In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries†
Kun
Chang
and
Weixiang
Chen
*
Department of Chemistry, Zhejiang University, Hangzhou, PR China 310027. E-mail: weixiangchen@zju.edu.cn; Fax: +86 571-87951895; Tel: +86 571-87952477
First published on 7th March 2011
Abstract
A facile process was developed to synthesize MoS2/graphene nanosheet (GNS) composites by a one-step in situ solution-phase method. These MoS2/GNS composites therefore exhibit extraordinary capacity, i.e., up to 1300 mA h g−1, and excellent rate capability and cycling stability as an anode material for lithium ion batteries.
The possibility of using a rechargeable lithium ion battery (LIB) for various stationary power storage applications has stimulated significant research activity aimed toward improving LIB energy, power density and cycling life. As a commercial anode material in LIBs, graphite suffers from a relatively small capacity (372 mA h g−1) and should be replaced by alternative anode materials with higher capacity.1 Recently, graphene, a flat one-atom-thick monolayer that is exfoliated from graphite, has been shown to exhibit outstanding electronic behavior, large surface area and high chemical tolerance, thus attracting much research interest.2,3 These advantages suggest that graphene sheets hold promise as matrices for improving the electrochemical performance of metal and metal oxides.4 To date, research concerning metal and metal oxides supported on graphene has greatly enhanced the electrochemical performance of graphene as an anode material for LIB.5,6 Metal sulfides such as MoS2, WS2 and SnS2 also deliver a higher theoretical capacity.7
As a typical layered transition metal sulfide, MoS2 has a structure analogous to that of graphene. This metal sulfide is composed of three atom layers (S–Mo–S) stacked together through van der Waals interactions.8,9 Due to this layered structure, Li+ ions can easily intercalate and exfoliate. Since the patent publication of the first lithium ion battery using MoS2 in 1980, several different MoS2 morphologies have been used in lithium ion batteries.10 Thus far, the highest specific capacity of MoS2 was reported by Xiao et al. and is 1131 mA h g−1 with a current of 50 mA g−1.11 Because graphene has extraordinary electronic behavior, MoS2 growth on the surface increases its electron conductivity and electrochemical performance.
Herein, we report a facile in situ solution-phase reduced method for growing MoS2 layers on a graphene nanosheet (GNS) to form MoS2/GNS composites. Electrochemical tests indicate that the MoS2/GNS composites exhibit very high capacity and excellent rate capability and cycling stability as anode materials for LIB.
The in situ synthesis of MoS2/GNS composites is shown schematically in Fig. 1a [also see ESI† for experimental details]. 0.035 g graphite powder was converted to graphene oxide (GO) by Hummers method.12 The as-prepared GO was transferred from the as-made suspension into a 200 ml beaker with the addition of 40 ml DI water. Then, 0.3 g Na2MoO4·2H2O was added. After ultrasonication and stirring for 20 min, 0.1 M NaOH was added to the solution until the pH value changed to 6.5. The mixture and 0.8 g NH2CSNH2 were dissolved in 80 ml DI water and then transferred into a 100 ml Teflon-lined stainless steel autoclave, sealed tightly, and heated at 240 °C for 24 h. After cooling naturally, the black precipitates were collected by centrifugation, washed with DI water and ethanol, and dried in a vacuum oven at 80 °C for 24 h. Due to the many functional groups on the surface of GO, such as carboxyl, hydroxyl and epoxy groups, as a result, MoO42− anions could tightly adsorb onto the GO surface and then be reduced by NH2CSNH2 in the solution at 240 °C to form MoS2/GNS composites. In this process, H2S, which is released from NH2CSNH2 at high temperatures, reduces MoO42− and GO to MoS2 and graphene (see ESI†, Fig. S1). From the analysis of EDAX, four kinds of elements can be observed, i.e., C, Mo, S and a small number of O, and it is calculated that the atomic ratio of Mo to C approaches to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
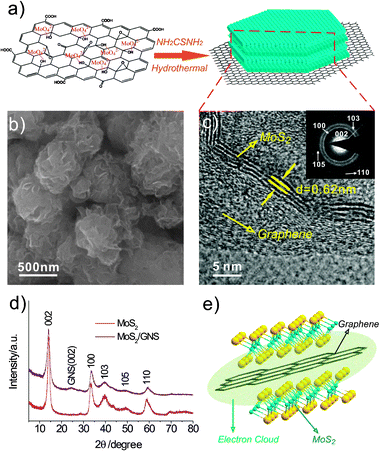 | ||
| Fig. 1 (a) Schematic diagram of the in situ synthesis of MoS2/GNS. (b) SEM image of MoS2/GNS composites. (c) HRTEM image of MoS2 layers on graphene. (d) XRD patterns of MoS2 and MoS2/GNS; inset shows the electron diffraction pattern of the MoS2 nanosheets on graphene. (e) Schematic illustration of the microstructure of MoS2/GNS. | ||
The general morphology of the MoS2/GNS composites is shown in Fig. 1b. The composites deliver a 2-dimensional nanoflake structure. The high-resolution TEM (HRTEM) image shows that MoS2 layers with an interlayer distance of 0.62 nm are grown on the surface of graphene (Fig. 1c). Comparing with the HRTEM image of pure MoS2 (see ESI†, Fig. S1b), it can be found that the thickness of MoS2 grown on the surface of graphene is about 4–5 layers, which is quite smaller than pure MoS2 (tens of layers). This factor indicates that during the process of synthesis of MoS2/GNS composites, MoS2 is in situ reduced on the surface of graphene layers, and the graphene sheets greatly inhibit the restacking of MoS2 layers. The same characteristic can be observed by XRD analysis (Fig. 1d). As shown in Fig. 1d, the MoS2/GNS composite shows the similar crystalline structure of pure MoS2, which are in good agreement with a hexagonal structure (JCPDS 37-1492). Additionally, the XRD patterns of the MoS2 and MoS2/GNS composites show that the growth of the (002) plane at 2θ = 14.2° was inhibited due to the presence of graphene. The other diffraction peaks are in accordance with the electron diffraction pattern in Fig. 1c. A weak diffraction peak can be observed at 2θ = 24.5°, which is attributed to the (002) plane of graphene nanosheets (see ESI†, Fig. S1d). If only pure graphene sheets were reduced by H2S in a hydrothermal process, it can be easy to restack for the graphene layers, and the interlayer distance of restacked graphene nanosheets is slightly large than that of graphite. Therefore, the increase of the interlayer of graphene nanosheets can provide larger space for Li ion intercalation, which also reduces the barriers to Li ion mobility, thereby facilitating lithium ion diffusion.7a In the composites, the electrons of the S atom layer and its adjacent carbon layer could form a co-electron cloud (Fig. 1e). The high concentration of electrons between the MoS2 layer and the graphene layer could greatly enhance the electronic conductivity of the composites and were beneficial to the diffusion of the Li+ ions during the charge and discharge in LIB.13
The MoS2/GNS composites were mixed with carbon black and polyvinyldifluoride (PVDF) in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The obtained slurry was spread on a copper foil with 14 mm in diameter and 0.02 mm in thickness, dried at 120 °C for 12 h under a vacuum atmosphere (−0.1 Mpa), and then pressed to form a working electrode with active material loading of 1.50 mg cm−2. The electrochemical tests were performed in a two-electrode cell with a Li foil as the counter electrode and a 1.0 M LiPF6 solution in a mixture of EC/DMC (1
10. The obtained slurry was spread on a copper foil with 14 mm in diameter and 0.02 mm in thickness, dried at 120 °C for 12 h under a vacuum atmosphere (−0.1 Mpa), and then pressed to form a working electrode with active material loading of 1.50 mg cm−2. The electrochemical tests were performed in a two-electrode cell with a Li foil as the counter electrode and a 1.0 M LiPF6 solution in a mixture of EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) as the electrolyte. Fig. 2c shows the first two charge and discharge curves of the MoS2/GNS composites at a current density of 100 mA g−1 and cycled between 3.0 and 0.1 V vs.Li+/Li. As shown in Fig. 2c, two potential plateaus at ∼1.2 and ∼0.6 V are observed for the composite electrode in the first discharge, which is in accordance with the findings for MoS2 (Fig. 2a). The plateau at 1.2 V is indicative of the formation of LixMoS2, and the plateau variation in the lithium intercalation is attributed to the different defect sites of MoS2. The discharge plateau at 0.6 V can be attributed to a conversion reaction process, which first entails the in situdecomposition of MoS2 into Mo particles embedded in a Li2S matrix and then the formation of a gel-like polymeric layer resulting from electrochemically driven electrolyte degradation.14 In the first charge (delithiation) process, two potential plateaus at about 1.7 and 2.3 V are displayed. This result can also be observed in the CV curves of the composites (Fig. 2b and d). During the second discharge, two inconspicuous potential plateaus at about 2.0 and 1.2 V, and the potential plateau observed at 0.6 V in the first discharge, disappeared, which is in accordance with the findings for MoS2. The reversible formation of Li2S at the nanoscale based on the reaction: MoS2 + 4Li ↔ Mo + 2Li2S, accompanying the redox of Mo nanoparticles and the reversible growth of the gel-like layer, should be the plausible reason for the reversible capacity and the sloping voltage regions in the subsequent cycles.15 In addition, during the first charge/discharge process, the lithiation capacity of MoS2/GNS composites is 2200 mA h g−1, and the delithiation capacity reaches 1300 mA h g−1, which is much higher than the capacity of free MoS2 and GNS (see ESI†, Fig. S2) obtained through the same method. As shown in Fig. 3a, MoS2/GNS composites exhibit extraordinary cycling behavior and still retain the reversible capacity of 1290 mA h g−1 after 50 cycles. On the contrary, the reversible capacity of the free MoS2 electrode reaches its maximum (835 mA h g−1) after several cycles, but only a capacity of 605 mA h g−1 is retained after 50 cycles.
1 in volume) as the electrolyte. Fig. 2c shows the first two charge and discharge curves of the MoS2/GNS composites at a current density of 100 mA g−1 and cycled between 3.0 and 0.1 V vs.Li+/Li. As shown in Fig. 2c, two potential plateaus at ∼1.2 and ∼0.6 V are observed for the composite electrode in the first discharge, which is in accordance with the findings for MoS2 (Fig. 2a). The plateau at 1.2 V is indicative of the formation of LixMoS2, and the plateau variation in the lithium intercalation is attributed to the different defect sites of MoS2. The discharge plateau at 0.6 V can be attributed to a conversion reaction process, which first entails the in situdecomposition of MoS2 into Mo particles embedded in a Li2S matrix and then the formation of a gel-like polymeric layer resulting from electrochemically driven electrolyte degradation.14 In the first charge (delithiation) process, two potential plateaus at about 1.7 and 2.3 V are displayed. This result can also be observed in the CV curves of the composites (Fig. 2b and d). During the second discharge, two inconspicuous potential plateaus at about 2.0 and 1.2 V, and the potential plateau observed at 0.6 V in the first discharge, disappeared, which is in accordance with the findings for MoS2. The reversible formation of Li2S at the nanoscale based on the reaction: MoS2 + 4Li ↔ Mo + 2Li2S, accompanying the redox of Mo nanoparticles and the reversible growth of the gel-like layer, should be the plausible reason for the reversible capacity and the sloping voltage regions in the subsequent cycles.15 In addition, during the first charge/discharge process, the lithiation capacity of MoS2/GNS composites is 2200 mA h g−1, and the delithiation capacity reaches 1300 mA h g−1, which is much higher than the capacity of free MoS2 and GNS (see ESI†, Fig. S2) obtained through the same method. As shown in Fig. 3a, MoS2/GNS composites exhibit extraordinary cycling behavior and still retain the reversible capacity of 1290 mA h g−1 after 50 cycles. On the contrary, the reversible capacity of the free MoS2 electrode reaches its maximum (835 mA h g−1) after several cycles, but only a capacity of 605 mA h g−1 is retained after 50 cycles.
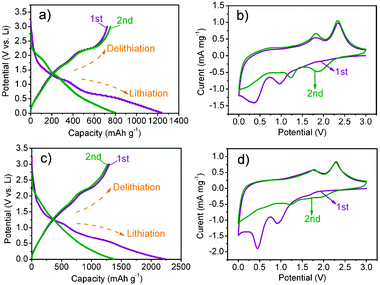 | ||
| Fig. 2 Electrochemical characterizations of a half-cell composed of MoS2vs.Li and MoS2/GNS vs.Li. (a) The first two charge and discharge curves of MoS2 at a current density of 100 mA g−1. (b) Cyclic voltammograms of MoS2 electrodes at a scan rate of 0.5 mV s−1 during the first two cycles. (c) The first two charge and discharge curves of MoS2/GNS at a current density of 100 mA g−1. (d) Cyclic voltammograms of MoS2/GNS electrodes at a scan rate of 0.5 mV s−1 during the first two cycles. | ||
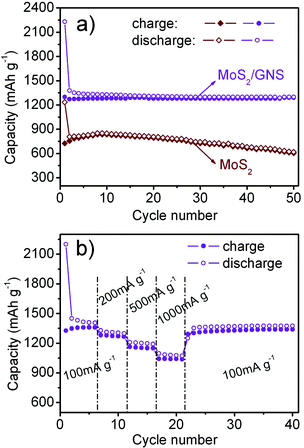 | ||
| Fig. 3 (a) Cycling behaviors of MoS2 and MoS2/GNS at a current density of 100 mA g−1. (b) Cycling behavior of MoS2/GNS at various current densities. | ||
Such a high reversible capacity and excellent cycling behavior of the MoS2/GNS composites are also exhibited in the rate capability. Fig. 3b shows the rate cycling behavior of the composites. The MoS2/GNS composites also demonstrate good rate performance. Even at a high current density of 1000 mA g−1, the specific capacity remains at 1040 mA h g−1, which is still higher than that of free MoS2 and GNS. Additionally, the extraordinary cycling stability of the composites is exhibited at various current densities. Even though the current changes from 1000 mA g−1 to 100 mA g−1, the specific capacity of the composites returns to 1300 mA h g−1 at once and does not ultimately change in the following cycles, indicating extraordinarily high cycling stability. The high capacity, cycling stability and rate capability of the MoS2/GNS composite could be attributed to the intimate interaction between the graphene substrates and MoS2 layers grown on them, which was facilitated to MoS2 electrochemically active since charge carriers could be effectively and rapidly conducted back and forth from MoS2 layers to graphene layers.5 In our experimental process, we found that the effect of the mole ratio of MoS2 to GNS on the electrochemical performance of the composites was also important. Here, we reported a MoS2/GNS composite with a Mo to C mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 that delivered the highest specific capacity (∼1300 mA h g−1). Although the specific capacity of samples with mole ratios of 1
2 that delivered the highest specific capacity (∼1300 mA h g−1). Although the specific capacity of samples with mole ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (i.e., 1001 mA h g-1 and 1132 mA h g-1, respectively) were inferior to that of a sample with a ratio of 1
4 (i.e., 1001 mA h g-1 and 1132 mA h g-1, respectively) were inferior to that of a sample with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, these samples still exhibited higher specific capacity and better cycling stability than pure MoS2 and GNS (see ESI†, Fig. S3), indicating that initial electrochemical performances of MoS2 grown on the surface of graphene were indeed greatly enhanced and these kinds of MoS2/GNS composites would find wide applications as a promising anode material for LIB.
2, these samples still exhibited higher specific capacity and better cycling stability than pure MoS2 and GNS (see ESI†, Fig. S3), indicating that initial electrochemical performances of MoS2 grown on the surface of graphene were indeed greatly enhanced and these kinds of MoS2/GNS composites would find wide applications as a promising anode material for LIB.
In summary, through the in situreduction of MoS2 nanoflakes on graphene nanosheets to form MoS2/GNS composites, we enabled the MoS2 anode material to achieve the highest specific capacity observed to date. The MoS2/GNS composites can be further explored for high-capacity, low-capacity and environment-friendly anode materials for LIB applications. We believe that our facile method can also be used for other metal sulfides (e.g., WS2, SnS2 and TiS2) supported on graphene to significantly improve their electrochemical performances.
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Y407030, Y4100119) and 973 Fundamental Research Program from the Ministry of Science and Technology of China (2010CB635116).
Notes and references
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS.
- (a) A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS; (b) E. J. Yoo, J. Kim, E. Hosono, H. S. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277 CrossRef CAS; (c) B. Wang, X. L. Wu, C. Y. Shu, Y. G. Guo and C. R. Wang, J. Mater. Chem., 2010, 20, 10661 RSC.
- (a) M. Pumera, Chem. Rec., 2009, 9, 211 CrossRef CAS; (b) M. Pumera, Energy Environ. Sci., 2011 10.1039/C0EE00295J.
- (a) Y. Li, W. Gao, L. Ci, C. Wang and P. M. Ajayan, Carbon, 2010, 48, 1124 CrossRef CAS; (b) H. L. Wang, H. S. Casalongue, Y. Y. Liang and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 7472 CrossRef CAS.
- H. L. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Y. Liang, Y. Cui and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 13978 CrossRef CAS.
- Z. S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 4, 3187 CrossRef CAS.
- (a) G. Du, Z. P. Guo, S. Wang, R. Zeng, Z. Chen and H. Liu, Chem. Commun., 2010, 46, 1106 RSC; (b) J. W. Seo, Y. W. Jun, S. W. Park, H. Nah, T. Moon, B. Park, J. G. Kim, Y. J. Kim and J. Cheon, Angew. Chem., Int. Ed., 2007, 46, 8828 CrossRef CAS; (c) J. W. Seo, J. T. Jang, S. W. Park, C. Kim, B. Park and J. Cheon, Adv. Mater., 2008, 20, 4269 CrossRef CAS.
- R. Tenne, L. Margulis, M. Genut and G. Hodes, Nature, 1992, 360, 444 CrossRef CAS.
- H. S. S. Ramakrishna Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. R. Rao, Angew. Chem., Int. Ed., 2010, 49, 4059.
- R. R. Haering, J. A. R. Stiles and K. Brandt, US Patent, 1980, No. 4224390 Search PubMed.
- J. Xiao, D. W. Choi, L. Cosimbescu, P. Koech, J. Liu and J. P. Lemmon, Chem. Mater., 2010, 22, 4522 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- C. R. German, P. Santiago, J. A. Ascencio, U. Pal, M. Perez-Alvarez, L. Rendon and D. Mendoza, J. Phys. Chem. B, 2005, 109, 17488 CrossRef.
- R. Dominko, D. Arcon, A. Mrzel, A. Zorko, P. Cevc, P. Venturini, M. Gaberscek, M. Remskar and D. Mihailovic, Adv. Mater., 2002, 14, 1531 CrossRef CAS.
- Q. Wang and J. H. Li, J. Phys. Chem. C, 2007, 111, 1675 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and figure. See DOI: 10.1039/c1cc10631g |
| This journal is © The Royal Society of Chemistry 2011 |
