Recent advances in colorimetric sensors based on nanozymes with peroxidase-like activity
Zhongmei
Chi
 *,
Qiong
Wang
and
Jiali
Gu
*
*,
Qiong
Wang
and
Jiali
Gu
*
College of Chemistry and Materials Engineering, Bohai University, Jinzhou, Liaoning Province 121013, P. R. China. E-mail: 980058161@qq.com
First published on 30th November 2022
Abstract
Nanozymes have been widely used to construct colorimetric sensors due to their advantages of cost-effectiveness, high stability, good biocompatibility, and ease of modification. The emergence of nanozymes greatly enhanced the detection sensitivity and stability of the colorimetric sensing platform. Recent significant research has focused on designing various sensors based on nanozymes with peroxidase-like activity for colorimetric analysis. However, with the deepening of research, nanozymes with peroxidase-like activity has also exposed some problems, such as weak affinity and low catalytic activity. In view of the above issues, existing investigations have shown that the catalytic properties of nanozymes can be improved by adding surface modification and changing the structure of nanomaterials. In this review, we summarize the recent trends and advances of colorimetric sensors based on several typical nanozymes with peroxidase-like activities, including noble metals, metal oxides, metal sulfides/metal selenides, and carbon and metal–organic frameworks (MOF). Finally, the current challenges and prospects of colorimetric sensors based on nanozymes with peroxidase-like activity are summarized and discussed to provide a reference for researchers in related fields.
1. Introduction
Nanomaterials with sizes of 1–100 nm may possess unique chemical, optical and electrical properties.1–3 Since the discovery of ferromagnetic nanoparticles through pioneering work in 2007,4 a growing number of nanomaterials have attracted extensive research. Nanozymes refer to nanomaterials showing intrinsic enzyme-like catalytic activities. They exhibit the advantages of low-cost, high stability, and ease of modification, which are expected to be the direct surrogates of natural enzymes.5–7 Furthermore, nanozymes are less sensitive to pH and temperature changes than natural enzymes, and thus generally lead to more robust analytical assays. Thus far, great efforts have been devoted to developing various nanozymes, such as noble metals,8,9 metal oxides,10,11 metal sulfides/metal selenides,12,13 carbon materials14,15 and metal–organic frameworks (MOFs).16–18 Various nanozymes have extremely broad application prospects in the fields of biosensing, immunoassay, and disease diagnosis.19–21Very recently, nanozyme-based sensors have been widely applied in electrochemical,22–24 chemiluminescence,25–27 luminescence,28–30 and colorimetric31–33 detection. Compared with other sensors, colorimetric sensors based on color change analysis can directly or indirectly achieve qualitative and quantitative detection of the target causing color change that is visible through naked eye observation34,35 or smartphone color readout device.36,37 Due to the merits of quick response, low cost, and no requirement of sophisticated instruments and professional operators, colorimetric sensors as a novel strategy have drawn tremendous attention.38–41 Thus, simple colorimetric assays-based nanozymes offer the unflinching biocatalytic potential for portable and inexpensive daily applications.
Nanozymes materials themselves show no light absorption properties in the constructed colorimetric sensors. After the introduction of a chromogenic substrate, nanozymes act as catalysts to catalyze the reaction of the substrate to trigger the light signal output, thus amplifying the signal as well as improving the sensitivity of colorimetric sensors.42,43 The types of nanozymes currently reported include peroxidase,44 oxidase,45 catalase,46 and superoxide dismutase.47 Among them, peroxidase is the most widely investigated nanozyme.48–50 For the peroxidase-mimetic system, nanozymes can efficiently catalyze the chromogenic substrates, such as 3,3′,5,5′-tetramethylbenzidine (TMB),51 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS),52 and o-phenylenediamine dihydrochloride (OPD),4 to generate chromogenic products also accompanied by colorimetric signals in the presence of hydrogen peroxide (H2O2). The mechanism of catalytic color involves nanozymes decomposing the O–O bond of H2O2 into hydroxyl radicals (˙OH) with high oxidative capacity and then generating ˙OH that further oxidizes chromogenic substrates.
Earlier reviews on nanozymes mainly focused on the aspects of fluorescent and electrochemical sensors. To date, almost few reviews have concentrated on nanozyme-based colorimetric assays. As a result, this review aims to highlight the recent advancements and applications of colorimetric sensors based on nanozymes with peroxidase-like activity, including noble metals, metal oxides, metal sulfides/metal selenides, carbon materials, and MOFs. Finally, we discuss the challenges faced in the nanozyme-based colorimetric sensors and look forward to the future development directions.
2. Metal-based nanozymes
The noble metal nanomaterials are stable and show catalytic performance similar to peroxidase. Their catalytic activity is mainly achieved by surface adsorption and rapid electron transfer. Thus, metal nanomaterials, including noble metals, transition metals, and alloys-based nanoparticles/nanoclusters (NPs/NCs) have been reported as enzyme mimics. For instance, gold (Au), platinum (Pt), palladium (Pd), and copper (Cu) NPs/NCs with intrinsic peroxidase-like activity have been successfully used in the colorimetric determination of Ag+, Hg2+, glucose, H2O2, dopamine, and xanthine, etc.2.1 Au-based nanozymes
In recent years, Au NPs have received more and more attention due to their simple preparation, good biocompatibility, and optoelectronic properties. In 2010, Jv Y. et al. discovered that positively charged Au NPs with peroxidase-like activity could be used to detect H2O2 and glucose.53 Au NPs could catalyze the oxidation of TMB-H2O2, which turned the colorless solution to blue. Based on this, a colorimetric sensor based on Au NPs was developed for the detection of H2O2 and glucose, and the detection limits (LODs) were 0.5 μM and 4 μM, respectively.Recently, paper-based sensors fabricated by modifying Au NPs on the paper have attracted considerable attention in improving colorimetric homogeneity and sensitivity.54 For example, Han N. K. et al. reported an Au NP-based paper chip for colorimetric detection of Hg2+.55 The combination of Hg2+ and Au NPs formed an Au–Hg alloy, which stimulated the peroxidase-like activity of Au NPs. The chromogenic substrates TMB were oxidized to blue stain on the cellulose paper chip by Au–Hg alloy in the presence of H2O2, with a LOD of 30 μg L−1. Subsequently, wicking pads (Whatman No. 1 filter paper) loaded with DNA-Au NPs were also developed to fabricate a paper device for colorimetric detection of Hg2+.56 DNA is an important substance that can control the peroxidase-like activity of various nanomaterials.57 Thus, DNA-Au NPs could combine with Hg2+ to produce a more stable DNA-Au–Hg alloy for enhancing peroxidase-like activity. Besides, wicking pads can adsorb a large volume of Hg2+ solution, therefore the color signal was significantly enhanced, and the lower LOD was 10 nM.
In addition, due to their specific identification function and easy modification, aptamers are mainly exploited as identification, signal conversion, and signal amplification elements in the design of colorimetric sensors.58 Recently, aptamer-based Au NP sensors have been used for competitive colorimetric analysis.59–61 The aptamers will inhibit the intrinsic peroxidase-mimicking activity of Au NPs by simply adsorbing onto the Au NPs surface. However, when there was a target, the target–aptamer will leave the Au NPs surface due to the high affinity and specificity to the target. Therefore, Au NPs restored their peroxidase-like activity for catalyzing TMB to produce a blue color. For example, an Au NP-based nanozyme aptasensor was proposed for ultrasensitive colorimetric sensing of murine norovirus (Fig. 1). An aptasensor probe was prepared by combining Au NPs with the high target specificity of a murine norovirus aptamer, which produced a blue color in the presence of a norovirus target.62 Similarly, other Au NP-based aptasensors were also used for colorimetric detection of Pseudomonas aeruginosa63 and multiple diarrheic shellfish poisons.64 More importantly, the peroxidase-like activity of Au NP-based aptasensors can be remarkably promoted by the hybridization chain reaction, enabling highly sensitive colorimetric detection of saxitoxin with a LOD as low as 42.46 pM.65 Thus, Au NP-based colorimetric aptasensors will be a promising direction for colorimetric sensor research.
 | ||
| Fig. 1 Schematic diagram of the nanozyme aptasensor prepared by adapting Au NPs to norovirus aptamer.62 | ||
Although the enzymatic catalytic activity has been greatly improved, Au NP-based nanozymes still face the challenges of difficult recovery and reuse. Alle M. et al. in situ synthesized Au NPs@cationic cellulose nanofibrils (C.CNF) nanozymes using C.CNF-loaded positively charged Au NPs, which could be reused by casting into a film.66 A sensitive colorimetric approach based on recyclable nanozymes was established for the detection of glucose in human serum with the help of the TMB substrate. Afterwards, the same group fabricated a reusable nanozyme via Au NPs immobilized on dialdehyde cellulose nanofibrils (DACNF).67 Au NPs@DACNF was modified onto a paper sensor for the colorimetric detection of cholesterol with ABTS as a substrate. Cholesterol oxidase (ChOx) catalyzed the oxidation of cholesterol to 4-cholestene-3-one and H2O2, and the resulting H2O2 further oxidized colorless ABTS to blue products in the presence of Au NPs@DACNF nanozymes. A smartphone application was employed to visually record and analyze color changes (Fig. 2). Additionally, some materials, such as quantum dots (QDs),68 β-cyclodextrin (β-CD),69 polyoxometalates (POMs),70 polyvinyl alcohol,71 hydrogel,72 cysteamine73 and tannic acid74 combined with Au NPs showed higher catalytic activity under neutral conditions, and were successfully utilized for colorimetric detection of metal ions and small biological molecules.
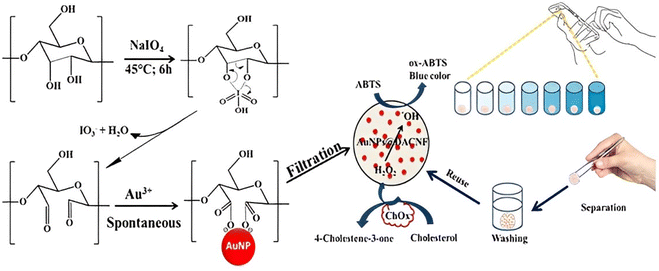 | ||
| Fig. 2 Schematic illustration of the preparation of paper-based Au NPs@DACNF sensor and the colorimetric detection of cholesterol assisted by smartphone.67 | ||
2.2 Pt-based nanozymes
In addition to Au nanomaterials, numerous investigations have proved that Pt nanomaterials can also be employed as peroxidase mimics for colorimetric assays. Compared with other metal-based nanozymes, Pt NPs possess much stronger activity for interacting with H2O2. However, due to the shortcomings of high polymerization cost and easy deactivation, the application scope of Pt NPs is greatly limited. To overcome these problems, Jin L. H. et al. prepared ultra-small Pt NCs for glucose analysis with the good catalytic ability and good biocompatibility via a one-pot hydrothermal approach.75 Besides precisely controlling the size and morphology of Pt nanomaterials, the catalytic activity of peroxidase-like enzymes can also be enhanced by surface modification. Li W. et al. selected bovine serum albumin (BSA) as the stabilizing agent to synthesize Pt-based nanozymes.76 BSA was regarded as an efficient template for the direct oriented growth of metal nanocrystals. BSA-stabilized Pt nanozymes, consisting of 57% Pt0 y with an average diameter of 2.0 nm have high peroxidase-like activity. Hg2+ was determined by label-free colorimetric strategy, which mainly reduced the enzyme activity of Pt NPs through the interaction with Pt0. This colorimetric sensor can detect Hg2+ in drinking water with a linear range of 0–120 nM and a LOD of 7.2 nM. Furthermore, the same group found that N-acety-L-cysteine (NAC) exhibited strong affinities to transition metal ions.77 They adopted NAC as the nucleation template to synthesize Pt NCs. This colorimetric assay of heparin was achieved by the interactions among Pt NCs, heparin and the TMB substrate, with a LOD of 2 × 10−3 μg mL−1. Such a colorimetric sensor has the advantages of high sensitivity, short sensing time, and is free of expensive instruments. In addition, nanozyme-linked immunoassays with Pt NPs offer a new platform for the colorimetric detection of parathion78 and triazophos.792.3 Pd-based nanozymes
Fu Y. et al. developed Pd-based nanozymes that were utilized for the colorimetric detection of Ag+.80 The synthesized Pd-based nanozymes using reduced glutathione (GSH) exhibited a high affinity for transition metal ions. They first reported that Ag+ can obviously inhibit the peroxidase-mimicking activity of Pd NPs. This inhibition was employed for highly sensitive colorimetric detection of Ag+ and Ag NPs in an aqueous solution by using TMB as a chromogenic substrate. Subsequently, the same group was inspired by GSH-capped Pd NPs, and they once again developed, for the first time, oligonucleotide-stabilized Pd NPs for the colorimetric detection of biothiols, with LODs of as low as 3.7 nM and 4.3 nM for cysteine and homocysteine, respectively.81 Thus, Pd-based nanozymes with simulated peroxidase-like activities show broad application prospects for quantitative colorimetric detection.2.4 Alloy-based nanozymes
Due to the synergistic effect of alloy-based nanozymes, their catalytic activities are generally stronger than those of single-metal nanomaterials. Among the various alloy nanomaterials, Pt-based alloy nanozymes are considered strong candidates for colorimetric analysis. For instance, Sun YH and coworkers have synthesized AuxPty nanozymes with high peroxidase mimicking activity.82 They first reported DNA-stabilized bimetallic AuxPty nanozymes for the colorimetric detection of biothiols using TMB as an indicator. Sun Y. et al. uniformly and rapidly grew Pt NPs on the surface of Au NRs using laser irradiation procedure.83 The prepared Au/Pt nanorods (NRs) possessed enhanced peroxide-like activity and surface-enhanced Raman scattering (SERS) efficiency, which realized the high sensitivity colorimetric detection of H2O2, with a LOD of 0.04 μM. Very recently, CuPt nanozymes with powerful catalytic ability have been extensively investigated. Lu Y. et al. fabricated three-dimensional (3D) layered porous CuPt dendrites (HPPtCuDs) by electrochemical displacement and chemical dealloying.84 Due to the electronic effect and geometric effect, HPPtCuDs showed enhanced peroxide-like activity, which was applied for colorimetric detection of H2O2 (Fig. 3). In addition, Wu L. L. et al. found that the catalytic performance of AgPt NCs could be inhibited by 3-mercaptopropionic acid (MPA), but could be recovered after catalyzed oxidation by Cu2+. Therefore, they successfully proposed a sensitive colorimetric method for detecting Cu2+via the TMB-H2O2 reaction system.85 Nevertheless, only a small fraction of Pt atoms were exposed when Pt-based nanozymes were synthesized by traditional methods. Therefore, the content of Pt atoms involved in the catalytic reaction was low, giving rise to poor catalytic performance. To overcome this limitation, Panferov V. G. et al. developed a new approach for efficient and uniform distribution of Pt atoms on nanozymes surfaces.86 Au core and Ag shell were applied to form Au@Ag NPs, and then the galvanic replacement of Ag with PtCl62− resulted in the formation of Au@Ag–Pt NPs. | ||
| Fig. 3 Schematic diagram of HPPtCuDs catalysis of the classical peroxidase substrate TMB by H2O2 and linear calibration plot for H2O2 detection.84 | ||
Besides Pt-based alloy nanozymes, Au-based alloy nanozymes with peroxidase-like activity are also attracting significant interest in colorimetric assays. Singh S. et al. explored the peroxide potential of Pd@Au NRs.87 The catalytic activity of Pd NPs dispersed on the surface of Au NRs was higher than that of Au NRs alone. Pd@Au NR-based nanozymes were employed for colorimetric sensing of malathion using OPD-H2O2 as substrates, with the lowest LOD of 60 ng mL−1. The peroxidase-mimicking activity of Ag–Au-based nanozymes was also demonstrated for visual colorimetric assays. Liu H. et al. covalently cross-linked Hemin (Hem) onto melamine (MA) through an EDC-NHS chemical bond to prepare a Hem-MA polymer matrix.88 MA-Hem/Au–Ag nanocomposites were fabricated by the bimetallic Au–Ag in-site mineralization into the Hem-MA polymer matrix (Fig. 4A). Moreover, the catalytic activity of complex enzymes obtained by combining MA-Hem/Au–Ag nanozymes with glucose oxidase (GOx) was investigated. As described in Fig. 4B, MA-Hem/Au–Ag-GOx complex enzymes catalyzed the oxidation of glucose-TMB to generate a blue signal. Complex enzymes exhibited significantly-enhanced catalytic activity due to the introduction of the Au–Ag bimetallic into the Hem-MA polymer matrix. It could not only be functionalized into “nanowires” to accelerate electron transfer but also had high adsorption capacity and affinity for the substrates. Similarly, Fang H. et al. proposed a colorimetric nanozyme-linked immunosorbent assay (NLSA) based on Au@Ag NPs nanocomposites.89 NLSA showed high sensitivity by M13 bacteriophage as a competing antigen assembled Au@Ag NPs for deoxynivalenol (DON) detection, with the LOD of 13.67 pg mL−1.
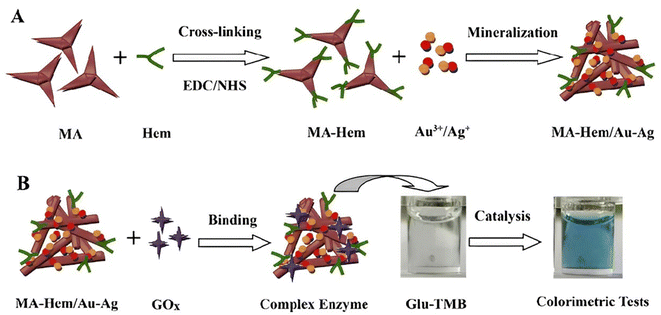 | ||
| Fig. 4 Schematic diagram of (A) MA-Hem/Au–Ag nanozymes prepared by MA-Hem cross-linking and Au–Ag mineralization, and (B) complex enzymes obtained by MA-Hem/Au–Ag nanozymes attached with GOx for colorimetric detection of glucose.88 | ||
3. Metal oxide-based nanozymes
Metal oxide nanomaterials are usually composed of transition metal oxide nanomaterials. The binding of such nanozymes to reaction substrates is usually accompanied by electron transfer and valence changes. Metal oxides nanozymes exhibiting peroxidase-like catalytic behavior possess the advantages of good stability, simple preparation process, and low cost, which have also been investigated for the colorimetric detection of various biomolecules, such as glucose, H2O2, and nucleic acids.3.1 Fe3O4-based nanozymes
In 2007, Gao et al. first reported that Fe3O4 nanomaterials showed peroxidase-like performance.4 Recently, a novel type of anti-aggregation nanozyme was synthesized containing Fe3O4 NPs immobilized monodispersed-porous SiO2 microspheres (Fe3O4 NP@pSiO2), which significantly enhanced the peroxidase-mimicking activity of Fe3O4.90 Previous studies have indicated that SiO2 can be grown in different NPs surfaces to form uniform core–shell nanostructures.91,92 For the first time, Fe3O4 NP@pSiO2 nanozymes were utilized to colorimetric determine human genomic DNA using OPD-H2O2 as substrates, and could also be applied for detecting other similar biological macromolecules. Subsequently, the same group selected an intracellular hyaluronic acid (HA) functionalized nanozyme as the ligand to obtain HA@Fe3O4@SiO2 microspheres by carbodiimide activation.93 A simple colorimetric protocol based on HA@Fe3O4@SiO2 nanozymes against TMB as the substrate was applied for the determination of tumor cells, including human cervical cancer (HeLa) and primary brain tumor (T98G) cells (Fig. 5). | ||
| Fig. 5 A schematic diagram for colorimetric determination of HeLa and T98G cell concentrations based on the difference in peroxidase-mimicking activity of HA@Fe3O4@SiO2 nanozymes.93 | ||
In addition, Yardimci B. et al. synthesized reusable and low-cost ethylenediamine (EDA)-modified Fe3O4 NPs in a one-pot reaction.94 The EDA@Fe3O4 nanozyme colorimetric sensors were designed to detect TNT/tetryl via catalyzed H2O2 oxidation to produce a blue-colored complex from oxidized TMB. The principle of the colorimetric sensors was that TNT and tetryl caused fading of blue color by inhibiting the catalytic performance of EDA@Fe3O4 nanozymes. Inspired by the principle of complementary coloring harmonics, Su M. et al. designed a bisubstrate multi-colorimetric system based on polyethylene glycol (PEG)@Fe3O4 NPs. According to the hue circle, TMB and dopamine were chosen to form the complementary colors of blue and orange.95 Thus, PEG@Fe3O4 NPs with peroxidase-like performance catalyzed the oxidation of TMB-dopamine for three-color threshold colorimetric detection of H2O2.
Although Fe3O4-based nanozymes colorimetry has been widely used in the field of biosensing, some limitations of nanozymes, such as the low catalytic ability and poor sensitivity to analytes remain unsolved. To address these shortcomings, multiple metal–metal oxide interfaces are good candidates for synergistic effects. Zeng's group rationally designed a Pd-Fe3O4 Janus nanozyme via a seed-mediated method.96 Due to the synergistic effect of Pd and Fe3O4 in dumbbell-like nanostructures, the ultrasensitive colorimetric detection of biothiols was realized. Meanwhile, the same group established an innovative colorimetric fluoride (F−) probe with high sensitivity and specificity based on AgPt-Fe3O4@SiO2.97
3.2 CeO2-based nanozymes
CeO2 displays strong redox behavior and enzyme-mimetic characteristics ascribed to its excellent O2 storage capacity and reversible valence switching between Ce(III) and Ce(IV). e Jiao X. et al. in 2012 reported that CeO2 NPs possessed peroxidase-like activity;98 how to improve their catalytic performance has become the focus of researchers. The current investigations demonstrated that surface functionalization and carrier loading can significantly improve the catalytic activity of CeO2. Lian J. J. et al. successfully fabricated functionalized CeO2 of perylene diimide (PDI) nanocomposites via a one-pot hydrothermal method.99 The combination of PDI and CeO2 maintained the excellent electron transfer ability of PDI and the strong redox ability of CeO2. Therefore, a highly sensitive and selective colorimetric sensor based on PDI-CeO2 nanocomposites was achieved for the detection of H2O2 and GSH, with LODs of 2.59 μM and 0.92 μM, respectively.Furthermore, due to the synergistic effects of bimetallic oxides and enzymatic cascade reactions, the obtained CeO2 has higher catalytic efficiency and stability. Chi M. Q. et al. prepared CeO2/Co3O4/poly(3,4-ethylenedioxythiophene) (PEDOT) composite nanofibers using an electrospinning and calcination process, followed by a redox reaction.100 They found that the enhanced peroxidase-mimicking activity of CeO2/Co3O4/PEDOT nanozymes was due to the synergistic interactions between multiple components. Therefore, a novel high-sensitivity colorimetric platform was established for the detection of H2O2 using CeO2/Co3O4/PEDOT composite nanofibers. Liu M. et al. synthesized a CeO2/GOx multi-enzyme complex via self-assembly.101 In this cascade catalytic system, GOx catalyzed the oxidation of glucose to produce H2O2, while CeO2 immediately degraded H2O2 with minimal diffusion (Fig. 6). Such an enzyme cascade reaction-based CeO2/GOx hybrid nanocomplex system was employed to establish a colorimetric sensor with high selectivity and sensitivity for glucose detection.
 | ||
| Fig. 6 Cascade reaction diagram of CeO2/GOx multi-enzyme complex catalyzed oxidation of glucose.101 | ||
Recently, the design and development of CeO2 nanozyme-based logic gates for colorimetric analysis have sparked extensive research efforts.102 Chishti B. et al. successfully integrated two and a three-input regulated AND logic circuit, and established a visual colorimetric sensor for the detection of melamine at acidic (4.5) and neutral (7.4) pH.103 The constructed logic gate sensor based on the intrinsic peroxidase-like activity of CeO2 NRs displayed a high affinity for melamine, producing outstanding visual output on a logic platform of TMB/H2O2 (Fig. 7). The developed sensor was successfully applied for the detection of melamine in raw milk and infant formula with picomolar sensitivity.
 | ||
| Fig. 7 Schematic illustration of the logic operation mechanism for melamine detection based on peroxidase-like activity of CeO2 NRs at acidic (4.5) and neutral (7.4) pH.103 | ||
3.3 MnO2-based nanozymes
MnO2 nanosheets as typical nanozymes possess appealing peroxidase-mimicking performance. Research on the peroxidase-like activity of MnO2 NPs has gradually deepened, and their catalytic activity can be improved by surface modification and loading. For example, Li Y. R. et al. established an immunosensor based on MnO2 NPs AND tyramine-triggered reaction for the amplification detection of alpha-fetoprotein (AFP) in human serum samples.104 Briefly, superparamagnetic beads (MBs)-Ab1 binding can specifically capture AFP in the sample solution. Then, a sandwich immune complex was formed with Ab2-MnO2 NPs through specific antibody–antigen interaction. Upon the introduction of H2O2, multiple MnO2 NPs can be conjugated to the surface of the immune complex through a tyramine-mediated reaction, resulting in signal amplification. Accordingly, MnO2 NPs with peroxidase-like activity can catalyze TMB from colorless to blue with repeated cycles of catalytic reactions.Likewise, Guan's group developed a colorimetric immunoassay based on MnO2 NRs for the detection of human chorionic gonadotropin (HCG).105 They synthesized MnO2 NRs with outstanding peroxidase-like activity using a hydrothermal method using EDTA as a template. The MnO2 NRs were first immobilized in the chitosan matrix of the BSA-modified 96-well plastic microplates, and then the microplates were further modified by streptavidin and biotinylated β-HCG antibodies. In the presence of HCG antigen, the specific recognition between HCG-antibodies on the sensing platform inhibited the peroxidase-like activity of MnO2 NRs through the steric hindrance effect. Therefore, based on this principle, as the HCG concentration was increased, the blue color generated by MnO2 NRs catalyzing TMB-H2O2 substrate gradually faded, which allowed for quantitative colorimetric sensing of HCG.
Although this review introduced the aforementioned metal oxides with peroxidase-like activity, the research is not limited to these. Other metal oxide-based nanozymes, such as copper(I) oxide (Cu2O),106 cobaltous oxide (CoO),107 molybdenum oxide (MoO3)108 and tricobalt tetraoxide (Co3O4)109 also have intrinsic peroxidase-like characteristics, forming a rich metal oxide-based nanozyme systems. These novel metal oxide-based nanozyme colorimetric platforms have the potential to be applied for sensing H2O2, glucose, acid phosphatase, and glyphosate.
4. Metal sulfide/metal selenide-based nanozymes
4.1 Metal sulfide-based nanozymes
A variety of metal sulfide-based nanomaterials have been widely used and studied because of their special structures and characteristics. Among them, the use of molybdenum disulfide (MoS2) with two-dimensional (2D) graphene-like layered materials have aroused remarkable interest in colorimetric assays. Lin T. R. et al. discovered that MoS2 nanosheets possessed intrinsic peroxidase-like properties, which can catalyze the oxidation of TMB by H2O2, resulting in a color reaction.110 Studies have shown that the surface modification of nanozymes can affect their catalytic performance. Based on this, Xian Z. Q. et al. formed a flower-like polymer MoS2@CoFe2O4 nanozyme based on CoFe2O4 modified with MoS2 for the colorimetric detection of cysteine (Cys) and GSH.111 TMB and H2O2 as substrates, MoS2@CoFe2O4 catalyzed oxidation of TMB to generate a blue color. The competition of TMB with Cys or GSH led to the gradual fading of the blue color of oxidized TMB with the increase of Cys or GSH content. It has been found that the favorable dispersion of MoS2 can reduce the aggregation effect of ferrite. Meanwhile, when interacting with ferrite, the peroxidase-mimicking activity of MoS2@CoFe2O4 was 4.4-fold and 1.8-fold to that of MoS2 and CoFe2O4, respectively.Inspired by the above work, Feng L. P. et al. first simply doped MoS2 nanosheets with Fe element via a one-pot hydrothermal route, obtaining a highly superior peroxidase-like catalytic Fe-MoS2 nanozymes (Fig. 8).112 Thus, Fe-MoS2 nanosheet-based colorimetric strategy realized the detection of glucose with satisfactory sensitivity. Except for Fe-based doping, Prussian blue NPs (CPBNPs) or other metals (i.e., Au, Ag, and Pd) NP hybridized MoS2 nanosheets can also increase the peroxidase-mimicking activities of MoS2. Zhu Z. Q. et al. prepared MoS2-CPBNPs nanozymes to present a probe for colorimetric detection of dopamine (DA), with a LOD of 0.09 μM.113 As expected, MoS2-CPBNPs nanozymes showed superior peroxidase-like activity and catalyzed TMB to produce a blue product and ˙OH in the presence of H2O2. While adding DA, the color of the solution gradually faded and the adsorption strength decreased with the consumption of ˙OH. Moreover, a series of polypyrrole (PPy)@MoS2-based nanozymes deposited on three typical metal NPs (Au, Ag, and Pd NPs) were synthesized via in situ reductions by exploiting the inherent reducibility of MoS2 (Fig. 9).114 The differential binding interactions of different biomolecules and metal NPs induced peroxidase-like property modulation of metal NP-decorated PPy@MoS2. On the basis of this, a cross-reactive colorimetric sensor array-based PPy@MoS2@Au/Ag/Pd was developed for the accurate and effective discrimination of 11 proteins and 5 oral bacteria with substrates of TMB and H2O2.
 | ||
| Fig. 8 Schematic diagram of enzyme activity comparison of MoS2 and Fe-MoS2 prepared using a hydrothermal method.112 | ||
 | ||
| Fig. 9 (A) Schematic illustration of Au, Ag, and Pd NP-decorated PPy@MoS2 nanocomposites prepared by in situ reduction and (B) PPy@MoS2@Au catalytic oxidation of TMB.114 | ||
In addition, other metal sulfide-based nanozymes, such as vanadium disulfide (VS2), copper sulfide (CuS), and platinum disulfide (PtS2), with specific morphologies also possess intrinsic peroxidase-like behavior. They were chosen as a useful and dependable colorimetric probe for the determination of H2O2 and glucose in real samples. For instance, Huang LJ and coworkers combined VS2 nanozymes and GOx to design a colorimetric cascade assay for the detection of glucose by employing the catalyzed TMB-H2O2 reaction.115 In brief, GOx was first incubated with glucose for catalytic oxidation to generate gluconic acid and H2O2 with the existence of O2. After that, the reaction between VS2 and TMB was initiated using the resultant H2O2, causing a blue color solution. Ultimately, the glucose concentration was indirectly detected using the resulting color as colorimetric signals. As with this colorimetric assay principle, Swaidan A. et al.116 and Zhang W. X. et al.117 integrated CuS/PtS2-based nanozymes with GOx to demonstrate a colorimetric cascade method for sensing H2O2 and glucose.
4.2 Metal selenide-based nanozymes
Molybdenum diselenide (MoSe2) possesses intrinsic peroxidase mimetic activity, which has emerged as a promising candidate in various colorimetric assays. Wu XJ and coworkers first pointed out that few-layer MoSe2 nanosheets displayed great potential as peroxidase mimics.118 Firstly, xanthine produced H2O2 and uric acid under the action of xanthine oxidase. Then, using a liquid exfoliation method, few-layer MoSe2 nanosheets were obtained for catalyzing the oxidation of TMB-H2O2 to a blue oxidative product in aqueous media. Based on this catalytic mechanism, a highly sensitive and selective colorimetric approach was developed for the determination of H2O2 and xanthine.Moreover, the large surface area of MoSe2 nanosheets was compatible with different surface engineering tactics. Chitosan was conjugated onto MoSe2 nanosheets (CS-MoSe2 nanosheets) surface through a one-step ionic liquid-assisted grinding approach for the colorimetric determination of Hg2+.119 When Hg2+ was introduced, chitosan molecules captured Hg2+, and partial Hg2+ was in situ reduced to Hg0 on the surface of CS-MoSe2 nanosheets. Hence, the peroxidase-like properties of CS-MoSe2 nanosheets were enhanced by the activating effect of Hg2+. Using TMB as a chromogenic dye, a sensitive and rapid platform for the colorimetric detection of Hg2+ was established by integrated low cytotoxicity CS-MoSe2 nanosheets sensor and a smartphone detection device, with a lower LOD of 8.4 nM (Fig. 10). Lin L. and coworkers recently synthesized MoSe2@Fe-based nanozymes via a one-pot hydrothermal strategy for the colorimetric sensing of Fe3+ and GSH.120 MoSe2@Fe with peroxidase mimetic property catalyzed the oxidation of TMB-H2O2 to form blue oxTMB solution. The enzymatic activity increased (or decreased) when Fe3+ (or GSH) was added, resulting in a deepening (or weakening) of the blue color of the system, allowing quantitative and selective recognition of Fe3+ and GSH in food samples.
 | ||
| Fig. 10 Versatile colorimetric detection diagram of Hg2+ based on CS-MoSe2 nanosheets.119 | ||
5. Carbon-based nanozymes
In 2010, Song Y. J. et al. showed that single-walled carbon nanotubes (SWCNTs) possessed intrinsic peroxidase-mimicking performance, catalyzing the reaction of substrate TMB-H2O2 to generate a blue color.121 Due to fascinating enzyme mimetic activity, low toxicity, high stability, and good biocompatibility, carbon-based nanomaterials, such as graphene oxides (GO), graphitic carbon nitride (g-C3N4), single atom nanozymes (SAzymes) and carbon dots have aroused growing interest in nanozyme-based colorimetric assays.5.1 GO-based nanozymes
GO is a 2D material with a honeycomb lattice composed of sp2 carbon atoms, which has a large specific surface area, high electron mobility, and mechanical strength. Qu's and Ren's groups reported that carboxy-modified GO (GO-COOH) possessed intrinsic peroxidase-like activity that catalyzed the oxidation of the TMB-H2O2 system to produce a blue color signal.122 Based on their findings, they established a glucose colorimetry assay using the catalytic activity of GOx and GO-COOH peroxidase (Fig. 11). Subsequently, the same group first synthesized GO-Au NCs hybrid (GA) by simply absorbing Au NCs on GO through the electrostatic interactions, which exhibited excellent peroxidase-like activity.123 Using folic acid conjugated GO-Au NCs hybrid, they designed a simple, low-cost, highly selective and sensitive colorimetric sensor for the detection of cancer cells. | ||
| Fig. 11 Schematic diagram of a colorimetric approach for the detection of glucose based on GOx and the GO-COOH catalyzed TMB-H2O2 system.122 | ||
However, the dispersion of metals and metal oxide NPs on GO nanosheets in a unique 2D layer assembly hinders their aggregation induction, leading to a decrease in enzyme-like activity. To solve this limitation, Sun H. Y. et al. prepared small-sized iridium oxide (IrO2) NPs supported on polyallylamine (PAH)-modified GO nanosheets via electrostatic interactions by employing a facile and environment-friendly pulsed laser ablation in liquid (PLAL) method.124 Due to the large surface area and anchor sites, the choice of GO modified with PAH as the carrier of IrO2 was beneficial to reduce the aggregation of GO while extending the reaction time. The high peroxidase-like activity of the IrO2@GO nanozymes was derived from accelerated electron transfer rather than dependent on ˙OH production. Upon the introduction of AA, the admirable peroxidase-mimicking properties of IrO2@GO nanozymes could be inhibited. Herein, a colorimetric probe based on the IrO2@GO-H2O2-TMB reaction system was constructed for sensing AA.
Additionally, using PEG derivatives as coupling linkers, Zhang S. T. and coworkers first synthesized strongly coupled Au/Fe3O4/GO hybrid materials.125 Owing to the abundant functional groups with remarkable hydrophilicity and high surface area in GO, the aggregation of Au and Fe3O4 NPs was reduced while the deposition of Hg0 on the Au surface was increased. Moreover, the use of superparamagnetic Fe3O4 as a carrier can facilitate the recovery of ternary hybrid materials. The authors further demonstrated that Hg2+ could stimulate the peroxidase-like property of Au/Fe3O4/GO hybrid materials for ultrasensitive colorimetric sensing of Hg2+ by catalyzing TMB with H2O2. Furthermore, Adegoke O. et al. successfully anchored Au NPs and CeO2 NPs onto GO nanosheet surfaces using a chemically modified means.126 In the presence of nitrite, nitrite drove the redox cycle of Au NP-CeO2 NP@GO nanozymes to rapidly catalyze the oxidation of colorless TMB to produce a unique green color signal without H2O2 (Fig. 12). Therefore, a colorimetric sensor based on AuNP-CeO2 NP@GO nanozymes was developed for the colorimetric determination of nitrite in tap water.
 | ||
| Fig. 12 Schematic illustration of the synthesis of Au NP-CeO2 NP@GO nanozymes and their use for colorimetric detection of nitrite.126 | ||
Compared with bimetallic NPs catalysts, bimetallic oxide NPs nanozymes are rarely investigated. Accordingly, Das's group reported low-cost Fe3O4–TiO2 decorated on the reduced GO (rGO) nanosheets through a simple hydrothermal method.127 rGO nanosheets could promote the interaction of composites with targeted molecules via π–π interactions. Benefiting from the synergistic effects of Fe3O4 NPs and TiO2 NPs, magnetic Fe3O4–TiO2/rGO-based nanozymes improved the activity of peroxidase-like catalysis for the colorimetric detection of atrazine pesticide by oxidizing TMB-H2O2 (Fig. 13). Similarly, a novel nanozyme for the modification of Fe3O4 NPs, CuO NPs, and cucurbit[6]uril (CB[6]) onto the surface of GO was fabricated by employing a facile sonochemical technique.128 In Fe3O4-CuO-CB[6]-GO nanozymes, CB[6] as a specific binding pocket equipped nanozymes with molecular recognition ability, and the synergistic effect of Fe3O4-CuO-GO facilitated the improvement of the peroxidase-mimicking activity. Attributable to the inhibition effect of homocysteine (Hcy) towards the oxidation of TMB-H2O2 catalyzed by Fe3O4-CuO-CB[6]-GO nanozymes, a sensitive and selective colorimetric approach was thereby established for the detection of Hcy, with a LOD of 1.8 μM. In addition, other GO-based nanozymes, such as Prussian blue (PB),129 cetyltrimethylammonium bromide (CTAB),130 ganoderma polysaccharide (GP),131 and quantum dot (QD)132 incorporated GO also possessed the excellent properties of simulating peroxidase with higher catalytic activity. Accordingly, the ultrasensitive colorimetric biosensor was established for the detection of apolipoprotein A1 (ApoA1), amphetamine (AMP), methamphetamine (MAMP), L-cysteine (L-Cys) and H2O2, respectively.
 | ||
| Fig. 13 Schematic illustration of (i) TMB adsorbed on the surface of Fe3O4–TiO2/rGO nanozymes, (ii) ˙OH generated by the Fenton reaction, and (iii) TMB oxidized by ˙OH radicals.127 | ||
5.2 g-C3N4-based nanozymes
g-C3N4, as a typical metal-free semiconductor, possesses a 2D graphene-layered structure, which exhibits enzyme-like activity due to its unique structure and property. However, compared with GO, the activity of g-C3N4 nanosheets appears very weak. Therefore, some metal ions or metal oxides were used to dope or modify g-C3N4 for remarkably enhancing the catalytic performance of g-C3N4. For example, Zhao X. T. et al. prepared spherical g-C3N4/CeO2 nanozymes by in situ growth of CeO2 NPs on g-C3N4 nanosheets.133 The synergistic introduction of g-C3N4 nanosheets facilitated the electron transfer of CeO2, which significantly improved the catalytic activities of g-C3N4/CeO2 nanozymes, and catalyzed the oxidation of TMB to give a blue signal. Surprisingly, due to the strong interaction between Hg2+ and the nitrogen of g-C3N4, g-C3N4/CeO2 nanozymes would be aggregated, resulting in a reasonable reduction of catalytic capacity in the presence of Hg2+. As a result, a selective and sensitive colorimetric platform based on g-C3N4/CeO2 nanozymes was developed for sensing Hg2+ in blood and wastewater samples containing complex matrices. Shen Y. Z. et al. introduced Cu2+ into g-C3N4 to fabricate Cu-doped-g-C3N4 nanocomposite-based nanozymes for evaluating residual antibiotics in food and agriculture-related matrices.134 Due to the synergistic coordination arising from abundant Cu2+ and g-C3N4 nanosheets, Cu-doped-g-C3N4 nanocomposite-based nanozymes exhibited impressive peroxidase-mimicking activity. As expected, it displayed a high affinity for catalyzing colorless TMB-H2O2 to generate a steel-blue oxTMB product. Upon the introduction of tetracycline (TC), the effect of Cu-doped-g-C3N4 nanozymes on the catalytic substrates was significantly blocked, which was due to the blocking effect of the π–π stacking interaction between the TC tetraphenyl skeleton and nanozymes. As a result, the residual concentration of TC increased, and the visual color could be observed from steel-blue to light-blue. Based on this principle, a smartphone-based colorimetric assay was built to perform the quantitative analysis of TC in milk, with a LOD of 86.27 nM.Moreover, g-C3N4 nanozymes have been shown to absorb single-stranded DNA (ssDNA). The high affinity between g-C3N4 and ssDNA enables g-C3N4/ssDNA sensing platforms to specifically recognize target molecules. However, the catalytic activity of g-C3N4 nanosheets against H2O2 is low. To break through this bottleneck, Fan Y. F. et al. prepared a novel nanozyme based on 3D branched carbon nitride nanoneedles (3DBC-C3N4).135 The 3D branched structures possessed a large specific surface area and plenty of active sites, which promoted electron transfer and induced a lightning-rod-like effect to accelerate the electron collection in the tip region for activating H2O2 (Fig. 14). Therefore, 3DBC-C3N4 nanozymes can adsorb more target molecules for the catalytic reaction and improve catalytic activity. With the activation of H2O2 to the formation of ˙OH, 3DBC-C3N4 with excellent peroxidase-like activity catalyzed the oxidation of TMB in the presence of H2O2. In addition, ssDNA accelerated the oxygenation of TMB through electrostatic interaction, thus improving the peroxidase-like activity of 3DBC-C3N4 nanozymes. The increased concentration and length of ssDNA further enhanced the catalytic oxidation of TMB by 3DBC-C3N4 nanozymes, which could also be employed for the colorimetric detection of oxytetracycline (OTC).
 | ||
| Fig. 14 Schematic diagram of the 3DBC-C3N4 nanozymes accelerating electron collection in the tip region to activate H2O2 to produce ˙OH.135 | ||
5.3 Single-atom nanozymes
Traditional nanozymes generally face enormous challenges of low activity and poor specificity ascribed to their different crystalline nanostructures and diverse surface configurations. Toward this end, single-atom nanozymes (SAzymes) with isolated metal atoms anchored on nitrogen-enriched carbon material supports have attracted extensive attention in catalysis and biosensing fields. SAzymes with uniformly dispersed active sites hold nearly 100% atom utilization efficiency and maximum specificity, which are reckoned as ideal nanozymes with favorable catalytic activity and selectivity.136,137Zhou X. B. et al. prepared Fe–N–C SAzymes showing exceedingly good peroxidase-mimicking activity and specificity via an isolation-pyrolysis approach.138 While galactose oxidase (Gal Ox) catalyzed the oxidation of galactose to generate H2O2, Fe–N–C SAzymes further catalyzed the oxidation of TMB-H2O2 to produce a blue signal. Such an enzymatic cascade reaction based on Gal Ox and Fe–N–C SAzymes realized the rapid and sensitive quantitative colorimetric detection of galactose. Similarly, Mao Y. and coworkers reported a novel colorimetric assay for the determination of Cr(VI) ions using Fe–N–C SAzymes.139 Using 8-hydroxyquinoline (8-HQ) as an inhibitor could prevent Fe–N–C SAzymes from catalyzing the oxidation of TMB to generate blue oxTMB products. When Cr(VI) ions were added, due to their strong chelating capability, the preferential binding of Cr(VI) ions to 8-HQ ultimately led to blue color recovery. According to this phenomenon, an ultrahigh sensitive and selective colorimetric platform based on Fe–N–C SAzymes was proposed for sensing Cr(VI) ions, with a lower LOD of 3 nM. However, many SAzymes have the disadvantages of low dispersion of aqueous solution and lack of recognition ability, which hinders their application in biological analysis. Therefore, Sun L. P. et al. synthesized DNA/Fe–N–C SAzymes conjugates by engineering DNA modification to Fe–N–C SAzymes.140 This significantly enhanced the dispersibility of Fe–N–C SAzymes without affecting the peroxidase-like activity of Fe–N–C SAzymes. A HePG2 cancer cell sensor was established based on a diblock DNA (one polyadenine and one aptamer DNA sequence) attached to Fe–N–C SAzymes in HEPES containing MgCl2 (Fig. 15A). Apt/Fe–N–C SAzymes, after binding to HePG2 cancer cells with aptamer-targeting receptors, catalyzed the oxidation of the TMB-H2O2 system to produce the blue signal (Fig. 15B).
