated microporous polymers (CMPs), covalent triazine frameworks (CTFs), etc. Many POPs have shown a wide range of applications in the fields of catalysis, photocatalysis, optoelectronics, sensing, gas storage, water remediation, and separation.2,7,8 Furthermore, the post-synthetic modification (PSM) of POPs in recent years has made them widely adaptable in many advanced applications, which are otherwise not feasible.9–12For biomedical applications, either the pre-synthetic modifications of building blocks or the PSM of COFs is necessary. However, sensitive functional groups may not be sustainable under harsh COF reaction conditions. For COFs, pre-modification of monomers increases steric hindrance and hampers crystallinity, while alternative PSM mostly retains the original COF's crystallinity. POPs have the advantage of high stability, and the functional group present on the building blocks makes them a convenient platform for PSM. So, PSM is an attractive strategy for tuning the properties of POPs to be more suitable for the intended purpose. In other words, the PSM of POPs broadens their chances of being utilized in biosystems by endowing functionality or enhancing the biocompatibility of POPs. In this context, recently developed PSM strategies for POPs have enhanced their potential and efficiency in biomedical applications. PSMs of POPs, such as organic transformation, metal chelation, and polyethylene glycol (PEG)ylation, have opened up tremendous opportunities. POPs have shown magnificent potential as evolving nanomedicine candidates for cancer treatment, including drug delivery, photothermal therapy (PTT), photodynamic therapy (PDT), and combined therapy.13,14 Beyond the field of cancer, biosensing, bioimaging, and POP-based catalysts are other promising biological application fields that POPs can achieve.15
First, the representative PSM strategy is functionalizing anchor sites or unreacted terminal sites through organic reactions.16 Modifying the inside pores of POPs is often achieved by amide coupling reaction with the residual functional groups and the desired compounds, such as enzymes, fluorophores and targeting agents. Given the nature of polymerization, residual functional groups are unavoidable, and these are attractive sites to confer additional functions by PSM.11 For example, the terminal aldehyde groups residing on COFs are good targets for attaching functional amine-containing molecules by Schiff-base condensation. Second, the strategy to modify the inner space by attaching the desired molecule to the large pores in POPs is a facile way to modify the POPs for biomedical applications. Metalation is a unique immobilization method that incorporates metal ions into organic frameworks capable of electrostatic interactions. Occasionally, functional molecules are impregnated inside the pores of POPs. The example molecules listed are drugs and photosensitizers for cancer therapy as well as enzymes for functioning as biocatalysts. Surface modification is another way to endow POPs with a function or enhance their biocompatibility. Surface immobilization of biomolecules, such as DNA, aptamers, and proteins, allows POPs to function as a biosensor or a polymer-based enzyme. The modification of POP surfaces with inorganic components is also an effective way to provide a specific function for bioapplicable POPs. Post-synthetic PEGylation is one of the most common and straightforward methods for preparing bioapplicable POPs. This is advantageous for enhancing their hydrophilicity, dispersibility, and stability required for application in cancer theranostics.
Various recent reviews have summarized the synthesis and PSM of POPs and COFs.17 In addition, many review papers covering COFs, focusing on the bioapplications, have been reported.18,19 In contrast, in this review, we have discussed the recent PSM strategies applied in the biomedical and related fields, which can be classified into functionalization through organic reactions, impregnation, and surface modification (Fig. 1 and Table 1). Since designing and manufacturing POPs for these biological applications mainly operates in aqueous media and must meet the demands of biocompatibility and competitive performance, PSM is a fundamental strategy to expand the usefulness of the polymer. We will cover how the initially synthesized POPs were given their functions depending on the type of further processing.
| POP | Detailed PSM method | Nanoparticle size/morphology | Crystallinity | Application | Ref. |
|---|---|---|---|---|---|
| COFHD-GOX | Amide coupling of GOX with COF | 1–2 μm, irregular particle packing structure | High | Enzyme immobilization and detection of glucose | 24 |
| TpASH-NPHS | Epoxide ring opening by glycidol and coupling of 3-aminopropyltriethoxysilane | 100–200 nm, rippled sheet-like morphology | High | Detection of intracellular H2S target bioimaging | 25 |
| TpASH-FA | Epoxide ring opening by glycidol and coupling of 3-aminopropyltriethoxysilane with folic acid | >1 μm, sheet-like morphology | High | Targeted drug delivery and bioimaging | 26 |
| H-MON-FA | Thiol–yne click reaction of L-cysteine and coupling with folic acid | 250 nm, hollow structure | Low | Targeted drug delivery | 27 |
| LZU-1-BODIPY-2X | Schiff-base condensation of BODIPY-2I and COF | ∼110 nm, spherical particles | High | Antitumor PDT | 28 |
| VONc@COF-Por | Schiff-base condensation of porphyrin and COF, and impregnation of VONc | 50–200 nm, spherical particles | High | Antitumor PDT and PTT | 29 |
| CaCO3@COF-BODIPY-2I@GAG | Schiff-base condensation of BODIPY-2I and COF, then coating with GAG and impregnation of CaCO3 NPs | ∼320 nm, spherical particles | High | PDT and Ca2+ overload synergistic tumor therapy | 30 |
| Lysozyme ⊂ COF 1 | Amide coupling of biomolecules with COF under Schiff-base | >1 μm, (SEM) filamentous morphology | High | Separation of racemic compounds | 31 |
| HCOF | Fe3+ coordination | 2–4 μm, flower-like sphere | High/moderate | Antitumor PTT | 33 |
| Zn/POF2 | ZnO coordination | <50 nm, irregular sphere | Low | Antibacterial and antiviral | 34 |
| TTI-COF | Quercetin impregnation | >1 μm, flake-like structures | High | Quercetin delivery | 39 |
| Que-POP | Drug impregnation | ∼70 nm, spherical particles | Low | pH-responsive drug delivery | 40 |
| Lipase@COF-ETTA-EDDA | Enzyme impregnation | 200–300 nm, rod-like shape | High | Enhanced reactant accessibility and higher activity of the enzyme | 44 |
| Probe/TpTta | Immobilization of DNA or aptamer and FRET acceptor for off–on probe | 2–3 μm, cluster like shape | High | Detection of DNA | 48 |
| Py-M-COF-based aptasensor | Immobilization of DNA aptamer via π–π stacking with COF | 0.6–1 μm, multilayered sheet | High | Detection of antibiotics | 49 |
| Apt/p-COF | Immerse p-COF-modified AE in the aptamer solution | 1 μm, sheet-like shape | Low | Aptasensor to bind EGFR and trace living cells | 51 |
| TPMM-COF | Immobilization of α-amylase | Strand-like shape | High | Enhancing reusability and storage stability of enzyme | 52 |
| COF-survivin | Immobilization of survivin via π–π stacking with COF | 100–200 nm, quasi sphere | High | PDT and diagnosis of cancer | 53 |
| IBU@DhaTph-membrane | Immobilization of ibuprofen | 150 μm in thickness (membrane) | High | Skin wound healing by drug and light-induced 1O2 | 54 |
| COF-TiO2 | Composite formation with TiO2 and HA coating | ∼300 nm, spherical particles | High | Antitumor SDT | 58 |
| COF−CuSe@PEG | CuSe composite formation and PEGylation | 200–600 nm, spherical particles | Moderate | Antitumor PDT and PTT | 56 |
| γ-SD/PLL | Dox impregnation, iron oxide composite formation, and PEGylation | ∼300 nm, sea-urchin shape | High | MRI and chemo-thermotherapy | 57 |
| DSPE-BCOP-5T | Carborane loading and DSPE-PEG-coating | ∼100 nm, spherical particles | Low | Antitumor boron neutron capture therapy | 66 |
| PFCE@THPPpf-PEG | PFCE loading and PEGylation | <100 nm, spherical particles | Low | Hypoxia relief and antitumor PTT | 68 |
| PEG-CCM@APTES-COF-1@DOX | Dox impregnation and fabrication with PEG-CCM | 150–250 nm, core–shell morphology | Moderate | Antitumor drug delivery | 69 |
| Se@COF-PEG | Se doping and PEGylation | 175 nm | High | Long-acting antitumor PDT | 70 |
2. PSM strategies
2.1. Functionalization through organic reactions
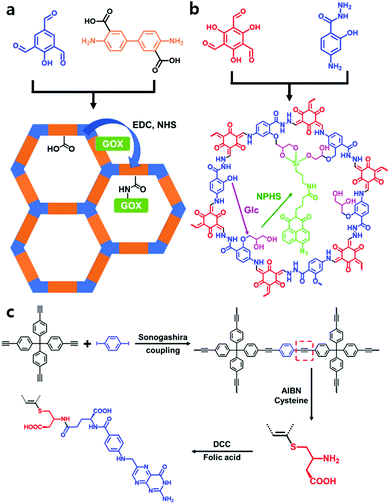
To diversify POPs for biomedical applications, further functionalization via organic reactions is a fascinating strategy among several post-synthetic approaches for designing biomedical materials. After covalently connecting well-known biomedical functional molecules to POPs, the functionalized material not only retains biomedical properties but also begins to show additional synergetic effects, such as stability and selectivity.20–23 Functionalization of components by covalent bonds requires available reactive sites for further reactions and can be classified into two types. The first is fabricating anchoring sites in the framework that have not participated as an ingredient in the construction of the framework. The second type is the reaction at the bonding defect site after framework formation. This section introduces some examples of post-modified POP materials through organic reactions that are functionalized in anchoring and bonding defect sites.A post-synthetically modified COF biosensor, TpASH-NPHS, was prepared for detecting intracellular H2S by Zhang et al.25 It was the first example of a two-photon fluorescent COF nanoprobe. TpASH, a stable COF, was fabricated through Schiff-base condensation and irreversible keto–enol tautomerization of 1,3,5-triformylphloroglucinol and 4-aminosalicylhydrazide in H2O at 90 °C for 12 h using p-toluenesulfonic acid as a catalyst. Then, the hydroxyl group in TpASH was successively functionalized with glycidol (Glc) by epoxide ring opening and coupling with TpASH and 4-amino-1,8-naphthalimide (NPHS) containing the 3-amino-propyltriethoxysilane (APTES) unit to obtain TpASH-NPHS (Fig. 2b). The azide group in NPHS could act as an H2S probe by enhancing the fluorescence through reduction to amine in the presence of H2S. However, intracellular enzymes could also reduce the azide group under hypoxic conditions; thus, the selective detection of H2S remains a challenge. But the small pore size of TpASH-NPHS prevented the intracellular enzymes from approaching the azide group in NPHS, and thus H2S selective detection was facilitated by applying the PSM strategy to stable and microporous TpASH. Additionally, TpASH-NPHS showed good photostability because of the intrinsic durability of TpASH. The cytotoxicity of TpASH-NPHS was determined by incubating human cervical carcinoma cells (HeLa) and human liver cancer cells (HepG2) with TpASH-NPHS for 24 h. Both HeLa cells and HepG2 cells showed high viability (>90%), which indicates the adequacy of its use as a biosensor.
On the other hand, Banerjee et al. reported a folic acid (FA)-decorated covalent organic nanosheet (CON), TpASH-FA, through PSM.26 They applied the CONs instead of COFs for drug delivery because the nanosheet system is more suitable for a targeted drug delivery system because of its large drug loading capacity, sustained release of the payload, and nontoxic nature. The TpASH COF was formed by salt-mediated synthesis, which was converted to functionalized targeted CONs by applying several PSM methods. The targeted CONs (TpASH-FA) were prepared by sequentially introducing Glc, APTES, and FA into the synthesized TpASH COFs. Compared to the non-targeted platform TpASH-APTES, TpASH-FA showed enhanced anticancer activity against the breast cancer cell line MDA-MB-231 after loading the anticancer drug 5-fluorouracil (5-FU).
Son et al. applied an analogous PSM strategy to microporous organic network (MON) nanoparticles for drug delivery.27 The MON nanoparticles were prepared through Sonogashira coupling reaction of tetrakis(4-ethynylphenyl)methane with 1,4-diiodobenzene using silica spheres as a template. The PSM hollow MON structures were able to enhance drug loading as an efficient drug delivery system due to their intrinsic amorphous structure. After etching the silica spheres with HF solution, hollow MONs (H-MONs) were obtained. Next, H-MON-CYS was synthesized by connecting the cysteine-included thiol reagent and alkyne in H-MON via a thiol–yne click reaction. To efficiently deliver the anticancer drug doxorubicin (DOX), FA was coupled with the amine residue of cysteine in H-MON-CYS to form H-MON-FA as a targeted drug delivery material (Fig. 2c). In terms of DOX delivery, DOX/H-MON-FA showed more effective delivery and controlled release than DOX/H-MON against cancer cells (MDA-MB-231 and A549 cell lines).
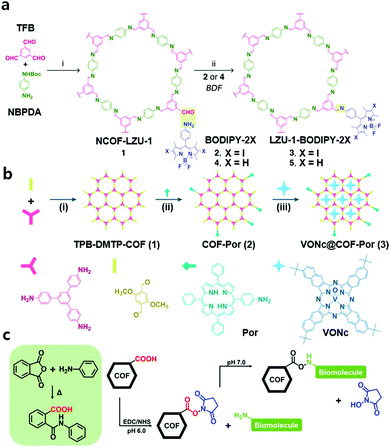 | ||
| Fig. 3 (a) Preparation of LZU-1-BODIPY-2X through the PSM strategy. Reproduced with permission from ref. 28. Copyright 2019, Elsevier Inc. (b) The preparation of VONc@COF-Por. Reproduced with permission from ref. 29. Copyright 2019, American Chemical Society. (c) Illustration of the covalent strategy to bond various biomolecules with COFs. | ||
The same group of authors designed another nCOF material by applying a similar PSM strategy and additional impregnation for combined PDT and PTT.29 They prepared TPB-DMTP-COF by Schiff-base condensation of 1,3,5-tris(4-aminophenyl)benzene and 2,5-dimethoxyterephthalaldehyde at room temperature for 12 h in CH3CN with acetic acid and polyvinylpyrrolidone. Subsequently, the free aldehyde groups of TPB-DMTP-COF were reacted with the amino group of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin (Por) to obtain COF-Por under reflux for 12 h in 1,4-dioxane. The porphyrin moiety of the framework functioned as a photosensitizer for 1O2 generation. The slightly decreased BET surface area (1217 m2 g−1 to 1180 m2 g−1) and the unchanged pore size (3.2 nm) indicated that the modification occurred only at the terminal site of TPB-DMTP-COF. Additionally, the sustained pore environment was suitable for impregnation of the photothermal agent (PTA), vanadyl 2,11,20,29-tetra(tert-butyl)-2,3-naphthalocyanine (VONc). After soaking COF-Por in a solution of N,N-dimethylacetamide and VONc at room temperature, the host–guest supramolecular system VONc@COF-Por was obtained (Fig. 3b). The PDT and PTT efficacy of VONc@COF-Por was tested in MCF-7 cells in vitro and in vivo. It showed high cell viability (83.8 ± 4.7%) in the dark, even at a high concentration (400 μg mL−1), and sharply decreased cell viability (16.5 ± 1.4%) after the combination of PDT and PTT. The antitumor effect of combined PDT + PTT was more intense than that of individual PDT or PTT, and its IC50 was 42 μg mL−1, much less than that of PDT (131 μg mL−1) and PTT (93 μg mL−1) alone. These results showed that combination therapy enabled by PSM was an effective strategy for the inhibition of tumors while minimizing side effects. The authors used the same nCOF for another synergistic tumor therapy by an analogous PSM strategy.30 They used glycosaminoglycan (GAG)-coated and CaCO3 nanoparticle (NP)-loaded nCOF materials (CaCO3@COF-BODIPY-2I@GAG) for combined PDT and Ca2+ overload therapy. The modified COF (COF-BODIPY-2I) was prepared by Schiff-base condensation of the amine group of BODIPY-2I and the unreacted aldehyde group at TPB-DMTP-COF and immersed in a CaCl2 aqueous solution with NH4HCO3. Then, CaCO3 NPs were loaded onto COF-BODIPY-2I to obtain CaCO3@COF-BODIPY-2I. Further modified CaCO3@COF-BODIPY-2I@GAG was formed by coating CaCO3@COF-BODIPY-2I with GAG in water at 25 °C for 24 h to enhance the targeting ability toward the CD44 receptor. It generated 1O2 under green LED irradiation (20 m W cm−2) owing to the BODIPY moiety. The generated 1O2 could cause cell death through direct cell oxidation and damage mitochondrial Ca2+ regulatory function, which induces cytotoxicity. Additionally, Ca2+ overload in the form of CaCO3 NPs in CaCO3@COF-BODIPY-2I@GAG enhanced antitumor synergetic therapy.
Ma et al. first demonstrated a covalently connected complex that was constructed from a series of chiral biomolecules (lysozyme, tripeptide, and L-lysine) and an achiral COF to utilize it as a chiral HPLC system to separate racemates by connecting biomolecules to the bonding defect site of the COF.31 A polyimide-linked achiral COF 1 was newly synthesized using benzene-1,2,4,5-tetracarboxylic dianhydride (PMDA) and 2,4,6-tris(4-aminophenyl)-1,3,5-triazine (TAPT) monomers by heating at 200 °C for 5 days. During the reaction, an amic acid was formed as an intermediate, and its carboxyl group was condensed with the amine group of biomolecules with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) (Fig. 3c). It exhibited excellent stability in solvents, such as pH 6.0 MES buffer and hot water. Its pore size was 3.6 nm, enough to contain biomolecules. As lysozyme is a suitable protein for chiral recognition and separation in terms of cost, thermal stability, size, and facile modification, it was bound with COF 1 to produce lysozyme ⊂ COF 1. It exhibited high chiral separation efficiency for all tested racemates, including DL-tryptophan and DL-leucine. However, pure COF 1, MCM-41, which is a protein that can load some lysozymes and lysozyme@MCM-41, showed no separation effect on any of the tested racemates. Additionally, they also used other biomolecules, such as the tripeptide Lys-Val-Phe and L-lysine. However, they exhibited low chiral separation efficiencies. The authors explained that this was caused by the relatively low structural order of these biomolecules from circular dichroism (CD) spectroscopic analysis.
2.2. Pore surface modification
The impregnation of various materials, such as metal ions, drugs, and enzymes, is a powerful strategy to endow biocompatibility, therapeutic effects, and catalytic performance. The POPs have the potential to contain enormous functional components because their large surface area and pore volume can firmly hold up the components because of the abundance of functional groups and linkers on the materials. Hence, POPs and COFs are attractive as new functional platforms for biomedical applications. In this section, we describe the inner space modified POPs and COFs for biomedical-related applications, depending on the type of impregnation component and the site of impregnation.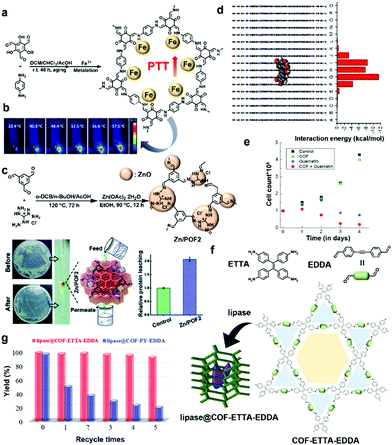 | ||
| Fig. 4 (a) Preparation and post-synthetic metalation of a hierarchical COF (HCOF) for PTT. (b) Whole-body photothermal images of mice after intratumoral injection of Fe–HCOF (0.1 mL, 1 mg mL−1) and exposure to a laser (808 nm, 1.5 W cm−2). Reproduced with permission from ref. 33. Copyright 2019, The Royal Society of Chemistry. (c) Synthetic schemes of POF2 and Zn/POF2 and their cleaning ability of bacteria-contaminated water by passing through a column packed with Zn/POF2. Reproduced with permission from ref. 34. Copyright 2020, The Royal Society of Chemistry. (d) Side view of the modeled COF pore showing interaction energies between the quercetin molecule and the model hexagon layers. (e) Proliferation assay of the cancer cells treated with the COF (green triangles), quercetin (blue stars), and quercetin-loaded COF (red dots) over a period of 4 days. Reproduced with permission from ref. 39. Copyright 2016, Wiley-VCH. (f) Schematic illustration of the synthesis of lipase@COF-ETTA-EDDA. (g) Recycling tests. Reaction conditions: soybean oil (20 mg), ethanol (100 μL), lipase@ETTA-EDDA (2.8 mg), or lipase@COF-PY-EDDA (3.4 mg), and 40 °C. Reproduced with permission from ref. 44. Copyright 2019, Wiley-VCH. | ||
Similarly, a COF-based antimicrobial agent (Zn/POFs) was prepared using the post-metalation method from triaminoguanidinium-based ionic porous organic frameworks (POFs) by Patra and Chande et al.34 They utilized triaminoguanidinium chloride, which is an N-rich moiety that binds metal ions and is well known for its positive antibacterial activity. Triaminoguanidinium chloride was reacted with terephthalaldehyde and benzene-1,3,5-tricarboxaldehyde through a Schiff-base polycondensation reaction to obtain POF1 and POF2, respectively (Fig. 4c). To increase remedial activity, ZnO, which is an inexpensive and antimicrobial active metal oxide, was loaded into the pores of POF2, resulting in Zn/POF2 with a high Zn(II) content of 47.2%. Based on the synergistic effect of the loaded ZnO and POF2 framework, the material showed potent antibacterial (Gram-positive, Staphylococcus aureus and Gram-negative, Escherichia coli) and antiviral (HIV-1 and VSV-G enveloped lentiviral particles) activity. As shown in Fig. 4c, Zn/POF2 was utilized in gravimetric column filtration to disinfect water, which was analyzed using colony-forming unit imaging. The analyses suggested that the metal-oxide-loaded COF has excellent potential for disinfection of water by eliminating the live bacterial load. The aforementioned studies by Pang, Patra, and Chande et al. suggest that the post-synthetic metalation method can be employed in various biomedical applications and is a promising strategy for the development of biomedical materials.
POPs are also convenient materials for the delivery of enzymes due to the strong intermolecular interactions between POPs and enzymes.41,42 Ma et al. introduced different enzymes into the pores of metal–organic frameworks, mesoporous silica, amorphous polymers, and COFs in their previous work.43 In succession of their previous research, they carefully devised non-blocked pores after enzyme immobilization, affecting the diffusion rates for reagents and products and high catalytic activity by the enzyme. Therefore, they attempted to incorporate an enzyme into the mesoporous COFs having a hydrophobic pore environment as host materials.44 They prepared two COFs (COF-ETTA-EDDA and COF-PY-EDDA) from [4,4′,4′′,4′′′-(ethene-1,1,2,2-tetrayl)tetraaniline, ETTA] and 4,4′,4′′,4′′′-(pyrene-1,3,6,8-tetrayl)tetraaniline (PY) with 4,4′-(ethyne-1,2-diyl)dibenzaldehyde (EDDA) through a condensation reaction, respectively (Fig. 4f). Compared to COF-PY-EDDA with a single pore environment (31.4 Å), COF-ETTA-EDDA with a dual pore environment (∼13.9 and 38.5 Å) was expected to have an accelerated diffusion of reagents and products in the framework. Lipase PS was incubated to obtain lipase@COF-ETTA-EDDA and lipase@COF-PY-EDDA with maintained crystallinity. The loaded amount of lipase PS in COF-ETTA-EDDA was evaluated to be 0.78 mg g−1 by bicinchoninic acid assay using UV-Vis spectra. Scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM), and energy dispersive X-ray (EDX) data suggested the homogeneous immobilization of the enzyme in the pores of the COFs. The enzyme-catalytic performances of lipase@COF and the free enzyme were evaluated using racemic secondary alcohols. Lipase@COF exhibited enhanced catalytic performance because the catalytic sites of the enzyme were more accessible in the porous framework. In particular, lipase@COF-ETTA-EDDA showed an improved catalytic performance about 1.5 times higher than that of lipase@COF-PY-EDDA despite the same enzyme amount. The enzymes in COF-ETTA-EDDA with a dual pore system exhibited superior performance compared to COF-PY-EDDA. The performance was verified by the rate parameter kcat/Km in the enzymatic reaction (where kcat indicates the catalytic reaction rate constant and Km refers to the Michaelis–Menten constant), and the performance difference between the two COFs originated from fast diffusion to and from the catalytic active sites. Surprisingly, in the recyclable test using soybean oil, the activity of lipase@COF-ETTA-EDDA was almost unchanged after five repeated measurements compared to lipase@COF-PY-EDDA (Fig. 4g). These results strongly suggest that the enzyme impregnation method into the pores of a COF has great potential for next-generation biocomposite catalysts with improved activity and stability.
2.3. Surface modification
The above discussion was related to PSM of the functional group via organic transformations and internal space metal coordination to improve the efficiency of biomedical applications. This section describes the PSM methods used to modify the surface of POP materials to make them usable under physiological conditions. Immobilization of biomolecules, such as aptamers and enzymes, on the surface is a standard way to confer this function.45–47 The other methods involve the formation of an inorganic nanocomposite and PEGylation via PSM after the synthesis of the POP.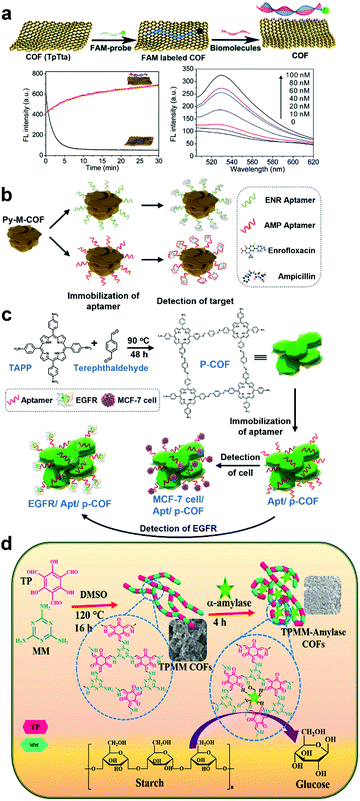 | ||
| Fig. 5 (a) Illustration of a versatile COF-based sensing platform for fluorescence turn-on detection of biomolecules. Time courses for the fluorescence quenching of the probe (50 nM) upon TpTta addition (10 mg) (black curve) and for the fluorescence recovery of the probe/TpTta upon target DNA addition (400 nM DNA) (red curve). Dependence of the fluorescence of the probe (50 nM) on the concentration of target DNA. Reproduced with permission from ref. 48. Copyright 2017, The Royal Society of Chemistry. (b) Scheme of electrochemical detection of ENR and AMP using the Py-M-COF-based aptasensors. Reproduced with permission from ref. 49. Copyright 2020, Elsevier Inc. (c) Schematic diagram of the p-COF-based aptasensor for detecting epidermal growth factor receptor (EGFR) or MCF-7 cells, including (i) preparation of p-COF, (ii) immobilization of the aptamer strands, and (iii) detection of EGFR or MCF-7 cells. Reproduced with permission from ref. 51. Copyright 2020, Elsevier Inc. (d) Schematic diagram of the synthesis of TPMM COFs and immobilization of α-amylase for catalytic application in starch hydrolysis. Reproduced with permission from ref. 52 Copyright 2020, Elsevier Inc. | ||
In addition to the surface-modified COF fluorescent sensor with DNA, COF materials have also been utilized in electrochemical sensor applications, as reported by Du and Zhang et al.49 An extended π-conjugation mesoporous framework (Py-M-COF) with a porosity of 3.9 nm was obtained from 1,3,6,8-tetrakis(4-formylphenyl)pyrene and melamine through condensation polymerization to form imine bonds at 150 °C for 48 h. To explore these unique properties, the authors developed COF-based electrochemical sensors using widely utilized antibiotics, such as enrofloxacin (ENR) and ampicillin (AMP). To selectively detect antibiotics, the COF surface was modified with aptamers, which are artificial single-stranded DNA or RNA molecules that bind targets through specific 3D structural recognition. Because aptamers have several advantages, including high selectivity, excellent stability, and simple synthetic processes, the molecules are beneficial in bioassays. Considering these characteristics, the authors fabricated Py-M-COF-based aptasensors (Fig. 5b). For the fabrication, the Py-M-COF suspension in Milli-Q water was dropped onto the Au electrode (AE), and then Py-M-COF/AE was dipped in the aptamer solutions of ENR and AMP (AptENR and AptAMP). After immersion in phosphate-buffered saline (PBS) solution and drying, AptENR/Py-M-COF/AE and AptAMP/Py-M-COF/AE were obtained. From electrochemical impedance spectroscopy data (EIS), the COF-based aptasensors exhibited outstanding electrochemical sensing properties with a remarkably low limit of detection (LOD) of 6.07 and 0.04 fg mL−1 for ENR and AMP detection, respectively. The sensors were also tested in human serum samples containing diluted ENR and AMP.
Similarly, the aptasensor using COFs for trace EGFR and living Michigan cancer foundation-7 (MCF-7) cells was investigated by Zhang and Zhou et al. EGFR is a transmembrane protein, and abnormal expression of EGFR (>75.3 mg L−1) may be affected by different cancers of the lung, ovary, and others.50 It is crucial to quantify proteins in situ in the cell membrane for cancer diagnosis and therapy. Simultaneously, elaborate detection of circulating tumor cells is required. However, there are limitations because of the deficient concentrations of tumor cells in the peripheral blood. To sense crucial biomolecules, such as EGFR, the authors prepared a porphyrin-based COF (p-COF) from 5,10,15,20-tetrakis(4-aminophenyl) porphyrin (TAPP) and terephthaldehyde (Fig. 5c). Through a similar procedure as in their previous work,49 the p-COF was located on the surface of the AE, and the aptamer was successfully immobilized onto the surface of p-COF.51 The COF was anticipated to show fast electron migration through a π-conjugated network. To investigate the detection of EGFR, the aptasensor was soaked in an EGFR solution and its performance was evaluated by EIS. As a result, the LODs of the sensor were determined to be 7.54 fg mL−1 of EGPR and 61 cells mL−1 of MCF-7, which reveal the excellent sensitivity of the sensor for favorable practicality in human serum. The sensor studies using the immobilization method on the surface of COFs suggest that this technique is an easy, facile, and powerful tool for fabricating highly effective COF-based sensors for biomedical applications.
On the other hand, the α-amylase-immobilized COF-based catalyst was investigated for starch hydrolysis by Sahu et al.52 As free enzymes are structurally unstable, a support which can stabilize enzymes has been considered essential for enhancing the catalytic activity of enzymes. In this context, COFs and POPs have considerable benefits, such as porosity, large surface area, and specific binding sites for enzymes. However, compared to the potential of COFs, only a few studies have been reported on enzyme immobilization onto the surface of a COF. A robust TPMM-COF was prepared by the condensation and tautomerization of melamine and triformylphloroglucinol, and α-amylase was attached to the surface of the COF to hydrolyze the α-bond of polysaccharides (e.g. starch), resulting in monosaccharides (e.g. glucose) (Fig. 5d). Using thermogravimetric analysis, the content of α-amylase in the COF was determined to be ∼55 wt%. The COF firmly captured α-amylase through physical adsorption because TPMM-COF has numerous binding sites for –NH, –OH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Compared to free α-amylase (∼30%), immobilized α-amylase exhibited enhanced catalytic performance (∼50%) even at 90 °C. Furthermore, the catalytic performance almost reached 74% after 10 repeated cycles, which suggests the usefulness of post-synthetic immobilization on the surface of COFs as an excellent catalytic support.
O. Compared to free α-amylase (∼30%), immobilized α-amylase exhibited enhanced catalytic performance (∼50%) even at 90 °C. Furthermore, the catalytic performance almost reached 74% after 10 repeated cycles, which suggests the usefulness of post-synthetic immobilization on the surface of COFs as an excellent catalytic support.
Recently, many POPs and COFs have been utilized in biomedical applications as multifunctional materials. Tang, Li and Pan et al. reported a COF-based photosensitizer with a TAMRA-labeled survivin antisense strand (TSAS) for PDT that employs reactive oxygen species (ROS) to attack malignant cells under light irradiation53 by synthesizing a porphyrin-based COF from 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphine and 2,5-dihydroxyterephthalaldehyde and collecting the small-sized COF nanoparticles (NPs) through differential centrifugation to remove large COF NPs (Fig. 6a). From UV-Vis experiments using the indicator 1,3-diphenylisobenzofuran (DPBF), the 1O2 generation ability of the COF was confirmed, which is one of the most widely utilized ROS in cancer treatments. Additionally, to diagnose tumor cells, TSAS was attached as a theranostic probe to the surface of the COF, resulting in COF-survivin. After attachment, the fluorescence of TAMRA of COF-survivin was quenched by the COF as the large planar structure of porphyrin-COF with a strong p-electron system can easily quench the fluorophore. In fluorescent images, a light-up fluorescent signal was observed only in the tumor tissue after injection of COF-survivin. The therapeutic effect of COF-survivin was investigated using in vitro experiments (Fig. 6b). While test cells still survived after laser irradiation alone, the cells with COF-survivin were killed under the same irradiation conditions, indicating a promising photodynamic effect. Furthermore, tumor inhibition by COF-survivin was observed in in vivo studies in mice (Fig. 6c).
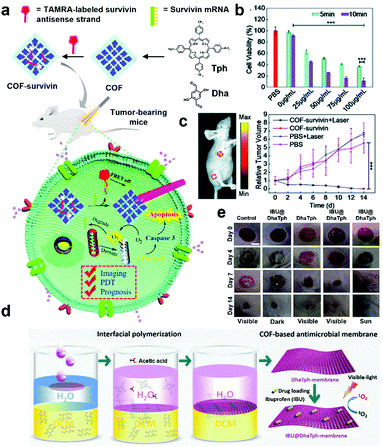 | ||
| Fig. 6 (a) Schematic illustration of the preparation of the theranostic nanoplatform COF-survivin for tumor imaging, PDT, and prognostic evaluation applications. (b) Cell viability of MCF-7 cells treated with different concentrations of COF-survivin followed by 5/10 min laser irradiation. (c) In vivo theranostic performance and prognostic evaluation of COF-survivin in tumor-bearing nude mice. 5 mL of COF-survivin (10 mg mL−1) was injected into the tumor tissue and leg muscle tissue as marked. Reproduced with permission from ref. 53. Copyright 2020, The Royal Society of Chemistry. (d) Schematic illustration of the synthesis of multifunctional antimicrobial IBU@DhaTph-membrane. (e) Photographs of wounds in mice infected with Staphylococcus aureus under various dressing treatments at intervals of 4, 7, and 14 days (scale bar: 5 mm). Reproduced with permission from ref. 54. Copyright 2021, Wiley-VCH. | ||
Similarly, Dong and Yao et al. fabricated an IBU-loaded self-standing COF membrane from 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphine and 2,5-dihydroxyterephthalaldehyde (Fig. 6d).54 Using in situ interfacial polymerization from water/dichloromethane with acetic acid, a membrane (DhaTph-membrane) was obtained, which exhibited 1O2 generation ability under visible light or natural sunlight irradiation. To load the nonsteroidal anti-inflammatory drug IBU, the membrane was immersed in a solution containing IBU. The content of the loaded drug was determined to be 31 wt% based on the UV-Vis spectrum. The prepared IBU@DhaTph-membrane was applied as a hollow medical tape for chronic wound healing studies in mice tests (Fig. 6e). As a result, a significant remedial effect by the membrane was visually observed in the photographs with decreased wound size. Only a small amount of secretion was found in IBU@DhaTph-membrane and DhaTph-membrane-treated cases compared to the control case. This effect is associated with the effective photodynamic and chemical antibacterial performances of IBU-loaded COF membranes. These studies represent the synthetic diversity of COFs for practicality because various biomolecules can be easily immobilized onto the surface of COFs to exhibit excellent synergetic biomedical effects.
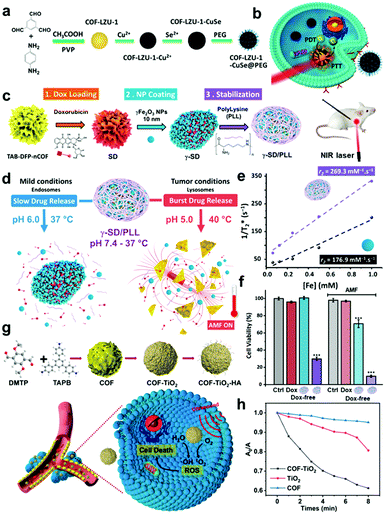 | ||
| Fig. 7 (a) Schematic illustration of the synthesis of COF-CuSe nanoparticles and (b) application for combined PTT and PDT. Reprinted with permission from ref. 56. Copyright 2019, American Chemical Society. (c) Schematic representation of γ-SD/PLL synthesis and (d) representative magnetically induced hyperthermia effect and acid triggered Dox release from γ-SD/PLL under the conditions of the cancer cell. (e) Comparison of R2* relaxation rates (1/T2*) plotted against iron concentrations of γ-SD/PLL (purple) and γ-Fe2O3 NP (black) solutions. (f) In vitro hyperthermia viability assays in U251-MG cells which were incubated for 4 h at 37 °C with each presented compound. The AMF treated groups was exposed to it for 1 h to induce hyperthermia. Reprinted with permission from ref. 57. Copyright 2020, American Chemical Society. (g) Schematic illustration of the synthetic process of COF-TiO2-HA and the therapeutic mechanism. (h) Comparison of the relative absorbance of DPBF (1O2 indicator) at 466 nm by TiO2, COF-TiO2, and COF under US irradiation which triggers a sonosensitizer to produce ROS. Reprinted with permission from ref. 58. Copyright 2021, American Chemical Society. | ||
Likewise, an imine-linked COF is useful because of appropriate stability, degradability, and high loading capacities achieved by an extended π-conjugated framework. Benyettou et al. developed γ-SD/PLL, a Fe2O3-nanoparticle-coated COF nanocomposite loaded with DOX, for cancer theragnosis (Fig. 7c).57 This functional multi-component COF composite was prepared by sequential PSM methods; first DOX was loaded onto the nCOF, which was followed by surface coating with magnetic γ-Fe2O3 NPs (9.8 nm in size), and then a poly(L-lysine) cationic polymer was incorporated by a simple impregnation procedure. The primary function of the imine COF is to provide a large surface area for high drug loading and provide sufficient stability for long-term circulation under physiological conditions. In particular, in the acidic cancer microenvironment, the imine bond hydrolyzes and the structure falls apart, rendering this NP selective toward cancer cells (Fig. 7d). The corresponding COF was synthesized by the condensation of 2,6-diformylpyridine (DFP) and 1,3,5-tris(4-aminophenyl)benzene (TAB) with a nanoparticle size of approximately 240 nm. The specific functions of loaded γ-Fe2O3 are as MRI probes, hyperthermia induction under an AMF, and magnetic targeting effect. Fig. 7e shows that the MRI contrast agent performance of γ-Fe2O3 is enhanced after forming the γ-SD/PLL composite as the transverse relaxivity (R2* = 1/T2*) is increased owing to the aggregation effect on proton relaxation processes. The effective drug release and synergistic effect of hyperthermia were investigated in U251-MG glioblastoma cells (Fig. 7f). As poly(L-lysine) (PLL) cationic polymers facilitate endocytosis into acidic lysosomes, tumor-selective activation of this nanocomposite becomes more favorable. In addition, the pH-responsive characteristic is provided by the PLL protonation of the amine residue and imine bond cleavage of the COF. Very recently, a new inorganic nanocomposite COF was prepared using the PSM strategy and utilized in sonodynamic cancer therapy (SDT). As US can overcome the limit of penetration depth in light-triggered phototherapy, Cai et al. developed a titanium oxide COF composite nanomaterial (COF-TiO2 NP) and applied it in SDT for the first time.58 The monodispersed imine COF is first synthesized through a condensation reaction between 1,3,5-tris(4-aminophenyl)benzene (TAPB) and 2,5-dimethoxyterephthaldehyde (DMTP). TiO2 NPs are grown in situ on the surface of the COF to form inorganic nanocomposites, and the outer shell is coated with hyaluronic acid (HA) to enhance biocompatibility. The inorganic nanocomposite's catalytic mechanism is based on the generation of holes and electrons,59 with ultrasound as an energy source for successful SDT (Fig. 7g). The COF-TiO2 nanocomposite formation is advantageous for ROS production under US irradiation by narrowing the bandgap and making electron and hole separation more favorable (Fig. 7h).
In a very recent study, Shi et al. modified nanoscale covalent organic polymers (nCOPs) with amino-PEG to efficiently deliver carborane drugs to the tumor site for boron neutron capture therapy (BNCT) (Fig. 8a).66 BNCT is one of the fastest-growing radiotherapies in which neutron beams destroy only boron isotope 10B bearing cells.67 To successfully demonstrate this strategy, the authors modified the aldehyde-bridged nCOPs with amino-PEG to enhance stability and dispersibility under physiological conditions. The PEGylated nCOPs selectively delivered the boron-10 drug within tumor cells in sufficiently high quantities for BNCT. An in vivo study on tumor-bearing mice demonstrated that neutron irradiation destroyed boron-bearing cancer cells to cure the tumor successfully. Tao et al. reported the one-step preparation of perfluorinated nCOPs and PEGylation for oxygen delivery and PDT of hypoxic tumors (Fig. 8b).68 As perfluoro-derived nanoscale POPs (nPOPs) are hydrophobic and non-dispersible in biological media, PEGylation stabilizes the colloidal solution and increases the blood circulation half-life for sufficient accumulation at the tumor site via the EPR effect. The PEGylated nCOPs were loaded with perfluoro-15-crown-5-ether (PFCE) and charged with oxygen to deliver it to the hypoxic tumor. This strategy successfully resulted in an enhanced PDT effect, as demonstrated by in vitro and in vivo experiments.
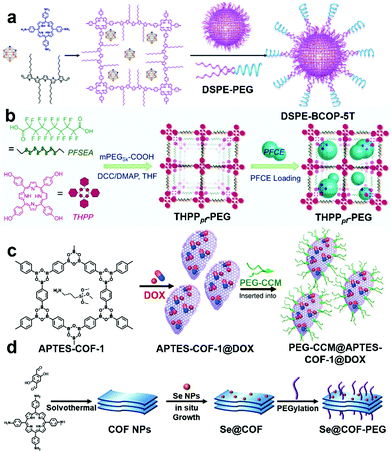 | ||
| Fig. 8 (a) Preparation of carborane-loaded nCOPs through post-synthetic PEGylation for BNCT. Reprinted with permission from ref. 66. Copyright 2020, American Chemical Society. (b) One step preparation of nCOPs and PEGylation and further PFCE loading for oxygen delivery. Reprinted with permission from ref. 68. Copyright 2018, Wiley-VCH. (c) DOX loading in nCOFs and stabilization by PEGylation. Adapted from ref. 69 with permission from Springer Nature, copyright 2018. (d) Preparation of COF nanosheets, Se NP loading and PEGylation. Reproduced with permission from ref. 70. Copyright 2021, The Royal Society of Chemistry. | ||
Zhang et al. in 2018 reported the PSM of a moisture-sensitive nCOF with monosubstituted polyethylene-glycol-curcumin (PEG-CCM) (Fig. 8c).69 The amine-functionalized COFs (APTES-COF-1) were modified via self-assembly with PEG-modified monofunctional curcumin, and were named PEG-CCM@APTES-COF-1. The authors effectively demonstrated high DOX loading, good blood circulation half-life, and selective delivery to tumor sites via in vitro and in vivo studies. PEGylation made the nCOF water dispersible, and the fluorescent curcumin helped track the circulation and delivery of PEG-CCM@APTES-COF-1. This strategy is highly promising for transforming nCOFs into a suitable candidate for cancer diagnosis and therapy. Another advantage of PEGylation of nPOPs is the stabilization of the sensitive nanoparticles on their surfaces. Wan and Wu et al. developed a new class of PSMs of nPOPs by decorating the in situ reduced selenium nanoparticles (Se NPs) (Fig. 8d) on the surface of nCOFs.70 The authors further modified the Se NP decorated nCOFs by PEGylation to make them biocompatible. The Se NPs were stabilized with this strategy, protected from aggregation, and successfully delivered to the tumor site. The modified Se@COF-PEG showed a high blood circulation half-life and sufficient accumulation at the tumor site due to the EPR effect. Under the low power of irradiation, Se NP decorated nCOFs generated ROS to kill the cancer cells in the hypoxic conditions, further inducing mitochondrial dysfunction to enhance cell apoptosis.
3. Conclusions and perspectives
In conclusion, recent studies have shown that the PSMs of POPs have opened a new horizon, especially for biomedical applications. Various PSM strategies, such as covalent modification, metalation, impregnation, immobilization, and surface modifications, have enhanced the efficiency of use of POPs in drug delivery, enzyme delivery, and cancer imaging PDT as well as other biological applications. The advantages of PSM of POPs are summarized as follows:(1) To make non-dispersible POPs applicable in biomedical applications, PSMs of POPs or the use of pre-modified building blocks is essential. However, the pre-modified building blocks with long amphiphilic chains significantly decrease the porosity and crystallinity, so PSM is most suitable for transforming POPs and making them advantageous for biomedical applications.
(2) The POPs can be converted into compatible nanomedicines via covalent modification. The covalently modified POPs are sufficiently stable for cancer therapy and ensure a higher blood circulation time to deliver the drug at the tumor location. This is the principal advantage of modified POPs in drug delivery and cancer therapy over other nanoparticles, which are restricted by covalent modifications.
(3) In another strategy, POPs are modified with covalent and non-covalent bonds with organic sensitizers to boost ROS generation and cancer cell apoptosis by chemo-, photo-, or radio-dynamic therapies. Suitably selected photosensitizers by engineering the band-edge positions substantially enhance the efficiency of dynamic therapies.
(4) To enhance EPR-guided NP accumulation at the tumor site, cell-targeted PSMs of POPs have been thoroughly researched and have shown great potential. However, many challenges still remain to be addressed in clinical trials.
(5) The POPs modified with a photosensitizer or photothermal agents show highly improved efficacy compared to non-modified ones in in vivo PDT and PTT studies. The combination of metals, organic sensitizers, and cancer cell-targeted PSMs will make them promising candidates for clinical trials.
(6) Crystallinity is the primary criterion for defining COFs. Developing large-scale synthesis protocols with sustainable crystallinity still remains a challenge; in contrast, for non-crystalline POPs, scaling-up processes are relatively straightforward. Therefore, the development of large-scale PSM methods will open new prospects for commercial applications of POPs.
PSMs of POPs offer multiple benefits, such as better dispersibility, stability, degradability, sustainable drug loading and delivery, extended blood circulation time, and engineering of band-edge positions for biomedical applications. Future research on post-synthetic modifications should be directed toward developing cancer cell targeting POP NPs to enhance cancer cell apoptosis significantly. Biomolecule modified POPs may have a constructive effect in the in vivo studies by increasing the biocompatibility. New non-toxic building blocks should be explored to develop POP NPs focusing on size optimization, uniformity and greater dispersibility in biological media. More in vivo studies are required to understand the POPs in targeted drug delivery and controlled release at the tumor locations. In recent years, there has been a significant increase in the large-scale synthesis of POPs and COFs. We anticipate that new methods for PSMs of POPs will be created at a similar scale. Therefore, the rapidly growing advanced applications, large-scale synthesis, and PSMs of POPs will soon lead them to be used in clinical trials and other clinical settings.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Basic Science Research Program (NRF-2021R1A2B5B03086313, CSH), the Priority Research Centers Program (NRF-2019R1A6A1A11044070, CSH), CRI project (No. 2018R1A3B1052702, JSK), and the global PhD fellowship (GPF) program (No. 2019H1A2A1074096, JHK) from the National Research Foundation of Korea (NRF) funded by the Ministry of Education. This research was supported by the Korea University Graduate School Junior Fellow Research Grant of JHK.References
- J.-S. M. Lee and A. I. Cooper, Chem. Rev., 2020, 120, 2171–2214 CrossRef CAS PubMed.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Chem. Soc. Rev., 2021, 50, 120–242 RSC.
- D. Taylor, S. J. Dalgarno, Z. Xu and F. Vilela, Chem. Soc. Rev., 2020, 49, 3981–4042 RSC.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- Y. Tian and G. Zhu, Chem. Rev., 2020, 120, 8934–8986 CrossRef CAS PubMed.
- J. Kang, S. Huang, K. Jiang, C. Lu, Z. Chen, J. Zhu, C. Yang, A. Ciesielski, F. Qiu and X. Zhuang, Adv. Funct. Mater., 2020, 30, 2000857 CrossRef CAS.
- B. Zhang, W. Wang, L. Liang, Z. Xu, X. Li and S. Qiao, Coord. Chem. Rev., 2021, 436, 213782 CrossRef CAS.
- C. Dai and B. Liu, Energy Environ. Sci., 2020, 13, 24–52 RSC.
- X. Zhao, B. Chen, W. Dong, S. Wang, Y. Xiang and H. Chen, J. Mater. Chem. A, 2021, 9, 10208–10216 RSC.
- A. H. Alahmed, M. E. Briggs, A. I. Cooper and D. J. Adams, J. Mater. Chem. A, 2019, 7, 549–557 RSC.
- J. L. Segura, S. Royuela and M. Mar Ramos, Chem. Soc. Rev., 2019, 48, 3903–3945 RSC.
- D. W. Kang, M. Kang and C. S. Hong, J. Mater. Chem. A, 2020, 8, 7474–7494 RSC.
- Q. Guan, L.-L. Zhou, W.-Y. Li, Y.-A. Li and Y.-B. Dong, Chem. – Eur. J., 2020, 26, 5583–5591 CrossRef CAS PubMed.
- S. Bhunia, K. A. Deo and A. K. Gaharwar, Adv. Funct. Mater., 2020, 30, 2002046 CrossRef CAS.
- Z. Li and Y.-W. Yang, J. Mater. Chem. B, 2017, 5, 9278–9290 RSC.
- S. M. Cohen, J. Am. Chem. Soc., 2017, 139, 2855–2863 CrossRef CAS PubMed.
- Q. Guan, G.-B. Wang, L.-L. Zhou, W.-Y. Li and Y.-B. Dong, Nanoscale Adv., 2020, 2, 3656–3733 RSC.
- A. Esrafili, A. Wagner, S. Inamdar and A. P. Acharya, Adv. Healthcare Mater., 2021, 10, 2002090 CrossRef CAS PubMed.
- F. Zhao, H. Liu, S. D. R. Mathe, A. Dong and J. Zhang, Nanomaterials, 2018, 8, 15 CrossRef.
- K. Zhao, P. Gong, J. Huang, Y. Huang, D. Wang, J. Peng, D. Shen, X. Zheng, J. You and Z. Liu, Microporous Mesoporous Mater., 2021, 311, 110713 CrossRef CAS.
- Z. Xie, Y. Yan, K. Tang and C.-F. Ding, Talanta, 2022, 236, 12283 CrossRef.
- C. Gao, J. Bai, Y. He, Q. Zheng, W. Ma and Z. Lin, ACS Sustainable Chem. Eng., 2019, 7, 18926–18934 CrossRef CAS.
- P. Gao, K. Tang, R. Lou, X. Liu, R. Wei, N. Li and B. Tang, Anal. Chem., 2021, 93, 12096–12102 CrossRef CAS.
- J.-Y. Yue, X.-L. Ding, L. Wang, R. Yang, J.-S. Bi, Y.-W. Song, P. Yang, Y. Ma and B. Tang, Mater. Chem. Front., 2021, 5, 3859–3866 RSC.
- P. Wang, F. Zhou, C. Zhang, S.-Y. Yin, L. Teng, L. Chen, X.-X. Hu, H.-W. Liu, X. Yin and X.-B. Zhang, Chem. Sci., 2018, 9, 8402–8408 RSC.
- S. Mitra, H. S. Sasmal, T. Kundu, S. Kandambeth, K. Illath, D. Diaz Diaz and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 4513–4520 CrossRef CAS PubMed.
- J. Y. Jang, H. T. T. Duong, S. M. Lee, H. J. Kim, Y.-J. Ko, J. H. Jeong, D. S. Lee, T. Thambi and S. U. Son, Chem. Commun., 2018, 54, 3652–3655 RSC.
- Q. Guan, D.-D. Fu, Y.-A. Li, X.-M. Kong, Z.-Y. Wei, W.-Y. Li, S.-J. Zhang and Y.-B. Dong, iScience, 2019, 14, 180–198 CrossRef CAS PubMed.
- Q. Guan, L.-L. Zhou, Y.-A. Li, W.-Y. Li, S. Wang, C. Song and Y.-B. Dong, ACS Nano, 2019, 13, 13304–13316 CrossRef CAS.
- Q. Guan, L.-L. Zhou, F.-H. Lv, W.-Y. Li, Y.-A. Li and Y.-B. Dong, Angew. Chem., Int. Ed., 2020, 59, 18042–18047 CrossRef CAS.
- S. Zhang, Y. Zheng, H. An, B. Aguila, C.-X. Yang, Y. Dong, W. Xie, P. Cheng, Z. Zhang, Y. Chen and S. Ma, Angew. Chem., Int. Ed., 2018, 57, 16754–16759 CrossRef CAS PubMed.
- W. Jiang, W.-R. Cui, R.-P. Liang and J.-D. Qiu, J. Hazard. Mater., 2021, 413, 125302 CrossRef CAS PubMed.
- Y. Shi, S. Liu, Z. Zhang, Y. Liu and M. Pang, Chem. Commun., 2019, 55, 14315–14318 RSC.
- M. W. Hussain, V. Bhardwaj, A. Giri, A. Chande and A. Patra, Chem. Sci., 2020, 11, 7910–7920 RSC.
- A. Rengaraj, P. Puthiaraj, Y. Haldorai, N. S. Heo, S.-K. Hwang, Y.-K. Han, S. Kwon, W.-S. Ahn and Y. S. Huh, ACS Appl. Mater. Interfaces, 2016, 8, 8947–8955 CrossRef CAS PubMed.
- L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng and Q. Wang, Nanoscale, 2021, 13, 10748–10764 RSC.
- L.-L. Zhou, Q. Guan, W.-Y. Li, Z. Zhang, Y.-A. Li and Y.-B. Dong, Small, 2021, 17, 2101368 CrossRef CAS.
- J. A. Ross and C. M. Kasum, Annu. Rev. Nutr., 2002, 22, 19–34 CrossRef CAS PubMed.
- V. S. Vyas, M. Vishwakarma, I. Moudrakovski, F. Haase, G. Savasci, C. Ochsenfeld, J. P. Spatz and B. V. Lotsch, Adv. Mater., 2016, 28, 8749–8754 CrossRef CAS PubMed.
- Z. Xu, L. Hu, J. Ming, X. Cui, M. Zhang, J. Dou, W. Zhang and B. Zhou, Microporous Mesoporous Mater., 2020, 303, 110259 CrossRef CAS.
- S. Kandambeth, V. Venkatesh, D. B. Shinde, S. Kumari, A. Halder, S. Verma and R. Banerjee, Nat. Commun., 2015, 6, 6786 CrossRef CAS PubMed.
- G. Zhang, Y. Ji, X. Li, X. Wang, M. Song, H. Gou, S. Gao and X. Ji, Adv. Healthcare Mater., 2020, 9, 2000221 CrossRef CAS PubMed.
- Q. Sun, C.-W. Fu, B. Aguila, J. Perman, S. Wang, H.-Y. Huang, F.-S. Xiao and S. Ma, J. Am. Chem. Soc., 2018, 140, 984–992 CrossRef CAS PubMed.
- Q. Sun, B. Aguila, P. C. Lan and S. Ma, Adv. Mater., 2019, 31, 1900008 CrossRef PubMed.
- L. Zhu, G. Liang, C. Guo, M. Xu, M. Wang, C. Wang, Z. Zhang and M. Du, Food Chem., 2022, 366, 130575 CrossRef CAS.
- P. Gao, K. Tang, R. Lou, X. Liu, R. Wei, N. Li and B. Tang, Anal. Chem., 2021, 93, 12096–12102 CrossRef CAS PubMed.
- R. Maleki, M. Khedri, S. Rezvantalab, F. Afsharchi, K. Musaie, S. Shafiee and M.-A. Shahbazi, ChemBioChem, 2021, 22, 2306–2318 CrossRef CAS PubMed.
- W. Li, C.-X. Yang and X.-P. Yan, Chem. Commun., 2017, 53, 11469–11471 RSC.
- M. Wang, M. Hu, J. Liu, C. Guo, D. Peng, Q. Jia, L. He, Z. Zhang and M. Du, Biosens. Bioelectron., 2019, 132, 8–16 CrossRef CAS PubMed.
- M. Hasanzadeh, N. Shadjou and M. de la Guardia, Trends Anal. Chem., 2017, 91, 67–76 CrossRef CAS.
- X. Yan, Y. Song, J. Liu, N. Zhou, C. Zhang, L. He, Z. Zhang and Z. Liu, Biosens. Bioelectron., 2019, 126, 734–742 CrossRef CAS PubMed.
- A. Samui and S. K. Sahu, Microporous Mesoporous Mater., 2020, 291, 109700 CrossRef CAS.
- P. Gao, M. Wang, Y. Chen, W. Pan, P. Zhou, X. Wan, N. Li and B. Tang, Chem. Sci., 2020, 11, 6882–6888 RSC.
- L. G. Ding, S. Wang, B.-J. Yao, F. Li, Y.-A. Li, G.-Y. Zhao and Y.-B. Dong, Adv. Healthcare Mater., 2021, 10, 2001821 CrossRef CAS PubMed.
- D. Wang, Z. Zhang, L. Lin, F. Liu, Y. Wang, Z. Guo, Y. Li, H. Tian and X. Chen, Biomaterials, 2019, 223, 119459 CrossRef CAS PubMed.
- C. Hu, Z. Zhang, S. Liu, X. Liu and M. Pang, ACS Appl. Mater. Interfaces, 2019, 11, 23072–23082 CrossRef CAS PubMed.
- F. Benyettou, G. Das, A. R. Nair, T. Prakasam, D. B. Shinde, S. K. Sharma, J. Whelan, Y. Lalatonne, H. Traboulsi, R. Pasricha, O. Abdullah, R. Jagannathan, Z. Lai, L. Motte, F. Gandara, K. C. Sadler and A. Trabolsi, J. Am. Chem. Soc., 2020, 142, 18782–18794 CrossRef CAS.
- L. Cai, C. Hu, S. Liu, Y. Zhou, Z. Liu and M. Pang, Bioconjugate Chem., 2021, 32, 661–666 CrossRef CAS PubMed.
- T. Zhang, G. Xing, W. Chen and L. Chen, Mater. Chem. Front., 2020, 4, 332–353 RSC.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed.
- L. Bai, S. Z. F. Phua, W. Q. Lim, A. Jana, Z. Luo, H. P. Tham, L. Zhao, Q. Gaoa and Y. Zhao, Chem. Commun., 2016, 52, 4128–4131 RSC.
- H. Wang, W. Zhu, L. Feng, Q. Chen, Y. Chao, Z. Dong and Z. Liu, Nano Res., 2018, 11, 3244–3257 CrossRef CAS.
- R. Xia, X. Zheng, C. Li, X. Yuan, J. Wang, Z. Xie and X. Jing, ACS Nano, 2021, 15, 7638–7648 CrossRef CAS PubMed.
- H. Wang, W. Zhu, J. Liu, Z. Dong and Z. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 14475–14482 CrossRef CAS.
- S. Liu, J. Yang, R. Guo, L. Deng, A. Dong and J. Zhang, Macromol. Rapid Commun., 2020, 41, 1900570 CrossRef CAS PubMed.
- Y. Shi, Q. Fu, J. Li, H. Liu, Z. Zhang, T. Liu and Z. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 55564–55573 CrossRef CAS.
- R. F. Barth, P. Mi and W. Yang, Cancer Commun., 2018, 38, 35 CrossRef PubMed.
- D. Tao, L. Feng, Y. Chao, C. Liang, X. Song, H. Wang, K. Yang and Z. Liu, Adv. Funct. Mater., 2018, 28, 1804901 CrossRef.
- G. Zhang, X. Li, Q. Liao, Y. Liu, K. Xi, W. Huang and X. Jia, Nat. Commun., 2018, 9, 2785 CrossRef PubMed.
- X. Wan, T. Wu, L. Song, W. Pan, N. Li and B. Tang, Chem. Commun., 2021, 57, 6145–6148 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |