Open Access Article
Open Access ArticleCatalytic intramolecular aminoarylation of unactivated alkenes with aryl sulfonamides†
Efrey A.
Noten
 ,
Rory C.
McAtee
,
Rory C.
McAtee
 and
Corey R. J.
Stephenson
and
Corey R. J.
Stephenson
 *
*
University of Michigan, Department of Chemistry, Willard Henry Dow Laboratory, 930 North University Ave., Ann Arbor, MI 48109, USA. E-mail: crjsteph@umich.edu
First published on 11th May 2022
Abstract
Arylethylamines are abundant motifs in myriad natural products and pharmaceuticals, so efficient methods to synthesize them are valuable in drug discovery. In this work, we disclose an intramolecular alkene aminoarylation cascade that exploits the electrophilicity of a nitrogen-centered radical to form a C–N bond, then repurposes the nitrogen atom's sulfonyl activating group as a traceless linker to form a subsequent C–C bond. This photoredox catalysis protocol enables the preparation of densely substituted arylethylamines from commercially abundant aryl sulfonamides and unactivated alkenes under mild conditions. Reaction optimization, scope, mechanism, and synthetic applications are discussed.
Introduction
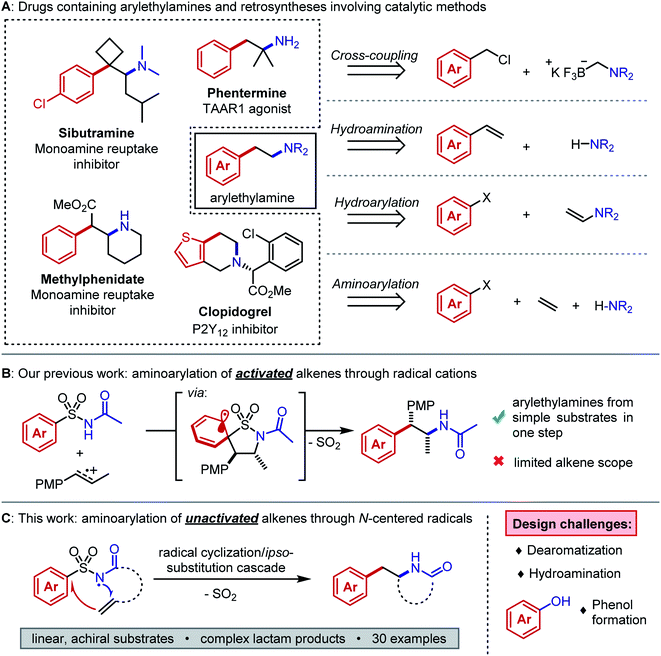
The arylethylamine pharmacophore is conserved across a range of biologically active natural products and drugs, particularly in molecules that act on the central nervous system (Fig. 1A, left).1 Conventional preparations of arylethylamines rely on linear, stoichiometric transformations to forge key C–C and C–N bonds.2 Such routes lack the combinatorial flexibility favored in early-stage medicinal chemistry campaigns and they restrict the accessible substitution patterns of the ethylene linker fragment. Substituents on the linker can drastically alter the molecule's lipophilicity, conformation, and elimination half-life.3,4 Modular preparations of complex arylethylamines from commercially available or easily synthesized substrates are therefore highly valuable, and considerable efforts have focused on this need (Fig. 1A, right).Recently, Murphy, Barrett, and coworkers published a method for arylethylamine synthesis by palladium-catalyzed Csp3–Csp3 cross-coupling of (chloromethyl)aryl electrophiles and aminomethyltrifluoroborate salts.5 A diverse library of compounds could be quickly produced in this manner; however, no products bearing linker substituents were reported. An alternative and succinct disconnection of an arylethylamine could be the difunctionalization of an alkene to incorporate (1) the C–N bond, (2) the aryl–Csp3 bond, or (3) both bonds at once. The first case describes anti-Markovnikov hydroamination of a styrene, and many methods exist to accomplish this transformation effectively with the aid of photoredox, lanthanide, or transition metal catalysts.6–9 The second case necessitates anti-Markovnikov hydroarylation of an enamine, which was only recently reported in good yields by Jui and coworkers.10 The third case entails aminoarylation of an unactivated alkene and is, in principle, the most modular of the three difunctionalization strategies. Because the substrate is decoupled from both the arene and the nitrogen atom, simple alkenes can be converted to arylethylamines in one step. Our interests in complex molecule synthesis by radical methods led us to question whether aminoarylation could be achieved with nitrogen-centered radicals. Such electrophilic intermediates can be accessed readily through photoredox catalysis.11,12 We perceived the advances by Knowles and coworkers in catalytic N-centered radical generation as particularly enabling towards our goal.13,14 Formal homolysis of N–H bonds via multiple-site concerted proton-electron transfer (MS-CPET) permits useful reactivity of N-centered radicals without the need for harsh oxidants or strong bases.15 If the N–H bond is sufficiently acidic, stepwise deprotonation/oxidation sequences can also give N-centered radicals under mild conditions.16
We first considered the state of the art in unactivated alkene aminoarylation to inform our reaction design. Palladium-catalyzed alkene aminoarylation was explored extensively by Wolfe and coworkers in the preparation of saturated nitrogen heterocycles.17,18 The Chemler and Antonchik groups have put forth copper-catalyzed19 and metal-free20 approaches to intramolecular carboamination. Engle and coworkers employed directing groups to orchestrate palladium- and nickel-catalyzed intermolecular aminoarylations of β,γ-unsaturated enamides and of homoallylic alcohols, respectively.21,22 The Molander and Leonori laboratories merged photoredox- and transition metal catalysis by trapping amidyl radical cyclization intermediates with nickel to accomplish C–C cross-coupling.23,24 Gaunt and coworkers achieved a three-component copper-catalyzed alkene azidoarylation using diaryliodonium triflates as aryl radical precursors.25 We envisioned a desulfonylative 1,4-aryl migration (Smiles–Truce rearrangement) as an unconventional disconnection of the C–C bond that could be induced by an N-centered sulfonamidyl radical addition to an alkene. Aryl migrations have become increasingly accessible to synthetic chemists as modern photochemical techniques have matured.26–28 In this case, the migration would grant entry to the arylethylamine scaffold from inexpensive sulfonamides.29,30 Mechanistically, this distinct cascade would not require a cross-coupling catalyst and may grant access to sterically congested products that are challenging to prepare through transition metal-mediated methods. Although Molander's approach to aminoarylation initiates by an N-centered radical cyclization from an N–H bond, the MS-CPET method chosen to generate the radical necessitates N-aryl amide precursors. Oxidative cleavage of the auxiliary arene in the product is therefore necessary to provide the free lactam. By contrast, the designed desulfonylative aryl migration in this work would function as an in situ deprotection of the nitrogen atom.
We previously reported an alkene aminoarylation that proceeded through alkene radical cation intermediates.31 These electrophilic species successfully coupled with sulfonamides, leading to a Smiles–Truce rearrangement that delivered the desired arylethylamine (Fig. 1B). However, only electron-rich, 1,2-disubstituted styrenes gave good yields. This restriction was attributed to the low oxidation potentials of the activated alkenes (1.28 V vs. SCE in CH3CN for trans-anethole) and the higher propensity of monosubstituted styrene radical cations to oligomerize.32,33 We expected our N-centered radical approach to circumvent this limitation as well, based on strong literature precedent describing anti-Markovnikov sulfonamidyl radical additions to unactivated alkenes.19,34–39 However, Smiles–Truce rearrangements following such radical additions have only been reported to occur by Deagostino and Liu in specially designed γ,δ-unsaturated arylsulfonyl hydrazones as applied to the synthesis of tetrahydropyridazines.40,41
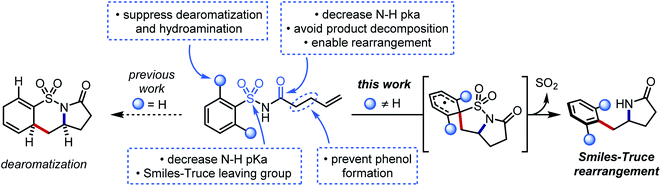
We hypothesized that a second electron-withdrawing group on the nitrogen atom could convert the sulfonamide into a better leaving group. This modification would also prevent a reactive free amine from forming after N-desulfonylation, and it would further increase the acidity of the N–H bond (pKa ≈ 5) such that stepwise N-centered radical generation could be feasible.42 However, intermolecular addition of N-acylsulfonamidyl radicals to unactivated alkenes was not observed. 1,4-Aryl migration to the carbonyl oxygen instead gave desulfonylated phenols.43,44 To avert this undesired Smiles rearrangement, we synthesized N-acylsulfonamides bearing tethered alkenes that would rapidly trap the N-centered radical in a 5-exo-trig cyclization. Desulfonylative aryl migration to the incipient alkyl radical would then provide the desired arylethylamine (Fig. 2, right).
 | ||
| Fig. 2 Structural features of the aminoarylation substrates that favor Smiles–Truce rearrangement and disfavor undesired side reactions. | ||
Results and discussion
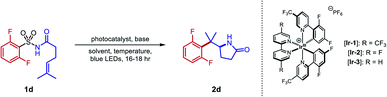
Our initial efforts to develop this reaction revealed that instances of the substrates with only meta or para substitution would selectively undergo dearomative addition of the alkyl radical ortho to the sulfonyl group, followed by radical-polar crossover and protonation to garner 1,4-cyclohexadiene-fused sultams (Fig. 2, left).45 We reasoned that substituents in the ortho positions could inhibit the dearomative cyclization. Thus, when 2,6-difluorobenzenesulfonyl enamide 1d was exposed to the dearomatization conditions from our previous work, the Smiles–Truce rearrangement occurred instead to give lactam 2d in 45% isolated yield. Notably, the new quaternary center in 2d is difficult to assemble through comparable transition metal-catalyzed methods.23,24,46 Compound 1d was therefore chosen as a model substrate for optimization, and key observations from this process are highlighted in Table 1.| Entry | Photocatalyst | Base | Solvent | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Asterisks (*) denote isolated yields. b Reaction conducted in a 17 mm diameter vial. | |||||
| 1 | Ir-1 | NBu4PO2(OnBu)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PhCF3/tBuOH (0.05 M) 1 PhCF3/tBuOH (0.05 M) |
35 | 45a |
| 2 | Ir-1 | NBu4PO2(OnBu)2 | PhCF3 (0.05 M) | 35 | 68 |
| 3 | Ir-1 | NBu4PO2(OnBu)2 | PhCF3 (0.1 M) | 35 | 39 |
| 4 | Ir-1 | NBu4PO2(OnBu)2 | CH3CN (0.05 M) | 35 | 70 |
| 5 | Ir-2 | NBu4PO2(OnBu)2 | CH3CN (0.05 M) | 35 | 44 |
| 6 | Ir-3 | NBu4PO2(OnBu)2 | CH3CN (0.05 M) | 35 | 43 |
| 7 | Ir-1 | NBu4PO2(OnBu)2 | CH3CN (0.05 M) | 60 | 63 |
| 8 | Ir-1 | NBu4PO2(OnBu)2 | PhCF3 (0.05 M) | 60 | 74 |
| 9 | Ir-1 | NBu4OBz | PhCF3 (0.05 M) | 60 | 67 |
| 10 | Ir-1 | KOBz | PhCF3 (0.05 M) | 60 | 71 |
| 11 | Ir-1 | p-OMeC6H4CO2Na | PhCF3 (0.05 M) | 60 | 45 |
| 12 | Ir-1 | p-OMeC6H4CO2K | PhCF3 (0.05 M) | 60 | 77 |
| 13 | Ir-1 | p-ClC6H4CO2K | PhCF3 (0.05 M) | 60 | 78 (72a) |
| 14 | Ir-1 | p-ClC 6 H 4 CO 2 K | PhCF 3 (0.05 M) | 60 | 70 (64 ) |
| 15 | None | p-ClC6H4CO2K | PhCF3 (0.05 M) | 60 | 0 |
| 16 | Ir-1 | None | PhCF3 (0.05 M) | 60 | <5% |
| 17 | Ir-1 (no light) | p-ClC6H4CO2K | PhCF3 (0.05 M) | 60 | 0 |
Although tBuOH was beneficial for the dearomative cyclization as part of a binary solvent mixture with PhCF3, yield of 2d improved when tBuOH was excluded (entry 2). The strong oxidizing properties of Ir-1 were crucial; less oxidizing iridium photocatalysts such Ir-2 and Ir-3 gave reduced yields. At ambient temperature, CH3CN was found to give the highest yield of 2d among all solvents evaluated (entry 4). However, at 60 °C, the yield decreased in CH3CN but improved further in PhCF3 (entries 7 and 8). We initially deemed tetrabutylammonium dibutylphosphate (NBu4PO2(OnBu)2) a suitable base, but it required several days to dry fully once prepared and was inconvenient to handle under ambient atmosphere due to its marked hygroscopicity. To allow a simpler reaction set-up, we sought alternative bases (entries 9–13). Ultimately, we identified potassium p-chlorobenzoate as a free-flowing powder that gave satisfactory yields of 2d despite its low solubility in PhCF3. Although isolated yields were slightly lower in larger vials (entry 14), the increased volume was necessary to conduct the reaction on a scale greater than 0.1 mmol. In this work, we opted to report the substrate scope on a 0.2 mmol scale. Control experiments excluding photocatalyst or light failed to generate detectable quantities of 2d, while only trace product was observed in the absence of a base (entries 15–17).
After identifying optimal conditions, the arene scope of the reaction was demonstrated on a variety of electron-neutral and electron-deficient mono- and bis-ortho-substituted benzene derivatives, as well as on a selection of heteroaromatic sulfonamides (Fig. 3). ortho-Halogenated arenes—many of which would be incompatible with palladium or nickel catalysts—and ortho-trifluoromethylated arenes were generally well-tolerated (2a–2k). (E)-Styrenyl sulfonamide 1l underwent a vinylogous Smiles–Truce rearrangement and alkene isomerization to give the aminoalkenylation product 2l as a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Z/E isomers. Heterocycles including pyridine (2m), furan (2n), thiophene (2o–2q), and thiazole (2r) could all undergo migration as well in modest to good yields. 2-Substituted thiophene 1o gave a 9
1 mixture of Z/E isomers. Heterocycles including pyridine (2m), furan (2n), thiophene (2o–2q), and thiazole (2r) could all undergo migration as well in modest to good yields. 2-Substituted thiophene 1o gave a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of lactam 2o with the 3-substituted thiophene regioisomer 2o′ arising from a C3-addition/β-elimination sequence (see ESI†).47 In the presence of an ortho ester substituent (1s), the amidyl anion liberated upon desulfonylation displaced the alkoxide to produce the tricyclic imide 2s. Next, we surveyed the scope of amenable alkenes. Monosubstituted (2t), disubstituted (2u–2z), trisubstituted (2aa–2ad), and tetrasubstituted (2ae) alkenes were all successfully functionalized, producing lactams with diverse carbon skeletons. The more electron-deficient trichlorophenyl ring in compound 1z was necessary to promote successful migration to the inherently less nucleophilic benzylic radical intermediate.
1 mixture of lactam 2o with the 3-substituted thiophene regioisomer 2o′ arising from a C3-addition/β-elimination sequence (see ESI†).47 In the presence of an ortho ester substituent (1s), the amidyl anion liberated upon desulfonylation displaced the alkoxide to produce the tricyclic imide 2s. Next, we surveyed the scope of amenable alkenes. Monosubstituted (2t), disubstituted (2u–2z), trisubstituted (2aa–2ad), and tetrasubstituted (2ae) alkenes were all successfully functionalized, producing lactams with diverse carbon skeletons. The more electron-deficient trichlorophenyl ring in compound 1z was necessary to promote successful migration to the inherently less nucleophilic benzylic radical intermediate.
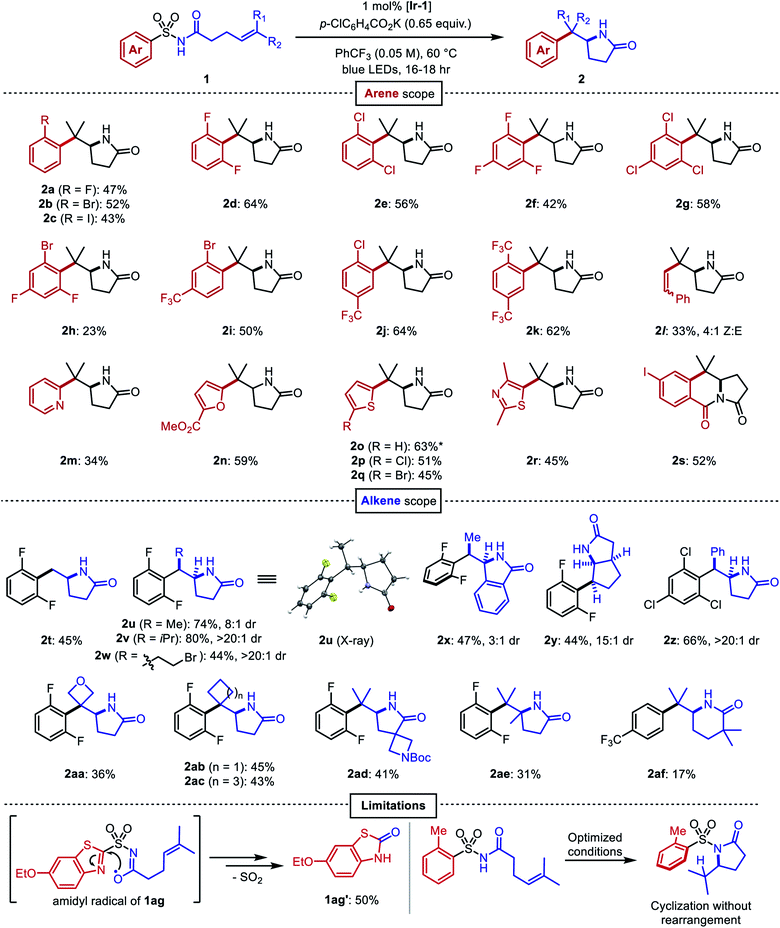 | ||
Fig. 3 Scope of the aminoarylation. All reactions performed on 0.2 mmol scale. All yields are from isolation. *Isolated as 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture with regioisomeric product 2o′, see ESI.† 1 mixture with regioisomeric product 2o′, see ESI.† | ||
Seemingly minor manipulations of the alkene tether in 1ad could greatly alter the reaction: when the tether was homologated and gem-dimethyl substitution was incorporated alpha to the carbonyl, the ensuing 6-exo trig cyclization triggered the migration of a 4-trifluoromethylphenyl ring lacking ortho substitution. This result was surprising because the same arene in our previous work on dearomative cyclization was not observed to undergo rearrangement.45 We believe that the Thorpe–Ingold effect in this substrate accelerates 6-exo-trig ring closure, and that the resultant alkyl radical is oriented closer to the ipso carbon of the sulfonamide than to the ortho carbons. This intriguing divergence invites further study of the impact that conformational biases may exert on the course of the reaction.
Certain limitations also became clear as we interrogated the scope of the reaction. Benzothiazole substrate 1ag degraded to benzothiazolone 1ag′ through the aforementioned desulfonylative arene oxygenation, which may be faster than sulfonamidyl radical cyclization in heterocycles with high migratory aptitudes.48 Electron-donating ortho substituents on the sulfonamide prohibited the desired aryl transfer. Consequently, hydrogen atom transfer (HAT) or reduction of the alkyl radical following 5-exo cyclization led to undesired hydroamination side products.
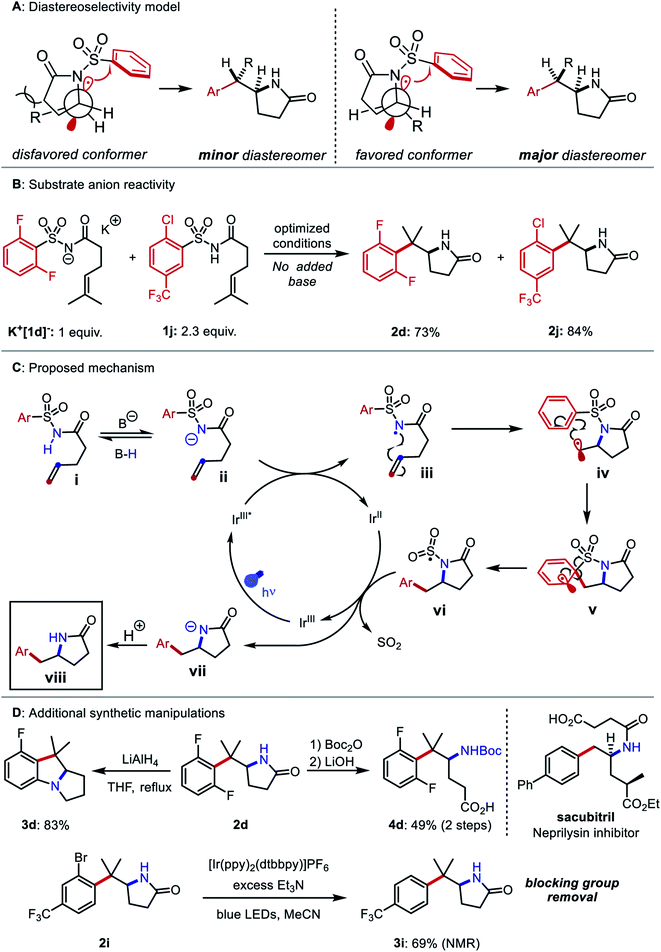
Compounds 1u–1z bearing 1,2-disubstituted olefins underwent aminoarylation with varying diastereoselectivities. The major diastereomer of product 2u (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) was isolated and its relative configuration was elucidated through X-ray crystallographic analysis. A possible model to rationalize the observed stereoselectivity is provided in Fig. 4A. Following 5-exo cyclization, a bond rotation positions the larger alkene substituent to minimize steric interaction with the newly formed lactam. When the alkene was substituted with larger groups, compounds 2v, 2w, and 2z were isolated as single diastereomers. In the aminoarylation of N-aroyl sulfonamide 1x (1
1 dr) was isolated and its relative configuration was elucidated through X-ray crystallographic analysis. A possible model to rationalize the observed stereoselectivity is provided in Fig. 4A. Following 5-exo cyclization, a bond rotation positions the larger alkene substituent to minimize steric interaction with the newly formed lactam. When the alkene was substituted with larger groups, compounds 2v, 2w, and 2z were isolated as single diastereomers. In the aminoarylation of N-aroyl sulfonamide 1x (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z), the sp2-hybridized carbon atoms at the lactam/arene ring fusion possess an attenuated steric influence on the methyl group. Therefore, diminished diastereoselectivity (3
1 E/Z), the sp2-hybridized carbon atoms at the lactam/arene ring fusion possess an attenuated steric influence on the methyl group. Therefore, diminished diastereoselectivity (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) was observed in the isomer distribution of 2x.
1 dr) was observed in the isomer distribution of 2x.
We then investigated the mechanism of N-centered radical generation. We considered three possibilities: (1) oxidation of benzoate by the photocatalyst, followed by HAT from the N–H bond of 1 to the resulting benzoyloxy radical; (2) oxidative MS-CPET involving the photocatalyst and a hydrogen-bonded substrate–benzoate complex, or (3) deprotonation of 1 by benzoate and subsequent oxidation of the N-acylsulfonamidyl anion. The first proposal seemed unlikely based on observations from the reaction optimization. Specifically, use of potassium o-methylbenzoate as the base gave 55% yield of product 2d, even though the benzoyloxy radical derived from this compound undergoes 1,5 HAT that would likely outcompete intermolecular HAT in the dilute reaction conditions.49 Use of pyridine, with an oxidation potential (2.2 V vs. SCE in CH3CN) well beyond that of the excited state of Ir-1 (1.68 V vs. SCE in CH3CN), still resulted in 12% yield of 2d.50,51 The second proposal was evaluated and rejected in our previous work based on Stern–Volmer luminescence quenching experiments, which indicated that tetrabutylammonium dibutylphosphate and N-acylsulfonamides do not form more easily oxidizable complexes in CH2Cl2 solution.45 The base and the solvent examined in the quenching studies differ from those employed in our optimized aminoarylation conditions, but both can be substituted successfully with only moderate yield reduction (see ESI†). To assess the third proposal, a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mixture of K+[1d−] and 1j was subjected to the optimized conditions without added base. In this case, full consumption of both compounds and good yields of their respective products 2d and 2j was observed (Fig. 4B). These data suggest a stepwise deprotonation-oxidation as the operative mechanism by which the N-centered radical forms. The data also imply that some reaction intermediate can deprotonate the starting material. Based on these findings, we posit a mechanism for the reaction detailed in Fig. 4C: The photoexcited iridium catalyst IrIII* oxidizes the deprotonated N-acylsulfonamide ii to the N-centered radical iii. The C–N bond is then formed via 5-exo-trig cyclization and the resultant alkyl radical iv adds to the arene to yield dearomatized spirocycle v. Elimination of the sulfonyl group from v restores the aromatic system and gives the N-sulfonyl radical vi. Desulfonylation from vi and reduction by IrII restores the ground state of the photocatalyst and produces amidyl anion vii. The anion irreversibly deprotonates either the benzoic acid or another equivalent of i to furnish the product viii.
7 mixture of K+[1d−] and 1j was subjected to the optimized conditions without added base. In this case, full consumption of both compounds and good yields of their respective products 2d and 2j was observed (Fig. 4B). These data suggest a stepwise deprotonation-oxidation as the operative mechanism by which the N-centered radical forms. The data also imply that some reaction intermediate can deprotonate the starting material. Based on these findings, we posit a mechanism for the reaction detailed in Fig. 4C: The photoexcited iridium catalyst IrIII* oxidizes the deprotonated N-acylsulfonamide ii to the N-centered radical iii. The C–N bond is then formed via 5-exo-trig cyclization and the resultant alkyl radical iv adds to the arene to yield dearomatized spirocycle v. Elimination of the sulfonyl group from v restores the aromatic system and gives the N-sulfonyl radical vi. Desulfonylation from vi and reduction by IrII restores the ground state of the photocatalyst and produces amidyl anion vii. The anion irreversibly deprotonates either the benzoic acid or another equivalent of i to furnish the product viii.
Finally, we performed additional diversification of the aminoarylation products that either leveraged the ortho substituents as functional handles to build additional complexity or converted the structures to molecules resembling other biologically active compounds (Fig. 4D). Reaction of 2d with LiAlH4 gave benzopyrrolizidine 3d through a sequential lactam reduction and SNAr of fluoride. A Boc protection/hydrolysis sequence yielded 3-(arylmethyl)-3-aminobutyric acid 4d, which mimics the carbon skeleton of the neprilysin inhibitor sacubitril. Using a strongly reducing photocatalyst52 and an amine reductant, we carried out the hydrodehalogenation of aryl bromide 2i, thus rendering the bromine atom a traceless blocking group in the synthesis of ortho-unsubstituted arylethylamine 3i.
Conclusion
In summary, we have developed a unique alkene aminoarylation that affords products containing privileged arylethylamine connectivity. The method is compatible with a broad selection of unactivated alkenes and is orthogonal to existing cross-coupling methods. We engineered the substrates to curb unproductive pathways en route to a Smiles–Truce rearrangement prompted by C–N bond construction from an N-centered radical. The substrates are easily synthesized from commercially abundant building blocks and the reaction set-up is performed under ambient atmosphere using conveniently handled reagents. The strategy disclosed herein will inform future efforts to revisit the recalcitrant intermolecular variant of this chemistry. This work is a testament to the complexity-building capabilities of N-centered radicals and the cascade reactivities that they can unleash when properly controlled.Data availability
The X-ray crystal structure of compound 2u is available free of charge from the Cambridge Crystallographic Data Centre under deposition number 2045499.Author contributions
EAN and RCM conceived the project with direction from CRJS. EAN and RCM performed the initial reaction discovery and optimization. EAN performed mechanistic studies as well as synthesis and characterization of all new compounds. EAN prepared the manuscript with input from RCM and CRJS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support for this research from the NIH (GM127774 and GM144286) and the University of Michigan. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship for R. C. M (Grant No. DGE 1256260). We thank Dr Jeff W. Kampf for collecting and analyzing X-ray crystallography data.References
- A. Zhang, J. L. Neumeyer and R. J. Baldessarini, Chem. Rev., 2007, 107, 274–302 CrossRef CAS PubMed.
- O. I. Afanasyev, E. Kuchuk, D. L. Usanov and D. Chusov, Chem. Rev., 2019, 119, 11857–11911 CrossRef CAS PubMed.
- J. S. Markowitz and K. S. Patrick, J. Child Adolesc. Psychopharmacol., 2017, 27, 678–689 CrossRef CAS PubMed.
- W. T. Garvey, Expert Opin. Drug Saf., 2013, 12, 741–756 CrossRef CAS PubMed.
- R. A. Lippa, D. J. Battersby, J. A. Murphy and T. N. Barrett, J. Org. Chem., 2021, 86, 3583–3604 CrossRef CAS PubMed.
- T. M. Nguyen, N. Manohar and D. A. Nicewicz, Angew. Chem., Int. Ed., 2014, 53, 6198–6201 CrossRef CAS PubMed.
- J.-S. Ryu, G. Y. Li and T. J. Marks, J. Am. Chem. Soc., 2003, 125, 12584–12605 CrossRef CAS PubMed.
- M. Utsunomiya, R. Kuwano, M. Kawatsura and J. F. Hartwig, J. Am. Chem. Soc., 2003, 125, 5608–5609 CrossRef CAS PubMed.
- M. Utsunomiya and J. F. Hartwig, J. Am. Chem. Soc., 2004, 126, 2702–2703 CrossRef CAS PubMed.
- A. J. Boyington, C. P. Seath, A. M. Zearfoss, Z. Xu and N. T. Jui, J. Am. Chem. Soc., 2019, 141, 4147–4153 CrossRef CAS PubMed.
- H. Jiang and A. Studer, CCS Chem., 2019, 1, 38–49 CAS.
- K. Kwon, R. T. Simons, M. Nandakumar and J. L. Roizen, Chem. Rev., 2022, 122, 2353–2428 CrossRef CAS PubMed.
- G. J. Choi and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 9226–9229 CrossRef CAS PubMed.
- D. C. Miller, G. J. Choi, H. S. Orbe and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 13492–13495 CrossRef CAS PubMed.
- W. D. Morris and J. M. Mayer, J. Am. Chem. Soc., 2017, 139, 10312–10319 CrossRef CAS PubMed.
- J. C. K. Chu and T. Rovis, Nature, 2016, 539, 272–275 CrossRef PubMed.
- J. E. Ney and J. P. Wolfe, Angew. Chem., Int. Ed., 2004, 43, 3605–3608 CrossRef PubMed.
- J. E. Ney and J. P. Wolfe, J. Am. Chem. Soc., 2005, 127, 8644–8651 CrossRef CAS PubMed.
- W. Zeng and S. R. Chemler, J. Am. Chem. Soc., 2007, 129, 12948–12949 CrossRef CAS PubMed.
- K. Matcha, R. Narayan and A. P. Antonchick, Angew. Chem., Int. Ed., 2013, 52, 7985–7989 CrossRef CAS PubMed.
- Z. Liu, Y. Wang, Z. Wang, T. Zeng, P. Liu and K. M. Engle, J. Am. Chem. Soc., 2017, 139, 11261–11270 CrossRef CAS PubMed.
- T. Kang, N. Kim, P. T. Cheng, H. Zhang, K. Foo and K. M. Engle, J. Am. Chem. Soc., 2021, 143, 13962–13970 CrossRef CAS PubMed.
- S. Zheng, A. Gutierrez-Bonet and G. A. Molander, Chem, 2019, 5, 339–352 Search PubMed.
- L. Angelini, J. Davies, M. Simonetti, L. Malet Sanz, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2019, 58, 5003–5007 CrossRef CAS PubMed.
- A. Bunescu, Y. Abdelhamid and M. J. Gaunt, Nature, 2021, 598, 597–603 CrossRef PubMed.
- A. R. Allen, E. A. Noten and C. R. J. Stephenson, Chem. Rev., 2022, 122, 2695–2751 CrossRef CAS PubMed.
- X. Wu and C. Zhu, Acc. Chem. Res., 2020, 53, 1620–1636 CrossRef CAS PubMed.
- D. M. Whalley and M. F. Greaney, Synthesis, 2022, 54, 1908–1918 CrossRef.
- M. Tada, H. Shijima and M. Nakamura, Org. Biomol. Chem., 2003, 1, 2499–2505 RSC.
- D. M. Whalley, H. A. Duong and M. F. Greaney, Chem. Commun., 2020, 56, 11493 RSC.
- T. M. Monos, R. C. McAtee and C. R. J. Stephenson, Science, 2018, 361, 1369–1373 CrossRef CAS PubMed.
- D. Nicewicz, H. Roth and N. Romero, Synlett, 2015, 27, 714–723 CrossRef.
- L. Wang, F. Wu, J. Chen, D. A. Nicewicz and Y. Huang, Angew. Chem., Int. Ed., 2017, 56, 6896–6900 CrossRef CAS PubMed.
- Q. Zhu, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 741–747 CrossRef CAS PubMed.
- Y.-X. Ji, J. Li, C.-M. Li, S. Qu and B. Zhang, Org. Lett., 2021, 23, 207–212 CrossRef CAS PubMed.
- K. Kaneko, T. Yoshino, S. Matsunaga and M. Kanai, Org. Lett., 2013, 15, 2502–2505 CrossRef CAS PubMed.
- D. Wang, L. Wu, F. Wang, X. Wan, P. Chen, Z. Lin and G. Liu, J. Am. Chem. Soc., 2017, 139, 6811–6814 CrossRef CAS PubMed.
- X. Wang, D. Xia, W. Qin, R. Zhou, X. Zhou, Q. Zhou, W. Liu, X. Dai, H. Wang, S. Wang, L. Tan, D. Zhang, H. Song, X.-Y. Liu and Y. Qin, Chem, 2017, 2, 803–816 CAS.
- J. Chen, H. M. Guo, Q. Q. Zhao, J. R. Chen and W. J. Xiao, Chem. Commun., 2018, 54, 6780–6783 RSC.
- E. Azzi, G. Ghigo, S. Parisotto, F. Pellegrino, E. Priola, P. Renzi and A. Deagostino, J. Org. Chem., 2021, 86, 3300–3323 CrossRef CAS PubMed.
- J.-L. Tu, W. Tang and F. Liu, Org. Chem. Front., 2021, 8, 3712–3717 RSC.
- P. Lassalas, B. Gay, C. Lasfargeas, M. J. James, V. Tran, K. G. Vijayendran, K. R. Brunden, M. C. Kozlowski, C. J. Thomas, A. B. Smith, D. M. Huryn and C. Ballatore, J. Med. Chem., 2016, 59, 3183–3203 CrossRef CAS PubMed.
- K. K. Irikura and N. G. Todua, Rapid Commun. Mass Spectrom., 2014, 28, 829–834 CrossRef CAS PubMed.
- Y. Liang, Y. Simón-Manso, P. Neta, X. Yang and S. E. Stein, J. Am. Soc. Mass Spectrom., 2021, 32, 806–814 CrossRef CAS PubMed.
- R. C. McAtee, E. A. Noten and C. R. J. Stephenson, Nat. Commun., 2020, 11, 2528 CrossRef CAS PubMed.
- H. Jiang, X. Yu, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2021, 60, 14399–14404 CrossRef CAS PubMed.
- W. E. Truce, B. VanGemert and W. W. Brand, J. Org. Chem., 1978, 43, 101–105 CrossRef CAS.
- H. Zhang, L. Kou, D. Chen, M. Ji, X. Bao, X. Wu and C. Zhu, Org. Lett., 2020, 22, 5947–5952 CrossRef CAS PubMed.
- J. Wang, M. Tsuchiya, K. Tokumaru and H. Sakuragi, Bull. Chem. Soc. Jpn., 1995, 68, 1213–1219 CrossRef CAS.
- Fundamentals and Applications of Organic Electrochemistry, ed. T. Fuchigami, S. Inagi and M. Atobe, 2014, pp. 217–222, DOI:10.1002/9781118670750.app2.
- G. J. Choi, Q. Zhu, D. C. Miller, C. J. Gu and R. R. Knowles, Nature, 2016, 539, 268–271 CrossRef CAS PubMed.
- T. U. Connell, C. L. Fraser, M. L. Czyz, Z. M. Smith, D. J. Hayne, E. H. Doeven, J. Agugiaro, D. J. D. Wilson, J. L. Adcock, A. D. Scully, D. E. Gómez, N. W. Barnett, A. Polyzos and P. S. Francis, J. Am. Chem. Soc., 2019, 141, 17646–17658 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2045499. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc01228f |
| This journal is © The Royal Society of Chemistry 2022 |