Open Access Article
Open Access ArticleAn activity-based fluorescent sensor for the detection of the phenol sulfotransferase SULT1A1 in living cells†
Regina A.
Baglia‡
 ,
Kira R.
Mills‡
,
Kira R.
Mills‡
 ,
Koushambi
Mitra‡
,
Koushambi
Mitra‡
 ,
Jasmine N.
Tutol‡
,
Jasmine N.
Tutol‡
 ,
Darby
Ball
,
Darby
Ball
 ,
Kierstin M.
Page
,
Jyothi
Kallu
,
Sriharika
Gottipolu
,
Sheena
D’Arcy
,
Kierstin M.
Page
,
Jyothi
Kallu
,
Sriharika
Gottipolu
,
Sheena
D’Arcy
 ,
Steven O.
Nielsen
,
Steven O.
Nielsen
 and
Sheel C.
Dodani
and
Sheel C.
Dodani
 *
*
Department of Chemistry and Biochemistry, The University of Texas at Dallas, Richardson, TX 75080, USA. E-mail: sheel.dodani@utdallas.edu
First published on 15th March 2021
Abstract
Human phenol sulfotransferases mediate the transfer of a sulfuryl moiety from the activated sulfate donor PAPS to hydroxy-containing substrates, altering substrate solubility and charge to affect phase II metabolism and cell signaling. Here, we present the development, computational modeling, in vitro enzymology, and biological application of STS-3, an activity-based fluorescent sensor for the SULT1A1 isoform.
Human phenol sulfotransferases (SULT1 family) are essential phase II metabolic enzymes that mediate sulfuryl group transfer from the activated sulfate donor 3′-phosphoadenosine-5′-phosphosulfate (PAPS) primarily to hydroxy-containing small molecules.1–6 Sulfurylation enhances the water solubility to increase the clearance of xenobiotics, to recycle endogenous metabolites (e.g. estrogen, dopamine), and, in some cases, to (in)activate drugs.2–4,6,7 As such, phenol sulfotransferases are not only linked to cellular signaling in normal physiology but also in disease states ranging from cancer to neurodegeneration.1,3,4,6–12 Our understanding of the substrate scope and activities of these enzymes in biological contexts has been significantly advanced by parallel efforts using computational modeling, structural characterization, mechanistic enzymology, and assay methods.10,13–23
Along these lines, one of the most widely used approaches to monitor phenol sulfotransferase activity with purified protein or cell lysates relies on radiolabeling of substrates, PAP35S, or sulfate (35SO2−4), coupled to chromatographic detection of the sulfurylated products.24,25 Alternatively, coupled-enzyme assays with colorimetric and fluorescent substrates provide a safer, cost-effective, and continuous readout of enzyme activity.24–28 If radiolabeling is not preferred or if the sulfurylated product does not have an optical signature, mass spectrometry or nuclear magnetic resonance spectroscopy can be used.20,24,25,29,30 A limited number of these approaches have been translated to living cells but do not provide a direct readout with a spatially and temporally-resolved map of activity.31 We envision that activity-based fluorescent sensors can address this gap.32–39 This strategy affords the ability to chemically tune and transform an enzyme's substrate into a fluorescent imaging platform for live-cell applications. To our knowledge, activity-based sensing has not been widely exploited for phenol sulfotransferases in living cells. The SULT1 family consists of 9 isoforms, all with a high degree of sequence and structural similarity (≥55%).15 Of these isoforms, SULT1A1 is the most widely expressed in the human body and promiscuous, thus making it an ideal target for this proof-of-concept study.40,41
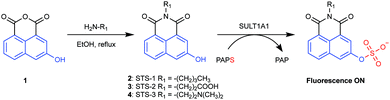
To generate an activity-based fluorescent sensor, we first selected the substrate 2-naphthol as it has been demonstrated to undergo sulfurylation by SULT1A1.15,18 Even though 2-naphthol and the resulting 2-naphthyl sulfate product are fluorescent in water, there are negligible differences in the emission spectra of these compounds at physiologically relevant pH.15,16 As such, our strategy to convert 2-naphthol into an activity-based fluorescent sensor relied on its structural similarity with 3-hydroxy-1,8-naphthalic anhydride (compound 1, Fig. 1). The latter can be readily functionalized with primary amines to generate naphthalimide fluorophores.42–47 Specifically, we selected three electronically distinct amines: butylamine (STS-1), 3-aminopropanoic acid (STS-2), and N,N-dimethylethylenediamine (STS-3) (Fig. 1). We reasoned that all three sensors would be weakly fluorescent because the non-bonding electrons on the oxygen atom of the phenol could quench the excited state of the naphthalimide fluorophore. However, the quenched state could be relieved upon sulfurylation to generate a turn-on or ratiometric fluorescence response, as previously reported for 3- and 4-substituted-1,8-naphthalimide-based sensors.42–44,48 We note that STS-1 has been evaluated as a fluorescent sensor for a plant glucosyltransferase in vitro,48 and STS-3 has been tested as an anti-cancer agent.45–47,49
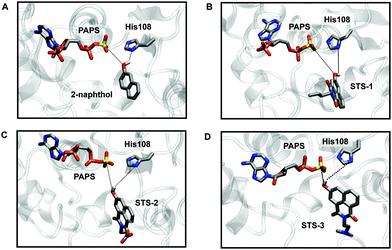
Preparatory docking calculations and constrained MD simulations were first carried out, followed by extensive equilibrium MD simulations to see if the sensors could bind SULT1A1 in a similar fashion to 2-naphthol.21,50 During the course of the MD simulations, two stabilizing interactions were monitored: one between the phenol group of the substrate and the sulfur atom of the PAPS cofactor (S–O distance) and one between the phenol group of the substrate and the δ-nitrogen atom (N–H distance) of the catalytic histidine residue (His108) in the active site (Fig. 2 and Fig. S9–S11, Movies smov1–smov8, ESI†). Like 2-naphthol, STS-1, STS-2, and STS-3 were maintained in catalytically productive orientations and had stabilizing interactions with PAPS and His108 (distances <6 Å).21,50 For each sensor, the functionalized naphthalimide backbone was exposed to the bulk water and did not interfere with the orientation of the phenol group in proximity to the site of catalysis. The importance of the phenol group for optimal substrate positioning and reactivity was further demonstrated with control substrates lacking the hydroxy functional groups, namely naphthalene and STS-3C (Fig. S9–S11, ESI†). The simulations revealed that water was able to interact with the PAPS sulfuryl group, the polar N and O atoms of the catalytic His108, and the control substrates in such a way that both naphthalene and STS-3C were separated from the binding pocket.
In parallel, the spectroscopic properties of STS-1, STS-2, and STS-3 were evaluated with purified SULT1A1. Spectral changes were observed when 10 μM of each sensor was incubated with 0.015 μg μL−1 (0.4 μM) of purified SULT1A1 and 60 μM of the PAPS cofactor at 37 °C in 50 mM Tris buffer at pH 7.4 (Fig. 3). At t = 0 min, the absorption spectrum for STS-1 was featureless, but STS-2 and STS-3 showed two broad maxima at ∼340 nm and ∼390 nm. Excitation at both absorption maxima resulted in no emission above the background of the buffer for all three sensors, which is consistent with a quenching mechanism. However, clear spectral changes were observed within t = 10 min of incubation with STS-2 and STS-3. Notably, the absorption maxima at ∼340 nm increased in intensity whereas the absorption maxima at ∼390 nm decreased in intensity, suggesting the formation of new products. Upon excitation at 340 nm, robust turn-on emission responses were observed at ∼415–420 nm within t = 1 h (average ± standard deviation): STS-1 (5.7 ± 0.9), STS-2 (6.3 ± 1.7), STS-3 (7.1 ± 0.2) (Fig. 3 and Table S1, ESI†). These spectral changes did not occur in the absence of the enzyme or PAPS cofactor (Fig. S14–S22, ESI†). Similarly, co-incubation with 10 μM of 2,6-dichloro-4-nitrophenol (DCNP), a substrate inhibitor for SULT1A1 (Ki = 2 μM)51 attenuated the turn-on fluorescence response of each sensor to varying degrees (Fig. S14–S22, ESI†).15,51–53 Encouraged by these results, we used liquid chromatography–mass spectrometry (LC–MS) to confirm that the turn-on fluorescence response for each sensor was attributed to the formation of the sulfurylated product (Fig. S23, ESI†).
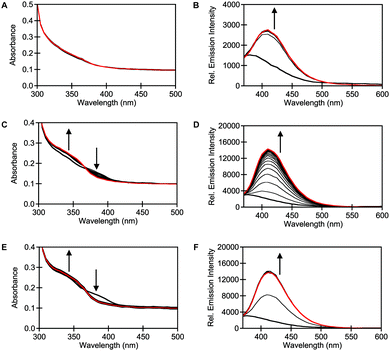 | ||
| Fig. 3 STS-1, STS-2, and STS-3 are turn-on fluorescent sensors for SULT1A1. Absorbance responses of 10 μM (A) STS-1, (C) STS-2, and (E) STS-3 to 0.015 μg μL−1 (0.4 μM) SULT1A1 at 0 (bold black) and 180 (red) min. Emission responses of 10 μM (B) STS-1, (D) STS-2, and (F) STS-3 to 0.015 μg μL−1 (0.4 μM) SULT1A1 at 0 (bold black) and 180 (red) min with 10 min intervals (black). Reactions were carried out in 50 mM Tris buffer at pH 7.5 with 60 μM PAPS at 37 °C. Excitation was provided at 340 nm, and the emission was collected from 370–600 nm. Arrow direction corresponds to increasing time. A representative data set from three technical replicates is shown (Fig. S14–S22, ESI†). | ||
To better understand the differences in the observed emission responses, we determined the kinetic parameters for each sensor with SULT1A1 using a Michaelis–Menten model (Fig. S24, ESI†). The low solubility of STS-1 above 10 μM prevented accurate measurements, so it was not further evaluated. The average KM values of STS-2 and STS-3 were determined to be 10 μM and 4 μM, respectively. Based on the kcat values (average ± standard deviation), STS-3 (21 ± 5 s−1) reacted faster than STS-2 (8 ± 4 s−1) with SULT1A1, thus allowing for the quick buildup of fluorescence signal (Table S1, ESI†). These differences also translated to the overall catalytic efficiencies. Based on these parameters and the robust turn-on fluorescence response of STS-3, we next established the ability of STS-3 to detect SULT1A1 activity in living cells.
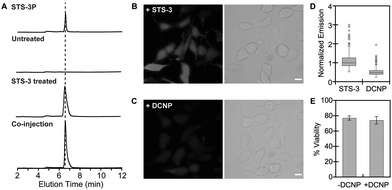
To validate STS-3, we selected the human SK-N-MC neuroepithelial cancer cell line, which has been previously reported to express SULT1A1.54,55 First, SK-N-MC cells were treated with either a DMSO vehicle control or 10 μM of STS-3 for 4 h at 37 °C in serum-free media, followed by lysis and analysis with fluorescence-based high-performance liquid chromatography (HPLC) (Fig. 4 and Fig. S25–S31, ESI†). Extracts from STS-3 treated cells revealed a single fluorescent product with emission at 420 nm, whereas extracts from DMSO treated cells did not. Co-injection with the authentic sulfurylated product standard, STS-3P, confirmed that STS-3 does indeed undergo sulfurylation in living cells. Encouraged by these results, we turned to live-cell fluorescence microscopy to visualize endogenous SULT1A1 activity (Fig. 4 and Fig. S32–S35, ESI†). Cells treated with STS-3 and excited at 340 nm showed measurable levels of fluorescence signal above the autofluorescence of cells treated with DMSO. Co-incubation with the substrate inhibitor DCNP attenuated the intracellular fluorescence signal by ∼51%. Moreover, this change did not arise from any cytotoxic effects of co-treatment with STS-3 and DCNP. It is not surprising that STS-3 lowered cell viability as related derivatives have been used as anti-cancer agents.46,47,49 Interestingly, DCNP is known to lower PAPS levels56 and inhibit the SULT1A1 isoform, but not the SULT1A3 isoform,15,51,57 which is also expressed in SK-N-MC cells.12,54,55 As such, this provides strong evidence that STS-3 is a reporter of SULT1A1 activity in this cell line. Indeed, the fluorescence imaging also highlights how the reported activity can vary from cell-to-cell. This could arise from differences in the uptake, distribution, or reactivity of DCNP or STS-3 with SULT1A1 or other sulfotransferases, and even cofactor availability, all of which will be the subject of future investigations.
In summary, we have presented the development, computational modeling, in vitro enzymology, and biological application of STS-3, a first-generation activity-based fluorescent sensor for SULT1A1. This proof-of-concept study sets the stage to further develop and apply activity-based fluorescent sensors to discover how phenol sulfotransferase activity can intersect competitive metabolic pathways to modify endogenous metabolites, xenobiotics, or drugs. Along these lines, efforts are currently underway to develop high-throughput screening methods with STS-3 and generate a palette of sensors with improved reaction kinetics and expanded isoform preferences for applications in a range of cell types.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We thank Dr Alexander Lippert, Dr Gabriele Meloni, Dr John Sibert, and members of the Dodani Lab for helpful discussions. We also thank the Lippert lab at SMU and the Gassensmith lab at UT Dallas for providing access to instrumentation. S. D. acknowledges support from the UT Proteomics Network and National Institute of General Medical Sciences of the National Institutes of Health (R35GM13375). S. C. D. acknowledges support from The University of Texas at Dallas startup funds, the Welch Foundation (AT-1918-20170325), and the National Institute of General Medical Sciences of the National Institutes of Health (R35GM128923).Notes and references
- S. Günal, R. Hardman, S. Kopriva and J. W. Mueller, J. Biol. Chem., 2019, 294, 12293–12312 CrossRef PubMed.
- A. W. Y. Leung, I. Backstrom and M. B. Bally, OncoTargets Ther., 2016, 7, 55811–55827 CrossRef PubMed.
- N. Gamage, A. Barnett, N. Hempel, R. G. Duggleby, K. F. Windmill, J. L. Martin and M. E. McManus, Toxicol. Sci., 2006, 90, 5–22 CrossRef CAS PubMed.
- R. Langford, E. Hurrion and P. A. Dawson, J. Genet. Genomics, 2017, 44, 7–20 CrossRef.
- M. W. H. Coughtrie, Pharmacogenomics J., 2002, 2, 297–308 CrossRef CAS.
- L.-Q. Wang and M. James, Curr. Drug Metab., 2005, 7, 83–104 CrossRef PubMed.
- C. A. Strott, Endocr. Rev., 2002, 23, 703–732 CrossRef CAS.
- N. J. Butcher, M. K. Horne, G. D. Mellick, C. J. Fowler, C. L. Masters and R. F. Minchin, Pharmacogenomics J., 2018, 18, 209–214 CrossRef CAS PubMed.
- M. Runge-Morris, T. A. Kocarek and C. N. Falany, Drug Metab. Rev., 2013, 45, 15–33 CrossRef CAS PubMed.
- M. Suiko, K. Kurogi, T. Hashiguchi, Y. Sakakibara and M.-C. Liu, Biosci., Biotechnol., Biochem., 2017, 81, 63–72 CrossRef CAS PubMed.
- S. Yasuda, T. Yasuda, Y. Hui, M. Y. Liu, M. Suiko, Y. Sakakibara and M. C. Liu, Neurosci. Res., 2009, 64, 273–279 CrossRef CAS PubMed.
- S. Yasuda, M.-Y. Liu, M. Suiko, Y. Sakakibara and M.-C. Liu, J. Neurochem., 2007, 103, 2679–2689 CrossRef CAS.
- I. Berger, C. Guttman, D. Amar, R. Zarivach and A. Aharoni, PLoS One, 2011, 6, e26794 CrossRef CAS PubMed.
- E. Chapman, M. D. Best, S. R. Hanson and C.-H. Wong, Angew. Chem., Int. Ed., 2004, 43, 3526–3548 CrossRef CAS PubMed.
- A. Allali-Hassani, P. W. Pan, L. Dombrovski, R. Najmanovich, W. Tempel, A. Dong, P. Loppnau, F. Martin, J. Thonton, A. M. Edwards, A. Bochkarev, A. N. Plotnikov, M. Vedadi and C. H. Arrowsmith, PLoS Biol., 2007, 5, e97 CrossRef PubMed.
- M. Arand, L. W. Robertson and F. Oesch, Anal. Biochem., 1987, 163, 546–551 CrossRef CAS.
- T. Wang, I. Cook and T. S. Leyh, J. Biol. Chem., 2017, 292, 20305–20312 CrossRef CAS.
- I. Cook, T. Wang and T. S. Leyh, Biochemistry, 2015, 54, 6114–6122 CrossRef CAS PubMed.
- C. Rakers, F. Schumacher, W. Meinl, H. Glatt, B. Kleuser and G. Wolber, J. Biol. Chem., 2016, 291, 58–71 CrossRef CAS.
- L. Yi, J. Dratter, C. Wang, J. A. Tunge and H. Desaire, Anal. Bioanal. Chem., 2006, 386, 666–674 CrossRef CAS PubMed.
- E. B. Yalcin, H. Stangl, S. Pichu, T. N. Mather and R. S. King, ACS Chem. Biol., 2011, 6, 176–184 CrossRef CAS PubMed.
- V. Y. Martiny, P. Carbonell, D. Lagorce, B. O. Villoutreix, G. Moroy and M. A. Miteva, PLoS One, 2013, 8, e73587 CrossRef CAS PubMed.
- E. E. Litsa, P. Das and L. E. Kavraki, Chem. Sci., 2020, 11, 12777–12788 RSC.
- M. W. Duffel, Comprehensive Toxicology, ed. C. A. B. T. McQueen, Elsevier, Oxford, 2nd edn, 2010, pp. 367–384 Search PubMed.
- P. Paul, J. Suwan, J. Liu, J. S. Dordick and R. J. Linhardt, Anal. Bioanal. Chem., 2012, 403, 1491–1500 CrossRef CAS PubMed.
- W. Zhou, B. P. Duckworth and R. J. Geraghty, Anal. Biochem., 2014, 461, 1–6 CrossRef CAS PubMed.
- W. Zhou, Y. Wang, J. Xie and R. J. Geraghty, Biochem. Biophys. Res. Commun., 2017, 482, 1207–1212 CrossRef CAS PubMed.
- T. Wang, I. Cook and T. S. Leyh, Drug Metab. Dispos., 2016, 44, 481–484 CrossRef CAS PubMed.
- C. Ballet, M. S. P. Correia, L. P. Conway, T. L. Locher, L. C. Lehmann, N. Garg, M. Vujasinovic, S. Deindl, J. M. Löhr and D. Globisch, Chem. Sci., 2018, 9, 6233–6239 RSC.
- I. Cook, T. Wang and T. S. Leyh, Biochem. Pharmacol., 2019, 159, 25–31 CrossRef CAS PubMed.
- G. B. Cole, G. Keum, J. Liu, G. W. Small, N. Satyamurthy, V. Kepe and J. R. Barrio, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6222–6227 CrossRef CAS PubMed.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- W. Chyan and R. T. Raines, ACS Chem. Biol., 2018, 13, 1810–1823 CrossRef CAS PubMed.
- K. Singh, A. M. Rotaru and A. A. Beharry, ACS Chem. Biol., 2018, 13, 1785–1798 CrossRef CAS PubMed.
- L. Feng, J. Ning, X. Tian, C. Wang, L. Zhang, X. Ma and T. D. James, Coord. Chem. Rev., 2019, 399, 213026 CrossRef CAS.
- J. Ning, W. Wang, G. Ge, P. Chu, F. Long, Y. Yang, Y. Peng, L. Feng, X. Ma and T. D. James, Angew. Chem., Int. Ed., 2019, 58, 9959–9963 CrossRef CAS PubMed.
- J. Zhang, X. Chai, X.-P. He, H.-J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2019, 48, 683–722 RSC.
- A. K. Yadav, C. J. Reinhardt, A. S. Arango, H. C. Huff, L. Dong, M. G. Malkowski, A. Das, E. Tajkhorshid and J. Chan, Angew. Chem., Int. Ed., 2020, 59, 3307–3314 CrossRef CAS.
- S. H. Gardner, C. J. Reinhardt and J. Chan, Angew. Chem., Int. Ed., 2021, 60, 5000–5009 CrossRef CAS PubMed.
- Z. Riches, E. L. Stanley, J. C. Bloomer and M. W. H. Coughtrie, Drug Metab. Dispos., 2009, 37, 2255–2261 CrossRef CAS PubMed.
- E. D. Salman, S. A. Kadlubar and C. N. Falany, Drug Metab. Dispos., 2009, 37, 706–709 CrossRef CAS PubMed.
- L. W. Zou, P. Wang, X. K. Qian, L. Feng, Y. Yu, D. D. Wang, Q. Jin, J. Hou, Z. H. Liu, G. B. Ge and L. Yang, Biosens. Bioelectron., 2017, 90, 283–289 CrossRef CAS PubMed.
- X. Lv, G.-B. Ge, L. Feng, J. Troberg, L.-H. Hu, J. Hou, H.-L. Cheng, P. Wang, Z.-M. Liu, M. Finel, J.-N. Cui and L. Yang, Biosens. Bioelectron., 2015, 72, 261–267 CrossRef CAS.
- H. Q. Dong, T. B. Wei, X. Q. Ma, Q. Y. Yang, Y. F. Zhang, Y. J. Sun, B. B. Shi, H. Yao, Y. M. Zhang and Q. Lin, J. Mater. Chem. C, 2020, 8, 13501–13529 RSC.
- L. K. E. Hardebeck, C. A. Johnson, G. A. Hudson, Y. Ren, M. Watt, C. C. Kirkpatrick, B. M. Znosko and M. Lewis, J. Phys. Org. Chem., 2013, 26, 879–884 CrossRef CAS.
- C. A. Johnson, G. A. Hudson, L. K. E. Hardebeck, E. A. Jolley, Y. Ren, M. Lewis and B. M. Znosko, Bioorg. Med. Chem., 2015, 23, 3586–3591 CrossRef CAS.
- M. F. Braña and A. Ramos, Curr. Med. Chem.: Anti-Cancer Agents, 2001, 1, 237–255 CrossRef.
- L. Feng, X. Tian, Z. Yu, X. Zhao, C. Sun, J. Cui, J. Ning, C. Wang, B. Zhang and X. Ma, Sens. Actuators, B, 2019, 282, 112–121 CrossRef CAS.
- R. K. Y. Zee-Cheng and C. C. Cheng, J. Med. Chem., 1985, 28, 1216–1222 CrossRef CAS PubMed.
- I. Cook, T. Wang, C. N. Falany and T. S. Leyh, J. Biol. Chem., 2013, 288, 34494–34501 CrossRef CAS.
- X. Li, D. L. Clemens, J. R. Cole and R. J. Anderson, J. Endocrinol., 2001, 171, 525–532 CAS.
- M. E. Veronese, W. Burgess, X. Zhu and M. E. McManus, Biochem. J., 1994, 302, 497–502 CrossRef CAS PubMed.
- H. Koster, E. Scholtens and G. J. Mulder, Aust. J. Exp. Biol. Med. Sci., 1979, 57, 340–344 CAS.
- N. P. Sidharthan, R. F. Minchin and N. J. Butcher, J. Biol. Chem., 2013, 288, 34364–34374 CrossRef CAS PubMed.
- M. Idris, D. J. Mitchell, R. Gordon, N. P. Sidharthan, N. J. Butcher and R. F. Minchin, Drug Metab. Dispos., 2020, 48, 337–344 CrossRef CAS PubMed.
- C. Gulat-Marnay, A. Lafitte, F. Vargas and J.-C. Schwartz, J. Neurochem., 1987, 49, 1443–1448 CrossRef CAS PubMed.
- G. J. Mulder and E. Scholtens, Biochem. J., 1977, 165, 553–559 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Methods, synthetic schemes and characterization, SDS-page, spectra for in vitro enzymology assays, LC–MS spectra, fluorescence-based HPLC spectra, fluorescence microscopy images, and cell viability. See DOI: 10.1039/d0cb00231c |
| ‡ R. A. B., K. R. M., K. M., and J. N. T. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |