Comparative study of sonication-assisted liquid phase exfoliation of six layered coordination polymers†
Jonas
Gosch‡
 a,
Kevin
Synnatschke‡
a,
Kevin
Synnatschke‡
 b,
Norbert
Stock
b,
Norbert
Stock
 *a and
Claudia
Backes
*a and
Claudia
Backes
 *c
*c
aChristian-Albrechts-Universität zu Kiel, Max-Eyth-Straße 2, Kiel 24118, Germany
bUniversity of Dublin, Trinity College, Dublin 2, SNIAM Building, Ireland
cUniversity of Kassel, Heinrich-Plett-Str. 40, Kassel D-34132, Germany
First published on 6th December 2022
Abstract
Sonication-assisted liquid phase exfoliation was applied to six different layered coordination polymers (CPs) in aqueous surfactant solution. The resulting nanosheets were investigated for structural and compositional integrity and microscopic analysis gives insights into the relationship between the crystal structure of the materials and their exfoliability. Larger open pores seem to favour the production of nanosheets with higher aspect ratio of lateral size to thickness.
The possibility of producing 2D porous CP nanosheets has attracted increasing attention in recent years.1,2 As observed for 2D nanomaterials, e.g. graphene3 or transition metal dichalcogenides (TMDs),4 they are expected to show unique structure and dimension related properties compared to their bulk counterparts. In particular, the exfoliation of bulk crystals into nanosheets increases the surface to volume ratio and consequently yields an improved accessibility of the pores.5 Therefore, porous CP nanosheets are of great interest for structure–property related studies but also for potential applications e.g. in gas separation,5,6 catalysis7,8 or sensing.9,10 For example, the CO2/CH4 separation performance was improved by the use of CP nanosheets dispersed in polymer membranes.6
However, the production of 2D CP nanosheets remains a great challenge. Direct synthesis requires the oppression of crystal growth in one dimension while leaving the lateral dimensions unaffected. Such bottom-up approaches were successfully applied for few CPs,6,9,11 but are not universally applicable to the vast structural variety of CPs. Top-down methods are more promising in this regard. Strong covalent or coordinative binding situations in two dimensions in combination with weaker interactions in the stacking direction (e.g. van der Waals forces-vdW, π–π-stacking or hydrogen bonds) are characteristic for layered CPs. Overcoming these interactions ultimately leads to 2D CP nanosheets. Until today, various top-down methods such as mechanical exfoliation,12 chemical exfoliation8 or Li-intercalation13 were successfully applied. While each technique has its advantages and limitations, a promising approach is sonication-assisted liquid phase exfoliation (LPE), due to the universal applicability of the technique, as demonstrated for a variety of different materials.14–16 In this process, layers are separated with the assistance of mechanical forces created by ultrasound followed by liquid stabilization of the nanosheets through noncovalent interactions with the medium. For traditional vdW crystals such as graphite and TMDs, the morphology of the nanosheets, i.e. their lateral size to thickness aspect ratio (and hence 2D character), is determined by the binding strength anisotropy in the crystal structure.15,16 The recent demonstration that molecular coordination compounds can be exfoliated with LPE into high aspect ratio nanosheets in spite of a relatively small binding strength anisotropy17 suggests that additional factors need to be taken into account for coordination compounds.
Here, we address this point in a comparative study. We demonstrate the successful exfoliation and characterization of six structurally different layered 2D CPs, including three porous CPs, five of which have not been exfoliated with LPE before. All materials were subjected to sonication in aqueous surfactant solution and the resulting nanosheets were studied by powder X-ray diffraction (PXRD), as well as Raman and IR spectroscopy to confirm the structural and compositional integrity. Furthermore, statistical atomic force microscopy (AFM) was applied to evaluate the nanosheet morphology.
The following six layered CPs were chosen: Ga-1,8-ndc (1),18 Sc-BPyDC (2),19 Sc-CAU-11 (3),20 Zr-FA-ODB (4), Zr-CAU-39 (5)21 and Zr-CAU-45 (6).22 Note that only compound (6) has been investigated in LPE before.22 Details on the composition of these CPs and their synthesis are summarized in the ESI,† Section S1.2. The bulk compounds were fully characterized and phase purity was confirmed using PXRD, scanning electron microscopy (SEM) combined with IR- and Raman spectroscopy as reference for the exfoliated nanomaterial species.
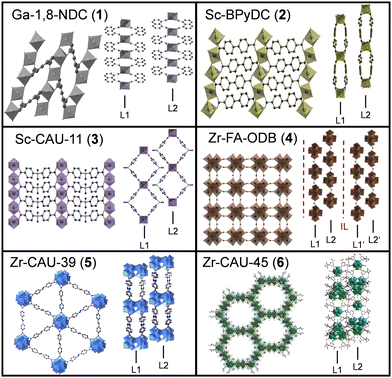 | ||
| Fig. 1 Crystal structures of the investigated 2D CPs from two different perspectives- top view and side view. Layers are labeled as layer 1 (L1), layer 2 (L2) and intercalation layer (IL). | ||
A simplified graphical representation of the crystal structures is given in Fig. 1. Further structural details can be found in literature18–22 or in Section S2 of the ESI.† The structures of Ga-1,8-ndc (1),18 Sc-BPyDC (2)19 and Sc-CAU-11 (3)20 contain trans-corner sharing [MO6]-octahedra as the inorganic building unit (IBU). The other compounds exhibit either hexanuclear (Zr-FA-ODB (4)), dodecanuclear (Zr-CAU-39 (5))21 or both (Zr-CAU-45 (6))22 Zr–O clusters as IBUs. The IBUs in all six CPs are interconnected through the respective dicarboxylate ions and layers are formed. The morphology of the bulk materials was examined with SEM (ESI,† Fig. S2) showing layered structures except for Sc-CAU-11 (3) where platelets of different crystallite sizes are observed.
Exfoliation was accomplished by bath sonication for 7 hours using aqueous solutions with sodium cholate as surfactant for the stabilization. After exfoliation, the samples were purified by centrifugation to remove unexfoliated material, as well as impurities. For details see ESI,† Section S1.3.
To confirm the structural integrity after exfoliation, PXRD, Raman- and IR spectroscopy on dried nanosheet samples were carried out and compared to the bulk material (ESI,† Section S4 and Fig. S3–S8). In the case of compounds 1, 2, 3, 5 and 6, no noticeable differences were observed between bulk and exfoliated material. Only the exfoliated compound 4 (Zr-FA-ODB) displays significant differences compared to the corresponding bulk material. As described in more detail in the ESI,† the bulk crystals contain intercalated molecules of 4,4-oxybis(benzoic acid) that may have been washed out upon material delamination resulting in a different crystal structure and composition.
The exfoliability of the compounds can in principle be assessed by yield and/or lateral size to thickness aspect ratio which gives a measure for the ease of exfoliation. The yield was determined gravimetrically after microfiltration and is shown as bar graph in Fig. 2A. It varies between less than 10% for Sc-CAU-11 (3) and >75% for Sc-BPyDC (2), i.e. by an order of magnitude. Such a striking difference cannot be rationalized based on the crystal structure and morphology of the starting material. However, we note that the yield will also be influenced by the buoyant density of the nanosheets due to the centrifugation-based purification. Thus, while it is an important parameter to assess in LPE, it cannot easily be interpreted.
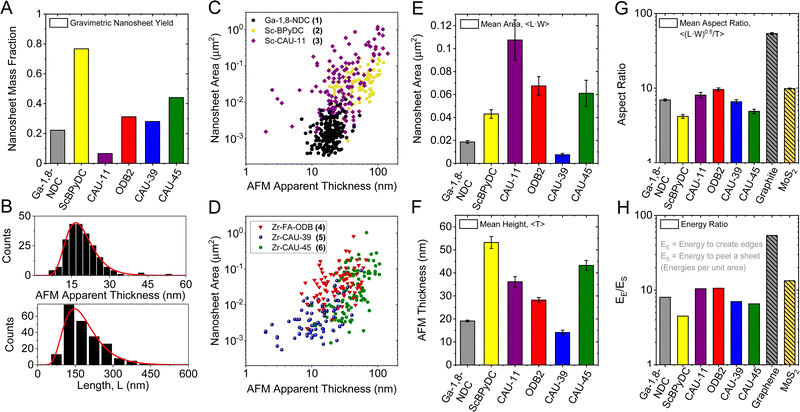 | ||
| Fig. 2 Quantitative analysis on the exfoliability for all six CPs. Gravimetric yield of exfoliated nanosheets after removal of unexfoliated material by low speed centrifugation (A). Length and thickness distribution histograms (B) from the statistical AFM analysis of Ga-1,8-ndc (1) nanosheets. A mean length, 〈L〉 of ∼150 nm with an average thickness, 〈T〉 of ∼19 nm is determined after measuring the dimensions of more than 100 individual nanosheets. Scatter plots of the nanosheet areas (approximated as length × width) over thickness of all six compounds (C and D). Bar charts of 2D CP nanosheet dimensions. Arithmetic mean apparent AFM area (E) and thickness (F). Square root of the average nanosheet area divided by the average thickness (G). This aspect ratio allows estimating the edge to surface energy ratio (H). Data on exfoliated graphite and MoS2 is shown for comparison.20 | ||
In this regard, the ease of exfoliation, i.e. lateral size to thickness aspect ratio, is an alternative metric for the LPE process. First, we examine the nanosheet morphology by SEM of networks (ESI,† Fig. S2) and AFM on individual platelets (Fig. S9–S14, ESI†). AFM length (L, longest dimension), width (W, perpendicular to L) and thickness (T) of >100 individual nanosheets were measured for each sample. The resulting T and L distribution histograms are exemplarily shown for Ga-1,8-ndc (1) in Fig. 2B. For all data see Fig. S9–S14, ESI.† To compare size and thickness across the samples, scatter plots of the nanosheet area (L × W) as function of thickness are presented in Fig. 2C and D.
A number of differences are observed across the six compounds. While the dimensions of Ga-1,8-ndc (1) nanosheets are clustered and are comparatively small laterally, nanosheet dimensions for Sc-BPyDC (2), Zr-FA-ODB (4) and Zr-CAU-45 (6) tend to be larger in a similar thickness range (15–80 nm). In contrast, nanosheets of Sc-CAU-11 (3) Zr-CAU-39 (5) show a wider distribution in both dimensions including nanosheets with thicknesses <15 nm. Note that the distribution width will also be affected by the centrifugation conditions used for the purification which limits comparability in this plot due to different (buoyant) densities across the materials.
For a better comparison of the nanosheet dimensions, bar charts are used to display the mean nanosheet area and apparent AFM thickness (Fig. 2E and F) of all six exfoliated 2D CPs. In addition, the respective length-to-width aspect ratios are given in Fig. S15 in the ESI.† The six exfoliated 2D CPs differ strongly in their nanosheet dimensions with the areas varying from 0.008 μm2 to 0.11 μm2 and average thicknesses ranging from 14 nm to 53 nm.
Further, the lateral size to thickness aspect ratio was calculated, represented by the square root of the average nanosheet area divided by the average thickness, Fig. 2G. For comparison, the aspect ratio data of graphite and MoS2 taken from literature are included in the same plot.16 While aspect ratios of the CPs are below 10 in all cases, i.e. the nanomaterials are rather nanobricks than nanosheets, all exfoliated materials exhibit aspect ratios above 3. Compound 4 with an intercalation layer in its bulk structure, exhibits the highest aspect ratio of 9.2 followed by Sc-CAU-11 (9.1), Ga-1,8-ndc (7.0), Zr-CAU-39 (6.1) and Zr-CAU-45 (5.7). The nanosheets (or nanobricks) of Sc-BPyDC show the lowest aspect ratio of only 3.9.
Understanding this difference in the ease of exfoliation is not trivial. In addition to the intrinsic factors which can be assessed computationally,23,24 it might be influenced by material properties such as particle size and morphology, the polarity of the surface and the interaction with the medium as well as the defect density. Some of these aspects have previously been investigated on other materials (for details see ESI† Section S6) and do not account for the variations. On a structural level, the ease of exfoliation of traditional vdW crystals is related to the ratio of in-plane to out-of-plane binding strength,15,16 which defines the ratio of scission and delamination events with equipartition of energy.16 Therefore, a high stability within a layer and weak interactions between those are expected to result in high aspect ratio nanosheets.
This model16 also suggests that it is possible to experimentally determine the energy ratio for creating new edges to the energy required for peeling off a sheet per unit based on the nanomaterials’ aspect ratios. This is useful, as this can in turn be compared to the expected structure–property relationship. Note that the in-plane to out-of-plane binding energy ratio is a better descriptor for the ease of exfoliation than the aspect ratio, since it takes into account different layer thicknesses that can vary significantly across crystal structures. A detailed calculation and discussion is described in Section S7 in the ESI.† The resultant data of the CPs is displayed in Fig. 2H, again in comparison to MoS2 and graphite from literature.16 According to the experimental energy ratio, the CPs are less exfoliable than both reference compounds with binding energy ratios of maximum ∼10. However, it should be noted that also other layered materials (e.g. layered hydroxides such as Ni(OH)2) can show area/thickness aspect ratios as low as 4.5 corresponding to energy ratios of ∼5.5.16
To test whether this experimental estimate of the binding energy ratio based on the nanomaterial's aspect ratio can indeed be rationalized based on the crystal structure, it is feasible to compare compounds where either the intralayer binding strength is approximately constant with different interlayer attraction or vice versa. Ga-1,8-ndc (1), Sc-BPyDC (2) and Sc-CAU-11 (3) fulfill the requirement of similar intralayer binding strengths. Their structures are built up by metal-oxide chains composed of trans corner-sharing MO6 polyhedra interconnected to two other chains via the same number of the respective linkers so that a similar in-plane binding strength is reasonable. However, while Ga-1,8-ndc (1) and Sc-BPyDC (2) are expected to exhibit only weak vdW interactions between the layers, the structure of Sc-CAU-11 (3) possesses comparatively strong interlayer hydrogen bonds between the sulfonyl-groups and the bridging hydroxyl groups of the IBUs of adjacent layers (Fig. S16, ESI†).20 Considering this, Sc-CAU-11 (3) is expected to be the least exfoliable and Ga-1,8-ndc (1) as well as Sc-BPyDC (2) being much more promising candidates for LPE. However this is not observed experimentally.
Instead, Sc-CAU-11 (3) exhibits the highest experimental energy ratio (10.5), followed by Ga-1,8-ndc (1) (8.0) and Sc-BPyDC (2) with only 4.4. This discrepancy indicates that additional parameters govern the ease of exfoliation of these layered materials. Therefore, one can only speculate why these different properties are observed. The compounds vary in particle size and morphology (Fig. S2, ESI†). While Ga-1,8-ndc (1) and Sc-BPyDC (2) crystallize in isolated platelets, the particles of Sc-CAU-11 (3) show a grainy morphology with less pronounced layered character. A greater particle size or grainy morphology might impede the exfoliation process, as there are fewer points of attack. While this is expected to have an influence on the kinetics of the exfoliation (i.e. the yield for a given sonication time), it has not unambiguously been linked to the platelet shape (see ESI,† Section S10). Here, it would not explain the higher aspect ratio of Sc-CAU-11 (3).
We suspect a combination of two factors. In contrast to the CPs Ga-1,8-ndc (1) and Sc-BPyDC (2), the structure of Sc-CAU-11 (3) exhibits intralayer channels.20 Considering the different exfoliabilities in these similar structures, we believe that the porosity of materials could be a decisive factor that favors the exfoliation of porous 2D CPs. In a simple picture, one can imagine that, instead of delamination of the layers by mechanical force only exerted at edges, a sufficient porosity might result in spaces within a particle to be partially filled with solvent during sonication. This leads to additional points of attack within the particles, potentially promoting the exfoliation process.
Another driving force could be the higher polarity of the linker in Sc-CAU-11 (3). Unlike the hydrophobic linker molecules of Ga-1,8-ndc (1) or Sc-BPyDC (2), the sulfonyl groups of the sulfonyldibenzoate linkers may additionally stabilize the resulting nanosheets during the exfoliation process in water. However, in this case, a higher yield would be expected which is not observed. To fully rationalize the complex relations that influence the ease of exfoliation of 2D CPs, more data on materials with preferentially similar structures is required.
Due to the significant difference in crystal structures, direct comparison of the ease of exfoliation to the porous CPs Zr-CAU-39 (5) and Zr-CAU-45 (6) is not appropriate. The presence of large pores (2.2 nm22 and 0.6 nm,21 respectively) and functional groups, however, might qualitatively explain the relatively good aspect ratios despite a strong interlayer interaction caused by hydrogen bonds. The role of porosity might also be one of the factors behind the remarkable aspect ratio of the molecular coordination compound reported recently.17
In summary, six different layered 2D CPs including three porous CPs were exfoliated in aqueous solution. The resulting nanosheets were characterized using PXRD, IR- and Raman spectroscopy. Comparison to the respective bulk samples implies that, except for Zr-FA-ODB (4), no changes of the material composition or crystallinity occurred during the exfoliation process. Statistical AFM was used to assess the nanosheet dimensions and particular aspect ratios of area to thickness which are characteristic for the ease of exfoliation. All materials are platelet-like implying successful (partial) exfoliation even though the CP nanomaterials are relatively thick and small compared to traditional vdW crystals such as graphite. Comparison of three most structurally related CPs indicates a complex relation between crystal structure and ease of exfoliation. The results imply that, in contrast to the more traditional vdW crystals, the nature of pores and polarity of the nanosheet surfaces could be decisive factors in exfoliation of CPs.
Conceptualization – all authors; experimental work and data analysis – J. G. and K. S.; writing – all authors; supervision – N. S. and C. B.
Conflicts of interest
There are no conflicts to declare.References
- M. Zhao, Y. Huang, Y. Peng, Z. Huang, Q. Ma and H. Zhang, Chem. Soc. Rev., 2018, 47, 6267–6295 RSC.
- P.-Z. Li, Y. Maeda and Q. Xu, Chem. Commun., 2011, 47, 8436 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- R. Lv, J. A. Robinson, R. E. Schaak, D. Sun, Y. Sun, T. E. Mallouk and M. Terrones, Acc. Chem. Res., 2014, 48, 56–64 CrossRef PubMed.
- X. Wang, C. Chi, K. Zhang, Y. Qian, K. M. Gupta, Z. Kang, J. Jiang and D. Zhao, Nat. Commun., 2017, 8, 14460 CrossRef CAS PubMed.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. Llabrés, I. Xamena and J. Gascon, Nat. Mater., 2015, 14, 48–55 CrossRef CAS PubMed.
- W. Shi, L. Cao, H. Zhang, X. Zhou, B. An, Z. Lin, R. Dai, J. Li, C. Wang and W. Lin, Angew. Chem., Int. Ed., 2017, 129, 9836–9841 CrossRef.
- Y. Ding, Y.-P. Chen, X. Zhang, L. Chen, Z. Dong, H.-L. Jiang, H. Xu and H.-C. Zhou, J. Am. Chem. Soc., 2017, 139, 9136–9139 CrossRef CAS PubMed.
- M. Zhao, Y. Wang, Q. Ma, Y. Huang, X. Zhang, J. Ping, Z. Zhang, Q. Lu, Y. Yu, H. Xu, Y. Zhao and H. Zhang, Adv. Mater., 2015, 27, 7372–7378 CrossRef CAS PubMed.
- M. Zhang, G. Feng, Z. Song, Y.-P. Zhou, H.-Y. Chao, D. Yuan, T. T. Y. Tan, Z. Guo, Z. Hu, B. Z. Tang, B. Liu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 7241–7244 CrossRef CAS PubMed.
- S. C. Junggeburth, L. Diehl, S. Werner, V. Duppel, W. Sigle and B. V. Lotsch, J. Am. Chem. Soc., 2013, 135, 6157–6164 CrossRef CAS PubMed.
- A. Abhervé, S. Mañas-Valero, M. Clemente-León and E. Coronado, Chem. Sci., 2015, 6, 4665–4673 RSC.
- H.-S. Wang, J. Li, J.-Y. Li, K. Wang, Y. Ding and X.-H. Xia, NPG Asia Mater., 2017, 9, e354–e354 CrossRef CAS.
- C.-X. Hu, Y. Shin, O. Read and C. Casiraghi, Nanoscale, 2021, 13, 460–484 RSC.
- L.-J. Ji, Y. Qin, D. Gui, W. Li, Y. Li, X. Li and P. Lu, Chem. Mater., 2018, 30, 8732–8738 CrossRef CAS.
- C. Backes, D. Campi, B. M. Szydlowska, K. Synnatschke, E. Ojala, F. Rashvand, A. Harvey, A. Griffin, Z. Sofer, N. Marzari, J. N. Coleman and D. D. O’Regan, ACS Nano, 2019, 13, 7050–7061 CrossRef CAS PubMed.
- J. Dong, L. Liu, C. Tan, Q. Xu, J. Zhang, Z. Qiao, D. Chu, Y. Liu, Q. Zhang, J. Jiang, Y. Han, A. P. Davis and Y. Cui, Nature, 2022, 602, 606–611 CrossRef CAS PubMed.
- T. Rabe, H. Pewe, H. Reinsch, T. Willhammar, E. Svensson Grape and N. Stock, Dalton Trans., 2020, 49, 4861–4868 RSC.
- P. Rönfeldt, N. Ruser, H. Reinsch, E. S. Grape, A. Ken Inge, M. Suta, H. Terraschke and N. Stock, Eur. J. Inorg. Chem., 2020, 2737–2743 CrossRef.
- P. Rönfeldt, E. S. Grape, A. K. Inge, D. V. Novikov, A. Khadiev, M. Etter, T. Rabe, J. Benecke, H. Terraschke and N. Stock, Inorg. Chem., 2020, 59, 8995–9004 CrossRef PubMed.
- S. Waitschat, H. Reinsch, M. Arpacioglu and N. Stock, CrystEngComm, 2018, 20, 5108–5111 RSC.
- S. Leubner, V. E. G. Bengtsson, K. Synnatschke, J. Gosch, A. Koch, H. Reinsch, H. Xu, C. Backes, X. Zou and N. Stock, J. Am. Chem. Soc., 2020, 142, 15995–16000 CrossRef CAS PubMed.
- X. Jia, Q. Shao, Y. Xu, R. Li, K. Huang, Y. Guo, C. Qu and E. Gao, npj Comput. Mater., 2021, 7, 211 CrossRef CAS.
- Y. An, Y. Hou, S. Gong, R. Wu, C. Zhao, T. Wang, Z. Jiao, H. Wang and W. Liu, Phys. Rev. B, 2020, 101, 075416 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis details, structural description of Zr-FA-ODB, SEM micrographs, details on the LPE process, PXRD patterns, Raman- and IR spectra, AFM characterization, calculation of Ee/ES. See DOI: https://doi.org/10.1039/d2cc03366f |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |
