Stabilizing photo-sensitive colchicine through rebalancing electron distribution of the reactive tropolone ring†
Xiaoyu
Ma
ab,
Bingqing
Zhu
a,
Zeen
Yang
ab,
Yuhang
Jiang
ab and
Xuefeng
Mei
 *ab
*ab
aPharmaceutical Analytical & Solid-State Chemistry ResearchCenter, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China. E-mail: xuefengmei@simm.ac.cn; Fax: +86 2150807088; Tel: +862150800934
bUniversity of Chinese Academy of Sciences, No.19A Yuquan Road, Beijing 100049, China
First published on 24th November 2020
Abstract
Colchicine is light-sensitive and undergoes electrocyclic reaction upon exposure to light. In this study, the photostability of colchicine was found to be significantly improved via rebalancing the electron density of the tropolone ring system after solid form changes. This work may provide a new approach for resolving photo-sensitive challenges during drug development.
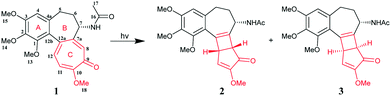
Colchicine (Col) is a kind of natural alkaloid extracted from autumn crocus, and the medicinal history of Col traces back thousands of years. Col was approved by the U.S. Food and Drug Administration (FDA) in 2009 for the prophylaxis and treatment of gout flares and familial Mediterranean fever (FMF) based on its multiple anti-inflammatory properties.1 The reported cumulative number of gout patients was 7.44 million around the world in 2017, with a prevalence of 41.22 million cases,2 and the global sales of Col were about $700 million each year during 2015–2019 (data from IMS Health Analytics Link; Value – Analytics Link). Recent studies have proved that Col is also beneficial to the treatment of other diseases, such as vasculitis,3 Sweet's syndrome, cardiovascular disease and cancer.4 Due to its broad application potential in clinical fields, many analogues and prodrugs have been synthesized and investigated.5 Although Col has been widely used around the world, there still exist many challenges that need to be overcome. Col has a narrow therapeutic window and the dosage is very low (0.5 mg tablet). Its bioavailability is highly variable, ranging from 24% to 88% in humans.6 The most common side-effect is gastrointestinal toxicity that is dose dependent. Hence, accurate dosage is particularly important in clinical application. Given the low dosage (0.5 mg) and narrow therapeutic window of Col, the presence of impurities, even in a small amount, would lower its therapeutic efficacy and even induce toxicity.7 Therefore, it is critical to minimize the degradation of Col during the manufacturing process and drug storage. It was reported that Col (1) was a highly photoreactive compound and could be easily oxidized into different isomers when exposed to ultraviolet radiation.8 As shown in Scheme 1,8 the structure of Col comprises an unsaturated tricyclic system, including a trimethoxy phenyl ring (A), a seven membered ring (B), and a highly photo-sensitive tropolone ring (C). When irradiated under UV light, Col undergoes an intramolecular 4π electrocyclic reaction on the tropolone moiety, forming impurities β- and γ-lumicolchicine (2 and 3) with a certain quantity. However, until now, no research has been done to overcome the photostability issue of this important drug. Our previous work has demonstrated that cocrystallization is an effective method to improve the photostability of a variety of different drugs by changing the molecular conformation or crystal packing patterns.9,10 In this particular case, we envisioned that the electrocyclic reaction of Col may be affected by the electronic environment of the tropolone ring, which could be potentially tuned by cocrystallization.
After careful examination of the chemical structure of Col, we would expect that the carbonyl group in ring C is a good hydrogen bond acceptor. Col is a very weak base with a pKa of 1.85, therefore, strong acids including benzenesulfonic acid (BSA), p-toluenesulfonic acid (PTA) and 2-naphthalenesulfonic acid (NSA) (structures shown in Table S1†) were initially selected to form cocrystals with Col. In the experiment, the solution of Col changes from colourless to yellow when equimolar amounts of sulfonic acids were added. Crystalline powders of Col–BSA, Col–PTA and Col–NSA complexes were obtained via slurry with equimolar amounts of Col and the corresponding acids in ethyl acetate solution for 24 hours. XRPD was used to confirm the formation of new phase. The results (Fig. S1†) show that the XRPD pattern of those newly formed Col complexes is different from either that of Col or the corresponding coformer (CCF). In addition, the experimental XRPD patterns are closely matched with the simulated ones derived from single crystal structure data, confirming the formation of highly pure phases. DSC and TGA profiles are also presented in Fig. S1,† indicating the high crystallinity and absence of solvent. It is generally accepted that salt formation will be expected if ΔpKa is greater than 3 and cocrystals will be exclusively formed if ΔpKa is less than 0.11 However, the extent of proton transfer cannot be accurately predicted in the solid state when ΔpKa is between 0 and 3. In this work, Col (protonated) has a pKa value of 1.85 and ΔpKa is between 1.15 and 2.45. Therefore, more evidence is needed to confirm whether the new complex is a cocrystal or salt.
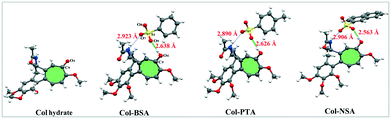
The distinction between salts and cocrystals is whether a proton transfer has occurred from an acid to a base.12 In the solid state, the extent of proton transfer can be evaluated from single-crystal X-ray diffraction analysis of the proton location and bond lengths involved. The single crystal structures of various Col complexes are exhibited in Fig. 1 and crystallographic data are summarized in Table S2.† Here, the single crystal of Col raw material, which was an unhydrated solid form, was not obtained. Considering the similar unionized state of Col hydrate with Col raw material, the single crystal of Col hydrate was used here to illustrate the difference of salts and neutral Col. On the one hand, structure determinations of the Col complexes show that the relevant protons are clearly located on the carbonyl oxygen atom O4 of Col in Col–BSA, Col–PTA and Col–NSA, implying that the protons have transferred from acids to Col. From the single crystal structures, we surprisingly observed that the carbonyl group could act as the protonation site, which has been rarely investigated before. On the other hand, the bond length of C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4 and S–O can also be used to determine the ionic state of the complex (listed in Table 1). In Col–BSA, Col–PTA and Col–NSA, the C9
O4 and S–O can also be used to determine the ionic state of the complex (listed in Table 1). In Col–BSA, Col–PTA and Col–NSA, the C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4 distances are found to be about 1.33 Å, longer than C
O4 distances are found to be about 1.33 Å, longer than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1.24 Å) in neutral Col, suggesting the ionized state of C
O (1.24 Å) in neutral Col, suggesting the ionized state of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. In free sulfonic acid, the S–O distances are about 1.58 Å and 1.42–1.48 Å, consistent with S–O and S
O. In free sulfonic acid, the S–O distances are about 1.58 Å and 1.42–1.48 Å, consistent with S–O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. However, when deprotonated, they have essentially identical S–O bond distances (1.44–1.47 Å), which can be observed in the Col complexes. Both the proton location and changes in the bond lengths involved imply the formation of Col salts rather than cocrystals. IR spectroscopy can further shed some light on the interactions between Col and acids. The stretching frequency corresponding to C9
O, respectively. However, when deprotonated, they have essentially identical S–O bond distances (1.44–1.47 Å), which can be observed in the Col complexes. Both the proton location and changes in the bond lengths involved imply the formation of Col salts rather than cocrystals. IR spectroscopy can further shed some light on the interactions between Col and acids. The stretching frequency corresponding to C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4 (ref. 13) of Col is red-shifted from 1615 cm−1 to 1596–1599 cm−1 (Fig. S3†). Changes in S
O4 (ref. 13) of Col is red-shifted from 1615 cm−1 to 1596–1599 cm−1 (Fig. S3†). Changes in S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration within 1400–1100 cm−1 also take place, which indicate the formation of salts.14 In summary, the X-ray crystallography and IR results confirmed the ionization of the carbonyl group of Col, which has been rarely reported before.
O stretching vibration within 1400–1100 cm−1 also take place, which indicate the formation of salts.14 In summary, the X-ray crystallography and IR results confirmed the ionization of the carbonyl group of Col, which has been rarely reported before.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4 and S–O/S
O4 and S–O/S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths in neutral Col and its salts
O bond lengths in neutral Col and its salts
| Bond type | Col hydrate | Col–BSA | Col–PTA | Col–NSA |
|---|---|---|---|---|
C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O4 (Å) O4 (Å) |
1.24 | 1.33 | 1.34 | 1.34 |
| S1–O7 (Å) | — | 1.47 | 1.47 | 1.47 |
| S1–O8 (Å) | — | 1.45 | 1.45 | 1.46 |
| S1–O9 (Å) | — | 1.44 | 1.44 | 1.44 |
The crystal structures reveal that the asymmetric units of the three salts consist of two molecules: one Col and one CCF, forming a salt pair. The salts are connected by a set of strong charge-assisted hydrogen-bonding interactions between Col and CCF, which are O4–H⋯O9 and N1–H⋯O7. As depicted in Fig. 1, the intermolecular H-bond distances of O4–H⋯O9 are about 2.64 Å, 2.63 Å and 2.56 Å in Col–BSA, Col–PTA and Col–NSA, respectively, shorter than that of N1–H⋯O7 (about 2.90 Å). We can also observe that both Col–PTA and Col–BSA are isomorphs with a similar packing mode, and crystallized in a monoclinic crystal system with the space group P21. Col–NSA crystalized in an orthorhombic crystal system with the space group P212121. However, looking along the a and b axis, we observed that there is no interaction between the salt pairs in the extended 3D structure (Fig. S2†) of the three Col salts.
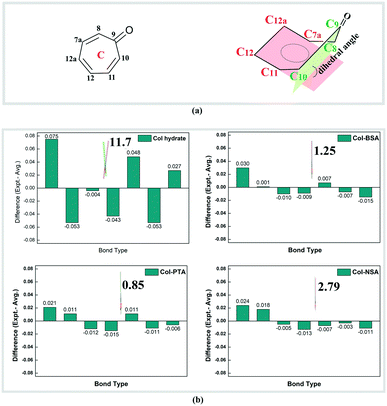
Besides the changes in the ionic state, we surprisingly found that the electronic environment of reactive ring C has also been changed dramatically. Bond length alternation existed in neutral Col, wherein the distance between adjacent carbons reflects the single- (1.43–1.49 Å) and double-bond (1.35–1.38 Å), respectively. After salt formation, there is a decrease in the bond lengths of the four single bonds (C9–C10, C11–C12, C12a–C7a, and C8–C9) and an increase in the three double bonds (C10–C11, C12–C12a, and C7a–C8). All the C–C bonds show an equalization with a bond length of 1.38–1.42 Å, which is an intermediate between a single and double bond. Deviation of the bond length from the average value in ring C of Col and its corresponding salts is displayed in Fig. 2 and the data are summarized in Table S6.† There are two Col molecules in an asymmetric unit, the bond lengths of which are similar although the configurations are different. Therefore, only one of the molecules was analysed and is shown in Fig. 2b. It is obvious that the deviation of each bond length within ring C from the average in salts (no more than 0.030) is much smaller than that of Col itself, implying a bond length equalization in ring C after salt formation. Upon salt formation, not only was the bond length equalization observed, but the conformational changes of ring C were also found. By carefully analysing the single crystal structure of neutral Col hydrate, it was found that seven-membered ring C is not an absolute plane because of the deviation of the carbonyl group. The deviation of the carbonyl group from planarity is evaluated by the dihedral angles between plane 1 (C8–C9–C10) and plane 2 (C7a–C12a–C12–C11), which denote the degree of coplanarity of ring C (depicted in Fig. 2). We can see that the dihedral angle in neutral Col is 11.73° and it was reduced to 1.25°, 0.85° and 2.79° in Col–BSA, Col–PTA and Col–NSA, respectively. The reduced dihedral angles indicate that reactive ring C becomes substantially planar in the salt. Both the bond length and dihedral angle changes were observed in the ionized carbonyl salts. Herein, we speculate that the conformational and electronic changes in reactive ring C may have a large influence on the photostability of Col in the solid state.
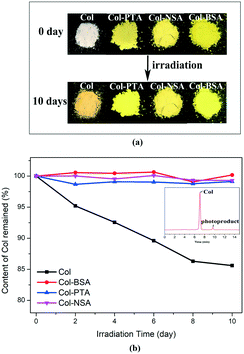
In order to investigate the impact of the physical form changes on the physicochemical properties, the photostability of the newly synthesized salts was studied and compared to that of Col itself. Samples were placed between two glass plates in a stability chamber with illumination of 5500 lx. The samples were collected at the intervals of 2, 4, 6, 8 and 10 days for analysis. It was observed that the color of Col changed from white to dark yellow after 10 day exposure under light. However, no obvious color change was observed in all the salt forms when subjected to the same conditions (Fig. 3a). Furthermore, time-dependent residual content of Col was evaluated by HPLC and the result is presented in Fig. 3b. The data for the Col salts were normalized and expressed as the percentage of Col component. For pure Col, it degrades at a linear rate, and the residual content decreased to 85% after 10 days under irradiation. The assay value was found to be quickly reduced to 90% at day 6 and 85% at day 10. However, no obvious degradation was observed for Col–BSA, Col–PTA and Col–NSA. The assay value of Col was maintained at almost 100% after 10 day stress treatment. The HPLC analysis also revealed the formation of an impurity peak (9.5 min) after Col (7.0 min) (inset in Fig. 3b). In the stability study with respect to humidity, no chemical degradation of salts was observed. The result demonstrates superior chemical stability of all the salts over Col itself. Therefore, we propose that the ionization of tropones plays an important role in the improvement of its stability under light.
To verify our proposal, we selected another three compounds with a tropolone ring (tropone, tropolone and hinokitiol; structures shown in Fig. S4†), which have also been reported to be light sensitive due to the electrocyclic ring-closing reaction.15 Strong sulfonic acids including PTA, NSA and DNSA (structures shown in Table S1†) were also selected to form salt products. As expected, single crystals of nine salts were obtained. The crystallographic data are summarized in Tables S3–S5† and their asymmetric units are shown in Fig. S5.† From the single crystal structures, it was found that the proton transfer existed in the nine new complexes, confirming the formation of salts. The bond lengths of the salts are presented in Table S7† and the deviation is illustrated in Fig. S6–S8.† As expected, equalization of the C–C bonds was observed after salt formation. Photostability study shows that the neutral tropones degrade rapidly under light (Fig. S9†). Specifically, Tr degrades to about 72% after 10 days, and the residual content of Tro and Hin is only about 50%. However, all the salts show superior stability and more than 90% remains after 10 day irradiation. The small amount of degradation of tropone salts may be caused by another chemical mechanism, like cycloaddition.16 A systematic increment of photostability of these tropone derivatives verifies our proposal that the ionized tropone has better photostability than its neutral form. Therefore, salt formation can be a general method to resolve the stability challenges of tropone containing compounds.
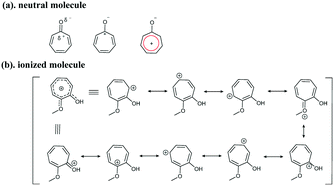
Based on the chemical structures of the tropolone ring, resonance theory can be used to understand the structure–property relationship. It is known that neutral tropones have aromatic properties. The carbon atom is polarized with a partial positive charge, forming an aromatic ring with 6 pi-electrons (Fig. 4a). After protonation, the dihedral angle was reduced to about 0° – a nearly planar structure (Fig. 2), compared to the non-coplanar aromatic tropolone ring in neutral Col (11.73°, illustrated in Fig. 2), suggesting the salts are more aromatic than the neutral ones. More resonance structures of ring C can be drawn according to the resonance theory developed by Linus Pauling.17 As depicted in Fig. 4b, the charge is distributed among ring C, including oxygen atoms, and shared on the atoms of ring C, resulting in a higher conjugated tropone system. The more aromatic system in salt forms should be responsible for the stability of tropones.
The natural bond orbital (NBO) analysis is an efficient method to study intramolecular bonding and interaction among bonds which helps to understand conjugative interactions between electron filled donor and empty acceptor orbitals. For each donor (i) and acceptor (j), the stabilization energy E(2) is associated with delocalization i → j.18,19 Larger E(2) values reflect stronger interactions between electron donors and electron acceptors, i.e., hyper conjugative interactions of the system. E(2) values of the tropolone rings were calculated and the stabilization energies larger than 20 kcal mol−1 were chosen for listing in Tables S8 and S9.† Table S8† shows that in the neutral tropolone ring the intra-molecular conjugative interactions are formed by the orbital overlap between π (C7a–C8) and π* (O4–C9), π (C10–C11) and π* (C12–C12a) and π (C12–C12a) and π* (C7a–C8). The interaction related to the conjugation in the neutral tropolone ring leads to the stabilization energies of 21.95 kcal mol−1, 21.30 kcal mol−1 and 20.26 kcal mol−1, respectively. However, stronger interactions have been observed between π (C7a–C8) and LP*1 (C9) with energies more than 75 kcal mol−1 in salts, reflecting higher conjugation of the system and thus causing the stabilization of the tropolone ring. According to Table S8,† the interactions between π (C10–C11) and LP*1 (C9) and π (C11–C12) and LP*1 (C10) are also responsible for the stabilization of Col. From Table S9,† larger E(2) values were also observed in the Tr, Tro and Hin salts, reflecting the higher conjugative effect of the tropolone ring after ionization.
In conclusion, a general approach for resolving stability challenges of Col was envisaged through rebalancing electron distribution within the tropolone ring system. This hypothesis was verified by examination of 12 model compounds with a common structure component. On the one hand, three Col salts were successfully prepared the and structure–property relationship was examined by a range of analysis tools, including single-crystal X-ray diffraction analysis, XRPD, DSC/TGA, IR, 1H-NMR, HPLC, etc. Photostability study showed that Col degraded rapidly under light, but no obvious degradation was observed in its salts after 10 day irradiation. On the other hand, sulfonates of the other nine reactive tropone derivatives were also studied, which showed a similar improvement of photostability in the solid state upon form changes. From these twelve salts, we also found that the carbonyl group can also act as a salt formation site. Resonance theory and NBO analysis revealed that a more aromatic tropolone system is the fundamental mechanism for the stability improvement. This finding may provide a new route for developing photo-sensitive drugs when facing stability challenges.
This research work was financially supported by the National Science and Technology Major Projects (no. 2017ZX09101001), the Natural Science Foundation of Shanghai (Grant No. 18ZR1447900), and the Youth Innovation Promotion Association CAS (Grant No. 2016257).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Slobodnick, B. Shah, S. Krasnokutsky and M. H. Pillinger, Rheumatology, 2018, 57, i4–i11 CrossRef CAS.
- C. Mattiuzzi and G. Lippi, Clin. Rheumatol., 2020, 39, 1061–1063 CrossRef.
- S. Yurdakul, C. Mat, Y. Tuzun, Y. Ozyazgan, V. Hamuryudan, O. Uysal, M. Senocak and H. Yazici, Arthritis Rheum., 2001, 44, 2686–2692 CrossRef CAS.
- F. Wang, C. Wang, Y. Liu, W. Lan, H. Han, R. Wang, S. Huang and C. Cao, Chem. Commun., 2020, 56, 2099–2102 RSC.
- A. A. Ghawanmeh, K. F. Chong, S. M. Sarkar, M. A. Bakar, R. Othaman and R. M. Khalid, Eur. J. Med. Chem., 2018, 144, 229–242 CrossRef CAS.
- A. Slobodnick, B. Shah, M. H. Pillinger and S. Krasnokutsky, Am. J. Med., 2015, 128, 461–470 CrossRef CAS.
- B. B. Levine, Arch. Biochem. Biophys., 1961, 93, 50–55 CrossRef CAS.
- I. Cacelli, M. D'Auria and V. Villani, J. Chem. Theory Comput., 2007, 3, 649–656 CrossRef CAS.
- B. Zhu, J.-R. Wang, Q. Zhang and X. Mei, Cryst. Growth Des., 2015, 16, 483–492 CrossRef.
- J. R. Wang, C. Zhou, X. Yu and X. Mei, Chem. Commun., 2014, 50, 855–858 RSC.
- A. J. Cruz-Cabeza, CrystEngComm, 2012, 14, 6362–6365 RSC.
- S. L. Childs, G. P. Stahly and A. Park, Mol. Pharmaceutics, 2007, 4, 323–338 CrossRef CAS.
- J. Coates, Encyclopedia of Analytical Chemistry, 2006, DOI:10.1002/9780470027318.a5606.
- Z. Yan-Min, J. Phys.: Conf. Ser., 2019, 1237, 022107 CrossRef.
- S. C. Coote, Eur. J. Org. Chem., 2020, 2020, 1405–1423 CrossRef CAS.
- T. Mukai, T. Tezuka and Y. Akasaki, J. Am. Chem. Soc., 1966, 88, 5025–5026 CrossRef CAS.
- H. He, Y. Huang, Q. Zhang, J.-R. Wang and X. Mei, Cryst. Growth Des., 2016, 16, 2348–2356 CrossRef CAS.
- M. Iwaoka, H. Komatsu, T. Katsuda and S. Tomoda, J. Am. Chem. Soc., 2004, 126, 5309–5317 CrossRef CAS.
- M. Prabhaharan, A. R. Prabakaran, S. Gunasekaran and S. Srinivasan, Spectrochim. Acta, Part A, 2015, 136 Pt B, 494–503 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data and crystallographic details. CCDC 2036740, 2027384–2027386 and 2027390–2027398. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce01551b |
| This journal is © The Royal Society of Chemistry 2021 |