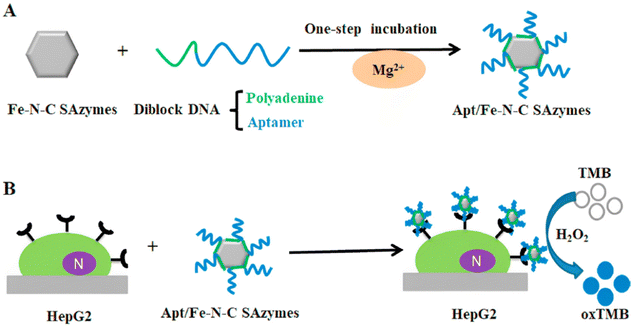 | ||
| Fig. 15 Schematic diagram of Apt/Fe–N–C SAzymes preparation (A) and colorimetric detection of HePG2 cancer cells (B).140 | ||
In recent years, sensors based on nanozymes with enzyme-like activity have been employed for the rapid and low-cost detection of antioxidants. However, most colorimetric sensors based on nanozymes are single sensors that can only detect one type of antioxidant, and also have very limited specificity. Herein, a colorimetric sensor array based on SAzymes was designed to recognize a variety of biological antioxidants. For instance, Jing W. J. et al. constructed a three-channel colorimetric sensor array based on Fe–N–C SAzymes for the simultaneous discrimination of five representative biological antioxidants including GSH, L-Cys, AA, uric acid (UA), and melatonin (MT).141 Fe–N–C SAzymes could catalyze the oxidation of TMB, OPD, and ABTS, respectively, generating blue oxTMB, yellow oxOPD, and green oxABTS. When different contents of GSH, L-Cys, AA, UA, and MT were added, the sensing probes of oxTMB, oxOPD, and oxABTS were reduced to diverse degrees, respectively, also being accompanied by different color responses. Thus, a three-channel colorimetric sensor array was fabricated to accurately identify five representatives as low as 100 nM in human serum samples through the linear discriminant analysis (LDA). Following the same detection principle, Liu B. and coworkers developed a Co–N–C SAzymes-based array sensor with a simpler design and more sensitive detection for distinguishing seven antioxidants: GSH, AA, Cys, tannin (TA), catechin (C), DA and UA, with the lowest LODs of 10 nM (Fig. 16).142 The identification and quantitative analysis of multiple target antioxidants were achieved using the colorimetric changes as fingerprints attributed to the different catalytic abilities of Co–N–C SAzymes at pH 3.6 and 4.8 as array receptors.
 | ||
| Fig. 16 Schematic diagram of identifying seven antioxidants based on the sensor array of Co–N–C SAzymes as sensor units at different pH values.142 | ||
5.4 Other carbon-based nanozymes
As a carbon nanostructure with a size of less than 10 nm, carbon dots that mimic the structure and function of natural enzymes have the advantages of easy modification, low toxicity, and good biocompatibility. Recently, C-dots/Mn3O4 nanocomposites were synthesized chemically and further verified with various techniques.143 Compared with Mn3O4 NPs, C-dots/Mn3O4 nanocomposites showed higher intrinsic oxidase-like activity. In the presence of O2, C-dots/Mn3O4 nanozymes catalyzed the oxidation of TMB to produce a blue color solution. Once ferrous ions (Fe2+) were introduced, the blue color faded to varying degrees due to the competition reaction between Fe2+ and TMB. In this way, a highly sensitive and selective colorimetric sensor based on a cheap C-dot/Mn3O4 nanozyme probe was established for sensing Fe2+, with a LOD of 0.03 μM.Additionally, enzymatic cascade reactions of other carbon-based nanozymes, such as carbon support material, carbon quantum dot (CQD), and graphdiyne oxide (GDYO), with natural enzymes have also been employed in colorimetric detection investigations. Ren H. et al. first synthesized MoO3 nanorod-supported carbon materials (MoO3/C) with outstanding peroxidase-mimicking activity utilizing a green tide biomass template (Fig. 17).144 As-synthesized MoO3/C combined with the GOx cascade reaction were applied for constructing colorimetric sensors for the sensitive detection of glucose by using TMB as chromogenic substrates. The process of applying MoO3/C nanozymes to detect glucose mainly consisted of two steps. Under the catalysis of GOx, glucose was initially oxidized by O2 to gluconic acid and H2O2, and then the synthesized H2O2 further oxidized TMB, inducing a color change in the presence of MoO3/C nanozymes. By the same principle, as described above, Mo, S-co-doped CQD (Mo-CQDs), and hemin/GDYO nanocomposites formed cascade reactions with ChOx and GOx, respectively, for colorimetrically sensing cholesterol145 and glucose.146 These studies have greatly broadened the application range of carbon-based nanozymes.
 | ||
| Fig. 17 Preparation of MoO3/C nanorods by a green tide biomass template.144 | ||
6. Metal–organic frameworks-based nanozymes
Metal–organic frameworks (MOFs) are self-assembled from common metal ions and organic cross-linking agents. Due to their unique porous structures and large specific surface areas, MOFs have been considered as flourishing research materials for the construction of nanozymes. The ordered arrangement of metal nodes and organic ligands provides MOFs with abundant accessible catalytic sites and endows MOFs with inherent enzyme-like activities for applications in colorimetric assays. The applications of MOFs in nanozymes mainly include the peroxidase-like activity of MOFs themselves and the use of MOFs as carriers for various enzymes. It is worth noting that one of the effective ways to further improve the catalytic performance is to modulate the composition of MOF-based nanozymes by metal doping (such as Fe, Ni, Co, and Mn).At present, Fe-doped MOFs with peroxidase-like activity have been demonstrated for the colorimetric detection of biomolecules. For example, Chen N. et al. developed a naked-eye and point-of-care testing sensor based on Fe-MOFs nanozymes for immunoassay colorimetric determination of Salmonella enteritidis (S. enteritidis).147 The basic principle of colorimetric determination was as follows. Ab1 (mouse anti-Salmonella monoclonal antibody) modified nanozymes will combine with Ab2-modified magnetic beads (Dynabeads™ anti-Salmonella) through a specific interaction between Ab1, Ab2, and S. enteritidis with the addition of target virus. In this manner, the formed sandwich structure “Ab1-modified nanozymes-S. enteritidis-Ab2 modified magnetic beads” were removed from the supernatant with an external magnet. After magnetic separation, residual Fe-MOFs nanozymes that were not bound to the target could not be removed due to the lack of the attached magnetic beads. Therefore, using TMB-H2O2 as colorimetric substrates, S. enteritidis can be measured indirectly and successfully by residual Fe-MOFs nanozymes promoting the oxidation of TMB to generate different degrees of blue color signals in the supernatant. Since the Fe-doped MOFs nanozymes enhanced the sensing signal, S. enteritidis can be detected with a lower LOD of 34 CFU mL−1. Furthermore, MIL-53(Fe) was chosen as MOFs-based nanozymes, serving to realize the colorimetric detection of salicylic acid (SA) in aspirin.148 MIL-53(Fe) prepared using a solvothermal method exhibited favorable peroxidase-mimicking activity, which catalyzed the oxidation of TMB in the presence of H2O2 and rendered it blue. Notably, the catalytic activity of MIL-53(Fe) was clearly suppressed after the introduction of SA, which displayed a lighter blue color due to the complexation reaction of SA with Fe3+ at the center of MIL-53(Fe) (Fig. 18). Accordingly, a high selectivity and sensitivity approach based on MIL-53(Fe) MOF nanozymes was proposed for the sensing of SA, with a LOD of 0.26 μM.
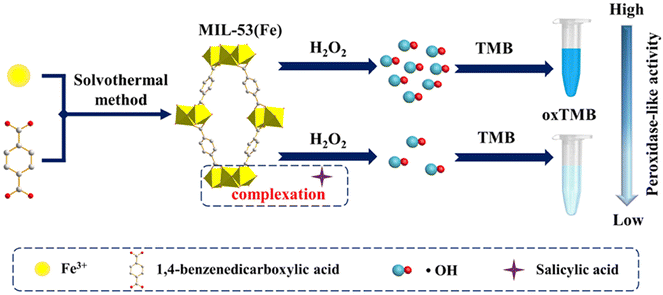 | ||
| Fig. 18 Schematic diagram of MIL-53(Fe) synthesized using the solvothermal method and the colorimetric detection of SA based on MIL-53(Fe) MOFs nanozymes.148 | ||
In recent years, Cu-MOF nanozymes systems have not been effectively applied due to the mutual interference between the properties of various enzymes. To address this issue, Ying M. H. and coworkers explored a novel Cu/fumaric acid (FMA)-MOFs with high bifunctional nanozyme activities and good anti-interference properties.149 Cu2+ and Cu+ active centers existed on the surface of Cu/FMA, which showed peroxidase and laccase-like activities under pH = 4 and 8, respectively. Accordingly, a dual-mode colorimetric platform based on Cu/FMA-MOFs was established for detecting glucose and epinephrine in human serum.
Relative to that of the single-metal MOFs, the bimetallic MOFs formed by the partial replacement of the original metal nodes with other metal ions possess higher nanozymes catalytic activity. In principle, doping two metal elements in the initial MOFs can enhance the catalytic activity due to the good synergistic electron transfer effects between various metals. For instance, 2D Ni/Fe-MOFs nanosheets were prepared using a simple two-step sonication method at room temperature.150 With strong intrinsic peroxidase-like activity, 2D Ni/Fe MOFs nanosheets could catalyze the oxidation of the TMB-H2O2 reaction system from colorless to blue. The addition of glutathione could markedly inhibit the peroxidase-like activity of 2D Ni/Fe-MOF nanosheets. Interestingly, due to the high specific affinity of Hg2+ for thiol groups in glutathione, further introduced Hg2+ could restore the suppressed TMB oxidation. According to the above principles, a colorimetric platform based on 2D Ni/Fe-MOFs nanosheets was constructed to recognize multiple targets, including H2O2, glutathione, and Hg2+, with LODs of 10 nM, 10 nM, and 100 nM, respectively. Due to the high density of active metal sites in 2D Ni/Fe-MOFs nanosheets, the synergistic effect between Ni and Fe strengthened the intrinsic catalytic behavior of each site. Meanwhile, as demonstrated from the UV-Vis spectra, compared with single-metal Ni-MOFs and Fe-MOFs, the peroxidase-like activities of the 2D Ni/Fe-MOFs nanosheets were enhanced by over 14-fold and 3-fold, respectively.
Subsequently, Zhao X. L. et al. designed a simple, rapid, and cost-effective colorimetric approach based on Co/Fe-MOFs-iodide (I−) nanozymes for detecting hydrogen sulfide (H2S) in real serum samples.151 In the presence of H2O2, Co/Fe-MOFs and I− could synergistically catalyze TMB to blue oxTMB, thereby enhancing the catalytic performance of Co/Fe-MOFs-I−. When H2S was present, the color rendering effect of TMB was suppressed and the system appeared a lighter blue (Fig. 19). A novel ultrahigh sensitivity H2S colorimetric platform was therefore established, with the lowest LOD of 0.33 nM. While in the absence of I−, the LOD was approximately 200 times higher than that from the amplified colorimetric assays.
 | ||
| Fig. 19 Schematic illustration of H2S detection by Co/Fe-MOFs-I− nanozyme-based colorimetric sensors.151 | ||
The synthesis conditions of most MOFs (i.e. Fe-MOF and Cu-MOF) are harsh, generally requiring high temperature and high pressure, as well as numerous organic reagents. Nonetheless, biomimetic zeolitic imidazolate frameworks (ZIF) have attracted extensive attention due to their promising biocompatibility, diverse enzyme-like activities, and facile synthetic conditions. Nataraj N. et al. reported a novel Co-ZIF-67 nanozyme via a simple co-precipitation method at room temperature.152 Co-ZIF-67 nanozymes exhibited intrinsic peroxidase-mimicking performance and effectively oxidized TMB to produce blue oxTMB in the presence of H2O2. Interestingly, the abundant availability of Co-ZIF-67 active sites binding acetaminophen provided enhanced catalytic properties. Different concentrations of acetaminophen could oxidize TMB to generate varying degrees of dark blue. As a result, Co-ZIF-67 nanozymes were employed for the colorimetric detection of acetaminophen. Furthermore, Ran F. P. et al. first constructed a novel nanozyme sensor by utilizing the self-assembly of Au NCs and ZIF-8/polyamine functionalized quantum dots (CQDs).153 They found that the peroxidase-like activity of Au NCs can be significantly enhanced by heparin in the physiological environment (pH 7.4). Accordingly, the catalytic activity of Au NCs-ZIF-8/CQD nanozmes on TMB-H2O2 substrate was significantly improved (Fig. 20). Once protamine was introduced, due to the specific binding of protamin to heparin, heparin was unable to adsorb on the surface of ZIF-8/CQDs, thus significantly inhibiting the enhancement of the catalytic activity of Au-NCs. Based on the above facts, a facile and efficient colorimetric method was proposed for the quantitative detection of heparin and protamine in biological samples. These studies provide valuable references for designing MOFs-based nanozymes in colorimetric assays.
 | ||
| Fig. 20 Schematic illustration of the colorimetric determination of heparin and protamine.153 | ||
7. Conclusion and prospects
This review outlines the research progress and applications of colorimetric sensors based on nanozymes with peroxidase-like activity in diverse fields over the past decade. Due to the advantages of nanozymes with more flexible structure, high stability, and ease of modification, nanozyme-based colorimetric sensors have emerged as promising alternative tools for the rapid, simple, and low-cost detection of analytes. Although remarkable progress has already been made in colorimetric sensors based on nanozymes with peroxidase-like activity, several challenges and obstacles remain in this frontier field.(1) Since nanomaterials are easily aggregated and unstable, how to improve the catalytic activity and stability of nanozymes has become an urgent problem to be solved. Hence, modifying the surface of nanomaterials to synthesize efficient nanozymes with high catalytic activity and high affinity will be the focus of future research.
(2) In the detection of real biological samples, the non-specific adsorption of proteins to nanozymes will affect the catalytic activity of nanozymes, resulting in unsatisfactory detection results. For the development of novel nanozymes with high utilization, it is necessary to further investigate the anti-interference of their enzyme-like activities.
(3) In recent years, the potential safety, environmental, and health concerns of nanozymes have drawn public and academic attention to their toxicity. Faced with this challenge, besides the nanomaterials themselves, surface coatings and modifications should also be considered.
(4) In addition, considering the diversity of natural enzymes, designing nanozymes with novel catalytic properties, such as hydrolases and synthases, to greatly improve the selectivity of nanozymes will also be the focus of future work.
To sum up, although nanozyme-based colorimetric biosensors show broad prospects, the practical applications of basic and leading scientific achievements in clinical diagnosis, food analysis, immunoassay, and environmental monitoring requires more effort. We hope that this review can provide a certain reference value for the synthesis of nanozymes and their application in colorimetric analysis, which is helpful for their future development.
Author contributions
Chi Zhongmei: Conceptualization, methodology, validation, investigation, resources, supervision, writing-original draft, writing-review & editing. Wang Qiong: Supervision. Gu Jiali: Conceptualization.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors gratefully acknowledge the financial support from the Initializing Foundation of Doctors in Bohai University (Grant No. 05013-0521bs018), Bohai University School-Level Scientific Research Project (Grant No. 05015-0522xn043), and the Doctoral Scientific Research Foundation of Liaoning Province (Grant No. 2023-BS-801).References
- N. Ullah, M. Mansha, I. Khan and A. Qurashi, TrAC, Trends Anal. Chem., 2018, 100, 155–166 CrossRef.
- A. Azzouz, S. K. Kailasa, P. Kumar, E. Ballesteros and K. H. Kim, TrAC, Trends Anal. Chem., 2019, 113, 256–279 CrossRef.
- M. A. Al Mamun and M. R. Yuce, Adv. Funct. Mater., 2020, 30, 2005703 CrossRef.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Y. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef.
- H. Wei and E. K. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Q. Q. Wang, H. Wei, Z. Q. Zhang, E. K. Wang and S. J. Dong, TrAC, Trends Anal. Chem., 2018, 105, 218–224 CrossRef CAS.
- J. J. X. Wu, X. Y. Wang, Q. Wang, Z. P. Lou, S. R. Li, Y. Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- Y. Y. Tian, Y. C. Chen, M. Chen, Z. L. Song, B. Xiong and X. B. Zhang, Talanta, 2021, 221, 121627 CrossRef CAS.
- B. Zhang, Y. J. Lv, C. G. Yu, W. Zhang, S. S. Song, Y. X. Li, Y. Chong, J. Huang and Z. J. Zhang, Biomater. Adv., 2022, 137, 212869 CrossRef CAS PubMed.
- Z. Zhou, Y. L. Wang, F. Peng, F. Q. Meng, J. J. Zha, L. Ma, Y. H. Du, N. Peng, L. F. Ma, Q. H. Zhang, L. Gu, W. Y. Yin, Z. J. Gu and C. L. Tan, Angew. Chem., Int. Ed., 2022, 61, e202115939 CAS.
- J. Q. Zha, W. G. Wu, P. Xie, H. H. Han, Z. Fang, Y. T. Chen and Z. F. Jia, Catalysts, 2022, 12, 614 CrossRef CAS.
- S. Zhang, P. Li, S. Chu, L. P. Feng, S. Li, J. T. Fan, S. J. Xie, Y. Zhang, G. J. Mao and H. Wang, Appl. Surf. Sci., 2022, 597, 153686 CrossRef CAS.
- L. M. Rao, X. Y. Lu, L. L. Xu, Y. F. Zhu, T. Xue, Y. Ge, Z. S. Duan, X. M. Duan, Y. P. Wen and J. K. Xu, Chemosphere, 2022, 289, 133116 CrossRef CAS.
- G. H. Jin, E. Ko, M. K. Kim, V. K. Tran, S. E. Son, Y. Geng, W. Hur and G. H. Seong, Sens. Actuators, B, 2018, 274, 201–209 CrossRef CAS.
- C. H. Zhang, C. X. Chen, D. Zhao, G. Kang, F. N. Liu, F. Yang, Y. Z. Lu and J. Sun, Anal. Chem., 2022, 94, 3485–3493 CrossRef CAS PubMed.
- T. X. Yu, Y. F. Wang, K. Yuan, Q. Guo and J. Ge, Sens. Actuators, B, 2022, 367, 132056 CrossRef CAS.
- F. P. Ran, Y. X. Xu, M. R. Ma, X. Y. Liu and H. X. Zhang, Talanta, 2022, 250, 123702 CrossRef.
- L. Zhang, Z. W. Liu, Q. Q. Deng, Y. J. Sang, K. Dong, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2021, 60, 3469–3474 CrossRef PubMed.
- Y. Y. Huang, J. S. Ren and X. G. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef PubMed.
- Y. Wei, S. Wu, Z. Q. Liu, J. S. Niu, Y. Zhou, J. S. Ren and X. G. Qu, Mater. Today, 2022, 56, 16–28 CrossRef.
- K. Dong, C. Xu, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2022, 61, e202208757 Search PubMed.
- Z. Wang, Y. C. Zhang, X. Z. Wang and L. Han, Biosens. Bioelectron., 2022, 206, 114120 CrossRef CAS PubMed.
- X. Y. Wang, S. J. Dong and H. Wei, Electroanalysis, 2022, 34, 1–13 CrossRef.
- G. Y. Li, G. X. Wu, J. D. Huang, B. Wang, H. M. Li, W. Chen, J. T. Liang, M. X. Tan and Z. D. Zhou, Bioelectrochemistry, 2022, 147, 108204 CrossRef CAS PubMed.
- D. Liu, C. H. Ju, C. Han, R. Shi, X. H. Chen, D. M. Duan, J. H. Yan and X. Y. Yan, Biosens. Bioelectron., 2021, 173, 112817 CrossRef CAS.
- Y. Q. Pei, L. J. Zeng, C. F. Wen, K. Wu, A. P. Deng and J. G. Li, Mikrochim. Acta, 2021, 188, 194–194 CrossRef CAS PubMed.
- Y. X. Jia, S. Q. Zhao, Q. S. Qu and L. Yang, Biosens. Bioelectron., 2022, 202, 114020 CrossRef.
- J. Y. Zhang and J. W. Liu, Luminescence, 2020, 35, 1185–1194 CrossRef PubMed.
- L. X. Yan, B. B. Wang, X. Zhao, L. J. Chen and X. P. Yan, ACS Appl. Mater. Interfaces, 2021, 13, 60955–60965 CrossRef.
- Q. J. Fu, X. B. Zhou, M. J. Wang and X. G. Su, Anal. Chim. Acta, 2022, 1216, 339993 CrossRef PubMed.
- F. Li, J. M. Jiang, H. Peng, C. X. Li, B. Li and J. B. He, Sens. Actuators, B, 2022, 369, 132334 CrossRef.
- Z. M. Li, X. L. Zhong, S. H. Wen, L. Zhang, R. P. Liang and J. D. Qiu, Sens. Actuators, B, 2019, 281, 1073–1079 CrossRef CAS.
- J. Zhu, J. Pan, Y. C. Li, J. Yang and B. C. Ye, Anal. Bioanal. Chem., 2022, 414, 6271–6280 CrossRef CAS PubMed.
- S. Shariati and G. Khayatian, New J. Chem., 2021, 45, 21788–21794 RSC.
- T. R. Bastami, M. Bayat and R. Paolesse, Sensors, 2022, 22, 2072 CrossRef.
- R. Saez-Hernandez, K. U. Antela, A. R. Mauri-Aucejo, A. Morales-Rubio and M. L. Cervera, Food Chem., 2022, 389, 133063 CrossRef CAS.
- J. Ge, L. K. Yang, Z. H. Li, Y. Wan, D. S. Mao, R. J. Deng, Q. Zhou, Y. Yang and W. H. Tan, J. Hazard. Mater., 2022, 436, 129199 CrossRef CAS.
- D. M. Liu, B. Xu and C. Dong, TrAC, Trends Anal. Chem., 2021, 142, 116320 CrossRef CAS.
- S. Melikishvili, I. Piovarci and T. Hianik, Chemosensors, 2021, 9, 281 CrossRef CAS.
- M. L. Xue, W. Mao, J. S. Chen, F. F. Zheng, W. H. Chen, W. Shen and S. Tang, Analyst, 2021, 146, 6726–6740 RSC.
- Q. Q. Fan, Y. Gao, F. Mazur and R. Chandrawati, Biomater. Sci., 2021, 9, 6983–7007 RSC.
- Q. Y. Liu, Q. Y. Jia, R. R. Zhu, Q. Shao, D. M. Wang, P. Cui and J. C. Ge, Mater. Sci. Eng., C, 2014, 42, 177–184 CrossRef.
- A. Hayat, W. Haider, Y. Raza and J. L. Marty, Talanta, 2015, 143, 157–161 CrossRef.
- X. Y. Wang and H. Wei, J. Nanopart. Res., 2020, 22, 22 CrossRef.
- Z. W. Gan, T. Zhang, X. X. An, Q. Tan, S. J. Zhen and X. L. Hu, Sens. Actuators, B, 2022, 368, 132181 CrossRef CAS.
- Z. F. Wang, Y. Liu, X. Y. Dong and Y. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 49974–49981 CrossRef.
- Y. F. Liu, Y. H. Zhang, Q. Y. Liu, Q. Wang, A. Q. Lin, J. Luo, Y. Du, Y. W. Lin and H. Wei, Analyst, 2021, 146, 1872–1879 RSC.
- Z. Xu, L. Li, K. Li, M. L. Chen, J. Tu, W. Chen, S. H. Zhu and Y. H. Cheng, Microchim. Acta, 2021, 188, 421 CrossRef PubMed.
- M. Razavi, A. Barras, M. Ifires, A. Swaidan, M. Khoshkam, S. Szunerits, M. Kompany-Zareh and R. Boukherroub, J. Colloid Interface Sci., 2022, 613, 384–395 CrossRef PubMed.
- S. Q. Li, D. Liu, B. Y. Wu, H. P. Sun, X. Y. Liu, H. X. Zhang, N. N. Ding and L. Wu, Talanta, 2022, 239, 123088 CrossRef PubMed.
- H. H. Ye, K. K. Yang, J. Tao, Y. J. Liu, Q. Zhang, S. Habibi, Z. H. Nie and X. H. Xia, ACS Nano, 2017, 11, 2052–2059 CrossRef PubMed.
- Z. J. Zhang, X. H. Zhang, B. W. Liu and J. W. Liu, J. Am. Chem. Soc., 2017, 139, 5412–5419 CrossRef PubMed.
- Y. Jv, B. X. Li and R. Cao, Chem. Commun., 2010, 46, 8017–8019 RSC.
- Y. F. Lee and C. C. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 2747–2754 CrossRef PubMed.
- K. N. Han, J. S. Choi and J. Kwon, Sci. Rep., 2017, 7, 2806 CrossRef PubMed.
- M. X. Mao, R. Zheng, C. F. Peng and X. L. Wei, Biosensors, 2020, 10, 211 CrossRef PubMed.
- B. W. Liu and J. W. Liu, Nanoscale, 2015, 7, 13831–13835 RSC.
- S. C. B. Gopinath, T. Lakshmipriya and K. Awazu, Biosens. Bioelectron., 2014, 51, 115–123 CrossRef.
- L. Qiao, H. Wang, J. L. He, S. M. Yang and A. L. Chen, Food Chem., 2021, 340, 128181 CrossRef PubMed.
- R. B. Liu, F. Y. Zhang, Y. X. Sang, M. X. Liu, M. H. Shi and X. H. Wang, Foods, 2022, 11, 1802 CrossRef.
- X. Y. Qi, Y. L. Zhao, H. P. Su, L. L. Wang, L. Li, R. Ma, X. C. Yan, J. A. Sun, S. Wang and X. Z. Mao, Talanta, 2022, 238, 123032 CrossRef PubMed.
- P. Weerathunge, R. Ramanathan, V. A. Torok, K. Hodgson, Y. Xu, R. Goodacre, B. K. Behera and V. Bansal, Anal. Chem., 2019, 91, 3270–3276 CrossRef PubMed.
- R. Das, A. Dhiman, A. Kapil, V. Bansal and T. K. Sharma, Anal. Bioanal. Chem., 2019, 411, 1229–1238 CrossRef PubMed.
- L. Li, R. Ma, Y. L. Zhao, L. L. Wang, S. Wang and X. Z. Mao, Talanta, 2022, 246, 123534–123534 CrossRef PubMed.
- Y. L. Zhao, L. Li, R. Ma, L. L. Wang, X. C. Yan, X. Y. Qi, S. Wang and X. Z. Mao, Anal. Chim. Acta, 2021, 1173, 338710 CrossRef PubMed.
- M. Alle, S. C. Park, R. Bandi, S. H. Lee and J. C. Kim, Carbohydr. Polym., 2021, 253, 117239 CrossRef PubMed.
- M. Alle, R. Bandi, G. Sharma, R. Dadigala, S. H. Lee and J. C. Kim, Int. J. Biol. Macromol., 2022, 201, 686–697 CrossRef PubMed.
- O. Adegoke, C. McKenzie and N. N. Daeid, Sens. Actuators, B, 2019, 287, 416–427 CrossRef.
- P. L. An, X. Xue, H. H. Rao, J. J. Wang, M. Gao, H. Q. Wang, M. Y. Luo, X. H. Liu, Z. H. Xue and X. Q. Lu, Anal. Chim. Acta, 2020, 1125, 114–127 CrossRef.
- T. R. Bastami and Z. Dabirifar, RSC Adv., 2020, 10, 35949–35956 RSC.
- F. Amourizi and M. Ghaedi, Polyhedron, 2021, 210, 115506 CrossRef.
- E. Ko, W. Hur, S. E. Son, G. H. Seong and D. K. Han, Microchim. Acta, 2021, 188, 382 CrossRef PubMed.
- M. M. Shah, W. Ren, J. Irudayaraj, A. A. Sajini, M. I. Ali and B. Ahmad, Sensors, 2021, 21, 8050 CrossRef PubMed.
- K. V. Serebrennikova, N. S. Komova, A. N. Berlina, A. V. Zherdev and B. B. Dzantiev, Chemosensors, 2021, 9, 332 CrossRef.
- L. H. Jin, Z. Meng, Y. Q. Zhang, S. J. Cai, Z. J. Zhang, C. Li, L. Shang and Y. H. Shen, ACS Appl. Mater. Interfaces, 2017, 9, 10027–10033 CrossRef PubMed.
- W. Li, B. Chen, H. X. Zhang, Y. H. Sun, J. Wang, J. L. Zhang and Y. Fu, Biosens. Bioelectron., 2015, 66, 251–258 CrossRef PubMed.
- X. X. Li, Q. W. Huang, W. Li, J. L. Zhang and Y. Fu, J. Anal. Test., 2019, 3, 277–285 CrossRef.
- G. Chen, M. J. Jin, M. M. Yan, X. Y. Cui, Y. S. Wang, W. J. Zheng, G. X. Qin, Y. D. Zhang, M. J. Li, Y. Liao, X. Y. Zhang, F. Y. Yan, A. M. Abd El-Aty, A. Hacimuftuoglu and J. Wang, Microchim. Acta, 2019, 186, 339 CrossRef PubMed.
- M. M. Yan, G. Chen, Y. X. She, J. Ma, S. H. Hong, Y. Shao, A. M. Abd El-Aty, M. Wang, S. S. Wang and J. Wang, J. Agric. Food Chem., 2019, 67, 9658–9666 CrossRef CAS PubMed.
- Y. Fu, H. X. Zhang, S. D. Dai, X. Zhi, J. L. Zhang and W. Li, Analyst, 2015, 140, 6676–6683 RSC.
- W. Li, X. Zhi, J. J. Yang, J. L. Zhang and Y. Fu, Anal. Methods, 2016, 8, 5111–5116 RSC.
- Y. H. Sun, J. Wang, W. Li, J. L. Zhang, Y. D. Zhang and Y. Fu, Biosens. Bioelectron., 2015, 74, 1038–1046 CrossRef CAS PubMed.
- Y. Sun, R. X. Wang, X. Liu, G. Y. Shan, Y. W. Chen, T. Tong and Y. C. Liu, Microchim. Acta, 2018, 185, 445 CrossRef.
- Y. Lu, W. C. Ye, Q. Yang, J. Yu, Q. Wang, P. P. Zhou, C. M. Wang, D. S. Xue and S. Q. Zhao, Sens. Actuators, B, 2016, 230, 721–730 CrossRef.
- L. L. Wu, Z. J. Qian, Z. J. Xie, Y. Y. Zhang and C. F. Peng, Chin. J. Anal. Chem., 2017, 45, 471–475 Search PubMed.
- V. G. Panferov, N. A. Byzova, A. V. Zherdev and B. B. Dzantiev, Microchim. Acta, 2021, 188, 309 CrossRef PubMed.
- S. Singh, P. Tripathi, N. Kumar and S. Nara, Biosens. Bioelectron., 2017, 92, 280–286 CrossRef PubMed.
- H. Liu, Y. Hua, Y. Y. Cai, L. P. Feng, S. Li and H. Wang, Anal. Chim. Acta, 2019, 1092, 57–65 CrossRef PubMed.
- H. Fang, S. N. Zhan, L. Feng, X. L. Chen, Q. Guo, Y. Q. Guo, Q. H. He and Y. H. Xiong, Sens. Actuators, B, 2021, 346, 130368 CrossRef.
- E. Ogut, C. Kip, B. Gokcal and A. Tuncel, J. Colloid Interface Sci., 2019, 550, 90–98 CrossRef PubMed.
- A. Grau-Carbonell, S. Sadighikia, T. A. J. Welling, R. J. A. van Dijk-Moes, R. Kotni, M. Bransen, A. van Blaaderen and M. A. van Huis, ACS Appl. Nano Mater., 2021, 4, 1136–1148 CrossRef PubMed.
- T. Feng, K. C. Chen, J. M. Zhong, Y. X. Cheng, H. L. Zhao and M. B. Lan, Sens. Actuators, B, 2022, 369, 132265 CrossRef.
- C. Kip, E. Akbay, B. Gokcal, B. O. Savas, M. A. Onur and A. Tuncel, Colloids Surf., A, 2020, 598, 124812 CrossRef.
- B. Yardimci, O. K. Koc, A. Uzer, J. Hizal and R. Apak, Microchim. Acta, 2021, 188, 228 CrossRef CAS PubMed.
- M. Su, H. D. Chen, H. Zhang and Z. X. Wang, Microchim. Acta, 2022, 189, 81 CrossRef CAS.
- W. Duan, Z. W. Qiu, S. F. Cao, Q. Guo, J. K. Huang, J. Y. Xing, X. Q. Lu and J. B. Zeng, Biosens. Bioelectron., 2022, 196, 113724 CrossRef CAS.
- Z. W. Qiu, W. Duan, S. F. Cao, T. Zeng, T. Y. Zhao, J. K. Huang, X. Q. Lu and J. B. Zeng, Environ. Sci. Technol., 2022, 56, 1713–1723 CrossRef.
- X. Jiao, H. J. Song, H. H. Zhao, W. Bai, L. C. Zhang and Y. Lv, Anal. Methods, 2012, 4, 3261–3267 RSC.
- J. J. Lian, P. Liu, C. Q. Jin, Z. Q. Shi, X. L. Luo and Q. Y. Liu, Microchim. Acta, 2019, 186, 1 CrossRef.
- M. Q. Chi, Y. Zhu, Z. Z. Yang, M. Gao, S. H. Chen, N. Song, C. Wang and X. F. Lu, Nanotechnology, 2017, 28, 295704 CrossRef PubMed.
- M. Liu, Z. H. Li, Y. X. Li, J. J. Chen and Q. Yuan, Chin. Chem. Lett., 2019, 30, 1009–1012 CrossRef.
- L. L. Fu, D. S. Yu, D. J. Zou, H. Qian and Y. H. Lin, Molecules, 2021, 26, 6494 CrossRef PubMed.
- B. Chishti, Z. A. Ansari, H. Fouad, O. Y. Alothman, M. Hashem and S. G. Ansari, Crystals, 2021, 11, 178 CrossRef CAS.
- Y. R. Li, J. Wu, C. Zhang, Y. P. Chen, Y. Wang and M. X. Xie, Microchim. Acta, 2017, 184, 2767–2774 CrossRef CAS.
- Z. Zhang, G. L. Xu, L. Xie and Y. P. Guan, Microchim. Acta, 2019, 186, 581 CrossRef PubMed.
- D. X. Dai, H. Y. Liu, H. Ma, Z. H. Huang, C. C. Gu and M. Z. Zhang, J. Alloys Compd., 2018, 747, 676–683 CrossRef CAS.
- Y. Y. Guo, L. Yan, R. Y. Zhang, H. Ren and A. H. Liu, Process Biochem., 2019, 81, 92–98 CrossRef CAS.
- Z. Lin, X. M. Zhang, S. J. Liu, L. L. Zheng, Y. M. Bu, H. H. Deng, R. T. Chen, H. P. Peng, X. H. Lin and W. Chen, Anal. Chim. Acta, 2020, 1105, 162–168 CrossRef CAS PubMed.
- D. Q. Luo, X. H. Huang, B. Y. Liu, W. Y. Zou and Y. G. Wu, J. Agric. Food Chem., 2021, 69, 3537–3547 CrossRef CAS.
- T. R. Lin, L. S. Zhong, L. Q. Guo, F. F. Fu and G. N. Chen, Nanoscale, 2014, 6, 11856–11862 RSC.
- Z. Q. Xian, L. Zhang, Y. Yu, B. X. Lin, Y. M. Wang, M. L. Guo and Y. J. Cao, Microchim. Acta, 2021, 188, 65 CrossRef CAS PubMed.
- L. P. Feng, L. X. Zhang, S. Chu, S. Zhang, X. Chen, Z. L. Du, Y. S. Gong and H. Wang, Appl. Surf. Sci., 2022, 583, 52496–52496 CrossRef.
- Z. Q. Zhu, L. B. Gong, X. Y. Miao, C. Y. Chen and S. Su, Biosensors, 2022, 12, 260 CrossRef CAS.
- Z. L. Lu, N. Lu, Y. Xiao, Y. Q. Zhang, Z. S. Tang and M. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 11156–11166 CrossRef.
- L. J. Huang, W. X. Zhu, W. T. Zhang, K. Chen, J. Wang, R. Wang, Q. F. Yang, N. Hu, Y. R. Suo and J. L. Wang, Microchim. Acta, 2018, 185, 7 CrossRef PubMed.
- A. Swaidan, A. Addad, J. F. Tahon, A. Barras, J. Toufaily, T. Hamieh, S. Szunerits and R. Boukherroub, Anal. Chim. Acta, 2020, 1109, 78–89 CrossRef PubMed.
- W. X. Zhang, X. P. Li, T. Y. Cui, S. C. Li, Y. Q. Qian, Y. Yue, W. Y. Zhong, B. Xu and W. Q. Yue, Microchim. Acta, 2021, 188, 174 CrossRef PubMed.
- X. J. Wu, T. M. Chen, J. X. Wang and G. W. Yang, J. Mater. Chem. B, 2018, 6, 105–111 RSC.
- L. J. Huang, Q. R. Zhu, J. Zhu, L. P. Luo, S. H. Pu, W. T. Zhang, W. X. Zhu, J. Sun and J. L. Wang, Inorg. Chem., 2019, 58, 1638–1646 CrossRef CAS PubMed.
- L. Lin, D. X. Chen, C. F. Lu and X. X. Wang, Microchem. J., 2022, 177, 107283 CrossRef CAS.
- Y. J. Song, X. H. Wang, C. Zhao, K. G. Qu, J. S. Ren and X. G. Qu, Chem. – Eur. J., 2010, 16, 3617–3621 CrossRef CAS.
- Y. J. Song, K. G. Qu, C. Zhao, J. S. Ren and X. G. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- Y. Tao, Y. H. Lin, Z. Z. Huang, J. S. Ren and X. G. Qu, Adv. Mater., 2013, 25, 2594–2599 CrossRef CAS PubMed.
- H. Y. Sun, X. L. Liu, X. H. Wang, Q. S. Han, C. Qi, Y. M. Li, C. Wang, Y. X. Chen and R. Yang, Microchim. Acta, 2020, 187, 110 CrossRef CAS PubMed.
- S. T. Zhang, H. Li, Z. Y. Wang, J. Liu, H. L. Zhang, B. D. Wang and Z. Y. Yang, Nanoscale, 2015, 7, 8495–8502 RSC.
- O. Adegoke, S. Zolotovskaya, A. Abdolvand and N. N. Daeid, Talanta, 2021, 224, 121875 CrossRef CAS PubMed.
- P. K. Boruah and M. R. Das, J. Hazard. Mater., 2020, 385, 121516 CrossRef CAS.
- Z. R. Song, C. R. Jiang, F. Q. Wang, L. L. Yu, S. J. Ye, P. Dramou and H. He, Mikrochim. Acta, 2021, 188, 207–207 CrossRef CAS PubMed.
- C. Peng, M. Y. Hua, N. S. Li, Y. P. Hsu, Y. T. Chen, C. K. Chuang, S. T. Pang and H. W. Yang, Biosens. Bioelectron., 2019, 126, 581–589 CrossRef CAS.
- O. Adegoke, S. Zolotovskaya, A. Abdolvand and N. N. Daeid, Talanta, 2020, 216, 120990 CrossRef PubMed.
- C. Liu, Y. M. Zhao, D. Xu, X. X. Zheng and Q. Huang, Anal. Bioanal. Chem., 2021, 413, 4013–4022 CrossRef CAS.
- Y. J. Wan, J. W. Zhao, X. C. Deng, J. Chen, F. N. Xi and X. B. Wang, Front. Chem., 2021, 9, 774486 CrossRef CAS.
- X. T. Zhao, S. Li, X. X. Yu, R. T. Gang and H. Wang, Nanoscale, 2020, 12, 21440–21446 RSC.
- Y. Z. Shen, Y. L. Wei, Z. M. Liu, C. Nie and Y. W. Ye, Microchim. Acta, 2022, 189, 233 CrossRef CAS PubMed.
- Y. F. Fan, W. C. Zhang, Y. M. Liu, Z. X. Zeng, X. Quan and H. M. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 17467–17474 CrossRef CAS PubMed.
- N. C. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. W. Xiao, R. Y. Li, T. K. Sham, L. M. Liu, G. A. Botton and X. L. Sun, Nat. Commun., 2016, 7, 13638 CrossRef CAS.
- J. Liu, M. G. Jiao, L. L. Lu, H. M. Barkholtz, Y. P. Li, Y. Wang, L. H. Jiang, Z. J. Wu, D. J. Liu, L. Zhuang, C. Ma, J. Zeng, B. S. Zhang, D. S. Su, P. Song, W. Xing, W. L. Xu, Y. Wang, Z. Jiang and G. Q. Sun, Nat. Commun., 2017, 8, 15938 CrossRef CAS PubMed.
- X. B. Zhou, M. K. Wang, J. Y. Chen, X. L. Xie and X. G. Su, Anal. Chim. Acta, 2020, 1128, 72–79 CrossRef CAS PubMed.
- Y. Mao, S. J. Gao, L. L. Yao, L. Wang, H. Qu, Y. Wu, Y. Chen and L. Zheng, J. Hazard. Mater., 2021, 408, 124898 CrossRef CAS.
- L. P. Sun, C. Li, Y. Yan, Y. Yu, H. Zhao, Z. J. Zhou, F. Wang and Y. Feng, Anal. Chim. Acta, 2021, 1180, 33856 Search PubMed.
- W. J. Jing, X. K. Cui, F. B. Kong, W. Wei, Y. C. Li, L. Z. Fan and X. H. Li, Analyst, 2021, 146, 207–212 RSC.
- B. Liu, Y. T. Xue, Z. Y. Gao, K. R. Tang, G. Wang, Z. B. Chen and X. Zuo, Colloids Surf., B, 2021, 208, 112060 CrossRef CAS PubMed.
- F. Honarasa, F. Peyravi and H. Amirian, J. Iran. Chem. Soc., 2020, 17, 507–512 CrossRef CAS.
- H. Ren, L. Yan, M. J. Liu, Y. B. Wang, X. Liu, C. Y. Liu, K. Liu, L. X. Zeng and A. H. Liu, Sens. Actuators, B, 2019, 296, 126517 CrossRef CAS.
- L. J. Zhao, Z. P. Wu, G. N. Liu, H. Y. Lu, Y. Gao, F. M. Liu, C. G. Wang, J. W. Cui and G. Y. Lu, J. Mater. Chem. B, 2019, 7, 7042–7051 RSC.
- Q. Q. Zhu, Y. H. Yuan, B. Yan, J. Zhou, J. L. Zuo and L. J. Bai, Analyst, 2021, 146, 7284–7293 RSC.
- N. Cheng, C. Z. Zhu, Y. L. Wang, D. Du, M. J. Zhu, Y. B. Luo, W. T. Xu and Y. H. Lin, J. Anal. Test., 2019, 3, 99–106 CrossRef.
- L. Liang, Y. J. Huang, W. R. Liu, W. Y. Zuo, F. G. Ye and S. L. Zhao, Front. Chem., 2020, 8, 671 CrossRef CAS.
- M. H. Ying, G. Z. Yang, Y. J. Xu, H. L. Ye, X. Lin, Y. Lu, H. B. Pan, Y. Bai and M. Du, Analyst, 2021, 147, 40–47 RSC.
- Q. T. Li, Q. Wang, Y. Li, X. D. Zhang and Y. M. Huang, Anal. Methods, 2021, 13, 2066–2074 RSC.
- X. L. Zhao, J. L. Liu, F. T. Xie, T. Yang, R. Hu and Y. H. Yang, Spectrochim. Acta, Part A, 2021, 262, 120117 CrossRef CAS PubMed.
- N. Nataraj, T. W. Chen, Z. W. Gan, S. M. Chen, M. R. Hatshan and M. A. Ali, Mater. Today Chem., 2022, 23, 100725 CrossRef CAS.
- F. P. Ran, Y. X. Xu, M. R. Ma, X. Y. Liu and H. X. Zhang, Talanta, 2022, 250, 123702 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |



