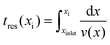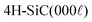Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Texture evolution in rhombohedral boron carbide films grown on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/h2_char_0031_0304.gif) ) and 4H-SiC(0001) substrates by chemical vapor deposition†
) and 4H-SiC(0001) substrates by chemical vapor deposition†
Laurent
Souqui‡
 ,
Sachin
Sharma
,
Sachin
Sharma
 ,
Hans
Högberg
,
Hans
Högberg
 and
Henrik
Pedersen
and
Henrik
Pedersen
 *
*
Department of Physics, Chemistry and Biology, Linköping University, SE-581 83 Linköping, Sweden. E-mail: henrik.pedersen@liu.se
First published on 30th September 2022
Abstract
Boron carbide in its rhombohedral form (r-BxC), commonly denoted B4C or B13C2, is a well-known hard material, but it is also a potential semiconductor material. We deposited r-BxC by chemical vapor deposition between 1100 °C and 1500 °C from triethylboron in H2 on 4H-SiC(0001) and 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). We show, using ToF-ERDA, that pure B4C was grown at 1300 °C, furthermore, using XRD that graphite forms above 1400 °C. The films deposited above 1300 °C on 4H-SiC(000
). We show, using ToF-ERDA, that pure B4C was grown at 1300 °C, furthermore, using XRD that graphite forms above 1400 °C. The films deposited above 1300 °C on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) were found to be epitaxial, with the epitaxial relationships B4C(0001)[10
) were found to be epitaxial, with the epitaxial relationships B4C(0001)[10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]‖4H-SiC(000
0]‖4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) )[10
)[10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] obtained from pole figure measurements. In contrast, the films deposited on 4H-SiC(0001) were polycrystalline. We suggest that the difference in growth mode is explained by the difference in the ability of the different surfaces of 4H-SiC to act as carbon sources in the initial stages of the film growth.
0] obtained from pole figure measurements. In contrast, the films deposited on 4H-SiC(0001) were polycrystalline. We suggest that the difference in growth mode is explained by the difference in the ability of the different surfaces of 4H-SiC to act as carbon sources in the initial stages of the film growth.
Introduction
Due to its high hardness of about 35 GPa,1 rhombohedral boron carbide, commonly denoted B4C or B13C2, here referred to as r-BxC (4 ≤ x ≤ 10.5), is mainly used as a super hard material in applications such as abrasive or plate armour.2 Additionally, owing to the self-healing capabilities of the boron icosahedra under energetic bombardment3 and due to the high neutron cross-section of the isotope 10B, it is a promising material for boron-based neutron detectors.4 Interestingly, and less explored, is that r-BxC is a semiconductor with electronic properties which can be tuned with the B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C stoichiometry.5–7 Its bandgap, for instance, ranges from 0.48 to 2.1 eV with increasing boron content.5–7 Semiconducting r-BxC could be used in combination with the wide bandgap materials boron subphosphide (B12P2)8 and boron subarsenide (B12As2),9 with which it shares structural similarities, and would enable the fabrication of novel radiation resistant heterostructures. In addition, r-BxC is interesting for thermoelectric devices due to its high temperature thermoelectric properties, with a high thermal conductivity (4–10 W m−1 K−1) and high Seebeck coefficient (150 to 300 μV K−1 between 500–1000 °C).4,10 Furthermore, another peculiarity of boron carbide is its ability to form crystals with five-fold symmetry due to crystal twinning.11,12 Controlled formation of these twinned crystals could be used to approximate and study boron-carbide-based quasicrystals. However, to fully explore r-BxC as a semiconductor, thin films with high crystalline quality, preferably epitaxially grown are needed, but the growth of epitaxial r-BxC crystals has proved to be challenging13 using vapor phase deposition techniques.
C stoichiometry.5–7 Its bandgap, for instance, ranges from 0.48 to 2.1 eV with increasing boron content.5–7 Semiconducting r-BxC could be used in combination with the wide bandgap materials boron subphosphide (B12P2)8 and boron subarsenide (B12As2),9 with which it shares structural similarities, and would enable the fabrication of novel radiation resistant heterostructures. In addition, r-BxC is interesting for thermoelectric devices due to its high temperature thermoelectric properties, with a high thermal conductivity (4–10 W m−1 K−1) and high Seebeck coefficient (150 to 300 μV K−1 between 500–1000 °C).4,10 Furthermore, another peculiarity of boron carbide is its ability to form crystals with five-fold symmetry due to crystal twinning.11,12 Controlled formation of these twinned crystals could be used to approximate and study boron-carbide-based quasicrystals. However, to fully explore r-BxC as a semiconductor, thin films with high crystalline quality, preferably epitaxially grown are needed, but the growth of epitaxial r-BxC crystals has proved to be challenging13 using vapor phase deposition techniques.
Using chemical vapor deposition (CVD) with the single-source precursor triethylboron (TEB) in an H2 ambient in the temperature range of 1100 to 1500 °C, we investigate the growth conditions for r-BxC films on the Si-terminated (4H-SiC(0001)) and C-terminated (4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) )) faces. We report epitaxial growth on the 4H-SiC(000
)) faces. We report epitaxial growth on the 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) at 1300 °C, while deposition on 4H-SiC(0001) yields polycrystalline films at the same temperature.
) at 1300 °C, while deposition on 4H-SiC(0001) yields polycrystalline films at the same temperature.
Experimental details
Film deposition
Thin films of r-BxC were deposited on 4H-SiC substrates in a hot-wall horizontal CVD reactor from pyrolysis of 0.7 SCCM triethylboron (TEB, B(CH2CH3)3, 99.99%, SAFC HiTech) in 5700 SCCM hydrogen gas (H2, Palladium membrane purified) for 180 min. The process pressure was regulated to 7000 Pa by a throttle valve and the deposition temperature of 1100–1500 °C, monitored by a pyrometer (Heitronics KT81R, calibrated by silicon melting). The influence of the residence time was studied by either changing the total flow or by utilizing the position of the substrates in the SiC-coated elliptical graphite susceptor.The temperature distribution and the residence times in the susceptor were evaluated using COMSOL Multiphysics (version 5.2). The susceptor was modelled using a 2D axisymmetric approximation. Since TEB is highly diluted in H2, a flow of pure H2 was considered in these calculations. The temperature profile inside the susceptor (without flow) was described by a parabolic function, as previously calculated for this reactor by Danielsson.14 The residence time tres at a given point xi was defined using the formula:
Prior to deposition, the 4H-SiC wafers were sawn into 10 × 10 mm2 pieces. The orientations of the 4H-SiC substrates used were 4H-SiC(0001) and 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) which conventionally refers to the Si-terminated and C-terminated sides, respectively. The offcut relative to the c-axis was ±0.5° (commonly referred as on-axis). Prior to deposition, the substrates were degreased in acetone for five minutes followed by ethanol for five minutes, both using an ultrasonic bath. This cleaning step was followed by a standard cleaning procedure for semiconductors with etching in oxidating alkaline solution (ammonia solution (25%), hydrogen peroxide solution (48%) and water in respective proportions 1
) which conventionally refers to the Si-terminated and C-terminated sides, respectively. The offcut relative to the c-axis was ±0.5° (commonly referred as on-axis). Prior to deposition, the substrates were degreased in acetone for five minutes followed by ethanol for five minutes, both using an ultrasonic bath. This cleaning step was followed by a standard cleaning procedure for semiconductors with etching in oxidating alkaline solution (ammonia solution (25%), hydrogen peroxide solution (48%) and water in respective proportions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) at 80 °C for five minutes followed by a rinse in deionized water and further treatment in hot oxidizing acidic solution (hydrochloric acid solution (37%), hydrogen peroxide (48%) and water in respective proportions 1
5) at 80 °C for five minutes followed by a rinse in deionized water and further treatment in hot oxidizing acidic solution (hydrochloric acid solution (37%), hydrogen peroxide (48%) and water in respective proportions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) at 80 °C for five minutes and finally a rinse in deionized water. Additionally, the substrates used for the lowest deposition (1100 °C) temperature were dipped in 2% HF solution to ensure the removal of the native silicon dioxide.
6) at 80 °C for five minutes and finally a rinse in deionized water. Additionally, the substrates used for the lowest deposition (1100 °C) temperature were dipped in 2% HF solution to ensure the removal of the native silicon dioxide.
Film characterization
X-ray diffraction (XRD) was performed using Cu Kα radiation. The 2θ/ω diffractograms were recorded using an X'Pert Pro diffractometer equipped with a Bragg–Brentano HD optics with 1/2° divergence slit as primary optics and a X'celerator detector with 5 mm anti-scatter slit, 0.4° Soller slits and Kβ Ni filter as secondary optics. XRD ω-scan (rocking curve measurements) are obtained using the PANAlytical Empyrean MRD and the measurements were performed by using capillary optics (X-ray lens) with a 2 × 2 mm mask on primary optics and a parallel plate collimator with 0.27° slit and a Ni filter as secondary optics. Since the X-Ray lens is not a monochromatic optic module, the use of a Ni filter was necessary to suppress the Cu Kβ line. The integral breadth was obtained by fitting the rocking curves with Pearson VII functions using the fityk software (version 1.3.1). XRD φ-scans and pole figures were recorded using an X'Pert MPD diffractometer equipped with 2 × 2 mm2 cross-slits and a Kβ Ni filter as primary optics and a proportional detector (PW1711/96) with a parallel plate collimator and a 0.27° slit as secondary optics.Fourier transform infrared spectroscopy (FTIR) reflectance spectra were measured in a Bruker VERTEX 70 equipment with incident s-polarized light at an angle of 60° with respect to the sample surface normal. The spectra were acquired in reflectance configuration at incidence angle of 30° with respect to the surface normal, a resolution of 2 cm−1 and s-polarization. The measurements were conducted at room temperature, after a 30 min N2 purge, with 2 cm−1 resolution and averaged over 50 scans. A thin film of gold was used as reference.
Scanning electron microscopy (SEM) was used to study the surface morphology of the films. The microscope used was a Zeiss Gemini. All the micrographs were acquired using an accelerating voltage of 5 kV and an in-lens detector. SEM was also used for thickness measurements of the BxC films deposited on the 4H-SiC(0001); these measurements were performed on the aligned cross sections of the samples. The samples were scratched using a diamond tipped pen and then cracked. The thickness of BxC film grown at 1100 °C was determined to be around 900 nm, those grown at 1200 °C, 1300 °C was around 1.5 μm, the film grown at 1400 °C was about 1.6 μm and the film grown at 1500 °C was determined around 3 μm.
The compositional analysis of selected films was performed using time-of-flight energy elastic recoil detection analysis (ToF-ERDA). The measurements were done using a 36 MeV 127I+8 beam. The incidence angle of primary ions and exit angle of recoils were both 67.5° to the sample surface normal constituting a recoil angle of 45°. The time-of-flight vs energy loss map is also utilized, this has better separation for lighter elements (B and C). The measured ToF-ERDA spectra data was then converted into relative atomic concentration profiles using the Potku code.15
Results
Boron carbide deposition on C-face 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/h3_char_0031_0304.gif) )
)
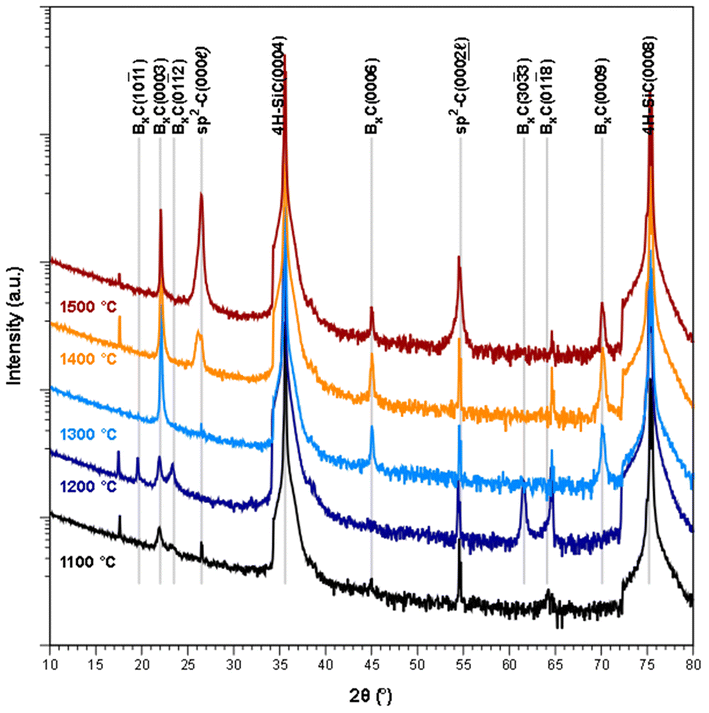
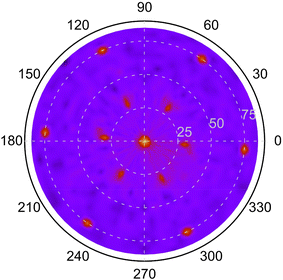
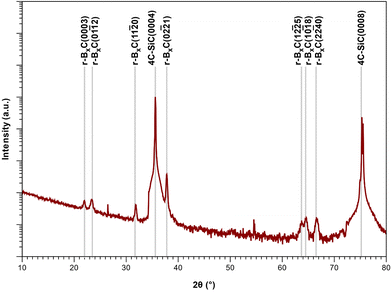
From the XRD diffractograms in Fig. 1, it is evident that films deposited at 1100 and 1200 °C are polycrystalline, as seen from the additional reflections from crystal planes other than (0003) and higher order diffractions. For higher temperatures, the films are oriented along [0003] since no reflections from crystal planes other than (0003) and higher order diffractions are visible in the diffractograms. As the information provided by the 2θ/ω scans is confined to planes that are parallel to the sample surface, XRD pole figure measurement was performed to assess the degree of in-plane orientation with respect to the substrate. The distinct poles in the pole figure measurement in Fig. 2, shows that the r-BxC films are not only c-axis oriented, but grown epitaxially on the 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). The epitaxial relationships were determined as r-BxC(0001)[10
). The epitaxial relationships were determined as r-BxC(0001)[10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]‖4H-SiC(000
0]‖4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) )[10
)[10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]. The six poles, separated by 60°, show that the films are twinned. This is expected due to the higher symmetry of the hexagonal 4H-SiC substrate compared to the r-BxC crystal of rhombohedral symmetry.
0]. The six poles, separated by 60°, show that the films are twinned. This is expected due to the higher symmetry of the hexagonal 4H-SiC substrate compared to the r-BxC crystal of rhombohedral symmetry.
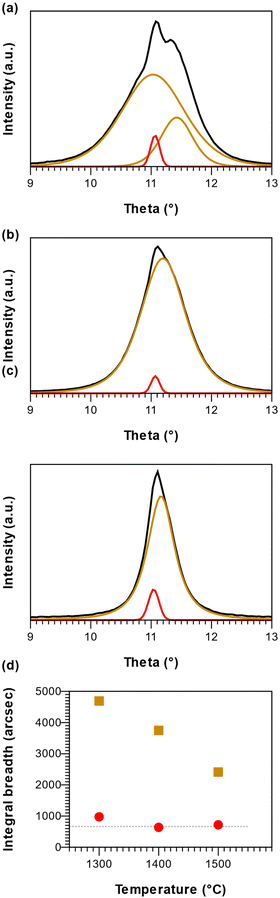
FTIR further supports the XRD data; as observed, the chain stretching mode disappears for r-BxC grown on the C-face compared to r-BxC grown on the Si-face in Fig. S1,‡ which is consistent with the triatomic chains being perpendicular to the electric field (s-polarized light). The onset of graphite formation is observed from the appearance of 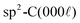 peaks at 1400 °C in Fig. 1. r-BxC and pyrolytic graphite where found to grow to a similar extent and in a similar fashion, as seen from a comparison of the intensity of r-BxC(0003) and
peaks at 1400 °C in Fig. 1. r-BxC and pyrolytic graphite where found to grow to a similar extent and in a similar fashion, as seen from a comparison of the intensity of r-BxC(0003) and 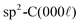 ) in Fig. 1, and from the top-view micrograph in Fig. 4. Rocking curve measurements of the (0003) planes for samples deposited above 1300 °C (Fig. 3) show a sharp component whose integral breadth is comparable to the substrate as well as a much broader component which decreases as the deposition temperature increases. Rocking curve measurements allows evaluation of the overall degree of alignments of the crystallites along the [001] and [101] directions. The dispersion [001] direction is associated to the tilting of the grains with respect to the vicinal surface normal while [101] comprises an additional rotational component referred as twisting. Further information about the differences in crystal quality between the films grown at the examined temperatures, as determined using rocking curves, is expressed in Fig. 3d
) in Fig. 1, and from the top-view micrograph in Fig. 4. Rocking curve measurements of the (0003) planes for samples deposited above 1300 °C (Fig. 3) show a sharp component whose integral breadth is comparable to the substrate as well as a much broader component which decreases as the deposition temperature increases. Rocking curve measurements allows evaluation of the overall degree of alignments of the crystallites along the [001] and [101] directions. The dispersion [001] direction is associated to the tilting of the grains with respect to the vicinal surface normal while [101] comprises an additional rotational component referred as twisting. Further information about the differences in crystal quality between the films grown at the examined temperatures, as determined using rocking curves, is expressed in Fig. 3d
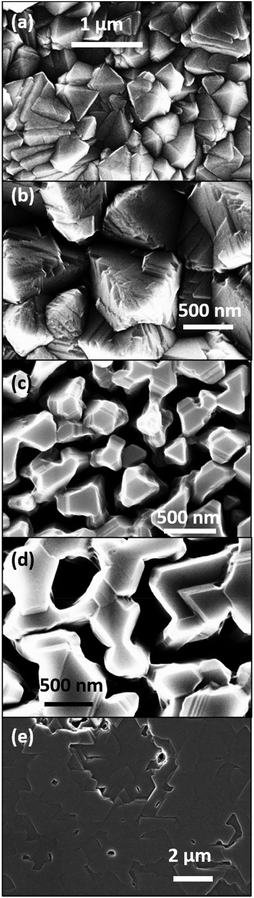 | ||
Fig. 4 Plan view SEM of rhombohedral boron carbide deposited on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ); at (a) 1100 °C, (b) 1200 °C, (c) 1300 °C, (d) 1400 °C and (e) 1500 °C. ); at (a) 1100 °C, (b) 1200 °C, (c) 1300 °C, (d) 1400 °C and (e) 1500 °C. | ||
Plan view SEM (Fig. 4) show that the films deposited on C-face 4H-SiC at 1100 °C (Fig. 4a) and 1200 °C (Fig. 4b) were highly facetted and polycrystalline while the films deposited at higher temperatures were smoother. The films deposited at 1300 °C (Fig. 4c) and 1400 °C (Fig. 4) constituted of percolated islands and continuous films were obtained at 1500 °C (Fig. 4e). The continuous films comprised distinct areas of boron carbide and graphite and hexagonal defects were observed for both materials (Fig. 5).
 | ||
Fig. 5 Plan view SEM of the defects observed (a) rhombohedral boron carbide film and (b) pyrolytic graphite co-deposited at 1500 °C on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). ). | ||
The sample deposited at 1300 °C was selected for compositional analysis as it was not contaminated with graphite (as seen from Fig. 1). ToF-ERDA yielded B to C ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (B – 79.2 at% ± 0.8 at%, C – 20.5 at% ± 0.4 at%) and with around 0.3 at% of O and H below the detection limit of the technique.
1 (B – 79.2 at% ± 0.8 at%, C – 20.5 at% ± 0.4 at%) and with around 0.3 at% of O and H below the detection limit of the technique.
Boron carbide deposition on Si-face 4H-SiC(0001)
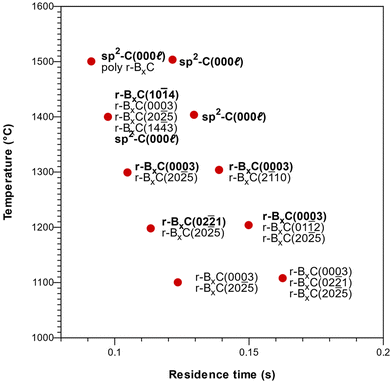
Deposition on Si-face 4H-SiC(0001) substrates resulted in polycrystalline rhombohedral boron carbide as seen from the 2θ/ω diffractogram (Fig. 6) and FT-IR reflectance spectra (Fig. S2‡) of a film deposited at 1400 °C. Pyrolytic graphite could also be detected under some conditions (Fig. 7). The orientation of the r-BxC crystals and formation of graphite were found to be strongly dependent on the growth temperature and on the residence time of the gas in the susceptor, as presented in Fig. 7. The residence time at a given temperature was varied by placing the sample at different positions in the susceptor.Regardless the deposition temperature, the BxC films deposited at low residence times (below 0.10 s) were polycrystalline with no preferred orientation in contrast with the films deposited at longer residence times which showed preferred orientation. For residence times between 0.10 and 0.12 s this orientation evolved from [02![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 1] at 1200 °C, to [0003] at 1300 °C to [10
1] at 1200 °C, to [0003] at 1300 °C to [10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4] at 1400 °C, with a minor contribution of the (20
4] at 1400 °C, with a minor contribution of the (20![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 5) planes in all cases, as shown in Fig. 7. For longer residence times (0.14 s), only the [0003] direction is favored at all temperatures. Finally, we found that the onset of pyrolytic graphite formation occurs at around 1400 °C and for residence times around 0.09 s and becomes the dominant phase at higher temperatures and residence times.
5) planes in all cases, as shown in Fig. 7. For longer residence times (0.14 s), only the [0003] direction is favored at all temperatures. Finally, we found that the onset of pyrolytic graphite formation occurs at around 1400 °C and for residence times around 0.09 s and becomes the dominant phase at higher temperatures and residence times.
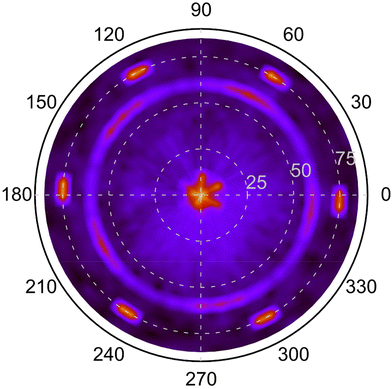
A pole figure of the {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4} planes (2θ = 35.08°) of a r-BxC film deposited at 1400 °C and 0.1 s residence time is shown in Fig. 8. This film is polycrystalline, mainly orientated along the [10
4} planes (2θ = 35.08°) of a r-BxC film deposited at 1400 °C and 0.1 s residence time is shown in Fig. 8. This film is polycrystalline, mainly orientated along the [10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4] direction, and graphite-contaminated, as seen in Fig. 7. The pole figure shows three concentric patterns. The outer most pattern, consists in 6 poles at ψ ≈ 75°, which as for Fig. 2, originates from the substrate and are the tails of 4H-SiC{10
4] direction, and graphite-contaminated, as seen in Fig. 7. The pole figure shows three concentric patterns. The outer most pattern, consists in 6 poles at ψ ≈ 75°, which as for Fig. 2, originates from the substrate and are the tails of 4H-SiC{10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1}. The second pattern at ψ ≈ 60° appears to be five-fold. This may seem counterintuitive considering two aspects: (1) the crystal structure of r-BxC which is rhombohedral and (2) the polycrystalline nature of the textured films, which would usually exhibit a fiber texture without symmetry. The five-fold symmetry originates from the tendency of r-BxC to form five-fold twinning (Fig. S3‡).11,12,16 However, upon closer inspection of the five broad poles at ψ ≈ 60°, reveals the further division into three distinct peaks within each of these five broad poles. This is consistent with the fact that five and six are non-divisible: for a given family of the directions of a five-fold crystal, can only be aligned with one of the 〈11
1}. The second pattern at ψ ≈ 60° appears to be five-fold. This may seem counterintuitive considering two aspects: (1) the crystal structure of r-BxC which is rhombohedral and (2) the polycrystalline nature of the textured films, which would usually exhibit a fiber texture without symmetry. The five-fold symmetry originates from the tendency of r-BxC to form five-fold twinning (Fig. S3‡).11,12,16 However, upon closer inspection of the five broad poles at ψ ≈ 60°, reveals the further division into three distinct peaks within each of these five broad poles. This is consistent with the fact that five and six are non-divisible: for a given family of the directions of a five-fold crystal, can only be aligned with one of the 〈11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0〉 directions of 4H-SiC. As such, an alignment along [11
0〉 directions of 4H-SiC. As such, an alignment along [11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0], [1
0], [1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10] and [
10] and [![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 110], will result in three adjacent 5-fold patterns as can be seen in Fig. 8. Furthermore, due to the lack of axial symmetry of five-fold objects (e.g., a pentagon) there are two ways two align a five-fold crystal along a given direction (e.g., the apex of a pentagon pointing towards either 4H-SiC[11
110], will result in three adjacent 5-fold patterns as can be seen in Fig. 8. Furthermore, due to the lack of axial symmetry of five-fold objects (e.g., a pentagon) there are two ways two align a five-fold crystal along a given direction (e.g., the apex of a pentagon pointing towards either 4H-SiC[11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0] or
0] or  ), so that one pole pattern is centrosymmetric with respect to the other. In our case, this is observed in Fig. 8 where poles of much fainter intensity can be found between the more intense poles of the five-fold patterns. As a result, the ring pattern comprises 5 (r-BxC twinning) × 3 (4H-SiC symmetry axes) × 2 (lack of axial symmetry of 5-fold objects) = 30 poles (Fig. S4‡). The fortuitous varying intensity of the pattern brings to light that these five-fold twinned crystals or domains grow along each 11
), so that one pole pattern is centrosymmetric with respect to the other. In our case, this is observed in Fig. 8 where poles of much fainter intensity can be found between the more intense poles of the five-fold patterns. As a result, the ring pattern comprises 5 (r-BxC twinning) × 3 (4H-SiC symmetry axes) × 2 (lack of axial symmetry of 5-fold objects) = 30 poles (Fig. S4‡). The fortuitous varying intensity of the pattern brings to light that these five-fold twinned crystals or domains grow along each 11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0 axes of the 4H-SiC surface in a heteroepitaxial fashion. The third pattern at the center of the pole figure is the superpositions of the last of the three BxC{10
0 axes of the 4H-SiC surface in a heteroepitaxial fashion. The third pattern at the center of the pole figure is the superpositions of the last of the three BxC{10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4} poles (ψ ≈ 7°) but are shadowed by the strong 4H-SiC(0001) pole (ψ ≈ 0°).
4} poles (ψ ≈ 7°) but are shadowed by the strong 4H-SiC(0001) pole (ψ ≈ 0°).
From top-view scanning electron microscopy (Fig. 9), the films deposited at 1100 °C appear to comprise nodular grains (Fig. 9a). The films deposited between 1200 and 1400 °C (Fig. 9b–d) show highly twinned grains with a more pronounced faceting with increasing temperature. The grain size is around 1 μm. The surface morphology of these films is consistent with the texture evolution of the film. The crystallites of the films deposited at 1200 °C (Fig. 9b) and 1300 °C (Fig. 9c) appear as square-based pyramids. This is associated with films with a strong [02![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 1] texture. At 1400 °C (Fig. 9d), the films appear porous and some of the grains with 5-fold twinning are visible, as expected from the pole figure of the [10
1] texture. At 1400 °C (Fig. 9d), the films appear porous and some of the grains with 5-fold twinning are visible, as expected from the pole figure of the [10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4] orientated films. At 1500 °C (Fig. 9e), the boron carbide grains do not show preferred orientation.
4] orientated films. At 1500 °C (Fig. 9e), the boron carbide grains do not show preferred orientation.
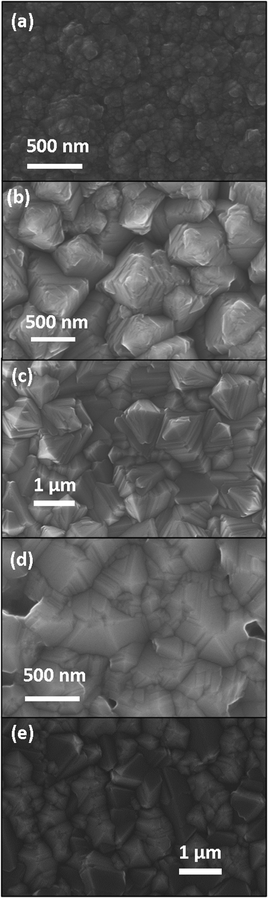 | ||
| Fig. 9 Plan view SEM of rhombohedral boron carbide deposited at (a) 1100 °C, (b) 1200 °C, (c) 1300 °C, (d) 1400 °C and, (e) 1500 °C on 4H-SiC(0001). | ||
Finally for comparison, the elemental composition of the film on the Si-face at 1300 °C determined using ToF-ERDA also yielded a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (B – 79.4% ± 0.7%, C – 20.5% ± 0.3%) with around 0.1% of O incorporation and the absence of H as it is below the detection limit of the instrument.
1 (B – 79.4% ± 0.7%, C – 20.5% ± 0.3%) with around 0.1% of O incorporation and the absence of H as it is below the detection limit of the instrument.
Discussion
As observed, the orientation of the boron carbide films on silicon carbide depends on the surface termination, the temperature, and the residence time. The demonstration of epitaxial growth of r-B4C on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) from 1300 °C supports this, while all films deposited on 4H-SiC(0001) were found to be polycrystalline at the studied temperatures.
) from 1300 °C supports this, while all films deposited on 4H-SiC(0001) were found to be polycrystalline at the studied temperatures.
In comparison to structurally similar materials such as rhombohedral boron subphosphide (B12P2) and subarsenide (B12As2), the threshold temperature for epitaxial growth for r-BxC is comparable to the one reported for the CVD of B12P2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and similar structural variants such as twinning was observed in B12As2
17 and similar structural variants such as twinning was observed in B12As2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18–20 and B12P2
18–20 and B12P2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 epitaxy, albeit on on-axis Si-face 4H-SiC(0001). A notable difference is observed in the evolution of crystalline quality with temperature as seen from rocking curves measurements: the crystalline quality of B12P2 and B12As2 was reported to degrade from 1350 °C (ref. 21) and 1450 °C,19 respectively, while the crystalline quality of r-BxC continues improving even up to 1500 °C. This could be attributed to the formation of volatile P or PHx (respectively As or AsHx) species from the deposited film, which is less prone to occur in the case of carbon, albeit at the cost of forming graphite. Finally, the presence of two components in our rocking curves measurements further suggests that the crystallites of the epitaxial r-B4C films initially grow well aligned with the substrate at the early stages of the growth and deviate slightly later on, as in ref. 22.
17 epitaxy, albeit on on-axis Si-face 4H-SiC(0001). A notable difference is observed in the evolution of crystalline quality with temperature as seen from rocking curves measurements: the crystalline quality of B12P2 and B12As2 was reported to degrade from 1350 °C (ref. 21) and 1450 °C,19 respectively, while the crystalline quality of r-BxC continues improving even up to 1500 °C. This could be attributed to the formation of volatile P or PHx (respectively As or AsHx) species from the deposited film, which is less prone to occur in the case of carbon, albeit at the cost of forming graphite. Finally, the presence of two components in our rocking curves measurements further suggests that the crystallites of the epitaxial r-B4C films initially grow well aligned with the substrate at the early stages of the growth and deviate slightly later on, as in ref. 22.
However, in spite of the similarities with B12P2 and B12As2, which are typically grown epitaxially on the Si-terminated face, epitaxial growth of r-B4C was not achieved on 4H-SiC(0001).17,19,23,24With the exception of their orientation on either SiC termination, the r-B4C films show identical crystal structures, composition at the same growth temperature (1300 °C) and temperature thresholds for graphite formation (1400 °C), which indicates that the observed differences can only be attributed to nucleation and not to the steady-state growth. To understand these observations, we consider the differences that are already known between the two terminations of silicon carbide and are relevant to the early stage of the deposition process, such as surface energy, and graphene formation or surface stability in H2.
From a thermodynamics perspective, the surface energy of the C-face of silicon carbides polytype is lower (7.5 J m−2) than that of the Si-face (18 J m−2).25 To our knowledge, there are no computed data of the surface energy for all r-B4C facets. Nevertheless, considering the surface energy for boron carbide for the (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1) planes (3.21 J m−2, (ref. 26) 3.27 J m−2 (ref. 27)) and the surface energy for α-boron facets (1.9 to 3.6 J m−2),28 having a similar structure as r-B4C, differing only in the absence the triatomic chains, one can conclude that they are lower than the surface energy of the C-face of 4H-SiC. Assuming the interfacial energy to be small for (0001)-oriented B4C crystals on SiC(0001) and SiC(000
1) planes (3.21 J m−2, (ref. 26) 3.27 J m−2 (ref. 27)) and the surface energy for α-boron facets (1.9 to 3.6 J m−2),28 having a similar structure as r-B4C, differing only in the absence the triatomic chains, one can conclude that they are lower than the surface energy of the C-face of 4H-SiC. Assuming the interfacial energy to be small for (0001)-oriented B4C crystals on SiC(0001) and SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) due to the small lattice mismatch, one would expect that both surfaces would allow epitaxial growth; although it can be argued that the surface energy of SiC(0001) is so high that multiple orientations of B4C could grow in-spite of a higher interfacial energy, hence leading to polycrystalline growth. The issue with these energetic considerations is that they might not be valid at our growth conditions.
) due to the small lattice mismatch, one would expect that both surfaces would allow epitaxial growth; although it can be argued that the surface energy of SiC(0001) is so high that multiple orientations of B4C could grow in-spite of a higher interfacial energy, hence leading to polycrystalline growth. The issue with these energetic considerations is that they might not be valid at our growth conditions.
A consequence of the high energy of the Si-face is that it is highly unstable in pure and carbon-enriched hydrogen ambient between 1400–1580 °C,29–31 which is similar to our experimental conditions. Under these conditions, the Si face tends to be subjected to heavy step-bunching, forming irregular macro steps and micro steps of variable height, as well as etch pits.29–31 In contrast, the C-face is reported to be etched smoothly at these conditions forming only micro steps with an average height of 2.5 nm,30 equivalent to 2.5 times the c-axis of a 4H-SiC unit cell and about twice the c-axis of a r-B4C unit cell (12.065–12.175 Å).32 These aspects could explain the different results obtained between the two SiC faces, the etching of the Si-face results in a complex topography, facilitating polycrystalline growth, while the steps after etching the C-face are close to lattice-matched to both the c- and a-axes of the r-B4C unit cell.
Besides thermodynamic and morphological aspects, the chemistry at the SiC surfaces is also relevant, especially the role of carbon. Our growth conditions (1100–1500 °C, 7000 Pa and hydrocarbon by-product to hydrogen ratio in the order of 135 to 406 ppm, assuming 1 to 3 mole of ethylene (C2H4) per mole of TEB33) seem to be adequate for the growth of graphene at the 4H-SiC(0001) surface.34 Formation of graphene is known to be even easier on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) surface due to the absence of the partially sp3-bonded interfacial layer (so-called buffer layer) between graphene and SiC.35 However, only the Si-face affords oriented graphene layers (from 1350 °C at our CVD conditions),34 while the graphene layers grown on the C-face are usually randomly rotated with respect to the substrate.35,36 If a few layers of graphene would be the enabler for epitaxial growth of r-BxC, one would expect epitaxial growth on the Si-face and not on the C-face, which is contrary to our observations, suggesting that graphene does not play a role in the process.
) surface due to the absence of the partially sp3-bonded interfacial layer (so-called buffer layer) between graphene and SiC.35 However, only the Si-face affords oriented graphene layers (from 1350 °C at our CVD conditions),34 while the graphene layers grown on the C-face are usually randomly rotated with respect to the substrate.35,36 If a few layers of graphene would be the enabler for epitaxial growth of r-BxC, one would expect epitaxial growth on the Si-face and not on the C-face, which is contrary to our observations, suggesting that graphene does not play a role in the process.
We instead consider the possibility that our deposition process operates in a window where the substrate, especially the C-face, may act as a carbon source but at a rate that prevents the formation of graphene. The second source of carbon in our process is evidently TEB, or rather, its main decomposition by-product, C2H4;33 the decomposition of which is kinetically slower but can ultimately lead to the formation of acetylene and in turn of pyrolytic graphite, if the temperature is high enough and/or the residence time long enough.37–39 From our results, we note that when the Si-terminated surface is used with a relatively short residence time, the texture of the films varied strongly with temperature, while for longer residence time the [0003] orientation prevails, until graphite forms. We suggest that this can be explained by the fact that a delicate balance of carbon concentration at the 4H-SiC(0001) surface may be needed to promote the growth of boron carbide along the c-axis. At lower temperatures (1200 °C and below), the Si-face is not an efficient carbon source on its own and the decomposition rate of the C2H4 byproducts is slow, leading to growth along the [0003] orientation only for the longest residence times. Around 1300 °C the carbon concentration is adequate to promote nucleation along the c-axis, although the films remain polycrystalline. Above 1300 °C, graphite forms and consumes the carbon provided by the C2H4 byproducts and again prevents the films from growing along the c-axis. In contrast, the C-face would be a more efficient C-source, making the growth less depending on the carbon coming from the gas phase and less sensitive to the formation of graphite. While the details of how carbon is involved in the nucleation mechanisms of boron carbide are yet to be understood, the carbon at the SiC surfaces might play a more significant role in r-BxC epitaxy than the surface energy considerations and step formation mentioned above, as epitaxial B12As2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19,23,24 and B12P2
19,23,24 and B12P2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 can be prepared on 4H-SiC(0001).
17 can be prepared on 4H-SiC(0001).
Conclusion
Our study shows that rhombohedral boron carbide with a composition B4C (ToF-ERDA) can grow epitaxially on 4H-SiC(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) at 1300 °C by CVD using triethylboron as single source precursor in H2. The epitaxial relationships were r-B4C(0001)[10
) at 1300 °C by CVD using triethylboron as single source precursor in H2. The epitaxial relationships were r-B4C(0001)[10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]‖4H-SiC(0001)[10
0]‖4H-SiC(0001)[10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] as seen from pole figure measurements. XRD and SEM revealed that higher deposition temperature results in co-deposition of graphite, and lower deposition temperature results in polycrystalline boron carbide. Deposition on 4H-SiC(0001) resulted in polycrystalline r-BxC with preferred orientation and amount of co-deposited graphite varying with the deposition temperature and the gas residence time in the CVD reactor. We suggest that these results are explained by the ability of the SiC surface to supply carbon for the initial growth of r-BxC. These results give further insight on the influence of growth conditions for this material system and enable the use of epitaxial boron carbide thin films for their intended applications in electronic and thermoelectric devices.
0] as seen from pole figure measurements. XRD and SEM revealed that higher deposition temperature results in co-deposition of graphite, and lower deposition temperature results in polycrystalline boron carbide. Deposition on 4H-SiC(0001) resulted in polycrystalline r-BxC with preferred orientation and amount of co-deposited graphite varying with the deposition temperature and the gas residence time in the CVD reactor. We suggest that these results are explained by the ability of the SiC surface to supply carbon for the initial growth of r-BxC. These results give further insight on the influence of growth conditions for this material system and enable the use of epitaxial boron carbide thin films for their intended applications in electronic and thermoelectric devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Swedish Foundation for Strategic Research (SSF), Contract No. IS14-0027, Carl Trygger's Foundation for Scientific Research, Contract No. CTS 14:189, and by the Swedish Research Council (VR) under contracts; No. 2018-05499 and 2017-04164. H. H. and H. P. further acknowledge financial support from the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant SFO-Mat-LiU No. 2009-00971). Accelerator operation was supported by Swedish Research Council VR-RFI (Contract No. 2019-00191).References
- H. Werheit, A. Leithe-Jasper, T. Tanaka, H. W. Rotter and K. A. Schwetz, Some properties of single-crystal boron carbide, J. Solid State Chem., 2004, 177, 575–579 CrossRef CAS.
- F. Thévenot, Boron Carbide A Comprehensive Review, J. Eur. Ceram. Soc., 1990, 6, 205–225 CrossRef.
- M. Carrard, D. Emin and L. Zuppiroli, Defect clustering and self-healing of electron-irradiated boron-rich solids, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 11270 CrossRef CAS.
- D. Emin, Unusual properties of icosahedral boron-rich solids, J. Solid State Chem., 2006, 179, 2791–2798 CrossRef CAS.
- H. Werheit, H. Binnenbruck and A. Hausen, Optical properties of boron carbide and comparison with β-rhombohedral boron, Phys. Status Solidi B, 1971, 47, 153–158 CrossRef CAS.
- H. Werheit, M. Laux, U. Kuhlmann and R. Telle, Optical Interband Transitions of Boron Carbide, Phys. Status Solidi B, 1992, 172, K81–K86 CrossRef CAS.
- H. Werheit, On excitons and other gap states in boron carbide, J. Phys.: Condens. Matter, 2006, 18, 10655–10662 CrossRef CAS.
- G. A. Slack, T. F. McNelly and E. A. Taft, Melt growth and properties of B6P crystals, J. Phys. Chem. Solids, 1983, 44, 1009–1013 CrossRef CAS.
- S. Bakalova, et al., Energy band structure and optical response function of icosahedral B12As2: A spectroscopic ellipsometry and first-principles calculational study, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 4–13 CrossRef.
- M. Bouchacourt and F. Thevenot, The correlation between the thermoelectric properties and stoichiometry in the boron carbide phase B4C-B10.5C, J. Mater. Sci., 1985, 20, 1237–1247 CrossRef CAS.
- B. Wei, et al., Massive icosahedral boron carbide crystals, J. Phys. Chem. B, 2002, 106, 5807–5809 CrossRef CAS.
- M. Imam, et al., Gas Phase Chemistry of Trimethylboron in Thermal Chemical Vapor Deposition, J. Phys. Chem. C, 2017, 121, 26465–26471 CrossRef CAS.
- P. D. Kervalishvili, S. O. Shalamberidze and Y. A. Bikovsky, Oriented boron carbide films produced by laser spraying, AIP Conf. Proc., 1991, 231, 524–527 CrossRef CAS.
- Ö. Danielsson, U. Forsberg, A. Henry and E. Janzén, Enlarging the usable growth area in a hot-wall silicon carbide CVD reactor by using simulation, Mater. Sci. Forum, 2001, 353–356, 99–102 Search PubMed.
- K. Arstila, et al., Potku – New analysis software for heavy ion elastic recoil detection analysis, Nucl. Instrum. Methods Phys. Res., Sect. B, 2014, 331, 34–41 CrossRef CAS.
- M. Karaman, Chemical Vapour Deposition of Boron Carbide, 2007 Search PubMed.
- C. D. Frye, C. K. Saw, B. Padavala, R. J. Nikoli and J. H. Edgar, Hydride CVD Hetero-epitaxy of B 12 P 2 on 4H-SiC, J. Cryst. Growth, 2017, 459, 112–117 CrossRef CAS.
- J. R. Michael, T. L. Aselage, D. Emin and P. G. Kotula, Structural variants in attempted heteroepitaxial growth of B12As2 on 6H-SiC (0001), J. Mater. Res., 2005, 20, 3004–3010 CrossRef CAS.
- R. Nagarajan, et al., Crystal growth of B12As2 on SiC substrate by CVD method, J. Cryst. Growth, 2005, 273, 431–438 CrossRef CAS.
- H. Chen, et al., Defect structures in B12As2epitaxial layers grown on (0001) 6H-SiC, J. Appl. Phys., 2008, 103, 123508 CrossRef.
- C. D. Frye, et al., Suppression of Rotational Twins in Epitaxial B12P2 on 4H-SiC, Cryst. Growth Des., 2018, 18, 669–676 CrossRef CAS.
- H. Miyake, C. H. Lin, K. Tokoro and K. Hiramatsu, Preparation of high-quality AlN on sapphire by high-temperature face-to-face annealing, J. Cryst. Growth, 2016, 456, 155–159 CrossRef CAS.
- R. H. Wang, et al., Chemical vapor deposition of B12As2 thin films on 6H-SiC, J. Electron. Mater., 2000, 29, 1304–1306 CrossRef CAS.
- W. M. Vetter, R. Nagarajan, J. H. Edgar and M. Dudley, Double-positioning twinning in icosahedral B12As2 thin films grown by chemical vapor deposition, Mater. Lett., 2004, 58, 1331–1335 CrossRef CAS.
- M. Syväjärvi, R. Yakimova and E. Janzén, Wetting properties and interfacial energies in liquid phase growth of α-SiC, Mater. Sci. Forum, 1998, 264–268, 159–162 Search PubMed.
- T. D. Beaudet, J. R. Smith and J. W. Adams, Surface energy and relaxation in boron carbide (101-1) from first principles, Solid State Commun., 2015, 219, 43–47 CrossRef CAS.
- J. D. Clayton, et al., Deformation and Failure Mechanics of Boron Carbide–Titanium Diboride Composites at Multiple Scales, JOM, 2019, 71, 2567–2575 CrossRef CAS.
- W. Hayami and S. Otani, The role of surface energy in the growth of boron crystals, J. Phys. Chem. C, 2007, 111, 688–692 CrossRef CAS.
- S. Soubatch, et al., Structure and Morphology of 4H-SiC Wafer Surfaces after H2-Etching, Mater. Sci. Forum, 2005, 483–485, 761–764 CAS.
- J. Hassan, J. P. Bergman, A. Henry and E. Janzén, In situ surface preparation of nominally on-axis 4H-SiC substrates, J. Cryst. Growth, 2008, 310, 4430–4437 CrossRef CAS.
- C. L. Frewin, C. Coletti, C. Riedl, U. Starke and S. E. Saddow, A comprehensive study of hydrogen etching on the major SiC polytypes and crystal orientations, Mater. Sci. Forum, 2009, 615–617, 589–592 CAS.
- T. L. Aselage and R. G. Tissot, Lattice Constants of Boron Carbides, J. Am. Ceram. Soc., 1992, 75, 2207–2212 CrossRef CAS.
- M. Imam, et al., Gas phase chemical vapor deposition chemistry of triethylboron probed by boron–carbon thin film deposition and quantum chemical calculations, J. Mater. Chem. C, 2015, 3, 10898–10906 RSC.
- A. Michon, et al., Effects of pressure, temperature, and hydrogen during graphene growth on SiC(0001) using propane-hydrogen chemical vapor deposition, J. Appl. Phys., 2013, 113, 203501 CrossRef.
- K. V. Emtsev, F. Speck, T. Seyller, L. Ley and J. D. Riley, Interaction, growth, and ordering of epitaxial graphene on SiC{0001} surfaces: A comparative photoelectron spectroscopy study, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 1–10 CrossRef.
- U. Starke and C. Riedl, Epitaxial graphene on SiC(0001) and : From surface reconstructions to carbon electronics, J. Phys.: Condens. Matter, 2009, 21, 134016 CrossRef CAS.
- G. D. Towell and J. J. Martin, Kinetic data from nonisothermal experiments: Thermal decomposition of ethane, ethylene, and acetylene, AIChE J., 1961, 7, 693–698 CrossRef CAS.
- A. Holmen, O. Olsvik and O. A. Rokstad, Pyrolysis of natural gas: chemistry and process concepts, Fuel Process. Technol., 1995, 42, 249–267 CrossRef CAS.
- K. Norinaga, V. M. Janardhanan and O. Deutschmann, Modeling of Pyrolysis of Ethylene, Acetylene, and Propylene at 1073–1373 K with a Plug-Flow Reactor Model, Int. J. Chem. Kinet., 2007, 199–208 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2dt02107b |
| ‡ Present address: Department of Materials Science and Engineering, University of Illinois at Urbana-Champaign, 1304 W. Green St. MC 246, Urbana, Illinois 61801, USA. |
| This journal is © The Royal Society of Chemistry 2022 |