Open Access Article
Open Access ArticleDiastereoselective construction of 4-indole substituted chromans bearing a ketal motif through a three-component Friedel–Crafts alkylation/ketalization sequence†
Jiao-Mei Guo‡
,
Wen-Bo Wang‡,
Jia Guo,
Yan-Shuo Zhu,
Xu-Guan Bai,
Shao-Jing Jin,
Qi-Lin Wang * and
Zhan-Wei Bu*
* and
Zhan-Wei Bu*
Institute of Functional Organic Molecular Engineering, College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, China. E-mail: wangqilin@henu.edu.cn; buzhanwei@henu.edu.cn
First published on 25th April 2018
Abstract
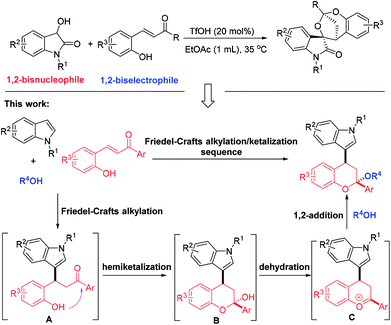
The first TfOH-catalyzed three-component Friedel–Crafts alkylation/ketalization sequence of indoles, alcohols and ortho-hydroxychalcones was developed to afford a wide range of 4-indole substituted chromans bearing a ketal motif in 77–99% yields. Notably, only a simple filtration was needed to purify them. By altering methanol to CHCl3, 2,4-bisindole substituted chroman with the same indole substituent at the C2 and C4 positions was afforded. Moreover, 2,4-bisindole substituted chromans with different indole substituents could be obtained by treatment of 4-indole monosubstituted chromans with another indole molecule.
Introduction
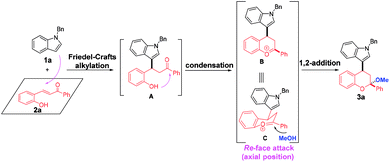
Multi-component reactions (MCRs), which show apparent advantages over conventional reactions with respect to operational simplicity, synthetic efficiency and convergence as well as atom- and step-economy, have emerged as an appealing and versatile synthetic tool to access bioactive heterocycles with structural and functional complexity and diversity.1 Chroman represents a kind of unique and privileged structural unit with wide occurrence in natural products and pharmaceuticals, and exhibit significant biological activities (Fig. 1).2 Thus, establishing convenient and practical strategies for assembly of this skeleton has been the focus of many chemists. On the other hand, indole is another one attractive structural motif prevalent in nature.3 Given the medicinal relevance and the intriguing scaffolds of chroman and indole, integration of them into one complex molecule would contribute greatly to medicinal chemistry, which not only inherit the structures and properties of both biologically active skeletons, but may grant a few new bioactivities. However, only limited methods were available for the synthesis of indole-substituted chromans and the developed approaches mainly involved cycloaddition of 2-vinyl-1H-indole with 3-nitro-2H-chromenes,4 substitution,5 domino Knoevenagel-hetero-Diels–Alder reactions,6 Friedel–Crafts alkylation of indoles with 3-nitro-2H-chromenes,7 Friedel–Crafts alkylation-inspired cyclizations,8 and others.9 Despite these elegant works, many of these methodologies suffer from limitations of substrate availability, synthetic efficiency and product diversity, which may limit their applicability in synthetic chemistry. As a result, development of an efficient and robust method for the rapid synthesis of indole-substituted chromans with diverse and complex structures is highly desirable and challenging.Recently, we have realized a TfOH-catalyzed highly diastereoselective Michael addition/ketalization sequence of 3-hydroxyoxindoles and ortho-hydroxychalcones, where 3-hydroxyoxindoles served as a bisnucleophile to react with competent biselectrophile ortho-hydroxychalcone by Michael addition-initiated tandem reaction (Scheme 1).10 Based on this, we envisioned that a three-component reaction among indole, ortho-hydroxychalcone and alcohol may also take place under suitable conditions, in which both indole and alcohol served as the nucleophiles. As shown in Scheme 1, this domino reaction was proposed to be initiated by a Friedel–Crafts alkylation between indole and ortho-hydroxychalcone to yield intermediate A. Followed by hemiketalization and sequential dehydration, an oxocarbenium ion C was afforded, which was trapped by an alcohol molecule to give the 4-indole substituted chromans bearing a ketal motif. However, there was a competition between indole and alcohol because the oxocarbenium ion C could also be captured by indole to deliver 2,4-bisindole substituted chromans. Thus, extra effort should be paid to control the chemoselectivity.
 | ||
| Scheme 1 Our synthetic design for the construction of 4-indole substituted chromans bearing a ketal motif. | ||
As part of our continuous interest in indole- and spirooxindole-related chemistry,11 herein, we wish to report a TfOH-catalyzed three-component reaction of indoles, ortho-hydroxychalcones and alcohols, which afforded a wide range of 4-indole substituted chromans bearing a ketal motif. In this transformation, the alcohol not only acted as a reactant, but served as a solvent. Notably, all of the products were precipitated from the homogeneous reaction system, and only a filtration was needed to purify them. These characteristics conferred our system with a prominent feature on meeting the requirement of green organic synthesis. Additionally, by switching the solvent to CHCl3, a novel 2,4-bisindole substituted chroman with the same indole substituents was afforded. Also, chromans with different 2,4-indole substituents could be obtained by reacting 4-indole substituted chromans with another distinct indole molecule.
Results and discussion
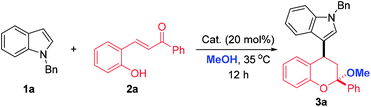
To test the feasibility of our synthetic design, N-benzyl indole 1a and ortho-hydroxychalcone 2a were initially chosen as the model substrates to react in methanol under the catalysis of FeCl3·6H2O. Gratifyingly, this reaction worked well to give the desired 4-indole substituted chroman with a ketal skeleton 3a in 54% yield (Table 1, entry 1). The ketal skeleton was also important structural unit, which not only exhibited significant bioactivities, but also served as a useful synthetic handle for various transformations.12 It should be mentioned that before our work Yin's group reported a similar reaction with iodine as catalyst under refluxing, but totally different products 4-indole chromenes were obtained in moderate yields with limited substrate scope.13 To improve the synthetic efficiency, some other catalysts, including Lewis acids and protonic acids, were examined (Table 1, entries 1–7). Generally, much better results were obtained when conducting the reactions in protic acids and TfOH turned out to be the catalyst of choice, in which the yield of 3a could increase to 97% (Table 1, entry 7). Then, the substrate ratios were evaluated and the results indicated that the ratios of 1a to 2a had some influence on the yield. When an excess of 1a or 2a were used, inferior yields were obtained (Table 1, entries 7 vs. 8–10). As a consequence, the following optimal conditions were recommended: 0.24 mmol 1a and 0.20 mmol 2a with 20 mol% TfOH in 1.0 mL MeOH at 35 °C for 12 h.| Entry | Cat. | Ratio (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) 2a) |
Yield (%) |
|---|---|---|---|
| a Unless otherwise noted, the reaction was carried out with 0.20 mmol of 2a and specified amount of 1a in 1.0 mL MeOH at 35 °C.b Isolated yield obtained by column chromatography.c Isolated yield obtained by filtration of the precipitate. | |||
| 1 | FeCl3·6H2O | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54b |
| 2 | Fe(OTf)3 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25b |
| 3 | Cu(OTf)2 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
n.r. |
| 4 | p-TSA | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
59c |
| 5 | TFA | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78c |
| 6 | MsOH | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70c |
| 7 | TfOH | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97c |
| 8 | TfOH | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96c |
| 9 | TfOH | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92c |
| 10 | TfOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
89c |
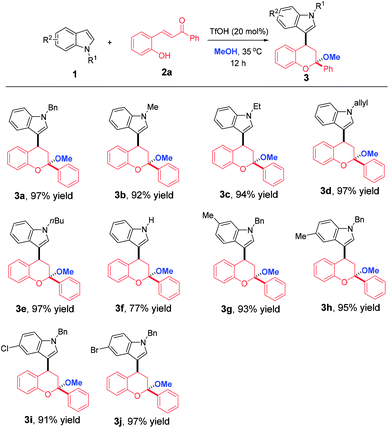
With the optimized reaction conditions in hand, the substrate scope with respect to indoles 1 bearing different substituents was initially explored. As shown in Table 2, the reaction tolerated a wide range of indoles, delivering 3a–j in 77–97% yields. At the outset, the effect of N-substituents was investigated and the results revealed that it had minimal impact on the yield. Remarkably, N–H indole was also a suitable reaction partner, delivering 3f in 77% yield. Subsequently, we turned our attention to study the effect of R2 substituents on the phenyl ring of indoles. When the R2 substituents were varied, irrespective of substitution positions and electronic nature, excellent yields (91–97%) were achieved for products 3g–j.
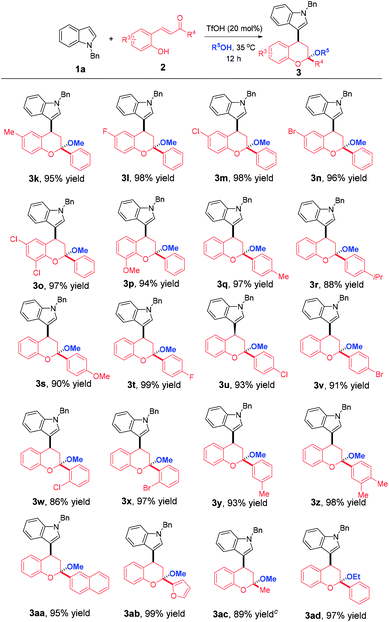
Then, a wide array of ortho-hydroxychalcones with various substitution patterns were employed to further extend the generality of this reaction. As highlighted in Table 3, all reactions proceeded smoothly, leading to the desired products 3k–ad in good to excellent yields. Firstly, ortho-hydroxychalcones with different R3 substitution patterns were examined. All of them participated in this multi-component domino reaction successfully with the formation of 3k–p in 94–98% yields. Afterwards, the R4 substituents effect was evaluated and the results implied that this reaction was amendable to a wide range of ortho-hydroxychalcones bearing different R4 substituents, regardless of the substitution positions and electronic nature, affording 3q–z in 86–99% yields. 2-Naphthyl and 2-furyl substituted ortho-hydroxychalcones were also compatible to generate 3aa and 3ab in 95% and 99% yields, respectively. Not only aromatic rings were tolerated in these reactions, but also a methyl group was successful, allowing the assembly of 3ac in 89% yield.
Besides methanol, ethanol could also participate in this reaction perfectly and the synthesis of 3ad was achieved in 97% yield. However, as the length of chain increases, n-propanol and n-butanol failed to give the desired products due to the more steric hindrance. It was worthy to note that all of the products were precipitated from the original homogeneous reaction system and only a filtration was needed to separate them, which not only greatly simplifies the purification procedure, but also reduces the synthetic cost. Furthermore, the reactions proceeded in a highly diastereoselective manner. Only one diastereoisomer was observed for all cases although the products contained two stereocenters. The structure and the relative configuration of 3o were definitely assigned by X-ray crystallization diffraction.14
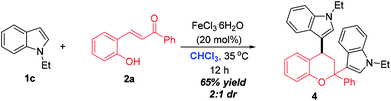
The synthesis of 2,4-bisindole substituted chroman was achieved by performing the reaction of N-ethyl indole 1c and ortho-hydroxychalcone 2a in CHCl3 under the catalysis of FeCl3·6H2O (Scheme 2). This reaction worked well and went completion within 12 h, delivering 4 in 65% yields with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr value.
1 dr value.
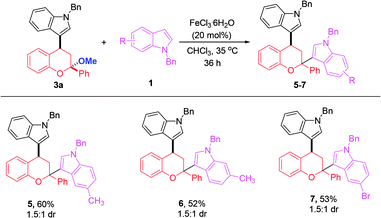
With monoindole substituted chromans bearing a ketal moiety 3, we could also convert them into chromans with two different indole substituents at the C2 and C4 positions. This work not only provides a supplement to the one-pot reaction in CHCl3 system, but also allows access to a highly congested quaternary carbon center linking with an oxygen atom and two aromatic rings. For instance, 3a could react with 5-Me, 6-Me and 5-Br substituted N-benzyl indoles, thus affording the products 5–7 in moderate yields (Scheme 3).
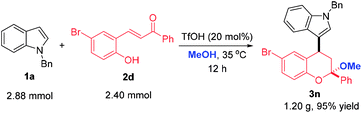
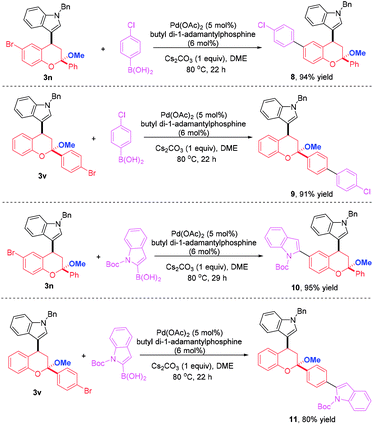
To highlight the synthetic applicability of this method, a gram-scale experiment was carried out with 2.88 mmol 1a and 2.40 mmol 2d. This reaction proceeded successfully to afford 3n in comparable yield (95% yield, Scheme 4). In addition, the presence of a bromide atom in 3n and 3v offer an opportunity for further decoration to generate molecules with more complex and diverse structures. Therefore, derivations of 3n and 3v with 4-chlorophenyl boronic acid were conducted under the condition of a palladium-catalyzed Suzuki coupling and a 4-chlorophenyl group was introduced to afford 8 and 9 in 94% and 91% yields, respectively. Likewise, a indole moiety could also be incorporated by reacting 3n and 3v with 2-indolyl boronic acid, thus giving 10 and 11 in 95% and 80% yields, which not only largely enriches the types of chromans, but provides more novel compounds for bioassay (Scheme 5).
On the basis of the experimental results and previous reports,8,10,11g,h a possible reaction mechanism was proposed to explain the reaction pathway and the stereochemistry (Scheme 6, taking the formation of 3a as an example). First, a Friedel–Crafts alkylation between N-benzyl indole 1a and ortho-hydroxychalcone 2a took place to deliver the intermediate A. Then, an intramolecular condensation occurred with the formation of oxocarbenium B, which was equal with intermediate C. Finally, methanol served as a nucleophile to attack the oxocarbenium from the re-face due to that axial position has more thermodynamic preference, thus affording the corresponding product 3a with more stable structure.
Conclusions
In summary, we have established the first TfOH-catalyzed highly diastereoselective three-component domino Friedel–Crafts alkylation/ketalization reaction of indoles, alcohols and ortho-hydroxychalcones, which provides an efficient and versatile approach for the construction of 4-indole substituted chromans bearing a ketal motif at the C2 position. In this transformation, the alcohol played dual roles: one was a reactant, and the other was a solvent. Moreover, the assembly of 2,4-bisindole substituted chromans and some chemical transformations were accomplished. The salient features of this reaction, including easily available starting materials, mild reaction conditions, simple procedures, broad scope, high yields and excellent diastereoselectivity, rendered our system possible for green synthesis. Further studies to extend the applicability of this method are ongoing in our lab.Experimental
General procedure for the synthesis of 4-indole substituted chromans 3a–ad
To a 5.0 mL vial were successively added indole 1 (0.24 mmol), ortho-hydroxychalcone 2 (0.20 mmol), TfOH (6.0 mg, 0.04 mmol) and 1.0 mL alcohol. The resulting mixture was stirred at 35 °C for 12 h. For all cases, the precipitate was generated from the homogenous reaction system, and only a simple filtration was needed to purify them.1-Benzyl-3-(2-methoxy-2-phenylchroman-4-yl)-1H-indole (3a)
White solid; 86.2 mg, 97% yield; reaction time = 12 h; mp 148.5–149.8 °C; 1H NMR (400 MHz, CDCl3), δ 7.65 (d, J = 8.0 Hz, 2H), 7.42–7.32 (m, 4H), 7.26–6.96 (m, 12H), 6.82 (t, J = 8.0 Hz, 1H), 5.25 (s, 2H), 4.82 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 3.17 (s, 3H), 2.52 (dd, J1 = J2 = 5.6 Hz, 1H), 2.31 (t, J = 13.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.1, 141.2, 137.6, 137.1, 129.1, 128.8, 128.4, 128.2, 127.6, 127.5, 127.4, 126.8, 126.7, 126.5, 126.3, 121.8, 121.3, 120.0, 119.1, 117.1, 117.0, 109.9, 100.7, 50.4, 50.0, 42.8, 30.2. IR (KBr) ν 3441, 3060, 3029, 2958, 2935, 2826, 1608, 1580, 1487, 1456, 1365, 1335, 1154, 1045, 1019, 908, 737, 699 cm−1. HRMS (ESI) calcd for C28H29NNaO2 [M + Na]+ 468.1934, found 468.1936.3-(2-Methoxy-2-phenylchroman-4-yl)-1-methyl-1H-indole (3b)
White solid; 68.3 mg, 92% yield; reaction time = 12 h; mp 71.9–73.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.57–7.55 (m, 2H), 7.31–7.28 (m, 2H), 7.25–7.22 (m, 2H), 7.16 (d, J = 8.0 Hz, 1H), 7.07–7.00 (m, 3H), 6.95–6.86 (m, 2H), 6.81 (s, 1H), 6.72–6.70 (s, 1H), 4.74–4.70 (m, 1H), 3.59 (s, 3H), 3.08 (s, 3H), 2.44–2.39 (m, 1H), 2.24–2.17 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 152.1, 141.2, 137.4, 129.2, 128.5, 128.2, 127.6, 127.4, 127.2, 126.7, 126.3, 121.6, 121.3, 119.9, 118.9, 117.2, 116.4, 109.4, 100.7, 50.4, 42.9, 32.7, 30.2. IR (KBr) ν 3425, 3058, 2935, 2831, 1721, 1612, 1582, 1483, 1452, 1236, 1155, 1045, 1019, 907, 743, 701 cm−1. HRMS (ESI) calcd for C25H23NNaO2 [M + Na]+ 392.1621, found 392.1607.1-Ethyl-3-(2-methoxy-2-phenylchroman-4-yl)-1H-indole (3c)
White solid; 72.2 mg, 94% yield; reaction time = 12 h; mp 113.7–115.2 °C; 1H NMR (400 MHz, CDCl3), δ 7.65 (d, J = 8.0 Hz, 2H), 7.39–7.27 (m, 5H), 7.18–7.09 (m, 3H), 7.04 (d, J = 8.0 Hz, 1H), 6.97–6.95 (m, 2H), 6.80 (t, J = 8.0 Hz, 1H), 4.83 (dd, J1 = J2 = 5.6 Hz, 1H), 4.05 (q, J = 6.4 Hz, 2H), 3.17 (s, 3H), 2.52 (dd, J1 = J2 = 5.6 Hz, 1H), 2.31 (t, J = 12.0 Hz, 1H), 1.38 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 152.2, 141.3, 136.5, 129.3, 128.5, 128.3, 127.6, 127.5, 126.8, 126.4, 125.6, 121.6, 121.4, 120.1, 118.9, 117.2, 116.5, 109.5, 100.8, 50.5, 43.0, 40.9, 30.3, 15.6. IR (KBr) ν 3426, 3056, 2965, 2935, 2832, 1732, 1612, 1582, 1483, 1452, 1233, 1155, 1047, 1019, 908, 743, 703 cm−1. HRMS (ESI) calcd for C26H25NNaO2 [M + Na]+ 406.1778, found 406.1777.1-Allyl-3-(2-methoxy-2-phenylchroman-4-yl)-1H-indole (3d)
White solid; 76.6 mg, 97% yield; reaction time = 12 h; mp 136.1–138.4 °C; 1H NMR (400 MHz, CDCl3), δ 7.57 (d, J = 8.0 Hz, 2H), 7.33–7.17 (m, 5H), 7.11–7.06 (m, 2H), 7.02 (t, J = 8.0 Hz, 1H), 6.94 (t, J = 8.0 Hz, 1H), 6.89 (t, J = 8.0 Hz, 2H), 6.72 (t, J = 8.0 Hz, 1H), 5.91–5.81 (m, 1H), 5.07 (d, J = 10.4 Hz, 1H), 4.95 (d, J = 16.0 Hz, 1H), 4.73 (dd, J1 = J2 = 5.6 Hz, 1H), 4.55 (d, J = 5.2 Hz, 2H), 3.09 (s, 3H), 2.43 (dd, J1 = J2 = 5.6 Hz, 1H), 2.22 (t, J = 13.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.1, 141.2, 136.9, 133.6, 129.2, 128.5, 128.2, 127.6, 127.4, 126.6, 126.4, 126.3, 121.7, 121.3, 120.0, 119.0, 117.3, 117.1, 116.8, 109.8, 100.7, 50.4, 48.8, 42.8, 30.1. IR (KBr) ν 3431, 3059, 2962, 2935, 2832, 1582, 1485, 1455, 1337, 1155, 1048, 1020, 912, 760, 738 cm−1. HRMS (ESI) calcd for C27H25NNaO2 [M + Na]+ 418.1778, found 418.1776.1-Butyl-3-(2-methoxy-2-phenylchroman-4-yl)-1H-indole (3e)
White solid; 80.2 mg, 97% yield; reaction time = 12 h; mp 145.1–146.5 °C; 1H NMR (400 MHz, CDCl3), δ 7.57 (d, J = 8.0 Hz, 2H), 7.31 (t, J = 8.0 Hz, 2H), 7.27–7.20 (m, 3H), 7.11–7.00 (m, 3H), 6.95 (d, J = 8.0 Hz, 1H), 6.89–6.86 (m, 2H), 6.72 (t, J = 8.0 Hz, 1H), 4.73 (dd, J1 = J2 = 8.0 Hz, 1H), 3.95 (t, J = 8.0 Hz, 2H), 3.09 (s, 3H), 2.42 (dd, J1 = J2 = 5.6 Hz, 1H), 2.21 (t, J = 13.2 Hz, 1H), 1.72–1.64 (m, 2H), 1.26–1.14 (m, 2H), 0.82 (t, J = 4.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 152.1, 141.2, 136.7, 129.2, 128.4, 128.2, 127.5, 127.3, 126.7, 126.3, 121.4, 121.3, 119.9, 118.7, 117.1, 116.2, 109.6, 100.7, (one carbon missing in the aromatic region), 50.4, 46.1, 42.9, 32.4, 30.1, 20.3, 13.8. IR (KBr) ν 3431, 3058, 2964, 2933, 2830, 1580, 1482, 1451, 1342, 1154, 1050, 1015, 906, 758, 736 cm−1. HRMS (ESI) calcd for C28H29NNaO2 [M + Na]+ 434.2091, found 434.2098.3-(2-Methoxy-2-phenylchroman-4-yl)-1H-indole (3f)
White solid; 55.0 mg, 77% yield; reaction time = 12 h; mp 188.3–189.4 °C; 1H NMR (400 MHz, DMSO-d6), δ 11.0 (s, 1H), 7.64 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 8.0 Hz, 2H), 7.41–7.35 (m, 3H), 7.17–7.08 (m, 3H), 7.03 (t, J = 8.0 Hz, 1H), 6.87–6.77 (m, 3H), 4.70 (dd, J1 = J2 = 8.0 Hz, 1H), 3.05 (s, 3H), 2.42 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.28 (t, J = 13.2 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 151.8, 140.9, 137.2, 129.0, 128.9, 128.8, 128.0, 127.1, 126.5, 126.4, 124.3, 121.6, 121.4, 119.2, 118.9, 117.4, 115.8, 112.2, 100.6, 50.2, 42.0, 30.1. IR (KBr) ν 3455, 3061, 3030, 2961, 2936, 2833, 1581, 1487, 1451, 1234, 1155, 1046, 1018, 907, 745, 702 cm−1. HRMS (ESI) calcd for C24H21NNaO2 [M + Na]+ 378.1465, found 378.1456.1-Benzyl-3-(2-methoxy-2-phenylchroman-4-yl)-6-methyl-1H-indole (3g)
White solid; 85.5 mg, 93% yield; reaction time = 12 h; mp 142.9–144.8 °C; 1H NMR (400 MHz, CDCl3), δ 7.65 (d, J = 8.0 Hz, 2H), 7.40 (t, J = 8.0 Hz, 2H), 7.34 (d, J = 8.0 Hz, 1H), 7.30–7.16 (m, 5H), 7.10–7.04 (m, 5H), 6.95 (s, 1H), 6.82 (t, J = 4.0 Hz, 2H), 5.23 (s, 2H), 4.78 (dd, J1 = J2 = 4.0 Hz, 1H), 3.17 (s, 3H), 2.50 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.39 (s, 3H), 2.30 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.0, 141.1, 137.8, 137.5, 131.6, 129.1, 128.8, 128.4, 128.2, 127.5, 127.4, 126.7, 126.6, 126.3, 126.1, 125.2, 121.2, 120.8, 119.7, 117.1, 116.9, 109.8, 100.7, 50.4, 49.8, 42.8, 30.2, 21.9. IR (KBr) ν 3433, 3064, 3028, 2937, 2828, 1607, 1580, 1488, 1448, 1233, 1184, 1155, 1045, 1023, 762, 702 cm−1. HRMS (ESI) calcd for C32H29NNaO2 [M + Na]+ 482.2091, found 482.2067.1-Benzyl-3-(2-methoxy-2-phenylchroman-4-yl)-5-methyl-1H-indole (3h)
White solid; 87.2 mg, 95% yield; reaction time = 12 h; mp 181.4–182.8 °C; 1H NMR (400 MHz, CDCl3), δ 7.68–7.65 (m, 2H), 7.42–7.34 (m, 3H), 7.27–7.20 (m, 6H), 7.15–7.07 (m, 5H), 6.97 (d, J = 4.0 Hz, 1H), 6.84 (t, J = 8.0 Hz, 1H), 5.24 (s, 2H), 4.82–4.79 (m, 1H), 3.18 (d, J = 4.0 Hz, 3H), 2.55–2.49 (m, 1H), 2.36 (d, J = 4.0 Hz, 3H), 2.28 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.0, 141.1, 137.7, 135.4, 129.0, 128.7, 128.4, 128.3, 128.2, 127.8, 127.5, 127.4, 126.7, 126.6, 126.3, 123.4, 121.2, 119.4, 117.0, 116.5, 116.4, 109.6, 100.6, 50.3, 50.0, 42.7, 29.9, 21.5. IR (KBr) ν 3425, 3063, 3031, 2933, 1616, 1582, 1487, 1450, 1230, 1162, 1045, 1024, 760, 702 cm−1. HRMS (ESI) calcd for C32H29NNaO2 [M + Na]+ 482.2091, found 482.2100.1-Benzyl-5-chloro-3-(2-methoxy-2-phenylchroman-4-yl)-1H-indole (3i)
White solid; 87.1 mg, 91% yield; reaction time = 12 h; mp 163.9–165.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.66 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 8.0 Hz, 2H), 7.35 (t, J = 8.0 Hz, 2H), 7.27 (t, J = 8.0 Hz, 3H), 7.20 (t, J = 8.0 Hz, 1H), 7.15–7.04 (m, 6H), 7.00 (d, J = 8.0 Hz, 1H), 6.84 (t, J = 8.0 Hz, 1H), 5.24 (s, 2H), 4.77 (dd, J1 = J2 = 8.0 Hz, 1H), 3.17 (s, 3H), 2.49 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.24 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 151.6, 138.8, 137.6, 137.1, 135.2, 129.8, 129.0, 128.8, 127.6, 127.5, 127.3, 126.8, 126.5, 121.8, 121.3, 120.7, 120.1, 119.1, 117.0, 116.8, 109.8, 100.8, one carbon missing in the aromatic region, 50.4, 50.0, 38.9, 29.7. IR (KBr) ν 3429, 3065, 3031, 2960, 2938, 2831, 1580, 1458, 1347, 1236, 1156, 1024, 908, 758, 701 cm−1. HRMS (ESI) calcd for C31H26ClNNaO2 [M + Na]+ 502.1544, found 502.1553.1-Benzyl-5-bromo-3-(2-methoxy-2-phenylchroman-4-yl)-1H-indole (3j)
White solid; 101.8 mg, 97% yield; reaction time = 12 h; mp 163.8–165.4 °C; 1H NMR (400 MHz, CDCl3), δ 7.58 (d, J = 4.0 Hz, 2H), 7.43 (s, 1H), 7.34 (t, J = 8.0 Hz, 2H), 7.28 (d, J = 4.0 Hz, 1H), 7.20–7.11 (m, 5H), 7.02 (d, J = 8.0 Hz, 2H), 6.96 (d, J = 4.0 Hz, 2H), 6.92 (d, J = 8.0 Hz, 2H), 6.77 (t, J = 8.0 Hz, 1H), 5.15 (s, 2H), 4.70 (dd, J1 = J2 = 4.0 Hz, 1H), 3.09 (s, 3H), 2.42 (dd, J1 = J2 = 5.6 Hz, 1H), 2.15 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.0, 140.9, 137.1, 135.6, 129.3, 128.9, 128.8, 128.4, 128.2, 127.9, 127.8, 127.7, 126.7, 126.3, 126.0, 124.7, 122.3, 121.3, 117.3, 116.8, 112.6, 111.4, 100.5, 50.3, 50.2, 42.7, 29.9. IR (KBr) ν 3430, 3067, 3031, 2936, 2835, 1608, 1581, 1477, 1450, 1355, 1308, 1157, 1046, 1022, 907, 759, 701 cm−1. HRMS (ESI) calcd for C31H26BrNNaO2 [M + Na]+ 546.1039, found 546.1036.1-Benzyl-3-(2-methoxy-6-methyl-2-phenylchroman-4-yl)-1H-indole (3k)
White solid; 87.6 mg, 95% yield; reaction time = 12 h; mp 175.0–176.3 °C; 1H NMR (400 MHz, CDCl3), δ 7.66 (t, J = 8.0 Hz, 2H), 7.43–7.38 (m, 3H), 7.35–7.23 (m, 5H), 7.17–7.08 (m, 3H), 7.02 (q, J = 8.0 Hz, 4H), 6.87 (d, J = 8.0 Hz, 1H), 5.27 (d, J = 4.0 Hz, 2H), 4.83–4.76 (m, 1H), 3.17 (d, J = 4.0 Hz, 3H), 2.54–2.47 (m, 1H), 2.33–2.24 (m, 1H), 2.14 (d, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 149.9, 141.2, 137.6, 137.0, 130.4, 129.3, 128.8, 128.4, 128.2, 128.1, 127.6, 127.5, 126.8, 126.7, 126.3, 126.2, 121.7, 120.0, 119.1, 117.3, 116.9, 109.8, 100.5, 50.3, 50.0, 43.1, 30.1, 20.8. IR (KBr) ν 3402, 3029, 2930, 2835, 1608, 1490, 1456, 1341, 1237, 1157, 1045, 927, 736, 708 cm−1. HRMS (ESI) calcd for C32H29NNaO2 [M + Na]+ 482.2091, found 482.2100.1-Benzyl-3-(6-fluoro-2-methoxy-2-phenylchroman-4-yl)-1H-indole (3l)
White solid; 90.7 mg, 98% yield; reaction time = 12 h; mp 148.1–149.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.55 (d, J = 8.0 Hz, 2H), 7.32–7.10 (m, 8H), 7.05–6.89 (m, 6H), 6.77 (d, J = 8.0 Hz, 1H), 6.66 (d, J = 8.0 Hz, 1H), 5.16 (s, 2H), 4.67 (s, 1H), 3.06 (s, 3H), 2.42–2.39 (m, 1H), 2.24–2.17 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 157.5 (d, J = 237.0 Hz, 1C), 148.0 (d, J = 2.0 Hz, 1C), 140.8, 137.5, 132.2, 128.9, 128.5, 128.3, 128.1 (d, J = 7.0 Hz, 1C), 127.7, 127.1, 126.8, 126.7, 126.3, 122.0, 119.9, 119.3, 118.4 (d, J = 8.0 Hz, 1C), 116.4, 115.2 (d, J = 23.0 Hz, 1C), 114.35 (d, J = 24.0 Hz, 1C), 110.0, 100.7, 50.4, 50.2, 42.4, 30.5. IR (KBr) ν 3429, 3038, 2936, 2832, 1615, 1488, 1249, 1187, 1153, 1020, 933, 739, 700 cm−1. HRMS (ESI) calcd for C31H26FNNaO2 [M + Na]+ 486.1840, found 486.1850.1-Benzyl-3-(6-chloro-2-methoxy-2-phenylchroman-4-yl)-1H-indole (3m)
White solid; 93.6 mg, 98% yield; reaction time = 12 h; mp 145.3–147.2 °C; 1H NMR (400 MHz, CDCl3), δ 7.63 (d, J = 8.0 Hz, 2H), 7.43–7.34 (m, 4H), 7.31–7.25 (m, 4H), 7.17–7.09 (m, 4H), 7.04–7.02 (m, 4H), 5.29 (s, 2H), 4.78 (dd, J1 = J2 = 4.0 Hz, 1H), 3.16 (s, 3H), 2.53–2.47 (m, 1H), 2.29 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 150.7, 140.6, 137.5, 137.1, 128.8, 128.7, 128.4, 128.3, 127.7, 127.6, 127.1, 126.8, 126.7, 126.2, 126.1, 121.9, 119.7, 119.2, 118.5, 116.2, 109.9, 100.9, 50.4, 50.0, 42.4, 30.2. IR (KBr) ν 3429, 3058, 3029, 2938, 2836, 1475, 1342, 1247, 1155, 1039, 1019, 909, 742, 705 cm−1. HRMS (ESI) calcd for C31H26ClNNaO2 [M + Na]+ 502.1544, found 502.1546.1-Benzyl-3-(6-bromo-2-methoxy-2-phenylchroman-4-yl)-1H-indole (3n)
White solid; 100.9 mg, 96% yield; reaction time = 12 h; mp 171.1–172.4 °C; 1H NMR (400 MHz, CDCl3), δ 7.62 (d, J = 8.0 Hz, 2H), 7.42–7.25 (m, 10H), 7.14 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.0 Hz, 2H), 7.03–6.97 (m, 2H), 5.28 (s, 2H), 4.78 (dd, J1 = J2 = 4.0 Hz, 1H), 3.15 (s, 3H), 2.50 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.28 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 151.3, 140.6, 137.5, 137.1, 131.6, 130.5, 129.0, 128.8, 128.4, 128.3, 127.7, 127.1, 126.8, 126.8, 126.2, 121.9, 119.7, 119.3, 119.0, 116.2, 113.6, 109.9, 100.9, 50.4, 50.0, 42.5, 30.2. IR (KBr) ν 3427, 3057, 3029, 2938, 2836, 1473, 1342, 1247, 1156, 1039, 1019, 908, 741, 703 cm−1. HRMS (ESI) calcd for C31H26BrNNaO2 [M + Na]+ 546.1039, found 546.1045.1-Benzyl-3-(6,8-dichloro-2-methoxy-2-phenylchroman-4-yl)-1H-indole (3o)
White solid; 100.0 mg, 97% yield; reaction time = 12 h; mp 186.2–187.7 °C; 1H NMR (400 MHz, CDCl3), δ 7.70–7.67 (m, 2H), 7.43 (t, J = 8.0 Hz, 2H), 7.38–7.25 (m, 7H), 7.16 (t, J = 8.0 Hz, 1H), 7.10 (d, J = 8.0 Hz, 2H), 7.03 (t, J = 8.0 Hz, 2H), 6.93–6.90 (m, 1H), 5.29 (s, 2H), 4.79 (dd, J1 = J2 = 4.0 Hz, 1H), 3.15 (s, 3H), 2.60–2.54 (m, 1H), 2.37–2.31 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 146.6, 139.9, 137.4, 137.1, 129.7, 128.8, 128.5, 128.4, 127.9, 127.7, 127.3, 126.9, 126.8, 126.3, 126.2, 125.7, 123.1, 122.0, 119.7, 119.4, 115.7, 110.0, 101.4, 50.5, 50.0, 42.0, 30.5. IR (KBr) ν 3427, 3032, 2938, 2832, 1452, 1341, 1255, 1154, 1021, 974, 922, 741, 699 cm−1. HRMS (ESI) calcd for C31H25Cl2NNaO2 [M + Na]+ 536.1155, found 536.1156.1-Benzyl-3-(2,8-dimethoxy-2-phenylchroman-4-yl)-1H-indole (3p)
White solid; 89.7 mg, 94% yield; reaction time = 12 h; mp 155.1–156.8 °C; 1H NMR (400 MHz, CDCl3), δ 7.61 (d, J = 8.0 Hz, 2H), 7.33–7.12 (m, 8H), 7.01 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 3H), 6.90 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 2H), 6.72 (d, J = 8.0 Hz, 1H), 6.67 (t, J = 8.0 Hz, 1H), 6.58 (d, J = 8.0 Hz, 1H), 5.15 (s, 2H), 4.74 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 3.84 (s, 3H), 3.10 (s, 3H), 2.45 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.24 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 147.8, 140.5, 140.0, 136.5, 135.9, 127.7, 127.3, 127.1, 126.5, 126.3, 125.7, 125.6, 125.3, 120.7, 119.9, 119.3, 118.9, 117.9, 116.1, 109.2, 108.7, 99.5, one carbon missing in the aromatic region, 55.3, 49.3, 48.9, 41.6, 29.2. IR (KBr) ν 3431, 3036, 2936, 2833, 1586, 1467, 1339, 1264, 1212, 1158, 1045, 1023, 945, 738, 704 cm−1. HRMS (ESI) calcd for C32H29NNaO3 [M + Na]+ 498.2040, found 498.2054.1-Benzyl-3-(2-methoxy-2-(p-tolyl)chroman-4-yl)-1H-indole (3q)
White solid; 88.8 mg, 97% yield; reaction time = 12 h; mp 168.2–169.3 °C; 1H NMR (400 MHz, CDCl3), δ 7.46 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 8.0 Hz, 1H), 7.22–7.07 (m, 8H), 7.05–7.00 (m, 3H), 6.98–6.89 (m, 3H), 6.74 (t, J = 8.0 Hz, 1H), 5.19 (s, 2H), 4.73 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 3.09 (s, 3H), 2.43 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.29 (s, 3H), 2.22 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 151.0, 137.1, 136.8, 136.5, 135.9, 128.0, 127.9, 127.7, 127.4, 126.8, 126.5, 126.4, 125.7, 125.6, 125.5, 125.1, 120.7, 120.1, 118.9, 118.0, 116.0, 108.7, 99.6, 49.2, 48.9, 41.7, 29.0, 20.1. IR (KBr) ν 3431, 3030, 2927, 1615, 1453, 1343, 1264, 1222, 1154, 1103, 1023, 977, 908, 812, 732 cm−1. HRMS (ESI) calcd for C32H29NNaO2 [M + Na]+ 482.2091, found 482.2086.1-Benzyl-3-(2-(4-isopropylphenyl)-2-methoxychroman-4-yl)-1H-indole (3r)
White solid; 85.4 mg, 88% yield; reaction time = 12 h; mp 173.5–174.4 °C; 1H NMR (400 MHz, CDCl3), δ 7.48 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 8.0 Hz, 1H), 7.17 (t, J = 8.0 Hz, 6H), 7.12–6.90 (m, 8H), 6.74 (t, J = 8.0 Hz, 1H), 5.18 (s, 2H), 4.73 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 3.11 (s, 3H), 2.88–2.81 (m, 1H), 2.43 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.23 (t, J = 12.0 Hz, 1H), 1.18 (d, J = 8.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 152.1, 148.8, 138.5, 137.6, 137.0, 129.1, 128.8, 128.7, 127.6, 127.5, 127.4, 126.8, 126.7, 126.6, 126.4, 126.2, 121.8, 121.2, 120.0, 119.0, 117.1, 109.8, 100.7, 50.3, 50.0, 42.8, 33.9, 30.1, 24.1. IR (KBr) ν 3435, 3033, 2963, 1582, 1480, 1456, 1340, 1264, 1220, 1156, 1107, 1025, 968, 908, 834, 736 cm−1. HRMS (ESI) calcd for C34H33NNaO2 [M + Na]+ 510.2404, found 510.2407.1-Benzyl-3-(2-methoxy-2-(4-methoxyphenyl)chroman-4-yl)-1H-indole (3s)
White solid; 85.8 mg, 90% yield; reaction time = 12 h; mp 81.1–82.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.50 (t, J = 8.0 Hz, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.19–6.84 (m, 14H), 6.74 (t, J = 8.0 Hz, 1H), 5.19 (s, 2H), 4.74 (s, 1H), 3.74 (d, J = 4.0 Hz, 3H), 3.09 (d, J = 8.0 Hz, 3H), 2.47–2.41 (m, 1H), 2.27–2.18 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 159.5, 152.1, 137.6, 137.0, 133.3, 129.1, 128.8, 127.6, 127.5, 127.5, 127.5, 127.4, 126.8, 126.7, 126.6, 121.8, 121.2, 120.0, 119.1, 117.1, 113.7, 109.9, 100.6, 55.3, 50.2, 50.0, 42.9, 30.2. IR (KBr) ν 3424, 3032, 2933, 2834, 1609, 1582, 1485, 1458, 1337, 1306, 1252, 1171, 1024, 970, 905, 835, 741 cm−1. HRMS (ESI) calcd for C32H29NNaO3 [M + Na]+ 498.2040, found 498.2030.1-Benzyl-3-(2-(4-fluorophenyl)-2-methoxychroman-4-yl)-1H-indole (3t)
White solid; 91.7 mg, 99% yield; reaction time = 12 h; mp 137.9–139.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.53 (t, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 1H), 7.17 (t, J = 8.0 Hz, 4H), 7.12–6.88 (m, 10H), 6.74 (t, J = 8.0 Hz, 1H), 5.16 (s, 2H), 4.72 (dd, J1 = J2 = 4.0 Hz, 1H), 3.07 (s, 3H), 2.41 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.20 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 162.7 (d, J = 245.0 Hz, 1C), 151.9, 137.6, 137.1, 129.1, 128.8, 128.3, 128.2, 127.7, 127.6, 127.4, 126.8, 126.7, 126.4, 121.9, 121.4, 120.0, 119.1, 117.1, 116.9, 115.3 (d, J = 21.0 Hz, 1C), 109.9, 100.4, 50.3, 50.0, 42.9, 30.2. IR (KBr) ν 3427, 3061, 3032, 2935, 2834, 1607, 1507, 1485, 1457, 1337, 1264, 1225, 1155, 1106, 1044, 1021, 908, 838, 809, 738, 698 cm−1. HRMS (ESI) calcd for C31H26FNNaO2 [M + Na]+ 486.1840, found 486.1839.1-Benzyl-3-(2-(4-chlorophenyl)-2-methoxychroman-4-yl)-1H-indole (3u)
White solid; 89.1 mg, 93% yield; reaction time = 12 h; mp 157.4–158.8 °C; 1H NMR (400 MHz, CDCl3), δ 7.50 (d, J = 8.0 Hz, 2H), 7.27 (dd, J1 = J2 = 8.0 Hz, 4H), 7.20–7.14 (m, 4H), 7.12–6.88 (m, 9H), 6.74 (t, J = 8.0 Hz, 1H), 5.16 (s, 2H), 4.72 (dd, J1 = J2 = 4.0 Hz, 1H), 3.06 (s, 3H), 2.39 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.19 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 150.7, 138.6, 136.5, 136.0, 133.0, 128.0, 127.7, 127.5, 127.4, 126.8, 126.5, 126.2, 125.7, 125.6, 125.3, 120.8, 120.3, 118.8, 118.0, 116.0, 115.7, 108.8, 99.2, 49.2, 48.9, 41.6, 29.0. IR (KBr) ν 3431, 3059, 3032, 2931, 2834, 1607, 1585, 1484, 1455, 1341, 1263, 1219, 1156, 1099, 1050, 1019, 969, 910, 815, 731 cm−1. HRMS (ESI) calcd for C31H26ClNNaO2 [M + Na]+ 502.1544, found 502.1555.1-Benzyl-3-(2-(4-bromophenyl)-2-methoxychroman-4-yl)-1H-indole (3v)
White solid; 95.7 mg, 91% yield; reaction time = 12 h; mp 163.4–164.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.44 (s, 4H), 7.25 (d, J = 8.0 Hz, 1H), 7.21–7.14 (m, 4H), 7.10–6.88 (m, 8H), 6.75 (t, J = 8.0 Hz, 1H), 5.18 (s, 2H), 4.72 (dd, J1 = J2 = 4.0 Hz, 1H), 3.07 (s, 3H), 2.39 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.19 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 150.7, 139.2, 136.5, 136.0, 130.5, 128.0, 127.7, 127.1, 126.6, 126.5, 126.2, 125.7, 125.6, 125.3, 121.3, 120.8, 120.3, 118.8, 118.0, 116.0, 115.7, 108.8, 99.2, 49.2, 48.9, 41.5, 29.0. IR (KBr) ν 3433, 3033, 2963, 1585, 1481, 1457, 1339, 1262, 1206, 1158, 1101, 1040, 805, 745 cm−1. HRMS (ESI) calcd for C31H26BrNNaO2 [M + Na]+ 546.1039, found 546.1052.1-Benzyl-3-(2-(2-chlorophenyl)-2-methoxychroman-4-yl)-1H-indole (3w)
White solid; 82.8 mg, 86% yield; reaction time = 12 h; mp 179.3–180.8 °C; 1H NMR (400 MHz, CDCl3), δ 7.89 (d, J = 8.0 Hz, 1H), 7.31 (t, J = 4.0 Hz, 1H), 7.27–7.13 (m, 7H), 7.11–6.97 (m, 7H), 6.91 (d, J = 8.0 Hz, 1H), 6.76 (d, J = 4.0 Hz, 1H), 5.19 (s, 2H), 4.74 (dd, J1 = J2 = 4.0 Hz, 1H), 3.14 (s, 3H), 2.87 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.31–2.23 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 151.6, 137.6, 137.3, 137.1, 132.1, 131.6, 129.7, 129.6, 129.0, 128.8, 127.6, 127.5, 126.9, 126.8, 126.6, 121.8, 121.3, 120.1, 119.1, 117.0, 116.9, 109.9, 100.5, 50.4, 50.0, 38.8, 29.7. IR (KBr) ν 3420, 3060, 2934, 1583, 1486, 1458, 1338, 1264, 1235, 1158, 1043, 1015, 906, 758, 744 cm−1. HRMS (ESI) calcd for C31H26ClNNaO2 [M + Na]+ 502.1544, found 502.1558.1-Benzyl-3-(2-(2-bromophenyl)-2-methoxychroman-4-yl)-1H-indole (3x)
White solid; 101.6 mg, 97% yield; reaction time = 12 h; mp 125.5–127.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.96 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 8.0 Hz, 1H), 7.41–7.36 (m, 2H), 7.31–7.24 (m, 4H), 7.20–7.18 (m, 2H), 7.12 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 4H), 7.06 (t, J = 8.0 Hz, 2H), 6.99 (t, J = 8.0 Hz, 1H), 6.83 (t, J = 8.0 Hz, 1H), 5.28 (s, 2H), 4.81 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 3.22 (s, 3H), 2.92 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.38 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.0, 140.9, 137.1, 135.4, 128.9, 128.8, 128.5, 128.4, 128.2, 128.0, 127.8, 127.7, 126.7, 126.3, 126.0, 124.9, 122.1, 121.3, 119.2, 117.2, 116.8, 110.9, 100.5, 50.3, 50.2, 42.7, 30.0. IR (KBr) ν 3440, 3061, 3030, 2961, 2933, 2829, 1584, 1487, 1458, 1337, 1265, 1235, 1207, 1157, 1117, 1038, 1012, 966, 906, 813, 744 cm−1. HRMS (ESI) calcd for C31H26BrNNaO2 [M + Na]+ 546.1039, found 546.1020.1-Benzyl-3-(2-methoxy-2-(m-tolyl)chroman-4-yl)-1H-indole (3y)
White solid; 85.5 mg, 93% yield; reaction time = 12 h; mp 149.0–150.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.45 (d, J = 8.0 Hz, 2H), 7.27 (d, J = 4.0 Hz, 2H), 7.17–7.10 (m, 6H), 7.03–6.95 (m, 5H), 6.91 (d, J = 12.0 Hz, 2H), 6.72 (t, J = 8.0 Hz, 1H), 5.15 (s, 2H), 4.73 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 3.08 (s, 3H), 2.42 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.27 (s, 3H), 2.21 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 151.0, 137.1, 136.8, 136.5, 135.9, 128.0, 127.9, 127.7, 127.4, 126.7, 126.6, 126.5, 126.4, 125.7, 125.6, 125.5, 125.1, 123.6, 120.7, 120.1, 118.9, 118.0, 116.0, 108.7, 99.6, 49.2, 48.9, 41.7, 29.0, 20.1. IR (KBr) ν 3431, 3032, 2932, 1730, 1611, 1481, 1457, 1340, 1264, 1235, 1157, 1107, 1042, 1023, 971, 906, 816, 742 cm−1. HRMS (ESI) calcd for C32H29NNaO2 [M + Na]+ 482.2091, found 482.2072.1-Benzyl-3-(2-(3,4-dimethylphenyl)-2-methoxychroman-4-yl)-1H-indole (3z)
White solid; 92.9 mg, 98% yield; reaction time = 12 h; mp 123.7–125.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.34 (s, 1H), 7.30 (d, J = 8.0 Hz, 2H), 7.22–7.14 (m, 4H), 7.10–6.89 (m, 9H), 6.72 (t, J = 8.0 Hz, 1H), 5.19 (s, 2H), 4.73 (dd, J1 = J2 = 4.0 Hz, 1H), 3.10 (s, 3H), 2.43 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.25–2.18 (m, 7H); 13C NMR (100 MHz, CDCl3) δ 151.1, 137.5, 136.5, 135.9, 135.5, 135.4, 128.6, 128.0, 127.7, 126.5, 126.4, 126.3, 125.7, 125.6, 125.5, 122.6, 120.7, 120.1, 118.9, 117.9, 116.1, 116.0, 115.9, 108.7, 99.6, 49.2, 48.9, 41.8, 29.0, 18.9, 18.5. IR (KBr) ν 3431, 3057, 3029, 2957, 2930, 2832, 1610, 1580, 1483, 1454, 1337, 1264, 1232, 1156, 1048, 1029, 909, 806, 737 cm−1. HRMS (ESI) calcd for C33H31NNaO2 [M + Na]+ 496.2247, found 496.2248.1-Benzyl-3-(2-methoxy-2-(naphthalen-2-yl)chroman-4-yl)-1H-indole (3aa)
White solid; 94.0 mg, 95% yield; reaction time = 12 h; mp 169.4–170.9 °C; 1H NMR (400 MHz, CDCl3), δ 8.21 (s, 1H), 7.91–7.82 (m, 3H), 7.69 (d, J = 8.0 Hz, 1H), 7.51–7.46 (m, 2H), 7.38 (d, J = 8.0 Hz, 1H), 7.25–7.17 (m, 6H), 7.13–6.96 (m, 6H), 6.84 (t, J = 8.0 Hz, 1H), 5.23 (s, 2H), 4.88 (dd, J1 = J2 = 4.0 Hz, 1H), 3.21 (s, 3H), 2.63 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.37 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.1, 138.6, 137.6, 137.1, 133.2 (2C), 129.2, 128.8, 128.5, 128.3, 127.7, 127.6, 127.5, 126.8, 126.7, 126.6, 126.4, 126.3, 125.8, 124.0, 121.8, 121.4, 120.0, 119.1, 117.2, 117.0, 109.9, 100.8, one carbon missing in the aromatic region, 50.5, 50.0, 42.7, 30.2. IR (KBr) ν 3435, 3056, 3029, 2932, 2835, 1609, 1581, 1452, 1342, 1271, 1226, 1157, 1044, 907, 862, 823, 741, 699 cm−1. HRMS (ESI) calcd for C35H30NO2 [M + H]+ 496.2271, found 496.2281.1-Benzyl-3-(2-(furan-2-yl)-2-methoxychroman-4-yl)-1H-indole (3ab)
White solid; 86.4 mg, 99% yield; reaction time = 12 h; mp 192.7–194.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.41 (s, 1H), 7.32–7.21 (m, 5H), 7.16–7.03 (m, 7H), 6.97 (t, J = 8.0 Hz, 1H), 6.81 (t, J = 8.0 Hz, 1H), 6.63 (d, J = 4.0 Hz, 1H), 6.39 (s, 1H), 5.27 (s, 2H), 4.76 (dd, J1 = J2 = 4.0 Hz, 1H), 3.23 (s, 3H), 2.73 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.40 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.7, 151.4, 142.5, 137.6, 137.1, 129.2, 128.8, 127.6 (2C), 127.3, 126.9, 126.8, 126.4, 121.8, 121.5, 120.1, 119.1, 117.1, 116.8, 110.2, 109.9, 108.5, 97.5, 50.8, 50.0, 39.5, 29.5. IR (KBr) ν 3434, 3038, 2940, 1609, 1584, 1456, 1343, 1265, 1233, 1157, 1039, 1007, 905, 815, 747, 698 cm−1. HRMS (ESI) calcd for C29H26NO3 [M + H]+ 436.1907, found 436.1901.1-Benzyl-3-(2-methoxy-2-methylchroman-4-yl)-1H-indole (3ac)
White solid; 68.3 mg, 89% yield; reaction time = 36 h; mp 158.7–160.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.31–7.19 (m, 5H), 7.17–7.09 (m, 4H), 6.99 (q, J = 8.0 Hz, 3H), 6.91 (d, J = 8.0 Hz, 1H), 6.75 (t, J = 8.0 Hz, 1H), 5.26 (s, 2H), 4.63 (dd, J1 = J2 = 4.0 Hz, 1H), 3.34 (s, 3H), 2.32–2.23 (m, 2H), 1.57 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 152.2, 137.7, 137.2, 129.1, 128.8, 127.7, 127.4, 127.3, 126.9, 126.8, 126.3, 121.8, 120.9, 120.1, 119.1, 117.5, 116.8, 109.9, 98.7, 50.0, 49.2, 40.7, 29.6, 23.2. IR (KBr) ν 3433, 3055, 3032, 2985, 2935, 1609, 1582, 1480, 1456, 1345, 1225, 1191, 1065, 885, 806, 742 cm−1. HRMS (ESI) calcd for C26H26NO2 [M + H]+ 384.1958, found 384.1957.1-Benzyl-3-(2-ethoxy-2-phenylchroman-4-yl)-1H-indole (3ad)
White solid; 89.1 mg, 97% yield; reaction time = 12 h; mp 154.5–155.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.67 (t, J = 8.0 Hz, 2H), 7.41–6.98 (m, 16H), 6.82 (d, J = 8.0 Hz, 1H), 5.27 (s, 2H), 4.86 (s, 1H), 3.54 (d, J = 8.0 Hz, 1H), 3.39 (d, J = 8.0 Hz, 1H), 2.51–2.25 (m, 2H), 1.09 (t, J = 8.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 152.3, 142.1, 137.6, 137.1, 129.0, 128.8, 128.4, 128.1, 127.6, 127.5, 127.4, 126.8, 126.7, 126.5, 126.2, 121.8, 121.1, 120.1, 119.0, 117.1, 117.0, 109.8, 100.6, 58.5, 50.0, 42.9, 30.2, 15.4. IR (KBr) ν 3428, 3062, 3031, 2975, 2929, 2886, 1611, 1581, 1487, 1456, 1337, 1264, 1233, 1161, 1058, 1018, 931, 896, 748, 702 cm−1. HRMS (ESI) calcd for C32H29NNaO2 [M + Na]+ 482.2089, found 482.2091.General procedure for the synthesis of 4 bearing two same indole substituents
To a 5.0 mL vial were successively added indole 1c (0.44 mmol), ortho-hydroxychalcone 2a (0.20 mmol), FeCl3·6H2O (10.8 mg, 0.04 mmol) and 1.0 mL CHCl3. The resulting mixture was stirred at 35 °C till almost full consumption of 2a monitored by thin layer chromatography, and then the reaction mixture was directly subjected to flash column chromatography on silica gel (petroleum ether/ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 80
1 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the corresponding products 4 in 65% yield with 2
1) to afford the corresponding products 4 in 65% yield with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
3,3′-(2-Phenylchromane-2,4-diyl)bis(1-ethyl-1H-indole) (4)
White solid; 64.5 mg, 65% yield; reaction time = 12 h; dr = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; mp 87.8–88.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.75–7.61 (m, 1H), 7.50–7.45 (m, 2H), 7.25–6.81 (m, 14H), 6.73–6.60 (m, 2H), 4.32–3.90 (m, 5H), 3.24–3.09 (m, 1H), 2.93–2.77 (m, 1H), 1.33–1.24 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 153.2, 144.9, 135.7, 135.2, 128.1, 127.3, 126.9, 126.6, 126.3, 125.8, 125.2, 125.1, 124.9, 124.6, 124.2, 124.0, 120.5, 120.0, 119.5, 119.0, 118.8, 118.3, 117.7, 116.1, 115.9, 108.3, 79.5, 39.8, 39.7, 30.6, 29.7, 14.4, 14.3. IR (KBr) ν 3428, 3055, 2975, 2932, 2878, 1610, 1581, 1546, 1477, 1456, 1346, 1232, 906, 742, 706 cm−1. HRMS (ESI) calcd for C35H32N2NaO [M + Na]+ 519.2407, found 519.2402.
1; mp 87.8–88.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.75–7.61 (m, 1H), 7.50–7.45 (m, 2H), 7.25–6.81 (m, 14H), 6.73–6.60 (m, 2H), 4.32–3.90 (m, 5H), 3.24–3.09 (m, 1H), 2.93–2.77 (m, 1H), 1.33–1.24 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 153.2, 144.9, 135.7, 135.2, 128.1, 127.3, 126.9, 126.6, 126.3, 125.8, 125.2, 125.1, 124.9, 124.6, 124.2, 124.0, 120.5, 120.0, 119.5, 119.0, 118.8, 118.3, 117.7, 116.1, 115.9, 108.3, 79.5, 39.8, 39.7, 30.6, 29.7, 14.4, 14.3. IR (KBr) ν 3428, 3055, 2975, 2932, 2878, 1610, 1581, 1546, 1477, 1456, 1346, 1232, 906, 742, 706 cm−1. HRMS (ESI) calcd for C35H32N2NaO [M + Na]+ 519.2407, found 519.2402.
General procedure for the synthesis of 5–7 bearing two different indole substituents
To a 5.0 mL vial were successively added mono-indole substituted chroman 3a (0.10 mmol), substituted indole (0.11 mmol), FeCl3·6H2O (5.4 mg, 0.02 mmol) and 1.0 mL CHCl3. The resulting mixture was stirred at 35 °C for 36 h, and then the reaction mixture was directly subjected to flash column chromatography on silica gel (petroleum ether/ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 80
1 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the corresponding products 5–7. Among them, the two isomers of 7 could be separated by gel column chromatography, while for 5–6, they could not be separated. The dr value was determined by 1H NMR.
1) to afford the corresponding products 5–7. Among them, the two isomers of 7 could be separated by gel column chromatography, while for 5–6, they could not be separated. The dr value was determined by 1H NMR.
1-Benzyl-3-(4-(1-benzyl-1H-indol-3-yl)-2-phenylchroman-2-yl)-5-methyl-1H-indole (5)
White solid; 38.3 mg, 60% yield; reaction time = 36 h; dr = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; mp 107.3–108.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.52 (d, J = 8.0 Hz, 2H), 7.24–6.80 (m, 25H), 6.63 (t, J = 8.0 Hz, 1H), 5.12 (dd, J1 = 12.0 Hz, J2 = 16.0 Hz, 4H), 4.34–3.98 (m, 1H), 3.28–3.07 (m, 1H), 2.94–2.74 (m, 1H), 2.28 (dd, J1 = J2 = 4.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 153.4, 142.9, 136.9, 136.7, 136.6, 136.4, 128.3, 127.8, 127.7, 127.5, 127.2, 126.8, 126.6, 126.5, 126.3, 126.0, 125.8, 125.7, 125.6, 125.5, 125.4, 125.2, 124.8, 124.6, 122.7, 122.5, 120.3, 119.9, 116.2, 116.0, 108.9, 108.7, 119.3, 118.2, 80.2, 57.6, 49.1, 39.3, 30.8, 20.7. IR (KBr) ν 3432, 3058, 3030, 2923, 2860, 1610, 1582, 1485, 1451, 1302, 1234, 1176, 1102, 1030, 907, 799, 739, 701 cm−1. HRMS (ESI) calcd for C46H38N2NaO [M + Na]+ 657.2876, found 657.2871.
1; mp 107.3–108.9 °C; 1H NMR (400 MHz, CDCl3), δ 7.52 (d, J = 8.0 Hz, 2H), 7.24–6.80 (m, 25H), 6.63 (t, J = 8.0 Hz, 1H), 5.12 (dd, J1 = 12.0 Hz, J2 = 16.0 Hz, 4H), 4.34–3.98 (m, 1H), 3.28–3.07 (m, 1H), 2.94–2.74 (m, 1H), 2.28 (dd, J1 = J2 = 4.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 153.4, 142.9, 136.9, 136.7, 136.6, 136.4, 128.3, 127.8, 127.7, 127.5, 127.2, 126.8, 126.6, 126.5, 126.3, 126.0, 125.8, 125.7, 125.6, 125.5, 125.4, 125.2, 124.8, 124.6, 122.7, 122.5, 120.3, 119.9, 116.2, 116.0, 108.9, 108.7, 119.3, 118.2, 80.2, 57.6, 49.1, 39.3, 30.8, 20.7. IR (KBr) ν 3432, 3058, 3030, 2923, 2860, 1610, 1582, 1485, 1451, 1302, 1234, 1176, 1102, 1030, 907, 799, 739, 701 cm−1. HRMS (ESI) calcd for C46H38N2NaO [M + Na]+ 657.2876, found 657.2871.
1-Benzyl-3-(4-(1-benzyl-1H-indol-3-yl)-2-phenylchroman-2-yl)-6-methyl-1H-indole (6)
White solid; 32.9 mg, 52% yield; reaction time = 36 h; dr = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; mp 106.6–108.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.63–7.49 (m, 3H), 7.24–6.73 (m, 24H), 6.63 (dd, J1 = J2 = 4.0 Hz, 1H), 5.17–5.07 (m, 4H), 4.34–3.94 (m, 1H), 3.22–3.03 (m, 1H), 2.92–2.74 (m, 1H), 2.30 (dd, J1 = J2 = 4.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 154.3, 146.0, 143.9, 137.8, 137.7, 137.4, 136.9, 131.9, 131.7, 129.2, 128.8, 128.4, 128.1, 127.7, 127.6, 127.3, 127.1, 126.9, 126.8, 126.7, 126.6, 126.3, 126.1, 125.6, 123.9, 121.9, 121.8, 121.5, 120.9, 120.2, 119.8, 117.7, 117.0, 109.8, 80.6, 58.5, 49.8, 40.4, 30.8, 21.9. IR (KBr) ν 3433, 3059, 3030, 2923, 2861, 1612, 1583, 1478, 1454, 1300, 1235, 1174, 1106, 1029, 906, 802, 736, 702 cm−1. HRMS (ESI) calcd for C46H38N2NaO [M + Na]+ 657.2876, found 657.2866.
1; mp 106.6–108.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.63–7.49 (m, 3H), 7.24–6.73 (m, 24H), 6.63 (dd, J1 = J2 = 4.0 Hz, 1H), 5.17–5.07 (m, 4H), 4.34–3.94 (m, 1H), 3.22–3.03 (m, 1H), 2.92–2.74 (m, 1H), 2.30 (dd, J1 = J2 = 4.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 154.3, 146.0, 143.9, 137.8, 137.7, 137.4, 136.9, 131.9, 131.7, 129.2, 128.8, 128.4, 128.1, 127.7, 127.6, 127.3, 127.1, 126.9, 126.8, 126.7, 126.6, 126.3, 126.1, 125.6, 123.9, 121.9, 121.8, 121.5, 120.9, 120.2, 119.8, 117.7, 117.0, 109.8, 80.6, 58.5, 49.8, 40.4, 30.8, 21.9. IR (KBr) ν 3433, 3059, 3030, 2923, 2861, 1612, 1583, 1478, 1454, 1300, 1235, 1174, 1106, 1029, 906, 802, 736, 702 cm−1. HRMS (ESI) calcd for C46H38N2NaO [M + Na]+ 657.2876, found 657.2866.
1-Benzyl-3-(4-(1-benzyl-1H-indol-3-yl)-2-phenylchroman-2-yl)-5-bromo-1H-indole (7)
White solid; 37.5 mg, 53% yield; reaction time = 36 h; dr = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; mp 215.7–212.3 °C (major isomer), 219.8–221.1 °C (minor isomer); 1H NMR (400 MHz, CDCl3) for major isomer δ 7.88 (s, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.28–7.01 (m, 17H), 6.92 (t, J = 8.0 Hz, 5H), 6.84 (d, J = 8.0 Hz, 1H), 6.73 (s, 1H), 6.65 (d, J = 4.0 Hz, 1H), 5.17 (s, 2H), 5.06 (s, 2H), 4.01 (t, J = 4.0 Hz, 1H), 3.13 (t, J = 12.0 Hz, 1H), 2.92 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) for major isomer δ 154.0, 143.4, 137.6, 137.0, 136.8, 136.0, 129.2, 128.9, 128.8, 128.6, 127.9, 127.8, 127.7, 127.6, 127.2, 127.1, 126.8, 126.7, 126.6, 126.5, 126.2, 125.4, 125.0, 124.1, 121.9, 121.1, 120.5, 119.9, 119.2, 117.4, 117.1, 113.2, 111.5, 109.9, 80.3, 58.6, 50.3, 40.4, 30.7. 1H NMR (400 MHz, CDCl3) for minor isomer δ 7.81 (s, 1H), 7.46 (d, J = 8.0 Hz, 2H), 7.18–7.11 (m, 12H), 7.08–6.98 (m, 6H), 6.96–6.92 (m, 2H), 6.89–6.86 (m, 3H), 6.81 (s, 1H), 6.63 (t, J = 8.0 Hz, 1H), 5.15 (d, J = 4.0 Hz, 4H), 4.35 (dd, J1 = J2 = 8.0 Hz, 1H), 3.01 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.73 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) for minor isomer δ 153.1, 144.5, 136.5, 135.8, 134.7, 128.1, 127.9, 127.8, 127.7, 127.5, 127.2, 126.9, 126.7, 126.6, 126.5, 126.3, 125.7, 125.5, 125.4, 125.3, 124.8, 124.2, 123.9, 122.6, 120.8, 119.4, 118.5, 118.2, 116.6, 116.4, 116.1, 112.2, 110.3, 108.8, 79.6, 57.4, 48.9, 41.0, 30.6. IR (KBr) for major isomer ν 3429, 3059, 3031, 2924, 2860, 1610, 1548, 1457, 1346, 1232, 1178, 1103, 1035, 906, 797, 735, 698 cm−1. IR (KBr) for minor isomer ν 3434, 3060, 3031, 2958, 2926, 2861, 1723, 1608, 1582, 1546, 1456, 1389, 1340, 1298, 1260, 1234, 1179, 1075, 1025, 800, 736, 698 cm−1. HRMS (ESI) calcd for C45H36BrN2O [M + H]+ 699.2006, found 699.1997.
1; mp 215.7–212.3 °C (major isomer), 219.8–221.1 °C (minor isomer); 1H NMR (400 MHz, CDCl3) for major isomer δ 7.88 (s, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.28–7.01 (m, 17H), 6.92 (t, J = 8.0 Hz, 5H), 6.84 (d, J = 8.0 Hz, 1H), 6.73 (s, 1H), 6.65 (d, J = 4.0 Hz, 1H), 5.17 (s, 2H), 5.06 (s, 2H), 4.01 (t, J = 4.0 Hz, 1H), 3.13 (t, J = 12.0 Hz, 1H), 2.92 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) for major isomer δ 154.0, 143.4, 137.6, 137.0, 136.8, 136.0, 129.2, 128.9, 128.8, 128.6, 127.9, 127.8, 127.7, 127.6, 127.2, 127.1, 126.8, 126.7, 126.6, 126.5, 126.2, 125.4, 125.0, 124.1, 121.9, 121.1, 120.5, 119.9, 119.2, 117.4, 117.1, 113.2, 111.5, 109.9, 80.3, 58.6, 50.3, 40.4, 30.7. 1H NMR (400 MHz, CDCl3) for minor isomer δ 7.81 (s, 1H), 7.46 (d, J = 8.0 Hz, 2H), 7.18–7.11 (m, 12H), 7.08–6.98 (m, 6H), 6.96–6.92 (m, 2H), 6.89–6.86 (m, 3H), 6.81 (s, 1H), 6.63 (t, J = 8.0 Hz, 1H), 5.15 (d, J = 4.0 Hz, 4H), 4.35 (dd, J1 = J2 = 8.0 Hz, 1H), 3.01 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.73 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) for minor isomer δ 153.1, 144.5, 136.5, 135.8, 134.7, 128.1, 127.9, 127.8, 127.7, 127.5, 127.2, 126.9, 126.7, 126.6, 126.5, 126.3, 125.7, 125.5, 125.4, 125.3, 124.8, 124.2, 123.9, 122.6, 120.8, 119.4, 118.5, 118.2, 116.6, 116.4, 116.1, 112.2, 110.3, 108.8, 79.6, 57.4, 48.9, 41.0, 30.6. IR (KBr) for major isomer ν 3429, 3059, 3031, 2924, 2860, 1610, 1548, 1457, 1346, 1232, 1178, 1103, 1035, 906, 797, 735, 698 cm−1. IR (KBr) for minor isomer ν 3434, 3060, 3031, 2958, 2926, 2861, 1723, 1608, 1582, 1546, 1456, 1389, 1340, 1298, 1260, 1234, 1179, 1075, 1025, 800, 736, 698 cm−1. HRMS (ESI) calcd for C45H36BrN2O [M + H]+ 699.2006, found 699.1997.
General procedure for the synthesis of 8
Under nitrogen atmosphere, compound 3n (104.9 mg, 0.20 mmol), 4-chlorophenyl boronic acid (1.5 equiv.), Cs2CO3 (2.0 equiv.), Pd(OAc)2 (0.05 equiv.) and butyl di-1-adamantylphosphine (0.06 equiv.) were successively added to a 15 mL dried tube, followed by adding 2.0 mL DME. The resulting mixture was stirred at 80 °C for 22 h till almost full consumption of 3n monitored by thin layer chromatography, and then the reaction mixture was directly subjected to flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford the corresponding product 8.1-Benzyl-3-(6-(4-chlorophenyl)-2-methoxy-2-phenylchroman-4-yl)-1H-indole (8)
White solid obtained by column chromatography (petroleum ether/ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 104.7 mg, 94% yield; reaction time = 22 h; mp 194.8–196.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.66 (d, J = 8.0 Hz, 2H), 7.44–7.31 (m, 5H), 7.27–7.15 (m, 10H), 7.12 (t, J = 8.0 Hz, 1H), 7.04–6.97 (m, 4H), 5.22 (s, 2H), 4.89 (dd, J1 = J2 = 8.0 Hz, 1H), 3.19 (s, 3H), 2.57 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.30 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.2, 140.9, 139.6, 137.7, 137.1, 133.0, 132.5, 128.8, 128.7, 128.5, 128.3, 128.0, 127.7, 127.4, 127.0, 126.8, 126.7, 126.3 (2C), 122.0, 119.8, 119.2, 117.8, 117.0, 109.9, 100.9, one carbon missing in the aromatic region, 50.5, 49.9, 43.1, 30.3. IR (KBr) ν 3432, 3031, 2961, 2931, 1610, 1476, 1337, 1251, 1209, 1156, 1042, 1015, 969, 909, 815, 735, 699 cm−1. HRMS (ESI) calcd for C37H31ClNO2 [M + H]+ 556.2038, found 556.2041.
1); 104.7 mg, 94% yield; reaction time = 22 h; mp 194.8–196.1 °C; 1H NMR (400 MHz, CDCl3), δ 7.66 (d, J = 8.0 Hz, 2H), 7.44–7.31 (m, 5H), 7.27–7.15 (m, 10H), 7.12 (t, J = 8.0 Hz, 1H), 7.04–6.97 (m, 4H), 5.22 (s, 2H), 4.89 (dd, J1 = J2 = 8.0 Hz, 1H), 3.19 (s, 3H), 2.57 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.30 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.2, 140.9, 139.6, 137.7, 137.1, 133.0, 132.5, 128.8, 128.7, 128.5, 128.3, 128.0, 127.7, 127.4, 127.0, 126.8, 126.7, 126.3 (2C), 122.0, 119.8, 119.2, 117.8, 117.0, 109.9, 100.9, one carbon missing in the aromatic region, 50.5, 49.9, 43.1, 30.3. IR (KBr) ν 3432, 3031, 2961, 2931, 1610, 1476, 1337, 1251, 1209, 1156, 1042, 1015, 969, 909, 815, 735, 699 cm−1. HRMS (ESI) calcd for C37H31ClNO2 [M + H]+ 556.2038, found 556.2041.
General procedure for the synthesis of 9
Under nitrogen atmosphere, compound 3v (104.9 mg, 0.20 mmol), 4-chlorophenyl boronic acid (1.5 equiv.), Cs2CO3 (2.0 equiv.), Pd(OAc)2 (0.05 equiv.) and butyl di-1-adamantylphosphine (0.06 equiv.) were successively added to a 15 mL dried tube, followed by adding 2.0 mL DME. The resulting mixture was stirred at 80 °C for 22 h till almost full consumption of 3v monitored by thin layer chromatography, and then the reaction mixture was directly subjected to flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford the corresponding product 9.1-Benzyl-3-(2-(4′-chloro-[1,1′-biphenyl]-4-yl)-2-methoxychroman-4-yl)-1H-indole (9)
White solid obtained by column chromatography (petroleum ether/ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 100.8 mg, 91% yield; reaction time = 22 h; mp 222.8–223.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.64 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 8.0 Hz, 2H), 7.30 (t, J = 8.0 Hz, 3H), 7.18–7.09 (m, 5H), 7.04–6.89 (m, 7H), 6.75 (t, J = 8.0 Hz, 1H), 5.17 (s, 2H), 4.76 (dd, J1 = J2 = 4.0 Hz, 1H), 3.13 (s, 3H), 2.46 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.25 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.0, 140.6, 139.8, 139.2, 137.6, 137.1, 133.6, 129.1, 129.0, 128.8, 128.4, 127.6 (2C), 127.4, 127.0, 126.9, 126.8, 126.7, 126.5, 121.8, 121.3, 120.0, 119.1, 117.1, 117.0, 109.9, 100.6, 50.4, 50.0, 42.8, 30.2. IR (KBr) ν 3434, 3032, 2962, 2931, 1610, 1581, 1480, 1457, 1388, 1345, 1297, 1263, 1225, 1154, 1100, 1040, 1017, 967, 906, 852, 816 cm−1. HRMS (ESI) calcd for C37H31ClNO2 [M + H]+ 556.2038, found 556.2042.
1); 100.8 mg, 91% yield; reaction time = 22 h; mp 222.8–223.6 °C; 1H NMR (400 MHz, CDCl3), δ 7.64 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 8.0 Hz, 2H), 7.30 (t, J = 8.0 Hz, 3H), 7.18–7.09 (m, 5H), 7.04–6.89 (m, 7H), 6.75 (t, J = 8.0 Hz, 1H), 5.17 (s, 2H), 4.76 (dd, J1 = J2 = 4.0 Hz, 1H), 3.13 (s, 3H), 2.46 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.25 (t, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 152.0, 140.6, 139.8, 139.2, 137.6, 137.1, 133.6, 129.1, 129.0, 128.8, 128.4, 127.6 (2C), 127.4, 127.0, 126.9, 126.8, 126.7, 126.5, 121.8, 121.3, 120.0, 119.1, 117.1, 117.0, 109.9, 100.6, 50.4, 50.0, 42.8, 30.2. IR (KBr) ν 3434, 3032, 2962, 2931, 1610, 1581, 1480, 1457, 1388, 1345, 1297, 1263, 1225, 1154, 1100, 1040, 1017, 967, 906, 852, 816 cm−1. HRMS (ESI) calcd for C37H31ClNO2 [M + H]+ 556.2038, found 556.2042.
General procedure for the synthesis of 10
Under nitrogen atmosphere, compound 3n (104.9 mg, 0.20 mmol), 2-indolyl boronic acid (1.7 equiv.), Cs2CO3 (2.0 equiv.), Pd(OAc)2 (0.05 equiv.) and butyl di-1-adamantylphosphine (0.06 equiv.) were successively added to a 15 mL dried tube, followed by adding 2.0 mL DME. The resulting mixture was stirred at 80 °C for 29 h till almost full consumption of 3n monitored by thin layer chromatography, and then the reaction mixture was directly subjected to flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford the corresponding product 10.tert-Butyl 2-(4-(1-benzyl-1H-indol-3-yl)-2-methoxy-2-phenylchroman-6-yl)-1H-indole-1-carboxylate (10)
White solid obtained by column chromatography (petroleum ether/ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 80
1 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 124.9 mg, 95% yield; reaction time = 29 h; mp 205.4–206.9 °C; 1H NMR (400 MHz, CDCl3), δ 8.14 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 2H), 7.45–7.33 (m, 5H), 7.27–7.14 (m, 7H), 7.11–7.04 (m, 5H), 7.01 (s, 1H), 6.96 (t, J = 8.0 Hz, 1H), 6.32 (s, 1H), 5.23 (s, 2H), 4.84 (dd, J1 = J2 = 4.0 Hz, 1H), 3.17 (s, 3H), 2.55 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.36 (t, J = 12.0 Hz, 1H), 1.32 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 151.9, 150.3, 140.9, 140.7, 137.5, 137.3, 137.2, 129.4, 129.2, 128.8, 128.4, 128.3, 128.2, 127.9, 127.6, 127.2, 126.8, 126.7, 126.3, 126.0, 124.0, 122.8, 121.9, 120.2, 119.8, 119.1, 116.7, 116.6, 115.2, 109.9, 109.3, 100.9, 83.1, 50.3, 49.9, 42.9, 30.4, 27.7. IR (KBr) ν 3425, 3058, 2974, 2933, 1726, 1610, 1486, 1455, 1365, 1330, 1257, 1217, 1161, 1126, 1042, 1022, 907, 813, 739, 703 cm−1. HRMS (ESI) calcd for C44H41N2O4 [M + H]+ 661.3061, found 661.3060.
1); 124.9 mg, 95% yield; reaction time = 29 h; mp 205.4–206.9 °C; 1H NMR (400 MHz, CDCl3), δ 8.14 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 2H), 7.45–7.33 (m, 5H), 7.27–7.14 (m, 7H), 7.11–7.04 (m, 5H), 7.01 (s, 1H), 6.96 (t, J = 8.0 Hz, 1H), 6.32 (s, 1H), 5.23 (s, 2H), 4.84 (dd, J1 = J2 = 4.0 Hz, 1H), 3.17 (s, 3H), 2.55 (dd, J1 = 8.0 Hz, J2 = 4.0 Hz, 1H), 2.36 (t, J = 12.0 Hz, 1H), 1.32 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 151.9, 150.3, 140.9, 140.7, 137.5, 137.3, 137.2, 129.4, 129.2, 128.8, 128.4, 128.3, 128.2, 127.9, 127.6, 127.2, 126.8, 126.7, 126.3, 126.0, 124.0, 122.8, 121.9, 120.2, 119.8, 119.1, 116.7, 116.6, 115.2, 109.9, 109.3, 100.9, 83.1, 50.3, 49.9, 42.9, 30.4, 27.7. IR (KBr) ν 3425, 3058, 2974, 2933, 1726, 1610, 1486, 1455, 1365, 1330, 1257, 1217, 1161, 1126, 1042, 1022, 907, 813, 739, 703 cm−1. HRMS (ESI) calcd for C44H41N2O4 [M + H]+ 661.3061, found 661.3060.
General procedure for the synthesis of 11
Under nitrogen atmosphere, compound 3v (104.9 mg, 0.20 mmol), 2-indolyl boronic acid (1.8 equiv.), Cs2CO3 (2.0 equiv.), Pd(OAc)2 (0.05 equiv.) and butyl di-1-adamantylphosphine (0.06 equiv.) were successively added to a 15 mL dried tube, followed by adding 2.0 mL DME. The resulting mixture was stirred at 80 °C for 22 h till almost full consumption of 3v monitored by thin layer chromatography, and then the reaction mixture was directly subjected to flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford the corresponding product 11.tert-Butyl 2-(4-(1-benzyl-1H-indol-3-yl)-2-methoxychroman-2-yl)phenyl-1H-indole-1-carboxylate (11)
White solid obtained by column chromatography (petroleum ether/ethyl acetate = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 80
1 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 105.8 mg, 80% yield; reaction time = 22 h; mp 229.8–230.9 °C; 1H NMR (400 MHz, CDCl3), δ 8.23 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.38 (d, J = 8.0 Hz, 1H), 7.35–6.98 (m, 14H), 6.83 (t, J = 8.0 Hz, 1H), 6.56 (s, 1H), 5.26 (s, 2H), 4.85 (dd, J1 = J2 = 4.0 Hz, 1H), 3.22 (s, 3H), 2.55 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.32 (t, J = 12.0 Hz, 1H), 1.28 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 152.0, 150.2, 140.6, 140.1, 137.6 (2C), 137.1, 134.9, 129.3, 129.1, 128.8, 127.7, 127.6, 127.5, 126.9, 126.7, 126.6, 125.8, 124.5, 123.0, 121.9, 121.3, 120.5, 120.0, 119.1, 117.1, 117.0, 115.3, 110.3, 109.9, 100.6, one carbon missing in the aromatic region, 83.5, 50.4, 50.0, 42.8, 30.2, 27.7. IR (KBr) ν 3427, 3071, 3033, 2979, 2935, 1721, 1583, 1456, 1363, 1330, 1264, 1227, 1161, 1037, 967, 905, 849, 816, 745 cm−1. HRMS (ESI) calcd for C44H41N2O4 [M + H]+ 661.3061, found 661.3074.
1); 105.8 mg, 80% yield; reaction time = 22 h; mp 229.8–230.9 °C; 1H NMR (400 MHz, CDCl3), δ 8.23 (d, J = 8.0 Hz, 1H), 7.69 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.38 (d, J = 8.0 Hz, 1H), 7.35–6.98 (m, 14H), 6.83 (t, J = 8.0 Hz, 1H), 6.56 (s, 1H), 5.26 (s, 2H), 4.85 (dd, J1 = J2 = 4.0 Hz, 1H), 3.22 (s, 3H), 2.55 (dd, J1 = 4.0 Hz, J2 = 8.0 Hz, 1H), 2.32 (t, J = 12.0 Hz, 1H), 1.28 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 152.0, 150.2, 140.6, 140.1, 137.6 (2C), 137.1, 134.9, 129.3, 129.1, 128.8, 127.7, 127.6, 127.5, 126.9, 126.7, 126.6, 125.8, 124.5, 123.0, 121.9, 121.3, 120.5, 120.0, 119.1, 117.1, 117.0, 115.3, 110.3, 109.9, 100.6, one carbon missing in the aromatic region, 83.5, 50.4, 50.0, 42.8, 30.2, 27.7. IR (KBr) ν 3427, 3071, 3033, 2979, 2935, 1721, 1583, 1456, 1363, 1330, 1264, 1227, 1161, 1037, 967, 905, 849, 816, 745 cm−1. HRMS (ESI) calcd for C44H41N2O4 [M + H]+ 661.3061, found 661.3074.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the financial support from National Natural Science Foundation of China (No. U1504206), the Foundation of He'nan Educational Committee (No. 18A150002), Henan University (No. yqpy20170008) and Kaifeng City.Notes and references
- For selected reviews: (a) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef PubMed; (b) J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300 CrossRef CAS PubMed; (c) B. Jiang, T. Rajale, W. Wever, S.-J. Tu and G.-G. Li, Chem.–Asian J., 2010, 5, 2318 CrossRef CAS PubMed; (d) J. Wu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156 CrossRef PubMed; (e) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 1958 RSC; (f) S. Hassan and T. J. J. Müller, Adv. Synth. Catal., 2015, 357, 617 CrossRef CAS.
- For selected reviews: (a) R. Pratap and V. J. Ram, Chem. Rev., 2014, 114, 10476 CrossRef CAS PubMedFor selected examples: (b) R. Hiessböck, C. Wolf, E. Richter, M. Hitzler, P. Chiba, M. Kratzel and G. Ecker, J. Med. Chem., 1999, 42, 1921 CrossRef PubMed; (c) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, C.-Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939 CrossRef CAS; (d) B. M. Trost, H.-C. Shen and J.-P. Surivet, J. Am. Chem. Soc., 2004, 126, 12565 CrossRef CAS PubMed; (e) S. Kumar, S. Deshpande, V. Chandra, S. Kitchlu, A. Dwivedi, V. L. Nayak, R. Konwar, Y. S. Prabhakar and D. P. Sahu, Bioorg. Med. Chem., 2009, 17, 6832 CrossRef CAS PubMed; (f) J. M. Ketcham, I. Volchkov, T.-Y. Chen, P. M. Blumberg, N. Kedei, N. E. Lewin and M. J. Krische, J. Am. Chem. Soc., 2016, 138, 13415 CrossRef CAS PubMed; (g) M. Kumar, P. Chauhan, A. Valkonen, K. Rissanen and D. Enders, Org. Lett., 2017, 19, 3025 CrossRef CAS PubMed.
- For selected reviews: (a) J. E. Saxton, Nat. Prod. Rep., 1997, 14, 559 RSC; (b) M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC; (c) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (d) S. E. O'Connor and J. J. Maresh, Nat. Prod. Rep., 2006, 23, 532 RSC; (e) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (f) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC.
- F. Tian, C. Xiao, H.-G. Cheng, W. Wu, K.-R. Ding and W.-J. Xiao, Chem.–Asian J., 2012, 7, 493 CrossRef PubMed.
- (a) S.-Y. Wang and S.-J. Ji, Synlett, 2007, 2222 CAS; (b) S.-Y. Wang and S.-J. Ji, Synth. Commun., 2008, 38, 465 CrossRef CAS; (c) H.-R. Li, W.-J. Li, W.-P. Liu, Z.-H. He and Z.-P. Li, Angew. Chem., Int. Ed., 2011, 50, 2975 CrossRef CAS PubMed.
- (a) K. C. Majumdar, A. Taher and K. Ray, Tetrahedron Lett., 2009, 50, 3889 CrossRef CAS; (b) M. Kiamehr and F. M. Moghaddam, Tetrahedron Lett., 2009, 50, 6723 CrossRef CAS; (c) K. C. Majumdar, A. Taher and S. Ponra, Tetrahedron Lett., 2010, 51, 147 CrossRef CAS; (d) M. Jha, S. Guy and T.-Y. Chou, Tetrahedron Lett., 2011, 52, 4337 CrossRef CAS.
- (a) P. M. Habib, V. Kavala, B. R. Raju, C.-W. Kuo, W.-C. Huang and C.-F. Yao, Eur. J. Org. Chem., 2009, 4503 CrossRef CAS; (b) Y. Jia, W. Yang and D.-M. Du, Org. Biomol. Chem., 2012, 10, 4739 RSC.
- (a) S. H. Gwon, S. Kim and S. G. Kim, Bull. Korean Chem. Soc., 2011, 32, 4163 CrossRef CAS; (b) C. Li, F.-L. Liu, Y.-Q. Zou, L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Synthesis, 2013, 45, 601 CrossRef CAS; (c) J.-H. Peng and D.-M. Du, Beilstein J. Org. Chem., 2013, 9, 1210 CrossRef CAS PubMed; (d) X.-Y. Hao, L.-L. Lin, F. Tan, S.-L. Ge, X.-H. Liu and X.-M. Feng, Org. Chem. Front., 2017, 4, 1647 RSC.
- (a) H.-F. Li, J.-Y. Yang, Y.-H. Liu and Y.-Z. Li, J. Org. Chem., 2009, 74, 6797 CrossRef CAS PubMed; (b) A. Taheri, B.-B. Lai, J. Yang, J. Zhang and Y.-L. Gu, Tetrahedron, 2016, 72, 479 CrossRef CAS.
- Y.-S. Zhu, J. Zhou, S.-J. Jin, H.-H. Dong, J.-M. Guo, X.-G. Bai, Q.-L. Wang and Z.-W. Bu, Chem. Commun., 2017, 53, 11201 RSC.
- (a) Q.-L. Wang, L. Peng, F.-Y. Wang, M.-L. Zhang, L.-N. Jia, F. Tian, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2013, 49, 9422 RSC; (b) Q.-L. Wang, T. Cai, J. Zhou, F. Tian, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2015, 51, 10726 RSC; (c) W.-B. Wang, Y.-S. Zhu, S.-Q. Guo, Q.-L. Wang and Z.-W. Bu, Org. Biomol. Chem., 2016, 14, 4420 RSC; (d) Y.-S. Zhu, W.-B. Wang, B.-B. Yuan, Y.-N. Li, Q.-L. Wang and Z.-W. Bu, Org. Biomol. Chem., 2017, 15, 984 RSC; (e) Y.-S. Zhu, B.-B. Yuan, J.-M. Guo, S.-J. Jin, H.-H. Dong, Q.-L. Wang and Z.-W. Bu, J. Org. Chem., 2017, 82, 5669 CrossRef CAS PubMed; (f) S.-J. Jin, J.-M. Guo, Y.-S. Zhu, Q.-L. Wang and Z.-W. Bu, Org. Biomol. Chem., 2017, 15, 8729 RSC; (g) Y.-S. Zhu, J. Guo, S.-J. Jin, J.-M. Guo, X.-G. Bai, Q.-L. Wang and Z.-W. Bu, Org. Biomol. Chem., 2018, 16, 1751 RSC; (h) J.-M. Guo, X.-G. Bai, Q.-L. Wang and Z.-W. Bu, J. Org. Chem., 2018, 83, 3679 CrossRef CAS PubMed.
- For selected reviews: (a) J.-G. Jun, Synlett, 2003, 1759 CrossRef CAS; (b) N. A. Harry, S. Saranya, K. K. Krishnan and G. Anilkumar, Asian J. Org. Chem., 2017, 6, 945 CrossRef CAS; (c) R. Quach, D. P. Furkert and M. A. Brimble, Org. Biomol. Chem., 2017, 15, 3098 RSC.
- G.-D. Yin, L. Fan, T.-B. Ren, C.-Y. Zheng, Q. Tao, A.-X. Wu and N.-F. She, Org. Biomol. Chem., 2012, 10, 8877 CAS.
- CCDC 1557519 (3o).
Footnotes |
| † Electronic supplementary information (ESI) available: CCDC 1557519. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra02481b |
| ‡ These two authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |