Open Access Article
Open Access ArticleGreening the pathways: a comprehensive review of sustainable synthesis strategies for silica nanoparticles and their diverse applications
Arighna Saha
ab,
Prashant Mishra
 c,
Goutam Biswas
*a and
Snehasis Bhakta
c,
Goutam Biswas
*a and
Snehasis Bhakta
 *b
*b
aDepartment of Chemistry, Cooch Behar Panchanan Barma University, Cooch Behar 736101, West Bengal, India. E-mail: snehasisbhakta@coochbeharcollege.org.in; goutam@cbpbu.ac.in
bCooch Behar College, Cooch Behar 736101, West Bengal, India
cDepartment of Biochemical Engineering and Biotechnology, Indian Institute of Technology Delhi, New Delhi 110016, India
First published on 8th April 2024
Abstract
Silica nanoparticles (SiNPs) have emerged as a multipurpose solution with wide-ranging applications in various industries such as medicine, agriculture, construction, cosmetics, and food production. In 1961, Stöber introduced a ground-breaking sol–gel method for synthesizing SiNPs, which carried a new era of exploration both in academia and industry, uncovering numerous possibilities for these simple yet multifaceted particles. Inspite of numerous reported literature with wide applicability, the synthesis of these nanoparticles with the desired size and functionalities poses considerable challenges. Over time, researchers have strived to optimize the synthetic route, particularly by developing greener approaches that minimize environmental impact. By reducing hazardous chemicals, energy consumption, and waste generation, these greener synthesis methods have become an important focus in the field. This review aims to provide a comprehensive analysis of the various synthetic approaches available for different types of SiNPs. Starting from the Stöber' method, we analyze other methods as well to synthesis different types of SiNPs including mesoporous, core–shell and functionalized nanoparticles. With increasing concerns with the chemical methods associated for environmental issues, we aim to assist readers in identifying suitable greener synthesis methods tailored to their specific requirements. By highlighting the advancements in reaction time optimization, waste reduction, and environmentally friendly precursors, we offer insights into the latest techniques that contribute to greener and more sustainable SiNPs synthesis. Additionally, we briefly discuss the diverse applications of SiNPs, demonstrating their relevance and potential impact in fields such as medicine, agriculture, and cosmetics. By emphasizing the greener synthesis methods and economical aspects, this review aims to inspire researchers and industry professionals to adopt environmentally conscious practices while harnessing the immense capabilities of SiNPs.
1. Introduction
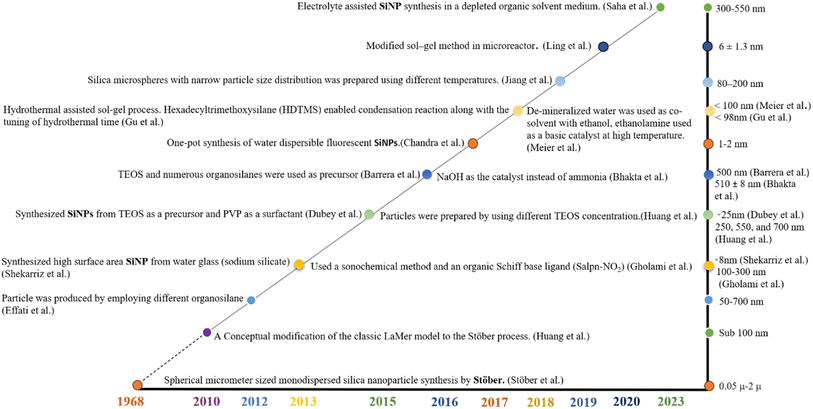
Nanotechnology is dedicated to crafting unique particles on the nanoscale, and it has witnessed substantial advancements in the creation of polymeric, liposomal, and inorganic nanoparticles, serving a wide array of industries including electronics, medicine, and consumer goods.1 Among these versatile nanoparticle formulations, silica-based nanoparticles (SiNPs) have emerged as a focal point of attention, showcasing significant promise across numerous applications. In recent years, SiNPs have experienced a surge in interest and exploration, solidifying their status as a highly promising frontier in the realm of nanotechnology.2Extensive research has yielded numerous reports detailing the synthesis of SiNPs. These reports showcase the successful synthesis achieved through the manipulation of various reaction conditions.3 Researchers have explored techniques such as varying reaction conditions, incorporating electrolytes, altering the base or catalyst, and utilizing different precursors to achieve desired SiNPs properties.4 Recent advances in SiNPs preparation, with a target of achieving sizes below 100 nm, have generated specific morphological architectures. These architectures include porous and mesoporous structures, spheres, hollow spheres, fibers, tubules, helical fibers, and more. The ability to tailor the morphology of SiNPs opens exciting possibilities for their application in diverse fields. Several synthesis techniques have been employed for SiNPs production, each offering distinct advantages. Methods such as flame spray pyrolysis, chemical vapor deposition, sol–gel process, micro-emulsion, and others have been explored. Among these techniques, the sol–gel process remains the most popular due to its exceptional ability to exert precise control over particle size, distribution, and morphology. This control is achieved through systematic monitoring and optimization of various reaction parameters.5 The sol–gel process enables researchers to finely tune SiNPs properties by manipulating several factors such as precursor composition, solvent composition, reaction temperature, and reaction time. This flexibility enables the customization of SiNPs for specific applications. Additionally, the sol–gel process allows for the introduction of dopants, functional groups, or surface modifications during synthesis, further expanding the potential applications of SiNPs. The sol–gel process offers a low-temperature method for synthesizing compounds that are either entirely inorganic or a combination of both inorganic and organic components. In recent times, significant advancements and developments have been reported, enhancing the effectiveness and versatility of the sol–gel process. These improvements encompass various aspects, such as the utilization of surfactants or polymers as templates, the exploration of different precursor materials, optimization of reaction settings, and advances in drying processes. These advancements collectively contribute to expanding the capabilities and applications of the sol–gel process, enabling the synthesis of a wide range of tailored nanoparticles with enhanced control and precision.6
Considering various applications such as drug delivery and waste management,7,8 SiNPs offer the flexibility to undergo chemical functionalization with a range of target substances. This involves the attachment of polymers, ligands, or specific functional compounds through physical adsorption or covalent conjugation, leveraging silane chemistry to augment or alter their chemical and physical characteristics. The main goal of this review is to explore diverse SiNPs synthetic approaches, spanning from the well-established and optimized Stöber method to more environmentally friendly and sustainable routes. Our prime objective is to offer a thorough overview of recent research progress in SiNPs synthesis. Alongside the analysis of these synthesis methods, this study also encompasses the fabrication of SiNPs with varying shapes and surface modifications. By delving into these facets, this review seeks to enhance the understanding of SiNPs synthesis towards future researchers, showcasing advancements in the field and laying the groundwork for future innovations in customized SiNPs manufacturing.
2. Synthesis of nonporous silica nanoparticle
The creation of impermeable silica nanoparticles (SiNPs) has long been acknowledged as a cornerstone in the field of nanomaterial science, establishing a vital basis for diverse scientific and industrial applications. These nanoparticles, with their well-defined and compact structures, have garnered significant attention due to their remarkable versatility and unique properties. In this section, we deep dive into the intricate world of SiNPs synthesis, tracing the journey from early methodologies like the Stöber method to more contemporary and environmentally conscious approaches. By comprehensively examining the evolving landscape of nonporous SiNPs synthesis, we aim to shed light on the rich history, the present state of art, and the future prospects of these essential nanomaterials in various fields, from material science to biotechnology and beyond.Various synthetic methodologies can be employed to produce nonporous SiNPs, which are widely acclaimed for their versatility in numerous applications. The synthesis of these nanoparticles commonly involves the adoption of both top-down and bottom-up methods, reflecting the diverse approaches used in nanoparticle production.9,10 A top-down method involves a nanoparticle synthesis approach that utilizes specific size reduction techniques to decrease the dimensions of the original material (a physical approach). This approach is known for its cost-effectiveness, however achieving a high degree of particle homogeneity poses a significant challenge.11 On the other hand, the bottom-up or chemical method is frequently employed to create SiNPs at the atomic or molecular level. This approach has gained substantial recognition as a promising avenue for nanoparticle synthesis, primarily because it is believed that conventional top-down methods cannot achieve size reduction at the subatomic or nano-scale level. However, it's important to note that bottom-up approaches often involve the use of a range of harsh chemicals and costly procedures, which may introduce potential environmental and biological risks.12 Thus, in the realm of SiNPs synthesis, an array of both physical and chemical processes have been harnessed, each with its own distinct advantages and limitations. Techniques such as the reverse microemulsion process, laser ablation, flame process, chemical vapor condensation, and sol–gel process offer promising avenues for SiNP production. However, it's important to recognize that these approaches are not without their drawbacks.13–17 In response to the growing need for more environmentally responsible practices, there is a resounding call for green and eco-friendly SiNPs synthetic methods. These sustainable approaches utilize natural silica precursors and environmentally sustainable catalysts. Silica, a fundamental component in nanoparticle synthesis, can be sourced from a wide array of materials, including minerals, rocks, clays, and various agricultural and industrial waste products such as rice hulls, husks, straw, and sugarcane bagasse.18 In the pursuit of eco-friendliness, alkaline catalysts derived from sources like leaf extracts, fruit pulps, and aqueous extracts of specific plant components have demonstrated promise in SiNP synthesis. This environmentally conscious approach not only proves efficient and cost-effective but also time-saving, aligning harmoniously with sustainable practices. Harnessing the potential of natural resources to produce SiNPs not only benefits science and industry but also upholds a commitment to environmentally responsible innovation.
This section comprehensively explores the evolution of modifications applied to the sol–gel process, focusing particularly on Stöber's method for synthesizing SiNPs. Additionally, it delves into alternative processes for producing SiNPs. Given the extensive applications of SiNPs across various fields, the imperative of adopting environmentally friendly synthesis methods is emphasized. The article adopts a historical perspective, tracing the development of synthesis methods over time. It critically evaluates diverse techniques for producing SiNPs with distinct shapes and surface characteristics. Furthermore, the review provides a consolidated examination of the applications of these nanoparticles in diverse domains.
2.1 SiNPs synthesis via. Stöber's sol–gel method
The Stöber method, initially introduced in the 1960s, involves a series of chemical reactions for synthesizing SiNPs. It begins with the hydrolysis and condensation of a silica alkoxide precursor, such as tetramethoxysilane (TMOS) or tetraethoxysilane (TEOS), conducted in the presence of a suitable solvent and catalyst, typically at room temperature. This process leads to the formation of colloidal silanol and siloxane compounds.19 In the Stöber method, SiNPs are crafted through a sequential hydrolysis and condensation process. During the hydrolysis step, a nucleophilic attack takes place on the tetraalkoxysilane molecule, facilitated by hydroxide anions, resulting in the formation of an anionic pentacoordinate intermediate and the removal of an alkoxide group (Fig. 1). The first step of the condensation process involves the extraction of a proton from the silanol by the hydroxide ion, leading to the generation of siloxide ions and water. Subsequently, the siloxide ion undergoes an SN2 attack on the core of other silanol molecules, resulting in the formation of siloxane bonds.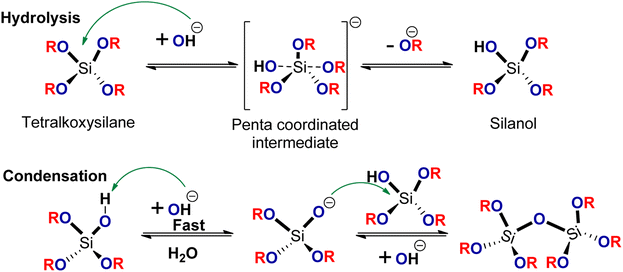 | ||
| Fig. 1 Scheme for SiNPs synthesis using the classical Stöber method. This method mainly comprises base catalyzed hydrolysis of the tetraalkylsilane followed by condensation of the silanol. | ||
Initially, Stöber's pioneering work focused on the synthesis of micrometer-sized SiNPs. Over time, numerous optimizations had been diligently carried out to refine the process, enabling the reproducibility of SiNPs with diverse sizes, shapes, and morphologies. To finely control the porosity, size, and uniformity of mesoporous silica nanoparticles (MNPs), commonly employed reactants include ammonia, serving as an alkaline catalyst, and a surfactant. These surfactants and segmented copolymers, play a pivotal role in achieving the desired structural characteristics of the nanoparticles. The absence of a surfactant during the synthesis process leads to the production of non-porous and non-monodisperse particles.
To precisely control over the dimensions, morphology, and uniformity of particles synthesized via the Stöber process, extensive research has been dedicated for understanding the kinetics and characterizing the steps involved. For instance, the study conducted by Nozawa et al., have delved into the intricate relationship between the rate of TEOS addition and the resulting particle size. These endeavors have contributed significantly to the advancement of nanoparticle synthesis.20 By carefully regulating the rate at which TEOS was added, researchers achieved a remarkable outcome—producing smaller and more uniformly sized particles. With precise control of the addition rate, they managed to achieve a significant 30% reduction in particle size. Pushing the boundaries further, an even faster addition rate resulted in an impressive 60% reduction in particle size. In pursuit of a streamlined approach for generating sub-100 nm particles, Y. Huang et al. introduced a novel reaction condition. This condition was based on a creative adaptation of the traditional LaMer model, tailored to the Stöber process. Their innovative approach aimed to find a set of optimized conditions that would yield particles of the desired sub-100 nm size.21 In contrast to various other synthetic approaches reported in the literature, this method stands out by notably diminishing the requisite number of test experiments essential for producing uniformly spherical silica particles below the threshold limit of 100 nm. Employing TEOS in conjunction with suitable modifier molecules, such as vinyltrimethoxysilane (VTMS) and 3-(methacryloyloxy) propyltrimethoxysilane (MPS), E. Effati et al. successfully synthesized silica nanoparticles (SiNPs) with vinyl and acrylate modifications.22 Through the fine-tuning of reaction conditions, this process yielded spherical SiNPs with average diameters that spanned a range from 50 to 700 nm. Gholami et al. synthesized spherical SiNPs using a sonochemical technique by reacting TEOS, methanol, and ethylenediamine (en) in the presence of an organic Schiff base ligand (Salpn-NO2)23 using water as solvent. To achieve the ideal conditions, the impacts of preparation parameters including ultrasonic power, ultrasonic irradiation time, and molar ratio of organic Schiff base ligand to TEOS were optimized. It was discovered that these characteristics could have an impact on the morphology, size, and phase of the products. Shekarriz and coworkers used the sol–gel process to make SiNPs with large surface area from water glass (sodium silicate), a cheap precursor.24 The response surface method (RSM) and central composite design (CCD) approaches were used to systematically design the synthesis. Particle surface area is regarded as the response or output parameter, and four important elements, including sodium silicate solution concentration, solution pH, reaction temperature, and reaction time, are designated as the key regulating parameters. It is possible to produce nanoparticles with a 630 m2 g−1 surface area and an 8 nm particle size. Huang et al.25 employed a novel precursor, 4-(chloromethyl) phenyltrichlorosilane, to introduce modifications at silica spheres. This innovative approach allowed for the creation of a highly sensitive and selective nanoparticle powder through a straightforward and cost-effective self-assembly process. The highly sensitive nanoparticle is utilized for latent fingerprint detection. Using the sol–gel process, Dubey et al. prepared silica nanoparticles (∼25 nm in diameter) from TEOS as a precursor and PVP as a surfactant.26 TEOS and numerous organosilanes were used by Barrera et al. to generate a range of functionalized, spherical, hydrophobic silica with a 0.5 μm size.27 From silicon tetrabromide (SiBr4) and 3-aminopropyltriethoxysilane (APTES), Chandra and coworkers described a one-pot synthesis of water dispersible fluorescent SiNPs functionalized with terminal amine groups.28 The NPs having a diameter between 1 and 2 nm, exhibit a strong blue fluorescence, and have a quantum yield (QY) in water of about 34%. Without using a base catalyst, the process of synthesis was also carried out in ethylene glycol and water medium. According to Gu et al. a two-step synthesis approach following the hydrothermal-assisted sol–gel method was adapted to produce superhydrophobic SiNPs.29 TEOS was hydrolyzed and condensed to produce SiNPs. The addition of hexadecyltrimethoxysilane (HDTMS) enabled the condensation reaction between the hydroxyl groups of silica and HDTMS, making the silica particles hydrophobic. A straightforward alteration to the traditional Stöber process was described by Meier et al., allowing for the high temperature synthesis of nanometric silica particles with diameters smaller than 100 nm in sealed reaction tubes.30 The improved synthetic method permits the production of smooth surface patterns and spherical SiNPs with mean diameters ranging from 28 to 647 nm. In this study, ethanolamine is used in sealed reaction tubes with ambient pressure to synthesize SiNPs instead of a traditional base catalyst. Jiang and co-workers reported silica microspheres with a narrow particle size distribution and an average diameter of 80–200 nm by varying the amounts of ammonia, water, TEOS, and temperature.31 Ling et al. designed the microfluidic reactor with a micro-droplets production junction where the reactants are combined to produce droplets with each droplet acting as a separate reactor.32 TEOS served as a precursor in the synthesis, acetic acid as a catalyst, and water as a hydrolyzing agent. With an average size of 6 ± 1.3 nm and flawless spherical structure, a highly monodispersed SiNPs were prepared in a microreactor. As per the findings of Bhakta et al., the substitution of ammonia with NaOH as the catalyst brought about a remarkable reduction in the reaction time, slashing it from 24 hours to a mere 90 minutes. Notably, the authors reported their ability to synthesize SiNPs of varying sizes simply by adjusting the NaOH concentration, all achieved at room temperature.33 In more recent research, it has been revealed that the incorporation of electrolytes in a low-alcohol environment (Fig. 2) offers a means to generate SiNPs within the desired size range.34
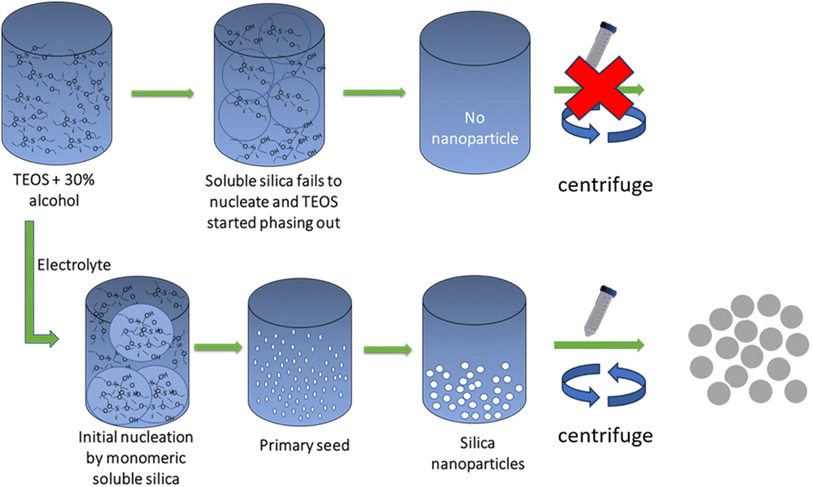 | ||
| Fig. 2 Graphical presentation of electrolyte assisted SiNP synthesis. This new finding helped to reduce the organic solvent use during the silica nanoparticles synthesis. | ||
These various optimizations in the Stöber method of SiNP synthesis not only present exciting prospects within the realm of research but also hold great promise for industrial-scale production. The scientific advancement in the synthesis of silica nanoparticles is pictured in the Fig. 3.
2.2 Reverse microemulsion process
The reverse microemulsion process, also known as water-in-oil (W/O) microemulsion, is a versatile and specialized technique used in nanoparticle synthesis and various chemical applications. In this process, tiny water droplets are dispersed within an organic solvent, forming a stable emulsion with the help of surfactants, with a drop diameter ranging from 10 to 100 nm.35 The roles of the components in reverse microemulsions are typically reversed compared to conventional microemulsions (oil-in-water). The reverse microemulsion process offers exceptional control over reaction conditions, making it particularly useful for nanoparticle synthesis. It allows for precise manipulation of particle size, shape, and uniformity, and it can be employed to encapsulate or coat nanoparticles with various materials, including silica, polymers, or metals. Schulman et al. (1959)36 first suggested the term “microemulsion”, which is a single optically isotropic and thermodynamically stable liquid solution. A system with low viscosity, transparency, isotropy, and high stability may arise from adding a co-surfactant to a coarse microemulsion made of water and surfactant in an amount sufficient to produce microdroplets. Reverse micelles are spheroidal aggregates created when surfactant molecules are dissolved in organic solvents.37 Indeed, the water-in-oil reverse microemulsion process is a commonly used method for producing SiNPs.38 In this process, water droplets, carefully stabilized by a surfactant, are introduced into an oil phase, effectively creating micelles that serve as nanoscale reactors. Within these micelles, the conditions are conducive for the nucleation and growth of SiNPs. TEOS, the precursor for silica, undergoes hydrolysis and condensation reactions as it diffuses into the aqueous phase, which also contains ammonia as a catalyst. Importantly, micelles interact by colliding and exchanging hydrolyzed monomers and/or nuclei, fostering the progressive development of SiNPs. This intricate interplay of reactions and nanoscale environments allows for precise control over SiNP formation, size, and properties.39 Osseo-Asare et al. have noted that the key factors influencing the ultimate size and distribution of nanoparticles are the ratios of water to surfactant, the concentration of ammonium hydroxide, and the concentration of TEOS.40In contrast to traditional Stöber techniques, the reverse microemulsion process offers a distinct advantage by producing monodisperse nanoparticles typically ranging in size from 15 to 50 nm. However, this method comes with a drawback: it relies heavily on organic solvents and surfactants throughout the synthesis, which subsequently complicates the purification process. Even after rigorous purification, these solvents and surfactants tend to linger on the nanoparticle surface, further exacerbating the issue. These limitations pose substantial challenges when contemplating the scalability of the process, primarily due to the requirement for costly solvents and extensive purification procedures. As a result, these challenges place significant constraints on the feasibility of large-scale production.7
3. Green and eco-friendly synthesis of SiNPs
Environmentally friendly methods for synthesizing SiNPs offer a host of advantages. Notably, SiNPs produced through green techniques exhibit at par quality with those NPs synthesized by using conventional chemical methods. These eco-friendly SiNPs find versatile applications across various technical domains, encompassing fields such as medicine, pharmaceuticals, electronics, paints, cosmetics, and many more.In green synthesis processes, environmentally safe reagents replace hazardous chemical counterparts, resulting in purer and less toxic nanoparticles compared to those generated through chemical means. The precursor employed in the synthesis of SiNPs often originates from agricultural waste, offering a substantial advantage due to its abundant availability at a remarkably low cost, particularly during the harvest season. An excellent example is rice husk, an agricultural byproduct rich in silica, which has been extensively documented in the literature as a valuable resource for producing high-quality SiNPs. For instance, Jansomboon et al. created silica micro- and nanoparticles by using rice shell for first time.41 In order to synthesize highly pure amorphous silica nano-discs, Lu et al. employed rice straw as a source of silica.42 Sankar et al. produced bio-generated SiNPs using sticky, red, and brown rice husk ash.43 In order to extract 52–78% silica from various agricultural wastes, Vaibhav et al. synthesized SiNPs from wastes such rice husk, bamboo leaves, sugarcane bagasse, and groundnut shell.44 Sorghum bicolor also produces agricultural waste, however, this waste is not properly disposed, therefore it causes pollution. Sorghum leftovers contain a significant amount of silica, making them suitable as a starting material to produce SiNPs. For a variety of uses in the food industry, Athinarayan et al. produced SiNPs using Sorghum bicolor leaves.45 Balamurugan et al. also used the seed head of Sorghum vulgare to produce nanosilica.46 As mentioned previously, various plant components can be used to create nanoparticles made of diverse materials and categories. For the synthesis of nanomaterials, the synthetic processes have employed extracts of diverse plant components from various origins as reducing or hydrolyzing agents. This environmentally friendly method helps to avoid using chemicals that are required for the synthesis of different nanomaterials. Many common plants can be used as a precursor for the synthesis of silica nanoparticles. Sethy et al. employed bamboo leaf ash to produce SiNPs and showed the application of this nanoparticles for the separation of polydimethylsiloxane (PDMS).47 In order to prepare SiNPs, Durairaj et al. used the well-known sol–gel technique using Bambusa vulgaris leaf ash.48 A green synthesis was reported by Jabeen et al. to synthesize SiNPs from TEOS and Azadirachta indica (Neem) leaf extract. In this method the neem leaf extract acts as reducing and capping agent.49
There have been several reports on the synthesis of SiNPs with the help of microbes. Vetchinkina et al. produced silicon nanospheres with sizes ranging from 5 to 250 nm using bacterial culture solutions.50 Singh et al. reported silica nanocomposites utilizing the bacterium actinobacter species.51 Yeasts and eukaryotic organisms can synthesize highly homogeneous nanoparticles. Using TEOS as a silicon source and diphenyl iodonium hexafuorophosphate as a super acid generator, Liu et al. produce SiNPs using a photochemical process.52 The initial hydrolysis of the silica source (TEOS) caused by the produced acid ultimately resulted in the formation of the particles. The use of fungi in the synthesis of SiNPs has been extensively reported in the literature. Verticillium sp. wereharvested for internal nanoparticle synthesis, and Fusarium, Aspergillus, and Penicillium have promising potential for extracellular bio-production of various metal nanoparticles. For instance, Pieła et al. used the Fusarium culmorum fungus to bioconvert corn cob husk into SiNPs of defined size and form.53 A few images of SiNPs via biosynthesis are given Fig. 4. From rice husk, Bansal et al. discovered a novel method to produce nanosilica. In this study, silica from rice husk is bioleached using the fungus Fusarium oxysporum. The naturally occurring biosilica found in rice husks can be quickly bio transformed by this fungus into crystalline SiNPs.54 In an intriguing study, Estevez et al. used Californian red worm to biodigest rice husk to produce silica nanoparticles. Rice husk and water are supplied to the worms, which then formed humus. After various treatments, SiNPs between 55 and 250 nm were obtained.55 Using several eukaryotic microorganisms such Actinomycetes, Fusarium oxysporum, Aspergillus niger, Trichoderma species, and Penicillium species, Kaur et al. synthesized SiO2 nanocomposites from rice husk and wheat bran. In the size range of 232 to 250 nm in which all these microorganisms biotransform amorphous silica from a natural source to crystalline silica in less than 24 hours.56 In comparison to SiNPs made using chemical procedures, the purity of the SiNPs produced using the various green methods discussed is noticeably higher and generate much lower amount hazardous byproducts. Although, the synthesis process may take longer time, there are many other advantages associated with those bio-synthesized nanoparticles. For instance, Albalawi et al.57 reported the SiNPs synthesized using. Aspergillus niger showed a promising antifungal activity against Alternaria solani. According to their studies, this biosynthesized SiNPs can be used useful to prevent early blight disease of eggplant as an alternative to chemical pesticides. Similarly, biosynthesized SiNPs in the range of 20–50 nm using Penicillium oxalicum exhibited photocatalytic properties and these particles decolorized Ribazol black b and Crystal violet dyes under a UV-light.58 Various naturally occurring precursor of SiNPs are summarized in Table 1.
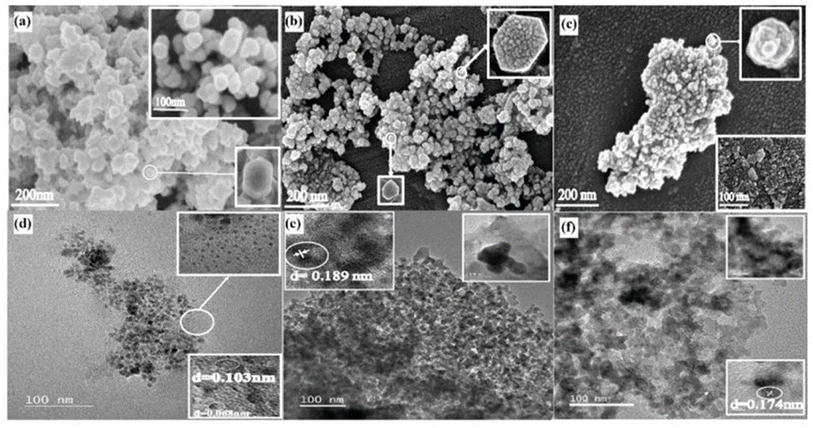 | ||
| Fig. 4 FESEM images of biosynthesized of SiNPs (a–c) HRTEM images of biosynthesized SiNPs (d–f).59 Copyright © 2021, Elsevier. | ||
| Author | Source/precursor | Ref. |
|---|---|---|
| Jansom Boon et al. | Rice shell | 41 |
| Lu et al. | Rice straw | 42 |
| Sankar et al. | Sticky, red, and brown rice husk ash | 43 |
| Vaibhav et al. | Rice husk, bamboo leaves, sugarcane bagasse, and groundnut shell | 44 |
| Athinarayan et al. | Sorghum bicolor leaves | 45 |
| Balamurugan et al. | Sorghum vulgare | 46 |
| Sethy et al. | Bamboo leaf ash | 47 |
| Durairaj et al. | Bambusa vulgaris leaf ash | 48 |
| Jabeen et al. | TEOS and Azadirachta indica (neem) leaf extract | 49 |
| Vetchinkina et al. | Bacteria cultured liquid | 50 |
| Singh et al. | Bacterium Actinobacter as reductase and oxidase | 51 |
| Liu et al. | Yeasts and eukaryotic organisms along with TEOS | 52 |
| Aleksandra Pieta et al. | Fusarium culmorum fungus as bio converter and corn cob husk | 53 |
| Bansal et al. | Rice husks and Fusarium oxysporum | 54 |
| Estevez et al. | Californian red worm and rice husk | 55 |
| Kaur et al. | Rice husk, wheat bran and eukaryotic microorganisms | 56 |
The synthesis of nonporous SiNPs has evolved significantly over the years, from classic methods like the Stöber approach to greener and more sustainable strategies. These compact nanoparticles, with their precise structures and tailored properties, continue to serve as fundamental building blocks in nanoscience and technology. With the development in the field of nanotechnology, it is evident that the progress of novel synthesis techniques and the optimization of existing ones will remain critical in unlocking the full potential of nonporous SiNPs across diverse applications. The journey from traditional to sustainable methods exemplifies not only the progress in materials science but also the commitment to environmentally responsible practices, making nonporous SiNPs a promising avenue for future innovations in nanotechnology.
4. Other silica nanoparticle
Beyond nonporous SiNPs, a diverse array of SiNPs, including mesoporous, hollow spheres, and core–shell structures, biologically compatible nanoparticles have gained widespread attention for their versatile applications and have been synthesized through various innovative routes (Fig. 6). These synthetic approaches for producing SiNPs, whether mesoporous, hollow spheres, or other distinct shapes, predominantly build upon the foundational Stöber process while incorporating additional structure-directing elements. Notably, alterations in nanoparticle shape play a pivotal role in shaping the in vivo properties, impacting critical factors such as biodistribution, bioavailability, and their potential for cellular endocytosis.60 The synthesis of nanoparticles can be systematically modified by introducing alternative dopants as precursors, making substantial adjustments to pH or temperature during the synthesis process, or commencing with a precisely engineered template. This subtopic explores the synthesis and implications of these varied SiNP structures in the realm of nanotechnology.4.1 Synthesis of mesoporous silica nanoparticles (MSNP)
The synthesis of MSNPs stands as a significant milestone in the realm of nanomaterials, offering a wealth of opportunities for applications in diverse fields. These nanoparticles, characterized by their intricate pore structures and high surface areas, have garnered substantial attention due to their exceptional versatility and potential to address various challenges in science and technology. In this section, we embark on a comprehensive exploration of the synthesis methodologies employed in crafting MSNPs, exploring into the ingenious strategies that enable precise control over their pore size, morphology, and surface functionality. By modifying the Stöber process with additional surfactants (such as cetyl trimethylammonium bromide [CTAB]), micelle-forming materials, polymers, and other dopants, mesoporous SiNPs can be obtained.61–63 The removal of the micelles causes the SiNPs to develop pores because the micelles serve as masks for the TEOS-based silica development (Fig. 5). Nooney et al. demonstrated that MSNPs with a wide range of diameters, varied from 65 to 740 nm, were synthesized by simply varying the TEOS-surfactant ratio under diluted conditions by using both cationic CTAB and non-ionic surfactants (n-dodecylamine) as templates.64 MSNPs can be made by changing a few synthesis variables, including the pH of the reaction mixture, the properties of the copolymers or surfactants utilized, the reagent concentrations, and the sources of silica.65 Möller and Bein discussed how to obtain functionalized mesoporous SiNPs through co-condensation methods with organosilane agents, with a focus on the spatially selective anchoring of various molecular functionalities within the nanoparticles. They also discussed the modification of the pore size and pore topology of the mesoporous system through templating and pore-swelling agents, as well as by adjusting the synthesis conditions.66 By adjusting the ethanol content, the pore diameter of the sphere was customized. To balance the hydrophilic–hydrophobic composition of the self-assembling system as well as the interaction between ethanol and the cationic surfactant, ethanol may function as a “spacing agent.” The team suggested a “Liquid Crystal Initiated Templating” mechanism for the micellar rods' hexagonal arrangement. In this particular method, the sequence of events unfolds in a specific manner. Initially, the surfactant undergoes a self-organization process, followed by the typical hydrolysis of alkoxides. Subsequently, the surfactant molecules become integrated with silica oligomers, forming an essential part of the nanoparticle synthesis process. The mesoporous silica MCM-41 were formed via this process. A thorough investigation on the formation and management of MCM-41 morphologies was published by Cai et al.67 utilizing the cationic surfactant CTAB as a template.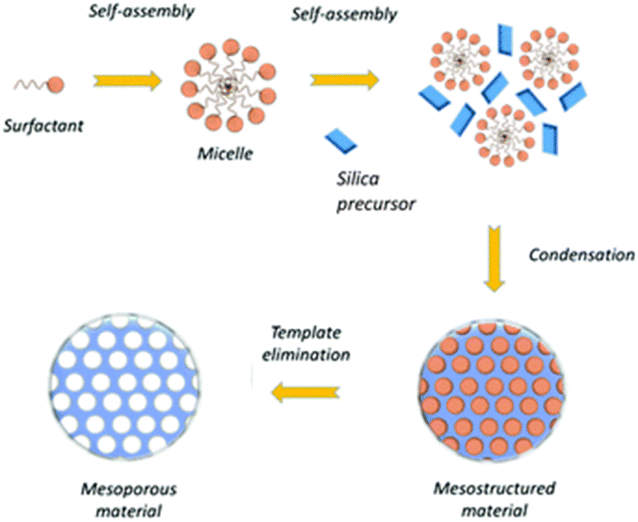 | ||
| Fig. 5 A step wise schematic diagram of the MSNP synthesis.68 Copyright © 2021, Royal Society of Chemistry. | ||
 | ||
| Fig. 6 Various types of silica nanoparticles with different shape and surface morphology. Each nanoparticles exhibited unique properties which can be used for specific applications. | ||
In both acidic and basic media, Boonamnuayvitaya et al. investigated the effects of surfactant type and concentration.69 As cationic, anionic, and non-ionic surfactants, they employed CTAB, sodium dodecyl sulphate (SDS), and Brij-56, respectively. In acidic circumstances, a large surface area (1282 m2 g−1) was produced utilizing CTAB as a template. With increasing surfactant content, pore volume and average pore diameter increased using CTAB as a template. The largest pore diameter in basic medium was found in Brij-56 templated silica (14.4 nm). The results of utilizing Brij-56 as a pore-forming agent followed the same general pattern as those shown with CTAB. Because SDS bind together to form a mass when the SDS content is increased, it is seen that the pore volume and average pore diameter decrease with increasing SDS content.70,71
The synthesis of MSNPs represents a remarkable intersection of science and engineering, yielding nanomaterials with intricately designed pores and vast surface areas. The diverse synthesis approaches explored in this section underscore the dynamic nature of MSNPs, offering researchers the tools to tailor these nanoparticles precisely for specific applications.
4.2 Synthesis of hollow sphere or core–shell SiNPs
The synthesis of hollow sphere and core–shell SiNPs marks a significant advancement in nanomaterial engineering, offering a unique and versatile platform for innovative applications. These specialized nanoparticles exhibit distinct structural characteristics that enable them to serve as dynamic carriers, catalysts, or multifunctional platforms in various fields of science and technology. In this section, we embark on a comprehensive journey into the world of hollow sphere and core–shell SiNPs synthesis, unraveling the ingenious techniques that enable the creation of these complex nanostructures. From carefully controlled etching processes to precise encapsulation strategies, we explore the diverse array of methods employed to craft these remarkable nanomaterials.A subclass of SiNPs known as hollow SiNPs (HSNs) is created when the core of core–shell of SiNPs is removed. The hollow interior of the HSNs is sealed off from the surrounding air by the silica shell.72 When compared to SiNPs, HSNs not only share similar characteristics, but also benefit from the hollow interior, which gives them a wide inner cavity, low density, improved optical performance, among other beneficial qualities.73,74 A review on the synthesis of HSNs using the three approaches of soft template, hard template, and dissolving and reconstructing SiNPs to HSNs were elaborated by Lin et al.75 Air bubbles, surfactant micelles, and liquid droplets all serve as soft templates in the synthesis of HSNs.76 EI-Said et al. effectively synthesized various new Gemini surfactants with various tail lengths and spacers that may create spherical micelles as soft templates in order to achieve size control of HSNs.77 On the proviso that the template is removed with an unbroken silica shell, the hard template approach is frequently used in the preparation of HSNs. Commonly, the silane coupling agent is hydrolyzed and condensed in the Stöber sol–gel technique to create HSNs. The major features of these HSNs are tunable size, template creation, and removal. In 2020, Nakashima et al. developed HSNs with diameters ranging from 10 nm to 21 nm using polyacrylic acid (PAA) as an adaptable template.78 The performance of hollow silica materials with various morphologies and their recent advancements in template synthesis were emphasized by Bao et al. in a variety of applications.79 By using polymer micelles as a template, Tao et al. were successful in producing hollow silica nanostructures with controllable shape. As it is well known, altering the concentration or chemical structure of the micellar template can alter its form.80 Based on a template of CaCO3 with various shapes, Yap Ang et al. created spherical, cubic, and rod-like hollow SiNPs that were placed in membranes. Membranes' hydrophilicity or hydrophilicity was enhanced using hollow silica particles.81 The synthesis of hollow sphere and core–shell SiNPs represents an exciting frontier in nanomaterial innovation, promising tailored solutions for a multitude of scientific and technological challenges.
4.3 Synthesis of functionalized SiNPs
The synthesis of functionalized SiNPs stands at the forefront of nanomaterial science, bridging the divide between traditional materials and cutting-edge nanotechnology. These nanoparticles, adorned with tailored functional groups, offer a dynamic platform for customizing their properties and applications across a multitude of fields. In this section, we embark on a comprehensive exploration of the strategies and methodologies employed to fabricate these versatile nanomaterials. From surface modification techniques to the incorporation of specific functionalities, we delve into the intricate world of functionalized SiNPs, uncovering the transformative potential they hold in areas ranging from targeted drug delivery to advanced sensing technologies. This section not only illuminates the principles of synthesis but also highlights the countless opportunities these nanomaterials present in shaping the landscape of materials science and nanotechnology.The most straightforward method for surface modification of silica particles is a reaction with one of the numerous alkoxysilanes or halosilanes that are commercially available. A general strategy for functionalization is described in Fig. 7 and several useful strategies are summarized in Table 2. Alkoxysilanes and halosilanes are offered by several chemical companies, including Sigma Aldrich, Gelest, Strem Chemicals, and many more. There are primarily two methods for functionalization: co-condensation or post-synthetic grafting.82,83 The most common method for covalently grafting organic functionalities onto the surface of SiNPs is post-synthetic grafting. On the other hand, the co-condensation approach, also known as the one-pot synthesis method, is a direct method for synthesizing nanoparticles. In this method, tetraalkoxysilanes [(R′O)4Si (TEOS or TMOS)] are co-condensed with the appropriate terminal trialkoxyorganosilane (R′O)3SiR (R′ is commonly -Et, -Me, and R is an organic group). In a condensation reaction with the surface silanol groups, alkoxysilanes will bind and create 1–3 Si–O–Si linkages. Alcohol groups can similarly go under condensation and produce 1–3 Si–O–Si linkages with surface silanol groups.84 In the similar way, anhydrous halosilanes directly interact with the surface silanol groups and form Si–O–Si linkage via. condensation reaction. Most typically, APTES, 3-mercaptopropyl trimethoxysilane (MPTS), 3-isocyanatopropyltriethoxysilane85 and other PEG-silanes are used to functionalize nanoparticles. The first two make it simple to combine linker chemistry with other frequently used linking compounds, such as isothiocyanates, maleimides, and molecules functionalized with n-hydroxysuccinide (NHS).86 To increase a nanoparticle's stability in biological fluids, biocompatibility, and in vivo circulation periods, pegylation is widely utilized.87–89
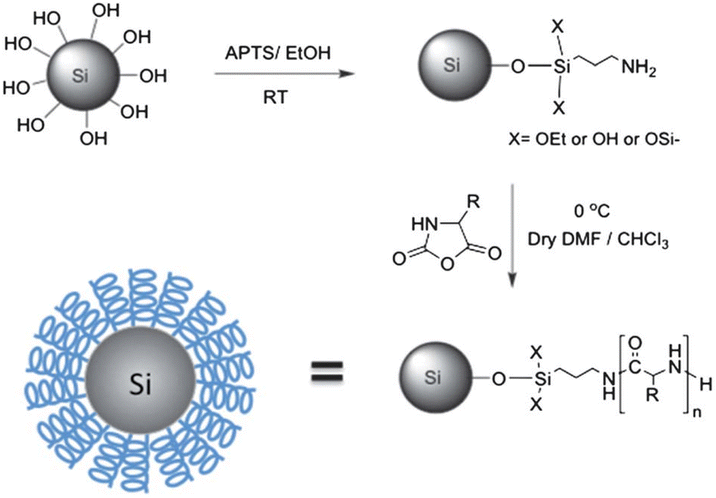 | ||
| Fig. 7 Synthesis of functionalized SiNP which is required for specific application.106 Copyright © 2012, Royal Society of Chemistry. | ||
| Functional group | Chemicals used | Advantage | Ref. |
|---|---|---|---|
| Amine (–NH2) | 3-Aminopropyltriethoxysilane (APTES) | Amide bond formation | 86 |
| Isocyanate (–NCO) | 3-Isocyanatopropyltriethoxysilane | Coupled with –OH, –NH2, –SH functionalized compounds | 80 |
| Thiol (–SH) | 3-Mercaptopropyl trimethoxy silane (MPTES) | Coupled with maleimides | 86 |
| Hydrophilic –OH | Polyethylene glycol (PEG) | Increase stability in biological mixtures | 87 |
| Polymers | Poly(methyl methacrylate) (PMMA) | Increase the colloidal stability | 90 |
| Poly(N-isopropylacrylamide) | Imparts thermo-responsivity | 82 |
There are a number of additional ways for APTES to adhere to or associate with a silica surface, mostly through hydrogen bonds, other non-covalent interactions, or vertical polymerization. Furthermore, uniform amine modification of the silica surface is often difficult due to the tendency of aminoalkoxysilanes in solution to spontaneously polymerize.91,92 Therefore, it is essential to carefully control reaction conditions and the amount of APTES utilized to produce consistent amine monolayers.93,94
Electrostatic repulsion can be used by post-synthesis coatings of SiNPs with amine-reactive moieties to increase colloidal stability, although it should be emphasized that this stability depends on other factors, such as pH. While the silica matrix is negatively charged under physiological pH, amine groups on the NP surface can be protonated (–NH3+) positively charged.95 As a result, the surface charge of the NP decreases, which causes NP–NP repulsion to decrease and leads to aggregation. In a significant work, Bagwe et al. demonstrated how negatively charged “inert” phosphonate groups and “reactive” amine groups can coexist on the surface of RuBpy-doped SiNPs to lessen particle aggregation while still providing the reactive sites required for later functionalization.96 Traditional “monopodal” APTMS has also been compared to “bipodal” bis amine[3-(triethoxysilyl) propyl], with the former producing more colloidally stable amine-coated SiNPs.97
The change in zeta potential, which often indicates the success of amine modification but does not quantify the functional groups surface density (essential for toxicological investigations because amine-modified materials might increase cytotoxicity), is a sign of successful amine modification98 Quantitative techniques to precisely characterize the presence of amine and other functional groups on the surface of SiNPs include acid–base titration,99 colorimetric or fluorescent reactions,100,101 thermogravimetric analysis,102 or solution103,104 or solid-state105 NMR.
5. Methods of characterization
In the field of synthetic chemistry, characterizing materials or products stands as a pivotal aspect in establishing the reliability of the work. Particularly, in the case of SiNPs, researchers employ various common characterization techniques to the post-synthesis. Based on the study of Brownian motion, Nanoparticle Tracking Analysis (NTA) is one of the few techniques for measuring and visualizing nanoparticles in suspension in the range of 10–1000 nm. The surface of a dispersed particle in a liquid medium is mostly coated with a thin layer of electric dipoles. Given that this layer affects the particle's motion, the so-called hydrodynamic particle diameter is measured using dynamic light scattering (DLS). The DLS size is always greater than the TEM size because of the solvent layer. Hence, more detailed information about individual particle size and morphology of SiNPs is obtained through imaging techniques such as Field Emission Scanning Electron Microscopy (FESEM), Transmission Electron Microscopy (TEM), and Scanning Electron Microscopy (SEM).The surface charge and electrokinetic behavior are assessed using zeta potential measurements, while Energy Dispersive X-ray Spectroscopy (EDX) analysis provides insights into the % of composition of the Si and O atoms and other impurities. To gain a comprehensive understanding, Powder X-ray Diffraction (XRD) analysis is employed to elucidate the material's texture, and Fourier Transform Infrared Spectroscopy (FTIR) is utilized to examine the bonding nature (specifically Si–O–Si bonds) and the presence of functional groups on the surface-modified SiNPs. In XRD analysis, the amorphous nature of SiNPs is confirmed by observing the maximum intensity at 2θ = 22°. Peaks at 21.8, 36, 40.3, 42.6, 46.7, 48.7, 53.9, 56.9, 61, 65.2, and 79° were connected to the (100), (110), (111), (200), (201), (112), (202), (210), (211), (300), and (213) planes, respectively, in the crystalline SiO2 XRD. These peaks are the identification of Crystalline-SiO2, as shown by the JCPD card (00-046-1045).107 In FTIR analysis, distinctive bands at 1058, 803, and 457 cm−1 are attributed to Si–O–Si asymmetric stretching, symmetric stretching, and bending vibrations, respectively. The band at 3422 cm−1 is associated with a hydrogen-bonded Si–OH stretching vibration.108 The silica particles are highly hygroscopic, according to FTIR measurements. A further identified peak in the infrared spectrum, located at 1635 cm−1, is ascribed to Si–H2O flexing.109 Furthermore, the surface area and pore size of MSNPs are quantified using BET adsorption isotherm, where nitrogen adsorption is measured. Thermal stability analysis is carried out through Thermogravimetric (TG) analysis to assess the stability of functionalized groups on the SiNPs.110 Fig. 8 shows some of the important characterization data obtained from the literature.
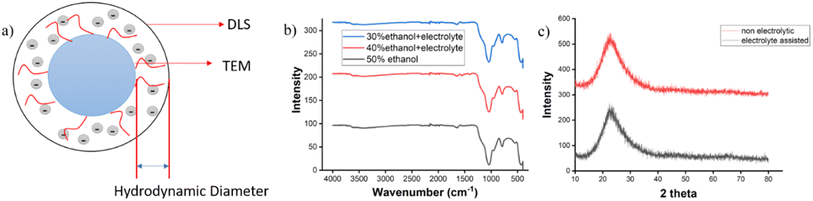 | ||
| Fig. 8 (a) Pictorial presentation of TEM, DLS size and hydrodynamic diameter; (b) characteristics FTIR diagram of Silica nanoparticles;34 Copyright © 2011, Royal Society of Chemistry, (c) powder XRD of amorphous Silica nanoparticle.34 Copyright © 2023, Royal Society of Chemistry. | ||
6. Major applications of SiNPs
6.1 Biomedical application
Over the past two decades, there has been a notable emphasis among scientists on leveraging the advantageous physicochemical properties of nanoparticles in biological research. The objective has been to enhance traditional immunoanalytical methods by harnessing nanoparticles' capabilities to enhance signal-to-noise ratios, reduce detection limits, heighten sensitivity, and decrease analysis time. Among the diverse array of nanomaterials available, silica particles have emerged as a leading material in this domain. This prominence is attributed to their predictable and adaptable chemistry, minimal cytotoxicity, and generous surface area, all of which facilitate various surface modifications and functionalization techniques. These attributes render silica particles highly suitable for a broad spectrum of biological applications.Within the realm of nanomaterials, the term “bioconjugation” pertains to the process of linking biomolecules (such as proteins, enzymes, oligonucleotides, etc.) to nanoparticle surfaces, thereby creating bio-modified surfaces crucial for in vivo drug delivery (Fig. 9). Nevertheless, the effectiveness of utilizing nanoparticles functionalized with targeting moieties remains a subject of ongoing debate.111 The process of synthesizing nanoparticles (NPs) with functionally active proteins on their surfaces has proven to be more challenging than initially expected, presenting unanticipated obstacles for researchers in this field. Bioconjugation employs two main approaches for this purpose: passive absorption and covalent attachment.112 The protein layers encasing the nanoparticle (NP) surface are bound through passive adsorption, a straightforward, cost-effective, and efficient method. Vertegel and her team also explored how the size of the particles influenced the structure and function of proteins adhered to SiNPs, examining sizes of 4 nm, 20 nm, and 100 nm.113 Similar trends were seen in the adsorption of cytochrome C to SiNPs of various sizes.114 Local pH affects a protein's isoelectric point, which in turn affects its electrostatic interactions with the NP surface and may ultimately lead to biomolecule desorption.115 Covalent bond formation, which is often aided by a linker molecule, is the approach that is most frequently employed to functionalize SiNPs with biomolecules.94,116 Linkers are typically utilized to bind to particular antibody amino acid residues. For instance, the total number of lysine residues in an IgG biomolecule is ∼83,117 and these primary amines can serve as covalent attachment sites when combined with the chemistry of ethyl(dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS).95,118 An increasingly common method of bioconjugation is “click” chemistry, which involves highly efficient and stereospecific reactions. Due to their high binding efficiencies, “click” reactions for bioconjugation provide the potential to utilize less antibody per reaction than standard EDC/NHS methods.119 Several frequently used organic dyes, including cyanine,120 rhodamine,121 and fluorescein,122 can be covalently attached on the particle surface or inside the particle core. As labels in an electrochemiluminescent sensor for the detection of prostate-specific antigen, Rusling and colleagues used Ru(bpy)-doped SiNPs made via the reverse microemulsion process.123 The capacity to detect Salmonella pullorum using 45 nm SiNPs doped with C.I. Reactive Blue 21 has been demonstrated by Sun and colleagues.124 Later, with a total assay time of one hour, this work was expanded to detect Salmonella pullorum and Salmonella gallinarum in powdered milk samples.125
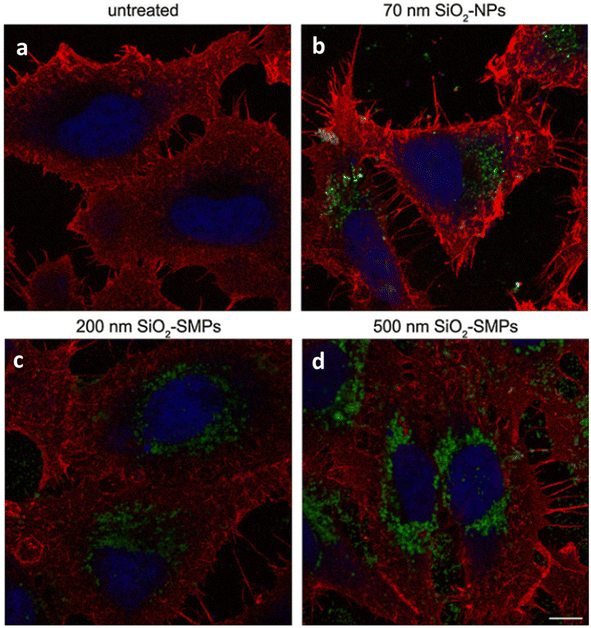 | ||
| Fig. 9 Figure showing the biomedical application of SiNPs as a potential drug-delivery agents. Internalization of FITC-SiNPs nanoparticles within HeLa fixed, stained with the nuclear marker DRAQ5 (blue) and the membrane marker TMR-WGA (red); (a) untreated and treated with (b) 70 nm (c) 200 nm and (d) 500 nm SiNPs.126 Copyright © 2011, Springer. | ||
A significant milestone under this development is the use of designed nanostructures for the targeted drug delivery to patients using SiNPs.127 The SiNP can be used as a potential carrier for hydrophobic drugs. Hydrophobic drugs have lower adsorption capacity when taken orally as compared to other drugs. These hydrophobic drugs can be conjugated with negatively charged silica nanoparticles and the adsorption capacity was found to be improved significantly during oral does.128,129 Furthermore, these nanoparticles' high loading efficiency is due to their biocompatibility and potential for functional group modification.130 SiNPs were utilized by Zhang et al.131 to transport the hydrophobic medication Telmisartan. It was discovered that SiNPs also increase the drug's permeability. Similarly, cancer treatment and colloidal delivery of therapeutic compounds to tumor sites via blood have received the majority of attention in drug delivery applications using SiNPs. Mashayekhi et al. and Elbialyl et al. demonstrated a notable enhancement in the functioning of curcumin (an anticancer medication) when conjugated with SiNPs.132,133 For instance, Roy et al. synthesized doxorubicin-loaded ORMOSILs with diameters of 30, 50, and 80 nm by utilizing an oil-in-water microemulsion to prepare vinyltriethoxysilane as an NP precursor. The 50 nm NPs were demonstrated to have optimum drug release to pancreatic MiaPaCa-2 cells give details (MIA PaCa-2 is an epithelial cell line that from tumor tissue of the pancreas) over a 2 weeks timeframe, while the NPs were declared non-toxic.134 Munoz et al. investigated the characteristics of MSNPs as Ibuprofen transporters for hydrophobic drugs. Ibuprofen's carboxylic acid group and the silanol groups in the silica matrix were shown to interact with each other, according to the authors, leading to interactions between the cargo Ibuprofen molecules and the SiNPs.135 Camptothecin (CPT) and paclitaxel (Taxol), two hydrophobic anti-cancer medicines, were examined as potential candidates for delivery through non-gated MSNPs to human cancer cells by Lu et al. According to reports, the MSNPs carry anticancer medications into human xenografts in mice and inhibit tumor growth.136
6.2 Environmental application
Due to the substantial negative impacts of heavy metals on both human health and the environment, the removal of these pollutants from water sources stands as a top priority. An essential goal in addressing this challenge involves harnessing the mesoporous nature of silica materials. This structural characteristic results in a considerable expansion of their surface area, which, in turn, enables a more substantial attachment of functional groups. This improved functionalization holds the promise of increasing the adsorption capacity of silica materials for binding metal ions. Making use of the benefits offered by mesoporous structures greatly amplifies the efficiency and effectiveness in the removal of heavy metals from water. In a specific application, Jang and colleagues, in 2020, synthesized amino-functionalized MSNPs using the Stöber method for the purpose of adsorbing Cr(VI) ions from aqueous environments.137 Mesoporous Organosilica Nanoparticles (MONs) have been applied to the adsorption of various targets. In 2019, Yang et al. developed ferrocene-incorporated MONs for Pb2+ adsorption; the results showed that MONs is superior Pb2+ absorbents when compared to ferrocene surface modified MSNs.138 There has been a lot of focus on the treatment of wastewater containing dyestuffs. Surface silanol groups and other functional entities formed complexes with dyes, or interactions including hydrogen bonding, electrostatic attraction, and hydrophobic forces between SiNPs and the dyes were employed for dye removal. In 2018, Lu and colleagues devised a straightforward one-step method to create MSNs for effectively eliminating various cationic dyes (such as rhodamine B, methylene blue, methyl violet, malachite green, and basic fuchsin) from aqueous solutions.139 Recently, TEOS, APTES, and N-isopropylacrylamide co-vinyltrimethoxysilane based microgels were used to create smart amine-functionalized MSNs using sol–gel techniques. Sabeela et al. demonstrate effective experimental adsorption capacity removal of Coomassie blue (CB-R250) from wastewater.140 In a recent study in this field, researchers led by El-Gazzar et al. employed rice husk for the biosynthesis of SiNPs. They utilized Trichoderma harzianum MF780864 to initiate the formation of these particles, which were subsequently employed for the removal of lead (Pb) from water.1416.3 Industrial and agricultural application
SiNPs find extensive industrial and agricultural applications due to their unique properties and versatility. Here are some notable examples:(a) Industrial coatings: SiNPs are utilized in industrial coatings to enhance scratch resistance, durability, and mechanical properties. They improve the adhesion and hardness of coatings, making them ideal for applications in automotive, aerospace, and other industrial sectors.142
(b) Carrier for catalysts or catalysis: SiNPs serve as supports or carriers for catalysts in various chemical reactions. Their high surface area and tunable porosity provide an excellent platform for catalyst immobilization, increasing reaction efficiency and selectivity in processes such as petroleum refining and chemical synthesis.143
(c) Agriculture: SiNPs are used in agriculture for soil improvement and crop protection. They can be incorporated into fertilizers to release nutrients gradually and improve nutrient uptake by plants. Additionally, SiNPs are employed as crop protectants, acting as carriers for agrochemicals and enhancing their targeted delivery to pests or diseases (Fig. 10).144
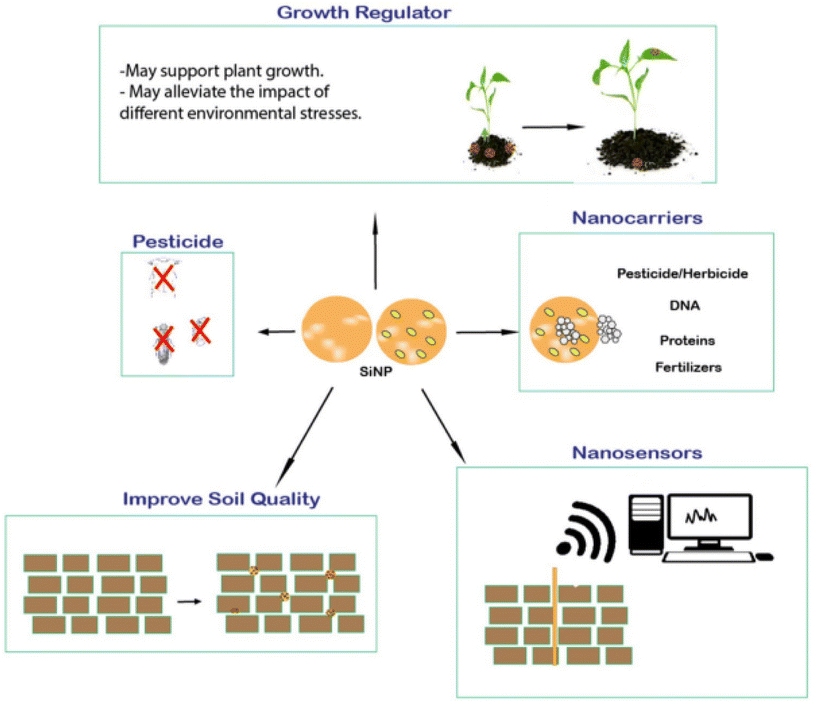 | ||
| Fig. 10 Figure shows the utility of SiNPs in agriculture. These nanoparticles can be used as a carrier for fertilizers, alternative to pesticides, sensors, and overall, this helps to improve soil quality.144 Copyright © 2019, Springer. | ||
(d) Food and beverage industry: SiNPs are utilized as food additives to improve stability, texture, and absorption of flavors or aromas. They find application as anticaking agents in powdered products, clarifying agents in beverages, and carriers for food encapsulation and controlled release of ingredients.145
(e) Energy Storage: SiNPs are employed in energy storage systems, particularly in lithium-ion batteries. They enhance battery performance by providing stable electrode structures, improving charge/discharge rates, and increasing overall energy storage capacity.146,147
(f) Water filtration: Functionalized-SiNPs are used in water treatment processes for the removal of environment contaminants. The high surface area and adsorption capacity make this particle effective adsorbents for heavy metals, organic pollutants, and other contaminants present in water sources.148
(g) Personal care products: SiNPs are used in cosmetics and personal care products. They contribute to the formulation of sunscreens, skincare products, and hair care items, providing properties like UV protection, improved texture, and controlled release of active ingredients.149
These examples represent a fraction of the various range of industrial and agricultural applications of SiNPs. The vast areas of application are summarized in Table 3. Ongoing research and technological advancements continue to uncover new possibilities for applying these nanoparticles across several areas, driving innovation and enhancing product performance in many fields.
| Application area | Reference | |
|---|---|---|
| Ru(bpy)-doped SiNPs | Electrochemiluminescent sensor for the detection of prostate-specific antigen | 123 |
| 45 nm SiNPs doped with C.I. Reactive blue 21 | Detect Salmonella pullorum and Salmonella gallinarum in powdered milk samples | 124 and 125 |
| Doxorubicin-loaded ORMOSILs | Drug release to pancreatic MiaPaCa-2 cells | 134 |
| Curcumin conjugated with SNPs | Anticancer medication | 132 and 133 |
| MSNPs | Ibuprofen transporters for hydrophobic drugs | 135 |
| MSNPs | Camptothecin (CPT) and paclitaxel (Taxol), two hydrophobic anti-cancer medicines delivery | 126 |
| Amino-functionalized MSNPs | Adsorption of Cr(VI) ions from aqueous environments | 137 |
| MSNPs | Removal of cationic dyes (rhodamine B, methylene blue, methyl violet, malachite green, and basic fuchsin) from aqueous solutions | 139 |
| Amino-functionalized MSNPs | Removal of Coomassie blue (CB-R250) from wastewater | 140 |
| Rice husk to biosynthesize SiNPs | Removal of Pb from water | 141 |
| Silica nanoparticles | Industrial coating | 142 |
| Catalyst | 143 | |
| Nano silica fertilizer | 144 | |
| Food and beverages | 145 | |
| Energy storage | 137 | |
| Personal care | 149 |
7. Industrial market value of SiNPs
The projected growth of nano silica is expected to reach ∼$9 billion in 2031. The wide range of usage of nano silica in many industries such as concrete, rubber, plastics, electronics, healthcare, coating, agriculture etc. make this industry grow rapidly in the coming years. Because of cementitious properties, nano silica is considered to be an extremely useful in concrete and rubber industry. Nano silica or SiNPs is used an additive material in ethylene acrylic rubber, silicon rubber, nitrile rubber styrene butadiene rubber and in natural rubber also. It can increase the tensile strength of the rubbers. In addition, this material can improve the durability and mechanical properties of cements, rubber and paints.In healthcare, SiNPs find applications in drug delivery systems, diagnostics, and therapeutics. Their large surface area and tunable properties enable efficient drug loading, controlled release, and targeted delivery, revolutionizing the field of personalized medicine.
SiNPs also play a crucial role in energy-related applications. They are used as catalyst supports in heterogeneous catalysis, enhancing efficiency in chemical processes and fuel cells. Additionally, SiNPs are utilized in energy storage systems, including lithium-ion batteries and supercapacitors, due to their high surface area and stability.
Many major industries, namely, Evonik industries, Akzonobel N.V., Dow Corning Corporation, Fuso Chemical Co. Ltd, Wacker Chemie AG, Bee Chems and many more play a major role to produce and supply nano silica to meet the global demand. Rapid growth in the construction activities in Asia pacific countries like India and China, contributed a massive demand in nano silica market. The toxicity associated with SiNPs sometimes hinders the growth of the materials. Thus, alternative greener and cheaper approach could be a massive success in this area.150,151 The industrial market value of SiNPs is expected to continue its upward trajectory as advancements in synthesis methods, surface modifications, and functionalization techniques enable tailored properties for specific applications. With ongoing research and development, SiNPs hold promise for further innovation and growth across multiple industries, driving their market value to new heights.
8. Future perspectives and conclusion
Silica nanoparticles have already found a significant spot in the nanotechnology market. Some of the unique properties like size-tunability, non-toxicity, pore size, biocompatibility, functionalization etc. make this nanoparticle an obvious alternative in many applications. SiNPs are widely used in biomedical applications, catalytic reactions, agriculture, the food industry, and more. Different types of silica nanoparticles e.g. mesoporous SiNPs, core–shell SiNPs offer a significant advantage in various applications due to its high surface area. Historically, Stöber introduced this particle using a simple sol–gel process. Many advancements in the synthetic procedure are reported in the literature. While the conventional synthesis route, following the Stöber method, has shown promising success, the use of harmful organic chemicals poses environmental concerns and increases material costs. Therefore, it is crucial to adopt safer and greener approaches to overcome these issues. To overcome this, various green methods including modified Stöber method using alternative base and electrolytes reduced the amount of harsh organic solvent significantly. Alternative basic catalysts during synthesis also have significantly reduced process time, and the use of microorganisms has enabled the production of highly homogeneous SiNPs without the need for expensive purification steps. Substantial progress has been made in producing SiNPs using alternative sources, such as agricultural waste materials, which addresses additional environmental concerns. However, these methods require further research to establish scalable solutions for the market. An electrolyte-based approach has emerged as a greener alternative, reducing the reliance on organic solvents. On the other hand, exploring alternative chemicals or green solvents like ionic liquids holds promise in this regard.After a thorough literature survey, it is evident that more extensive research is needed in this field to establish a truly environmentally friendly approach that can be universally adopted. The authors highlight a few key insights for future research:
(a) Further exploration of electrolytes to better understand their role in the synthesis process and reduce the concentration of organic solvents.
(b) Examination of alternative naturally available bases/acids for greener synthesis routes.
(c) Optimization of methods to easily obtain desired nanoparticle sizes by adjusting physical conditions such as temperature, pH, or pressure.
(d) Extensive research on utilizing agricultural waste for energetically favorable alternative routes.
By addressing these insights and pursuing further research, the development of environmentally sustainable synthesis methods for SiNPs can be advanced, contributing to their widespread application in various industries.
Conflicts of interest
The authors claim no conflict of interest for this work.Acknowledgements
The authors would like to acknowledge Department of Chemistry, Cooch Behar College and Cooch Behar Panchanan Barma University and Cooch Behar College (The project reference number 15(ii)/71–2021) for providing the necessary support for this article.References
- W. J. Stark, P. R. Stoessel, W. Wohlleben and A. Hafner, Chem. Soc. Rev., 2015, 44, 5793–5805 RSC.
- C. Binns, Introduction to Nanoscience and Nanotechnology, John Wiley & Sons., USA, 2nd edn, 2021 Search PubMed.
- K. S. Rao, K. El-Hami, T. Kodaki, K. Matsushige and K. Makino, J. Colloid Interface Sci., 2005, 289, 125–131 CrossRef CAS PubMed.
- I. A. Rahman and V. Padavettan, J. Nanomater., 2012, 2012, 1–15 CrossRef.
- C. J. Brinker and G. W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, Academic Press, Cambridge, USA, 2013 Search PubMed.
- M. Keshavarz and N. Ahmad, J. Nanopart., 2013, 2013, 1–4 CrossRef.
- V. Gubala, G. Giovannini, F. Kunc, M. P. Monopoli and C. J. Moore, Cancer Nanotechnol., 2020, 11, 1 CrossRef CAS.
- F. Akhter, A. A. Rao, M. N. Abbasi, S. A. Wahocho, M. A. Mallah, H. Anees-ur-Rehman and Z. A. Chandio, Silicon, 2022, 14, 8295–8310 CrossRef CAS.
- Nanoscale Materials in Chemistry, ed. K. J. Klabunde and R. Richards, Wiley, Hoboken, N.J, 2nd edn, 2009 Search PubMed.
- E. Reverchon and R. Adami, J. Supercrit. Fluids, 2006, 37, 1–22 CrossRef CAS.
- N. Abid, A. M. Khan, S. Shujait, K. Chaudhary, M. Ikram, M. Imran, J. Haider, M. Khan, Q. Khan and M. Maqbool, Adv. Colloid Interface Sci., 2022, 300, 102597 CrossRef CAS PubMed.
- P. Yugandhar, R. Haribabu and N. Savithramma, 3 Biotech, 2015, 5, 1031–1039 CrossRef PubMed.
- S. A. Jadhav, H. B. Garud, S. S. Thoravat, V. S. Patil, P. S. Shinde, S. H. Burungale and P. S. Patil, Biointerface Res. Appl. Chem., 2020, 11, 8599–8607 Search PubMed.
- C. J. Choi, X. L. Dong and B. K. Kim, Scr. Mater., 2001, 44, 2225–2229 CrossRef CAS.
- R. Yue, D. Meng, Y. Ni, Y. Jia, G. Liu, J. Yang, H. Liu, X. Wu and Y. Chen, Powder Technol., 2013, 235, 909–913 CrossRef CAS.
- N. O. San, C. Kurşungöz, Y. Tümtaş, Ö. Yaşa, B. Ortaç and T. Tekinay, Particuology, 2014, 17, 29–35 CrossRef CAS.
- C.-H. Lin, J.-H. Chang, Y.-Q. Yeh, S.-H. Wu, Y.-H. Liu and C.-Y. Mou, Nanoscale, 2015, 7, 9614–9626 RSC.
- S. D. Karande, S. A. Jadhav, H. B. Garud, V. A. Kalantre, S. H. Burungale and P. S. Patil, Nanotechnol. Environ. Eng., 2021, 6, 29 CrossRef CAS.
- R. J. P. Corriu and D. Leclercq, Angew Chem. Int. Ed. Engl., 1996, 35, 1420–1436 CrossRef.
- K. Nozawa, H. Gailhanou, L. Raison, P. Panizza, H. Ushiki, E. Sellier, J. P. Delville and M. H. Delville, Langmuir, 2005, 21, 1516–1523 CrossRef CAS PubMed.
- Y. Huang and J. E. Pemberton, Colloids Surf., A, 2010, 360, 175–183 CrossRef CAS.
- E. Effati and B. Pourabbas, Powder Technol., 2012, 219, 276–283 CrossRef CAS.
- T. Gholami, M. Salavati-Niasari, M. Bazarganipour and E. Noori, Superlattices Microstruct., 2013, 61, 33–41 CrossRef CAS.
- M. Shekarriz, R. Khadivi, S. Taghipoor and M. Eslamian, Can. J. Chem. Eng., 2014, 92, 828–834 CrossRef CAS.
- W. Huang, X. Li, H. Wang, X. Xu, H. Liu and G. Wang, Anal. Lett., 2015, 48, 1524–1535 CrossRef CAS.
- R. S. Dubey, Y. B. R. D. Rajesh and M. A. More, Mater. Today: Proc., 2015, 2, 3575–3579 CAS.
- E. G. Barrera, P. R. Livotto and J. H. Z. D. Santos, Powder Technol., 2016, 301, 486–492 CrossRef CAS.
- S. Chandra, G. Beaune, N. Shirahata and F. M. Winnik, J. Mater. Chem. B, 2017, 5, 1363–1370 RSC.
- H. Gu, Q. Zhang, J. Gu, N. Li and J. Xiong, J. Sol-Gel Sci. Technol., 2018, 87, 478–485 CrossRef CAS.
- M. Meier, J. Ungerer, M. Klinge and H. Nirschl, Colloids Surf., A, 2018, 538, 559–564 CrossRef CAS.
- X. Jiang, X. Tang, L. Tang, B. Zhang and H. Mao, Ceram. Int., 2019, 45, 7673–7680 CrossRef CAS.
- F. W. M. Ling, H. A. Abdulbari and S.-Y. Chin, Mater. Today: Proc., 2021, 42, 1–7 CAS.
- C. K. Dixit, S. Bhakta, A. Kumar, S. L. Suib and J. F. Rusling, Nanoscale, 2016, 8, 19662–19667 RSC.
- A. Saha, K. Narula, P. Mishra, G. Biswas and S. Bhakta, Nanoscale Adv., 2023, 5, 1386–1396 RSC.
- I. Danielsson and B. Lindman, Colloids Surf., 1981, 3, 391–392 CrossRef CAS.
- J. H. Schulman, W. Stoeckenius and L. M. Prince, J. Phys. Chem., 1959, 63, 1677–1680 CrossRef CAS.
- G. Platz, Ber. Bunsenges. Phys. Chem., 1991, 95, 103–104 CrossRef.
- S. Bonacchi, D. Genovese, R. Juris, M. Montalti, L. Prodi, E. Rampazzo and N. Zaccheroni, Angew. Chem., Int. Ed., 2011, 50, 4056–4066 CrossRef CAS PubMed.
- K. S. Finnie, J. R. Bartlett, C. J. A. Barbé and L. Kong, Langmuir, 2007, 23, 3017–3024 CrossRef CAS PubMed.
- F. J. Arriagada and K. Osseo-Asare, J. Colloid Interface Sci., 1999, 211, 210–220 CrossRef CAS PubMed.
- W. Jansomboon, K. Boonmaloet, S. Sukaros and P. Prapainainar, Key Eng. Mater., 2016, 718, 77–80 Search PubMed.
- P. Lu and Y.-L. Hsieh, Powder Technol., 2012, 225, 149–155 CrossRef CAS.
- S. Sankar, S. K. Sharma, N. Kaur, B. Lee, D. Y. Kim, S. Lee and H. Jung, Ceram. Int., 2016, 42, 4875–4885 CrossRef CAS.
- V. Vaibhav, U. Vijayalakshmi and S. M. Roopan, Spectrochim. Acta, Part A, 2015, 139, 515–520 CrossRef CAS PubMed.
- J. Athinarayanan, S. A. A. H. Jaafari, V. S. Periasamy, T. N. A. Almanaa and A. A. Alshatwi, Silicon, 2020, 12, 2829–2836 CrossRef CAS.
- M. Balamurugan and S. Saravanan, Powder Technol., 2012, 224, 345–350 CrossRef CAS.
- N. K. Sethy, Z. Arif, P. K. Mishra and P. Kumar, J. Polym. Eng., 2019, 39, 679–687 CrossRef CAS.
- K. Durairaj, P. Senthilkumar, P. Velmurugan, K. Dhamodaran, K. Kadirvelu and S. Kumaran, J. Sol-Gel Sci. Technol., 2019, 90, 653–664 CrossRef CAS.
- N. Jabeen, Q. Maqbool, S. Sajjad, A. Minhas, U. Younas, S. Anwaar, M. Nazar, R. Kausar and S. Z. Hussain, IET Nanobiotechnol., 2017, 11, 557–561 CrossRef PubMed.
- E. Vetchinkina, E. Loshchinina, M. Kupryashina, A. Burov and V. Nikitina, Ind. Eng. Chem. Res., 2019, 58, 17207–17218 CrossRef CAS.
- S. Singh, U. M. Bhatta, P. V. Satyam, A. Dhawan, M. Sastry and B. L. V. Prasad, J. Mater. Chem., 2008, 18, 2601 RSC.
- F. Liu, W. Yang, Y. Wang, X. Xue and H. Yang, Chem. Lett., 2014, 43, 720–722 CrossRef CAS.
- A. Pieła, E. Żymańczyk-Duda, M. Brzezińska-Rodak, M. Duda, J. Grzesiak, A. Saeid, M. Mironiuk and M. Klimek-Ochab, Bioorg. Chem., 2020, 99, 103773 CrossRef PubMed.
- V. Bansal, A. Ahmad and M. Sastry, J. Am. Chem. Soc., 2006, 128, 14059–14066 CrossRef CAS PubMed.
- M. Estevez, S. Vargas, V. M. Castaño and R. Rodriguez, J. Non-Cryst. Solids, 2009, 355, 844–850 CrossRef CAS.
- T. Kaur, G. P. Singh, G. Kaur, S. Kaur and P. K. Gill, Mater. Res. Express, 2016, 3, 085026 CrossRef.
- M. A. Albalawi, A. M. Abdelaziz, M. S. Attia, E. Saied, H. H. Elganzory and A. H. Hashem, Antioxidants, 2022, 11, 2323 CrossRef CAS PubMed.
- H. E. Kaabo, E. Saied, S. E. D. Hassan, H. M. Mahdy and M. H. Sultan, Biomass Convers. Biorefin., 2024, 1–18 Search PubMed.
- D. Sachan, A. Ramesh and G. Das, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100467 CAS.
- X. Huang, X. Teng, D. Chen, F. Tang and J. He, Biomaterials, 2010, 31, 438–448 CrossRef CAS PubMed.
- Q. Cai, W.-Y. Lin, F.-S. Xiao, W.-Q. Pang, X.-H. Chen and B.-S. Zou, Microporous Mesoporous Mater., 1999, 32, 1–15 CrossRef CAS.
- D. R. Radu, C.-Y. Lai, J. Huang, X. Shu and V. S.-Y. Lin, Chem. Commun., 2005, 1264 RSC.
- T. Yokoi, H. Yoshitake and T. Tatsumi, J. Mater. Chem., 2004, 14, 951 RSC.
- R. I. Nooney, D. Thirunavukkarasu, Y. Chen, R. Josephs and A. E. Ostafin, Chem. Mater., 2002, 14, 4721–4728 CrossRef CAS.
- S. H. Wu, C.-Y. Mou and H.-P. Lin, Chem. Soc. Rev., 2013, 42, 3862 RSC.
- K. Möller and T. Bein, Chem. Mater., 2017, 29, 371–388 CrossRef.
- Q. Cai, Z.-S. Luo, W.-Q. Pang, Y.-W. Fan, X.-H. Chen and F.-Z. Cui, Chem. Mater., 2001, 13, 258–263 CrossRef CAS.
- F. Olivieri, R. Castaldo, M. Cocca, G. Gentile and M. Lavorgna, Nanoscale, 2021, 13, 9091–9111 RSC.
- V. Boonamnuayvitaya, C. Tayamanon, S. Sae-ung and W. Tanthapanichakoon, Chem. Eng. Sci., 2006, 61, 1686–1691 CrossRef CAS.
- Surfactants: a practical handbook, ed. K. R. Lange, Hanser Gardner Publications, Munich: Cincinnati, 1999 Search PubMed.
- G. Cheng and C. Liu, Mater. Chem. Phys., 2003, 77, 359–364 CrossRef CAS.
- J. Hu, M. Chen, X. Fang and L. Wu, Chem. Soc. Rev., 2011, 40, 5472–5491 RSC.
- A. Khanal, Y. Inoue, M. Yada and K. Nakashima, J. Am. Chem. Soc., 2007, 129, 1534–1535 CrossRef CAS PubMed.
- Y. Li and J. Shi, Adv. Mater., 2014, 26, 3176–3205 CrossRef CAS PubMed.
- S. H. Wu and H. P. Lin, Chem. Soc. Rev., 2013, 42, 3862–3875 RSC.
- F. Zeng, Z. Z. Pan, M. Zhang, Y. Z. Huang, Y. N. Cui and Q. Xu, Prog. Chem., 2015, 27, 1356–1373 CAS.
- W. A. El-Said, A. S. Moharram, E. M. Hussein and A. M. El-Khawaga, Mater. Chem. Phys., 2018, 211, 123–136 CrossRef CAS.
- Y. Nakashima, C. Takaib, H. Razavi-Khosroshahia and M. Fuji, Colloids Surf., A, 2020, 593, 124582 CrossRef CAS.
- Y. Bao, C. Shi, T. Wang, X. Li and J. Ma, Microporous Mesoporous Mater., 2016, 227, 121–136 CrossRef CAS.
- C. Y. Tao, H. W. Yan, X. D. Yuan, C. Z. Yao, Q. Yin, J. Y. Zhu, W. Ni, L. H. Yan and L. Zhang, Colloids Surf., A, 2016, 501, 17–23 CrossRef CAS.
- M. B. M. Yap Ang, S. H. Huang, S. J. Tsai, M. R. De Guzman, K. R. Lee and J. Y. Lai, J. Membr. Sci., 2020, 611, 118333 CrossRef CAS.
- S. A. Jadhav, I. Miletto, V. Brunella, G. Berlier and D. Scalarone, Polym. Adv. Technol., 2015, 26, 1070–1075 CrossRef CAS.
- Y. R. Han, J. W. Park, H. Kim, H. Ji, S. H. Lim and C. H. Jun, Chem. Commun., 2015, 51, 17084–17087 RSC.
- R. Banga, J. Yarwood, A. M. Morgan, B. Evans and J. Kells, Langmuir, 1995, 11, 4393–4399 CrossRef CAS.
- S. Zanarini, E. Rampazzo, L. D. Ciana, M. Marcaccio, E. Marzocchi, M. Montalti, F. Paolucci and L. Prodi, J. Am. Chem. Soc., 2009, 131, 2260–2267 CrossRef CAS PubMed.
- G. T. Hermanson, Bioconjugate Techniques, Academic Press, San Diego, CA, 1996 Search PubMed.
- N. Insin, J. B. Tracy, H. Lee, J. P. Zimmer, R. M. Westervelt and M. G. Bawendi, ACS Nano, 2008, 2, 197–202 CrossRef CAS PubMed.
- J. S. Kim, W. J. Rieter, K. M. L. Taylor, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2007, 129, 8962–8963 CrossRef CAS PubMed.
- S. Santra, R. P. Bagwe, D. Dutta, J. T. Stanley, G. A. Walter, W. Tan, B. M. Moudgil and R. A. Mericle, Adv. Mater., 2005, 17, 2165–2169 CrossRef CAS.
- K. Ueno, K. Hata, T. Katakabe, M. Kondoh and M. Watanabe, J. Phys. Chem. B, 2008, 112, 9013–9019 CrossRef CAS PubMed.
- M. Zhu, M. Lerum and W. Chen, Langmuir, 2012, 28, 416–423 CrossRef CAS PubMed.
- A. J. Sivaram, A. Wardiana, C. B. Howard, S. M. Mahler and K. J. Thurecht, Adv. Healthcare Mater., 2018, 7, 1700607 CrossRef PubMed.
- A. Schulz, R. Woolley, T. Tabarin and C. McDonagh, Analyst, 2011, 136, 1722–1727 RSC.
- D. R. Hristov, L. Rocks, P. M. Kelly, S. S. Thomas, A. S. Pitek, P. Verderio, E. Mahon and K. A. Dawson, Sci. Rep., 2015, 5, 17040 CrossRef CAS PubMed.
- R. P. Bagwe, C. Yang, L. R. Hilliard and W. Tan, Langmuir, 2004, 20, 8336–8342 CrossRef CAS PubMed.
- R. P. Bagwe, L. R. Hilliard and W. Tan, Langmuir, 2006, 22, 4357–4362 CrossRef CAS PubMed.
- S. M. Kelleher, R. I. Nooney, S. P. Flynn, E. Clancy, M. Burke, S. Daly, T. J. Smith, S. Daniels and C. McDonagh, Nanotechnology, 2015, 26, 365703 CrossRef CAS PubMed.
- S. Shahabi, L. Treccani, R. Dringen and K. Rezwan, ACS Appl. Mater. Interfaces, 2015, 7, 13821–13833 CrossRef CAS PubMed.
- H. S. Jung, D. S. Moon and J. K. Lee, J. Nanomater., 2012, 48 Search PubMed.
- Y. Chen and Y. Zhang, Anal. Bioanal. Chem., 2011, 399, 2503–2509 CrossRef CAS PubMed.
- S. Noel, B. Liberelle, L. Robitaille and G. De Crescenzo, Bioconjugate Chem., 2011, 22, 1690–1699 CrossRef CAS PubMed.
- H. T. Lu, Colloid J., 2013, 75, 311–318 CrossRef CAS.
- D. R. Hristov, L. Rocks, P. M. Kelly, S. S. Thomas, A. S. Pitek, P. Verderio, E. Mahon and K. A. Dawson, Sci. Rep., 2015, 5, 17040 CrossRef CAS PubMed.
- F. Kunc, V. Balhara, A. Brinkmann, Y. Sun, D. M. Leek and L. J. Johnston, Anal. Chem., 2018, 90, 13322–13330 CrossRef CAS PubMed.
- Y. Sun, F. Kunc, V. Balhara, B. Coleman, O. Kodra, M. Raza, M. Chen, A. Brinkmann, G. P. Lopinski and L. J. Johnston, Nanoscale Adv., 2019, 1, 1598–1607 RSC.
- T. Borase, M. Iacono, S. I. Ali, P. D. Thornton and A. Heise, Polym. Chem., 2012, 3, 1267–1275 RSC.
- A. Ali, S. Saeed, R. Hussain, G. Afzal, A. B. Siddique, G. Parveen, M. Hasan and G. Caprioli, ACS Omega, 2023, 8, 20900–20911 CrossRef CAS PubMed.
- E. Moncada, R. Quijada and J. Retuert, Nanotechnology, 2007, 18, 335606 CrossRef.
- H. El Rassy and A. C. Pierre, J. Non-Cryst. Solids, 2005, 351, 1603–1610 CrossRef CAS.
- V. Purcar, V. Rădiţoiu, C. Nichita, A. Bălan, A. Rădiţoiu, S. Căprărescu, F. M. Raduly, R. Manea, R. Şomoghi, C.-A. Nicolae, I. Raut and L. Jecu, Materials, 2021, 14, 2086 CrossRef CAS PubMed.
- Z. Cheng, A. Al Zaki, J. Z. Hui, V. R. Muzykantov and A. Tsourkas, Science, 2012, 338, 903–910 CrossRef CAS PubMed.
- V. Gubala, L. J. Johnston, Z. Liu, H. Krug, C. J. Moore, C. K. Ober, M. Schwenk and M. Vert, Pure Appl. Chem., 2018, 90, 1283–1324 CrossRef CAS.
- A. A. Vertegel, R. W. Siegel and J. S. Dordick, Langmuir, 2004, 20, 6800–6807 CrossRef CAS PubMed.
- W. Shang, J. H. Nuffer, V. A. Muñiz-Papandrea, W. Colón, R. W. Siegel and J. S. Dordick, Small, 2009, 50, 470–476 CrossRef PubMed.
- B. Korzeniowska, R. Nooney, D. Wencel and C. McDonagh, Nanotechnology, 2013, 24, 442002 CrossRef CAS PubMed.
- C. J. Moore, H. Monton, R. O'Kennedy, D. E. Williams, C. Nogues, C. Crean and V. Gubala, J. Mater. Chem. B, 2015, 3, 2043–2055 RSC.
- Y. H. Tan, M. Liu, B. Nolting, J. G. Go, J. Gervay-Hague and G. Y. Liu, ACS Nano, 2008, 2, 2374–2384 CrossRef CAS PubMed.
- F. Kunc, C. J. Moore, R. E. Sully, A. J. Hall and V. Gubala, Langmuir, 2019, 35, 4909–4917 CrossRef CAS PubMed.
- A. J. Sivaram, A. Wardiana, C. B. Howard, S. M. Mahler and K. J. Thurecht, Adv. Healthcare Mater., 2018, 7, 1700607 CrossRef PubMed.
- R. Nooney, C. O'Connell, S. Roy, K. Boland, G. Keegan, S. Kelleher, S. Daniels and C. McDonagh, Sens. Actuators, B, 2015, 221, 470–479 CrossRef CAS.
- A. Dhir and A. Datta, J. Phys. Chem. C, 2016, 120, 20125–20131 CrossRef CAS.
- C. J. Moore, G. Giovannini, F. Kunc, A. J. Hall and V. Gubala, J. Mater. Chem. B, 2017, 5, 5564–5572 RSC.
- N. Sardesai, S. Pan and J. Rusling, Chem. Commun., 2009, 33, 4968–4970 RSC.
- Q. Sun, G. Zhao and W. Dou, Anal. Methods, 2015, 7, 8647–8654 RSC.
- Q. Sun, G. Zhao and W. Dou, Sens. Actuators, B, 2016, 226, 69–75 CrossRef CAS.
- M. Al-Rawi, S. Diabaté and C. Weiss, Arch. Toxicol., 2011, 85, 813–826 CrossRef CAS PubMed.
- M. Manzano and M. Vallet-Regí, Adv. Funct. Mater., 2020, 30, 1902634 CrossRef CAS.
- S. Zhao, G. Siqueira, S. Drdova, D. Norris, C. Ubert, A. Bonnin, S. Galmarini, M. Ganobjak, Z. Pan, S. Brunner and G. Nyström, Nature, 2020, 584, 387–392 CrossRef CAS PubMed.
- Y. Liu, Y. Mi, J. Zhao and S. S. Feng, Int. J. Pharm., 2011, 421, 370–378 CrossRef CAS PubMed.
- R. Narayan, U. Y. Nayak, A. M. Raichur and S. Garg, Pharmaceutics, 2018, 10, 118 CrossRef CAS PubMed.
- Y. Zhang, J. Wang, X. Bai, T. Jiang, Q. Zhang and S. Wang, Mol. Pharmaceutics, 2012, 9, 505–513 CrossRef CAS PubMed.
- S. Mashayekhi, S. Rasoulpoor, S. Shabani, N. Esmaeilizadeh, H. Serati-Nouri, R. Sheervalilou and Y. Pilehvar-Soltanahmadi, Int. J. Pharm., 2020, 587, 119656 CrossRef CAS PubMed.
- N. S. Elbialy, S. F. Aboushoushah, B. F. Sofi and A. Noorwali, Microporous Mesoporous Mater., 2020, 291, 109540 CrossRef CAS.
- I. Roy, P. Kumar, R. Kumar, T. Y. Ohulchanskyy, K. T. Yong and P. N. Prasad, RSC Adv., 2014, 4, 53498–53504 RSC.
- B. Munoz, A. Ramila, J. Perez-Pariente, I. Diaz and M. Vallet-Regi, Chem. Mater., 2003, 15, 500–503 CrossRef CAS.
- J. Lu, M. Liong, J. I. Zink and F. Tamanoi, Small, 2007, 3, 1341–1346 CrossRef CAS PubMed.
- E.-H. Jang, S. P. Pack, I. Kim and S. Chung, Sci. Rep., 2020, 10, 5558 CrossRef CAS PubMed.
- S. Yang, S. Chen, J. Fan, T. Shang, D. Huang and G. Li, J. Colloid Interface Sci., 2019, 554, 565–571 CrossRef CAS PubMed.
- P. Qin, Y. Yang, X. Zhang, J. Niu, H. Yang, S. Tian, J. Zhu and M. Lu, Nanomaterials, 2018, 8, 4 CrossRef PubMed.
- N. I. Sabeela, T. M. Almutairi, H. A. Al-Lohedan, A. O. Ezzat and A. M. Atta, Nanomaterials, 2019, 9, 1721 CrossRef CAS PubMed.
- N. El-Gazzar, T. N. Almanaa, R. M. Reda, M. N. El Gaafary, A. A. Rashwan and F. Mahsoub, Saudi J. Biol. Sci., 2021, 28, 5119–5130 CrossRef CAS PubMed.
- F. Olivieri, R. Castaldo, M. Cocca, G. Gentile and M. Lavorgna, Nanoscale, 2021, 13, 9091–9111 RSC.
- E. J. Cueto-Díaz, A. Castro-Muñiz, F. Suárez-García, S. Gálvez-Martínez, M. C. Torquemada-Vico, M. P. Valles-González and E. Mateo-Martí, Nanomaterials, 2021, 11, 2893 CrossRef PubMed.
- A. Rastogi, D. K. Tripathi, S. Yadav, D. K. Chauhan, M. Živčák, M. Ghorbanpour, N. I. El-Sheery and M. Brestic, 3 Biotech, 2019, 9, 1–11 CrossRef PubMed.
- M. Younes, P. Aggett, F. Aguilar, R. Crebelli, B. Dusemund, M. Filipič, M. J. Frutos, P. Galtier, D. Gott and U. Gundert-Remy, EFSA J., 2018, 16, 05088 Search PubMed.
- J. He, Y. Shen, B. Li, X. Xiang, S. Li, X. Fang, H. Xiao, X. Zu and L. Qiao, Opt. Mater., 2021, 111, 110611 CrossRef CAS.
- L. Qiao and X. Bi, J. Cryst. Growth, 2008, 310, 5327–5330 CrossRef CAS.
- C. I. Covaliu, G. Paraschiv, O. Stoian and A. Vişan, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 572, 012074 CAS.
- G. Fytianos, A. Rahdar and G. Z. Kyzas, Nanomaterials, 2020, 10, 979 CrossRef CAS PubMed.
- Nano Silica Market Size, Share, Industry Forecast 2031, https://www.alliedmarketresearch.com/nano-silica-market, accessed on 20th December, 2023.
- M. Ayaz, A. Shahbaz, M. Zahid, H. Ajaz, F. Saeed, U. B. Khalid, S. Zahid and L. Liaqat, Environ. Eng. Manage. J., 2023, 22, 465 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |