Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterobimetallic multi-site concerted proton electron transfer (MS-CPET) promotes coordination-induced O–H bond weakening†
Julia
Feresin
a,
Brett A.
Barden
a,
Jayden A.
Reyes
a,
Preshit C.
Abhyankar
 a,
Seth M.
Barrett
a,
Seth M.
Barrett
 *b and
Christine M.
Thomas
*b and
Christine M.
Thomas
 *a
*a
aDepartment of Chemistry & Biochemistry, The Ohio State University, 100 W.18th Ave, Columbus, OH 43210, USA. E-mail: thomasc@chemistry.ohio-state.edu
bDepartment of Chemistry, Muskingum University, 260 Stadium Drive, New Concord, OH 43762, USA. E-mail: sbarrett@muskingum.edu
First published on 5th June 2025
Abstract
Coordination-induced bond weakening of X–H bonds (X = O, N, C) has been observed in a number of low-valent transition metal compounds. However, the impact of an appended electron reservoir on the bond dissociation free energy of the O–H bond (BDFEO–H) of a substrate bound to a d0 metal is poorly understood. To gain insight into the ability of separated deprotonation and oxidation sites to decrease the BDFEO–H during proton-coupled electron transfer (PCET) reactions, a bimetallic system in which the sites of proton and electron loss are two distinct metal sites is described. Herein, the interconversion of tris(phosphinoamide) Zr/Co complexes HO–Zr(MesNPiPr2)3CoCNtBu and O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr(MesNPiPr2)3CoCNtBu via hydrogen atom addition/abstraction was studied. Since the Zr center remains in the d0 ZrIV state throughout these transformations, the electron transfer process is mediated by the appended redox-active Co0/I center. A series of open-circuit potential (OCP) measurements on the HO–Zr(MesNPiPr2)3CoCNtBu and O
Zr(MesNPiPr2)3CoCNtBu via hydrogen atom addition/abstraction was studied. Since the Zr center remains in the d0 ZrIV state throughout these transformations, the electron transfer process is mediated by the appended redox-active Co0/I center. A series of open-circuit potential (OCP) measurements on the HO–Zr(MesNPiPr2)3CoCNtBu and O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr(MesNPiPr2)3CoCNtBu complexes was performed, from which the BDFEO–H was found to be 64 ± 1 kcal mol−1. The BDFEO–H value was further verified through a series of stoichiometric H atom transfer reactions, stoichiometric protonation/deprotonation reactions, and computational studies.
Zr(MesNPiPr2)3CoCNtBu complexes was performed, from which the BDFEO–H was found to be 64 ± 1 kcal mol−1. The BDFEO–H value was further verified through a series of stoichiometric H atom transfer reactions, stoichiometric protonation/deprotonation reactions, and computational studies.
Introduction
Coordination-induced bond weakening describes the diminished energy required to homolytically cleave the element-hydrogen (X–H; X = O, N, C) bonds of a metal-bound substrate. Homolytic bond cleavage typically involves one-electron oxidation of the coordinating metal complex in a proton-coupled electron transfer (PCET) reaction.1 Coordination-induced bond weakening has been shown to promote the dehydrogenation of feedstock molecules such as water and ammonia, reactions that have potential applications in renewable chemical fuel storage systems.1 Chirik and coworkers reported an example of a redox-active molybdenum complex that lowered the bond dissociation free energy of the N–H bonds in ammonia (BDFEN–H) to 45.8 kcal mol−1 (Fig. 1A), suggesting potential applications of coordination-induced bond weakening in the fields of catalysis, bioinorganic chemistry, and alternative energy.2 However, the first example of coordination-induced bond weakening in early transition metals was observed by Cuerva et al. with a titanocene(III) system that mediated radical reductions using water as a hydrogen-atom source (Fig. 1A).3 Coordination-induced bond weakening has also been demonstrated with main group compounds and transition metal clusters.4,5 Although coordination-induced bond weakening has been established for transition metal compounds, accessing the requisite low-valent state can be challenging. Thus, redox-active ligands have been employed as a strategy to promote coordination-induced bond weakening of substrates bound to redox-inactive metals, as examined by Mankad and coworkers using an aluminum-containing compound (Fig. 1B).6 A report from Chirik et al. subsequently demonstrated the potential for redox-active ligands to serve as electron-reservoirs for N–H bond cleavage/formation in vanadium amido/imido complexes using redox-active pyridine diimine ligands (Fig. 1B).7 More recently, the Abbenseth group demonstrated the weakening of aniline and anilide N–H bonds in a series of d0 TaV compounds ligated by a redox-active NNN pincer ligand (Fig. 1B).8 The use of redox-active ligands spawns a different subset of PCET reactions, namely multi-site concerted proton electron transfer (MS-CPET), a term used to describe a process where the proton and electron originate and terminate at different sites within a molecule.9,10MS-CPET mechanisms are employed in a variety of biological systems.11–20 Understanding the relationship between separated electron transfer (ET) and proton transfer (PT) sites promotes deeper insight into the mechanisms by which MS-CPET enables reactions that would otherwise be thermodynamically unfavorable.9,10 Thus, studying the impacts of extensive separation of proton and electron donor/acceptor sites in well-defined molecular species can help clarify the roles of these sites in complex biological or materials-based systems. The Mayer group previously reported examples of MS-CPET mechanisms in molecular transition metal model systems.21–26
For example, they measured the BDFEO–H of a carboxylic acid functionality appended to a Ru-bound terpyridine ligand and the impact of increasing the separation between proton (O–H) and electron (Ru) transfer sites by inserting a phenyl ring between the terpyridine ligand and the carboxylate fragment, ultimately finding the O–H bond to be weakened by the appended redox-active Ru center even with a separation of 11.2 Å between the proton and electron transfer sites (Fig. 1C).26 In related work, Peters and coworkers have leveraged the reducing nature of the cobaltacene fragment to generate potent H-atom transfer reagents, where the H+ originates at an ammonium site more than 7 Å away from the redox-active cobalt center (Fig. 1C).27 Herein, we set out to explore the impact of MS-CPET in a multimetallic system in which the proton and electron transfer steps occur at different metal centers and the extent to which coordination-induced bond weakening can be realized in substrates bound to a d0 metal center with the aid of a pendent redox-active metal atom (Fig. 1D).
During the course of our previous studies on metal–metal cooperativity in early/late heterobimetallic compounds,28 we described a tris(phosphinoamide) ZrIV/Co−I complex, (THF)Zr(MesNPiPr2)3CoCNtBu, in which a redox-active Co−I center is appended to a d0 ZrIV center.29 By sterically blocking the Co site with a tightly binding tBuNC ligand, substrate binding can only occur at the redox-inactive Zr site and Co plays the role of an electron reservoir. This strategy permitted oxidative group transfer at the formally d0 Zr center to generate a terminal Zr-imido compound and two-electron reduction of O2 to generate an η2-peroxo compound.29,30 The addition of one equivalent of water to (THF)Zr(MesNPiPr2)3CoCNtBu afforded a transient intermediate H2O–Zr(MesNPiPr2)3CoCNtBu (A) with a sufficiently low BDFEO–H that H2 is spontaneously released to afford the ZrIV/Co0 hydroxide compound HO–Zr(MesNPiPr2)3CoCNtBu (1).31 Treatment of (THF)Zr(MesNPiPr2)3CoCNtBu with pyridine-N-oxide (py-O) afforded the ZrIV/CoI oxo species O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Zr(MesNPiPr2)3CoCNtBu (2) (Scheme 1).30 Hydrogen atom abstraction from 1 to generate 2 would rely on loss of a proton from the Zr-bound hydroxide ligand and an electron from the Co center, sites that are separated by 2.69 Å (Scheme 1). Herein, we report the first example, to our knowledge, of a MS-CPET process in which the electron transfer and the proton transfer steps occur at two different metal centers, allowing a d0 metal to undergo a PCET reaction. To better examine the impact of separated oxidation and deprotonation sites on the coordination-induced weakening of the O–H bond, we measure the O–H bond dissociation free energy (BDFEO–H) within compound 1.
Zr(MesNPiPr2)3CoCNtBu (2) (Scheme 1).30 Hydrogen atom abstraction from 1 to generate 2 would rely on loss of a proton from the Zr-bound hydroxide ligand and an electron from the Co center, sites that are separated by 2.69 Å (Scheme 1). Herein, we report the first example, to our knowledge, of a MS-CPET process in which the electron transfer and the proton transfer steps occur at two different metal centers, allowing a d0 metal to undergo a PCET reaction. To better examine the impact of separated oxidation and deprotonation sites on the coordination-induced weakening of the O–H bond, we measure the O–H bond dissociation free energy (BDFEO–H) within compound 1.
 | ||
| Scheme 1 Reported synthetic procedures to generate 1 and 2 and sequential H-atom removal from Zr-OHx (x = 2, 1) fragments in a Zr/Co heterobimetallic system. | ||
Results & discussion
Compounds 1 and 2 were synthesized using reported procedures,30,31 followed by electrochemical experiments to determine the BDFEO–H of the hydroxide ligand in 1. In 2020, the Mayer group reported a method to calculate the BDFE of polar X–H bonds (O–H and N–H) in nonaqueous solvents using open-circuit potential (OCP) measurements of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the two compounds that differ by one H atom in a buffered electrolyte solution.32 The
1 mixture of the two compounds that differ by one H atom in a buffered electrolyte solution.32 The  values are referenced against the H+/H2 couple and the BDFEX–H can be calculated using eqn (1).32–36 The
values are referenced against the H+/H2 couple and the BDFEX–H can be calculated using eqn (1).32–36 The  is measured using a solution containing equal concentrations of both the oxidized and reduced species (X/XH) and
is measured using a solution containing equal concentrations of both the oxidized and reduced species (X/XH) and  is a solvent-related constant describing the free energy of H2 homolytic cleavage.32
is a solvent-related constant describing the free energy of H2 homolytic cleavage.32 | (1) |
To obtain the BDFEO–H of 1 using OCP measurements, a series of buffered electrolyte solutions in THF (100 mM [nBu4N][PF6], 50 mM lutidine (lut), and 50 mM [Hlut][BPh4]) containing both the oxidized (2) and reduced (1) species were prepared. OCP measurements (referenced to the ferrocenium/ferrocene redox couple, Fc+/0) were collected using five different ratios of hydroxide (1): oxo (2) to plot the OCP vs. log([1]/[2]) and find the y-intercept that represents the  of the 1
of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between the oxidized (2) and reduced (1) species needed in eqn (1). The OCP measurements of five varying ratios of 1 and 2 also provide insight into whether the MS-CPET behaves as an ideal, Nernstian system (eqn (2)).32 Three overall trials at each hydroxide (1)
1 ratio between the oxidized (2) and reduced (1) species needed in eqn (1). The OCP measurements of five varying ratios of 1 and 2 also provide insight into whether the MS-CPET behaves as an ideal, Nernstian system (eqn (2)).32 Three overall trials at each hydroxide (1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxo (2) ratio were performed to confirm reproducibility (ESI Section 2.2†).
oxo (2) ratio were performed to confirm reproducibility (ESI Section 2.2†).
 | (2) |
The measured OCP was then referenced to the H+/H2 couple in the same buffered solution (100 mM [nBu4N][PF6], 50 mM lutidine, and 50 mM [Hlut][BPh4] in THF) in the presence of H2 (ESI, Section 2.1†). The  (H+/H2) was referenced vs. Fc+/0, allowing the OCP measurements of each hydroxide (1)
(H+/H2) was referenced vs. Fc+/0, allowing the OCP measurements of each hydroxide (1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxo (2) ratio to be referenced to H+/H2 prior to plotting the OCP (V vs. H2) vs. log [1]/[2] (Fig. 2). Fig. 2A shows an example of one of the three trials performed, in which the y-intercept provides information about the
oxo (2) ratio to be referenced to H+/H2 prior to plotting the OCP (V vs. H2) vs. log [1]/[2] (Fig. 2). Fig. 2A shows an example of one of the three trials performed, in which the y-intercept provides information about the  (1/2) at the 1
(1/2) at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The average y-intercept for the three trials was found to be 0.503 V vs. H2. When all three trials are plotted together (Fig. 2B), minimal deviation in the OCP is observed between trials, yielding a consistent OCP value within 5 mV. Furthermore, the slope of the OCP (V vs. H2) vs. log [1]/[2] plot provides information about the behavior of the system. In an ideal system for a one-electron process, the OCP should decrease by 0.0592 V dec−1 for each order of magnitude change in ratio between hydroxide and oxo, as described by eqn (2).32 The slopes obtained in all three trials ranged from −0.0274 V dec−1 to −0.0502 V dec−1, which is in reasonable agreement with ideal Nernstian behavior considering the use of a low dielectric constant non-aqueous solvent (THF).
1 ratio. The average y-intercept for the three trials was found to be 0.503 V vs. H2. When all three trials are plotted together (Fig. 2B), minimal deviation in the OCP is observed between trials, yielding a consistent OCP value within 5 mV. Furthermore, the slope of the OCP (V vs. H2) vs. log [1]/[2] plot provides information about the behavior of the system. In an ideal system for a one-electron process, the OCP should decrease by 0.0592 V dec−1 for each order of magnitude change in ratio between hydroxide and oxo, as described by eqn (2).32 The slopes obtained in all three trials ranged from −0.0274 V dec−1 to −0.0502 V dec−1, which is in reasonable agreement with ideal Nernstian behavior considering the use of a low dielectric constant non-aqueous solvent (THF).
 | ||
| Fig. 2 (A) Representative trial of the OCP (V vs. H2) vs. the log of varying ratios of 1 and 2. (B) OCP (V vs. H2) vs. the log of varying ratios of 1 and 2 for all three trials. | ||
By referencing the OCP of the equimolar mixture of 1 and 2 to the OCP of the H+/H2 solution, a direct route was used to calculate the BDFEO–H.32 By substituting the  (V vs. H2), which was found to be 0.503 V vs. H2, into eqn (1), the BDFEO–H obtained was 63.6 (64 ± 1) kcal mol−1, since the value for the
(V vs. H2), which was found to be 0.503 V vs. H2, into eqn (1), the BDFEO–H obtained was 63.6 (64 ± 1) kcal mol−1, since the value for the  is known to be 52.0 kcal mol−1 in THF.32 For further sample calculations and error analysis, consult ESI Sections 3.1 and 3.3,† respectively.
is known to be 52.0 kcal mol−1 in THF.32 For further sample calculations and error analysis, consult ESI Sections 3.1 and 3.3,† respectively.
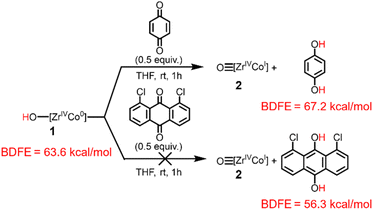
To provide further support for the BDFEO–H value of the hydroxide compound 1 determined via the OCP measurements, the stoichiometric reactivity of 1 towards H atom abstractors was investigated (Scheme 2). Further reactivity studies of 1 with additional H atom abstractors and of oxo (2) towards H atom donors is described in the ESI, Section 4.† When complex 1 (BDFEO–H = 63.6 kcal mol−1) was treated with 2,4,6-tri-tert-butylphenoxyl radical (BDFEO–H = 74.4 kcal mol−1)37 generation of 2 was observed (ESI, Section 4.1†). Moreover, when 2 was treated with 9,10-dihydroanthracene (BDFEC–H = 72.9 kcal mol−1)37 no reaction occurred (ESI, Section 4.2†), confirming that the BDFEO–H must be less than 73 kcal mol−1. Treatment of complex 1 with p-benzoquinone (BDFEO–H = 67.2 kcal mol−1)37 resulted in the formation of 2, but no reactivity was observed between 1 and 1,8-dichloroanthraquinone (BDFEO–H = 56.3 kcal mol−1)37 (Scheme 2). Thus, this series of H atom transfer reactions provides a BDFEO–H range of 67.2 > BDFEO–H > 56.3, which agrees with the BDFEO–H = 64 ± 1 determined via the OCP measurements.
Following experimental verification of the BDFEO–H value determined via OCP measurements, the pKa values of the neutral hydroxide complex 1 and the cationic hydroxide complex [HO–Zr(MesNPiPr2)3CoCNtBu]+ (1+) could be estimated using the square scheme shown in Scheme 3, the Bordwell equation (eqn (3)),38 the established solvent-specific constant (Cg,sol = 59.9 kcal mol−1) for THF,37 and the previously reported redox potentials for complexes 1 and 2 determined via cyclic voltammetry (CV)30,31 (ESI, Section 3.2†). The pKa values for complexes 1 and 1+ were calculated to be 31.5 and 21.7, respectively (Scheme 3). It is important to note that the pKa values calculated using this method represent the pKa values in a buffered electrolyte solution. As such, the calculated pKa values can be considered estimates, not exact values.
| BDFE(X−H) = 23.06E°(X0/−) + 1.37pKa(X−H) + Cg,sol | (3) |
 | ||
| Scheme 3 Square scheme showing the stepwise and concerted interconversion of 1 and 2. The BDFEO–H and E° values were experimentally determined using OCP and CV measurements[31] and the pKa values are estimated from the BDFEO–H and E° values using eqn (3). | ||
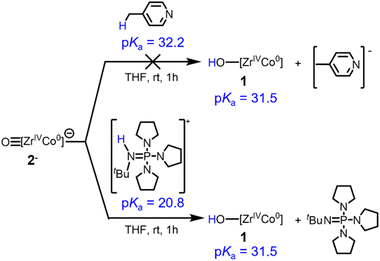
To provide support for the estimated pKa values and further verify the BDFEO–H determined via OCP measurements, the reactivity of previously reported anionic oxo complex [OZr(MesNPiPr2)3CoCNtBu]− (2−) toward acids was investigated (Scheme 4). The estimated pKa value for 1 of 31.5 is consistent with the observed lack of reaction between 2− and 4-methylpyridine (pKa = 32.2).39 In contrast, 2− was found to react with [tBuHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P(pyrr)][BPh4] (pyrr = pyrrolidinyl, pKa = 20.8)40 to generate 1. Thus, the stoichiometric protonation/deprotonation reactions provided a pKa range for complex 1 of 32.2 > pKa > 20.8, which agrees with the estimated value of 31.5.
P(pyrr)][BPh4] (pyrr = pyrrolidinyl, pKa = 20.8)40 to generate 1. Thus, the stoichiometric protonation/deprotonation reactions provided a pKa range for complex 1 of 32.2 > pKa > 20.8, which agrees with the estimated value of 31.5.
 | ||
| Scheme 4 Reactivity of complex 2− with different acids to estimate the upper and lower bounds of the pKa of complex 1. | ||
To verify the estimated pKa value of 21.7 for the cationic hydroxide compound 1+, the protonation of 2 was investigated (Scheme 5). No reaction occurs between complex 2 and (Me3Si)2NH (pKa = 25.8).39 Oxo complex 2 was, however, readily protonated with [HNEt3][BPh4] (pKa = 12.5)40 to afford the previously reported cationic hydroxide compound 1+. The pKa range for the cationic hydroxide 1+ was therefore experimentally determined to be 25.8 > pKa > 12.5, which agrees with the estimated pKa value of 21.7.
 | ||
| Scheme 5 Reactivity of complex 2 with different acids to estimate the upper and lower bounds of the pKa of complex 1+. | ||
After further verification of the BDFEO–H value via BDFE and pKa test reactions, density functional theory (DFT) was used to compute BDFE and pKa values to provide additional support. Eight H atom donors were used to construct a BDFE calibration curve using known BDFE values and computed free energy values (ESI Section 6.3†), yielding a calculated BDFEO–H value of 60 ± 4 kcal mol−1 for the hydroxide complex 1. The calculated and experimental BDFEO–H are within error of one another. Thus, the same computational method was used to calculate the BDFEO–H for the unobservable H2O-bound transient intermediate complex A, yielding a BDFEO–H of 42.9 ± 4 kcal mol−1. This value is consistent with the spontaneous loss of H˙ and formation of H2 (BDFEH–H = 104 kcal mol−1),41 which is presumed to proceed via a bimolecular mechanism. The pKa values of 1 and 1+ were also computed using DFT. Nine organic acids were used to construct a pKa calibration curve using known pKa values and computed free energy values (ESI Section 6.4†), yielding a pKa value of 27.9 for the neutral hydroxide 1 and 21.8 for the cationic hydroxide 1+. The computed BDFEO–H and pKa values agree well with experimentally determined values.
Conclusions
In summary, the analysis of OCP measurements referenced to H+/H2 has been shown to be a facile technique for determining the BDFE of the terminal hydroxide functionality of the Zr/Co complex 1. It was found that the O–H bond in complex 1 has a BDFE of 64 ± 1 kcal mol−1, and this value has been supported by both DFT computations and reactions with stoichiometric H-atom transfer reagents. Using the BDFEO–H value of 64 ± 1 kcal mol−1 and a square scheme analysis, the pKa of complexes 1 and 1+ were estimated to be 31.5 and 21.7, respectively.Through this study, it can be concluded that in both Zr–OH and Zr–OH2 compounds, the BDFEO–H is dramatically decreased by the presence of an appended redox-active metal center. Importantly, this demonstrates the viability of the multimetallic system to facilitate element–hydrogen bond cleavage, as significant coordination-induced bond weaking was observed despite the separation between the proton and electron transfer sites: Although the d0 ZrIV center to which the hydroxide ligand is directly bound is redox-inactive, the electron-transfer capacity of the appended Co center in complex 1 results in a similar degree of coordination-induced bond weakening as would be expected if the substrate were directly bound to a redox-active metal. The low BDFEO–H value of 64 ± 1 kcal mol−1 within HO–ZrIV/Co0 complex 1 is at the low end of the range of BDFEO–H values reported for terminal CoII, FeIII, FeII, and MnII hydroxide compounds (64–85 kcal mol−1).42–44 Although the corresponding H2O–ZrIV/Co−I compound A cannot be isolated, the spontaneous release of H2 from this aquo intermediate suggests a BDFEO–H value lower than half the BDFEH–H of H2 (52 kcal mol−1), which is substantiated by a DFT-calculated BDFEO–H of 43 kcal mol−1. This represents a coordination-induced weakening of the O–H bond of ∼70 kcal mol−1 when compared to the BDFEO–H of free H2O (BDFE = 115.8 kcal mol−1).37 The extent of coordination-induced O–H bond weakening in 1 and A is similar to the effect expected if the H2O and OH− ligands were directly bound to a redox-active metal, demonstrating that a redox-active metal appended to the substrate binding site is a viable strategy to facilitate element–hydrogen bond activation. The estimated pKa values for 1 and 1+ are unremarkable compared to monometallic terminal hydroxide compounds45 indicating that the weakening of the O–H bond is, indeed, driven by the low CoI/0 redox potential. Future studies will explore whether the coordination-induced bond weakening phenomenon can be generalized across other element-hydrogen bond-containing substrates and bimetallic combinations and seek to establish applications for the resulting H-atom transfer processes.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
C. M. T. supervised and acquired funding for the project. J. F., S. M. B., and J. A. R. conducted the experiments and data analysis. B. A. B. and C. M. T. conceptualized the goals and aims of the project and B. A. B. collected the preliminary data on which this study is based. P. C. A. performed and analyzed the DFT calculations. J. F., S. M. B., and C. M. T. contributed to writing, reviewing, and editing the manuscript and all authors gave approval to the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Catalysis Science program for providing financial support for this project under Award No. DE-SC0019179. The authors thank Maria C. Seith for her contribution in designing the graphic for the Table of Contents. S. M. B. thanks the Faculty Development Grant Program of the Office of Academic Affairs at Muskingum University.References
- N. G. Boekell and R. A. Flowers, Chem. Rev., 2022, 122, 13447–13477 CrossRef CAS PubMed
.
- M. J. Bezdek, S. Guo and P. J. Chirik, Science, 2016, 354, 730–733 CrossRef CAS PubMed
.
- J. M. Cuerva, A. G. Campaña, J. Justicia, A. Rosales, J. L. Oller-López, R. Robles, D. J. Cárdenas, E. Buñuel and J. E. Oltra, Angew. Chem., Int. Ed., 2006, 45, 5522–5526 CrossRef CAS PubMed
.
- X. Yang, E. J. Reijerse, K. Bhattacharyya, M. Leutzsch, M. Kochius, N. Nöthling, J. Busch, A. Schnegg, A. A. Auer and J. Cornella, J. Am. Chem. Soc., 2022, 144, 16535–16544 CrossRef CAS PubMed
.
- S. E. Cooney, A. A. Fertig, M. R. Buisch, W. W. Brennessel and E. M. Matson, Chem. Sci., 2022, 13, 12726–12737 RSC
.
- S. Sinhababu, R. P. Singh, M. R. Radzhabov, J. Kumawat, D. H. Ess and N. P. Mankad, Nat. Commun., 2024, 15, 1315 CrossRef CAS PubMed
.
- C. Milsmann, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2014, 136, 12099–12107 CrossRef CAS PubMed
.
- S. Pavlidis, J. Alasadi, A. Opis-Basilio and J. Abbenseth, Dalton Trans., 2025, 54, 2421–2429 RSC
.
- M. H. V. Huynh and T. J. Meyer, Chem. Rev., 2007, 107, 5004–5064 CrossRef CAS PubMed
.
- J. W. Darcy, B. Koronkiewicz, G. A. Parada and J. M. Mayer, Acc. Chem. Res., 2018, 51, 2391–2399 CrossRef CAS PubMed
.
- J. L. Dempsey, J. R. Winkler and H. B. Gray, Chem. Rev., 2010, 110, 7024–7039 CrossRef CAS PubMed
.
- W. Lubitz, H. Ogata, O. Rüdiger and E. Reijerse, Chem. Rev., 2014, 114, 4081–4148 CrossRef CAS PubMed
.
- B. M. Hoffman, D. Lukoyanov, Z.-Y. Yang, D. R. Dean and L. C. Seefeldt, Chem. Rev., 2014, 114, 4041–4062 CrossRef CAS PubMed
.
- N. A. Arnet, T. R. Dugan, F. S. Menges, B. Q. Mercado, W. W. Brennessel, E. Bill, M. A. Johnson and P. L. Holland, J. Am. Chem. Soc., 2015, 137, 13220–13223 CrossRef CAS PubMed
.
- J. T. Kleinhaus, F. Wittkamp, S. Yadav, D. Siegmund and U.-P. Apfel, Chem. Soc. Rev., 2021, 50, 1668–1784 RSC
.
- S. He, F. Huang, Q. Wu, P. Zhang, Y. Xiong, J. Yang, R. Zhang, F. Wang, L. Chen, T. L. Liu and F. Li, Angew. Chem., Int. Ed., 2021, 60, 25839–25845 CrossRef CAS PubMed
.
- M. J. Knapp, K. Rickert and J. P. Klinman, J. Am. Chem. Soc., 2002, 124, 3865–3874 CrossRef CAS PubMed
.
- B. Meunier, S. P. De Visser and S. Shaik, Chem. Rev., 2004, 104, 3947–3980 CrossRef CAS PubMed
.
- J. Rittle and M. T. Green, Science, 2010, 330, 933–937 Search PubMed
.
- C. Sommer, C. P. Richers, W. Lubitz, T. B. Rauchfuss and E. J. Reijerse, Angew. Chem., Int. Ed., 2018, 57, 5429–5432 Search PubMed
.
- E. A. Mader, A. S. Larsen and J. M. Mayer, J. Am. Chem. Soc., 2004, 126, 8066–8067 Search PubMed
.
- J. P. Roth and J. M. Mayer, Inorg. Chem., 1999, 38, 2760–2761 CrossRef CAS PubMed
.
- J. P. Roth, S. Lovell and J. M. Mayer, J. Am. Chem. Soc., 2000, 122, 5486–5498 CrossRef CAS
.
- E. A. Mader, E. R. Davidson and J. M. Mayer, J. Am. Chem. Soc., 2007, 129, 5153–5166 CrossRef CAS PubMed
.
- V. W. Manner, A. G. DiPasquale and J. M. Mayer, J. Am. Chem. Soc., 2008, 130, 7210–7211 CrossRef CAS PubMed
.
- V. W. Manner and J. M. Mayer, J. Am. Chem. Soc., 2009, 131, 9874–9875 Search PubMed
.
- M. J. Chalkley, P. Garrido-Barros and J. C. Peters, Science, 2020, 369, 850–854 CrossRef CAS PubMed
.
- N. H. Hunter and C. M. Thomas, Dalton Trans., 2024, 53, 15764–15781 RSC
.
- J. P. Krogman, M. W. Bezpalko, B. M. Foxman and C. M. Thomas, Dalton Trans., 2016, 45, 11182–11190 Search PubMed
.
- H. Zhang, G. P. Hatzis, C. E. Moore, D. A. Dickie, M. W. Bezpalko, B. M. Foxman and C. M. Thomas, J. Am. Chem. Soc., 2019, 141, 9516–9520 CrossRef CAS PubMed
.
- H. Zhang, G. P. Hatzis, D. A. Dickie, C. E. Moore and C. M. Thomas, Chem. Sci., 2020, 11, 10729–10736 Search PubMed
.
- C. F. Wise, R. G. Agarwal and J. M. Mayer, J. Am. Chem. Soc., 2020, 142, 10681–10691 Search PubMed
.
- S. Mondal, W. Zhang and S. Zhang, J. Am. Chem. Soc., 2024, 146, 15036–15044 CrossRef CAS PubMed
.
- T. Wu, A. Puri, Y. L. Qiu, D. Ye, R. Sarma, Y. Wang, T. Kowalewski, M. A. Siegler, M. Swart and I. Garcia-Bosch, Inorg. Chem., 2024, 63, 9014–9025 CrossRef CAS PubMed
.
- A. M. Smith and A. J. M. Miller, Organometallics, 2024, 43, 3163–3170 Search PubMed
.
- B. D. Groff, M. Cattaneo, S. C. Coste, C. A. Pressley, B. Q. Mercado and J. M. Mayer, Inorg. Chem., 2023, 62, 10031–10038 CrossRef CAS PubMed
.
- R. G. Agarwal, S. C. Coste, B. D. Groff, A. M. Heuer, H. Noh, G. A. Parada, C. F. Wise, E. M. Nichols, J. J. Warren and J. M. Mayer, Chem. Rev., 2022, 122, 1–49 CrossRef CAS PubMed
.
- F. G. Bordwell, J. P. Cheng and J. A. Harrelson, J. Am. Chem. Soc., 1988, 110, 1229–1231 CrossRef CAS
.
- R. R. Fraser, T. S. Mansour and S. Savard, J. Org. Chem., 1985, 50, 3232–3234 CrossRef CAS
.
- I. Kaljurand, T. Rodima, A. Pihl, V. Mäemets, I. Leito, I. A. Koppel and M. Mishima, J. Org. Chem., 2003, 68, 9988–9993 CrossRef CAS PubMed
.
- J. J. Warren, T. A. Tronic and J. M. Mayer, Chem. Rev., 2010, 110, 6961–7001 CrossRef CAS PubMed
.
- D. Usharani, D. C. Lacy, A. S. Borovik and S. Shaik, J. Am. Chem. Soc., 2013, 135, 17090–17104 CrossRef CAS PubMed
.
- R. Gupta and A. S. Borovik, J. Am. Chem. Soc., 2003, 125, 13234–13242 CrossRef CAS PubMed
.
- M. K. Goetz, E. A. Hill, A. S. Filatov and J. S. Anderson, J. Am. Chem. Soc., 2018, 140, 13176–13180 CrossRef CAS PubMed
.
- P. E. VanNatta, D. A. Ramirez, A. R. Velarde, G. Ali and M. T. Kieber-Emmons, J. Am. Chem. Soc., 2020, 142, 16292–16312 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: For detailed experimental procedures, descriptions of additional experiments, and computational details. See DOI: https://doi.org/10.1039/d5sc03298a |
| This journal is © The Royal Society of Chemistry 2025 |