Open Access Article
Open Access ArticleRecent applications of coinage metal nanoparticles passivated with salicylaldehyde and salicylaldehyde-based Schiff bases
Mamta
Sahu
,
Mainak
Ganguly
 * and
Priyanka
Sharma
* and
Priyanka
Sharma
Department of Chemistry, Solar Energy Conversion and Nanomaterials Laboratory, Manipal University Jaipur, Dehmi Kalan, Jaipur 303007, Rajasthan, India. E-mail: humansense2009@gmail.com
First published on 27th July 2024
Abstract
Salicylaldehyde (SD) and its derivatives are effective precursors for generating coinage metal (gold, silver, and copper) nanoparticles (NPs). These NPs have a variety of potential environmental applications, such as in water purification and sensing, and those arising from their antibacterial activity. The use of SD and its derivatives for synthesizing coinage NPs is attractive due to several factors. First, SD is a relatively inexpensive and readily available starting material. Second, the synthetic procedures are typically simple and can be carried out under mild conditions. Finally, the resulting NPs can be tailored to have specific properties, such as size, shape, and surface functionality, by varying the reaction conditions. In an alkaline solution, the phenolate form of SD was converted to its quinone form, while ionic coinage metal salts were converted to zero-valent nanoparticles. The capping in situ produced quinone of coinage metal nanoparticles generated metal-enhanced fluorescence under suitable experimental conditions. The formation of iminic bonds during the formation of Schiff bases altered the properties (especially metal-enhanced fluorescence) and applications.
1. Motivation
The study highlights the advantages of using SD for synthesizing and capping coinage metal nanoparticles (CNPs). Cost-effectiveness, small size, ease of use, and capability of manipulating the plasmonic behavior made SD an interesting research material. SD is commonly used to form chelating metal complexes.1 Maher and Mohammed reviewed SD and SD derivatives for metal complex formation. However, no review article is available on SD-capped NPs with versatile applications including metal-enhanced fluorescence (due to manipulation of plasmonic mode density). CNPs with SD capping have been well studied. The condensation reaction with various amines produced Schiff bases with iminic bonds contributing a new milestone in the application of CNPs. Under these circumstances, we concentrated on writing a critical review article on SD and SD-based Schiff bases in nanoscience.2. Introduction
Biotechnology, materials science, computer science, health, pharmacy, and engineering are all involved in nanotechnology, which focuses on materials at 10−9 meter scales. Due to their widespread applications in commercial settings, NPs in both crystalline and amorphous forms have drawn much attention worldwide. This has encouraged several research institutes to focus on creating and growing a range of nano-applications.2,3 A capping agent can function as both a “poison” restricting access to active sites and a “promoter” increasing yields and controlling selectivity unexpectedly. These effects can be attributed to the formation of an interphase between metals and ligands, which have special characteristics that drive catalysis. Thus, it is crucial to comprehend this interphase structure to optimize the design of customized nanocatalysts.4,5 SD, also known as 2-hydroxybenzaldehyde, is a simple, naturally occurring reagent that is inexpensive and easily available. The price per kilogram at Sigma-Aldrich is $341. SD fluoresces very faintly because of its comparatively small π-conjugated benzene ring structure.6 SD is a capping agent that contributes both directly and indirectly to the production of nanoparticles.7,8 SD is used as a sensing probe and for elimination of toxic materials, with CNPs. Nowadays, SD is also used for metal enhanced fluorescence (MEF), placing CNPs in perfect proximity.8With little change in the capping agent, we can get drastic changes in the physicochemical properties of NPs. SD can be converted into a Schiff base via the condensation reaction of amines with the formation of iminic bonds. Schiff base (obtained from SD) capped NPs are used for many applications, for example sensing and antibacterial activity.
CNPs, which include gold, silver, and copper, exhibit special optical characteristics due to localized surface plasmon resonance (LSPR), which makes them a great option for sensor applications.9 In addition to causing higher local electromagnetic fields close to the nanoparticle's surface, LSPR is the cause of the strong absorption band that the particle exhibits. Because LSPR is dependent on both the nanoparticle and the medium in which it is disseminated, it may be adjusted by adjusting the composition, size, form, and dielectric characteristics of the surrounding medium of the nanoparticles.10 The interparticle distance between pairs of NPs affects the surface plasmon characteristics of metal nanoparticles as well. As NPs approach one another under the influence of an analyte, their electric fields interact, causing interparticle plasmon coupling, which in turn causes a coupling-induced LSPR shift and perceptible color changes.10
This review emphasizes the synthesis process of SD and SD-based Schiff-capped CNPs, their applications, and the fate of the involved elements. This suggests that the current study might be filling a gap in existing knowledge.
3. Coinage metal nanoparticles
Three nonradioactive elements from group 11 of the periodic table—Cu, Ag, and Au—have historically been used to manufacture the majority of coinage metals (or alloys). Cu is often augmented with Sn and other metals to create bronze. The ancient world's money was made of gold, silver, bronze, or copper, as was the majority of medieval coinage. Around 650 BC, Aegina Island, Ephesus, and Lydia were the starting points of all Western currency histories. In approximately the sixth century BC, ancient India became among the first issuers. Coins have been the most widely used representation of money ever since. To create the first currency, electrum, a naturally occurring creamy mixture of Ag and Au, was doped with a different metal.11,12The term “coinage”, however, is not entirely defined because other metals have already been used to make “demonstration coins”, which have never been used to make any monetized nation-state currencies. They could come in handy later on. Although some of these elements (like zirconium) would make excellent coins in theory, it is unclear where these elements stand in terms of coin metals. Because coin metal is fundamentally costly, there is a significant challenge. The twenty-first century has seen several initiatives throughout the world advocating for the use of the least expensive and uncommon types of coinage metals. Tee and Ye provided an overview of the latest advancements in coinage metals within the nanoscale realm and their biological applications.7,11
From a historical standpoint, coinage metals are most renowned for their monetary, ornamental, and metallurgical merits; nevertheless, as nanotechnology's potential has only just come to light, their metal nanostructures and uses may be viewed as creations of modern science. Notable characteristics of coinage metal nanostructures include visual, electrical, chemical, and catalytic qualities that depend on form and size. Considerable research has been conducted on the creation and synthesis of coinage metal nanostructures and their possible uses because of each of these characteristics.11 Numerous publications on using Cu, Ag, and Au catalysts to produce heterocycles can be found in the literature.13 When formed as nanostructures, the metals Ag, Au, and Cu are known to display localized surface plasmon resonance (LSPR) peaks in the visible range.14 Antibacterial metals like Cu, Ag, and Au may generally be employed as a kind of antibacterial agent.15–17
4. Salicylaldehyde
SD is a widely used, highly functionalized analyte, frequently used as a precursor to other chemicals. In analytical chemistry, SD is used for many different applications. For example, it may detect hydrazine and be used to make saligenin, coumarin, and salicylaldoxime, important analytical reagents. Moreover, SD contributes to taste and is an essential building block for several chelating agents.7 Preservatives like SD and its derivatives are frequently found in perfumes, cosmetics, and essential oils.7 SD is a widely used important chemical in many industrial processes, particularly in the large-scale manufacturing of medicines.It has two distinct active functional groups, an aldehyde group and a hydroxy group. Due to their commercial availability and ease of access, SD and most of its derivatives are perfect starting materials for multicomponent reactions, primarily in pseudo-three and four-component ones that result in a wide variety of heterocyclic systems.7 Before the introduction of a torsional twist of the substituent groups, the SD molecule was thought to have Cs symmetry.18
SD is frequently employed to produce chelating metal complexes.1 Maher and Mohammed studied SD and its derivatives for metal complex formation. When SD condenses with amines, it produces chelating ligands. It condenses with ethylenediamine to provide the ligand ‘salen’. Salicylaldoxime is produced with hydroxylamine. Catechol (1,2-dihydroxybenzene) is produced by oxidation with hydrogen peroxide (Dakin reaction) {Dakin, 1923 #47}. An aldol condensation produces the derivative of the heterocycle coumarin upon condensation with diethyl malonate.1
4.1 Fluorescence properties of salicylaldehyde
Properties of protonated SD have been studied both theoretically and experimentally using ab initio calculations. This aromatic molecule has two functional groups: a hydroxyl group (OH as in phenol) and an aldehyde group (CHO as in benzaldehyde). With intramolecular hydrogen bonding between the hydroxyl and carbonyl groups, neutral SD is a model molecule that displays an excited-state intramolecular proton transfer (ESIPT) reaction. Experimental methods and theoretical methods have been used extensively to study the intramolecular proton transfer process in the lowest singlet19 states (S0, S1, and S2).20,21 The rotation of the aldehyde group around the C–C bond and the hydroxyl group's cis–trans location about the aldehyde group result in a variety of conformers for protonated SD.20 Many functionalized SD derivatives have been synthesized under a range of conditions. Their photophysical properties and solid-state/solution phase fluorescence intensities are quite promising.22Synthetic intermediates known as SD derivatives have been extensively documented in the literature as the first steps towards developing Schiff bases,7 salen-based probes,19 and other heterocycles.23 They benefit from being readily accessible commercial reagents at a low cost or being attainable through a restricted number of synthesis steps. Moreover, a recent case brought out the use of unsubstituted deprotonated SD as a photocatalyst relying on visible light.6 However, unlike acetophenone or benzophenone analogs,22 SD derivatives display lower chelating capacity towards metal or metalloid ions, unless an additional chelating group is introduced in the vicinity.24 However, there have been reports of some coordination complexes involving SD derivatives. Because SD derivatives have a 6-membered H-bonded ring, they may exhibit weak emission in solution due to the potential of excited-state intramolecular proton transfer (ESIPT) emission.22,25 The photophysical process known as ESIPT is made up of photo-tautomerism that depends on the location and kind of electronic substitution on the molecular scaffold.22
Though the fluorescence of SD is weak,26 some studies reported SD-made dyes via coupling reactions with significant fluorescence. Stoerkler et al. synthesized 4-extended SD derivatives 1a–d, 2a–b, and 3a–b starting with 4-bromosalicylaldehyde. Strong naked-eye fluorescence could be induced by substituting certain SD derivatives at the 4-position. Electronic substitution and the kind of π-conjugated spacer affected the optical characteristics of the final dyes. Three distinct spacers (vinyl, ethynyl, or a direct link between SD and different aryl groups) were examined and the emissions were changed according to the π-conjugated spacer (Fig. 1 and Table 1).22
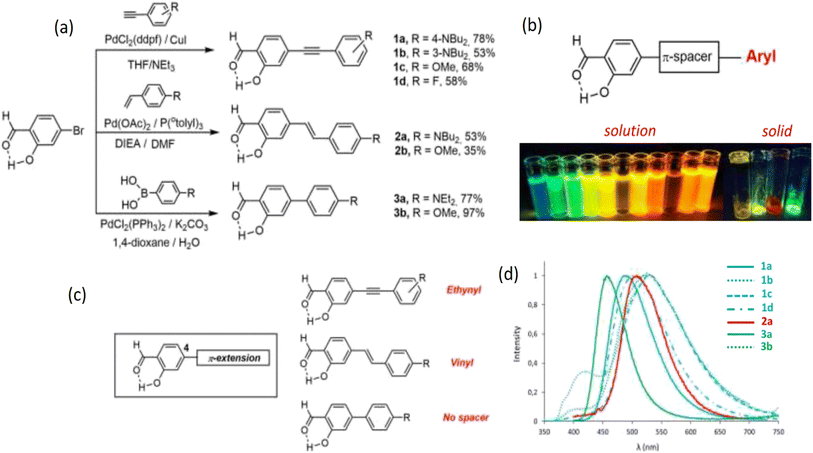 | ||
| Fig. 1 (a) Synthesis protocol of 4-extended SD derivatives 1a–d, 2a–b, and 3a–b. (b) Compounds of SD that exhibit intense fluorescence in multiple environments in solution and solid state. (c) 4-Extended SD derivatives. (d) SD's emission spectra in aerated solutions of toluene at room temperature are as follows: 1a (plain blue), 1b (dotted blue), 1c (dashed blue), 1d (dotted dashed blue), 2a (plain red), 2b (dotted red), 3a (plain green), and 3b (dotted green). Reproduced with permission from ref. 22, copyright 2024, Eur. J. Org. Chem. | ||
| Dye | λ abs (nm) | ε (M−1 cm−1) | λ em (nm) | ΔSb (cm−1) | Φ F | τ (ns) | K r | K nr | Solvent |
|---|---|---|---|---|---|---|---|---|---|
| a Relative quantum yield determined in solution using Rhodamine 6G as a reference (λexc = 488 nm, Φ = 0.88 in ethanol). b Stokes shift. c k r (108 s−1) and knr (108 s−1) were calculated using: kr = ΦF/σ, knr = (1 − ΦF)/σ where σ is the lifetime. d Cyclohexane. e Non fluorescent. | |||||||||
| 1a | 412 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
424 | 690 | 0.53 | 1.3 | 4.08 | 3.62 | CHd |
| 1a | 407 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
490 | 4200 | 0.68 | 3.1 | 2.19 | 1.03 | Toluene |
| 1a | 398 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
508 | 5400 | 0.60 | 3.4 | 1.76 | 1.18 | Et2O |
| 1a | 401 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
555 | 6900 | 0.11 | 2.5 | 0.44 | 3.56 | THF |
| 1a | 411 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
605 | 7800 | 0.02 | 0.6 | 0.33 | 16.30 | CH2Cl2 |
| 1a | 351 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
533 | 9700 | 0.01 | 0.4 | 0.25 | 24.80 | CH2Cl2/H+ |
| 1a | 400 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
Acetone | ||||||
| 1a | 400 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
CH3CN | ||||||
| 1a | 401 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
442/594 | 2300 | 0.01 | 0.5 | 0.20 | 19.80 | DMSO |
| 1a | 400 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
587 | 8000 | 0.02 | 0.4 | 0.50 | 24.50 | DMF |
| 1b | 393 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
445 | 3000 | 0.12 | 2.3 | 0.53 | 3.83 | CHd |
| 1b | 388 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
506 | 6000 | 0.29 | 10.0 | 0.29 | 0.71 | Toluene |
| 1b | 389 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
410/605 | 9200 | 0.06 | 4.1 | 0.15 | 2.29 | THF |
| 1b | 394 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
654 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.02 | 1.7 | 0.12 | 5.76 | CH2Cl2 |
| 1b | 348 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
533 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.01 | 0.8 | 0.13 | 12.40 | CH2Cl2/H+ |
| 1c | 346 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
424/532 | 5300 | 0.01 | 0.5 | 0.20 | 19.80 | Toluene |
| 1d | 320 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
411/533 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.01 | 0.4 | 0.25 | 24.80 | Toluene |
| 2a | 416 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
423 | 3980 | 0.04 | 0.4 | 1.00 | 24.00 | CHd |
| 2a | 425 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
508 | 3800 | 0.10 | 0.8 | 1.25 | 1130 | Toluene |
| 2a | 415 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
522 | 4900 | 0.19 | 0.8 | 2.38 | 10.10 | Et2O |
| 2a | 425 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
558 | 5600 | 0.29 | 1.6 | 1.81 | 4.44 | THF |
| 2a | 436 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
593 | 6100 | 0.64 | 2.2 | 2.91 | 1.64 | CH2Cl2 |
| 2a | 331 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
418/532 | 6300 | 0.04 | 0.4 | 1.00 | 24.00 | CH2Cl2/H+ |
| 2a | 425 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
600 | 6900 | 0.44 | 2.4 | 1.83 | 2.33 | Acetone |
| 2a | 427 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
631 | 7600 | 0.47 | 2.0 | 2.35 | 2.65 | CH3CN |
| 2a | 426 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
643 | 7900 | 0.09 | 0.5 | 1.80 | 18.20 | EtOH |
| 2a | 425 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
602 | 6900 | 0.22 | 2.4 | 0.92 | 3.25 | DMSO |
| 2a | 430 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
617 | 7100 | 0.70 | 2.4 | 2.92 | 1.25 | DMF |
| 2b | 367 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
Toluene | ||||||
| 3a | 383 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
410/568 | 1700 | 0.24 | 0.2 | 12.0 | 3.80 | CHd |
| 3a | 392 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
459 | 3700 | 0.48 | 1.3 | 3.69 | 4.00 | Toluene |
| 3a | 383 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
464 | 4600 | 0.38 | 2.1 | 1.81 | 2.95 | Et2O |
| 3a | 389 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
504 | 5900 | 0.76 | 2.4 | 3.17 | 1.00 | THF |
| 3a | 396 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
532 | 6500 | 0.56 | 2.3 | 2.43 | 1.91 | CH2Cl2 |
| 3a | 332 | 7800 | CH2Cl2/H+ | ||||||
| 3a | 391 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
548 | 7300 | 0.09 | 0.09 | 1.80 | 18.20 | Acetone |
| 3a | 387 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
546 | 7500 | 0.03 | 0.03 | 1.50 | 1.50 | EtOH |
| 3a | 390 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
533 | 6900 | 0.58 | 0.58 | 1.93 | 1.93 | DMSO |
| 3a | 385 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
520 | 6700 | 0.27 | 0.27 | 1.93 | 0.93 | DMF |
| 3b | 321 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
400/527 | 6200 | 0.01 | 0.2 | 0.10 | 49.50 | Toluene |
4.2 Synthesis of salicylaldehyde
Through a Reimer–Tiemann reaction, SD could be made from phenol and chloroform.27 By electrolytically reducing salicylic acid on a mercury electrode, SD is produced.28 The effective conversion of salicylic alcohols to SD under moderate conditions is facilitated by lipase. High yields (81–95%) of the SD from salicylic alcohol under ideal reaction conditions suggest that the technique has a lot of promise for SD production (Fig. 2).29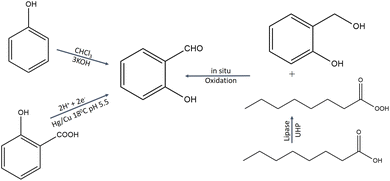 | ||
| Fig. 2 Synthesis protocol of salicylaldehyde.27–29 | ||
4.3 Synthesis of salicylaldehyde-capped coinage metal nanoparticles
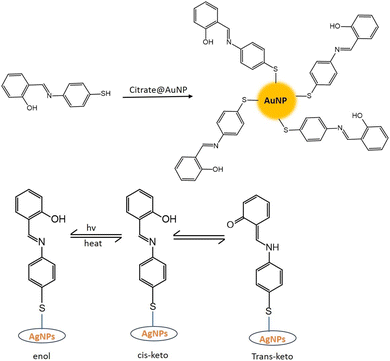
SD is a hydrophobic organic compound and insoluble in water. That is why it is dissolved in an alkaline solution. In an alkaline solution, SD converted to its phenolate form. However, in the presence of coinage metal ions, the phenolate form of SD was oxidized to the quinone form. On the other hand, coinage metal ions were reduced to zero valent nanoparticles, capped with oxidized SD (OSD).7,30CNPs with OSD capping were prepared under various experimental conditions. Sahu et al.8 synthesized OSD-capped AgNPs at room temperature. Ganguly et al. synthesized OSD-capped AgNPs in the presence of UV light for one hour with strong fluorescence. The same group synthesized gold and silver nanoparticles at room temperature with simple aging (without any exposure).31,32 Sharma et al.7 produced copper nanoparticles from SD after 8 h of simple aging. OSD-capped coinage metal nanoparticles exhibited strong metal-enhanced fluorescence.
OSD-capped CNPs were quite stable. Under ambient conditions, the emission of SD-AgNP hydrosol exhibited a high degree of stability (quantum yield of 7% using quinine sulfate as a reference). After aging for 30 days, the highest emission wavelength of SD-AgNPs was around 419 nm, having previously been approximately 424 nm on day 0. There was only a 9% decrease in fluorescence after 30 days of aging.8 In comparison to AgNPs, CuNPs were less stable due to the susceptibility of aerial oxidation.7
4.4 Generation of metal-enhanced fluorescence (MEF) from salicylaldehyde
SD-passivated CNPs were found to be highly fluorescent exhibiting myriad applications in environmental remediation. SD, being oxidized in its quinone form, maintained a perfect distance from in situ-produced CNPs to generate MEF.Extensive research has been conducted on fluorescence-based biosensor platforms to enhance biosensor performance and sensitivity. Fluorescence signals may be enhanced or quenched in near-optical fields using a variety of structures that make use of metal's properties from the macroscopic to the nanoscopic level. Proximal fluorophores (those in the 5–90 nm range) interact well with metallic surfaces, leading to favorable optical features including shortened fluorophore lifetime, enhanced photostability, and higher quantum yield. The term MEF describes this phenomenon.9 In 2002, Geddes came up with the term MEF.33 The excited metal emitted photons, causing MEF. Light interactions with freely movable electrons in a metal particle can increase the incident light field. Such an effect (lightning rod effect) is associated with an extra excitation field with a tremendous effect, causing MEF. Near a silver particle, the local electric field could be multiplied by 140, resulting in a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold increase in local intensity and rate of excitation8,34,35 (Fig. 3).
000-fold increase in local intensity and rate of excitation8,34,35 (Fig. 3).
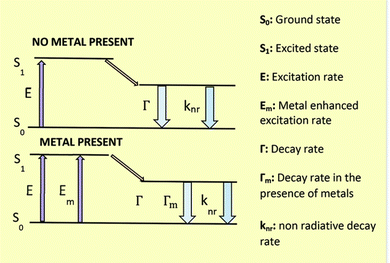 | ||
| Fig. 3 Energy diagram for fluorescence in the presence and absence of a metal surface. Reproduced with permission from ref. 36, copyright 2024, Nanoscale. | ||
Fluorophores' quantum efficiency and photostability may both be enhanced by MEF. The free electrons in the metal surface (surface plasmon) pair with the electrons in the fluorophore when it is in close contact with a metallic nanostructure. Here, the metal may be considered an optical antenna that confines concentrated hot areas of electromagnetic radiation in progress. This confinement depends on the metallic nanoparticle's form, composition, and surrounding environment since it is at the particle's surface.36,37
Fluorescent materials are often used in biosensing. Much lower quantities of biomarkers utilized in biosensing or bioimaging may be detected with MEF. When exposed to the right wavelength of light, the free surface electrons on metallic nanoparticles (MNPs) resonate. Localized surface plasmon resonance (LSPR) is the name for the charge density oscillations resulting from the metal's electrons being dispersed relative to the particle's core due to this light.36
Because each of these factors will alter the electromagnetic field density at the metal nanoparticle surface, the resonance condition depends on the size, shape, distance, and dielectric properties of the metallic nanoparticles and the surrounding medium. It is possible to alter a fluorophore's emission and excitation properties by subjecting it to the near field of a metallic surface. Because of the fluorophore's increased exposure to the local electric field and the subsequent coupling between it and neighboring MNPs, MEF permits an increase in the excitation rate (Fig. 3).34,36
OSD-capped AgNPs aggregated in nature. It was clear from the SEM pictures that the particles were clumped together. The metalized surface was formed by the aggregation of particles measuring around 90 nm, displaying MEF.8 The oxidized SD was used as a fluorophore and in situ produced AgNP, AuNP, and CuNP enhanced fluorescence due to the high photonic mode density associated with the lightning rod effect. After two days of simple aging, the aqueous alkaline solution of SD produced by Ganguly et al. showed greatly increased fluorescence at room temperature (λem ∼ 420 nm, Stokes shift 120 nm, stability >a year), when Ag(I) or Au(III) was added. Such extraordinary fluorescence enhancement caused by Ag and Au particles was attributed to the increased scattering cross-section of the in situ-produced aggregated metal particles and the lightning rod effect of the metal aggregates, which concentrated the electric field around the fluorophore (i.e., alkaline solutions of SD).12,32
After one hour of UV irradiation, the lifetime of free SD in an alkaline medium was 0.39 nanoseconds. However, the OSD-AgNP hydrosol had a lifetime of 3.85 nanoseconds. The lightning rod effect, which concentrates the electric field surrounding the fluorophore in the presence of Ag0 owing to LSP, was the reason for the extended fluorescence lifetime in the presence of AgNPs.31
4.5 Application of salicylaldehyde-capped coinage metal nanoparticles
Several colors are being investigated for adsorption with nanoparticles. Methyl blue (MB) is the focus of infrequent studies. Rubber, paints, pharmaceuticals, dyes, and varnishes all include MB as a disinfectant.41 After mixing several dyes with OSD-AgNPs and letting them mature for 20 minutes, no recognizable color change was seen. Only MB produced an immediate blue precipitate, known as MB-Ag0@Zn(OH)2, with the addition of zinc acetate solution. The supernatant of this precipitate was almost colorless when it formed. Except for Zn2+, no discernible changes occurred when other metal ions (K+, Al3+, Co2+, Hg2+, Fe3+, Na+, Ba2+, Pb2+, and Cu2+) were introduced one at a time to the OSD-AgNP hydrocolloid in addition to MB. The blue solid MB-Ag0@Zn(OH)2 was only formed by Zn2+, suggesting that MB was selectively adsorbed onto Ag0@Zn(OH)2. The highest possible absorption level was 1065 mg·g−1. To regenerate MB, hot water and ethyl alcohol were applied to the solid MB-Ag0@Zn(OH)2. But there was insufficient MB regeneration. When deionized water was used to suspend solid MB-Ag0@Zn(OH)2, and other metal ions were introduced one at a time, only Fe3+ produced an instant blue color that indicated MB regeneration. In the presence of Fe3+, around 97% of the MB was recovered. No other metal ion showed a noteworthy MB recovery.30
4.5.2.1 Colorimetric pathway. The unique characteristics of noble metal nanoparticles are referred to as LSPR. The conduction electrons of metal nanoparticles (MNPs) collectively and tenaciously vibrate, when a certain frequency of electromagnetic radiation interacts with them; this resonance is known as LSPR or SPR. MNPs and their surrounding conditions were essential to LSPR. Changes in the size, shape, and composition of the nanoparticles, as well as their sensitivity to the dielectric properties of their surroundings, may be used to adjust the LSPR frequency.43
Two kinds of sensors emerge when MNPs interact with the target analyte; one is an aggregation sensor (due to inter-particle plasmon coupling of NPs) and another is a refractive index sensor (due to a change in local refractive index – plasmon shift of the medium). This gives rise to the LSPR shift from source frequency. Besides, other classes of sensors also exist, and they are based on Surface Enhanced Raman Spectroscopy (SERS) and MEF. A sensor under focus in the current discussion is an aggregation sensor. Aggregation of MNPs leads to broad and red shifting of the LSPR band. This makes it simple, quick, reliable, and inexpensive to detect the metal ions. The said sensor is called a colorimetric sensor, which detects small molecules, proteins, DNA, pollutants, and noxious metal ions.44–47
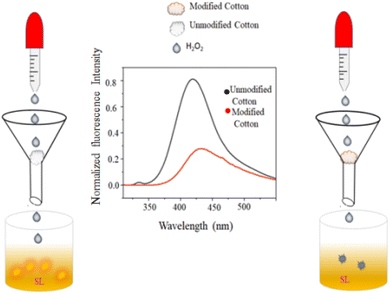
4.5.2.1.1 H2O2 colorimetric sensing. Hydrogen peroxide is widely used in food processing, environmental monitoring, and several chemical sectors due to its strong oxidizing capacity. Furthermore, a variety of biological functions require H2O2, a substantial species of reactive oxygen. Imbalanced H2O2 concentrations have been linked to Alzheimer's disease, myocardial infarction, and human atherosclerosis.48–50 H2O2 is also considered to be a significant water pollutant. H2O2 detection is essential to analytical and biological science. Muralidharan51 amperometrically sensed H2O2 using a glassy carbon electrode infused with copper nanoparticles. Many fluorescence techniques (involving DNA-AgNCs,50 urea-formaldehyde resin passivated ZnO nanoparticles52) were also used. Iridium(III) complex-AgNPs were used as a turn-on fluorescent probe in the presence of H2O2.53 When Sharma et al.7 introduced H2O2 to the alkaline solution of SD, the light-yellow color of SD instantly turned colorless. Then a dark brown color was achieved by adding Cu2+ solution. The impact of various competing molecules (Na+, K+, Cu2+, Fe3+, Zn2+, fructose, ascorbic acid, and urea) was also investigated. However, only H2O2-induced oxidation of SD produced a dark coloration in comparison to other competitive molecules. They experimented with different H2O2 concentrations to find the sensor's sensitivity and discovered that colorimetric detection was impossible below 10−3 M.7
4.5.2.1.2 Fe3+ colorimetric sensing. One of the most common transition metals in biological systems is iron. The dual character of iron in biological systems is its vital function in several processes such as electron transport and oxygen metabolism, but it may also be harmful if present in excess. This dichotomy highlights the significance of identifying and regulating the body's iron levels, namely Fe3+ ions, to avert related illnesses such as cancer, Parkinson's disease, and Alzheimer's.8N-Acetyl-L-cysteine,54p-phenylenediamine,55Poria cocos extract,56 and Sonchus arvensis leaf extract57 passivated CNPs were used as a colorimetric sensor for Fe3+.
In the previously discussed paragraph (dye removal and recycling), in the presence of Fe3+, around 97% of MB was recovered. No other metal ion has shown a noteworthy MB recovery. Differences in iron's oxidation states (2/3) and counter anions did not affect the amount of MB recovered. This Fe3+ sensing technique used a colorimetric route. From the adsorbed solid MB-Ag0@Zn(OH)2, iron selectively recovered MB, certifying the ionic iron detecting platform. MB-Ag0@Zn(OH)2 developed a darker blue color as the concentration of Fe increased. The blue color was visible to the naked eye at iron concentrations between 10−2 M and 10−7 M, and the absorption spectra revealed that the λmax was 565 nm. Nevertheless, a linear detection range of 10−4 M to 10−6 M was found, and 10−6 M LOD. Other metal ions (Co2+, Cu2+, K+, Ni2+, Ba2+, Sn2+, Al3+, Na+, Hg2+, Pb2+), introduced separately with Fe3+, did not alter the restoration of the blue hue, nor did they affect the sensing efficiency.30
4.5.2.2 Fluorometric pathway. Light emission is the outcome of the electronic excitation phenomenon during the fluorophore's light absorption process. After light absorption, the fluorophore remains excited, and fluorescence emission is produced with a wavelength longer and energy lower than the excitation wavelength.58 Fluorophore and ionophore units, which make up fluoroionophores, are used for producing the fluorescence sensor.33 When constructing a fluorescence sensor, the primary focus should be on two fundamental components: the recognition and signaling units. The former is responsible for the binding's efficiency and selectivity, while the latter transforms the data into an optical signal.59
4.5.2.2.1 Ag+ sensing. Ag ions are regarded as hazardous heavy metals, even though silver compounds are widely used in many sectors (such as electronics, photography, and mirrors). Ag ions accumulate in the body and deactivate sulphydryl enzymes. About 2500 tonnes of silver are discharged into the environment each year as a result of emissions and industrial wastes; of these, 150 tonnes end up in wastewater sludge and 80 tonnes are combined with surface waters. For the detection of Ag at the trace level in aqueous systems, electrochemical techniques, plasma emission spectroscopy, atomic absorption, and optical tactics have been used.60–62
Fluorescence sensing devices for Ag+ species have been developed in various forms, including small molecules, macromolecules, quantum dots, and nanoparticles.63 Mehta et al.64 provided an overview of Ag+ sensing using various current techniques. As an aggregation-induced emission fluorophore, benzoimidazolyl-cyanovinylene (1) was developed and a fluorescent peptidyl probe (2 and 3) containing this fluorophore was created. The fluorescent peptidyl probes exhibited a ratiometric response to Ag+ in pure aqueous solutions by preferentially coordinating with Ag+ among different metal ions.
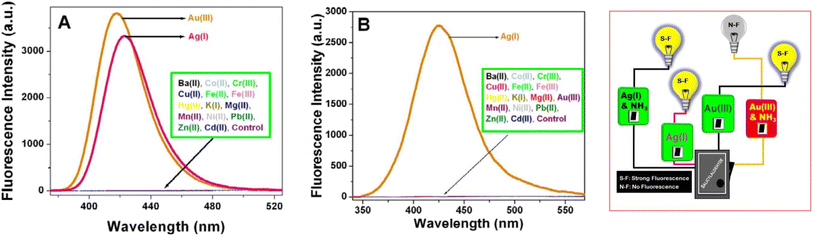
Gold and silver exhibited the highest fluorescence intensity in the alkaline solution of SD. No other metals show a similar increase in fluorescence. When –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in SD is transformed to –CH
O in SD is transformed to –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (via iminic bond formation), gold-enhanced fluorescence was selectively suppressed. Thus, selective and massively amplified silver-enhanced fluorescence was proposed for a sensitive Ag(I) sensor. The LOD was 10 × 10−8 M (far lower than the United States Environmental Protection Agency (EPA) permissible level).32
N– (via iminic bond formation), gold-enhanced fluorescence was selectively suppressed. Thus, selective and massively amplified silver-enhanced fluorescence was proposed for a sensitive Ag(I) sensor. The LOD was 10 × 10−8 M (far lower than the United States Environmental Protection Agency (EPA) permissible level).32
4.5.2.2.2 Fe3+ sensing. Fe3+ could be detected via coinage metal nanoparticles with different methods such as colorimetric and fluorometric. The majority of Fe3+ sensing techniques rely on an organic chemosensor that either experiences a “turn-on” process65 or fluorescence quenching66 due to the paramagnetic nature of ferric ions. AgNPs were used widely in a fluorometric route for Fe3+ sensing. Calix[4]resorcinarene polyhydrazide-capped AgNPs were developed by Makwana et al.67 and exhibited respectable fluorescence. The fluorescence was selectively quenched by Fe3+, and Fe3+ sensing was achieved by this approach. A novel Schiff base generated from rhodamine was developed and fluorescence spectroscopy methods were used to detect Fe3+. “Off–on” fluorescence response in semi-aqueous media helped to detect Fe3+.68
Fluorescent OSD-AgNPs, synthesized at room temperature, showed quenching in the presence of Fe3+. No other metal ion displayed such kind of quenching. The complexation of the capping agent SD with Fe was ascribed to be the cause of the quenching of silver-enhanced fluorescence. Therefore, iron might be detected with high selectivity and sensitivity using silver-enhanced fluorescence (limit of detection: 2 × 10−9 M).8,69
4.5.2.2.3 Cysteine sensing. Cysteine is a common biothiol in biological systems.70 It is essential for reversible cell redox reactions, including several metabolic and detoxifying biological processes.70,71 Cysteine has also been shown to be a possible neurotoxin and a biomarker for certain illnesses—inadequate amounts of cysteine cause weakness, edema, skin problems, slow development, and hair depigmentation.31
With the aid of binding, the fluorophore emissions may be turned on and off. To recover the fluorescence emission used in the construction of the fluorescence probe, a particular analyte takes the place of the metal ion ligand.72,73
Rajamanikandan used highly fluorescent glutathione decorated copper nanoclusters (GSH-CuNCs) as a fluorescence probe to study a very sensitive and specific detection of L-cysteine (Cys) in human urine samples. With an excitation wavelength of 380 nm, GSH-CuNCs show an emission peak at 613 nm. GSH-CuNCs' emission intensity decreased when Cys was added, indicating that the metal–thiol interaction between the GSH-CuNCs surface and the thiol group containing the Cys analyte may be the cause of the emission intensity quenching.74
The OSD-AgNP fluorescence was influenced by the presence of certain amino acids in distinct ways. Tryptophan exhibited increased fluorescence because of its intrinsic fluorescence, whereas cysteine, histidine, and arginine efficiently suppressed fluorescence. Furthermore, the OSD-AgNPs' fluorescence gradually diminished as the cysteine content increased. A strong linear association between (I0 − I)/I0 and cysteine concentration was found for the concentration range of 0 to 10 mm. The lower detection limit for cysteine was found to be 50 nm. Other amino acids including histidine, arginine, tryptophan, and methionine don't change the OSD-AgNPs' fluorescence. Because of its inherent fluorescence, tryptophan was the only substance in the OSD-AgNPs to exhibit any increased fluorescence. The fluorescence of OSD-AgNPs was selectively quenched by cysteine, and other amino acids did not affect the quenched fluorescence in the OSD-AgNP hydrosol solution caused by cysteine. This phenomenon was explained by changes in the scattering/absorption cross-section and the “lightning rod effect”, a change in the electric field density surrounding the fluorophore.31
4.5.2.2.4 Cu2+ sensing. At high concentrations, copper—a crucial trace element in biological systems—becomes a dangerous and deadly heavy metal.31 Current advances in emissive sensors for copper, with mechanistic ground and bio-applications, were examined by Li et al.75
As previously discussed (in the sub-section Cysteine sensing), cysteine was the only amino acid that could specifically quench the fluorescence of OSD-AgNPs. Other amino acids did not quench the fluorescence in the hydrosol solution of OSD-AgNPs (unlike cysteine). Cu2+, a metal ion, could completely restore lost fluorescence. Cu2+ ions and cysteine might be detected selectively and sensitively in a single pot using this mechanism of quenching and fluorescence recovery. The off/on fluorescence was caused by changes in the electric field density surrounding the fluorophore and the scattering/absorption cross-section. The restoration of the quenched fluorescence was caused by the formation of Cu(0). The selective quenching and regeneration of fluorescence were largely dependent on the interaction between the fluorophore and the metal ions.31
4.5.2.2.5 Pb2+ sensing. Although lead poisoning was initially identified as early as 2000 BC, Pb2+ is a persistent and highly hazardous environmental pollutant. The Centres for Disease Control (CDC) state that even in the USA, 250
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 children between the ages of one and five have blood lead levels exceeding 10 micrograms per deciliter of blood, which is potentially dangerous.1–5 Since lead does not degrade, exposure to high levels of lead in children can delay their mental and physical development and result in irreparable brain damage.76,77
000 children between the ages of one and five have blood lead levels exceeding 10 micrograms per deciliter of blood, which is potentially dangerous.1–5 Since lead does not degrade, exposure to high levels of lead in children can delay their mental and physical development and result in irreparable brain damage.76,77
Pb ion sensing with coinage metal nanoparticles is frequently found in the literature Ganguly et al. showed lead, DMSO, and cysteine sensing via synergistically evaluating a gold–silver giant cluster.78,79 Kang et al.80 designed an electrochemical sensing platform of lead ions based on copper, while Anambiga et al. designed a colorimetric Pb2+ sensor with glutathione-capped silver nanoparticles.81
The interaction of Pb2+ with the dye molecule MB in the presence of the fluorescent OSD-AgNP hydrosol was the process by which Pb2+ turned on fluorescence. MB first quenched the fluorescence of OSD-AgNPs-MB. However, the introduction of Pb2+ caused MB to be removed from the vicinity of the silver surface, resulting in the formation of PbS and the release of the quenching action. As a consequence, fluorescence was restored, enabling sensitive and focused Pb2+ fluorometric monitoring. The method of turning on the fluorescence was mostly dependent on the interaction of Pb2+ with MB and the silver surface.30 Fluorometric Pb2+ sensing (linear detection range: 10−5 to 5 × 10−8 M; limit of detection: 5 × 10−8 M) was made possible (Fig. 4). Lead in various natural water sources such as rainfall, tap water, and the Ganga River was measured using the spiking method.30
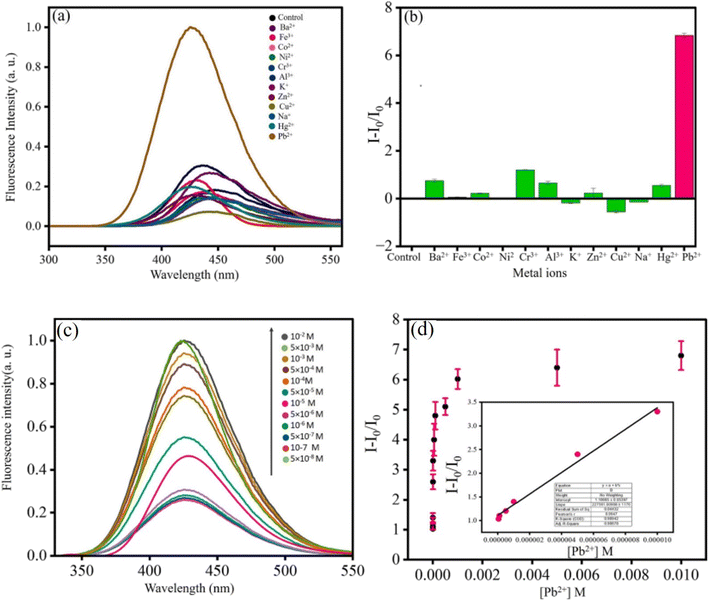 | ||
| Fig. 4 Effect of metal ions on (OSD-AgNPs-MB); (a) fluorescence spectra and (b) bar diagram; (c) fluorescence spectra of OSD-AgNPs-MB at different [Pb2+] and (d) plot of I/I0vs. [Pb2+] and linear detection range of Pb2+ detection. Reproduced with permission from ref. 30, copyright 2024, Spectrochim. Acta, Part A. | ||
4.5.2.2.6 H2O2 sensing. SD itself has weak fluorescence with an emission peak at 500 nm. Adding H2O2 increased the overall fluorescence intensity. As the H2O2 concentration increased, a new emission peak appeared at 440 nm. The original peak at 500 nm gradually disappeared. A final peak at 420 nm became dominant. The phenolate form was responsible for the weak 500 nm emission. H2O2 oxidized the phenolate to the quinone form. The quinone form had a stronger fluorescence with a peak at 420 nm (blue-shifted compared to phenolate). At low H2O2 concentrations, only a partial conversion of phenolate to quinone occurred, resulting in a mix of both forms. This explained the initial blue shift (appearance of the 440 nm peak) with some residual 500 nm emission. At high H2O2 concentrations, most of the phenolate got converted to quinone, leading to the disappearance of the 500 nm peak and a dominant 420 nm emission.7 Combination with H2O2 and the other competing chemicals (Na+, K+, Cu2+, Fe3+, Zn2+, fructose, ascorbic acid, and urea) had negligible effects on the fluorescence of the alkaline SD. This resulted in the selective amplification of fluorescence generated by H2O2. The fluorescence amplification effect was not linear in its dependence on H2O2 concentration.
H2O2 was added gradually in OSD-CuNPs and the effect on the emission was observed. A continuous decrease in fluorescence was noticed with the successive increment of concentration of H2O2, without any significant change in λex and λem (a linear detection was obtained in the range of 10−4 M to10−6 M with a limit of detection of 10−6 M). The primary cause of fluorescence quenching was the oxidation of Cu0 by H2O2, destroying MEF in the absence of a metal surface.7
 | ||
| Fig. 6 Displayed imine brings selectivity for silver-enhanced fluorescence (A) without imminic bond, (B) with imminic bonds. Reproduced with permission from ref. 32, copyright 2024, Dalton Trans. | ||
The absorption spectra of the yellow-colored alkaline SD solution showed two peaks at 330 and 373 nm, which were attributed to the phenolate and quinone forms. The quinone form (λmax 381 nm) was the only form found in OSD-CuNPs, whereas the phenolate form (λmax 330 nm) entirely disappeared. OSD-CuNP formation significantly reduced the absorbance at 381 nm (Table 2).
| Salicylaldehyde-capped nanoparticles | Synthesis conditions | λ ex and λem (nm) | Size (nm) | Shape | Oxidation state | Lattice spacing (nm) |
|---|---|---|---|---|---|---|
| AgNPs8 | Room temperature | 290 and 424 | 90 | Aggregated | Zero | 0.232 |
| AgNPs31 | UV light | 290 and 424 | — | Aggregated | Zero | 0.236 |
| CuNPs7 | Room temperature | 290 and 420 | — | Aggregated | Zero | 0.20 |
The values of 2θ at 38°, 44°, 64°, and 77° correspond to (111), (200), (220), and (311) for zero-valent Ag in OSD-AgNPs, respectively.8 The TEM image reflected an aggregated structure, while the HR-TEM image indicated a zero valent lattice fringe (Fig. 7).
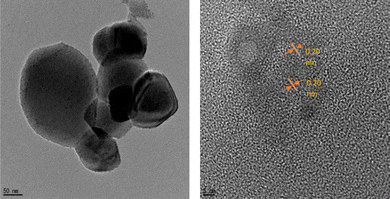 | ||
| Fig. 7 TEM and HRTEM digital images of CuNPs. Reproduced with permission from ref. 7, copyright 2024, New J. Chem. | ||
XPS and lattice fringes both show that Ag is in the zero-oxidation state. The (111) plane of Ag0 was indicated by a fringe spacing of 0.236 nm. Binding energies of 365.81 and 371.82 eV corresponded to Ag0 3d5/2 and Ag0 3d3/2 respectively.31
5. Mono-iminic Schiff base
Schiff bases resemble aldehyde or ketone derivatives but have an imine or azomethine group instead of the carbonyl group.82 First described by Hugo Schiff in 1864, Schiff bases are adaptable ligands produced by condensing an amino molecule with carbonyl compounds.83,84 Schiff bases are often formed by heat, acid or base catalysis, or both. The crystalline solids that are the typical Schiff bases are weakly basic, but some may also form insoluble salts with strong acids.84 Usually, a condensation reaction between an aldehyde or ketone (also called the carbonyl component) and a primary or secondary amine produces Schiff bases. A carbon–nitrogen double bond, which is the defining property of Schiff bases, is formed during the condensation reaction due to the amine group's nucleophilic assault on the carbonyl carbon of the aldehyde or ketone. R1(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)R2, where R1 and R2 are organic groups, can be used to illustrate the overall structure of a Schiff base. One important structural component is the double bond between carbon and nitrogen in the center. Schiff bases can have several isomeric configurations, such as E/Z isomers, contingent on the configuration of substituents surrounding the carbon–nitrogen double bond.85 Schiff bases are susceptible to tautomerism, a phenomenon in which the nitrogen's hydrogen atom migrates to the carbon atom, producing distinct structural isomers.86
N)R2, where R1 and R2 are organic groups, can be used to illustrate the overall structure of a Schiff base. One important structural component is the double bond between carbon and nitrogen in the center. Schiff bases can have several isomeric configurations, such as E/Z isomers, contingent on the configuration of substituents surrounding the carbon–nitrogen double bond.85 Schiff bases are susceptible to tautomerism, a phenomenon in which the nitrogen's hydrogen atom migrates to the carbon atom, producing distinct structural isomers.86
Schiff bases are frequently employed in nanoparticle production. These substances are adaptable and can function as stabilizing agents or ligands throughout the nanoparticle manufacturing process.87 Schiff bases are frequently used in the formation of nanoparticles because of their capacity to chelate metal ions. Metal nanoparticle production and stabilization are aided by this chelation process. Metal ions and the Schiff base may coordinate, resulting in the reduction and nucleation of metal nanoparticles. Schiff bases can also function as capping or reducing agents, which can affect the stability, size, and form of the resultant nanoparticles.88 Applications for synthesizing nanoparticles with Schiff bases include drug delivery, sensing, catalysis, and other areas of nanotechnology. Scholars frequently investigate various Schiff bases and their metal complexes to customize the characteristics of nanoparticles for certain uses.88
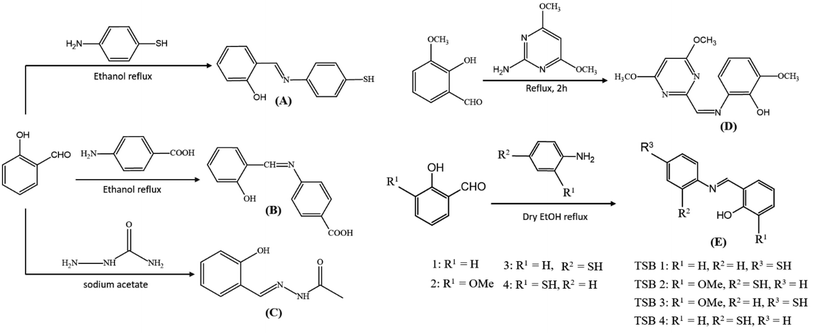
5.1 Synthesis of salicylaldehyde-derived mono-iminic Schiff bases
Ample Schiff bases are synthesized via condensation reaction with amines. Fig. 8 displays schematic representations of mono-iminic Schiff bases, reported for passivating CNPs.5.2 Design of mono-iminic Schiff base capping on CNPs
| Capping agent | Reducing agent | Salt precursor | Conditions | NPs | Shape | Size (nm) |
|---|---|---|---|---|---|---|
| Thiolated Schiff base ligand89 (A) | Trisodium citrate | HAuCl4·3H2O | Room temperature | AuNPs | Spherical | 8–10 |
| 4-((2-Hydroxybenzylidene) amino) benzoic acid91 (B) | Maltose sugar | AgNO3 | 60 °C | AgNPs | Spherical | 6–45 |
| Semi-carbazone oxidized form (C)92 | Semi-carbazone | AgNO3 + CuSO4 | Aged for 8 h | Ag–Cu hydrosol | Aggregated spheroid | ∼30 |
| 2-((4,6-Dimethoxypyrimidine-2-yl)methyleneenamino)-6-methoxyphenol (DPMM)93 (D) | NaBH4 | HAuCl4·3H2O | Modified Brust–Schiffrin method | AuNPs | Granular | 38.14 ± 4.5 |
| 2-(((4-Mercaptophenyl)imino)methyl)phenol (TSB 1)94 (E) | NaBH4 | AgNO3 | Room temperature with N2 gas | AgNPs | Spherical | <40 |
| 2-(((2-Mercaptophenyl)imino)methyl)-6-methoxyphenol (TSB 2)94 (E) | NaBH4 | AgNO3 | Ice bath with N2 gas | AgNPs | — | — |
| 2-(((4-Mercaptophenyl)imino)methyl)-6-methoxyphenol (TSB 3)94 (E) | NaBH4 | AgNO3 | Room temperature with N2 gas | AgNPs | — | — |
| 2-(((2-Mercaptophenyl)imino)methyl)phenol (TSB 4)94 (E) | NaBH4 | AgNO3 | Ice bath with N2 gas | AgNPs | — | — |
| Cinnamaldehyde90 (F) | Starch | AgNO3 | 60 °C, pH 13 | AgNPs | Spherical | 5–10 |
| Chitosan-SD Schiff base95 (G) | Chitosan-SD Schiff base | AgNO3 | 60 °C with magnetic stirring continued for 12 h | AgNPs | Spherical | 5 to 20 nm |
5.3 Manipulation of metal-enhanced fluorescence
Sharma et al.92 reported MEF involving SC (a mono-iminic Schiff base). Through a sequence of redox reactions and energy transfer processes, the interaction between CuO and Ag nanoparticles in an alkaline SC solution could result in significant MEF. Cu2+ ions first formed chelates in an alkaline SC solution, which quenched the fluorescence of SC. Electrons go from a full orbital in the ligand to an empty orbital in the metal centers as a result of ligand-to-metal charge transfer, or LMCT, which causes this quenching. High-valent metal centers like Cu(II), which contain low-energy empty d orbitals appropriate for LMCT, are commonly involved in this transition. Because electron-rich ligands (such as halides and carboxylates) make it easier for electrons to transfer to the metal, LMCT states are more likely to occur. This bond dissociation causes non-radiative decay processes.Cu2+ ions were reduced to Cu0 and then oxidized to generate CuO nanoparticles over an 8 hour aging period. At the same time, SC oxidized to quinone (OSC). Fluorescence quenching was caused by energy trapping between the fluorophore (OSC) and CuO nanoparticles upon excitation with 290 nm photons. Heat energy, which was not radiative, was created from this stored energy. This quenching was facilitated by the “lossy surface waves” phenomena that Lakowicz described.96 Owing to a mismatch in the wavevector, generated electron oscillations could not radiate effectively, giving rise to these waves. Ag0 nanoparticles were developed in the CuO-OSC system when Ag+ was added. Cu0 and CuO were also formed. Due to the lightning rod effect, the Ag0 nanoparticles, which had plasmon bands, released the energy that was previously held at 420 nm, greatly increasing the fluorescence. As a result, the quantum yield increased to 6.5% as compared to quinine sulfate. Strong MEF was produced when Cu and Ag nanoparticles were present together because they efficiently transmitted energy from the fluorophore to the metallic nanoparticles. This system's heightened fluorescence was a result of the synergy between Ag and CuO nanoparticles (Fig. 10).92
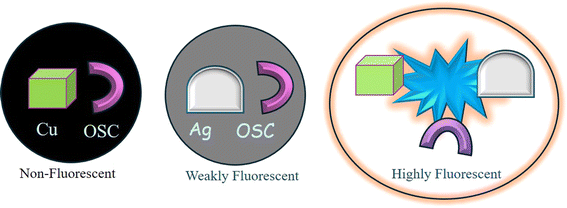 | ||
| Fig. 10 Synergism of Cu and Ag to generate strong metal-enhanced fluorescence.92 Reproduced with permission from ref. 92, copyright 2024, Appl. Nanosci. | ||
5.4 Applications of salicylaldehyde derived mono-iminic Schiff base capped coinage metal nanoparticles
5.4.1.1 Colorimetric pathway.
5.4.1.1.1 Ag+ sensing. The addition of Ag+ to a solution of SC caused a visible color change, along with the formation of AgNPs. At a high concentration of Ag+ (10−2 M), the yellow color of SC turned white with the formation of a precipitate. At a lower concentration of Ag+ (10−3 M), the solution turned a distinct red color. Other metal ions tested (Na+, K+, Pb2+, Hg2+, Zn2+, Cu2+, Fe3+, Ba2+, Cd2+, Co3+, Al3+) at the same concentration (10−3 M) did not cause any significant color change. This indicated good selectivity of SC for Ag+ detection. The red color arose from two factors. The plasmonic band of AgNPs formed by the interaction of Ag+ and SC exhibited a specific light absorption pattern called the plasmonic band. This band typically appears in the red region for aggregated AgNPs. Aggregation of AgNPs in the presence of SC further intensified the red color in the plasmonic band.92
5.4.1.2 Fluorometric pathway.
5.4.1.2.1 Fe3+ sensing. Emissive Schiff base ligand-capped AuNPs showed quenching in the presence of Fe3+. No other metal ions (Al3+, Ni2+, Cu2+, Co2+, Hg2+, Zn2+, Na+, K+, Ca2+, and Rb+) showed such type of quenching. The quenching was attributed to chelation-enhanced quenching because Fe3+ is paramagnetic. Because Fe3+ has a strong thermodynamic affinity for phenolic-C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –OH groups—a combination of the oxygen of the phenol ring and the imino nitrogen of the amine—the AuNP chemosensor showed selectivity for Fe3+ ions. The great propensity of phenol to deprotonate during complex formation resulted in the development of this hybrid, which had quick metal-to-ligand binding kinetics, not achievable with other transition-metal ions. For Fe3+, the AuNP estimated detection limit was 1.2 μM, and the fluorescence turn-on method outperformed the switch-off method. Furthermore, a 1
N and –OH groups—a combination of the oxygen of the phenol ring and the imino nitrogen of the amine—the AuNP chemosensor showed selectivity for Fe3+ ions. The great propensity of phenol to deprotonate during complex formation resulted in the development of this hybrid, which had quick metal-to-ligand binding kinetics, not achievable with other transition-metal ions. For Fe3+, the AuNP estimated detection limit was 1.2 μM, and the fluorescence turn-on method outperformed the switch-off method. Furthermore, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode for Fe3+ was shown by the absorption and emission spectrum analyses, with robust binding constants of 2.9 × 105 M−1 and 8.5 × 105 M−1, respectively. The AuNP chemosensor had a high selectivity for Fe3+ ions, which made it useful for detecting Fe3+ in a variety of applications, particularly fluorescence sensing.89
1 binding mode for Fe3+ was shown by the absorption and emission spectrum analyses, with robust binding constants of 2.9 × 105 M−1 and 8.5 × 105 M−1, respectively. The AuNP chemosensor had a high selectivity for Fe3+ ions, which made it useful for detecting Fe3+ in a variety of applications, particularly fluorescence sensing.89
5.4.1.2.2 Ag+ and Cu2+ dual sensing. (E)-1-((Pyridin-4-yl)methylene),97 1-(4-(pyridine-4-yl)benzylidene)thiosemicarbazide,98 and L-cysteine capped CdTe quantum dots99 were used for the fluorimetric and/or colorimetric sensing of silver and copper simultaneously.
Cu2+ showed quenching in the presence of emissive SC (a Schiff base synthesized from semicarbazide and SD) due to the formation of copper oxide nanoparticles in situ. With a limit of detection of 13 μM, this chemical showed a linear detection range from 10−4 to 5 × 10−8 M. Copper oxide nanoparticles were formed as a result of the complexation–reduction technique, used to achieve the detection. Moreover, the addition of Ag+ ions to the solution produced Ag0 and Cu0 nanoparticles, which had a much higher fluorescence. This made it possible to detect Ag+ ions with a linear detection range of 10−3 to 10−7 M and a detection limit of 7.7 μM. This made the detection of Ag+ ions sensitive and selective. Using turn-on/off fluorescence, the study showed how to detect Cu2+ and Ag+ ions simultaneously in a single reaction vessel.92
Ganguly et al. reported102 the antibacterial activity of gold core–silver shell giant nanoclusters, applied to form antibacterial cotton and papers. Al-Hakkani103 reviewed the antibacterial activity of copper-based nanoparticles. Tang and Zheng100 and Mobed104 also reviewed antibacterial activity from silver and gold nanoparticles. Here we concentrated on the antibacterial activity of coinage metal nanoparticles passivated with SD.
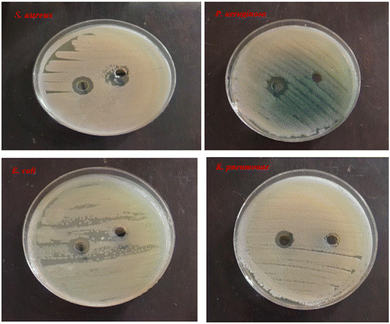
Antibacterial and antioxidant studies of DPMM indicated that DPMM-AuNPs were more active than DPMM. In vitro, anticancer results showed that the DPMM-AuNPs had significant cytotoxic activity against cancer cell lines and the least toxic effect on the normal cell line.93 AgNPs were tested against S. aureus, P. aeruginosa, K. pneumoniae, and E. coli and found to have significant inhibitory activity against these bacteria species (Fig. 11).91
5.5 Fate of coinage metals, passivated with salicylaldehyde-derived monoiminic Schiff bases
SD-derived Schiff base (A) capped AuNPs existed in a zero-oxidation state. The peaks in the XRD pattern at 2θ = 38.2, 44.4, 64.5, 77.5, and 81.7° were the same as those documented in the literature and correlated to the (111), (200), (220), (311), and (222) planes in the AuNPs. The TEM image displayed spherical particles that were evenly dispersed and had an average diameter of 8–10 nm.89 TEM analysis results show that the particle size of the DPMM-AuNPs was found to be approximately 38.14 ± 4.5 nm.93Semicarbazide and SD were condensed to produce SC, an imine derivative. SC's FTIR spectrum was captured. The O–H bond's symmetric and asymmetric stretching vibrations were represented by the band at 3168 cm−1. The existence of a band at 1696 cm−1 suggested that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond had several bending vibration modes. Different modes of the N–H bond were indicated by the existence of a band at 3478 cm−1.
O bond had several bending vibration modes. Different modes of the N–H bond were indicated by the existence of a band at 3478 cm−1.
Cu and Ag were clearly in a zero oxidation state in CuSCAg92 based on the XPS spectrum. Cu's zero oxidation state was confirmed by the binding energies of Cu, which were 932.56 eV and 962.40 eV, respectively, with Cu 2p3/2 and Cu 2p5/2. In the zero oxidation state, Ag binding energies of 374.15 eV and 368.12 eV were confirmed by Ag 3d3/2 and Ag 3d5/2, respectively. Nonetheless, zero-valent silver and CuO were detected in the XRD pattern. Surface oxidation of zero-valent copper in CuOSCAg was most likely detected by X-ray radiation. The 2θ values for CuO nanoparticles were 32.36°, 35.42°, 38.1°, 48.82°, and 61.52°, representing (110), (111), (200), (202), and (311), respectively; for Ag0 in the CuOSCAg hydrosol, the 2θ values were 38.12°, 44.18°, 64.46°, and 77.42°.92
6. Diiminic Schiff base (DSB)
DSB, such as salen and salen-like compounds, are derived from diamines and SD. These days, a lot of emphasis is focused on the chemistry of salen due to its many uses. Nowadays, “salen” is a sophisticated term in synthetic chemistry. It's been a long trip since Pfeiffer first reported “salen” in 1933. Salen and its derivatives have been widely used in synthetic, analytical, and biological chemistry.19Condensation products of SD and different diamines constitute an important class of diiminic Schiff bases (DSBs). This class of compounds has been rediscovered as reducing as well as capping agents. Two iminic bonds (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) are present in the DSB. DSBs are the result of a condensation process involving one diamine and two SD molecules. A process when a small molecule, such as water or methanol, is lost at the same time that two or more molecules join to produce a larger molecule is called a condensation reaction. Salen is produced by condensing SD and ethylenediamine. It has been demonstrated that these salen-like molecules are an excellent candidate for coinage metal-enhanced fluorescence.12,105 Different DSBs were formed by adjusting the diamines, or the distance between two imines while maintaining a constant aldehyde. Variable spacers were present in these DSBs between two iminic linkages. Six distinct DSBs were made from SD and various diamines.12,105
N–) are present in the DSB. DSBs are the result of a condensation process involving one diamine and two SD molecules. A process when a small molecule, such as water or methanol, is lost at the same time that two or more molecules join to produce a larger molecule is called a condensation reaction. Salen is produced by condensing SD and ethylenediamine. It has been demonstrated that these salen-like molecules are an excellent candidate for coinage metal-enhanced fluorescence.12,105 Different DSBs were formed by adjusting the diamines, or the distance between two imines while maintaining a constant aldehyde. Variable spacers were present in these DSBs between two iminic linkages. Six distinct DSBs were made from SD and various diamines.12,105
6.1 Salicylaldehyde-derived diiminic Schiff bases for the evaluation of coinage metal nanoparticles
DSB compounds were insoluble and dissolved in an alkaline aqueous solution. HAuClO4, AgNO3, and CuSO4 were introduced individually under different experimental conditions under UV light (365 nm) exposure in the alkaline aqueous medium of DSB. As a result, pale yellow-colored copper nanoparticles, wine-red colored gold nanoparticles, and yellowish-brown colored silver nanoparticles were produced.12,106 DSBs were utilized to stabilize nanoparticles.Different diamines and SD were used to create different Schiff bases. Triethylenetetramine was condensed with SD to form another Schiff base. The Schiff base and the appropriate metal salts were used to form coinage metal nanoparticles (Cu, Ag, and Au) in an aqueous alkaline solution. AgNPs and AuNPs did not require UV light to be developed, while CuNPs did. During the production of the nanoparticles, the Schiff base underwent oxidation to produce an oxidized condensation product (OCP), which served as a fluorophore and stabilizer for the nanoparticles.
The DSB family of compounds was found to be effectively capping and reducing agents under UV light. Ethylenediamine was used in the synthesis of C1, or salen. Similarly, 1,3-propylenediamine, 1,4-butanediamine, o-phenylenediamine, m-phenylenediamine, and p-phenylenediamine were used in place of ethylenediamine to produce C2 (salprn), C3 (salben), C4, C5, and C6. To form the Schiff base C1 (salen), ethylenediamine and methanolic SD react with condensation. Using other diamines, identical steps were taken to produce the additional Schiff bases (C2 to C6).19 AgNO3 in an alkaline solution oxidized the DSB to produce stable Ag0 at room temperature under 1 h of 365 nm UV light. At λem of 412 nm, the solution displayed strong AgNP enhanced fluorescence with an emission wavelength.105–107 Ionic copper salt in an alkaline solution oxidized the DSB to produce stable Cu0 at room temperature under 9 h of 365 nm UV light. At λem of 429 nm, the solution displayed strong CuNP enhanced fluorescence with an emission wavelength (Fig. 12).105
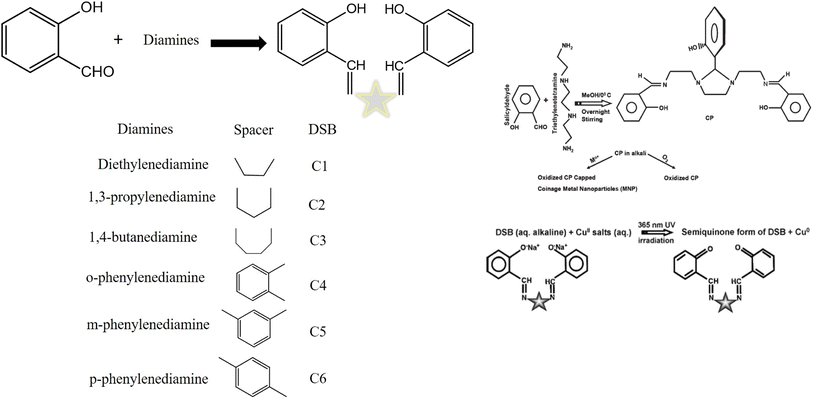 | ||
| Fig. 12 Distinct diamines and salicylaldehyde-derived synthetic DSBs105 and the mechanism of the formation of metal nanoparticles.19 | ||
6.2 Manipulation of metal-enhanced fluorescence
The type of spacer between the two iminic bonds in the DSB affected the fluorescence behavior of the CNPs. It was discovered that DSBs had a great candidacy for MEF. Because they were hydrophobic organic molecules, DSBs were insoluble in water. In situ generated metal nanoparticles with oxidized DSB as the fluorophore showed interesting fluorescence behavior. The metals and spacer were required for both quenching and amplification of the fluorescence. For this reason, such MEF was utilized to identify chemical molecules and environmentally hazardous metal ions.12In the case of DSB-capped AuNP fluorescence behavior,19 a radiation-free transfer of the fluorophore's excitation energy to the quencher molecule was observed. While the photoexcited electrons of the quinones returned to the ground state, the electrons of the quencher Au0 went to a higher energy state. The Au0 molecule lost its obtained energy when it equilibrated with the solvent. Plotting the I0/I, where I0 is the fluorescence intensity of the free DSB following irradiation and I is the fluorescence intensity of the exposed DSB with varying Au0 concentrations, Stern–Volmer graphs depicted both the static and dynamic quenching. Compared to C2, the slope of these plots for C5 is steeper. It suggested a stronger interaction between quencher Au0 and C5. Compared to C2, the bigger size of C5 stabilized AuNPs had a lower surface area (or surface-to-volume ratio). For the latter, there should be a greater quenching magnitude. The observable fact, however, was the opposite. The two structures' stiffness was most likely the cause of this occurrence. C5 was not as flexible as C2. It was the presence of a meta-substituted phenyl ring that stopped free rotation between the C5's two iminic bonds. Conversely, the aliphatic chain that stretches between two iminic bonds is in charge of assuming different rotameric conformations, which resulted in reduced fluorophore contact with AuNPs. There was a strong correlation between the fluorescence intensity of the capping agent and the size of the nanoparticles. The surfaces of homogeneous colloids were not as useful for MEF as aggregates, according to Lakowicz et al.108 As a result, photoproduced AuNPs with a narrow size distribution quenched fluorescence by reducing the fluorophore's dipole oscillations.
On the other hand, DSB-capped AgNPs exhibited intriguing fluorescence behaviors. The “lightning rod effect” was responsible for the silver colloids' remarkable enhancement of fluorescence.109 AgNPs improved the rate of excitation by concentrating the local field on the fluorophore. The inherent radiative decay rate of a fluorophore, or how quickly it emits photons, may likewise be altered by the silver metal. Radiative decay engineering (RDE) is the process of linking the molecular dipole of the fluorophore with the metal's surface plasmon band to increase the rate of radiative decay and produce a higher quantum yield fluorescence emission. The capping agent's fluorescence intensity and the size of the NPs are highly correlated.110
When compared to just exposed DSB, exposed DSB solutions with in situ photoproduced AgNPs exhibited a significantly different fluorescence characteristic. Except for C5, all of the DSBs exhibited a sudden rise in fluorescence intensity after being exposed to silver nitrate for three hours. The intensity of fluorescence was enhanced to varying degrees by various DSBs. Fluorescence damping only happened for C5. AgNPs maximally increased the fluorescence intensity for C3. In this context, it was also clear that the amplification of fluorescence is noticeably modest and that, occasionally, quenching (C5) of fluorescence is also observed when two iminic bonds are separated by an aromatic group. DSBs are a novel class of fluorophores that exhibit metal-enhanced fluorescence in an alkaline aqueous solution. For the extreme variation in fluorescence behavior, the distance between the metal nanoparticles and the capping agent, or fluorophore, may be crucial. Nearby, metallic surfaces greatly damped fluorophore emission up to ∼50 Å. The probe experienced maximum enhancement at a distance of around 100 Å from the metallic surface. Sokolov et al.111 found that the quenching zone of the fluorophore (capping agent) occurs at a distance of approximately 600 Å. AuNPs are more likely to remain closer, while larger AgNPs are at a suitable distance from their capping agent, exhibiting enhanced fluorescence.
When the DSB was exposed to UV radiation in the presence of copper salt, the fluorescence (λem 429 nm) of each exposed reaction mixture, for example, C1, C2, C3, and C4, was dramatically increased, because in situ Cu0 was produced. Remarkably, the quenching phenomena were noted for C5 and C6. An enormous increase in emission intensity was seen when aliphatic groups (C1–C3) were present between the two iminic bonds. In contrast, when photoproduced Cu0 was present, an aromatic group positioned between the two iminic bonds showed a slight increase in fluorescence (C4) and occasionally quenching (C5 and C6). Thus, in the presence of CuNPs, generated in situ in the reaction mixture as a result of UV light irradiation, the spacer between the iminic bonds significantly tuned the fluorescence behavior of the exposed DSBs. This illustrated the extent of varying MEF in the presence of different spacers when CuSO4 was used. Of the DSBs, the C2-capped CuNPs had the highest fluorescence amplification, while the C6-capped CuNPs had the most effective quenching ability (Fig. 13).105
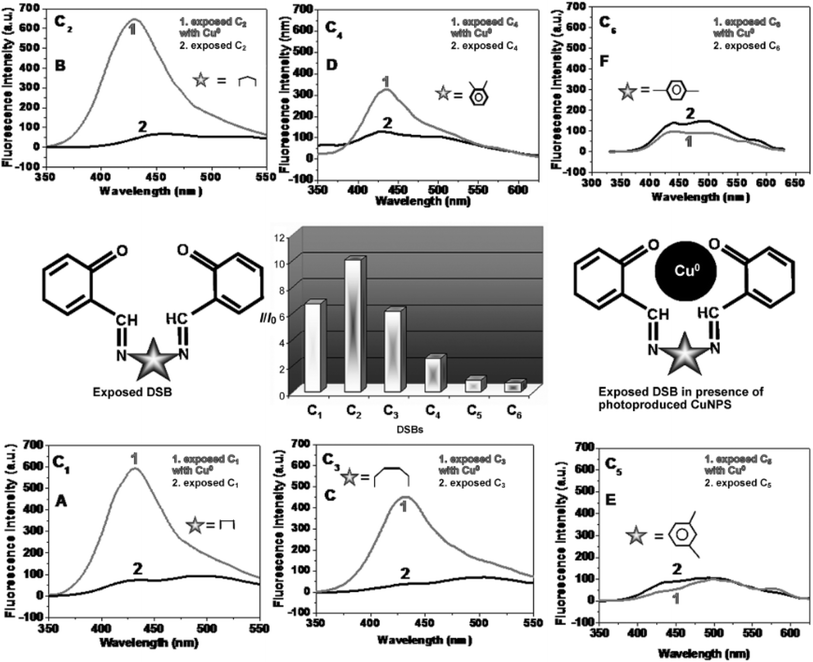 | ||
| Fig. 13 Fluorescence spectra (A–F) showing enhancement/quenching of exposed DSBs in the presence of photoproduced CuNPs. The bar diagram represents different degrees of enhancement from the exposed DSBs in the presence of photoproduced CuNPs (I = fluorescence intensity of exposed C2 in the presence of photogenerated CuNPs; I0 = fluorescence intensity of exposed C2 solution without copper). Reproduced with permission from ref. 105, copyright 2024, Chem.–Eur. J. | ||
6.3 Application
6.3.1.1 Fluorometric pathway.
6.3.1.1.1 Dopamine sensing. Dopamine (DA) is a catecholamine neurotransmitter that is recognized to be essential in the mammalian central nervous system. A deviation of the usual concentration of dopamine in the brain can lead to Parkinson's disease and other nervous system diseases.107,112 DA and melamine binding to AuNPs dominated their aptamer-based label-free colorimetric sensing.113 Rostami et al.114 designed a colorimetric sensor of DA using hexagonal AgNPs, decorated by the task-specific pyridinium-based ionic liquid.
Ganguly et al. reported on the use of DSB as a fluorophore in the chemosensory detection of dopamine. The fluorescence of DSB-AgNPs was selectively quenched, enabling the detection of DA at nanomolar concentrations. A variety of allied interfering molecules were investigated, including glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, 4-hydroxyproline, serine, threonine, tyrosine, valine, glucose, lactose, Zn2+, and K+. The DA detection range of 0–300 nM was observed. The LOD was determined to be 16 nM.107
As a result, the 3.64 ns lifetime component of the DSB-AgNP solution was changed to 3.97 ns for the DSB-AgNPs-DA solution. This increase in lifetime was an indication of the slightly decreased radiative decay rate. DA-induced fluorescence quenching was quite high, which signified that the decrease of radiative decay was not the major factor for quenching. Therefore, DA effectively trapped the plasmon (lossy surface wave) that radiated as heat and produced ∼10 nm AgNPs from highly aggregated microparticles in the DSB-AgNPs. As a result, the scattering cross section was greatly reduced (according to Mie theory) and efficient quenching was observed.107
6.3.1.1.2 Hg2+ sensing. One of the most hazardous elements is mercury, and it may be difficult to diagnose low mercury concentrations. Animals and plants as well as people are harmed by mercury. It is discovered that mercury contamination predominantly affects waterways. Gold mining is the main cause of mercury contamination in coastal seas globally, among other sources of the metal. Moreover, fossil fuels are a significant source. The Minamata convention included the formulation of strategies to use less mercury as well as emissions of mercury into the atmosphere.115,116
Sahu et al.117 provided an overview of ionic mercury sensing utilizing AgNPs and AgNCs, both calorimetrically and fluorometrically. Additionally, they demonstrated the need for detection, what happens to analytes and silver particles, how sensing works, actual sample analysis, spot detection for prototype applications, and the impact of adulteration. Kraithong et al. used a tripodal ligand connected to three 7-nitrobenzol-2-oxa-1,3-diazolyl (NBD) molecules (TNBD) to build a selective fluorescence silver-based sensor for Hg2+. This sensor demonstrated good Hg2+ selectivity against other interfering ions, with a LOD of 12.5 ppb.118 For the selective detection of Hg2+ and Cu2+, Zhang et al.119 developed bovine serum albumin-capped fluorescent bimetallic alloying gold–silver NCs.
Hg2+ was able to restore the quenched fluorescence (as was mentioned in the dopamine sensing paragraph above) of DSB-AgNPs, caused by DA selectively. Thirteen metal ions were examined in this context: Ba2+, Cd2+, Co2+, K+, Mg2+, Na+, Ni2+, Zn2+, Pb2+, Mn2+, Cu2+, Fe2+, Fe3+, and Hg2+. Hg2+ alone restored fluorescence due to the complex formation with dopamine. Other interfering basic radials, such as Ba2+, Cd2+, Co2+, K+, Mn2+, Na+, Ni2+, Zn2+, and Pb2+, when added separately to AgNPs-dopamine, decreased fluorescence further, while Mn2+, Cu2+, Fe2+, and Fe3+ had no influence.107
6.3.1.1.3 Ag+ sensing. After exposure to UV light for one hour in an AgNO3 + DSB solution, the DSB was oxidized to its semiquinone form. In the vicinity of the oxidized DSB, in situ-produced Ag nanostructures exhibited very efficient MEF. Various basic radicals (Na+, K+, Cu2+, Hg2+, Pd2+, Co3+, Cd2+, Ba2+, and Au3+) were used in place of Ag+ in the same experiment. Ag+ was the only metal ion that might increase fluorescence. Therefore, silver in an aqueous medium can be detected using DSB as a platform.
It was possible to attain different detection ranges by adjusting the DSB concentrations. The detection ranges were determined to be 0–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM, 0–250
000 nM, 0–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM, 0–66
000 nM, 0–66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nM, and 0–22
000 nM, and 0–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 nM, respectively, at concentrations of 2.86 × 10−4 M, 1.43 × 10−4 M, 1.43 × 10−5 M, and 1.43 × 10−6 M.106
400 nM, respectively, at concentrations of 2.86 × 10−4 M, 1.43 × 10−4 M, 1.43 × 10−5 M, and 1.43 × 10−6 M.106
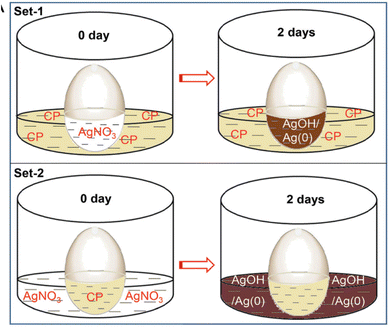
Ganguly et al.106 pierced a small hole (0.5 mm diameter) on the pointed edge of a chicken egg, to remove the egg white and yolk. Both the interior semipermeable membrane and the hard egg wall remained intact. Triple-distilled water was used to repeatedly clean the inside of the empty egg membrane. After that, the portion of the hard exterior CaCO3 shell was dissolved from the blunt end in mild HCl to expose the semipermeable membrane within for diffusion testing. The egg membrane and the residual hard shell were retained in a beaker filled with an aqueous alkaline CP solution. Next, the egg cavity was filled with AgNO3 (set 1, quantity of solution). The eggshell with the membrane was then placed in a beaker for a second testing session. This arrangement involved placing the CP solution in the egg cavity and the AgNO3 solution in the beaker (set 2, with the amount of silver being significantly more than CP). For hydrostatic pressure equalization, the solutions (in the hollow and the beaker) stayed at roughly the same height in all of these cases. After three hours, the generation of silver hydroxide caused the colorless silver nitrate solution (sets 1 and 2: internal solution inside the egg membrane and external solution outside the egg membrane) to become blackish brown. After two days, the light yellow solution outside the membrane exhibited less fluorescence intensity than the brown fluid inside the set 1 egg. Set 2 yielded a dark brown solution with reduced light intensity inside the membrane and no fluorescence outside due to the exceptionally high silver concentration. This demonstrated that the silver ion diffused more slowly through the membrane.12,106
The experiment of Ganguly et al. demonstrated a useful application of silver-enhanced fluorescence in detecting trace levels of silver ions and provided insightful information on the diffusion dynamics of various compounds through semi-permeable membranes. The color variations in the solutions indicated that the silver ions and the CP underwent chemical reactions. The observed color changes were caused by the creation of silver hydroxide, and the intensity of the fluorescence provided information about the concentration and distribution of silver ions. Variations in the amounts of silver ions and CP were suggested by the differences in fluorescence intensity between the solutions inside and outside the egg membrane. The heightened fluorescence observed within the membrane indicated effective CP transport and subsequent interaction with trace silver ions. The experiment showed how silver-enhanced fluorescence could be used to detect traces of silver in biological systems. Silver ion detection and quantification, even at low concentrations, had potential applications in biological research and environmental monitoring, among other domains106 (Fig. 14).
 | ||
| Fig. 14 An illustration of the permeation experiment showing how easily the alkaline CP passed through the egg membrane. Reproduced with permission from ref. 106, copyright 2024, Dalton Trans. | ||
6.4 Fate of DSB-capped coinage metal nanoparticles
Ag0 3d5/2 and 3d3/2 were represented by the binding energies of 366.21 eV and 372.16 eV, respectively. The peak shift for Ag0 towards a lower binding energy indicated strict OCP capping. Similarly, Cu0 2p3/2 and Cu0 2p1/2 were represented by the binding energies of 932.74 eV and 952.77 eV. In support of their zero oxidation state, the lattice fringes of AgNPs and CuNPs were measured to be 0.234 and 0.211 nm, respectively [for the (111) plane]. The zero oxidation state of gold was confirmed by the XPS spectra (binding energies of 81.58 eV and 85.19 eV, respectively, corresponding to Au0 4f7/2 and Au0 4f5/2), as well as the lattice fringe (0.236 nm for the (111) plane).106The photoproduced CuNPs capped with exposed C2 had spherical sizes ranging from 3 to 6 nm. Copper was in a zero oxidation state. The peaks at 932.66 and 952.72 eV were assigned to be 2p3/2 and 2p1/2, respectively.105 Ag+ was reduced to Ag0, as shown by XPS measurements, XRD, and lattice fringes. The HRTEM scan yielded a lattice fringe of 0.236 nm, which was consistent with the Ag0 (111) plane. Ag0 3d5/2 and Ag0 3d3/2 were confirmed by ∼368.14 and 374.12 eV binding energies, respectively (Fig. 15).107
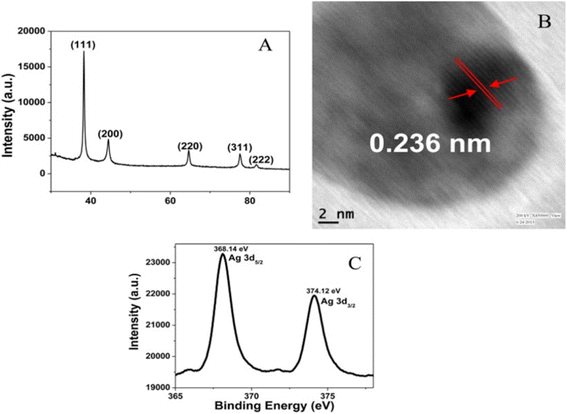 | ||
| Fig. 15 (A) Powder XRD pattern, (B) lattice fringe, and (C) XPS spectra for the element Ag of DSB capped AgNPs. Reproduced with permission from ref. 107, copyright 2024, Langmuir. | ||
7. Salicylaldehyde-decorated coinage metal nanoclusters
Formally, a metal particle having a diameter of less than 2 nm is called a “nanocluster” (NCs). NCs have shown notable catalytic activity and unique selectivity in several catalytic processes because of their incredibly small size, abundance of unsaturated active sites, and unique electronic structure that varies from that of ordinary NPs. More importantly, because of their distinct structure and monodispersity, they act as model catalysts to illustrate the connection between atomic-scale structure and catalytic activity. With dimensions typically under 2 nm, noble metal NCs such as silver and gold have attracted attention due to their unique capacity to bridge the “gap” between atomic and NP behavior. Recent research focusing on their quantum electronic properties includes studies on ferromagnetism, chirality, quantum behavior, single molecule optoelectronics, photoluminescence (PL), bioassay, and sensing. The particle size of NCs is smaller than the exciton Bohr radius, similar to semiconductor quantum dots with tight confinement of the quantum-size range (CdSe quantum dots are 4–5 nm in size).102,120,121The literature contains few reports on SD-conjugated nanoclusters: lysozyme-stabilized AuNCs122 and trypsin-stabilized AuNCs.123 The red-emitting AuNCs were stabilized by lysozyme, and the trypsin-stabilized AuNCs rapidly changed to yellow and green after the addition of SD. This was due to the formation of an imine linkage between the –CHO group of SD and the –NH2 groups of the lysozyme and trypsin coated on the surface of AuNCs. Zn2+ ions could be detected at concentrations as low as 1.37 × 10−7 mM in SD-Lyso-AuNCs, which showed a selective turn-on fluorescence increase at 452 nm. On the other hand, SD-Tryp-AuNCs interacted with Cd2+ preferentially and showed a fluorescence enhancement at 660 nm (with a detection limit of 98.1 nM). Sang et al.124 developed a Schiff base (an aldimine, formed between the aldehyde group of SD and the amino groups of methionine) fluorescent probe based on methionine-protected gold nanoclusters for the detection of Al3+. In the literature, no report is available regarding copper and silver nanoclusters involving SD capping.
The TEM analysis of well-dispersed SD-Tryp-AuNCs revealed that the particles were spherical with a size of approximately 4.8 ± 0.95 nm. The XPS analysis of the SD-Tryp-AuNC survey file revealed peaks for the Au, C, and O atoms. The deconvolution of the C 1s peak produced four binding energy peaks at 284.3, 285.4, 287.7, and 288 eV. The deconvoluted C 1s peaks were attributed to C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl), COO–, and C
O (carbonyl), COO–, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionalities, which supported the Schiff base formation between functionalized Tryp and SD. The two peaks at 86.9 eV (Au 4f5/2) and 83.3 eV (Au 4f7/2) were attributed to the presence of Au metal.123
N functionalities, which supported the Schiff base formation between functionalized Tryp and SD. The two peaks at 86.9 eV (Au 4f5/2) and 83.3 eV (Au 4f7/2) were attributed to the presence of Au metal.123
8. Detection of salicylaldehyde
SD is widely used in the chemical industry and environmental processes, making its detection crucial. Naturally, SD is present in buckwheat (Filipendula vulgaris) as an aroma component.123Liu et al.125 synthesized bovine serum albumin-protected gold nanoclusters (BSA-AuNCs) for the detection of SD. In the presence of fluorescent BSA-AuNCs SD showed enhancement, while no other competitive aldehyde (benzaldehyde, p-nitrobenzaldehyde, and p-dimethylaminobenzaldehyde, and aliphatic aldehydes, such as formaldehyde, glutaraldehyde, heptaldehyde, and paraformaldehyde) showed such type of enhancement at 500 nm at the same concentration. This finding indicated that the fluorescence switching technique had high selectivity for SD (LOD 0.19 Mm). SD interacted with the BSA-AuNCs, causing two things to happen: the formation of a fluorescent Schiff base due to increased fluorescence, and fluorescence quenching of AuNCs.
9. Conclusions and future perspective
A key component in the production of coinage metal nanoparticles with a variety of environmental applications is salicylaldehyde and its derivatives. These nanoparticles' special qualities make them useful for environmental sensing, catalysis, and antibacterial activity. As studies continue, overcoming obstacles and guaranteeing the sustainability of these procedures will help salicylaldehyde-derived metal nanoparticles find broad applications in environmental technology. All things considered, the incorporation of environmentally friendly synthesis techniques and the adaptable character of these nanoparticles demonstrate their potential as powerful instruments for future environmental concerns.Salicylaldehyde and its monoiminic and diiminic Schiff bases are used for capping coinage metal nanoparticles. They are used extensively in various sensing applications. However, antibacterial activity is reported for monoiminic Schiff base capped coinage metal nanoparticles. Pure salicylaldehyde and salicylaldehyde-based diiminic Schiff base capped coinage metal particles have not been utilized so far for their antibacterial activity.
Generation of ultra-small nanoclusters with strong emissive properties (due to inter and intraband transition) is a challenge with finding suitable capping agents and experimental conditions. A few reports are available for gold nanocluster formation involving salicylaldehyde. However, copper and silver nanocluster formation with salicylaldehyde has not been found so far. Owing to the extraordinary demand for fluorescent nanoclusters in analytical and biological science, more research is warranted for the experimental manipulation of salicylaldehyde and its derivatives.
One weak fluorophore and a metal surface (nanoparticles) are needed for MEF, whereas in the case of a cluster, just one entity is needed for the emissive characteristic. Suitable research is required for both cases to enrich the fundamental concept. The position of –OH and –CHO in an aromatic ring is crucial for MEF. Meta-hydroxy benzaldehyde and para-hydroxy benzaldehyde are unable to generate MEF. For the case of diiminic Schiff bases the spacer between two iminic bonds is responsible for the extent of MEF. Thus, manipulation of MEF can be made possible for prototype applications.
A small molecule exhibiting MEF and manipulation of MEF with its derivatives are the pivotal focus of this review article. This paper is expected to be an asset for the young scientists venturing into the field of nanoscience and nanotechnology from analytical and biological points of view.
Data availability
No data was used for the research described in the article.Author contributions
Mamta Sahu: writing – original draft; Mainak Ganguly: writing – review & editing, conceptualization; Priyanka Sharma: review & editing.Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- K. A. Maher and S. R. Mohammes, Int. J. ChemTech Res., 2015, 8, 937–943 Search PubMed.
- M. Das, K. H. Shim, S. S. A. An and D. K. Yi, Toxicol. Environ. Health Sci., 2011, 3, 193–205 CrossRef.
- P. Khandel and S. K. Shahi, J. Nanostruct. Chem., 2018, 8, 369–391 CrossRef CAS.
- S. Campisi, M. Schiavoni, C. E. Chan-Thaw and A. Villa, Catalysts, 2016, 6, 1–21 CrossRef.
- Z. Niu and Y. Li, Chem. Mater., 2014, 26, 72–83 CrossRef CAS.
- Y. J. Zhuang, J. P. Qu and Y. B. Kang, J. Org. Chem., 2020, 85, 4386–4397 CrossRef CAS PubMed.
- P. Sharma and M. Ganguly, New J. Chem., 2023, 47, 7481–7485 RSC.
- M. Sahu, M. Ganguly and A. Doi, ChemistrySelect, 2023, 8, e202301017 CrossRef CAS.
- Y. Jeong, Y. M. Kook, K. Lee and W. G. Koh, Biosens. Bioelectron., 2018, 111, 102–116 CrossRef CAS PubMed.
- M. Sui, S. Kunwar, P. Pandey and J. Lee, Sci. Rep., 2019, 9, 1–14 CrossRef CAS PubMed.
- S. Y. Tee and E. Ye, Mater. Adv., 2021, 2, 1507–1529 RSC.
- P. Sharma and M. Ganguly, Int. J. Environ. Sci. Technol., 2023, 21, 3345–3364 CrossRef.
- N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395–3442 CrossRef CAS PubMed.
- M. Jin, G. He, H. Zhang, J. Zeng, Z. Xie and Y. Xia, Angew. Chem. Int. Ed., 2011, 50, 10560–10564 CrossRef CAS PubMed.
- M. Ganguly, J. Pal, S. Das, C. Mondal, A. Pal, Y. Negishi and T. Pal, Langmuir, 2013, 29, 10945–10958 CrossRef CAS PubMed.
- S. Sen and K. Sarkar, Microb. Drug Resist., 2021, 27, 616–627 CrossRef CAS PubMed.
- M. Sahu, M. Ganguly and A. Doi, J. Cluster Sci., 2024, 1–19 Search PubMed.
- K. B. Borisenko, C. W. Bock and I. Hargittai, J. Phys. Chem., 1996, 100, 7426–7434 CrossRef CAS.
- M. Ganguly, A. Pal and T. Pal, J. Phys. Chem. C, 2011, 115, 22138–22147 CrossRef CAS.
- I. Alata, R. Omidyan, M. Broquier, C. Dedonder and C. Jouvet, Chem. Phys., 2012, 399, 224–231 CrossRef CAS.
- A. I. H. Cholilalah and R. Arifin, J. Math. Chem., 1967, 10, 82–95 Search PubMed.
- T. Stoerkler, D. Frath, D. Jacquemin, J. Massue and G. Ulrich, Eur. J. Org Chem., 2021, 2021, 3726–3736 CrossRef CAS.
- A. Karawek, P. Mayurachayakul, T. Santiwat, M. Sukwattanasinitt and N. Niamnont, J. Photochem. Photobiol., A, 2021, 404, 112879 CrossRef CAS.
- S. Arumugam, B. Shankar and K. C. Mondal, Eur. J. Inorg. Chem., 2020, 2020, 4127–4136 CrossRef CAS.
- M. Durko-Maciag, G. Ulrich, D. Jacquemin, J. Mysliwiec and J. Massue, Phys. Chem. Chem. Phys., 2023, 15085–15098 RSC.
- P. Sharma, M. Ganguly and M. Sahu, Nanoworld J., 2023, 9, 487–495 Search PubMed.
- E. W. F Brühne, J. Chem. Educ., 2005, 82, 1770 CrossRef.
- N. R. K. Vilambi and D. -T. Chin, J. Electrochem. Soc., 1987, 134, 3074–3077 CrossRef CAS.
- Z. Zhao, L. Zhang, F. Li, X. Tang, Y. Ma, C. Wang, Z. Wang, R. Zhao and L. Wang, Catalysts, 2017, 7, 1–9 Search PubMed.
- M. Sahu, M. Ganguly and P. Sharma, Spectrochim. Acta, Part A, 2024, 311, 123981 CrossRef CAS PubMed.
- M. Ganguly, J. Pal, C. Mondal, A. Pal and T. Pal, Chem.–Eur. J., 2014, 20, 12470–12476 CrossRef CAS PubMed.
- M. Ganguly, J. Pal, C. Mondal, A. Pal and T. Pal, Dalton Trans., 2015, 44, 4370–4379 RSC.
- C. D. Geddes, J. Fluoresc., 2002, 12, 121–129 CrossRef.
- C. D. Geddes, Phys. Chem. Chem. Phys., 2013, 15, 19537 RSC.
- M. T. Yaraki and Y. N. Tan, Chem.–Asian J., 2020, 15, 3180–3208 CrossRef CAS PubMed.
- S. M. Fothergill, C. Joyce and F. Xie, Nanoscale, 2018, 10, 20914–20929 RSC.
- K. Aslan, I. Gryczynski, J. Malicka, E. Matveeva, J. R. Lakowicz and C. D. Geddes, Curr. Opin. Biotechnol., 2005, 16, 55–62 CrossRef CAS PubMed.
- A. Doi, M. Ganguly and M. Sahu, Adsorption, 2024, 1–28 Search PubMed.
- P. Sharma, M. Ganguly and M. Sahu, RSC Adv., 2024, 14, 11411–11428 RSC.
- S. M. Padre, S. Kiruthika, S. Mundinamani, Ravikirana, S. Surabhi, J. R. Jeong, K. M. Eshwarappa, M. S. Murari, V. Shetty, M. Ballal and S. C. Gurumurthy, ACS Omega, 2022, 7, 35023–35034 CrossRef CAS PubMed.
- F. Zhang, J. Lan, Y. Yang, T. Wei, R. Tan and W. Song, J. Nanopart. Res., 2013, 15, 1–10 Search PubMed.
- M. R. Willner and P. J. Vikesland, J. Nanobiotechnol., 2018, 16, 1–16 CrossRef PubMed.
- N. C. Pomal, K. D. Bhatt, K. M. Modi, A. L. Desai, N. P. Patel, A. Kongor and V. Kolivoška, J. Fluoresc., 2021, 31, 635–649 CrossRef CAS PubMed.
- F. Zarlaida and M. Adlim, Microchim. Acta, 2017, 184, 45–58 CrossRef CAS.
- D. K. Ban and S. Paul, Appl. Surf. Sci., 2018, 458, 245–251 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- C. Caro, P. M. Castillo, R. Klippstein, D. Pozo and A. P. Zaderenko, Silver Nanopart., 2010, 201–225 CAS.
- W. Chen, S. Cai, Q. Q. Ren, W. Wen and Y. Di Zhao, Analyst, 2012, 137, 49–58 RSC.
- G. He, F. Gao, W. Li, P. Li, X. Zhang, H. Yin, B. Yang, Y. Liu and S. Zhang, Anal. Methods, 2019, 11, 1651–1656 RSC.
- X. Lin, D. Xuan, F. Li, C. Liu, P. Fan, F. Xiao, H. Liang and S. Yang, Spectrochim. Acta, Part A, 2020, 229, 117894 CrossRef CAS PubMed.
- J. Sophia and G. Muralidharan, Mater. Res. Bull., 2015, 70, 315–320 CrossRef CAS.
- P. Sharma, M. Ganguly and M. Sahu, RSC Adv., 2024, 14, 14606–14615 RSC.
- J. Liu, Z. Z. Dong, C. Yang, G. Li, C. Wu, F. W. Lee, C. H. Leung and D. L. Ma, Sci. Rep., 2017, 7, 4–10 CrossRef PubMed.
- P. Prosposito, L. Burratti and I. Venditti, Chemosensors, 2020, 8, 1–29 CrossRef.
- S. Bothra, J. N. Solanki and S. K. Sahoo, Sens. Actuators, B, 2013, 188, 937–943 CrossRef CAS.
- V. D. Doan, T. L. Phan, V. T. Le, Y. Vasseghian, L. O. Evgenievna, D. L. Tran and V. T. Le, Chemosphere, 2022, 286, 131894 CrossRef CAS PubMed.
- S. K. Chandraker, M. Lal, R. Shukla, M. K. Ghosh and T. K. Ghorai, New J. Chem., 2019, 43, 18175–18183 RSC.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS.
- L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Coord. Chem. Rev., 2000, 205, 59–83 CrossRef CAS.
- H. T. Ratte, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
- Y. Wen, F. Xing, S. He, S. Song, L. Wang, Y. Long, D. Li and C. Fan, Chem. Commun., 2010, 46, 2596–2598 RSC.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416–3429 RSC.
- S. Singha, D. Kim, H. Seo, S. W. Cho and K. H. Ahn, Chem. Soc. Rev., 2015, 44, 4367–4399 RSC.
- P. K. Mehta, L. N. Neupane, S. H. Park and K. H. Lee, J. Hazard. Mater., 2021, 411, 125041 CrossRef CAS PubMed.
- M. She, Z. Yang, B. Yin, J. Zhang, J. Gu, W. Yin, J. Li, G. Zhao and Z. Shi, Dyes Pigm., 2012, 92, 1337–1343 CrossRef CAS.
- J. L. Bricks, A. Kovalchuk, C. Trieflinger, M. Nofz, M. Büschel, A. I. Tolmachev, J. Daub and K. Rurack, J. Am. Chem. Soc., 2005, 127, 13522–13529 CrossRef CAS PubMed.
- B. A. Makwana, D. J. Vyas, K. D. Bhatt, V. K. Jain and Y. K. Agrawal, Spectrochim. Acta, Part A, 2015, 134, 73–80 CrossRef CAS PubMed.
- V. K. Gupta, N. Mergu and L. K. Kumawat, Sens. Actuators, B, 2016, 223, 101–113 CrossRef CAS.
- X. Gao, Y. Lu, S. He, X. Li and W. Chen, Anal. Chim. Acta, 2015, 879, 118–125 CrossRef CAS PubMed.
- E. Weerapana, C. Wang, G. M. Simon, F. Richter, S. Khare, M. B. D. Dillon, D. A. Bachovchin, K. Mowen, D. Baker and B. F. Cravatt, Nature, 2010, 468, 790–797 CrossRef CAS PubMed.
- K. G. Reddie and K. S. Carroll, Curr. Opin. Chem. Biol., 2008, 12, 746–754 CrossRef CAS PubMed.
- R. Asghar, Y. Li, F. Huo and C. Yin, Chem. Biomed. Imaging, 2024, 2, 250–269 CrossRef CAS.
- Y. Wang, X. Wang, Q. Meng, H. Jia, R. Zhang, P. Zhu, R. Song, H. Feng and Z. Zhang, Tetrahedron, 2017, 73, 5700–5705 CrossRef CAS.
- R. Rajamanikandan, B. Aazaad, S. Lakshmipathi and M. Ilanchelian, Microchem. J., 2020, 158, 105253 CrossRef CAS.
- Z. Li, J.-T. Hou, S. Wang, L. Zhu, X. He and J. Shen, Coord. Chem. Rev., 2022, 469, 214695 CrossRef CAS.
- A. K. Singh, R. Kanchanapally, Z. Fan, D. Senapati and P. C. Ray, Chem. Commun., 2012, 48, 9047–9049 RSC.
- H.-J. Lee, K.-B. Lee, K.-C. Noh and Y.-S. Nam, Analyst, 2015, 140, 8209–8216 RSC.
- M. Ganguly, J. Jana, C. Mondal, A. Pal and T. Pal, Phys. Chem. Chem. Phys., 2014, 16, 18185–18197 RSC.
- M. Ganguly, C. Mondal, J. Jana, A. Pal and T. Pal, Langmuir, 2014, 30, 348–357 CrossRef CAS PubMed.
- W. Kang, X. Pei, C. A. Rusinek, A. Bange, E. N. Haynes, W. R. Heineman and I. Papautsky, Anal. Chem., 2017, 89, 3345–3352 CrossRef CAS PubMed.
- I. V. Anambiga, V. Suganthan, N. Arunai Nambi Raj, G. Buvaneswari and T. S. Sampath Kumar, Int. J. Sci. Eng. Res., 2013, 4, 710–715 Search PubMed.
- C. M. Da Silva, D. L. Da Silva, L. V. Modolo, R. B. Alves, M. A. De Resende, C. V. B. Martins and Â. De Fátima, J. Adv. Res., 2011, 2, 1–8 CrossRef.
- N. Saikumari, Mater. Today: Proc., 2021, 47, 1777–1781 CAS.
- N. R. Bader, Rasayan J. Chem., 2010, 3, 660–670 CAS.
- E. Raczuk, B. Dmochowska, J. Samaszko-Fiertek and J. Madaj, Molecules, 2022,(3), 787 CrossRef CAS PubMed.
- R. F. Martínez, M. Ávalos, R. Babiano, P. Cintas, J. L. Jiménez, M. E. Light and J. C. Palacios, Org. Biomol. Chem., 2011, 9, 8268–8275 RSC.
- C. S. Marvel and N. Tarköy, J. Am. Chem. Soc., 1958, 80, 832–835 CrossRef CAS.
- G. S. Bhagyasree, B. Nithyaja, V. N. Reena, K. Subin Kumar, T. Shilpa and R. Aswati Nair, J. Fluoresc., 2023, 33, 1927–1940 CrossRef PubMed.
- A. A. Jimoh, A. Helal, M. N. Shaikh, M. Abdul Aziz, Z. H. Yamani, A. Al-Ahmed and J. P. Kim, J. Nanomater., 2015, 2015, 101694 CrossRef.
- V. A. S. Kannaiyan, Easwaramoorthy, K. Kannan and V. Andal, Nanotechnol. Russ., 2020, 15, 828–836 CrossRef.
- F. A. Almashal, Z. T. Al-Abdullah and A. M. Jassem, Egypt. J. Chem., 2020, 63, 813–821 Search PubMed.
- P. Sharma, M. Ganguly and A. Doi, Appl. Nanosci., 2024, 14, 739–751 CrossRef CAS.
- M. Sankarganesh, P. Adwin Jose, J. Dhaveethu Raja, M. P. Kesavan, M. Vadivel, J. Rajesh, R. Jeyamurugan, R. Senthil Kumar and S. Karthikeyan, J. Photochem. Photobiol., B, 2017, 176, 44–53 CrossRef CAS PubMed.
- N. O. Mahmoodi, N. Aghajani and A. Ghavidast, J. Mol. Struct., 2017, 1128, 21–29 CrossRef CAS.
- S. S. Alharthi, T. Gomathi, J. John Joseph, J. Rakshavi, J. Annie Kamala Florence, P. N. Sudha and G. Rajakumar, J. King Saud Univ., Sci., 2022, 34, 102177 CrossRef.
- J. R. Lakowicz, Anal. Biochem., 2005, 337, 171–194 CrossRef CAS PubMed.
- M. Sahu, A. Kumar Manna, K. Rout, J. Mondal and G. K. Patra, Inorg. Chim. Acta, 2020, 508, 119633 CrossRef CAS.
- A. K. Manna, J. Mondal, R. Chandra, K. Rout and G. K. Patra, J. Photochem. Photobiol., A, 2018, 356, 477–488 CrossRef CAS.
- T. Gong, J. Liu, X. Liu, J. Liu, J. Xiang and Y. Wu, Food Chem., 2016, 213, 306–312 CrossRef CAS PubMed.
- S. Tang and J. Zheng, Adv. Healthcare Mater., 2018, 7, 1–10 Search PubMed.
- C. Su, K. Huang, H.-H. Li, Y. G. Lu and D. L. Zheng, J. Nanomater., 2022, 2020, 5616379 Search PubMed.
- M. Sahu, M. Ganguly and P. Sharma, Nanoworld J., 2023, 9, 496–504 Search PubMed.
- M. F. Al-Hakkani, SN Appl. Sci., 2020, 2, 505 CrossRef CAS.
- A. Mobed, M. Hasanzadeh and F. Seidi, RSC Adv., 2021, 11, 34688–34698 RSC.
- M. Ganguly, A. Pal, Y. Negishi and T. Pal, Chem.–Eur. J., 2012, 18, 15845–15855 CrossRef CAS PubMed.
- M. Ganguly, C. Mondal, J. Chowdhury, J. Pal, A. Pal and T. Pal, Dalton Trans., 2014, 43, 1032–1047 RSC.
- M. Ganguly, C. Mondal, J. Jana, A. Pal and T. Pal, Langmuir, 2014, 30, 4120–4128 CrossRef CAS PubMed.
- J. R. Lakowicz, C. D. Geddes, I. Gryczynski, J. Malicka, Z. Gryczynski, K. Aslan, J. Lukomska, E. Matveeva, J. Zhang, R. Badugu and J. Huang, J. Fluoresc., 2004, 14, 425–441 CrossRef CAS PubMed.
- I. Gryczynski, J. Malicka, Y. Shen, Z. Gryczynski and J. R. Lakowicz, J. Phys. Chem. B, 2002, 106, 2191–2195 CrossRef CAS PubMed.
- J. R. Lakowicz, Y. Shen, S. D'Auria, J. Malicka, J. Fang, Z. Gryczynski and I. Gryczynski, Anal. Biochem., 2002, 301, 261–277 CrossRef CAS PubMed.
- K. Sokolov, G. Chumanov and T. M. Cotton, Anal. Chem., 1998, 70, 3898–3905 CrossRef CAS PubMed.
- R. M. Wightman, L. J. May and A. C. Michael, Anal. Chem., 1988, 60, 769–779 CrossRef PubMed.
- X. Liu, F. He, F. Zhang, Z. Zhang, Z. Huang and J. Liu, Anal. Chem., 2020, 92, 9370–9378 CrossRef CAS PubMed.
- S. Rostami, A. Mehdinia, A. Jabbari, E. Kowsari, R. Niroumand and T. J. Booth, Sens. Actuators, B, 2018, 271, 64–72 CrossRef CAS.
- M. Liu, Q. Zhang, T. Maavara, S. Liu, X. Wang and P. A. Raymond, Nat. Geosci., 2021, 14, 672–677 CrossRef CAS.
- A. C. (Thanos) Bourtsalas and N. J. Themelis, Waste Manage., 2019, 85, 90–94 CrossRef CAS PubMed.
- M. Sahu, M. Ganguly and P. Sharma, RSC Adv., 2024, 14, 22374–22392 RSC.
- S. Kraithong, N. Chailek, J. Sirirak, K. Suwatpipat, N. Wanichacheva and P. Swanglap, J. Photochem. Photobiol., A, 2021, 407, 113064 CrossRef CAS.
- N. Zhang, Y. Si, Z. Sun, L. Chen, R. Li, Y. Qiao and H. Wang, Anal. Chem., 2014, 86, 11714–11721 CrossRef CAS PubMed.
- H. Huang, H. Li, J. J. Feng, H. Feng, A. J. Wang and Z. Qian, Sens. Actuators, B, 2017, 241, 292–297 CrossRef CAS.
- G.-X. Duan, Y.-P. Xie, Y.-L. Shen, X. Lu, J. Han and L.-P. Zhang, Mater. Chem. Front., 2020, 4, 2205–2222 RSC.
- V. Bhardwaj, T. Anand, H. J. Choi and S. K. Sahoo, Microchem. J., 2019, 151, 104227 CrossRef CAS.
- A. Tripathi, V. Bhardwaj and S. K. Sahoo, J. Fluoresc., 2023, 1–9 Search PubMed.
- H. Shi, Y. Zhao, F. Sang, T. Xiong, W. Wang and J. Pan, J. Fluoresc., 2022, 33, 177–184 Search PubMed.
- X. Liu, C. Fu, X. Ren, H. Liu, L. Li and X. Meng, Biosens. Bioelectron., 2015, 74, 322–328 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |