 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Review of recent progress in the development of electrolytes for Cd/Pb-based quantum dot-sensitized solar cells: performance and stability
Bayisa Batu Kasaye
 ,
Megersa Wodajo Shura
* and
Solomon Tiruneh Dibaba
,
Megersa Wodajo Shura
* and
Solomon Tiruneh Dibaba
Department of Applied Physics, School of Natural and Applied Sciences, Adama Science and Technology University, Adama, Oromia, Ethiopia. E-mail: megersawodajo@gmail.com
First published on 20th May 2024
Abstract
Quantum dot-sensitized solar cells (QDSSCs) represent an exciting advancement in third-generation photovoltaic solar cells owing to their ability to generate multiple electron–hole pairs per photon, high stability under light and moisture exposure, and flexibility in size and composition tuning. Although these cells have achieved power conversion efficiencies exceeding 15%, there remains a challenge in enhancing both their efficiency and stability for practical large-scale applications. Therefore, in this review, we aimed to investigate recent progress in improving the long-term stability, analyzing the impact of advanced quantum dot properties on charge-transport optimization, and assessing the role of interface engineering in reducing recombination losses to maximize QDSSC performance and stability. Additionally, this review delves into key elements such as the electrolyte composition, ionic conductivity, and compatibility with counter electrodes and photoanodes to understand their influence on power conversion efficiencies and stability. Finally, potential directions for advancing QDSC development in future are discussed to provide insights into the obstacles and opportunities for achieving high-efficiency QDSSCs.
1. Introduction
Fossil fuels are non-renewable sources of energy that causes toxic emissions, global warming, energy crises, and environmental pollution. Therefore, fossil fuels must be replaced with renewable energy sources such as solar cells, wind energy and hydroelectric power.1,2 Among these, solar cells use sunlight radiation to generate electricity. Based on both the material used and level of economic maturity, solar cells are often divided into three generations, namely, silicon-based materials (first generation), thin film solar cells (second generation), and third-generation solar cells (low cost and high efficiency). Compared to the first- and second-generation solar cells, the third-generation solar cells are characterized by low cost, high efficiency, environmentally friendly, transparent, and plastic substrates.3 Among the quantum dot-based solar cells, quantum dot-sensitized solar cells (QDSSCs) harvest light using nanostructured quantum dots.4 Quantum dots are unique in their optical, electronic, and chemical properties such as tuneable band gap, multiple exciton generation, and high absorption coefficients.5A QDSSC works by absorbing photons using quantum dots as absorbers. The QDs absorb photons, then electrons are excited from the ground state (VB) to higher energy (CB) states when the photon energy exceeds the bandgap of the QD. Electron–hole pairs are formed as a result of Coulomb attraction between opposite charges. Because of the thermal energy exceeds the binding energy, these electron–hole pairs dissociate. Then electrons are injected into the conduction band of the wide band gap of the semiconductor, and diffuse toward the counter electrode through an external circuit. As the holes moves toward the electrolyte, the oxidation species diffuse toward the counter electrode and are reduced by receiving electrons from the external circuit.6 To increase electron injection into a wide semiconductor, the conduction band (CB) of the wide semiconductor must be lower than that of a quantum dot.7 A typical QDSSC device structure and its working principles are shown in Fig. 1a, and an energy level diagram of a quantum dot sensitized solar cell is depicted in Fig. 1b.
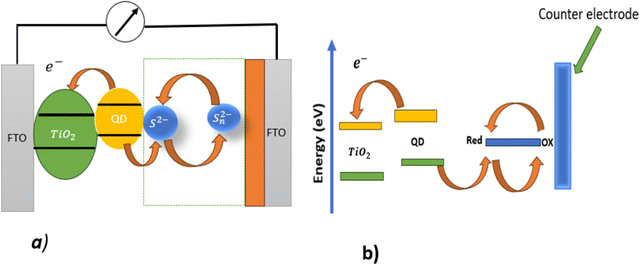 | ||
| Fig. 1 (a) Diagram of a quantum dot-sensitized solar cell; (b) energy level diagram for a quantum dot-sensitized solar cell. | ||
For a QDSSC to be highly efficient, the QD must have a high conduction band edge, wide absorption range, and large QD number (loading amount onto a wide band gap). QDSSCs consist of a working electrode (e.g. TiO2 or Zano) counter electrode (e.g. rGO or CuS), and electrolyte (e.g. polysulfide).
A counter electrode receives electrons from an external circuit and promotes them to the electrolyte to catalyze the reduction reaction of the oxidized electrolyte at the interface between the counter electrode and electrolyte. For QDSSCs, high conductivity, high catalytic activity, and excellent corrosion resistance are crucial. However, QDSSCs based on platinum electrodes exhibit low power conversion because the polysulfide electrolyte reacts with platinum, in contrast to other materials such as CoS, CuS, and NiS,8–11 or combinations of carbon compounds with other compounds, such as graphene hydrogel/CuS or graphene/PbS.12
In recent years, the use of carbon materials and composites has increased. As a result of their excellent catalytic activity towards polysulfide redox couples, the use of such materials has enabled QDSSC to become more efficient.
Electrolytes are also crucial for QDSSCs. The electrolytes in QDSSCs are responsible for regenerating oxidized quantum dots after the electrons are injected into the semiconductor, transporting the positive charge to the counter electrode, and transferring the charge efficiently between the anode and counter electrolyte. QDSSCs require electrolytes with high solubility, high ion mobility, and fast electron-transfer kinetics.
QDSSCs have been investigated using several electrolytes, including liquid electrolytes, which exhibit higher performance but lower stability. In contrast, quasi-solid-state and solid-state electrolytes have higher stability but lower power conversion than liquids. This review paper focuses on strategies to improve the stability and performance of quantum dot-sensitized solar cells as a result of electrolyte improvements.
2. Electrolytes
High corrosive resistance, good redox potential for regeneration of the oxidized quantum dots, and high ionic conductivity for hole transfer10 are crucial requirements for an electrolyte in a solar cell. The electrolyte is located between the cathode and anode to exchange charge carriers or separate the positive and negative electrodes to maintain the long-term stability of QDSSCs.The use of highly wettable solvents, with low surface tensions and low dielectric constants make nanostructured films highly permeable. Ethanol, methanol, alcohol, and water are solvents with high wettability, low surface tension, and low dielectric constant, respectively. Generally, solvents with low surface tension exhibit high wettability.13
In general, to improve the performance of QDSSCs, it is important that electrolytes are dissolved in solvents that are highly wettable, and highly permeable to promote redox versus diffusion and reduce the charge-recombination kinetics.14,15 However, low-surface-tension solvents have low ion-dissociation capabilities, and instead, the use of high-surface-tension and low-surface-tension co-solvents is advantageous.16 Based on the phase, there are three possible electrolytes: liquid, quasi-solid-state, and solid electrolytes.
An electrolyte in a QDSSC works based on eqn (1) and (2).
The hole moves from the oxidized QD to the electrolyte, then the electrolyte is oxidized:16
| S2− + 2h+ → S | (1) |
An oxidized electrolyte accepts electrons from the counter electrode and is reduced:
| Sx2− + 2e− → Sx−12− + S2− | (2) |
2.1 Liquid electrolytes
As conductive pathways for photovoltaic redox reactions, liquid electrolytes in QDSSCs contain oxidizing and reducing components in an aqueous or organic medium (solvent). Due to their non corrosive and non-photodegradation properties, polysulfide electrolytes (containing Na2S and S) can be corrosive and non-photodegradation properties, polysulfide electrolytes (containing Na2S and S) can be used.17 Despite their high redox potential, such electrolytes reduce the open circuit voltage and have a poor fill factor, which reduces their power conversion efficiency (PCE).18 The performance of the device can deteriorate due to oxygen permeation and volatility. Liquid electrolyte cations adsorb strongly on nanoporous photoanodes with a downshift of the TiO2 conduction band (CB) and a decrease in the open circuit voltage. To reduce charge recombination at the photoanode and at the electrolyte interface, as well as at the electrolyte and counter electrode interface, several additives are used.In polysulfide electrolytes, additives are adsorbed onto wide band gaps and form molecular complexes that block the surface trap states of photoanodes.15,19 This increases the short circuit current, open circuit voltage, and power conversion efficiency of QDSSCs. The use of polyethylene glycol (PEG) as a polymer additive to modify the polysulfide electrolyte in a ZCISe QDSSC reduced charge recombination and enabled achieving a PCE of 10.20%.15 PEG molecules containing hydroxyl and oxygen atoms coordinate strongly with TiO2. To prevent charge recombination, these molecules are used as a passivation layer. Reportedly, some additives, like PEG,20 TEOS,19 and PVP,21 in the polysulfide electrolyte solution do not change the CB edge or the electron density of TiO2, as depicted in Fig. 2a. These additives do not change the energy level of TiO2, rather they act as a coating or layer to improve its stability by reducing electron recombination at the photoanode/electrolyte interface. The effect can be strengthened by increasing the concentration of the additive, as shown in Fig. 2b. It can be seen that as the Mw of the PEG additives in the electrolyte increases, the diameter of EIS semicircles increases systematically (Fig. 2c).
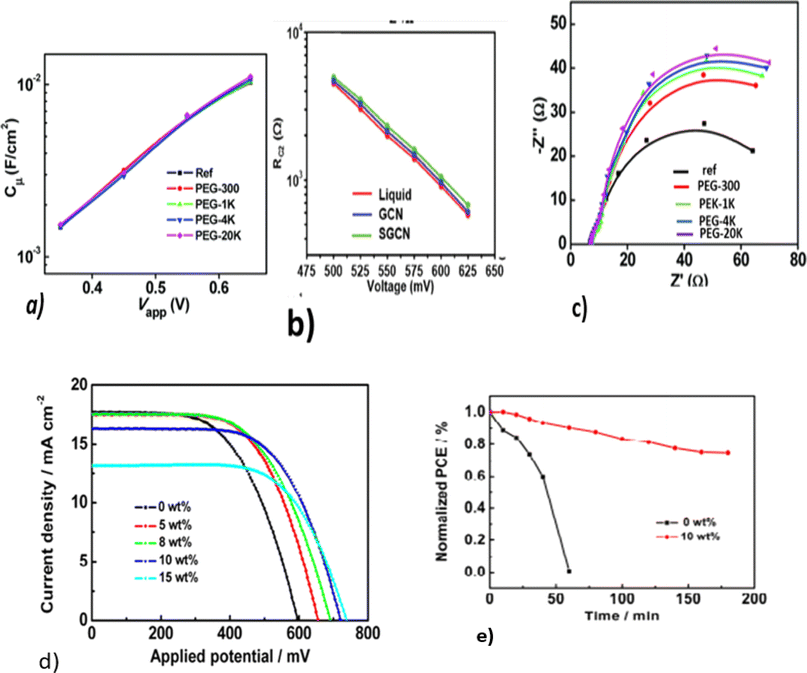 | ||
| Fig. 2 EIS characterizations of ZCISe QDSCs with electrolytes containing different PEG additives. (a) Chemical capacitance. Reprinted from ref. 15 with permission from the Royal Society of Chemistry, copyright 2018; (b) recombination resistance. Reprinted from ref. 18 with permission from the Royal Society of Chemistry, copyright 2021; (c) Nyquist plots under a forward bias of −0.65 V; (d) J–V curves for CdS/CdSe co-sensitized QDSCs with different electrolyte contents. Reprinted from ref. 15 and 24; (e) stability tests of a QDSSC fabricated with a polysulfide electrolyte. Reprinted from ref. 24. | ||
Meng and colleagues modified polysulfide electrolytes with fumed SiO2 nanoparticles to reduce electron recombination and increase the electron lifetime in a CdSeTe QDSSC. Silica nanoparticles were dispersed using liquid polysulfide to achieve a PCE of 11.23%. Gels were formed when silica nanoparticles were dispersed in polysulfide and formed hydrogen bonds with water molecules silanol (Si–OH),22 which was hydrolyzed under high pH conditions, leading to a degradation of the silica nanoparticles and ultimately the degradation of the device.23 Additionally, the insertion of silica nanoparticles into a polysulfide electrolyte requires additional fabrication steps and increases the fabrication cost.
With NH2-rich silica nanoparticles (A-SiO2) as an additive, Pin Ma's group produced stable ionic-liquid gel-based electrolytes with an improved PCE of 7.11% for CdS/CdSe QDSSCs, and the stability of the device was improved through the use of additives,24 as shown in Fig. 2d and e, respectively. NH2-rich silica nanoparticles, also called amino-functionalized silica nanoparticles, have also been tested.25 These NH2 amino group, however, can affect the electrolyte additives, since it can accept (H+) from water and form NH4+ and OH−. Hydroxide ions can form strong bases that can degrade silica nanoparticles, which in turn will reduce device stability.
Beiraghdar's group modified a polysulfide electrolyte using different additives, such as amine, thiourea, and urea, in CdS/CdSe QDSSCs, and reported a best PCE of 3.42% using a thiourea additive. By adding thiourea to liquid polysulfide, electrons are transferred to TiO2 through the amine groups and sulfur atoms, resulting in a longer electron lifetime.26 However, at high temperatures and UV exposure, amines may undergo degradation, resulting in cell component degradation and reduced performance.23
According to Rasal, Akash S. et al., the addition of graphitic carbon nitride (GCN) and sulfur-rich graphitic carbon nitride (SGCN) into the polysulfide electrolyte enhanced the PCE of CIS QDs as sensitizers in QDSSCs with liquid, GCN, and SGCN contents of 6.16%, 6.73% and 7.13%, respectively. Additionally, the charge-recombination resistance between the TiO2/QDs/electrolyte interfaces was reduced by adding the additive,18 resulting in a longer electron lifetime.
Table 1 summarizes the results from studies on the solar cell parameters of QDSSCs based on liquid electrolytes.
| QD | Electrolyte | CE | Jsc | Voc | FF | η% | Ref. |
|---|---|---|---|---|---|---|---|
| a CB – carbon black, C/S – core/shell, Cu@CNR – Cu nanoparticle@carbon nanorod, NPCN – nitrogen-doped porous carbon nanoribbon. | |||||||
| CdSe | Na2S, S, KCl | NPCN | 7.51 | 0.71 | 0.50 | 2.7 | 27 |
| CdS | Na2S, S, NaOH | Cu2S/CP | 10.52 | 0.606 | 05.57 | 2.85 | 25 and 28 |
| CdSe | Na2S, S | Pt | 7.51 | 0.71 | 0.50 | 2.7 | 29 |
| CdSeTe | Na2S, S, KCl | GH/CuS | 20.69 | 0.786 | 0.66 | 10.71 | 12 |
| CdS/CdSe/ZnS | Na2S, 2S | g-C3N4/CuS | 0.63 | 19.35 | 30.17 | 3.65 | 30 |
| CdS | Na2S, S, KCl | CB/CuS | 0.43 | 6.36 | 47 | 1.3 | 31 |
| PbS/CdS–C/S | Na2S, S, KCl | Cu2S | 627.7 | 14.85 | 43.90 | 4.16 | 32 |
| CdSeTe- | Na2S, S, KCl | CuS | 638 | 13.90 | 0.51 | 4.60 | 33 |
| ZCISe | Na2S, S, KCl | Cu@CNR | 0.628 | 26.46 | 0.593 | 9.50 | 34 |
| CdSeTe/ZnS | Na2S, S | Cu2−xS | 20.78 | 0.702 | 0.636 | 9.28 | 35 |
| CdS | Na2S, S | PbS | 0.59 | 8.59 | 0.58 | 2.91 | 36 |
| CdS | Na2S, S, KCl | Cu2S-SiW12/MoS2 | 15.78 | 0.622 | 0.431 | 4.28 | 37 |
| CdSe | Na2S, S, KCl | Au | 502.6 | 11.66 | 0.49 | 2.9 | 38 |
| CdSxSe1−x | Na2S, S | Cu2S | 12.5 | 0.57 | 0.55 | 3.69 | 39 |
| CdSeTe | Na2S, S, KCl | CNT@rGO@MoCuSe | 0.633 | 20.54 | 0.636 | 8.28 | 40 |
| CdSeTe | Na2S, S KCl | CuxSe | 0.654 | 20.82 | 60.13 | 8.72 | 41 |
| Zn–Cu–In–Se | Na2S, S | CNT/GH/CuS | 0.782 | 26.87 | 66.70 | 14.02 | 42 |
| CdSe/CdS | Na2S, S | CuS-MoS2 | 0.62 | 26.25 | 41.22 | 7.03 | 43 |
| CdSeTe | Na2S, S, NaOH | Cu2S | 10.05 | 0.64 | 53.67 | 3.46 | 44 |
| CdSe | Na2S, S, KCl | PbS/CuS | 14.52 | 0.607 | 47.43 | 4.18 | 45 |
| CdS/CdSe/ZnS | Na2S, S, KCl | NiCO2O4 | 22.49 | 0.574 | 0.43 | 5.55 | 46 |
Table 2 summarizes the parameters and performances of some electrolyte additives used in studies of QDSSCs.
| QD | Electrolyte | Additive | CE | Voc | Jsc | FF | η% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a NMC – N-doped mesoporous carbon, AC– activated carbon, SGCN – sulfur-rich graphitic carbon nitride, PVP – poly(vinyl pyrrolidone), 1,5-NPTD – 1,5-naphtalenediamine, ETH – ethylamine carbon. | ||||||||
| ZCISe | Na2S, S, KCl | Se | Cu2S | 0.601 | 26.34 | 0.624 | 9.88 | 47 |
| CdS | Na2S, S, KCl | TEOS | Pt/CuS | 0.543 | 19.33 | 0.523 | 5.5% | 48 |
| CdS/CdSe | Na2S, S | SC(NH2)2 | Cu2S | 0.50 | 18.98 | 0.36 | 3.42 | 48 |
| ZCISe | Na2S, S, KCl | Se | Cu2S | 0.601 | 26.34 | 0.624 | 9.88 | 47 |
| ZCISe | Na2S, S | PEG | Cu2S | 0.632 | 26.76 | 61.13 | 10. 29 | 15 |
| CdSeTe | Na2S, S KCl | SiO2 | Cu2S | 710 | 22.21 | 0.712 | 11.23 | 22 |
| CdSeTe | Na2S, S | PVP | Cu2−xS | 0.723 | 20.49 | 0.66 | 9.77 | 21 |
| CdSeTe | Na2S, S | TEOS | Cu2S | 0.77 | 26.36 | 0.61 | 12.34 | 19 |
| Cu–In–Se | Na2S thiourea | SGCN | Cu2S | 616 | 21.52 | 53.6 | 7.11 | 18 |
| ZCISSe | Na2S, S, KCl | PVP | NMC | 26.52 | 0.802 | 0.720 | 15.31 | 49 |
2.2 Quasi-solid-state electrolytes (gel electrolytes)
The main drawback of liquid electrolytes is their short-term stability. A liquid electrolyte evaporates easily, leaks through the electrode, has a high surface tension that reduces contact between the metal oxide and electrolyte, is volatile, flammable, difficult to handle, and is not easily filled with pore water.50Instead of liquid-based electrolyte, quasi-solid-state electrolytes (QSSEs) may be used that contain a composite liquid electrolyte (e.g. polysulfide) and a solid matrix (e.g. polymer), whose polymer chain network is swollen by polysulfide salt solution,51 which may reduce the device stability of QDSSCs. In these cases, the liquid facilitates ion transport52 by serving as a percolation pathway and the solid matrix enhances electrolytic capability by resisting the voltage between the anode and counter electrodes. To make it easier to compare, digital images for each electrolyte as shown in Fig. 3a.
 | ||
| Fig. 3 (a) Digital images of gel electrolytes with various compositions. Reprinted from ref. 57 with permission from ACS; (b) colour change of the electrolytes. Reprinted from ref. 50 with permission from the Royal Society of Chemistry, copyright 2016. | ||
An electrolyte with a higher mechanical strength can withstand anode–cathode voltages50,53 while maintaining ionic conductivity.54
Silica nanoparticles can be used as a filler to gel and solidify the polysulfide electrolyte. The ion-adsorption centres (polar functional groups) of the polymers make silica nanoparticles a good liquid electrolyte gelling agent, which can penetrate porous TiO2 films and display excellent conductivity. As a thin film, it is easy to fabricate, inexpensive, and mechanically flexible.55 It has a low vapor pressure, excellent contact and filling properties between the nanostructured electrode and the counter electrode, good ionic conductivity, and excellent thermal stability when compared with conventional polymer electrolytes.
Liquid electrolytes are absorbed by polymers, which can improve the long-term stability of QDSSCs by preventing evaporation.56 Fig. 3b shows there was a gradual change in colour of the liquid electrolyte over 30 days from brown to colourless, indicating that the gel polymer electrolyte was more stable than the liquid electrolyte based on these results.
A QSSE based on PGE can also act as a surface passivation to reduce charge recombination, consequently enhancing the power conversion efficiency of the QSSC.58 Fig. 4a depicts the working principles and recombination pathways in a QDSSC gel electrolyte: (1) from TiO2 to the QD; (2) from TiO2 to the electrolyte, which happens because the polysulfide species can diffuse into the TiO2 film and react with photogenerated electrons, resulting in electron recombination back to the electrolyte; and (3) from the QD to the electrolyte, which is due to the gel network obstructing ion movement. From Fig. 4b, it can be seen that the current density values of the co-additives (Se and PDA) are greater than that of the liquid, suggesting a faster charge-transfer kinetics at the electrolyte and electrode.
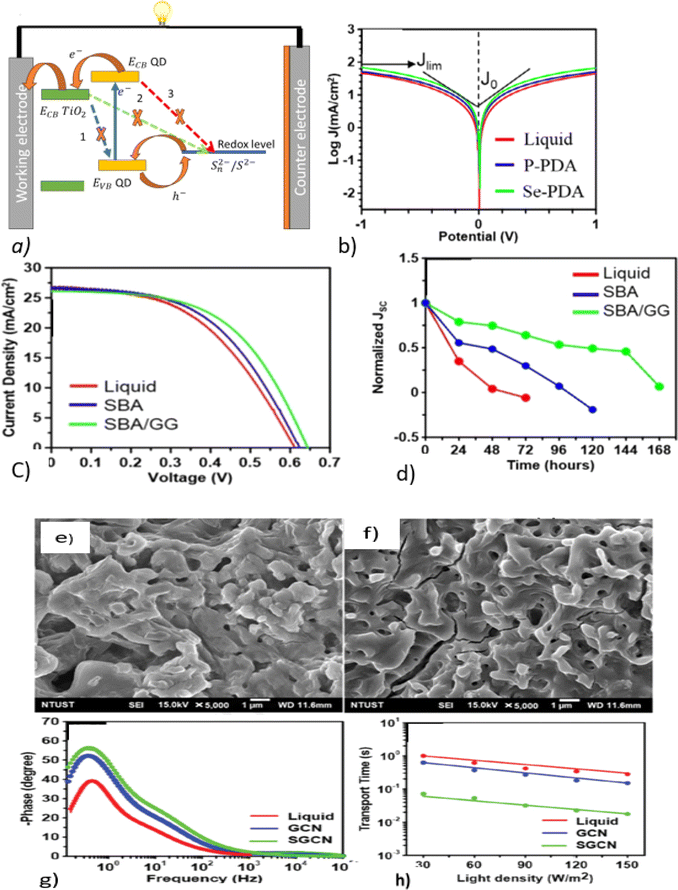 | ||
| Fig. 4 (a) Recombination pathways in XG/NP gel-electrolyte-fabricated QDSSCs; reproduced from ref. 58. (b) Tafel polarization curves; reprinted from ref. 61. (c) I–V curve, and (d) stability of a QDSSC based on a guar gum (GG) biopolymer; reprinted from ref. 60. SEM images of (e) XG gel and (f) XG/NP gel electrolytes. (g) Bode plot curves of Cu–In–S QDDSCs with different electrolytes; (h) electron-transport time obtained from IMPS and an ordered nanopores silica (SBA) gel electrolyte; adopted from ref. 58. | ||
There are several disadvantages of QSSEs to note, such as structural rigidity, which makes it difficult for ions to move freely, the complex ion-diffusion pathway due to the gel-like structure, which creates a complex network for ion diffusion, and the limited solvation capabilities, which limits ion mobility. As a result, gel electrolytes have lower ionic conductivities than liquid electrolytes. The addition of ionic liquids and fillers to gel electrolytes containing many ionic carriers can help overcome these problems.59
Owing to their improved charge recombination from a porous structure that allows ions to freely migrate, and hydroxyl groups that form good contact with TiO2, the stability and PCEs of guar gum (GG) and ordered nanoporous silica (SBA)-based gel electrolytes for QDSSCs were found to be higher than those of SBA and liquid polysulfide electrolytes,60 as shown in Fig. 4c and d.
Rasal et al. added TiO2 to an XG-polysulfide gel electrolyte, which showed improve ionic conductivity, which in turn, increased the free volume of the polymer matrix, and thus improved the availability of ions through the growth and diffusion of the polymer.58 SEM images of the XG gel and XG/NP gel electrolytes are shown in Fig. 4e and f, respectively, where it can be seen that XG/NP formed a microporous framework, allowing electrolyte permeation and fast redox coupling. From Fig. 4g and h, it can be seen that the electron-transport time and recombination lifetime decreased with the increase in light intensity.
Some reported solar cell parameters based on gel electrolytes for QDSSCs are summarized in Table 4.
Improving the temperature of the electrolyte
Polymer chains become more mobile as the electrolyte temperature increases because the electrolytes are more mobile, molecular distances are larger, secondary valence forces are smaller, and the ionic conduction resistance decreases.62 Fig. 5a and b show the temperature-dependent ionic conductivity of a gel electrolyte. The temperature dependence of the electrolytes can be calculated using the Arrhenius equation:51
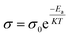 | (3) |
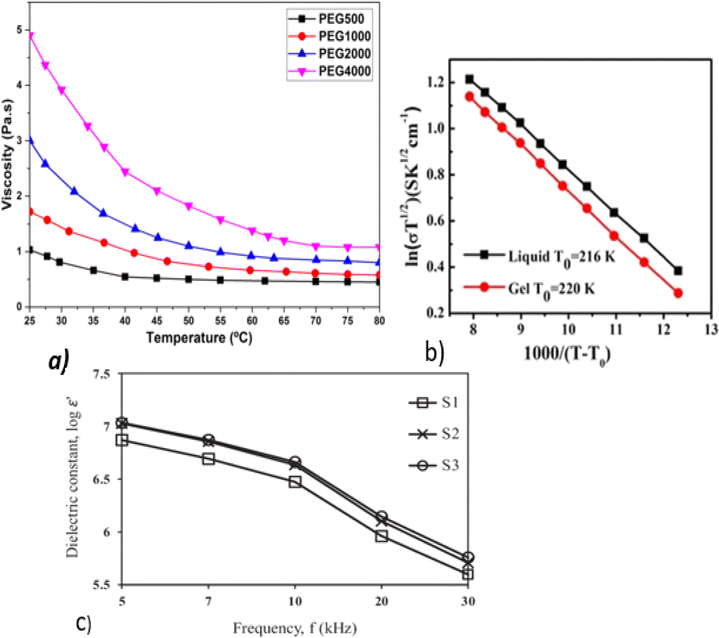 | ||
| Fig. 5 (a) Viscosity as a function of temperature; reproduced from ref. 67 with permission from Springer Nature. (b) Temperature dependence of the ionic conductivity of GE electrolytes; adopted from ref. 69. (c) Dielectric constant as a function of frequency.66 | ||
Research on polyelectrolytes has recently focused on those with polar functional groups, superior adsorption, and a water holding capacity, making them suitable electrolyte hosts for QDSSCs.70
Prakash and coworkers used graphene oxide (GO) to functionalize polyurethane (FPU). For the QDSSC gel electrolyte, they used co-sensitized CdS/CdSe as a sensitizer, resulting in a PCE of 1.71%. This may be due to the higher conductivity and reduced recombination. Polyurethanes represent a group of polyelectrolytes comprising hard di-isocyanate and chain extenders that provide strength, hardness, and high-temperature performance, as well as soft segments of polyether or polyester polyol, which provide flexibility, toughness, and resilience.71 The chain on a hard segment is used to increase the chain length and molecular weight, thereby reducing crystallization. The functionalization of polyurethane provides additional active pendant anion on the hard segment content.70 Using MPA-capped CdS as a sensitizer and FTO/rGO/TiO2 as a photoanode, Kumar and coworkers functionalized PU with graphene, a carboxylate, and a sulfonate segment as pendant anions (FTO/rGO/TiO2 photoanode and FTO/Pt counter electrode), exhibiting power conversion.70 As an electrolyte for a CdS quantum dot sensitizer, Ravi, and Maiti functionalized polyurethane using 3-mercapto propionic acid.72 Kumar and coworkers, in another work, used PU as a gel electrolyte in QDSSCs with TiO2/CdS photoanodes and achieved a PCE of 1.25%.73
Table 3 lists shows some polyelectrolytes gel electrolytes used with QDSSCs.
| CE | QD | Electrolyte | Voc | Isc | FF | PC | Ref. |
|---|---|---|---|---|---|---|---|
| a gPU – polyurethane, PANi – polyaniline, PUI-GO – functionalized polyurethane ionomers-graphene oxide, SPU-GO-CC – sulfonation of polyurethane graphene oxide conductive carbon, S(PU + CB)-PCL-EDA – sulfonic, polyurethane, carbon black, ethylene diamine, and PCL composite ionomer. | |||||||
| Pt | CdS/CdSe | PU | 4.66 | 0.74 | 0.44 | 1.51 | 72 |
| Pt | CdS | PANi | 2.2 | 0.60 | 0.78 | 1.25 | 73 |
| Pt | CdS/CdSe/ZnSe | SPU-GO-CC | 0.73 | 5.09 | 0.46 | 1.71 | 20 |
| Pt | CdS | PUI-GO | 0.594 | 6.44 | 0.43 | 1.63 | 70 |
| Pt | CdS | S(PU + CB)-PCL-EDA | 0.51 | 5.95 | 0.38 | 1.16 | 74 |
| CE | El. comp | Gelator | QD | Voc | Isc | FF | η% | Ref. |
|---|---|---|---|---|---|---|---|---|
| a 12-HDXA – 12-hydroxystearic acid, PVP – poly(vinyl pyrrolidone), PEG – polyethylene glycol, XG/NP – TiO2 incorporated into xanthan gum, SBA/GG – nanoporous silica (SBA) incorporated into guar gum (GG), PbS-CnF – lead quantum dot cellulose acetate nanofiber, A-SiO2 – NH2-rich silica nanoparticle, Se-PDA – Se-doped polydopamine, KGM – Konjac glucomannan, PAM–MBA – polyacrylamide-bis-acrylamide, PAAm-G – graphene-implanted polyacrylamide, PAAm/GO – graphene oxide-tailored polyacrylamide, CMS-Na – sodium carboxymethyl starch, PAAm-PAA – poly(acrylamide-co-acrylic acid). | ||||||||
| CoSe | Na2S, S | PAAm/GO | CdS/CdSe | 0.513 | 12.18 | 65.6 | 4.10 | 56 |
| Cu2−xS | Na2S, S | CMS-Na | CdSe | 0.615 | 15.63 | 65.8 | 6.32 | 69 |
| Pt | Na2S, S | PAAm-PAA | CdS | 14.77 | 0.37 | 0.44 | 2.24 | 66 |
| CZTS/Pt | Na2S, S | PEG | CdS | 0.59 | 11.47 | 60.5 | 4.09 | 50 |
| Pt | Na2S, S | PAAm-G | CdS | 0.590 | 9.63 | 39.4 | 2.24 | 75 |
| Cu2S | Na2S, S | PAM-MBA | CdS/CdSe | 534 | 12.4 | 0.601 | 4.0 | 11 |
| Pt | Na2S, S | Dextran | CdS/CdSe | 399 | 2.45 | 0.56 | 4.58 | 76 |
| Cu2S | Na2S, S | KGM | CdS/CdSe | 12.76 | 503 | 0.63 | 4.06 | 77 |
| Pt | Na2S, S | 12-HDXA | CdS/CdSe | 0.47 | 12.18 | 0.42 | 2.40 | 78 |
| CuS | Na2S, S | Se-PDA | Cu–In–S | 659 | 23.15 | 56.3 | 8.59 | 61 |
| Cu2S/RGO | Na2S, S | Agar | CdS | 0.578 | 14.13 | 0.39 | 3.09 | 2 |
| Cu2−xS | Na2S, S | PVP | CdSeTe | 0.723 | 20.49 | 0.66 | 9.77 | 21 |
| CuS | Na2S, S, KCl | XG/TiO2 | Cu–In–Se | 667 | 22.9 | 53.7 | 8.19 | 58 |
| Pt | Na2S, S | PbS-CnF | CdS | 446.1 | 9.95 | 34.75 | 1.51 | 79 |
| Pt | Na2S, S, KCl | A-SiO2 | CdS/CdSe | 625 | 18.95 | 0.6 | 7.11 | 24 |
| Cu@CNR | Na2S, S, KCl | CM-Na | ZCISe | 0.628 | 26.46 | 0.59 | 9.50 | 34 |
2.3 Solid-state electrolyte for QDSSCs
QSSE are limited by the leakage of organic solvents and liquid parts from the polymer host, which affects the stability, ionic conductivity, and electrode performance.80 Rather than QSSEs or liquid electrolytes, researchers have focused on solid electrolytes, which are simple to manufacture and can improve the stability of the devices. In contrast, these have poor permeability and low ion mobilization, which results in low ionic conductivity, and poor penetration into the nanostructure,81 which results in a low power conversion efficiency compared to liquids and gels.In solid-state electrolytes, the electrons cannot move quickly. Rapid electron mobilization leads to higher ionic conductivity, which facilitates the redox cycle and accelerates, the injection of electrons from quantum dots to TiO2. These factors affect the difference between the Fermi level of electrons in TiO2 and the redox potential of the electrolyte, or the open circuit voltage.82 These factors improve the ionic conductivity of the electrolyte, improving the generation of photocurrents and the overall PCE.
Ma. Chengfeng et al. developed a solid-state electrolyte from spiro-OMeTAD for QDSSCs using Pb/S/1,2-ethanedithiol QD as an absorber onto TiO2 nanorods, as shown in Fig. 6a, with a PCE of 4.51%.83 Spiro-OMeTAD is mostly used as a solid-state electrolyte in QDSSCs because of its high ionic conductivity, high stability, and compatibility with other device structures.84
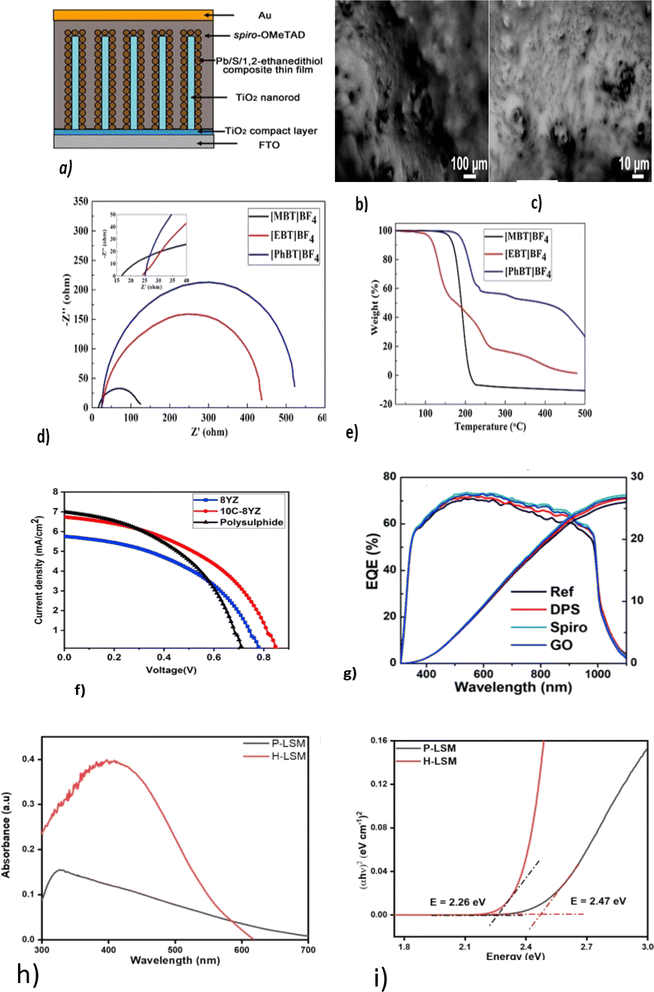 | ||
| Fig. 6 (a)Schematic diagram of Pb/S/1,2-ethanedithiol composite thin-film-sensitized TiO2 nanorod array solar cells; reproduced from ref. 83 with permission from Springer Nature copyright 2018. (b and c) SEM image of a solid electrolyte at low and high magnifications; reproduced with permission from the American Chemical Society, copyright 2019.88 (d) Nyquist plots of [DHexBIm][X] (X = Br, BF4 and SCN)-based electrolytes; reproduced from ref. 89, with permission from the Royal Society of Chemistry, copyright 2019. (e) TGA curves of OICs [MBT]BF4, [EBT]BF4 and [PhBT]BF4; reproduced from ref. 90. (f) J–V curves for the Pechini method and hydrothermal method. (g) EQ and integrated Jsc curves; reprinted from ref. 85. (h) UV-visible spectra and (i) Tauc plots of H-LSM and P-LSM to determine the optical bandgap; reproduced from ref. 97. | ||
According to Yu Lin and colleagues,85 the quantum yield (EQE) of 2,2,7,7-tetrakis[N,N-di(4-methoxyphenyl)-amino]-9,9-spiro-bifluorene-based HTMs is higher than that of graphene oxide (GO), and diphenyl sulfide (DPS), as shown in (Fig. 6g). Based on this, it can be concluded that spiro-based solid-state electrolytes (HTM) can improve the overall stability and Voc of devices more than DPS and GO. The higher the EQE, the better the long-term stability, possibly due to more photons being absorbed, which reduces the stress on materials and interfaces. As a result, device degradation based on HTMs may be improved.86
There are several strategies to deal with ionic conductivity issues in solid-state electrolytes, including the use of plastic crystals, such as succinonitrile polymers and ammonium salts. Succinitrile polymers are the plastic crystalline phases that can be used as additives in polymer electrolytes to improve their ionic conductivity of electrolytes.87 These plasticizers are used to improve electrolyte leakage and decrease device degradation through resisting corrosion of the device by the absorption of liquid polysulfide on the counter electrode, resulting in a good compatibility of the electrolyte with the sensitizer.
Kokal, Ramesh K. et al. developed a solid-state electrolyte based on succinonitrile/Na2S for a CdS QDSSC with a maximum PCE of 6.3%, Voc of 753 V, Jsc of 13 mA cm−2, and FF of 61.88 According to their work, solid-state electrolytes have increased thermal, electrical, and ionic conductivity. Raising the electrolyte temperature increases the electron density and electron mobility. In turn, this improves the performance of the device. Fig. 6(b and c) depict SEM images at low and high magnifications for succinonitrile and Na2S solid electrolytes. There has been research done to enable solid-state electrolytes to have good conductivity and a good contact between the electrolyte and the electrodes.89 By substituting benzothiophenium with S. Wang and coworkers developed a solid-state electrolyte that co-sensitized CdS/CdSe quantum dots. As a result of the saturated carbon chain, they were able to obtain the highest optical absorption and ionic conductivity from S-methyl benzothiophenium tetrafluoroborate ([MBT]BF4) with a voltage of 0.71 V and PCE of 5.49% (ref. 90) (Fig. 6d). From the TGA results, it was found that ([MBT]BF4) had good thermal stability at an operating temperature of 100 °C, as depicted in Fig. 6e.
It is possible to improve the adsorption and diffusion of electrolytes by using oxide nanoceramics, which are inexpensive, easy to process, and can form highly porous solid-state electrolytes.
Kusuma and colleagues developed solid-state electrolytes for QDSSCs using yttria-stabilized zirconia and ceria instead of polysulfides. Ceramic dopants can be used to create oxygen vacancies that result in band shifting, reduced electron recombination, and increased device stability. Using a ceramic oxide, the stability of one device lasted 60 days, whereas with polysulfide it lasted only 5 days.91
As reported by Akhil S. et al., it is possible to increase the stability of a device by using a perovskite material in a solid electrolyte. This might be because perovskite materials are chemically stable and can resist light, heat, and moisture, which could otherwise degrade and reduce the stability of devices. In addition they have high ion conductivity, therefore they are ideal solid electrolytes for QDSSCs.92 Moreover perovskite materials can be used in conjunction with other components of the devices, such as counter electrodes and photoanodes.93
The synthesis method can affect the optical properties of perovskite-based solid-state electrolytes. Akhil and coworkers used hydrothermal (HLSM) and Pechini (PLSM) methods to synthesize a solid-state electrolyte for a QDSSC with an improved PCE of 1.73%. An electrolyte containing ceramic particles generally improves ionic conductivity at high temperatures, allowing efficient ion transport to improve the power conversion efficiency, as shown in Fig. 6f. Using HLSM, a lower band gap and higher absorption maxima of 2.26 eV and 410 nm, respectively, were obtained, as shown in Fig. 6(h and i), indicating that the hydrothermal synthesis of perovskites enables better charge-transport properties at the electrolyte/electrode interface. Hydrothermal synthesis can produce smoother, more uniform surfaces, a slower growth rate, more controlled nucleation and growth of the crystal, and lower defects94 than the Pechini method. This results in a narrower band gap and high UV-visible absorption. In addition, surface and interface effects can contribute to the observed higher UV-visible peak in the absorbance spectrum of hydrothermally synthesized95 perovskites.
Quantum dot-sensitized solar cells based on solid-state electrolytes can perform better with an increased quantum dot loading amount,96 which can provide more number active sites, doping to optimize the interfacial band alignment, and insertion of a passivation layer to reduce the charge-recombination resistance and increase the PCE. In addition, several plastic crystalline and p-type semiconductors have received attention because of their low cost, high stability, and high-performance solid-state hole-transport properties. In Table 5, the parameters of some quantum dot-sensitized solar cells fabricated using solid-state electrolytes are summarized, showing that QDSSCs with solid-state electrolytes currently still have lower efficiency.
| Electrolyte | QD | CE | Voc | Jsc | FF | PCE | Ref |
|---|---|---|---|---|---|---|---|
| a PVP/Na2S–S – polyvinylpyrrolidone/polysulfide, spiro-OMeTAD – 9,9′-spirobifluoride, [MBT]BF4-S – methyl benzthiophenium tetrafluoroborate, NaCMC – sodium-carboxymethylcellulose, (P3HT) – poly(3-hexylthiophene). | |||||||
| PVP/Na2S–S | CdS | CoSe | 0.67 | 2.84 | 28.9 | 0.55 | 98 |
| [MBT]BF4 | CdS/CdSe | PbSe | 0.71 | 20.73 | 37.30- | 5.49 | 90 |
| NaCMC | CdS | Pt | 0.42 | 5.27 | 0.50 | 0.96 | 82 |
| succinonitrile/Na2S | CdS | CoSe | 0.670 | 3.65 | 52.7 | 1.29 | 99 |
| Spiro-OMeTAD | (Pb,Cd)S | Au | 0.49 | 1.79 | 0.36 | 0.32 | 100 |
| Spiro-OMeTAD | PbS | Au | 0.56 | 13.00 | 64.8 | 4.75 | 101 |
| Spiro-OMeTAD | CuInS2 | Au | 0.48 | 4.21 | 0.37 | 0.75 | 96 |
| Spiro-OMeTAD | CuInS2 | Ag | 0.64 | 5.34 | 0.41 | 1.41 | 102 |
| NaCMC | CdS/ZnS | Pt | 5.27 | 0.45 | 0.50 | 0.96 | 82 |
| Succinonitrile | CdS/ZnS | C | 753 | 13 | 61 | 6 | 88 |
| Spiro-OMeTAD | CIS | Au | 0.68 | 11.33 | 0.41 | 3.13 | 96 |
| Spiro-OMeTAD | Pb/S/1,2-ethanedithiol | Au | 0.56 | 11.84 | 0.68 | 4.51 | 83 |
| Spiro-OMeTAD | PbS | Au | 0.49 | 14.47 | 59.65 | 4.25 | 101 |
| Spiro-OMeTAD | Sb | Ag | 9.84 | 0.523 | 0.600 | 3.09 | 103 |
| P3HT | PbS[CuS] | Au | 0.6 | 20.7 | 65 | 8.07 | 104 |
| Spiro-OMeTAD | PbS | Au | 0.52 | 13.56 | 57.88 | 4.10 | 105 |
| P3HT | PbS/CuS | Au | 0.58 | 20.1 | 60.5 | 7.0 | 106 |
3. Future prospects and conclusion
In this review, we examined quantum dot-sensitized solar cells in terms of their current state and future prospects, especially regarding the electrolyte stability. We focused the reader's attention on the state-of-the-art efforts and advancements in improving the stability and performance of QDSSCs that have been occurred over the last decade, which have promoted their unique properties, like facile fabrication, low cost, tuneable bandgap, and generation of multiple excitons, and that have continually sought to increase their power conversion efficiency. Their potential is recognized as a part of all future energy technologies. A variety of electrolytic liquids, gels, and solid electrolytes have been used to enhance the PCE and stability. The PCE of QDSSCs has now reached over 15%. The electrolytes need to be modified for improving the QDSSC stability and PCE even more. Liquid electrolytes have a higher power conversion efficiency and are less stable than quasi-solid-state electrolytes and solid electrolytes. QDSSC needs to have a high power conversion and stability to be commercially successful. Solid-state QDSSCs are expected to be a promising direction for solving the stability problem of QDSSCs. Liquid junction devices are susceptible to electrolyte leakage and anodic corrosion. Several polymers are used to gel and solidify polysulfide electrolytes in QDSSCs. However, their power conversion is still lower than that of liquid electrolytes. Adding redox couples with low redox potentials is necessary to improve the efficiency of QDSSCs.Author contributions
Bayisa Batu Kasaye: conceptualization and writing – original draft. Megersa Wodajo Shura: conceptualization, supervision and editing. Solomon Tiruneh Dibaba: provisioning of resources.Conflicts of interest
There are no conflicts to declare.References
- I. Capellán-Pérez, M. Mediavilla, C. de Castro, Ó. Carpintero and L. J. Miguel, Energy, 2014, 77, 641–666 CrossRef.
- E. Raphael, D. H. Jara and M. A. Schiavon, RSC Adv., 2017, 7(11), 6492–6500 RSC.
- N. Shah, A. Shah, P. Leung, S. Khan, K. Sun, X. Zhu and Q. Liao, Processes, 2023, 11(6), 1852 CrossRef CAS.
- A. M. Bagher, M. M. A. Vahid and M. Mohsen, Am. J. Optom. Physiol. Opt., 2015, 3(5), 94–113 Search PubMed.
- W. A. Mohamed, H. Abd El-Gawad, S. Mekkey, H. Galal, H. Handal, H. Mousa and A. Labib, Nanotechnol. Rev., 2021, 10(1), 1926–1940 CrossRef CAS.
- K. E. Jasim, KnE Engineering, 2018, 171–187 CrossRef.
- D. Liu, J. Liu, J. Liu, S. Liu, C. Wang, Z. Ge, X. Hao, N. Du and H. Xiao, Phys. E, 2020, 115, 113669 CrossRef CAS.
- H. Yuan, J. Lu, X. Xu, D. Huang, W. Chen, Y. Shen and M. Wang, J. Electrochem. Soc., 2013, 160(9), H624 CrossRef CAS.
- M. P. A. Muthalif, C. D. Sunesh and Y. Choe, J. Colloid Interface Sci., 2019, 534, 291–300 CrossRef CAS PubMed.
- Z. Pan, H. Rao, I. Mora-Seró, J. Bisquert and X. Zhong, Chem. Soc. Rev., 2018, 47(20), 7659–7702 RSC.
- Z. Yu, Q. Zhang, D. Qin, Y. Luo, D. Li, Q. Shen, T. Toyoda and Q. Meng, Electrochem. Commun., 2010, 12(12), 1776–1779 CrossRef CAS.
- H. Zhang, C. Yang, Z. Du, D. Pan and X. Zhong, J. Mater. Chem. A, 2017, 5(4), 1614–1622 RSC.
- C. Rosiles-Perez, A. Cerdán-Pasarán, S. Sidhik, D. Esparza, T. López-Luke and E. De la Rosa, Sol. Energy, 2018, 174, 240–247 CrossRef CAS.
- J. Tian, T. Shen, X. Liu, C. Fei, L. Lv and G. Cao, Sci. Rep., 2016, 6(1), 23094 CrossRef CAS PubMed.
- Y. Sun, G. Jiang, M. Zhou, Z. Pan and X. Zhong, RSC Adv., 2018, 8(52), 29958–29966 RSC.
- Z. Yang, C.-Y. Chen, P. Roy and H.-T. Chang, Chem. Commun., 2011, 47(34), 9561–9571 RSC.
- I. Hwang and K. Yong, ChemElectroChem, 2015, 2(5), 634–653 CrossRef CAS.
- A. S. Rasal, K. Dehvari, G. Getachew, C. Korupalli, A. V. Ghule and J.-Y. Chang, Nanoscale, 2021, 13(11), 5730–5743 RSC.
- J. Yu, W. Wang, Z. Pan, J. Du, Z. Ren, W. Xue and X. Zhong, J. Mater. Chem. A, 2017, 5(27), 14124–14133 RSC.
- J. Du, X. Meng, K. Zhao, Y. Li and X. Zhong, J. Mater. Chem. A, 2015, 3(33), 17091–17097 RSC.
- G. Jiang, Z. Pan, Z. Ren, J. Du, C. Yang, W. Wang and X. Zhong, J. Mater. Chem. A, 2016, 4(29), 11416–11421 RSC.
- H. Wei, G. Wang, J. Shi, H. Wu, Y. Luo, D. Li and Q. Meng, J. Mater. Chem. A, 2016, 4(37), 14194–14203 RSC.
- E. Choi and S. Kim, J. Colloid Interface Sci., 2019, 533, 463–470 CrossRef CAS PubMed.
- P. Ma, Y. Fang, H. Cheng, Y. Wang, X. Zhou, S. Fang and Y. Lin, Electrochim. Acta, 2018, 262, 197–205 CrossRef CAS.
- L. Hsu-Tung, Colloid J., 2013, 75(3), 343 Search PubMed.
- N. Beiraghdar, H. Dehghani and M. Afrooz, Sol. Energy, 2021, 220, 384–393 CrossRef CAS.
- W. Dong, J. Liu and G. Wang, Int. J. Energy Res., 2020, 44(8), 6522–6530 CrossRef CAS.
- Q. Zhang, Y. Zhang, S. Huang, X. Huang, Y. Luo, Q. Meng and D. Li, Electrochem. Commun., 2010, 12(2), 327–330 CrossRef CAS.
- L. J. Diguna, Q. Shen, J. Kobayashi and T. Toyoda, Appl. Phys. Lett., 2007, 91(2), 023116 CrossRef.
- X. Chang, N. Xu, Z. Liu, S. Tian, D. Wen, W. Zheng and D. Wang, Process. Appl. Ceram., 2022, 16(2), 167–174 CrossRef CAS.
- S. Monika, M. Mahalakshmi, K. Veerathangam, M. Senthil Pandian and P. Ramasamy, Appl. Phys. A: Mater. Sci. Process., 2022, 128(3), 228 CrossRef CAS.
- M. Dissanayake, T. Jaseetharan, G. Senadeera and J. Kumari, J. Solid State Electrochem., 2020, 24, 283–292 CrossRef CAS.
- M. Marandi and S. H. Abadi, Sol. Energy, 2020, 209, 387–399 CrossRef CAS.
- Y. Wang, M. Yao, L. Zhao, W. Wang, W. Xue and Y. Li, J. Mater. Chem. A, 2019, 7(5), 2210–2218 RSC.
- Z. Ren, J. Wang, Z. Pan, K. Zhao, H. Zhang, Y. Li, Y. Zhao, I. Mora-Sero, J. Bisquert and X. Zhong, Chem. Mater., 2015, 27(24), 8398–8405 CrossRef CAS.
- Y. Chen, X. Zhang, Q. Tao, W. Fu, H. Yang, S. Su, Y. Mu, L. Zhou and M. Li, RSC Adv., 2015, 5(3), 1835–1840 RSC.
- L. Li, Z. Jin, R. Tao, F. Li, Y. Wang, X. Yang and L. Xu, J. Photochem. Photobiol., A, 2019, 377, 101–108 CrossRef CAS.
- Y.-L. Lee, B.-M. Huang and H.-T. Chien, Chem. Mater., 2008, 20(22), 6903–6905 CrossRef CAS.
- R. Zhou, L. Wan, H. Niu, L. Yang, X. Mao, Q. Zhang, S. Miao, J. Xu and G. Cao, Sol. Energy Mater. Sol. Cells, 2016, 155, 20–29 CrossRef CAS.
- C. V. M. Gopi, S. Singh, A. Eswar Reddy and H.-J. Kim, ACS Appl. Mater. Interfaces, 2018, 10(12), 10036–10042 CrossRef CAS PubMed.
- H. Zhang, C. Wang, W. Peng, C. Yang and X. Zhong, Nano Energy, 2016, 23, 60–69 CrossRef CAS.
- H. Zhang, X. Ji, N. Liu and Q. Zhao, Electrochim. Acta, 2019, 327, 134937 CrossRef CAS.
- J. Kusuma, S. Akash and R. G. Balakrishna, Sol. Energy, 2020, 201, 674–681 CrossRef CAS.
- E. Elibol, Sol. Energy, 2020, 206, 741–750 CrossRef CAS.
- X. Song, X. Liu, Y. Yan, J. Deng, Y. Wang, X. Dong, Z. Mo and C. Xia, Sol. Energy Mater. Sol. Cells, 2018, 176, 418–426 CrossRef CAS.
- Q. Zhang, T.-T. Zhang, F.-Y. Li and L. Xu, Tungsten, 2023, 5(2), 235–246 CrossRef.
- M. Zhou, G. Shen, Z. Pan and X. Zhong, J. Energy Chem., 2019, 38, 147–152 CrossRef.
- T. Archana, G. Subashini, A. N. Grace, M. Arivanandhan and R. Jayavel, J. Appl. Electrochem., 2021, 51, 1111–1122 CrossRef CAS.
- H. Song, Y. Lin, Z. Zhang, H. Rao, W. Wang, Y. Fang, Z. Pan and X. Zhong, J. Am. Chem. Soc., 2021, 143(12), 4790–4800 CrossRef CAS PubMed.
- W. Feng, L. Zhao, J. Du, Y. Li and X. Zhong, J. Mater. Chem. A, 2016, 4(38), 14849–14856 RSC.
- M. Mingsukang, M. Buraidah and M. Careem, Ionics, 2017, 23, 347–355 CrossRef CAS.
- N. K. Farhana, F. S. Omar, N. Mohamad Saidi, G. Z. Ling, S. Bashir, R. Subramaniam, R. Kasi, J. Iqbal, S. Wageh and H. Algarni, Polymers, 2022, 14(16), 3426 CrossRef CAS PubMed.
- C. W. Liew, R. Durairaj and S. Ramesh, PLoS One, 2014, 9(7), e102815 CrossRef PubMed.
- A. Mauger, M. Armand, C. Julien and K. Zaghib, J. Power Sources, 2017, 353, 333–342 CrossRef CAS.
- T. Winie, A. K. Arof and S. Thomas, Polymer Electrolytes: Characterization Techniques and Energy Applications, John Wiley & Sons, 2019 Search PubMed.
- X. Jin, C. Chang, Z. Chen and Q. Li, Electrochim. Acta, 2018, 283, 597–602 CrossRef CAS.
- H. Kim, I. Hwang and K. Yong, ACS Appl. Mater. Interfaces, 2014, 6(14), 11245–11253 CrossRef CAS PubMed.
- A. Rasal, Y.-H. Chen, K. Dehvari, G. Getachew, P.-J. Tseng, K. Waki, S. Bela and J.-Y. Chang, Mater. Today Energy, 2021, 19, 100615 CrossRef CAS.
- N. Pullanjiot and S. Swaminathan, Sol. Energy, 2019, 186, 37–45 CrossRef CAS.
- A. S. Rasal, M.-H. Wu, A. V. Ghule, G. Gatechew, A. A. Kashale, I.-W. P. Chen and J.-Y. Chang, Chem. Eng. J., 2022, 446, 137293 CrossRef CAS.
- A. S. Rasal, C. Korupalli, G. Getachew, T.-H. Chou, T.-Y. Lee, A. V. Ghule and J.-Y. Chang, Electrochim. Acta, 2021, 391, 138972 CrossRef CAS.
- C. Johannes, M. Hartung and H.-P. Heim, Polymers, 2022, 14(13), 2636 CrossRef CAS PubMed.
- Y. Mallaiah, V. R. Jeedi, R. Swarnalatha, A. Raju, S. N. Reddy and A. S. Chary, J. Phys. Chem. Solids, 2021, 155, 110096 CrossRef CAS.
- R. Singh, B. Bhattacharya, H.-W. Rhee and P. K. Singh, Int. J. Hydrogen Energy, 2015, 40(30), 9365–9372 CrossRef CAS.
- M. J. Reddy and P. P. Chu, J. Power Sources, 2002, 109(2), 340–346 CrossRef.
- Y. Lee, M. Buraidah and H. Woo, High Perform. Polym., 2020, 32(2), 183–191 CrossRef CAS.
- S. B. Patel and J. V. Gohel, J. Solid State Electrochem., 2019, 23(9), 2657–2666 CrossRef CAS.
- I. Zakariya’u, B. Gultekin, V. Singh and P. K. Singh, High Perform. Polym., 2020, 32(2), 201–207 CrossRef.
- X. Wang, W. Feng, W. Wang, W. Wang, L. Zhao and Y. Li, Res. Chem. Intermed., 2018, 44, 1161–1172 CrossRef CAS.
- S. Kumar, P. K. Yadav, R. Prakash, A. Santra and P. Maiti, J. Alloys Compd., 2022, 922, 166121 CrossRef CAS.
- L. Jiang, Z. Ren, W. Zhao, W. Liu, H. Liu and C. Zhu, R. Soc. Open Sci., 2018, 5(7), 180536 CrossRef PubMed.
- R. Prakash and P. Maiti, Energy Fuels, 2020, 34(12), 16847–16857 CrossRef CAS.
- S. Kumar, I. C. Maurya, O. Prakash, P. Srivastava, S. Das and P. Maiti, ACS Appl. Energy Mater., 2018, 1(9), 4641–4650 CrossRef CAS.
- S. Kumar, R. Prakash and P. Maiti, Sol. Energy, 2022, 231, 985–1001 CrossRef CAS.
- J. Duan, Q. Tang, R. Li, B. He, L. Yu and P. Yang, J. Power Sources, 2015, 284, 369–376 CrossRef CAS.
- H.-Y. Chen, L. Lin, X.-Y. Yu, K.-Q. Qiu, X.-Y. Lü, D.-B. Kuang and C.-Y. Su, Electrochim. Acta, 2013, 92, 117–123 CrossRef CAS.
- S. Wang, Q.-X. Zhang, Y.-Z. Xu, D.-M. Li, Y.-H. Luo and Q.-B. Meng, J. Power Sources, 2013, 224, 152–157 CrossRef CAS.
- Z. Huo, L. Tao, S. Wang, J. Wei, J. Zhu, W. Dong, F. Liu, S. Chen, B. Zhang and S. Dai, J. Power Sources, 2015, 284, 582–587 CrossRef CAS.
- M. Dissanayake, T. Liyanage, T. Jaseetharan, G. Senadeera and B. Dassanayake, Electrochim. Acta, 2020, 347, 136311 CrossRef CAS.
- S. Gupta and P. K. Varshney, Ionics, 2017, 23(6), 1613–1617 CrossRef CAS.
- H. Song, H. Rao and X. Zhong, J. Mater. Chem. A, 2018, 6(12), 4895–4911 RSC.
- N. Baharun, M. Mingsukang, M. Buraidah, H. Woo, L. Teo and A. Arof, Ionics, 2020, 26, 1365–1378 CrossRef CAS.
- C. Ma, C. Shi, K. Lv, Z. Shao and W. Chen, J. Mater. Sci.: Mater. Electron., 2018, 29, 11783–11789 CrossRef CAS.
- Y. Oda, H. Shen, L. Zhao, J. Li, M. Iwamoto and H. Lin, Sci. Technol. Adv. Mater., 2014, 15(3), 035006 CrossRef PubMed.
- Y. Lin, H. Song, J. Zhang, H. Rao, Z. Pan and X. Zhong, J. Mater. Chem. A, 2021, 9(2), 997–1005 RSC.
- M. Kramarenko, C. G. Ferreira, G. Martinez-Denegri, C. Sansierra, J. Toudert and J. Martorell, Sol. RRL, 2020, 4(4), 1900554 CrossRef CAS.
- V. van Laack, F. Langer, A. Hartwig and K. Koschek, ACS Omega, 2023, 8(10), 9058–9066 CrossRef CAS PubMed.
- R. K. Kokal, A. R. Bredar, B. H. Farnum and M. Deepa, ACS Appl. Nano Mater., 2019, 2(12), 7880–7887 CrossRef CAS.
- R. Dang, Y. Wang, J. Zeng, Z. Huang, Z. Fei and P. J. Dyson, J. Mater. Chem. A, 2017, 5(26), 13526–13534 RSC.
- Y. Wang, J. H. Zeng and G. Zhao, Sol. Energy, 2019, 194, 286–293 CrossRef CAS.
- J. Kusuma and R. G. Balakrishna, Sol. Energy Mater. Sol. Cells, 2020, 209, 110445 CrossRef CAS.
- B. He, C. Wang, J. Li, Z. Su, G. Xing, X. Gao and S. Chen, Nanomaterials, 2023, 13(13), 1983 CrossRef CAS PubMed.
- T. A. Chowdhury, M. A. B. Zafar, M. S.-U. Islam, M. Shahinuzzaman, M. A. Islam and M. U. Khandaker, RSC Adv., 2023, 13(3), 1787–1810 RSC.
- Y. Zhao, L. Hu, H. Liu, M. Liao, X. Fang and L. Wu, Sci. Rep., 2014, 4(1), 6847 CrossRef CAS PubMed.
- S. Promnopas, W. Promnopas, W. Maisang, S. Wannapop, T. Thongtem, S. Thongtem and O. Wiranwetchayan, Sci. Rep., 2023, 13(1), 4742 CrossRef CAS PubMed.
- B. Fu, C. Deng and L. Yang, Nanoscale Res. Lett., 2019, 14(1), 198 CrossRef PubMed.
- S. Akhil, J. Kusuma, S. Akash and R. G. Balakrishna, Sol. Energy, 2021, 224, 355–360 CrossRef CAS.
- J. Duan, Q. Tang, Y. Sun, B. He and H. Chen, RSC Adv., 2014, 4(105), 60478–60483 RSC.
- J. Duan, Q. Tang, B. He and H. Chen, RSC Adv., 2015, 5(42), 33463–33467 RSC.
- X. Mao, J. Yu, J. Xu, J. Zhou, C. Luo, L. Wang, H. Niu, J. Xu and R. Zhou, New J. Chem., 2020, 44(2), 505–512 RSC.
- K. Lv, C. Shi, C. Ma, Q. Wang and W. Chen, J. Nanopart. Res., 2019, 21, 1–6 CrossRef CAS.
- L. Yang, Y. Ma, J. Liu and Y. Mai, RSC Adv., 2016, 6(95), 92869–92873 RSC.
- C. Zhang, Y. Li, P. Zhang, M. Qiu, X. Jiang and H. Zhang, Sol. Energy Mater. Sol. Cells, 2019, 189, 11–20 CrossRef CAS.
- J. P. Park, J. H. Heo, S. H. Im and S.-W. Kim, J. Mater. Chem. A, 2016, 4(3), 785–790 RSC.
- Z. Zhang, C. Shi, J. Chen, G. Xiao and L. Li, Appl. Surf. Sci., 2017, 410, 8–13 CrossRef CAS.
- O.-H. Kwon, J. H. Heo, S. Park, S.-W. Kim and S. H. Im, J. Ind. Eng. Chem., 2019, 75, 164–170 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |



