Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Neocuproine-functionalized silica-coated magnetic nanoparticles for extraction of copper(II) from aqueous solution†‡
Ashfaq
Afsar
a,
Laurence M.
Harwood
*a,
Michael J.
Hudson
a,
Mark E.
Hodson
b and
Elizabeth J.
Shaw
c
aSchool of Chemistry, University of Reading, Whiteknights, Reading, Berkshire RG6 6AD, UK. E-mail: l.m.harwood@reading.ac.uk
bEnvironment Department, University of York, Heslington, York, YO10 5DD, UK
cDepartment of Geography and Environmental Science, University of Reading, Whiteknights, Reading, Berkshire RG6 6AD, UK
First published on 27th May 2014
Abstract
Neocuproine has been covalently bound to silica-coated maghemite (γ-Fe2O3) magnetic nanoparticles (MNPs) by a phenyl ether linkage. The resulting MNPs are able to remove Cu(II) from 12 ppm aqueous solution with an extraction efficiency of up to 99% at pH 2.
Magnetic nanoparticles (MNPs) combine high surface area with ease of separation1 and the use of iron oxide particles has opened fascinating separation applications.2–7 However, iron oxide based MNPs in combination with complexing agents cannot be used in acidic media, which would dissolve the particles.8 A way to solve this problem is to use silica to provide a chemically unreactive surface whilst not affecting the core.8 Furthermore, the free Si–OH surface groups can allow effective covalent binding of organic functional groups onto the surface of the SiO2-coated MNPs. Commonly, surface modification with alkoxysilanes of the general formula X–(CH2)n–Si(OR)3, is used where X represents the head-group functionality, (CH2)n that acts as a flexible spacer, and Si(OR)3 the anchor group that can attach to the free Si–OH groups on the surface of the MNPs.9,10 The present work combines the stability of SiO2-coated MNPs and the complexing power of neocuproine11 in order to study the capabilities of such materials for the effective removal of Cu(II) from aqueous solutions at different pH.
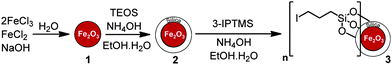
Iron oxide (γ-Fe2O3) MNPs were prepared by co-precipitation8,12–14 from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aqueous mixture of FeCl2 and FeCl3 with sodium hydroxide. The external silica layer was then coated onto the surface of the MNPs by a sol–gel method using tetraethyl orthosilicate (TEOS).8,12–14 After this, the silica surface was further modified with 3-iodopropyltrimethoxysilane (3-IPTMS) (Scheme 1).
2 aqueous mixture of FeCl2 and FeCl3 with sodium hydroxide. The external silica layer was then coated onto the surface of the MNPs by a sol–gel method using tetraethyl orthosilicate (TEOS).8,12–14 After this, the silica surface was further modified with 3-iodopropyltrimethoxysilane (3-IPTMS) (Scheme 1).
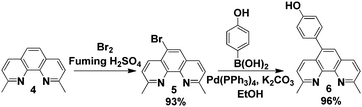
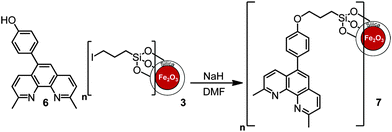
In order to introduce functionality at the 5-position of neocuproine 4, bromination15,16 was carried out with bromine (0.6 equivalents) in the presence of fuming H2SO4 (20% SO3). Replacement of the bromine with a 4-hydroxyphenol linking group was successfully achieved via Suzuki coupling17 with 4-hydroxyphenylboronic acid (Scheme 2). This phenol functionalized ligand could then be immobilized onto the MNPs by nucleophilic substitution of the iodo-substituent (Scheme 3).
Each functionalization step of the MNPs was followed by infrared spectroscopy. Fig. 1 depicts the FT-IR spectra of uncoated (γ-Fe2O3) MNPs 1, SiO2-coated MNPs 2 and iodoalkyl-functionalized SiO2-coated MNPs 3. The γ-Fe2O3 MNPs 1 cause two strong absorptions at 630 and 580 cm−1. The introduction of silica on the surface of the γ-Fe2O3 MNPs 1 results in an additional absorption band at 1080 cm−1 owing to Si–O stretching. After functionalization with 3-iodopropyltrimethoxysilane, bands at 2930 cm−1 and 688 cm−1 were observed, assigned to the C–H stretching and C–I stretching modes of iodoalkyl-functionalized SiO2-coated MNPs 3, respectively.
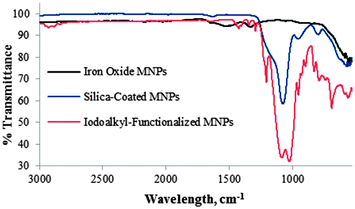 | ||
| Fig. 1 FR-IR spectra of Fe2O31, SiO2-coated Fe2O32, and iodoalkyl-functionalized SiO2-coated MNPs 3. | ||
The FT-IR spectra shown in Fig. 2 demonstrate a clear distinction between iodoalkyl-functionalized SiO2-coated MNPs 3 and neocuproine-functionalized SiO2-coated MNPs 7. Absence of the C–I stretching absorption at 688 cm−1 and presence of bands at 1500–1600 cm−1 owing to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic vibrations are indicative of the covalent incorporation of neocuproine onto the MNPs.
C aromatic vibrations are indicative of the covalent incorporation of neocuproine onto the MNPs.
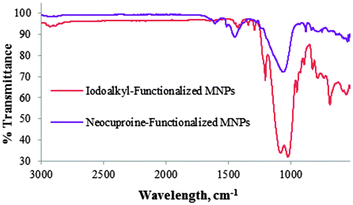 | ||
| Fig. 2 FR-IR spectra of iodoalkyl-functionalized SiO2-coated MNPs 3 and neocuproine-functionalized SiO2 coated MNPs 7. | ||
The unfunctionalized γ-Fe2O3 MNPs 1 were characterized by X-ray powder diffraction18 and were found to consist of pure maghemite or γ-Fe2O3;19 whereas diffraction data of the composite nanoparticles after modification with silica did not show any well defined peaks (ESI‡), indicating that the silica coating is thick enough to interfere with the iron oxide diffraction, or the iron oxide is more amorphous than before (smaller particles, smaller diffracting domains or more disordered structure) allowing the silica pattern to dominate.
Representative TEM images of γ-Fe2O3 MNPs 1, iodoalkyl-functionalized SiO2-coated MNPs 3 and neocuproine-functionalized SiO2-coated MNPs 7, shown in Fig. 3, were recorded on the particles dispersed in methanol and dried on a copper grid at room temperature.
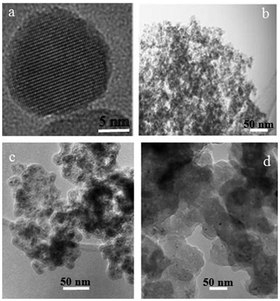 | ||
| Fig. 3 TEM images of (a, b) γ-Fe2O3 MNPs 1, (c) iodoalkyl-functionalized SiO2-coated MNPs 3 and (d) neocuproine functionalized MNPs 7. | ||
Fig. 3(a) shows the predominantly spherical morphology of the γ-Fe2O3 MNPs 1 with an average diameter of ca. 15 nm. These tended to form aggregates as shown in Fig. 3(b). In the case of iodoalkyl-functionalized SiO2-coated MNPs 3 [Fig. 3(c)], the diameter of the particles was found to be ca. 20–25 nm, while Fig. 3(d) reveals that neocuproine-functionalized SiO2-coated MNPs 7 have diameter of ca. 50–55 nm.
To complement the TEM images that only provide information on the size of the MNP cores, DLS measurements (ESI‡) were also carried out. This indicated the γ-Fe2O3 MNPs 1, iodoalkyl-functionalized SiO2-coated MNPs 3 and neocuproine-functionalized SiO2-coated MNPs 7 to have narrow particle size distributions and average diameter values of 15, 48, and 72 nm, respectively. The values measured for the iodoalkyl-functionalized SiO2-coated MNPs 3 and neocuproine-functionalized SiO2-coated MNPs 7 are larger compared to the TEM images; this phenomenon has been observed previously.20,21
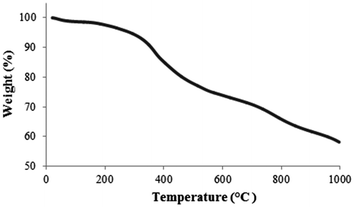
The organic content on the MNPs was investigated using thermal gravimetric analysis (TGA) under nitrogen. The weight loss for the SiO2-coated MNPs 2 was about 2.5% over the temperature range from 60–200 °C, presumably due to the loss of residual water; whereas the TGA curve of iodoalkyl-functionalized SiO2-coated MNPs 3 showed a sharp weight loss at 250–300 °C, proposed to correspond to the loss of iodoalkyl coating (ESI‡). The TGA curve (Fig. 4) of neocuproine-functionalized SiO2-coated MNPs 7 shows three weight-loss steps. Below 200 °C, the weight loss is quite small, probably resulting from the removal of absorbed water. After that, there is a significant weight loss from 300–500 °C corresponding to the decomposition of the organic components. From this, it can be estimated that the amount of neocuproine bound onto the MNPs is about ∼15% w/w (ESI‡). Further weight loss at 700–900 °C can be attributed to the formation of iron carbide.22,23
The extraction of Cu(II) from aqueous media was tested by weighing about 12 mg of functionalized MNPs 7 into plastic tubes containing 10 mL solutions of 12 ppm of Cu(II) at different pH. The mixtures were sonicated for 5 min and then shaken overnight. A neodymium permanent magnet was placed for 60 seconds beneath the tube to move the MNPs to the tube wall allowing the supernatant liquid to be decanted. After separation, the supernatant was subjected to quantitative elemental analysis by atomic absorption spectrometry.
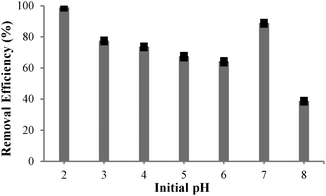
Acidity of an aqueous solution is known to exert a profound influence on extraction efficiency of various ligands.5 The effect of solution pH on Cu(II) extraction was investigated in the range pH 2–8. As shown in Fig. 5, close to 99% of Cu(II) was removed at pH 2 (initial concentration 12.33 ppm, final concentration 0.13 ppm); although the extraction efficiency decreased with increasing the pH. The lowest pH was then selected for kinetic studies where extraction was effectively complete after 5 min (ESI‡).
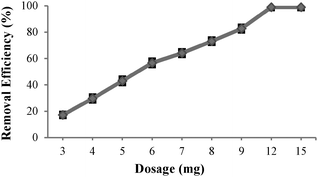
The percentage of Cu(II) extraction from a 12 ppm initial concentration with different amounts of neocuproine-functionalized MNPs 7 at pH 2 is presented in Fig. 6 and shows an almost linear correlation of Cu(II) extraction with the amount of MNPs 7 added.
To assess the chelating effect of the bound neocuproine in functionalized MNPs 7, the extraction efficiencies of SiO2-coated Fe2O3 MNPs 2 and iodoalkyl-functionalized SiO2-coated MNPs 3 were also investigated at pH 2 and compared with the extraction efficiency of neocuproine functionalized MNPs 7 (Table 1).
| Removal (%) | ||
|---|---|---|
| SiO2-coated MNPs | Iodoalkyl-functionalized SiO2-coated MNPs | Neocuproine functionalized MNPs |
| 17 | 30 | 99 |
The extraction efficiencies of SiO2-coated Fe2O3 MNPs 2 and iodoalkyl-functionalized SiO2-coated MNPs 3 were found to be 17% and 30%, respectively; compared to the 99% extraction efficiency observed for neocuproine functionalized MNPs 7. Thus, the majority of the extraction capacity of functionalized MNPs 7 is due to the bound neocuproine.
In summary, neocuproine functionalized MNPs 7 have been prepared and characterised. These MNPs have the advantages of high surface area and their ability to be separated from aqueous media magnetically. Their ability to extract Cu(II) from aqueous media has been investigated at varying pH, with the maximum extraction efficiency of Cu(II) by the MNPs being found to be 99% at pH 2. Extraction was complete within 5 min and about 12 mg of these MNPs were able to alter the concentration of Cu(II) in 10 mL of solution from 12.33 ppm to 0.13 ppm.
The authors acknowledge the EPSRC for financial support (A.A.). Use of the Chemical Analysis Facility (CAF) and Centre for Advanced Microscopy (CfAM) at the University of Reading is gratefully acknowledged. We also would like to thank Mr Michael Andrews, Dr Peter Harris and Miss Anne Dudley for their assistance with X-ray diffraction (XRD), Transmission Electron Microscopy (TEM) and Atomic Absorption (AA) measurements, respectively.
Notes and references
- A.-F. Ngomsik, A. Bee, D. Talbot and G. Cote, Sep. Purif. Technol., 2012, 86, 1–8 CrossRef CAS PubMed.
- Y. Sun, X. Ding, Z. Zheng, X. Cheng, X. Hu and Y. Peng, Chem. Commun., 2006, 2765–2767 RSC.
- F. M. Koehler, M. Rossier, M. Waelle, E. K. Athanassiou, L. K. Limbach, R. N. Grass, D. Gunther and W. J. Stark, Chem. Commun., 2009, 4862–4864 RSC.
- X. Liu, Q. Hu, Z. Fang, X. Zhang and B. Zhang, Langmuir, 2009, 25, 3–8 CrossRef CAS PubMed.
- Y. Liu, M. Chen and H. Yongmei, Chem. Eng. J., 2013, 218, 46–54 CrossRef CAS PubMed.
- M. Kaur, A. Johnson, G. Tian, W. Jiang, L. Rao, A. Paszczynski and Y. Qiang, Nano Energy, 2013, 2, 124–132 CrossRef CAS PubMed.
- M. H. Mashhadizadeh and Z. Karami, J. Hazard. Mater., 2011, 190, 1023–1029 CrossRef CAS PubMed.
- J. H. Jang and H. B. Lim, Microchem. J., 2010, 94, 148–158 CrossRef CAS PubMed.
- I. J. Bruce and T. Sen, Langmuir, 2005, 21, 7029–7035 CrossRef CAS PubMed.
- M. Yamaura, R. L. Camilo, L. C. Sampaio, M. A. Macêdo, M. Nakamura and H. E. Toma, J. Magn. Magn. Mater., 2004, 279, 210–217 CrossRef CAS PubMed.
- P. Ashtari, K. Wang, X. Yang and S. J. Ahmadi, Anal. Chim. Acta, 2009, 646, 123–127 CrossRef CAS PubMed.
- A. L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson and M. Simonoff, ACS Nano, 2008, 2, 847–856 CrossRef CAS PubMed.
- M. Nazrul Islam, L. Van Phong, J.-R. Jeong and C. Kim, Thin Solid Films, 2011, 519, 8277–8279 CrossRef PubMed.
- F. W. Zhang, Z. Z. Zhu, Z. P. Dong, Z. K. Cui, H. B. Wang, W. Q. Hu, P. Zhao, P. Wang, S. Y. Wei, R. Li and J. T. Ma, Microchem. J., 2011, 98, 328–333 CrossRef CAS PubMed.
- J. Mlochowski, Rocz. Chem., 1974, 48, 2145–2155 CAS.
- A. Afsar, D. M. Laventine, L. M. Harwood, M. J. Hudson and A. Geist, Chem. Commun., 2013, 49, 8534–8536 RSC.
- J. P. W. Eggert, U. Luning and C. Nather, Eur. J. Org. Chem., 2005, 1107–1112 CrossRef CAS.
- A. Afsar, D. M. Laventine, L. M. Harwood, M. J. Hudson and A. Geist, Heterocycles, 2014, 88, 613–620 CrossRef CAS PubMed.
- T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. B. Na, J. Am. Chem. Soc., 2001, 123, 12798–12801 CrossRef CAS PubMed.
- L. Sun, C. Huang, T. Gong and S. Zhou, Mater. Sci. Eng., C, 2010, 30, 583–589 CrossRef CAS PubMed.
- J. Lim, S. Yeap, H. Che and S. Low, Nanoscale Res. Lett., 2013, 8, 381 CrossRef PubMed.
- R. Snovski, J. Grinblat, M.-T. Sougrati, J.-C. Jumas and S. Margel, J. Magn. Magn. Mater., 2014, 349, 35–44 CrossRef CAS PubMed.
- M. Sharma, S. Mantri and D. Bahadur, J. Magn. Magn. Mater., 2012, 324, 3975–3980 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Richard Taylor, a colleague and valued friend, to celebrate his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental details and characterization of compounds. See DOI: 10.1039/c4cc01250j |
| This journal is © The Royal Society of Chemistry 2014 |