Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transforming colloidal Cs4PbBr6 nanocrystals with poly(maleic anhydride-alt-1-octadecene) into stable CsPbBr3 perovskite emitters through intermediate heterostructures†
Dmitry
Baranov
 *a,
Gianvito
Caputo
*a,
Gianvito
Caputo
 a,
Luca
Goldoni
b,
Zhiya
Dang
a,
Riccardo
Scarfiello
c,
Luca
De Trizio
a,
Luca
Goldoni
b,
Zhiya
Dang
a,
Riccardo
Scarfiello
c,
Luca
De Trizio
 a,
Alberto
Portone
a,
Alberto
Portone
 d,
Filippo
Fabbri
d,
Andrea
Camposeo
d,
Filippo
Fabbri
d,
Andrea
Camposeo
 d,
Dario
Pisignano
d,
Dario
Pisignano
 de and
Liberato
Manna
de and
Liberato
Manna
 *a
*a
aNanochemistry Department, Istituto Italiano di Tecnologia, Via Morego 30, 16163 Genova, Italy. E-mail: dmitry.baranov@iit.it; liberato.manna@iit.it
bAnalytical Chemistry Lab, Istituto Italiano di Tecnologia, Via Morego 30, 16163 Genova, Italy
cCNR NANOTEC, Institute of Nanotechnology, c/o Campus Ecotecne, via Monteroni, 73100 Lecce, Italy
dNEST, Istituto Nanoscience-CNR, Piazza S. Silvestro 12, I-56127 Pisa, Italy
eDipartimento di Fisica “Enrico Fermi”, Università di Pisa, Largo Bruno Pontecorvo 3, I-56127 Pisa, Italy
First published on 20th March 2020
Abstract
The preparation of strongly emissive CsPbBr3 perovskite nanocrystals with robust surface passivation is a challenge in the field of lead halide perovskite nanomaterials. We report an approach to prepare polymer-capped CsPbBr3 perovskite nanocrystals by reacting oleylammonium/oleate-capped Cs4PbBr6 nanocrystals with poly(maleic anhydride-alt-1-octadecene) (PMAO). PMAO contains succinic anhydride units that are reactive towards the oleylamine species present on the Cs4PbBr6 nanocrystals' surface and produces polysuccinamic acid, which, in turn, triggers the Cs4PbBr6 to CsPbBr3 conversion. The transformation occurs through the formation of Cs4PbBr6–CsPbBr3 heterostructures as intermediates, which are captured because of the mild reactivity of PMAO and are investigated by high-resolution electron microscopy. The Cs4PbBr6–CsPbBr3 heterostructures demonstrate a dual emission at cryogenic temperature with an indication of the energy transfer from Cs4PbBr6 to CsPbBr3. The fully-transformed CsPbBr3 NCs have high photoluminescence quantum yield and enhanced colloidal stability, which we attribute to the adhesion of polysuccinamic acid to the NC surface through its multiple functional groups in place of oleate and alkylammonium ligands. The PMAO-induced transformation of Cs4PbBr6 NCs opens up a strategy for the chemical modification of metal halide NCs initially passivated with nucleophilic amines.
Introduction
Nanocrystals (NCs) of cesium lead halides have recently emerged as a class of semiconductor materials promising for light-emitting applications.1–3 The chemical reactivity of these NCs and the interconversion between the NCs of the two most studied bromides in this class, Cs4PbBr6 and CsPbBr3 perovskite, have been of interest since these NCs were first synthesized in the colloidal form.4–8 The Cs4PbBr6 → CsPbBr3 conversion, which can be triggered using various reagents (for example, Prussian blue,9 oleic acid,10 PbBr2,7,11 and water)12–14 is an interesting approach to prepare emissive CsPbBr3 NCs. For example, Yin's group exploited heterogeneous water-mediated CsBr extraction from Cs4PbBr6 NCs in hexane as a method for making luminescent CsPbBr3/SiO2 or CsPbBr3/Ta2O5 Janus-type heterostructures,13 and branched CsPbBr3 dodecapods.15 Despite several reports on Cs4PbBr6 → CsPbBr3 transformation at the nanoscale, the nanocrystal intermediates of this reaction and the surface passivation and stability of the resulting CsPbBr3 NCs have not been investigated.Designing the Cs4PbBr6 → CsPbBr3 NC transformation in such a way that it delivers encapsulated CsPbBr3 NCs with an enhanced stability is a promising approach for exploiting the Cs4PbBr6 NC reactivity, as shown by the above mentioned studies of Yin's group.13 The use of an organic polymer instead of an inorganic oxide (e.g. SiO2 or Ta2O5) shell would yield a NC-polymer blend which can be drop-cast, spin-coated or electrospun, widening the range of available applications.16 More generally, polymer encapsulation of CsPbX3 perovskite NCs (X = Cl, Br, I, and their mixtures) is promising because it has been shown to enhance the shelf-time of NCs by providing enhanced stability against moisture and photodegradation.17 Interestingly, stability enhancement has been reported irrespective of whether polymer chains preserve the native CsPbX3 NC surface ligands as in the case of polystyrene17–19 and poly(styrene–ethylene–butylene–styrene),17 or whether the polymer adheres to the surface of CsPbX3 NCs as in the case of ammonium bromide-terminated polystyrene20 or poly(n-butyl methacrylate) modified with zwitterionic sulfobetaine or phosphorylcholine functional groups.21 Arguably, an ideal NC transformation of Cs4PbBr6 → CsPbBr3 in this context could be caused by a polymer which acts both as a reactant and a macromolecular surfactant,20 minimizing the number of reagents and preparatory steps involved in the process.
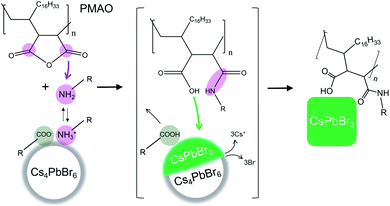
In this work, we demonstrate that poly(maleic anhydride-1-alt-octadecene) (PMAO) can simultaneously trigger the Cs4PbBr6 → CsPbBr3 NC transformation and provide enhanced surface passivation to the resulting CsPbBr3 NCs. PMAO is a widely available co-polymer of 1-octadecene and maleic anhydride and has been extensively used for the surface functionalization of NCs.22–24 In our experiments, upon mixing PMAO with oleylammonium/oleate-capped Cs4PbBr6 NCs, the cyclic anhydride groups of PMAO react with oleylamine species, forming polysuccinamic acid (Fig. 1). Polysuccinamic acid destabilizes the NC surface by displacing both the amine and the oleate ligands and acidifies the reaction environment, thus triggering the formation of CsPbBr3 NCs (Fig. 1). The core chemistry of the NC transformation is summarized by the following chemical equation: Cs4PbBr6 + nRNH2 + (–R′(CHCO)2O–)n → CsPbBr3 + (–R′(CHCOOH)(CHCONHR)–)n + 3Cs+(solvated) + 3Br−(solvated), where R = oleyl, R′ = octadecenyl, and the ratio between oleylamine molecules and anhydride units is assumed to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for simplicity. The extent of the transformation is tunable by varying the amount of added PMAO, enabling the investigation of the transformation intermediates, which consist of Cs4PbBr6–CsPbBr3 heterostructures. The fully-transformed CsPbBr3 NCs are bright emitters and retain their green emission for four weeks of storage under ambient conditions in air, even after one washing cycle with ethyl acetate (a solvent which typically causes the degradation of oleylammonium/oleate-capped CsPbBr3 NCs within hours or days). The increase in the stability of CsPbBr3 NCs synthesized from Cs4PbBr6 and PMAO is attributed to the adhesion of polysuccinamic acid to the NC surface through its multiple functional groups.
1 for simplicity. The extent of the transformation is tunable by varying the amount of added PMAO, enabling the investigation of the transformation intermediates, which consist of Cs4PbBr6–CsPbBr3 heterostructures. The fully-transformed CsPbBr3 NCs are bright emitters and retain their green emission for four weeks of storage under ambient conditions in air, even after one washing cycle with ethyl acetate (a solvent which typically causes the degradation of oleylammonium/oleate-capped CsPbBr3 NCs within hours or days). The increase in the stability of CsPbBr3 NCs synthesized from Cs4PbBr6 and PMAO is attributed to the adhesion of polysuccinamic acid to the NC surface through its multiple functional groups.
Results and discussion
Cs4PbBr6 NCs and their transformation with PMAO in solution
The synthesis of the initial Cs4PbBr6 NCs was performed in air, via the hot injection of cesium oleate into the solution of lead(II) bromide dissolved in a mixture of oleylamine and oleic acid in 1-octadecene,7 as detailed in Section S1 of the ESI.† The synthesis is similar to that of CsPbBr3 NCs,5 except that it is performed at a higher concentration of oleylamine and oleic acid with respect to lead ([oleylamine] : [oleic acid] : [PbBr2] ∼0.63 : 0.31 : 0.027![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M). Such reaction conditions favor the formation of a Pb-poor Cs4PbBr6 phase over the CsPbBr3 phase, as detailed previously.25 The synthesis delivers batches of Cs-rich rhombohedral Cs4PbBr6 NCs with a narrow size distribution and an average diameter in the range from 10 to 16 nm (Fig. 2a, b, and S1–S5†). 1H and 1H–13C heteronuclear single quantum coherence (HSQC) nuclear magnetic resonance (NMR) experiments established that Cs4PbBr6 NCs are passivated by a mixture of oleylammonium oleate and neutral oleylamine with a ligand ratio of ∼3
M). Such reaction conditions favor the formation of a Pb-poor Cs4PbBr6 phase over the CsPbBr3 phase, as detailed previously.25 The synthesis delivers batches of Cs-rich rhombohedral Cs4PbBr6 NCs with a narrow size distribution and an average diameter in the range from 10 to 16 nm (Fig. 2a, b, and S1–S5†). 1H and 1H–13C heteronuclear single quantum coherence (HSQC) nuclear magnetic resonance (NMR) experiments established that Cs4PbBr6 NCs are passivated by a mixture of oleylammonium oleate and neutral oleylamine with a ligand ratio of ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 between (oleylamine + oleylammonium)
2 between (oleylamine + oleylammonium)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oleate species (see Section S3 and Fig. S6–S9 of the ESI for details†).
oleate species (see Section S3 and Fig. S6–S9 of the ESI for details†).
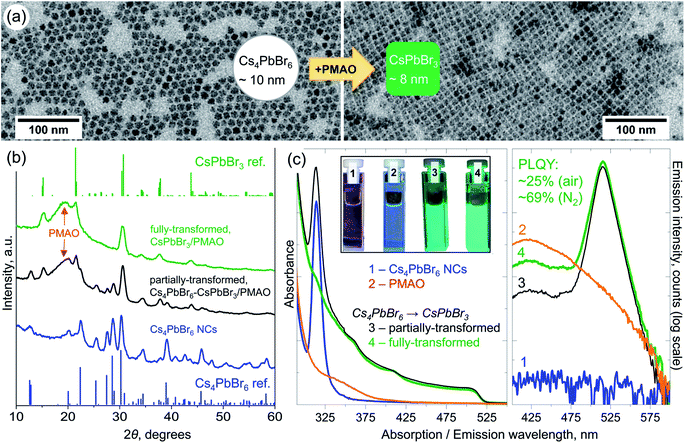 | ||
| Fig. 2 (a) Low-magnification TEM images of the initial Cs4PbBr6 NCs (average diameter 10 ± 1.5 nm) and fully-transformed CsPbBr3 NCs (average edge length 8 ± 0.4 nm) after their reaction with PMAO in toluene. (b) XRD patterns of the initial Cs4PbBr6 NCs and of the partially- and fully-transformed ones. Top and bottom stick patterns are those of the reference bulk compounds: rhombohedral Cs4PbBr6 (pattern ID 04-015-9683, ICSD code 162158)27 and orthorhombic CsPbBr3 (pattern ID 96-451-0746, COD code 4510745).28 The broad peak at ∼20° is due to PMAO. (c) Optical absorption (left panel) and emission (right panel) spectra of toluene solutions of initial Cs4PbBr6 NCs (blue curve), PMAO (orange curve), and partially- (black curve), and fully-transformed (green curve) NCs. The inset in the left panel shows photographs of the samples under excitation with a 365 nm lamp demonstrating visible green PL of partially- and fully-transformed NC samples. | ||
These Cs4PbBr6 NCs can react with PMAO in toluene, fully or partially transforming into CsPbBr3 NCs, depending on the amount of the added polymer (Fig. 2c). The reaction of Cs4PbBr6 NCs with PMAO typically starts within a few minutes after the addition of PMAO at room temperature (Movie S1 and Fig. S10†) and can be accelerated by mild heating of the reaction mixture (up to 80 °C). It is important to highlight here that heating up the NCs alone to 80 °C without the addition of PMAO does not trigger any transformation (Fig. S11†). The fully-transformed CsPbBr3 NCs have a narrow size distribution, as inferred from their self-organization into ordered close-packed monolayers on a carbon-coated TEM copper grid (Fig. 2a). The XRD patterns of the initial NCs, partially- and completely-transformed samples are shown in Fig. 2b. Following the transformation, the XRD reflections of the rhombohedral Cs4PbBr6 crystal structure gradually disappeared, and peaks characteristic of the orthorhombic CsPbBr3 perovskite phase emerged (Fig. 2b, see Fig. S12† for peak assignment). The progression of the reaction was monitored by steady-state UV-Vis absorption spectroscopy (Fig. 2c, left panel, Fig. S10†) in which the disappearance of the ∼314 nm peak characteristic of Cs4PbBr67 and the appearance of the ∼510 nm band edge absorption of CsPbBr3 are evident. The transformation was also tracked by steady-state photoluminescence (PL) spectroscopy, through the appearance of a cyan emission (λmax ∼475–480 nm) in the early stages of the reaction (Fig. S10†). The absolute PL quantum yield (PLQY) of the samples transformed in air was measured to be ∼19% (partially transformed), and ∼25% (fully transformed). On the other hand, when the transformation was performed under an inert atmosphere, the sample had a 69% PLQY (Fig. S13–S15†). Such a value is comparable to those reported for other Cs4PbBr6 → CsPbBr3 chemical transformations of NCs: 47% (ref. 7) and 62% (ref. 11) via the addition of solid PbBr2 at elevated temperatures, and 75% (ref. 12) upon reaction with H2O. The lower PLQY of the samples transformed in air is attributed to the presence of electron traps formed as a result of sample exposure to atmospheric O2. Similar results have been reported by Rodà et al. who observed PL dimming in oxygen-exposed CsPbBr3 nanocubes.26
Rationalization of the observed reactivity between PMAO and Cs4PbBr6 NCs
PMAO is a copolymer of octadecene-1 and maleic anhydride, and it consists of repeating units composed of a saturated hydrocarbon chain and a cyclic succinic anhydride ring (Fig. 1). PMAO has a negligible reactivity towards inorganic salts such as Cs4PbBr6, as confirmed in a control experiment on finely ground powder of bulk Cs4PbBr6 (Fig. S16 and S17†). However, the succinic anhydride rings of PMAO feature acyl groups that are reactive towards nucleophilic reagents such as water and primary amines (yielding, in the latter case, either succinamic acid at room temperature23,29–31 or cyclic imides at high temperatures31–33). The presence of a significant amount of water as a potential reactant towards PMAO in the Cs4PbBr6 NC samples was ruled out based on FTIR and NIR characterization (Fig. S18 and S19†). On the other hand, the ligand shell of Cs4PbBr6 NCs contains partially-protonated oleylamine (Section S3 and Fig. S6–S9†). In analogy with the widely studied oleylammonium/oleate-capped CsPbBr3 NCs,25,34–36 the ligands on the surface of Cs4PbBr6 NCs are likely to exist in a dynamic equilibrium between neutral and protonated species (oleylamine and oleylammonium, respectively). Thus neutral oleylamine is always available in the NC solution. Neutral oleylamine is a nucleophile with a documented reactivity towards linear and cyclic anhydrides,37,38 and polymaleic anhydride derivatives.39,40 The reaction between neutral oleylamine and PMAO in the absence of NCs causes broadening of the vinyl hydrogen resonance of the oleyl chain in the 1H NMR spectrum due to the attachment of small oleylamine molecules to PMAO macromolecules (the specified Mw of PMAO is ∼30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, which roughly corresponds to ∼80–150 succinic anhydride-octadecene subunits) (Fig. S20–S22†). The addition of neutral oleylamine to cyclic anhydride produces a succinamic acid derivative, as was confirmed by 1H and 1H–13C HSQC NMR in a control reaction (Fig. S23†). Therefore, the formation of polysuccinamic acid (Fig. 1) is expected upon mixing of PMAO with oleylammonium/oleate capped Cs4PbBr6 NCs. The key role of oleylamine species in the Cs4PbBr6 → CsPbBr3 NC transformation was further verified by a control reaction between PMAO and oleylamine-free Cs4PbBr6 NCs (synthesized with tri-n-octylphosphine oxide (TOPO) and oleic acid41). The Cs4PbBr6 NCs synthesized with TOPO and oleic acid were found to be unreactive towards PMAO (Fig. S27 and S28†).
000 g mol−1, which roughly corresponds to ∼80–150 succinic anhydride-octadecene subunits) (Fig. S20–S22†). The addition of neutral oleylamine to cyclic anhydride produces a succinamic acid derivative, as was confirmed by 1H and 1H–13C HSQC NMR in a control reaction (Fig. S23†). Therefore, the formation of polysuccinamic acid (Fig. 1) is expected upon mixing of PMAO with oleylammonium/oleate capped Cs4PbBr6 NCs. The key role of oleylamine species in the Cs4PbBr6 → CsPbBr3 NC transformation was further verified by a control reaction between PMAO and oleylamine-free Cs4PbBr6 NCs (synthesized with tri-n-octylphosphine oxide (TOPO) and oleic acid41). The Cs4PbBr6 NCs synthesized with TOPO and oleic acid were found to be unreactive towards PMAO (Fig. S27 and S28†).
In summary, the removal of oleylamine from the surface of Cs4PbBr6 NCs destabilizes them, while polysuccinamic acid acidifies the reaction environment. Surface destabilization and acidic environments are both general conditions that are known to cause the Cs4PbBr6 → CsPbBr3 transformation.10,25,42 The stoichiometry of the transformation is balanced by a nominal removal of 3 equivalents of CsBr from 1 equivalent of Cs4PbBr6, yet we have not experimentally detected crystalline CsBr by XRD or high-resolution TEM (HRTEM). This discrepancy is tentatively rationalized by solvation of Cs+ and Br− ions by oleate and polysuccinamic acid species, similar to the previously reported dissociation of CsBr in dimethylformamide in the presence of the polyacrylic acid co-polymer.43 Eventually, our 1H NMR analysis also revealed that the final NCs were capped solely by polysuccinamic acid, indicating the displacement of both the oleate and amine/ammonium ligands from the NC surface upon transformation (see the discussion in Section S9 and Fig. S20–S26†).
Enhanced stability of the CsPbBr3/PMAO NCs
The fully-transformed CsPbBr3 NCs formed an optically clear solution in toluene. These CsPbBr3 NCs possessed an enhanced stability compared to the polymer-free, ligand-capped CsPbBr3 NCs directly synthesized by following the cesium oleate/lead(II) bromide route.5,25 Such enhanced stability was demonstrated by the fact that the NCs retained their green emission after four weeks of storage under ambient conditions in air (Fig. S29†), even after undergoing a washing cycle of precipitation/redispersion with ethyl acetate (Fig. S30 and S31†), while the polymer-free CsPbBr3 NCs aggregated within hours or days after undergoing a similar washing procedure. Another indicator of the increased stability is the observation that the CsPbBr3/PMAO NCs could be concentrated or diluted over ∼5 orders of magnitude range of concentrations, from ∼26 mg ml−1 to ∼1 × 10−4 mg ml−1, without any loss of optical transparency or PL emission (Fig. S32†). The increase in the stability of the fully-transformed CsPbBr3/PMAO NCs is in agreement with prior reports on CsPbBr3 NCs blended with PMAO44,45 or with the related dodecyl-grafted-poly(isobutylene-alt-maleic-anhydride).46 Our hypothesis is that the binding of polysuccinamic acid through its multiple functional groups to the NC surface, in place of the standard ligands used in the direct synthesis of CsPbBr3 NCs (as discussed above and in Section S9, Fig. S20–S26†), is the origin of this enhancement. To test this hypothesis, we compared the solvodynamic diameters of PMAO and CsPbBr3/PMAO NCs (washed once with ethyl acetate) determined by dynamic light scattering (∼1.7 ± 1.2 nm and ∼11.2 ± 0.9 nm, respectively, Fig. S33 and S34†) with the sizes of the inorganic CsPbBr3 cores from the TEM analysis of the same sample (∼7 nm edge length, Fig. S35†). The larger solvodynamic diameter of CsPbBr3/PMAO NCs in solution compared to the CsPbBr3 NC edge length from TEM is explained by the PMAO wrapping and NC tumbling in solution (the diagonal of a cube with a 7 nm edge length is ∼12 nm). The lack of a substantial increase in the solvodynamic diameter of CsPbBr3/PMAO NCs is interpreted as an indicator of PMAO wrapping around NCs, supporting the hypothesis about the origin of increased NC stability. In addition, the relatively small solvodynamic diameter of CsPbBr3/PMAO NCs indicates that PMAO molecules do not bind multiple NCs together.Cs4PbBr6–CsPbBr3 heterostructures
The Cs4PbBr6 → CsPbBr3 NC transformation with PMAO is relatively slow at room temperature. This enabled the observation of NC intermediates consisting of Cs4PbBr6–CsPbBr3 heterostructures (Fig. 3), which were investigated by HRTEM (Fig. 3a–e). In one of the partially-transformed samples we observed NCs with different degrees of conversion (Fig. 3a–e). The heterostructures displayed a variety of interfaces between Cs4PbBr6 and CsPbBr3, some adopting an epitaxial relationship, some not (analysis of the cases is shown in Fig. S36†). For example, the heterostructure shown in Fig. 3c, analyzed in detail in Fig. 3f–h, is characterized by an epitaxial relationship adopted by the two domains, as indicated by the overlap of the spots from the planes of the two crystal structures in fast Fourier transform (FFT, Fig. 3g) of the real space image. The <5% mismatch between the atomic spacing of the two domains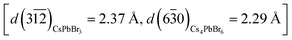 leads to a slight bending of the planes, as labeled by the dashed lines in Fig. 3f. This bending also indicates that the atomic planes of Cs4PbBr6 domains on the two sides of the CsPbBr3 domain are rotated by a small angle. The rotation gives rise to extended diffraction spots in the FFT image, instead of single sharp spots that would otherwise appear for a single crystal. Considering an orthorhombic phase for CsPbBr3 (ICSD: 97851, a = 8.207 Å, b = 8.255 Å, c = 11.759 Å), the epitaxial relationship between the two domains can be described as follows: CsPbBr3 [02
leads to a slight bending of the planes, as labeled by the dashed lines in Fig. 3f. This bending also indicates that the atomic planes of Cs4PbBr6 domains on the two sides of the CsPbBr3 domain are rotated by a small angle. The rotation gives rise to extended diffraction spots in the FFT image, instead of single sharp spots that would otherwise appear for a single crystal. Considering an orthorhombic phase for CsPbBr3 (ICSD: 97851, a = 8.207 Å, b = 8.255 Å, c = 11.759 Å), the epitaxial relationship between the two domains can be described as follows: CsPbBr3 [02![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ]‖Cs4PbBr6 [001], and CsPbBr3 (112)‖Cs4PbBr6 (030) (see Fig. 3h).
]‖Cs4PbBr6 [001], and CsPbBr3 (112)‖Cs4PbBr6 (030) (see Fig. 3h).
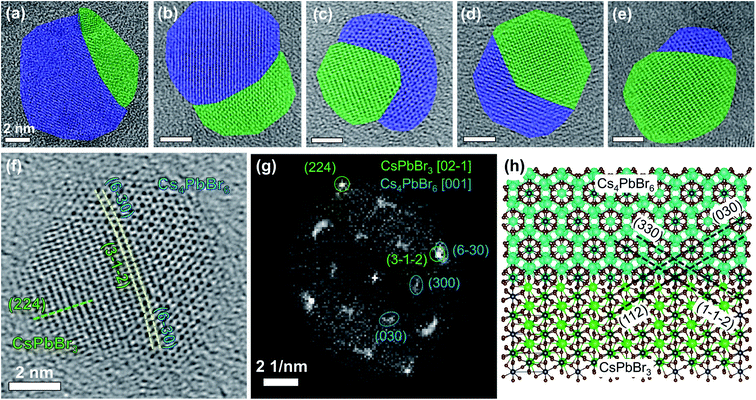 | ||
| Fig. 3 (a–e) HRTEM images of Cs4PbBr6–CsPbBr3 heterostructures formed upon partial conversion of Cs4PbBr6 NCs with PMAO (scale bars are 2 nm). Cs4PbBr6 domains are shaded in blue, and CsPbBr3 domains are shaded in green; (f) a magnified view of (c) and (g) the corresponding FFT image; and (h) ball-and-stick atomic model of the epitaxial interface built using VESTA software (ver. 3.4.6, the atoms are depicted as spheres with radii corresponding to 40% of actual atomic radii).47 Cs atoms in the model are colored in two different colors for clarity: in cyan for Cs4PbBr6 and green for CsPbBr3. | ||
The low-magnification TEM images of the two NC samples were analyzed to quantify changes in the NC dimensions before and after the transformation (Fig. S37–S42†). For example, a sample of 10.1 nm ± 1.4 nm diameter Cs4PbBr6 NCs transformed into 8 nm ± 0.4 nm edge length CsPbBr3 NCs (Fig. 2a). The Scherrer analysis of the XRD patterns of the same sample before and after the transformation indicated a reduction in the crystallite size from 16.1 ± 1.8 nm to 12.5 ± 2.6 nm, in agreement with the TEM analysis (larger dimensions from XRD as compared to TEM are due to the differences between techniques and analyses). In another sample, 15.7 nm ± 2.6 nm Cs4PbBr6 NCs transformed into 12 nm ± 1.9 nm NCs (dimensions from TEM). If one assumes that such transformation does not proceed by dissolution–recrystallization, but simply by the gradual removal of CsBr from each individual spherical NC of Cs4PbBr6, converting it to a cube-shaped NC of CsPbBr3, then by volume contraction the resulting CsPbBr3 NCs should have an edge length of 6 nm in TEM (9.5 nm in the second sample), which is ∼2 nm smaller than the obtained value (Table S1†). Hence, dissolution–recrystallization processes should also play an important role in this transformation. A similar mechanism has been previously invoked to rationalize the inverse NC transformation (from CsPbBr3 to Cs4PbBr6).8,10
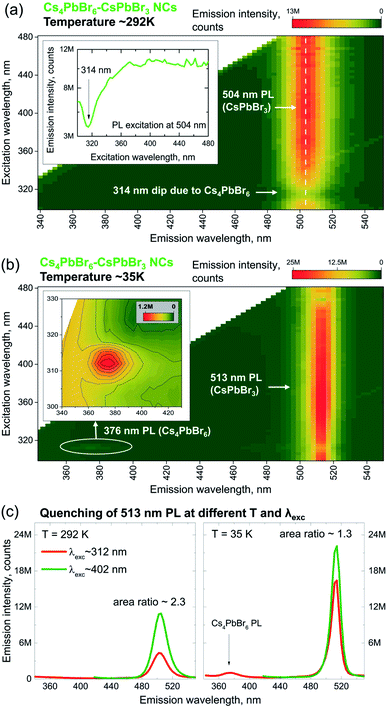
The PL of the partially-converted sample containing Cs4PbBr6–CsPbBr3 heterostructures was surveyed at room and cryogenic temperatures (see Section S14 of the ESI† for experimental details) because their optical properties are unknown to date. The results are presented in Fig. 4a and b as excitation-emission maps (PL maps). The room temperature (T ∼292 K) PL map of the partially-converted sample contains a single emission peak of CsPbBr3 at ∼504 nm (Fig. 4a). The CsPbBr3 emission has a broad PL excitation spectrum (inset in Fig. 4a) with a dip at ∼314 nm characteristic of Cs4PbBr6 absorption. Upon cooling to T ∼ 35 K, the PL map shows two emission peaks (Fig. 4b): an intense peak at ∼513 nm and a weak peak at ∼376 nm (inset in Fig. 4b). The ∼513 nm peak is an emission feature of CsPbBr3, red-shifted from ∼504 nm as a result of cooling.48,49 The ∼376 nm emission with narrow excitation at ∼313 nm is assigned to Cs4PbBr6 because it matches with previously reported cryogenic PL spectra of bulk Cs4PbBr6 (ref. 50) and Cs4PbBr6 aggregates in CsBr.51 This assignment was further confirmed by collecting the PL map of the as-synthesized Cs4PbBr6 NCs at T ∼ 27 K (Fig. S43†). At 27 K, the emission of the as-synthesized Cs4PbBr6 NCs is dominated by a peak at ∼376 nm surrounded by weaker features due to various electronic transitions in Pb2+ ions.52–54 The as-synthesized Cs4PbBr6 NCs are not emissive at room temperature and, besides the discussed ∼376 nm emission, are non-emissive up to the detection limit of 1600 nm when cooled (Fig. S44†).
The dual emission of partially-transformed NCs provides an opportunity to probe the energy transfer between Cs4PbBr6 and CsPbBr3. Fig. 4c shows a comparison between pairs of emission spectra for the partially-converted sample collected at two different temperatures (292 K and 35 K) and two different excitation energies: one matching with Cs4PbBr6 absorption (λexc ∼ 312 nm) and one below it (λexc ∼ 402 nm, only CsPbBr3 absorbs). At 292 K (Fig. 4c, left panel), only CsPbBr3 emits, regardless of excitation energy, and its emission is quenched by a factor of ∼2.3 after changing the excitation energy from ∼402 nm to ∼312 nm. This quenching is attributed to the attenuation of ∼312 nm excitation due to absorption by Cs4PbBr6 and an excitation-dependent PL efficiency.55 At 35 K, both materials emit, and the CsPbBr3 emission is quenched by a smaller factor of ∼1.3 (Fig. 4c, right panel). We can assign the lower quenching of CsPbBr3 emission at 35 K to the energy transfer from Cs4PbBr6, which indeed is favored due to the overlap between the emission of the donor (Cs4PbBr6) and the absorption of the acceptor (CsPbBr3). These initial observations make Cs4PbBr6–CsPbBr3 NCs a promising platform for future spectroscopic studies of the energy flow between lead halide perovskites and related compounds.
Reactivity of Cs4PbBr6 NC samples with PMAO in drop-cast films
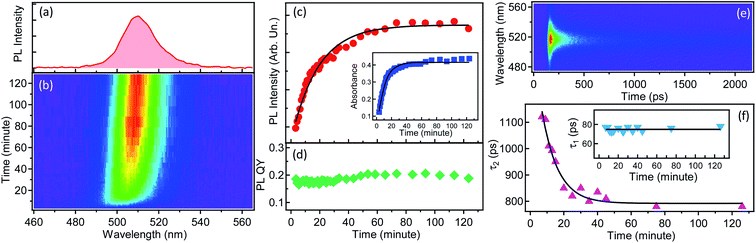
The reaction described above can also proceed inside a polymer film (as was confirmed by in situ Raman spectroscopy, see Fig. S45†), which makes its investigation relevant for the emerging application of blends between PMAO and oleylammonium/oleate-capped perovskite NCs in light-emitting diodes.44,45 From this point of view, the Cs4PbBr6 to CsPbBr3 transformation is an indicator of amine-anhydride reactivity, and its kinetics can be studied in situ by steady-state and time-resolved PL. Fig. 5 shows the results of the in situ PL measurements from a macroscopic area (∼2 mm excitation spot size) of the film made by quick drop-casting of a freshly prepared PMAO–Cs4PbBr6 NCs blend. Green PL develops within the first few minutes in the drop-cast film, and reaches a stable intensity and position (∼510 nm, full width at half maximum of 18 nm) after ∼2 hours (Fig. 5a and b), indicating the timescale of the complete conversion. Both the PL intensity and the absorbance of the film (at 405 nm, the wavelength of the CW laser used for excitation) increase over the course of the transformation, with a characteristic time constant of about ∼10 minutes (Fig. 5c). Similar kinetics were obtained by in situ micro-PL performed with a confocal fluorescence microscope (Fig. S46†), suggesting that the transformation proceeds uniformly across the blend. The PLQY in the film remains almost constant at ∼20% throughout the transformation, similar to the values measured in the solution (Fig. 5d).The evolution of PL during the transformation was also monitored by in situ spectrally-resolved transient PL. The temporal PL decay is sub-ns and contains two main components, the shorter (∼70 ps) and longer (950 ps) ones (Fig. 5e and S47†). The shorter decay component varies little over the course of the transformation while the longer decay component decreases from ∼1.1 ns to ∼800 ps with a time constant of ∼10 minutes (Fig. 5f). The PL decay of the emitting NCs in the film is much shorter than that of the NCs in solution (∼4–5 ns), the polymer-free CsPbBr3 NCs5,35,56–58 (∼2–10 ns), and polymer-encapsulated single CsPbBr3 NCs (∼6 ns).18 It is definitely much shorter than that of MAPbBr3 NCs/polymer blends (>100 ns).59 The fast PL decay of CsPbBr3/PMAO NCs in the drop-cast film can be attributed to various possible causes, including: (i) the appearance of a new non-radiative carrier recombination channel, ascribable to oxygen molecules (as the samples were prepared in air) which act as traps for electrons;26 (ii) electron hopping between neighboring nanocrystals in the film;60 (iii) a more defective surface of NCs formed in films, due to reduced mobility of ions and molecules (preventing efficient passivation of surface sites in comparison to the solution case). The sub-ns PL decay of NCs in blends with PMAO, combined with a reasonable PLQY, should be of interest for applications in scintillators, where ultrafast and efficient emission is required for fast timing capability of imaging detectors.61,62
Conclusions
Chemical transformation of colloidal Cs4PbBr6 NCs to perovskite CsPbBr3 NCs induced by the organic co-polymer PMAO is presented as a promising strategy to prepare stable and bright CsPbBr3 NC emitters. The PMAO reactivity towards oleylammonium/oleate-capped Cs4PbBr6 NCs favors an addition reaction of oleylamine ligands from the NC surface to the succinic anhydride groups of the polymer. This destabilizes the NCs and acidifies the reaction environment through the formation of polysuccinamic acid, a PMAO–oleylamine adduct, which binds to the surface of the NCs in lieu of the original ligands. These two factors – ligand replacement and in situ acid formation – drive the Cs4PbBr6 to CsPbBr3 NC transformation. The lower reactivity of PMAO, as compared to that of the previously reported reagents, enabled the investigation of Cs4PbBr6–CsPbBr3 intermediate heterostructures by HRTEM. The heterostructures feature a variety of epitaxial and non-epitaxial relationships between the two structurally dissimilar domains. At cryogenic temperature, Cs4PbBr6–CsPbBr3 NCs display dual emission at ∼376 nm and 513 nm with evidence of energy transfer from Cs4PbBr6 to CsPbBr3. The PMAO-induced transformation proceeds both in solutions and in drop-cast films, producing CsPbBr3 NCs with a narrow size distribution and attractive photoluminescence properties (up to 69% PLQY in solution and a sub-ns PL lifetime in the drop-cast films). The resulting CsPbBr3/PMAO NCs demonstrate enhanced stability by retaining their green emission for several weeks in air. The increased stability of CsPbBr3/PMAO NCs is attributed to the adhesion of polysuccinamic acid through its multiple functional groups to the NC surface. The PMAO-induced transformation of Cs4PbBr6 NCs opens up a general strategy for chemical modification of inorganic NCs passivated with nucleophilic amines.Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Abbreviations
| COD | Crystallography open database |
| EDS | Energy dispersive X-ray spectroscopy |
| FFT | Fast Fourier transform |
| FTIR | Fourier transform infrared spectroscopy |
| HRTEM | High resolution TEM |
| HSQC | Heteronuclear single quantum coherence |
| ICSD | Inorganic crystal structure database |
| NIR | Near infrared |
| NMR | Nuclear magnetic resonance |
| NC | Nanocrystal |
| PL | Photoluminescence |
| PMAO | Poly(maleic anhydride-alt-1-octadecene) |
| QY | Quantum yield |
| STEM | Scanning TEM |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
Acknowledgements
We thank Simone Lauciello and Dr Rosaria Brescia (IIT Electron Microscopy Facility) for the assistance with EDS measurements; Mr Aniruddha Ray and Dr Ahmed Abdelhady for providing samples of bulk Cs4PbBr6 powders for the control experiments; Dr Urko Petralanda, Dr Ivan Infante, and Mr Stefano Toso for helpful discussions; Dr Luana Persano for support in optical measurements and film sample preparation. The work of Dmitry Baranov was supported by the European Union's Horizon 2020 Research and Innovation Programme under Marie Sklodowska-Curie grant agreement No 794560 (RETAIN). Liberato Manna acknowledges funding from the European Union under grant agreement No 614897 (ERC Grant TRANS-NANO). Riccardo Scarfiello acknowledges financial support by the Progetto FISR – C.N.R,“Tecnopolo di nanotecnologia e fotonica per la medicina di precisione” – CUP B83B17000010001. Andrea Camposeo and Dario Pisignano acknowledge funding from the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (Grant Agreement n. 682157, “xPRINT”), and from MIUR (project “3D-Phys”, PRIN 2017PHRM8X).References
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Properties and potential optoelectronic applications of lead halide perovskite nanocrystals, Science, 2017, 358(6364), 745–750 CrossRef CAS PubMed.
- Q. A. Akkerman, G. Rainò, M. V. Kovalenko and L. Manna, Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals, Nat. Mater., 2018, 17(5), 394–405 CrossRef CAS PubMed.
- J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties, Chem. Rev., 2019, 119(5), 3296–3348 CrossRef CAS PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I), Nano Lett., 2015, 15(8), 5635–5640 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut, Nano Lett., 2015, 15(6), 3692–3696 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions, J. Am. Chem. Soc., 2015, 137(32), 10276–10281 CrossRef CAS PubMed.
- Q. A. Akkerman, S. Park, E. Radicchi, F. Nunzi, E. Mosconi, F. De Angelis, R. Brescia, P. Rastogi, M. Prato and L. Manna, Nearly Monodisperse Insulator Cs4PbX6 (X = Cl, Br, I) Nanocrystals, Their Mixed Halide Compositions, and Their Transformation into CsPbX3 Nanocrystals, Nano Lett., 2017, 17(3), 1924–1930 CrossRef CAS PubMed.
- Z. Liu, Y. Bekenstein, X. Ye, S. C. Nguyen, J. Swabeck, D. Zhang, S.-T. Lee, P. Yang, W. Ma and A. P. Alivisatos, Ligand Mediated Transformation of Cesium Lead Bromide Perovskite Nanocrystals to Lead Depleted Cs4PbBr6 Nanocrystals, J. Am. Chem. Soc., 2017, 139(15), 5309–5312 CrossRef CAS PubMed.
- F. Palazon, C. Urso, L. De Trizio, Q. Akkerman, S. Marras, F. Locardi, I. Nelli, M. Ferretti, M. Prato and L. Manna, Postsynthesis Transformation of Insulating Cs4PbBr6 Nanocrystals into Bright Perovskite CsPbBr3 through Physical and Chemical Extraction of CsBr, ACS Energy Lett., 2017, 2(10), 2445–2448 CrossRef CAS PubMed.
- T. Udayabhaskararao, L. Houben, H. Cohen, M. Menahem, I. Pinkas, L. Avram, T. Wolf, A. Teitelboim, M. Leskes, O. Yaffe, D. Oron and M. Kazes, A Mechanistic Study of Phase Transformation in Perovskite Nanocrystals Driven by Ligand Passivation, Chem. Mater., 2018, 30(1), 84–93 CrossRef CAS.
- Y. Li, H. Huang, Y. Xiong, S. V. Kershaw and A. L. Rogach, Reversible transformation between CsPbBr3 and Cs4PbBr6 nanocrystals, CrystEngComm, 2018, 20(34), 4900–4904 RSC.
- L. Wu, H. Hu, Y. Xu, S. Jiang, M. Chen, Q. Zhong, D. Yang, Q. Liu, Y. Zhao, B. Sun, Q. Zhang and Y. Yin, From Nonluminescent Cs4PbX6 (X = Cl, Br, I) Nanocrystals to Highly Luminescent CsPbX3 Nanocrystals: Water-Triggered Transformation through a CsX-Stripping Mechanism, Nano Lett., 2017, 17(9), 5799–5804 CrossRef CAS PubMed.
- H. Hu, L. Wu, Y. Tan, Q. Zhong, M. Chen, Y. Qiu, D. Yang, B. Sun, Q. Zhang and Y. Yin, Interfacial Synthesis of Highly Stable CsPbX3/Oxide Janus Nanoparticles, J. Am. Chem. Soc., 2018, 140(1), 406–412 CrossRef CAS PubMed.
- L. Yang, T. Wang, Q. Min, B. Liu, Z. Liu, X. Fan, J. Qiu, X. Xu, J. Yu and X. Yu, High Water Resistance of Monoclinic CsPbBr3 Nanocrystals Derived from Zero-Dimensional Cesium Lead Halide Perovskites, ACS Omega, 2019, 4(3), 6084–6091 CrossRef CAS.
- M. Chen, H. Hu, Y. Tan, N. Yao, Q. Zhong, B. Sun, M. Cao, Q. Zhang and Y. Yin, Controlled growth of dodecapod-branched CsPbBr3 nanocrystals and their application in white light emitting diodes, Nano Energy, 2018, 53, 559–566 CrossRef CAS.
- Y. Wei, Z. Cheng and J. Lin, An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs, Chem. Soc. Rev., 2019, 48(1), 310–350 RSC.
- S. N. Raja, Y. Bekenstein, M. A. Koc, S. Fischer, D. Zhang, L. Lin, R. O. Ritchie, P. Yang and A. P. Alivisatos, Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization, ACS Appl. Mater. Interfaces, 2016, 8(51), 35523–35533 CrossRef CAS PubMed.
- G. Rainò, A. Landuyt, F. Krieg, C. Bernasconi, S. T. Ochsenbein, D. N. Dirin, M. I. Bodnarchuk and M. V. Kovalenko, Underestimated Effect of a Polymer Matrix on the Light Emission of Single CsPbBr3 Nanocrystals, Nano Lett., 2019, 19(6), 3648–3653 CrossRef PubMed.
- Y. Wang, Y. Zhu, J. Huang, J. Cai, J. Zhu, X. Yang, J. Shen, H. Jiang and C. Li, CsPbBr3 Perovskite Quantum Dots-Based Monolithic Electrospun Fiber Membrane as an Ultrastable and Ultrasensitive Fluorescent Sensor in Aqueous Medium, J. Phys. Chem. Lett., 2016, 7(21), 4253–4258 CrossRef CAS PubMed.
- H. Kim, S. So, A. Ribbe, Y. Liu, W. Hu, V. V. Duzhko, R. C. Hayward and T. Emrick, Functional polymers for growth and stabilization of CsPbBr3 perovskite nanoparticles, Chem. Commun., 2019, 55(12), 1833–1836 RSC.
- H. Kim, N. Hight-Huf, J.-H. Kang, P. Bisnoff, S. Sundararajan, T. Thompson, M. Barnes, R. Hayward and T. S. Emrick, Polymer Zwitterions for Stabilization of CsPbBr3 Perovskite Nanoparticle and Nanocomposite Films, Angew. Chem., Int. Ed. DOI:10.1002/anie.201916492 , accepted article.
- T. Pellegrino, L. Manna, S. Kudera, T. Liedl, D. Koktysh, A. L. Rogach, S. Keller, J. Rädler, G. Natile and W. J. Parak, Hydrophobic Nanocrystals Coated with an Amphiphilic Polymer Shell: A General Route to Water Soluble Nanocrystals, Nano Lett., 2004, 4(4), 703–707 CrossRef CAS.
- C.-A. J. Lin, R. A. Sperling, J. K. Li, T.-Y. Yang, P.-Y. Li, M. Zanella, W. H. Chang and W. J. Parak, Design of an Amphiphilic Polymer for Nanoparticle Coating and Functionalization, Small, 2008, 4(3), 334–341 CrossRef CAS PubMed.
- R. Di Corato, A. Quarta, P. Piacenza, A. Ragusa, A. Figuerola, R. Buonsanti, R. Cingolani, L. Manna and T. Pellegrino, Water solubilization of hydrophobic nanocrystals by means of poly(maleic anhydride-alt-1-octadecene), J. Mater. Chem., 2008, 18(17), 1991–1996 RSC.
- G. Almeida, L. Goldoni, Q. Akkerman, Z. Dang, A. H. Khan, S. Marras, I. Moreels and L. Manna, Role of Acid–Base Equilibria in the Size, Shape, and Phase Control of Cesium Lead Bromide Nanocrystals, ACS Nano, 2018, 12(2), 1704–1711 CrossRef CAS PubMed.
- C. Rodà, A. L. Abdelhady, J. Shamsi, M. Lorenzon, V. Pinchetti, M. Gandini, F. Meinardi, L. Manna and S. Brovelli, O2 as a molecular probe for nonradiative surface defects in CsPbBr3 perovskite nanostructures and single crystals, Nanoscale, 2019, 11(16), 7613–7623 RSC.
- M. Velázquez, A. Ferrier, S. Péchev, P. Gravereau, J.-P. Chaminade, X. Portier and R. Moncorgé, Growth and characterization of pure and Pr3+-doped Cs4PbBr6 crystals, J. Cryst. Growth, 2008, 310(24), 5458–5463 CrossRef.
- C. C. Stoumpos, C. D. Malliakas, J. A. Peters, Z. Liu, M. Sebastian, J. Im, T. C. Chasapis, A. C. Wibowo, D. Y. Chung, A. J. Freeman, B. W. Wessels and M. G. Kanatzidis, Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection, Cryst. Growth Des., 2013, 13(7), 2722–2727 CrossRef CAS.
- R. Kluger and J. C. Hunt, Aminolysis of maleic anhydride. Kinetics and thermodynamics of amide formation, J. Am. Chem. Soc., 1984, 106(19), 5667–5670 CrossRef CAS.
- R. Kluger and J. C. Hunt, Circumventive catalysis: contrasting reaction patterns of tertiary and primary amines with cyclic anhydrides and the avoidance of intermediates, J. Am. Chem. Soc., 1989, 111(9), 3325–3328 CrossRef CAS.
- Z. Jin, L. Du, C. Zhang, Y. Sugiyama, W. Wang, G. Palui, S. Wang and H. Mattoussi, Modification of Poly(maleic anhydride)-Based Polymers with H2N–R Nucleophiles: Addition or Substitution Reaction?, Bioconjugate Chem., 2019, 30(3), 871–880 CrossRef CAS PubMed.
- L. Coleman, J. Bork and H. Dunn, Notes. Reaction of Primary Aliphatic Amines with Maleic Anhydride, J. Org. Chem., 1959, 24(1), 135–136 CrossRef CAS.
- I. Vermeesch and G. Groeninckx, Chemical modification of poly(styrene-co-maleic anhydride) with primary N-alkylamines by reactive extrusion, J. Appl. Polym. Sci., 1994, 53(10), 1365–1373 CrossRef CAS.
- J. De Roo, M. Ibáñez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. Van Driessche, M. V. Kovalenko and Z. Hens, Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals, ACS Nano, 2016, 10(2), 2071–2081 CrossRef CAS PubMed.
- M. I. Bodnarchuk, S. C. Boehme, S. ten Brinck, C. Bernasconi, Y. Shynkarenko, F. Krieg, R. Widmer, B. Aeschlimann, D. Günther, M. V. Kovalenko and I. Infante, Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals, ACS Energy Lett., 2019, 4(1), 63–74 CrossRef CAS PubMed.
- D. Quarta, M. Imran, A.-L. Capodilupo, U. Petralanda, B. van Beek, F. De Angelis, L. Manna, I. Infante, L. De Trizio and C. Giansante, Stable Ligand Coordination at the Surface of Colloidal CsPbBr3 Nanocrystals, J. Phys. Chem. Lett., 2019, 10(13), 3715–3726 CrossRef CAS PubMed.
- S. Watanabe, H. Kawahara and T. Kuramochi, Adducts of cyclic acid anhydrides and fatty amines as anti-rust additives in water-based cutting fluids, J. Am. Oil Chem. Soc., 1991, 68(2), 92–94 CrossRef CAS.
- M. Abbas and C. Slugovc, Optimized reaction conditions for the cross-metathesis of methyl oleate and oleylamine with ethyl acrylate, Monatsh. Chem., 2012, 143(4), 669–673 CrossRef CAS.
- S. Percec, L. Howe, J. Li and S. Bair, Chemical modification of poly(ethylene-co-methyl acrylate-co-maleic anhydride) for cathodic electrodepositions, J. Polym. Sci., Part A: Polym. Chem., 2012, 50(2), 261–270 CrossRef CAS.
- C. Zhang, C. Gao, F. Gao, J. Wang, D. Zhang, Y. Wang and D. Xu, Synthesis of comb bipolymers and their pour point depressing properties, Pet. Sci., 2014, 11(1), 155–160 CrossRef CAS.
- G. Almeida, O. J. Ashton, L. Goldoni, D. Maggioni, U. Petralanda, N. Mishra, Q. A. Akkerman, I. Infante, H. J. Snaith and L. Manna, The Phosphine Oxide Route toward Lead Halide Perovskite Nanocrystals, J. Am. Chem. Soc., 2018, 140(44), 14878–14886 CrossRef PubMed.
- S. Park, N. M. An, G. Almeida, F. Palazon, D. Spirito, R. Krahne, Z. Dang, L. De Trizio and L. Manna, CsPbX3/SiOx (X=Cl, Br, I) Monoliths Prepared via a Novel Sol-gel Route Starting from Cs4PbX6 Nanocrystals, Nanoscale, 2019, 11(40), 18739–18745 RSC.
- Y. Liu, Z. Wang, S. Liang, Z. Li, M. Zhang, H. Li and Z. Lin, Polar Organic Solvent-Tolerant Perovskite Nanocrystals Permanently Ligated with Polymer Hairs via Star-like Molecular Bottlebrush Trilobe Nanoreactors, Nano Lett., 2019, 19(12), 9019–9028 CrossRef CAS PubMed.
- M. Meyns, M. Perálvarez, A. Heuer-Jungemann, W. Hertog, M. Ibáñez, R. Nafria, A. Genç, J. Arbiol, M. V. Kovalenko, J. Carreras, A. Cabot and A. G. Kanaras, Polymer-Enhanced Stability of Inorganic Perovskite Nanocrystals and Their Application in Color Conversion LEDs, ACS Appl. Mater. Interfaces, 2016, 8(30), 19579–19586 CrossRef CAS PubMed.
- H. Wu, S. Wang, F. Cao, J. Zhou, Q. Wu, H. Wang, X. Li, L. Yin and X. Yang, Ultrastable Inorganic Perovskite Nanocrystals Coated with a Thick Long-Chain Polymer for Efficient White Light-Emitting Diodes, Chem. Mater., 2019, 31(6), 1936–1940 CrossRef CAS.
- C. Carrillo-Carrión, P. del Pino and B. Pelaz, Aqueous stable luminescent perovskite-polymer composites, Applied Materials Today, 2019, 15, 562–569 CrossRef.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011, 44(6), 1272–1276 CrossRef CAS.
- A. D. Wright, C. Verdi, R. L. Milot, G. E. Eperon, M. A. Pérez-Osorio, H. J. Snaith, F. Giustino, M. B. Johnston and L. M. Herz, Electron–phonon coupling in hybrid lead halide perovskites, Nat. Commun., 2016, 7(1), 11755 CrossRef PubMed.
- Y. Guo, O. Yaffe, T. D. Hull, J. S. Owen, D. R. Reichman and L. E. Brus, Dynamic emission Stokes shift and liquid-like dielectric solvation of band edge carriers in lead-halide perovskites, Nat. Commun., 2019, 10(1), 1175 CrossRef PubMed.
- M. Nikl, E. Mihokova, K. Nitsch, F. Somma, C. Giampaolo, G. P. Pazzi, P. Fabeni and S. Zazubovich, Photoluminescence of Cs4PbBr6 crystals and thin films, Chem. Phys. Lett., 1999, 306(5), 280–284 CrossRef CAS.
- V. Babin, P. Fabeni, E. Mihokova, M. Nikl, G. P. Pazzi, N. Zazubovich and S. Zazubovich, Luminescence of Cs4PbBr6 Aggregates in As-Grown and in Annealed CsBr:Pb Single Crystals, Phys. Status Solidi B, 2000, 219(1), 205–214 CrossRef CAS.
- S. Radhakrishna and K. P. Pande, Lead Centers in Cesium Halides, Phys. Rev. B, 1973, 7(1), 424–431 CrossRef CAS.
- P. W. M. Jacobs, Alkali halide crystals containing impurity ions with the ns2 ground-state electronic configuration, J. Phys. Chem. Solids, 1991, 52(1), 35–67 CrossRef CAS.
- J. Yin, Y. Zhang, A. Bruno, C. Soci, O. M. Bakr, J.-L. Brédas and O. F. Mohammed, Intrinsic Lead Ion Emissions in Zero-Dimensional Cs4PbBr6 Nanocrystals, ACS Energy Lett., 2017, 2(12), 2805–2811 CrossRef CAS.
- J. Hoy, P. J. Morrison, L. K. Steinberg, W. E. Buhro and R. A. Loomis, Excitation Energy Dependence of the Photoluminescence Quantum Yields of Core and Core/Shell Quantum Dots, J. Phys. Chem. Lett., 2013, 4(12), 2053–2060 CrossRef CAS PubMed.
- M. Imran, V. Caligiuri, M. Wang, L. Goldoni, M. Prato, R. Krahne, L. De Trizio and L. Manna, Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals, J. Am. Chem. Soc., 2018, 140(7), 2656–2664 CrossRef CAS PubMed.
- M. Imran, P. Ijaz, D. Baranov, L. Goldoni, U. Petralanda, Q. Akkerman, A. L. Abdelhady, M. Prato, P. Bianchini, I. Infante and L. Manna, Shape-Pure, Nearly Monodispersed CsPbBr3 Nanocubes Prepared Using Secondary Aliphatic Amines, Nano Lett., 2018, 18(12), 7822–7831 CrossRef CAS PubMed.
- M. Imran, P. Ijaz, L. Goldoni, D. Maggioni, U. Petralanda, M. Prato, G. Almeida, I. Infante and L. Manna, Simultaneous Cationic and Anionic Ligand Exchange For Colloidally Stable CsPbBr3 Nanocrystals, ACS Energy Lett., 2019, 4(4), 819–824 CrossRef CAS.
- Y. Wang, J. He, H. Chen, J. Chen, R. Zhu, P. Ma, A. Towers, Y. Lin, A. J. Gesquiere, S.-T. Wu and Y. Dong, Ultrastable, Highly Luminescent Organic–Inorganic Perovskite–Polymer Composite Films, Adv. Mater., 2016, 28(48), 10710–10717 CrossRef CAS PubMed.
- S. J. Yoon, Z. Guo, P. C. dos Santos Claro, E. V. Shevchenko and L. Huang, Direct Imaging of Long-Range Exciton Transport in Quantum Dot Superlattices by Ultrafast Microscopy, ACS Nano, 2016, 10(7), 7208–7215 CrossRef CAS PubMed.
- C. Dujardin, E. Auffray, E. Bourret-Courchesne, P. Dorenbos, P. Lecoq, M. Nikl, A. N. Vasil’ev, A. Yoshikawa and R. Zhu, Needs, Trends, and Advances in Inorganic Scintillators, IEEE Trans. Nucl. Sci., 2018, 65(8), 1977–1997 CAS.
- K. Tomanová, V. Čuba, M. G. Brik, E. Mihóková, R. M. Turtos, P. Lecoq, E. Auffray and M. Nikl, On the structure, synthesis, and characterization of ultrafast blue-emitting CsPbBr3 nanoplatelets, APL Mater., 2019, 7(1), 011104 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and procedures, EDS-STEM data, 1H and 1H–13C HSQC NMR spectra and discussion, annotated XRD patterns, PLQY spectra, FTIR and NIR absorbance spectra, tests of PMAO reactivity with powders of bulk Cs4PbBr6 and amine-free Cs4PbBr6 NCs, stability tests of CsPbBr3/PMAO NCs, HRTEM images of Cs4PbBr6–CsPbBr3 heterostructures, low-resolution TEM size analysis, PL maps and spectra of Cs4PbBr6 NCs at 27 K, and time-resolved PL, micro-PL, and Raman spectra for the NC-PMAO blend (PDF). A video showing transformation of non-luminescent Cs4PbBr6 NCs into green-emitting CsPbBr3 NCs after addition of PMAO (MP4). See DOI: 10.1039/d0sc00738b. |
| This journal is © The Royal Society of Chemistry 2020 |