Open Access Article
Open Access ArticleNanomaterials for small diameter vascular grafts: overview and outlook
Nuoxin
Wang
*abcd,
Haoyuan
Wang
aef,
Dong
Weng
ad,
Yanyang
Wang
ad,
Limei
Yu
abc,
Feng
Wang
efg,
Tao
Zhang
abc,
Juan
Liu
abc and
Zhixu
He
*abcdh
aKey Laboratory of Cell Engineering of Guizhou Province, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou, China. E-mail: wangnuoxin@foxmail.com
bThe Clinical Stem Cell Research Institute, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou, China
cCollaborative Innovation Center of Chinese Ministry of Education, Zunyi Medical University, Zunyi 563003, Guizhou, China. E-mail: hzx@gmc.edu.cn
dThe First Clinical Institute, Zunyi Medical University, Zunyi 563003, Guizhou, China
eDepartment of Cardiothoracic Surgery, The Second Affiliated Hospital of Zunyi Medical University, Zunyi 563006, Guizhou, China
fThe Second Clinical Institute, Zunyi Medical University, Zunyi 563003, Guizhou, China
gDepartment of Cardiovascular Surgery, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou, China
hDepartment of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi 563003, Guizhou, China
First published on 6th November 2023
Abstract
Small-diameter vascular grafts (SDVGs) cannot meet current clinical demands owing to their suboptimal long-term patency rate. Various materials have been employed to address this issue, including nanomaterials (NMs), which have demonstrated exceptional capabilities and promising application potentials. In this review, the utilization of NMs in different forms, including nanoparticles, nanofibers, and nanofilms, in the SDVG field is discussed, and future perspectives for the development of NM-loading SDVGs are highlighted. It is expected that this review will provide helpful information to scholars in the innovative interdiscipline of cardiovascular disease treatment and NM.
Introduction
The fast-developing era has witnessed the prevalence of cardiovascular diseases (CVDs), mostly originating from unhealthy lifestyles. CVDs have become the leading cause of global mortality and morbidity, heavily burdening society and patient families.1 A consensus has been reached that maintaining a healthy lifestyle greatly benefits the prevention and control of the onset and progression of CVDs, while appropriate medical measures should be taken for people based on the progressive stages of diseases, including drug medication, interventional therapy, and vascular replacement.1,2 In the suffering populations, one that should be of special concern is those who urgently need small-diameter vascular grafts (SDVGs) with an inner diameter of less than or equal to 6 mm to save their lives because this kind of graft on demand is still considerably insufficient in quantity.3Every year, more than 1 million people urgently need SDVG surgeries globally to cure severe CVDs, such as coronary/carotid artery diseases, critical limb ischemia, and vascular trauma.4 The current gold standard for the treatment is grafting autologous vessels in which the great saphenous veins, internal mammary arteries, and radial arteries are mostly used.4 However, over 30% of these people cannot obtain their arteries/veins because of old age, existing diseases (e.g., diabetes mellitus), vascular injuries, secondary vascular acquisition, and other reasons. In this context, pioneers have employed VGs as substitutions for natural blood vessels. Unlike large diameter VGs with an inner diameter larger than 6 mm used to replace large vessels (e.g., aorta) prepared by materials, such as ePTFE or PET, their small diameter counterparts with the same materials tend to have stenosis or occlusion.3,4 This is mainly attributed to the fact that slow blood flow in small vessels makes thrombogenic chemicals and cells more prone to aggregate on the lumen. Additionally, SDVGs experience complex biochemical and biomechanical environments under a blood flowing physiological condition, further increasing their challenges in maintaining long-term patency.
To date, substantial advances have been made in the development of an eligible SDVG.5 Nonetheless, the venue for success is rather slushy, and only few have crossed pre-clinical stages to enter clinical trials, yet none of them have hit the market so far.5 The main reasons are ascribed to thrombus formation, intimal hyperplasia (IH), and mechanical mismatches.6 Intensive strategies have been adopted to resolve or mitigate these issues, such as surface modification, cell coverage, and new substrate material usage.7 With the advancement of nanotechnology, NMs have entered the horizons of SDVG researchers and have exhibited enormous potential to improve SDVG performance.8 NMs are a set of materials with a scale ranging from one to several hundred nanometers in at least one dimension. They represent a cutting-edge direction of materials whose attractive properties include a high surface-to-volume ratio, high adsorption, and high reactive/catalytic activity, basically stemming from their ultra-small scale.9 NMs with various forms in shape, such as particles, fibers, and films, can be distinct in derivations from the inorganically synthetic to the organically synthetic and to the biological, such as AuNPs, PLGA NPs, and EVs. Various NMs have enhanced the performance of SDVGs by their beneficial functions,8 which include improving EC adhesion, regulating inflammation, mimicking vascular ECM, controllably releasing vasoactive drugs, and refining mechanical properties. Although the application of NMs in SDVGs is generally underexplored, they have shown great promise in boosting the development of SDVGs. In this review, we summarize the state-of-the-art applications of NMs in SDVGs and discuss future perspectives of this new field.
Application of nanomaterials in SDVGs
To date, various types of NMs have been employed to improve the performance of SDVGs (Fig. 1 and Table 1).8 Grossly classified by the nano-scale dimensions that the materials possess, they include nanoparticles (NPs), nanofibers, and nanofilms. NPs, also called zero dimensional (0D) NMs, have three dimensions (anterior-posterior, left-right, up-down directions) in a nano scale; nanofibers, also called one dimensional (1D) NMs, have two dimensions in a nano scale but the third in a micro/macro scale, which typically form interconnected mats or matrices; nanofilms, also called two dimensional (2D) NMs, have only one dimension in a nano scale yet the other two in micro/macro. In this section, we summarize the progress of representative NMs that have been applied to fabricate SDVGs (vascular patches with similar application scenarios are also included).| VG No. | NM-bearing VG constitution | VG assessment stage | Merits of NMs | Flaws or needed improvement of NMs | Ref. |
|---|---|---|---|---|---|
| 1 | AuNP bearing decellularized porcine aorta | Longitudinal arteriotomy model of pig for 2 to 6 months | ● AuNP | ● AuNPs | 12 and 13 |
| - Minor inflammation | - Uncertain long-term metabolism behavior and safe concentration range | ||||
| - Enhanced EC and SMC regeneration | - Photothermal/electronic, drug delivering and anti-bacterial ability need exploitation in VGs | ||||
| ● Decellularized tissue nanofiber | - NP size, shape and surface modification on tissue reaction need evaluation | ||||
| - Biomimetic and high biocompatibility | ● Decellularized tissue nanofiber | ||||
| - Easy-to-fabricate | - Remove more immunogenic substances | ||||
| - Abundant-in-source | - Add pro-regenerative coatings | ||||
| - Off-the-shelf and non-invasive acquisition of xenogenous VGs | - Optimize fabrication approach | ||||
| - Mildly invasive for autologous VGs | |||||
| 2 | Fe3O4 NP loading HUVECs seeded on PTFE VG | In vitro | ● Fe3O4 NP | ● Fe3O4 NP | 19 |
| 3 | LbL assembles Fe3O4 NPs loading vascular cell sheets | In vitro | - Superparamagnetic | - In vivo performance of participant VGs | 20 |
| 4 | Construct Fe3O4 NPs loaded with vascular cell spheroids, form cell sheets, and then LbL constructs VGs | In vitro | - Homogeneous and rapid cell layer formation and biomimetic multi-layer VG construction | - Long-term and in vivo safety | 21 |
| - Noninvasive imaging of cell distribution | - Measures to lower the risk of toxicity | ||||
| 5 | ZnO NP coated P(VDF-TrFE) ES VGs | In vitro and subcutaneous implantation | ● ZnO NP | ● ZnO NP | 24 |
| - Economic- and biomedical-friendly | - Measures to lower the risk of toxicity | ||||
| - Promoted HUVEC adhesion and proliferation | - Evaluate in vivo reactivity related to their sizes, concentrations, and surface modifications | ||||
| - Enhanced subcutaneous angiogenesis | ● ES P(VDF-TrFE) nanofiber | ||||
| - ROS production | - Optimize fiber size and orientation | ||||
| ● ES P(VDF-TrFE) nanofiber | - Decorate proper coatings and/or seeding cells | ||||
| - Low cost, controllable parameter, simple equipment, and scalable | - Elongated in vivo test | ||||
| - ECM-like substrates | |||||
| - Piezoelectric | |||||
| 6 | BaTiO3 NP doped PU-PDMS VGs | In vitro | ● BaTiO3 NP | ● BaTiO3 NP | 26 |
| - High biocompatibility, non-linear optical property, piezoelectric property, drug loading capacity, and elasticity | - In vivo performance of participant VGs | ||||
| - Refined mechanical property of VG | - Exploit optical property and drug loading capacity in VGs | ||||
| 7 | RSV-carrying SWCNTs coated onto the surface of rat-decellularized carotid | Rat common carotid artery replacement model for 90 days | ● SWCNT | ● SWCNT | 29 |
| - Outstanding mechanical, thermal, electrical, and adsorptive properties | - In vivo toxicity | ||||
| - Stable coating and controlled drug release-Stabilize | - Exploit mechanical, thermal, and electrical properties in VGs | ||||
| - SMCs in a contractile state | ● Decellularized tissue nanofiber | ||||
| - Transit macrophages to the M2 state | - Idem VG No. 1 | ||||
| ● Decellularized tissue nanofiber | |||||
| - Idem VG No. 1 | |||||
| 8 | Covalently immobilize FD-loaded PLGA NPs on ePTFE VGs | In vitro | ● PLGA NP | ● PLGA NP | 32 |
| - FDA-approved | - Elongated in vivo test | ||||
| - Excellent biocompatibility, biodegradability, non-immunogenicity, and drug-loading ability | - Improve drug loading efficiency | ||||
| - Stable immobilization on VGs | - Reduce burst release and side effects of degradation products | ||||
| 9 | Coat miR-145 loading PLGA NPs on rabbit jugular veins | Rabbit carotid artery replacement model for 2 weeks | ● PLGA NP | ● PLGA NP | 33 and 34 |
| - Idem VG No. 8 | - Idem VG No. 8 | ||||
| - Controlled release of miR-145 | ● Decellularized tissue nanofiber | ||||
| - Stabilize SMCs' contractile phenotype-inhibit IH | - Idem VG No. 1 | ||||
| ● Decellularized tissue nanofiber | |||||
| - Idem VG No. 1 | |||||
| 10 | MK2i loading PPAA NPs coated on fresh rabbit external jugular veins | Rabbit common carotid artery replacement model for 4 weeks | ● PPAA NP | ● PPAA NP | 36 |
| - Superior biocompatibility and drug carrying ability | - Reduced EC coverage | ||||
| - pH-dependent membrane disruptive activity | - Undesirable hemolysis | ||||
| - Increase MK2i cellular uptake | - Establish-optimized concentration in VGs | ||||
| - Loaded MK2i peptides have anti-inflammatory, anti-migratory, and anti-proliferating properties | - Broaden pH-responsive range by molecular structure design | ||||
| - Triggered the signalling of inflammation and proliferation inhibition | |||||
| - Strong reduction in IH | |||||
| 11 | Bilayered vascular patch; outer layer: SF/gelatin hydrogel; inner layer: SMV-loaded micelles in gelatin hydrogel | Rat carotid artery replacement model for 2 weeks | ● Micelle | ● Micelle | 39 |
| - Excellent drug carriers | - Elongated in vivo test | ||||
| - Lower blood lipids, promote EPC adhesion and proliferation | - Optimize application conditions for each kind of micelle NP based on its carried drug and formulation | ||||
| - Inhibit SMC migration and proliferation | |||||
| - Rapid endothelialization | |||||
| 12 | Human placental MSC-derived EV and heparin-modified ES PCL VGs | Rat abdominal artery replacement model in hyperlipidemia conditions for 3 months | ● EV | ● EV | 42 |
| - Abundant sources, easy acquisition, ultralow immunity, high para-secretion and immunomodulation, and low ethical risk | - Long-term implantation in VG | ||||
| - Off-the-shelf | - Detailed the therapeutic mechanism | ||||
| - Improve VG patency | ● ES PCL nanofiber | ||||
| - Inhibit thrombosis and calcification, enhance regeneration, and transit macrophages to the M2 state | - Idem VG No. 5 | ||||
| ● ES PCL nanofiber | - Slow degradation of PCL polymers may cause calcification and degeneration of neotissues | ||||
| - Idem VG No. 5 (except for “piezoelectric”) | |||||
| 13 | Human adipose MSC-derived EV-seeded silk-based VGs | Rat aortic replacement model for 8 weeks | ● EV | ● EV | 43 |
| - Idem VG No. 12 | - Idem VG No. 12 | ||||
| 14 | Decellularized fibrotic conduits with heparin coating | Carotid artery replacement model of rats for 6 months and that of mini-pigs for 1 month | ● Decellularized tissue nanofiber | ● Decellularized tissue nanofiber | 49 |
| - Idem VG No. 1 | - Idem VG No. 1 | ||||
| 15 | CNP loaded with ES PCL VGs | Rabbit AV-shunt model for 1.5 hours and rat abdominal aorta replacement model for 1 month | ● ES PCL nanofiber | ● ES PCL nanofiber | 56 |
| - Idem VG No. 12 | - Idem VG No. 12 | ||||
| - CNP loading showed higher EC coverage, NO production, VEGF secretion, M2-type macrophage polarization, contractile SMC transition, and ECM deposition | |||||
| - 100% patency rate | |||||
| 16 | 20% NaOH treatment of tubular BC hydrogel | Rat abdominal aorta replacement model for 5 months | ● BC nanofiber | ● BC nanofiber | 62 |
| - ECM-like and high biocompatibility | - Long degradation term in vivo | ||||
| - Convenient fabrication | - Proper surface modification to improve its performance in VGs | ||||
| - NaOH treatment improved the mechanical property, lessened platelet adhesion and activation, and promoted EC proliferation | |||||
| - Low immunity and high patency rate | |||||
| 17 | Nano-thick Ta film on ePTFE VGs | Canine aortic replacement model for 4 weeks | ● Ta nanofilm | ● Ta nanofilm | 67 |
| - Abundant lightweight metal with considerable material strength, corrosion resistance, and biocompatibility | - Improve rigidity, abrasive resistance, and reactivity with other metals | ||||
| - Refine hydrophobicity of the VG surface | |||||
| - Improve EC coverage, and suppress platelet adhesion/activation | |||||
| 18 | A bilayer VG, inner layer: freeze-casting SF/gelatin to form high aspect ratio lamellar nanofilms; outer layer: ES PCL | Rabbit carotid artery replacement model for 3 months | ● Lamellar nanofilms | ● Lamellar nanofilms | 70 |
| - Refine the VG surface by physical method without changing the property of materials | - Precise manufacturing techniques | ||||
| - Guide EC alignment, inhibit platelet adhesion, reduce blood flow disturbance, and induce a high patency rate | ● ES PCL nanofiber | ||||
| - Computer-aided design | - Idem VG No. 12 | ||||
| ● ES PCL nanofiber | |||||
| - Idem VG No. 12 | |||||
| - Reinforce the VG |
Nanoparticles
NPs used in SDVGs can be synthetic or biological in origin and are inorganic or organic in composition. The inorganically synthetic ones include metallic NPs (e.g., AuNPs), metallic oxide NPs (e.g., Fe3O4 NPs, ZnO NPs, and BaTiO3 NPs), and inorganic nonmetal NPs (e.g. carbon nanotubes (CNTs)). The organically synthetic ones mainly contain polymeric NPs (e.g., PLGA NPs and poly(propylacrylic acid) NPs) and micelles (e.g., L-lactic acid oligomer (Lo)-grafted gelatin micelles). The biological ones used in SDVGs are specifically referred to as EVs in this review.Gold NPs
Gold NPs (AuNPs), usually existing stably in the form of colloidal suspension in aqueous solutions, are a kind of noble metallic NM that may be prepared using various methods, including sodium citrate reduction, crystal seed growth, and electrochemistry. Owing to their unique physicochemical properties, they have been extensively used in biomedicine.10,11 In tissue engineering (TE), AuNPs are typically incorporated into scaffold matrices to uprate scaffold performance. For VGs, it has been reported that vascular patches made of decellularized porcine aorta conjugated with mercaptoethylamine-functionalized AuNPs showed fine patency in longitudinal arteriotomy models of pigs. The AuNPs enhanced the tissue integration and EC and SMC regeneration, as well as decreased inflammation and scaffold degradation, compared with the commercially available bovine pericardium material control after the patches were implanted in carotis12 or thoracic aorta13 from 2 to 6 months. This is partially because AuNPs hinder collagenase binding sites.11,14,15 The possible disadvantage of AuNPs in VGs may arise from their undetermined long-term metabolism behavior and safe concentration range, which needs to be determined. The utilization of AuNPs in VGs is in a very early stage because important properties, such as anti-bacteria, photothermal/electronic effect, and drug delivery of AuNPs, are still unexploited. Furthermore, AuNP sizes, shapes (e.g., nanospheres, nanorods, and nanostars), and surface modifying groups highly affect their tissue reactions, which should therefore be carefully evaluated in VG applications.Fe3O4 NPs
Pure Fe3O4 NPs, appearing as black powders, are complex ionic crystals bonded by Fe2+, Fe3+, and O2− ions. Owing to their special crystal structure, ultrasmall size, and the characteristic feature of transition metal Fe, Fe3O4 NPs are famous because of their superparamagnetic properties and thus have been widely used in magnetic control and imaging in biomedicine.16–18 These advantages have also shown benefits in the SDVG field. For instance, human umbilical vein endothelial cells (HUVECs) were incubated with carboxydextran-coated Fe3O4 NPs and then seeded on a polytetrafluorethylene (PTFE) VG lumen homogeneously under the guidance of an annular magnetic field. In addition to the rapidness of construction, cell arrangements of the cell-seeded VG could be sensed non-invasively using magnetic resonance imaging (MRI).19 In another study, vascular ECs, SMCs, and fibroblasts (FBs) were separately incubated with Fe3O4 NPs encapsulated in cationic liposomes to form cell sheets on flat magnets, and then the sheets were rolled layer-by-layer around a cylindrical magnet to construct a cell distribution mimicking multilayered VG.20 This method can rapidly and conveniently prepare structurally mimetic VGs. In a third study, researchers first established magnetic tissue spheroid units around 200 μm in diameter, each of which contained two coterminal domains: one was cell aggregates assembled by one type of vascular cells (ECs, SMCs, or FBs), while the other was collagen gel encapsulating Fe3O4 NPs.21 To rapidly complete a biomimetic VG, the magnetic units first formed flat rectangle stripes under the guidance of flat magnets, and the stripes were then wrapped layer by layer around cylinder magnets. However, all the three VGs were proof of concept models, and their in vivo performances had not been validated. In addition, the long-term and in vivo safety of Fe3O4 NPs in these VGs were still questionable although a safe dose range of 10–200 μg mL−1 was identified by short-time cell tests and measures; for example, separating cell and NP containing domains were used to lower the risk of toxicity in the above cases.ZnO NPs
ZnO NPs are nanosized hexagonal crystals with white powders, which are mainly fabricated using solid, liquid, or vapor phase methods. ZnO NPs are considered a novel type of economic- and biomedical friendly inorganic NM with broad application potentials.22,23 Researchers have fabricated ZnO NP-decorated VGs rapidly using the electrospinning (ES) method when ZnO NPs are dispersed into ES solutions constituted by a piezoelectric polymer poly(vinylidene fluoridetrifluoroethylene) P(VDF-TrFE).24 Below 2% concentration, ZnO NP-decorated ES VGs promote HUVEC proliferation in in vitro tests, and subcutaneous implantation further demonstrated that they increased microvessel formation compared to undecorated ones. The mechanism was attributed to ZnO NP-induced moderate H2O2 molecule release and piezoelectric properties (the ability to transform energies between mechanical stress and electrical potential) of P(VDF-TrFE). These results demonstrate the biocompatibility and preliminary feasibility of ZnO ES VGs. However, the in vivo performance of VGs in vascular replacement models has not been tested. Moreover, the biochemical activity and in vivo reaction of ZnO NPs are highly related to their sizes, concentrations, and surface modifications, which should therefore be carefully evaluated.BaTiO3 NPs
BaTiO3 NPs, originating from a perovskite-like oxide-based elastomeric ceramic, can be prepared using coprecipitation, sol–gel, or emulsion methods. BaTiO3 NPs are very promising NMs in biomedical applications owing to their high biocompatibility, non-linear optical property, piezoelectric property, drug loading capacity, and elasticity.25 Hence, BaTiO3 NPs are favorable for incorporating materials into VGs although currently the application of these NPs is limited. Researchers have doped glycol chitosan (GC)-coated BaTiO3 NPs into VGs. Porous VGs were fabricated by phase inversion spraying of polyurethane (PU)/polydimethylsiloxane (PDMS) solutions when the NPs were dissolved in spray solutions before being doped into the microfibers that were sprayed out. Owing to the elasticity of the NPs, the mechanical property of the VGs was greatly refined.26 After NP doping, the burst strength of VGs increased greatly from ∼800 kPa to ∼1100 kPa; the cytocompatibility with human fibroblasts was greatly improved, and the VGs acquired piezoelectric properties. These in vitro tests exhibited excellent potential for doped VGs, and further in vivo tests were warranted. In addition, the powerful advantages of BaTiO3 NPs, such as their unique optical property and drug loading ability, are still underused.Carbon nanotubes
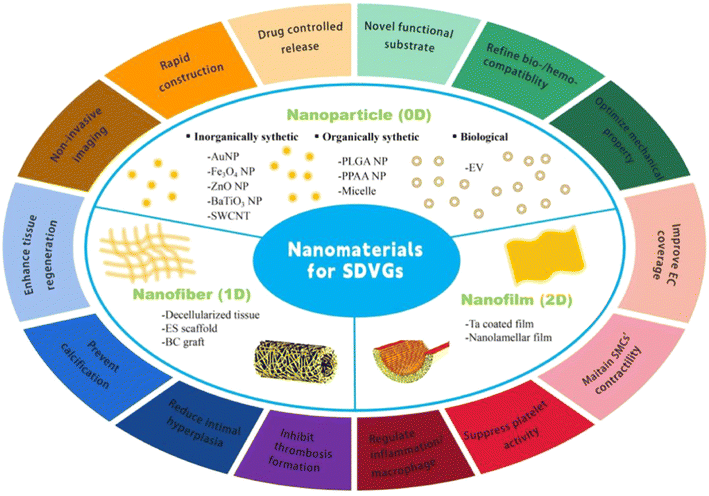
Single-walled carbon nanotubes (SWCNTs) are tubular nano-sized graphite crystals formed by a single graphite sheet curled around the central axis at a definitive spiral angle. Their particular structure endows SWCNTs with outstanding mechanical, thermal, electrical, and adsorptive properties, making them a multifaceted material in biomedical applications.27,28 In a novel VG design, resveratrol (RSV), a star natural small molecular drug, was conjugated to SWCNTs, and the drug-carrying SWCNTs were then coated as irregular mesh onto the surface of decellularized rat carotids (Fig. 2).29 During 90 days of assessment in a rat common carotid artery replacement model, the SWCNTs showed a well-controlled drug release profile and potent resistance to physiological blood flow shear. The released drug prohibited the proliferating state but stabilized the contractile state of the SMCs to reduce IH formation and sustain VG elasticity. In addition, SWCNTs engulfed by macrophages together with resveratrol released inside cells shifted the macrophage state from M1 pro-inflammatory to M2 anti-inflammatory. These overall benefits enhanced the VG patency and regeneration compared to the group without coating RSV-carrying SWCNTs, the group coated with SWCNTs without RSV carrying, and the group carrying RSV but without SWCNTs. However, as far as we know, the toxicity of SWCNTs to cells and organisms remains an unresolved issue. Additionally, valuable features of SWCNTs, including their mechanical, thermal, and electrical properties, should be developed in VG applications.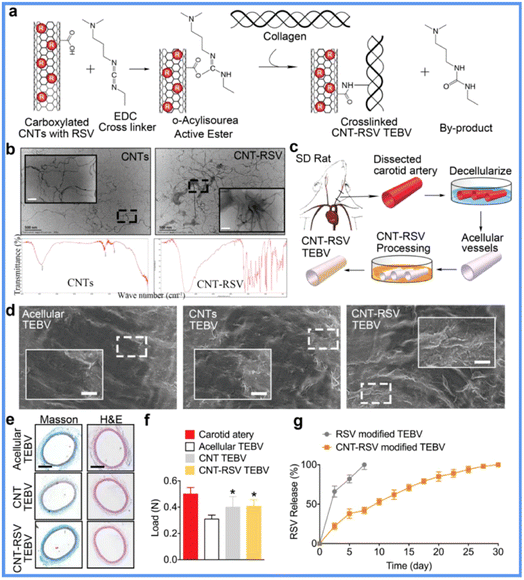 | ||
| Fig. 2 Application of CNTs in SDVGs. (a) Scheme of surface modification of SDVGs with collagen and CNTs. (b) Transmission electron microscopy and infrared spectrum detection of CNTs. (c) Procedure for preparing SDVGs. (d) Scanning electron microscopy images showing the lumen of SDVGs. (e) H&E and Masson staining of SDVGs. (f) Tension test for SDVGs. (g) In vitro release profiles of RSV from SDVG. Reprinted with permission from ref. 29. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA. | ||
PLGA NPs
PLGA NPs are nanoscale aggregates of PLGA polymers. They are considered the most widely applied type of organic NPs in the biomedical field and have been approved by the FDA owing to their biocompatibility, biodegradability, non-immunogenicity, and drug-loading ability.30,31 PLGA NPs have shown promise in VGs. For instance, researchers covalently immobilized PLGA NPs loaded with a model molecule, fluorescein isothiocyanate-dextran (FD), on ePTFE VGs using wet chemistry.32 The mobilization was blood flow resistant and promoted L929 cell adhesion and growth. This approach provided a model of drug loading PLGA for improving VG patency. To propel the application of the model, researchers further loaded microRNA-145 (miR-145) into PLGA NPs (Fig. 3), which were then coated on the jugular veins of male Japanese white rabbits to construct a new VG.33,34 The miR-145 enabled the alteration of the phenotype of SMCs from proliferative states to contractile states. After the VGs were implanted into the carotid arteries of rabbits for 2 weeks, the controlled release of miR-145 from PLGA NPs stabilized the SMC contractile phenotype and suppressed SMC proliferation and inflammation, resulting in reduced IH formation. A follow-up mechanism study indicated that these NPs could act on SMCs by regulating the CD40 and NF-κB signals. Despite the large potentials of PLGA NPs in VGs, relevant reports are still rare, and the in vivo implantation period of the VGs should be further elongated. Scientists are also working on improving PLGA NP drug loading efficiency and reducing burst release and the influence of their degradation products on in vivo circulation.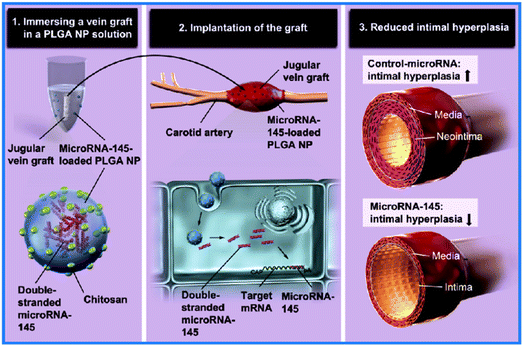 | ||
| Fig. 3 Application of PLGA NPs in SDVGs. SDVGs modified by PLGA NP loading miR-145 showed significantly reduced IH. Reprinted with permission from ref. 33. Copyright 2019, Elsevier. | ||
Poly(propylacrylic acid) NPs
Poly(propylacrylic acid) (PPAA) NPs are novel anionic polymer NPs with pH-responsive features. They have superior biocompatibility, drug carrying ability, and, particularly, membrane destabilizing ability at pH ∼6.7, rendering them a quite useful material in biomedicine.35 In VGs, researchers have fabricated vasoactive drug-loading PPAA NPs by mixing PPAA homopolymers and MAPK-activated protein kinase 2 inhibitor (MK2i) peptides via electrostatic complexation.36 The external jugular veins were then coated with PPAA NPs that delivered MK2i peptides possessing anti-inflammatory, anti-migratory, and anti-proliferating properties into vascular cells. When the VGs were implanted into rabbit common carotid arteries for 28 days, they showed high patency and potent IH suppression, increased MK2i peptide cellular uptake, endosomal escape, and intracellular stability induced by PPAA encapsulation. The mechanism could be explained by MK2i-PPAA NPs depressing phosphorylation of the transcription factor cAMP element-binding protein, the chaperone heat shock protein 27, and the gene regulator heterogeneous nuclear ribonucleoprotein A0, triggering signaling on the inhibition of inflammation and proliferation.36 However, the reduced EC coverage linked to MK2i-PPAA NPs may be a hurdle in VG applications. Although PPAA NPs have shown promise as an easy-fabricated drug-loading environmentally responsive nano vehicle, they may cause undesirable hemolysis, their optimized concentration has not been established, and their pH-responsive range can be broadened by designing molecular structures and functional groups to expand their applications.Micelles
Micelles are nanoscale spheroids self-assembled by amphiphilic block copolymers, with a hydrophobic core and hydrophilic shell. They are excellent drug carriers that increase the solubility of indissolvable drugs and well protect the inner contents.37,38 The micelles have been widely used in other biomedical fields but are still rarely reported in VGs. Researchers have constructed a micelle-containing double-layered vascular patch, whose outer layer comprises Thai silk fibroin/gelatin, and inner layer comprises gelatin hydrogel incorporating L-lactic acid oligomer (Lo)-grafted gelatin micelles loaded with simvastatin (SM). The controlledly released SM played roles that included lowering blood lipids, promoting the adhesion and proliferation of endothelial progenitor cells (EPCs), and inhibiting the migration and proliferation of SMCs. After the vascular patches were implanted in rat carotid arteries for 2 weeks, they achieved complete endothelialization and were well regenerated. However, the long-term performance of the design in real tissue-engineered VGs should be evaluated in the future. Additionally, the drug and formulation-specific therapeutic effects should be evaluated for each kind of micelle NP.Extracellular vesicles
Extracellular vesicles (EVs) secreted by cells are nanoscale disk-like vesicles containing complex RNAs and proteins, which are widely distributed within the cell micro-environment and body liquids. EVs are regarded to possess the same regulatory functions as corresponding cells yet with off-the-shelf properties.40,41 With the development of cellular therapy, accompanying EV therapy has demonstrated great application potential. In a recent study, heparin-modified ES polycaprolactone (PCL) tubular scaffolds were decorated by human placental MSC-derived EVs to construct VGs, which were then evaluated in a rat abdominal artery replacement model of hyperlipidemia for 3 months (Fig. 4A).42 The patency of the graft was great because the MSC-derived EVs effectively inhibited thrombosis and calcification, enhanced the regeneration of ECs and SMCs, and switched the phenotype of macrophages from pro-inflammatory M1 to anti-inflammatory M2. Further mechanism studies illustrated that this partially contributed to the indigenous presence of bioactive factors in EVs, such as miR-145, miR-126, and VEGF. It is noteworthy that even in a high-cholesterol pathological state, the MSC-derived EVs still exhibited powerful regenerative capacities in VGs. More recently, EVs derived from human adipose MSCs were loaded into porous silk-based tubular scaffolds by vacuum seeding to establish a new VG (Fig. 4B).43 After implantation in a rat aortic replacement model for 8 weeks, high patency of the VGs was observed. The EV-loading VGs showed higher endothelization and smooth muscle regeneration but reduced macrophage numbers compared to the non-loading ones. The merits of these EVs for VGs include the basic properties of placental and adipose MSCs, such as abundant sources, easy acquisition, ultralow immunity, high para-secretion and immunomodulation, as well as low ethical risk. However, long-term implantation should be performed continuously to accelerate the translational process, and the detailed therapeutic mechanism of EVs should be elucidated.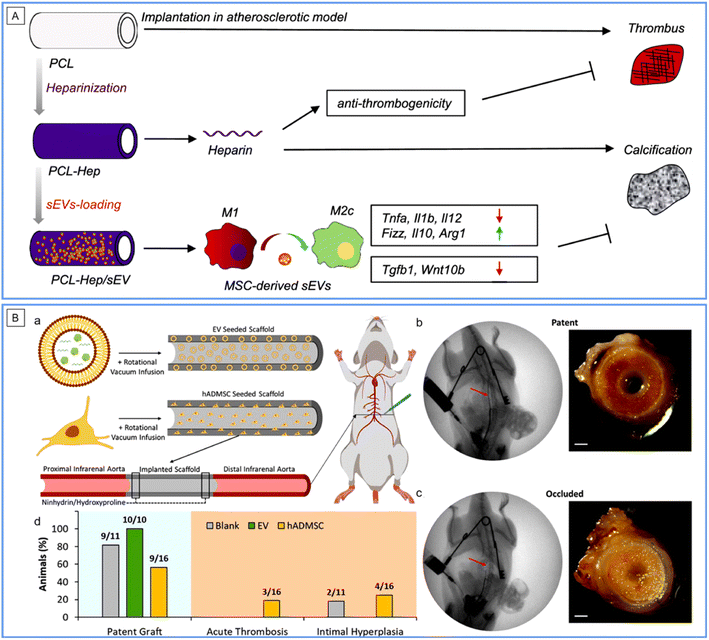 | ||
| Fig. 4 Application of EVs in SDVGs. (A) Schematic mechanism of the improved performance of PCL SDVGs loaded with human placenta-derived MSC EVs in a rat model of hyperlipidemia. Reprinted with permission from ref. 42. Copyright 2019, Elsevier. (B) Graft patency of silk SDVGs loaded with human adipose-derived MSC EVs in a rat model. (a) Schematic representation of scaffold seeding and implantation. (b and c) Angioplasty and gross imaging of a representative patent (b) and occluded (c) explant. Scale bar: 500 μm. (d) Patency rates of SDVGs and manner of graft occlusion. Reprinted with permission from ref. 43. Copyright 2020, American Chemical Society. | ||
Nanofibers
Nanofibers are the second form of NMs used in VGs. The fibers are usually interconnected to form 3D tubular matrices or 2D films (which can then be rolled up to form 3D tubular structures), which are then used as scaffold materials for VGs. There are three main kinds of widely used nanofiber interlaced materials in VGs: decellularized tissue matrices, electrospinning scaffolds, and bacterial cellulose scaffolds.Decellularized tissue matrices
When tissues are decellularized, the remnant parts are their extracellular matrix (ECM) mainly constituted by nano/micro-scale extracellular protein fibers, which provide a very biomimetic (both constitutively and mechanically) substrate for cell in-growth. Many decellularized tissues, such as blood vessels, ureters, small intestinal submucosas, and amnions, have been introduced into VG applications, and excellent reviews have been proposed on this topic (see ref. 44–48). In the above-mentioned VG examples in this review, some adopted decellularized tissues, e.g., vascular patches by AuNP conjugated decellularized porcine aorta12,13 and VGs by resveratrol-carrying SWCNT-coated rat decellularized carotid.29 Here, we highlight one more of the latest developments in interest. In this study, researchers first subcutaneously implanted the ePTFE mandrel into the abdominal wall of rats for 4 weeks, and fibrotic tissue tubes were formed around the mandrel. The VGs were then developed by decellularizing the fibrotic tubes with an anti-thrombogenic heparin coating (Fig. 5A).49 After evaluation in a carotid artery replacement model of rats for 6 months and in that of mini-pigs for 1 month, the researchers found that the decellularization greatly improved the patency and regenerative performance, compared to non-decellularized fibrotic tubes made using the same method. Furthermore, the decellularized autologous grafts exhibited an even higher patency rate compared to the decellularized allogeneic fibrotic tubes, indicating that a foreign body reaction still exists. The decellularized tissue matrix-based VGs represent an easy-to-fabricate and abundant-in-source kind of VG approach, showing off-the-shelf and non-invasive features for allogeneic VGs and mildly invasive features for autologous ones. However, to further improve the VG quality and fabrication for allogeneic/xenogenic ones, measures such as removing immunogenic substances and coating immuno-suppressive/regeneration promoting molecules should be taken; meanwhile, for autologous ones, the period for fibrotic tube formation should be reduced.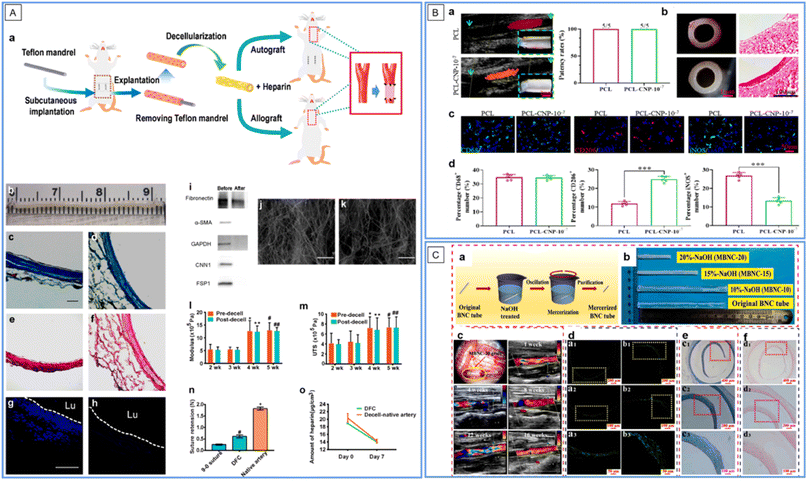 | ||
| Fig. 5 Application of nanofibers in SDVGs. (A) Fabrication and characterization of decellularized fibrotic tissue-based VGs (DFC in the figure). (a) Schematic showing the fabrication, modification and implantation of the VG. (b) Gross appearance of the VG before implantation. (c and d) Masson's trichrome staining of VGs before (c) and after (d) decellularization. (e and f) Verhoeff's staining of VGs before (e) and after (f) decellularization. (g and h) DAPI staining of cross sections of VGs before (g) and after decellularization (h). Scale bars: 100 μm in (c)–(h). (i) Western blotting analysis of major cellular proteins before and after decellularization. (j and k) SEM images of the inner (j) and outer (k) surfaces of VGs. Scale bars: 50 μm. (l and m) Young's modulus (l) and ultimate tensile strength (UTS) (m) before and at 2–5 weeks after decellularization. (n) Suture retention of VGs at 4 weeks after decellularization. (o) Content of heparin conjugated on VG and the decellularized native artery on days 0 and 7 under in vitro flow conditions. Reprinted with permission from ref. 49. Copyright 2021, Elsevier. (B) Evaluation of the patency and macrophage polarization of the explanted grafts 1 month after implantation. (a) Patency of VGs. (b) Representative stereomicroscope and HE images of cross sections of VGs. (c) Distribution of total M2 and M1 macrophages. (d) Quantitative analysis of the number of macrophages per field. Reprinted with permission from ref. 56. Copyright 2022, Elsevier. (C) Preparation and implantation of mercerized BC VGs. (a) Schematic illustration of the alkaline treatment process. (b) Tuning the morphology of VGs using various NaOH concentrations. (c) VGs during transplantation and ultrasonic analysis after implantation for 1–16 weeks. (d–f) Analysis of VGs by fluorescence staining of ECs (d), HE staining (e), and Masson's staining (f) after implantation for 5 months. Reprinted with permission from ref. 62. Copyright 2022, Elsevier. | ||
Electrospinning scaffolds
Electrospinning (ES) is an electrical field-controlled, useful technology for fabricating ECM-like nano/micro-diameter fibers with various synthetic or natural polymers. It is well known in biomedical and other fields owing to its low cost, process controllability, simple equipment, diversiform spinnable substances, and scalability. By tuning the working conditions, ES can well control the fiber size and orientation, scaffold configuration (2D films or 3D geometry in designated shapes) and surface thickness. ES substrates are typically versatile scaffold materials in VGs, which can load drugs and control cell behaviors.50,51 There have been several examples of ES-based VGs in the previous texts in this review, e.g., VGs by ZnO NP-coated P(VDF-TrFE) ES conduits24 and by human placental MSC-derived EV-modified ES PCL conduits.42 Intensive and comprehensive reviews on this topic have been published (see ref. 52–55). Herein, we provide another latest example of interest. The VG was fabricated by decorating a C-type natriuretic peptide (CNP), a vasodilator, on PCL ES tubular scaffolds (Fig. 5B).56 The VG showed great blood compatibility in a rabbit arterial venous (AV)-shunt model for 1.5 hours of the test. One month post-implantation in a rat abdominal aorta replacement model, compared to its counterpart without CNPs, the CNP loaded with VGs showed significantly more EC coverage, NO production, VEGF secretion, M2 type macrophage polarization, contractile SMC transition, and ECM deposition, along with a parallel yet also 100% patency rate. Therefore, the CNP decoration further improved the regenerative capacity of the VG, which may play an important role in long-term VG patency. However, the in vivo test should be performed at an elongated time. In addition, the slow degradation of PCL polymers may cause the calcification and degeneration of vascular neotissues. To achieve ideal VGs, it is a critical issue for ES-based VGs to select the proper polymer material, construct the proper fiber size and orientation, and decorate proper seeding cells and/or coatings.Bacterial nanocellulose scaffolds
Bacterial nanocellulose (BC) is another kind of nanofiber material for VG applications. It is a natural polysaccharide produced by several bacteria, such as Acetobacter xylinum and Gluconacetobacter sucrofermentans, within a short period of around 10 days.57 The nanofibrous networks of BC are similar to those of ECM cells, thus providing superior biocompatibility. BC can be conveniently fabricated into 2D films, hollow tubes, and other 3D shapes. Numerous reviews on the application of BCs in VGs have been presented (see ref. 58–61). Herein, we also underline one of the latest examples of interest. The tubular BC hydrogel was treated with 20% NaOH to construct a novel VG (Fig. 5C).62 The treatment greatly increased the smoothness, burst strength, and compliance of the VGs. Compared with untreated BC VGs, the treated ones showed lessened platelet adhesion and activation and promoted EC proliferation in vitro. After the subcutaneous implantation of the VGs in a rat for 6 months, both the treated and untreated BC showed low inflammation and good tissue growth, indicating their superior biocompatibility. After 5 months of implantation in a rat abdominal aorta replacement model, the treated BC VG showed normal blood circulation and satisfactory patency. By treating NaOH with different concentrations, the length, diameter, and wall thickness of the VG could become tunable, which may fit multiple applications. However, the long degradation term in vivo may be a limitation of BC's application in VGs. Moreover, more surface modification of BC could be attempted to improve its performance in VGs.Nano-films
Nanoscale films, the third type of NMs used in VGs, are a novel but effective strategy to enhance the performance of VGs as well. Nano-films can be formed by surface coating to construct uniform nano-thick films (e.g., tantalum-coated films), or surface patterning to construct films with designated nano-scale geometrics (e.g., nanolamellar films). Reports on nano-film bearing VGs are rare to date. Here, we provide two representative cases.Tantalum nanofilms
Tantalum (Ta) is an abundant lightweight metal with considerable material strength, corrosion resistance, and biocompatibility; thus, it is one of the major metals for biomedical use. Ta and its alloys have played important roles in surface and structural modification in current clinical devices, including cardiovascular ones.63–66 Recently, nano-thick Ta surface coatings have been employed in VGs. Commercially available ePTFE vascular scaffolds were coated with a nano-thick Ta layer via a sputtering-based plasma immersion ion implantation technique within 1 min.67 The Ta coating was found to refine the hydrophobicity of ePTFE, improve EC growth, and suppress platelet adhesion and activation, thereby rendering the graft more biocompatible and anti-thrombogenic. Assessing a canine aortic replacement model for 4 weeks demonstrated thrombosis suppression and rapid EC layer formation of the VG. Although Ta films have shown great merits in VGs, they are mainly limited by their low rigidity, abrasive resistance, and reactivity with other metals.Nanolamellar films
The second strategy for obtaining nanofilms is to design nanoscale topographic cues for device surfaces. This strategy does not need to introduce new materials to the devices but only needs to fabricate the device surface using physical methods without changing the properties of the materials. It has been reported that the nano topographic lumen plays pivotal roles in tuning cell fate, device integrity, and tissue remodeling fate.68,69 In VG application, researchers have fabricated a double-layered graft, whose inner layer was fabricated by freeze-casting silk fibroin and gelatin solutions on a cylinder template with pre-designed nano patterns to eventually form longitudinally aligned high aspect ratio lamellar nanotopography on the lumen, and whose outer layer was reinforced by ES PCL (Fig. 6).70 The nanolamellar surface, mimicking the groove-valley topography of the native vascular inner layer, guided EC alignment, inhibited platelet adhesion, and reduced blood flow disturbance, thereby maintaining a high patency rate of the VG after 3 months in a rabbit carotid artery replacement model. It is noteworthy that in this VG, numerical simulation was successfully employed to predict the performance of its surface nanopatterns. Therefore, more efficient nanopatterns can be designed through computers. The major difficulty of this strategy arises from precise manufacturing techniques.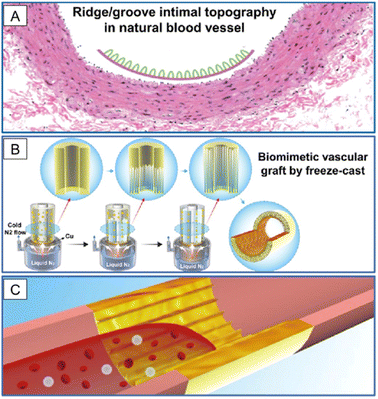 | ||
| Fig. 6 Application of nanolamellar films in SDVGs. (A) Natural blood vessel shows a ridge/groove nanotopography on the intimal surface. (B) SDVG with a biomimetic structure is prepared by applying a freeze-cast technique with lamellar nanotopography on the inner surface. (C) VG is used to replace the injured vessel to promote fast endothelialization and to maintain long-term patency. Reprinted with permission from ref. 70. Copyright 2019, American Chemical Society. | ||
Summary and outlook
The major efforts of SDVGs are to reverse the dilemma that surgeons clearly know the gold treatment to cure diseases but lack available grafts to save more lives. NMs have opened a new door for enhancing the performance of vascular grafts although the application of NMs in SDVGs is still in its infancy. As listed in Table 1, NMs in the forms of nanoparticles, nanofibers, nanofilms, or some of these in combination have been utilized for SDVG patency improvement, regeneration promotion, rapid construction, non-invasive imaging, and so forth. Each kind of material has its advantages and disadvantages. Thus, it is important to rationally use a specific material according to the target goal. Because all these NMs have represented a certain effectiveness in VGs, safety is the fundamental issue to be considered in the VG application. In this context, the Ta nanofilms, nanopatterns, and distinct nanofibers tested practically or experimentally perform well. For NPs, in general, the safest ones are biologically derived EVs with the highest biocompatibility; the safety of organically synthetic ones takes second place, which should be further identified; and the safety of inorganically synthetic ones is even lower, which should be carefully evaluated for further applications, although combining inorganic NPs and organic substrates demonstrates a new direction that improves VG performance. High safety means high translational potential. However, the effectiveness of the NMs in VGs could be continuously improved by structural redesign, surface modification, chemical treatment, a combination of various NMs, and so forth. Moreover, it is worth noting that the aforementioned VGs include not only the fabrication or refinement of newly designed synthetic VGs (the major efforts in the SDVG field currently) but also the amelioration of gold-standard and traditional material-based ones.By scanning the huge NM pool, many NMs potentially applicable to SDVGs are to be discovered. For example, silver NPs were linked to porous PCL films, which showed potent effects in inhibiting platelet adhesion and aggregation in vitro, simultaneously retaining the inherent antimicrobial properties of the NPs.72–74 An alternative nanomaterial, plasma-modified TiO2 nanotube coating, has been widely used in cardiovascular stents and other biomedical devices. Researchers have found that the coating can enhance endothelial coverage but reduce platelet adhesion and unfavorable SMC propagation. Interestingly, the coating also exhibited good anti-infective capacity.75 Although they have not been directly applied, NMs have shown powerful potential in improving SDVG patency.76 It is noteworthy that owing to the shared targeting organ systems and disease mechanisms with small diameter vascular diseases, NMs used in the management of atherosclerosis and other cardiovascular diseases (e.g., NMs in vascular or heart stents) could enhance the utilization of NMs in SDVGs (see several excellent reviews on this topic77–80).
In our opinion, there are several future studies on improving NM-bearing SDVGs. First, as aforementioned, only very few NMs have been tested in the SDVGs in the context of the exploding development of thousands of NMs.71 An item that could be immediately executed is the discovery of more currently available NMs that can be used in SDVGs. Second, owing to the strong designability of NMs, we can construct new NMs for SDVG based on our particular goal. It is suggested that in the design, the target should be considered systematically because the reasons for the low patency are closely interconnected. Multi-cargo carrying and precisely targeting NMs may be helpful. Third, the long-term and systematic impact of NMs on SDVGs should be evaluated to obtain more reliable conclusions to promote clinical translation. NM functions, distributions, and toxicities are all essential parts of the safety and efficacy of the NM-bearing VG. In the meantime, intrinsic limitations on each material should be overcome with the development of science and technology. Finally, we noticed that computer-based numerical stimulation has been applied in the design and evaluation of the efficacy of NMs in nanolamallar surface bearing VGs.70 Therefore, NMs in conjugation with other advanced technologies may significantly accelerate the development of SDVGs, and the fabrication and application of NMs are a collection of advanced technologies. Although the incorporation of NMs into SDVGs may not be dominant, we believe that the outstanding particularity and utilization potential of NMs will illuminate the way ahead for achieving commercially available SDVGs and directing the SDVG into a new era.
Abbreviations
| CVD | Cardiovascular disease |
| VG | Vascular graft |
| SDVG | Small diameter vascular graft |
| NP | Nanoparticle |
| AuNP | Gold nanoparticle |
| EC | Endothelial cell |
| SMC | Smooth muscle cell |
| HUVEC | Human umbilical cord vascular EC |
| PTFE | Polytetrafluorethylene |
| ePTFE | Expanded polytetrafluorethylene |
| LbL | Layer by layer |
| P(VDF-TrFE) | Poly(vinylidene fluoridetrifluoroethylene) |
| ES | Electrospinning |
| PU | Polyurethane |
| PDMS | Polydimethylsiloxane |
| RSV | Resveratrol |
| SWCNT | Single walled carbon nanotube |
| FD | Fluorescein isothiocyanate-dextran |
| miR | microRNA |
| PLGA | Poly(lactic-co-glycolic acid) |
| PPAA | Poly(propylacrylic acid); |
| MK2i | MAPK-activated protein kinase 2 inhibitor peptide |
| IH | Intimal hyperplasia |
| SF | Silk fibroin |
| SMV | Simvastatin |
| MSC | Mesenchymal stem cell |
| EV | Extracellular vesicle |
| CNP | C-Type natriuretic peptide |
| PCL | Polycaprolactone |
| AV | Arterial venous |
| BC | Bacterial nanocellulose |
| Ta | Tantalum |
| 0D/1D/2D/3D | zero/one/two/three dimensional, respectively |
| Lo | L-Lactic acid oligomer |
| TE | Tissue engineering |
| GC | Glycol chitosan |
Author contributions
Conceptualization, N. W. & Z. H.; resources, N. W., H. W. & D. W.; data curation, N. W. & H. W.; writing – original draft preparation, N. W.; writing – review & editing, D. W., L. Y., Y. W., H. W., F. W., T. Z., J. L. & Z. H.; visualization, N. W., H. W. & D. W.; supervision, Z. H.; project administration, N. W. & Z. H.; funding acquisition, F. W., N. W. & Z. H.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is financially supported by Science and Technology Foundation of Guizhou (No. QKH-JC-[2020]1Z017, QKH-JC-ZK[2023]YB586, QKH-PT-[2019]5406, and QKH-ZC-[2020]4Y192), Excellent Scientific and Technological Innovation Youth Training Program of Zunyi (No. ZSKH-HZ-(2019)48), Science and Technology Foundation of Guizhou Health Commission (No. gzwkj2023-250), The PhD Scientific Research Start-Up Fund of Affiliated Hospital of Zunyi Medical University (No. (2018)13), Guizhou Province High Level Innovative Talent Training Program-“1000” Talents (to N. W.), National Training Program of Innovation and Entrepreneurship for Undergraduates and Training Program of Innovation and Entrepreneurship for Undergraduates of Guizhou Province and Zunyi Medical University (No. ZYDC202202003 and 202310661184), Postgraduate Research Project of Education Department of Guizhou Province (No. QJH-YJSKYJJ-(2021)145), National Natural Science Foundation of China (No. 32270848), Collaborative Innovation Center of Chinese Ministry of Education (No. 2020-39), and University Scientific Research Project of Guizhou Provincial Department of Education. We thank Qingqing Hu (from Class 1, Psychologic Medicine), Jiajing Chen, Yunfeng He, Pu Shen, and Dingkun Yang (from Class 2, Psychologic Medicine) in the First Clinical Institute, Zunyi Medical University for useful discussions.References
- D. Zhao, Epidemiological features of cardiovascular disease in Asia, J. Am. Coll. Cardiol., 2021, 1(1), 1–13 Search PubMed.
- B. Şahin and G. İlgün, Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries, Health Soc. Care Community, 2022, 30(1), 73–80 CrossRef PubMed.
- P. Gupta and B. B. Mandal, Tissue-Engineered Vascular Grafts: Emerging Trends and Technologies, Adv. Funct. Mater., 2021, 31(33), 2100027 CrossRef CAS.
- P. Mallis, A. Kostakis, C. Stavropoulos-Giokas and E. Michalopoulos, Future perspectives in small-diameter vascular graft engineering, Bioengineering, 2020, 7(4), 160 CrossRef CAS PubMed.
- S. Fang, D. G. Ellman and D. C. Andersen, Tissue engineering of small-diameter vascular grafts and their in vivo evaluation in large animals and humans, Cells, 2021, 10(3), 713 CrossRef CAS PubMed.
- H. H. G. Song, R. T. Rumma, C. K. Ozaki, E. R. Edelman and C. S. Chen, Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise, Cell Stem Cell, 2018, 22(3), 340–354 CrossRef CAS PubMed.
- D. Radke, W. Jia, D. Sharma, K. Fena, G. Wang and J. Goldman, et al., Tissue engineering at the blood-contacting surface: a review of challenges and strategies in vascular graft development, Adv. Healthcare Mater., 2018, 7(15), 1701461 CrossRef PubMed.
- S. Agrawal, S. K. Nooti, H. Singh and V. Rai, Nanomaterial-mediated theranostics for vascular diseases, J. Nanotheranostics, 2020, 2(1), 1–15 CrossRef.
- L. A. Kolahalam, I. K. Viswanath, B. S. Diwakar, B. Govindh, V. Reddy and Y. Murthy, Review on nanomaterials: synthesis and applications, Mater. Today: Proc., 2019, 18, 2182–2190 Search PubMed.
- N. Elahi, M. Kamali and M. H. Baghersad, Recent biomedical applications of gold nanoparticles: a review, Talanta, 2018, 184, 537–556 CrossRef CAS PubMed.
- M. Yadid, R. Feiner and T. Dvir, Gold Nanoparticle-Integrated Scaffolds for Tissue Engineering and Regenerative Medicine, Nano Lett., 2019, 19(4), 2198–2206 CrossRef CAS PubMed.
- A. M. Ostdiek, J. R. Ivey, S. A. Hansen, R. Gopaldas and S. A. Grant, Feasibility of a nanomaterial-tissue patch for vascular and cardiac reconstruction, J. Biomed. Mater. Res. B Appl. Biomater., 2016, 104(3), 449–457 CrossRef CAS PubMed.
- A. M. Ostdiek, J. R. Ivey, D. A. Grant, J. Gopaldas and S. A. Grant, An in vivo study of a gold nanocomposite biomaterial for vascular repair, Biomaterials, 2015, 65, 175–183 CrossRef CAS PubMed.
- S. A. Grant, C. S. Spradling, D. N. Grant, D. B. Fox, L. Jimenez and D. A. Grant, et al., Assessment of the biocompatibility and stability of a gold nanoparticle collagen bioscaffold, J. Biomed. Mater. Res., Part A, 2014, 102(2), 332–339 CrossRef PubMed.
- S. A. Grant, J. Zhu, J. Gootee, C. L. Snider, M. Bellrichard and D. A. Grant, Gold Nanoparticle-Collagen Gels for Soft Tissue Augmentation, Tissue Eng., Part A, 2018, 24(13–14), 1091–1098 CrossRef CAS PubMed.
- A. Baki, F. Wiekhorst and R. Bleul, Advances in Magnetic Nanoparticles Engineering for Biomedical Applications-A Review, Bioengineering, 2021, 8(10), 134 CrossRef CAS PubMed.
- M. Monteserin, S. Larumbe, A. V. Martinez, S. Burgui and L. Francisco Martin, Recent Advances in the Development of Magnetic Nanoparticles for Biomedical Applications, J. Nanosci. Nanotechnol., 2021, 21(5), 2705–2741 CrossRef CAS PubMed.
- V. F. Cardoso, A. Francesko, C. Ribeiro, M. Banobre-Lopez, P. Martins and S. Lanceros-Mendez, Advances in Magnetic Nanoparticles for Biomedical Applications, Adv. Healthcare Mater., 2018, 7(5), 1700845 CrossRef PubMed.
- H. Perea, J. Aigner, J. T. Heverhagen, U. Hopfner and E. Wintermantel, Vascular tissue engineering with magnetic nanoparticles: seeing deeper, J. Tissue Eng. Regener. Med., 2007, 1(4), 318–321 CrossRef CAS PubMed.
- A. Ito, K. Ino, M. Hayashida, T. Kobayashi, H. Matsunuma and H. Kagami, et al., Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force, Tissue Eng., 2005, 11(9–10), 1553–1561 CrossRef CAS PubMed.
- B. M. Mattix, T. R. Olsen, M. Casco, L. Reese, J. T. Poole and J. Zhang, et al., Janus magnetic cellular spheroids for vascular tissue engineering, Biomaterials, 2014, 35(3), 949–960 CrossRef CAS PubMed.
- R. Verma, S. Pathak, A. K. Srivastava, S. Prawer and S. Tomljenovic-Hanic, ZnO nanomaterials: green synthesis, toxicity evaluation and new insights in biomedical applications, J. Alloys Compd., 2021, 876, 160175 CrossRef CAS.
- V. N. Kalpana and V. D. Rajeswari, A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs, Bioinorg. Chem. Appl., 2018, 2018, 3569758 CAS.
- R. Augustine, P. Dan, A. Sosnik, N. Kalarikkal, N. Tran and B. Vincent, et al., Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation, Nano Res., 2017, 10(10), 3358–3376 CrossRef CAS.
- G. G. Genchi, A. Marino, A. Rocca, V. Mattoli and G. Ciofani, Barium titanate nanoparticles: promising multitasking vectors in nanomedicine, Nanotechnology, 2016, 27(23), 232001 CrossRef PubMed.
- A. Cafarelli, P. Losi, A. R. Salgarella, M. C. Barsotti, I. B. Di Cioccio and I. Foffa, et al., Small-caliber vascular grafts based on a piezoelectric nanocomposite elastomer: Mechanical properties and biocompatibility, J. Mech. Behav. Biomed. Mater., 2019, 97, 138–148 CrossRef CAS PubMed.
- S. K. Prajapati, A. Malaiya, P. Kesharwani, D. Soni and A. Jain, Biomedical applications and toxicities of carbon nanotubes, Drug Chem. Toxicol., 2022, 45(1), 435–450 CrossRef CAS PubMed.
- T. U. Wani, R. Mohi-ud-din, T. A. Wani, R. H. Mir, A. M. Itoo and F. A. Sheikh, et al., Green Synthesis, Spectroscopic Characterization and Biomedical Applications of Carbon Nanotubes, Curr. Pharm. Biotechnol., 2021, 22(6), 783–797 Search PubMed.
- N. Ding, C. Dou, Y. X. Wang, F. L. Liu, G. Guan and D. Huo, et al., Antishear Stress Bionic Carbon Nanotube Mesh Coating with Intracellular Controlled Drug Delivery Constructing Small-Diameter Tissue-Engineered Vascular Grafts, Adv. Healthcare Mater., 2018, 7(11), 1800026 CrossRef PubMed.
- E. M. Elmowafy, M. Tiboni and M. E. Soliman, Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles, J. Pharm. Invest., 2019, 49(4), 347–380 CrossRef CAS.
- K. K. Chereddy, V. L. Payen and V. Preat, PLGA: from a classic drug carrier to a novel therapeutic activity contributor, J. Controlled Release, 2018, 289, 10–13 CrossRef CAS PubMed.
- B. M. Al Meslmani, G. F. Mahmoud and U. Bakowsky, Development of expanded polytetrafluoroethylene cardiovascular graft platform based on immobilization of poly lactic-co-glycolic acid nanoparticles using a wet chemical modification technique, Int. J. Pharm., 2017, 529(1–2), 238–244 CrossRef CAS PubMed.
- H. Nishio, H. Masumoto, K. Sakamoto, K. Yamazaki, T. Ikeda and K. Minatoya, MicroRNA-145-loaded poly(lactic-co-glycolic acid) nanoparticles attenuate venous intimal hyperplasia in a rabbit model, J. Thorac. Cardiovasc. Surg., 2019, 157(6), 2242–2251 CrossRef CAS PubMed.
- H. Nishio, H. Masumoto, H. Kanemitsu, K. Ueyama, K. Yamazaki and T. Ikeda, et al., MicroRNA-145-loaded Poly Lactic-co-glycolic Acid Nanoparticles Attenuate Venous Intimal Hyperplasia: A Basic Study for Preventing Vein Graft Diseases, Circulation, 2017, 136, A14221 Search PubMed.
- B. C. Evans, R. B. Fletcher, K. V. Kilchrist, E. A. Dailing, A. J. Mukalel and J. M. Colazo, et al., An anionic, endosome-escaping polymer to potentiate intracellular delivery of cationic peptides, biomacromolecules, and nanoparticles, Nat. Commun., 2019, 10(1), 1–19 CrossRef CAS PubMed.
- B. C. Evans, K. M. Hocking, M. J. Osgood, I. Voskresensky, J. Dmowska and K. V. Kilchrist, et al., MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia, Sci. Transl. Med., 2015, 7(291), 291ra295 Search PubMed.
- A. Figueiras, C. Domingues, I. Jarak, A. I. Santos, A. Parra and A. Pais, et al., New Advances in Biomedical Application of Polymeric Micelles, Pharmaceutics, 2022, 14(8), 1700 CrossRef CAS PubMed.
- M. H. Lin, Y. Dai, F. Xia and X. J. Zhang, Advances in non-covalent crosslinked polymer micelles for biomedical applications, Mater. Sci. Eng. C, 2021, 119, 111626 CrossRef CAS PubMed.
- P. Thitiwuthikiat, M. Ii, T. Saito, M. Asahi, S. Kanokpanont and Y. Tabata, A Vascular Patch Prepared from Thai Silk Fibroin and Gelatin Hydrogel Incorporating Simvastatin-Micelles to Recruit Endothelial Progenitor Cells, Tissue Eng., Part A, 2015, 21(7–8), 1309–1319 CrossRef CAS PubMed.
- J. Shi, Y. C. Zhao, Z. F. Niu, H. J. Fan, S. K. Hou and X. Q. Guo, et al., Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: progress and prospect, World J. Stem Cell., 2021, 13(1), 49–63 CrossRef PubMed.
- F. Shekari, A. Nazari, S. A. Kashani, E. Hajizadeh-Saffar, R. Lim and H. Baharvand, Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: a systematic review, Cytotherapy, 2021, 23(4), 277–284 CrossRef CAS PubMed.
- Y. Z. Wei, Y. F. Wu, R. X. Zhao, K. Y. Zhang, A. C. Midgley and D. L. Kong, et al., MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia, Biomaterials, 2019, 204, 13–24 CrossRef CAS PubMed.
- E. M. Cunnane, K. L. Lorentz, A. K. Ramaswamy, P. Gupta, B. B. Mandal and F. J. O'Brien, et al., Extracellular Vesicles Enhance the Remodeling of Cell-Free Silk Vascular Scaffolds in Rat Aortae, ACS Appl. Mater. Interfaces, 2020, 12(24), 26955–26965 CrossRef CAS PubMed.
- Z. H. Syedain, R. Maciver and R. T. Tranquillo, Vascular grafts and valves that animate, made from decellularized biologically-engineered tissue tubes, J. Cardiovasc. Surg., 2020, 61(5), 577–585 Search PubMed.
- C. H. Lin, K. Hsia, H. Ma, H. Lee and J. H. Lu, In Vivo Performance of Decellularized Vascular Grafts: A Review Article, Int. J. Mol. Sci., 2018, 19(7), 2101 CrossRef PubMed.
- A. Porzionato, E. Stocco, S. Barbon, F. Grandi, V. Macchi and R. De Caro, Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives, Int. J. Mol. Sci., 2018, 19(12), 4117 CrossRef PubMed.
- M. Kimicata, P. Swamykumar and J. P. Fisher, Extracellular Matrix for Small-Diameter Vascular Grafts, Tissue Eng., Part A, 2020, 26(23–24), 1388–1401 CrossRef PubMed.
- M. Carrabba and P. Madeddu, Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts, Front. Bioeng. Biotechnol., 2018, 6, 41 CrossRef PubMed.
- X. Qiu, B. L.-P. Lee, S. Y. Wong, X. Ding, K. Xu and W. Zhao, et al., Cellular remodeling of fibrotic conduit as vascular graft, Biomaterials, 2021, 268, 120565 CrossRef CAS PubMed.
- J. Xue, D. Pisignano and Y. Xia, Maneuvering the Migration and Differentiation of Stem Cells with Electrospun Nanofibers, Adv. Sci., 2020, 7(15), 2000735 CrossRef CAS PubMed.
- M. M. Stevens and J. H. George, Exploring and engineering the cell surface interface, Science, 2005, 310(5751), 1135–1138 CrossRef CAS PubMed.
- A. P. Rickel, X. Deng, D. Engebretson and Z. Hong, Electrospun nanofiber scaffold for vascular tissue engineering, Mater. Sci. Eng. C, 2021, 129, 112373 CrossRef CAS PubMed.
- B. B. Leal, N. Wakabayashi, K. Oyama, H. Kamiya, D. I. Braghirolli and P. Pranke, Vascular tissue engineering: polymers and methodologies for small caliber vascular grafts, Front. Cardiovasc. Med., 2021, 7, 592361 CrossRef PubMed.
- S. Bai, X. Zhang, L. Zang, S. Yang, X. Chen and X. Yuan, Electrospinning of biomaterials for vascular regeneration, Chem. Res. Chin. Univ., 2021, 37(3), 394–403 CrossRef CAS.
- A. Hasan, A. Memic, N. Annabi, M. Hossain, A. Paul and M. R. Dokmeci, et al., Electrospun scaffolds for tissue engineering of vascular grafts, Acta Biomater., 2014, 10(1), 11–25 CrossRef CAS PubMed.
- J. Li, N. Zhuo, J. Zhang, Q. Sun, J. Si and K. Wang, et al., The loading of C-type natriuretic peptides improved hemocompatibility and vascular regeneration of electrospun poly(ε-caprolactone) grafts, Acta Biomater., 2022, 151, 304–316 CrossRef CAS PubMed.
- A. F. Jozala, L. C. de Lencastre-Novaes, A. M. Lopes, V. de Carvalho Santos-Ebinuma, P. G. Mazzola and A. Pessoa Jr, et al., Bacterial nanocellulose production and application: a 10-year overview, Appl. Microbiol. Biotechnol., 2016, 100(5), 2063–2072 CrossRef CAS PubMed.
- C. Weber, S. Reinhardt, K. Eghbalzadeh, M. Wacker, M. Guschlbauer and A. Maul, et al., Patency and in vivo compatibility of bacterial nanocellulose grafts as small-diameter vascular substitute, J. Vasc. Surg., 2018, 68(6), 177S–187S CrossRef PubMed.
- Z. Goli-Malekabadi and S. Pournaghmeh, Nanocellulose for Vascular Grafts and Blood Vessel Tissue Engineering, Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications, Springer, 2022, pp. 1–24 Search PubMed.
- S. E. Lee and Y. S. Park, The role of bacterial cellulose in artificial blood vessels, Mol. Cell. Toxicol., 2017, 13(3), 257–261 CrossRef CAS.
- D. Klemm, D. Schumann, U. Udhardt and S. Marsch, Bacterial synthesized cellulose—artificial blood vessels for microsurgery, Prog. Polym. Sci., 2001, 26(9), 1561–1603 CrossRef CAS.
- G. Hu, L. Chen, S. Zhao and F. F. Hong, Mercerization of tubular bacterial nanocellulose for control of the size and performance of small-caliber vascular grafts, Chem. Eng. J., 2022, 428, 131104 CrossRef CAS.
- S. Huang, S. L. Sing, G. de Looze, R. Wilson and W. Y. Yeong, Laser powder bed fusion of titanium-tantalum alloys: compositions and designs for biomedical applications, J. Mech. Behav. Biomed. Mater., 2020, 108, 103775 CrossRef CAS PubMed.
- G. Maccauro, P. R. Iommetti, F. Muratori, L. Raffaelli, P. F. Manicone and C. Fabbriciani, An overview about biomedical applications of micron and nano size tantalum, Recent Pat. Biotechnol., 2009, 3(3), 157–165 CrossRef CAS PubMed.
- R. A. Silva, I. P. Silva and B. Rondot, Effect of surface treatments on anodic oxide film growth and electrochemical properties of tantalum used for biomedical applications, J. Biomater. Appl., 2006, 21(1), 93–103 CrossRef CAS PubMed.
- R. A. Silva, M. Walls, B. Rondot, M. Da Cunha Belo and R. Guidoin, Electrochemical and microstructural studies of tantalum and its oxide films for biomedical applications in endovascular surgery, J. Mater. Sci. Mater. Med., 2002, 13(5), 495–500 CrossRef CAS PubMed.
- C. Park, S. Park, J. Kim, A. Han, S. Ahn and S.-K. Min, et al., Enhanced endothelial cell activity induced by incorporation of nano-thick tantalum layer in artificial vascular grafts, Appl. Surf. Sci., 2020, 508, 144801 CrossRef CAS.
- Y. Zhuang, C. Zhang, M. Cheng, J. Huang, Q. Liu and G. Yuan, et al., Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts, Bioact. Mater., 2021, 6(6), 1791–1809 CAS.
- P. Mohindra and T. A. Desai, Micro-and nanoscale biophysical cues for cardiovascular disease therapy, Nanomed. Nanotechnol. Biol. Med., 2021, 34, 102365 CrossRef CAS PubMed.
- Z. Wang, C. Liu, Y. Xiao, X. Gu, Y. Xu and N. Dong, et al., Remodeling of a cell-free vascular graft with nanolamellar intima into a neovessel, ACS Nano, 2019, 13(9), 10576–10586 CrossRef CAS PubMed.
- Z. Ji, W. Guo, S. Sakkiah, J. Liu, T. A. Patterson and H. Hong, Nanomaterial databases: data sources for promoting design and risk assessment of nanomaterials, Nanomaterials, 2021, 11(6), 1599 CrossRef CAS PubMed.
- V. Ragaseema, S. Unnikrishnan, V. K. Krishnan and L. K. Krishnan, The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces, Biomaterials, 2012, 33(11), 3083–3092 CrossRef CAS PubMed.
- R. V. Madhavan, M. J. Rosemary, M. A. Nandkumar, K. V. Krishnan and L. K. Krishnan, Silver nanoparticle impregnated poly (ε-Caprolactone) scaffolds: optimization of antimicrobial and noncytotoxic concentrations, Tissue Eng., Part A, 2011, 17(3–4), 439–449 CrossRef CAS PubMed.
- S. Shrivastava, T. Bera, S. K. Singh, G. Singh, P. Ramachandrarao and D. Dash, Characterization of antiplatelet properties of silver nanoparticles, ACS Nano, 2009, 3(6), 1357–1364 CrossRef CAS PubMed.
- I. Junkar, M. Kulkarni, M. Benčina, J. Kovač, K. Mrak-Poljšak and K. Lakota, et al., Titanium dioxide nanotube arrays for cardiovascular stent applications, ACS Omega, 2020, 5(13), 7280–7289 CrossRef CAS PubMed.
- I. Junkar, M. Kulkarni, P. Humpolíček, Z. Capáková, B. Burja, A. Mazare, et al., Could titanium dioxide nanotubes represent a viable support system for appropriate cells in vascular implants?, Advances in Biomembranes and Lipid Self-Assembly, Elsevier, 2017, vol. 25, pp. 1–39 Search PubMed.
- Z. Li, C. Jiang, L. Chai, T. Fan, C. Li and Z. Chen, et al., New insights to atherosclerosis management: Role of nanomaterials, Appl. Mater. Today, 2022, 27, 101466 CrossRef.
- P. Ambesh, U. Campia, C. Obiagwu, R. Bansal, V. Shetty and G. Hollander, et al., Nanomedicine in coronary artery disease, Indian Heart J., 2017, 69(2), 244–251 CrossRef PubMed.
- M. Iafisco, A. Alogna, M. Miragoli and D. Catalucci, Cardiovascular nanomedicine: the route ahead, Nanomedicine, 2019, 2391–2394 CrossRef CAS PubMed.
- I. Cicha, C. Chauvierre, I. Texier, C. Cabella, J. M. Metselaar and J. Szebeni, et al., From design to the clinic: practical guidelines for translating cardiovascular nanomedicine, Cardiovasc. Res., 2018, 114(13), 1714–1727 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |