Open Access Article
Open Access ArticleHighly microporous SbPO4/BCx hybrid anodes for sodium-ion batteries†
Huiqi
Wang
 *a,
Li
Gou
a,
Weifeng
Jing
a,
Duo
An
a,
Ying
Li
a,
Mei
Wang
a,
Ning
Li
*a,
Li
Gou
a,
Weifeng
Jing
a,
Duo
An
a,
Ying
Li
a,
Mei
Wang
a,
Ning
Li
 a,
Shengliang
Hu
a,
Shengliang
Hu
 *a and
Yan-Bing
He
*a and
Yan-Bing
He
 *b
*b
aSchool of Materials Science and Engineering & School of Energy and Power Engineering, North University of China, Taiyuan 030051, P. R. China. E-mail: hqiwang@163.com; hsliang@yeah.net
bShenzhen Geim Graphene Center, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, 518055, P. R. China. E-mail: he.yanbing@sz.tsinghua.edu.cn
First published on 1st April 2020
Abstract
The current anode materials greatly restrict the electrochemical performance of sodium-ion batteries. Herein, we propose a highly microporous SbPO4/BCx hybrid anode for sodium-ion batteries, exhibiting a high initial reversible capacity of 871 mA h g−1 at 50 mA g−1, a good rate capability of around 300 mA h g−1 even at 5 A g−1 as well as an excellent cycling stability of 500 cycles. The excellent rate capability and cyclability with high capacity are probably due to the novel BCx structure and stable PO43− anions. The abundant micropores serve as reservoirs for storing the sodium ions and shorten the diffusion distance. The high surface area contributes to ample contact area between the electrode and electrolyte, thus achieving a rapid charge-transfer reaction. XPS analysis reveals that the BCx matrix consists of three B/C structures of BC3, BC2O, and BCO2, and contains around 12.93 at% substitutional boron. Since valence band holes are created by the B/C structures, more sodium ions would be captured easily, which motivates more sodium ions to intercalate electrochemically. Additionally, both the robust BCx matrix and stable PO43− anions as buffers could accommodate the volumetric expansion during the sodium ion insertion, thus optimizing the cycling performance. The strong attachment between SbPO4 and the BCx matrix would benefit mutual charge transfer between them and keep the integrity of the electrode during the sodiation/desodiation processes, which are favorable for sodium-ion transport and play a crucial role in enhancing the rate performance. Accordingly, the SbPO4/BCx composite is expected to become a promising anode for advanced SIBs.
1. Introduction
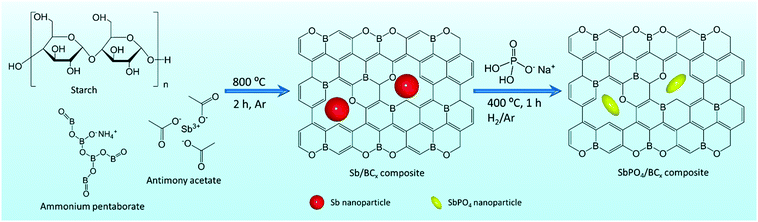
With regard to large-scale energy storage systems, sodium-ion batteries (SIBs) have gained extensive attention as potential alternatives to lithium-ion batteries (LIBs) due to the natural abundance and low cost of sodium and similar principles to LIBs.1–5 Currently, the largest challenge of SIBs for practical applications is exploiting suitable electrode materials that can absorb/release sodium ions with enough reversible capacity and diffusion kinetics.6,7 SIBs are not as attractive as LIBs, mostly because of the lack of anode materials with comparable performance to graphite anodes in LIBs. Among the current anode materials for SIBs, an alloy-type Sb anode is a promising candidate to enable high capacity (660 mA h g−1, Na3Sb),1,8 high electronic conductivity, and considerably safe operating potential (0.6 V vs. Na+/Na).9,10 However, metallic Sb anodes are often subjected to large volume changes (390%) upon cycling,11–13 which would easily lead to rapid capacity decay.To alleviate the large volume changes upon cycling of the Sb anodes, carbon buffer was proposed and various composites, such as Sb/hard carbon,13–18 Sb/carbon black,11 Sb/carbon fibers,19 Sb/carbon nanotubes,20 and Sb/graphene,7,21–24 have been reported. These carbon matrices, especially hard carbon, also contribute to the sodium storage capacity of about 300 mA h g−1,25–27 yet more than 0.5 times capacity of the carbon matrix was obtained at a low plateau (0.1 V vs. Na/Na+), further triggering security issues during the fast charging processes.21,27,28 Additionally, the carbon matrix suffers from slow ion diffusion kinetics.29,30 These deficiencies of metallic Sb anodes and the carbon matrix thus severely hamper their practical applications in SIBs, which urges the exploitation of novel and robust anode materials for SIBs. Recently, layered-structure SbPO4 was explored, for the first time, by Qian et.al.,7 exhibiting hopeful potential as an anode material for SIBs, because bulky and stable PO43− anions can buffer the volume change upon cycling and facilitate the cycling stability. Also, Na3PO4 generated by the discharge reaction, as an ionic conductor, may reduce the diffusion barrier of sodium ions and promote the reaction kinetics. Accordingly, if the conversion of Sb to SbPO4 over the Sb/C anode material was achieved, it would greatly improve the electrochemical performances of pristine metallic Sb anodes. On the other hand, the heteroatom doping and structure engineering of carbon is beneficial to increasing the sodium storage capacity of Sb/C anodes at the expense of the initial Coulombic efficiency and cycling stability.31–34 Most typically, some documents35–38 were aimed at the novel graphite-like BC3 structure as an anode material for alkali metal ion batteries. Many simulation results35,39 suggest that sodium could effectively intercalate this structure with the maximum sodium concentration, and the much lower potential barrier of BC3 promotes the sodium transportation, which is helpful in increasing the diffusion rates of sodium atoms inside the BC3 structure. Thus, these excellent features of BC3 encourage further experimental studies that the BC3 structure was used to prepare the volume buffer of the Sb anodes.
Herein, we propose the preparation of SbPO4/BCx hybrid anodes for sodium-ion batteries by a facile conversion of Sb to SbPO4 through one-step annealing of a solid mixed powder of Sb/BCx and NH4H2PO4, in which the Sb/BCx composites were fabricated by pyrolyzing antimony acetate impregnated ammonium pentaborate/starch xerogels at 800 °C under an argon atmosphere. The SbPO4/BCx hybrid electrode could provide stable PO43− anions and sufficient void space from the BCx matrix, which can perfectly accommodate the volumetric expansion during the sodium ion insertion, and preserve the structural stability of anodes. As a result, the SbPO4/BCx composite as an anode of SIBs exhibits a high initial reversible capacity of 871 mA h g−1 at 50 mA g−1, a good rate capability of around 300 mA h g−1 even at 5 A g−1 and an excellent cycling stability of 500 cycles. Hence, the SbPO4/BCx composite is expected to have promising application in future high-performance SIBs.
2. Material and methods
2.1. Material preparation
The Sb/BCx composite was first synthesized by pyrolyzing antimony acetate impregnated ammonium pentaborate/starch xerogels (Fig. 1). Briefly, 0.7 g of Sb(CH3COO)3 and 0.15 g of NH4B5O8 were dissolved in turn in 20 mL of deionized water. Next, 0.3 g of soluble starch was added to form a white solution. The mixed solution was heated at 85 °C for 1 h under vigorous stirring to obtain a transparent hydrogel. After freeze-drying, the resultant xerogels were carbonized at 800 °C for 2 h under an argon atmosphere, forming the Sb/BCx composites (denoted as Sb/BCx). The Sb/C composites and Sb were prepared using a similar method, respectively, and used as the control sample. Afterwards, 0.5 g of the as-prepared Sb/BCx was fully mixed with 5 g of NaH2PO4 powder through hand-grinding, and then the mixture was heated to 400 °C at a rate of 10 °C min−1, and annealed for 1 h in a H2/Ar (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) atmosphere. The calcined products were washed with deionized water and alcohol a few times, and dried at 60 °C (denoted as SbPO4/BCx).
95) atmosphere. The calcined products were washed with deionized water and alcohol a few times, and dried at 60 °C (denoted as SbPO4/BCx).
2.2. Structural characterization
X-ray diffraction (XRD) of all the samples was performed on a Bruker AXS D8 with Cu-Kα as a radiation source (λ = 1.5406 Å). Raman spectra were obtained on inVia instrument (Renishaw) with a 532 nm Ar-ion laser as an excitation source. Their morphologies and microstructure were analysed by scanning electron microscopy (SEM, S4800, Hitachi) and transmission electron microscopy (TEM, FEI Tecnai G20). X-ray photoelectron spectroscopy (XPS) was determined on a Thermo spectrometer using Al-Kα radiation (Thermo Scientific, ESCALAN 250Xi). N2 sorption isotherms were performed on a gas sorptometer at 77 K (Micrometrics ASAP 2020 analyser). Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 6700 FTIR spectrophotometer over the wavenumber range of 4000–500 cm−1.2.3. Electrochemical measurements
The electrochemical performance of SbPO4/BCx as an anode material in SIBs was studied using CR2025-type coin cells. In each test, the working electrode was prepared by mixing 80 wt% active material, 10 wt% acetylene black, and 10 wt% sodium alginate to form a black slurry, which was then coated on a clean copper foil and dried at 60 °C overnight under vacuum conditions. The mass loading of active materials was about 1.0–2.0 mg cm−2. The cells were assembled in an Ar-filled glove box (MIKROUNA, O2 < 1 ppm, H2O < 1 ppm) with sodium foil as the reference and counter electrodes, 1 M NaClO6 solution in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) solvent with 5% fluoroethylene carbonate as the electrolyte, and the GF/D glass microfiber filter (Whatman) as the separator. Galvanostatic discharge/charge tests were performed on a battery test system (NEWARE CT4008) with a voltage range from 0.01 to 3.0 V. All the capacity values were determined based on the total weight of the active material in the working electrodes. Cyclic voltammetry (CV) was carried out with coin cells at a scan rate of 0.1 mV s−1 using a CHI 760E electrochemical workstation. Electrochemical impedance spectroscopy (EIS) of coin cells was performed using an SP200 electrochemical workstation in the frequency range of 10−2-105 Hz by applying an AC voltage of 1 mV amplitude. The Nyquist curves are fitted by Zview software. All the tests were performed at room temperature.
1 by volume) solvent with 5% fluoroethylene carbonate as the electrolyte, and the GF/D glass microfiber filter (Whatman) as the separator. Galvanostatic discharge/charge tests were performed on a battery test system (NEWARE CT4008) with a voltage range from 0.01 to 3.0 V. All the capacity values were determined based on the total weight of the active material in the working electrodes. Cyclic voltammetry (CV) was carried out with coin cells at a scan rate of 0.1 mV s−1 using a CHI 760E electrochemical workstation. Electrochemical impedance spectroscopy (EIS) of coin cells was performed using an SP200 electrochemical workstation in the frequency range of 10−2-105 Hz by applying an AC voltage of 1 mV amplitude. The Nyquist curves are fitted by Zview software. All the tests were performed at room temperature.
3. Results and discussion
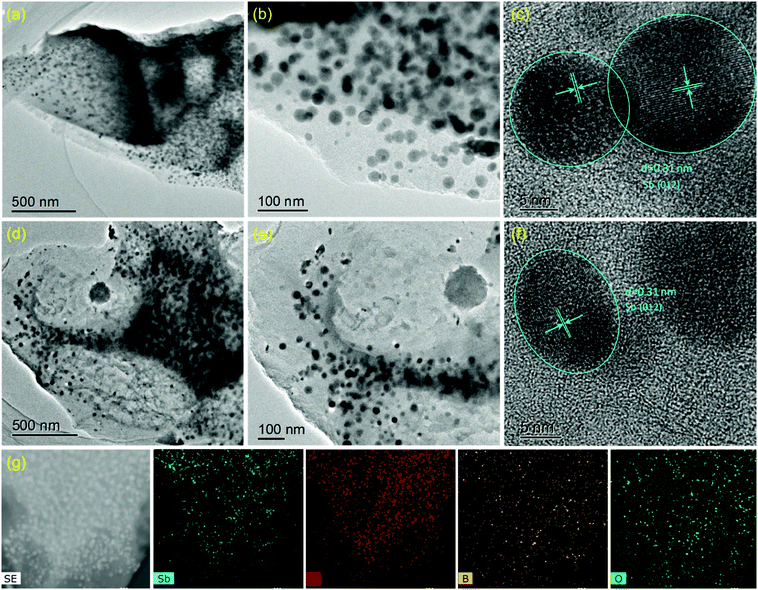
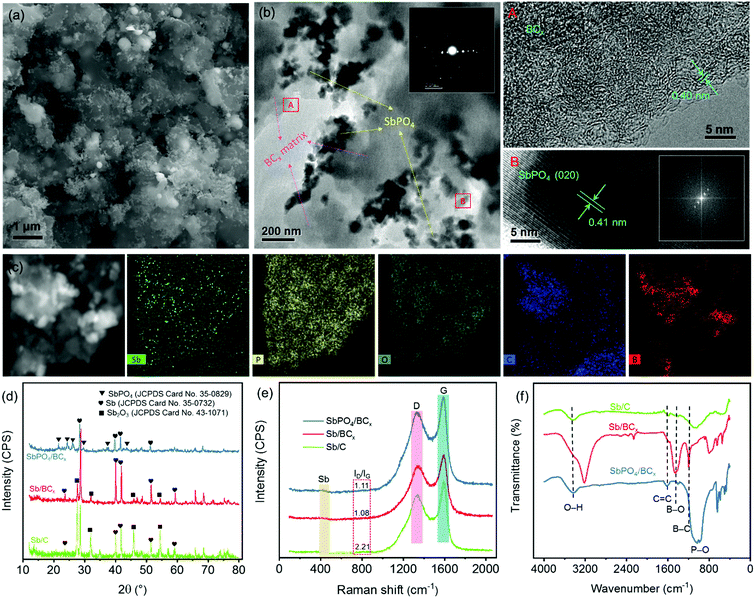
The SbPO4/BCx composite was prepared by a solid-phase annealing process (Fig. 1). The morphological characteristics of the precursor Sb/BCx after pyrolysis at 800 °C under an argon atmosphere are shown in Fig. 2. The spherical Sb nanoparticles with a mean size of 22 nm are uniformly anchored within the BCx matrix as presented by TEM images (Fig. 2a and b). The HRTEM image in Fig. 2c reveals clear lattice fringes with a d-spacing of around 0.31 nm corresponding to the (021) lattice planes of hexagonal Sb. For comparison, the TEM images of the Sb/C composite are shown in Fig. 2d and e, where the Sb nanoparticles with a mean size of 24 nm are unevenly embedded within the carbon matrix, and some cavities can be clearly seen since some Sb nanoparticles are shed from the carbon matrix, in contrast to the Sb nanoparticles in the Sb/BCx composite that are attached closely to the BCx matrix. Besides, the HRTEM image of Fig. 2f presents lattice fringes with a d-spacing of around 0.31 nm corresponding to the (021) lattice plane for hexagonal Sb, which is consistent with the results from the HRTEM image of the Sb/BCx composite. The distribution of Sb in the BCx matrix was confirmed by a scanning transmission electron microscopy (STEM) image, where Sb is identified as white nanodots. The TEM-EDS elemental mappings of Sb, C, B and O correspond to the area shown in Fig. 2g, and the four elements are uniformly distributed over the entire sample. The presence of the O element may be due to the highly porous structure of the Sb/BCx composite, which can easily absorb oxygen from air on the surface of the material.The characteristics of the SbPO4/BCx composite formed by one-step annealing of the solid powder of Sb/BCx and NH4H2PO4 are shown in Fig. 3. The SEM image in Fig. 3a reveals that the SbPO4 nanoparticles are randomly dispersed on the BCx matrix. The TEM image in Fig. 3b also indicates that there are numerous short rod-like nanoparticles with a diameter of about 50 nm and a length of up to 100 nm. Also, there are a small number of spindles in the SbPO4/BCx composite. The selected area electron diffraction (SAED) and the corresponding fast Fourier transform (FFT) patterns (the inset of Fig. 3b) can be indexed to monoclinic SbPO4,40,41 indicating the presence of a pure phase of SbPO4 in the material. The lattice-resolved HRTEM image of a single nanoparticle in Fig. 3b shows clear lattice fringes with a d-spacing of 0.41 nm, assigned to the (020) planes of SbPO4.7 In the HRTEM image of the BCx matrix, no obvious long-range-order structure exists, suggesting the amorphous nature of the material. A large number of defects and pores are distributed within the BCx matrix. The STEM image of the SbPO4/BCx composite and the corresponding TEM-EDS element mappings display the good distribution of the Sb, P, O, C and B elements in the material (Fig. 3c). All the above results confirm that the SbPO4 nanoparticles adhere well to the BCx matrix. The strong attachment would benefit the charge transfer between SbPO4 and the BCx matrix and retain the integrity of the electrode upon cycling, thereby improving the cycling stability and rate capability.14,15,25 The XRD patterns in Fig. 3d confirm the phase constituents of the material during each stage of the preparation of the SbPO4/BCx composite. The precursor Sb/BCx displays two phases consisting of Sb (JCPDS Card No. 85-1322) and Sb2O3 (JCPDS Card No. 43-1071). Compared with the Sb/C sample, a majority of the Sb2O3 phase in Sb/BCx is reduced to form metallic Sb after doping boron into the carbon matrix, since boron atoms can boost the carbon graphitization process, which largely accelerates the conversion from Sb2O3 to Sb. The resultant SbPO4/BCx composite displays multiphase components assigned to SbPO4 (JCPDS Card No. 78-1791) and Sb (JCPDS Card No. 85-1322). In addition to the six diffraction peaks of SbPO4 at 21.5°, 24.3°, 26.1°, 29.6°, 37.5° and 43.7°, it shows the diffraction peaks of Sb at 28.6°, 40.1°, 41.8°, and 51.6°. Additionally, no impurity peaks were detected, indicating that the Sb2O3 phase was completely converted into the SbPO4 phase. The phase conversion of Sb to SbPO4 can thus be easily achieved on the BCx matrix through one-step annealing of the solid mixed powder of Sb/BCx and NH4H2PO4. The ratios of Sb species were obtained by quantitative analysis through the Rietveld refinement of the XRD pattern (Fig. S1, ESI†), and the results are summarized in Table S1 (ESI†). The ratio of Sb to SbPO4 in SbPO4/BCx and the ratio of Sb to Sb2O3 in Sb/BCx and Sb/C were about 11.4/88.6, 78.1/21.9, and 28.4/71.6, respectively. The proportion of the Sb phase in SbPO4/BCx is much lower than that of the precursor Sb/BCx, hinting that metallic Sb is also partially converted to SbPO4.
To examine the crystallinity of the BCx matrix, Raman spectra of the SbPO4/BCx, Sb/BCx and Sb/C samples are recorded (Fig. 3e). Except for the peaks around 456 cm−1, corresponding to typical metallic Sb,25 all the Raman spectra exhibit two prominent peaks at 1340 and 1580 cm−1 assigned to the disordered carbon with sp3 hybridization (D-band) and graphitic carbon with sp2 hybridization (G-band), respectively.29,36 The integrated intensity ratio of the D and G bands (ID/IG) for the Sb/BCx composite is 1.08, which is lower than that (2.21) of Sb/C, revealing that the BCx matrix has a higher graphitization degree.23,42 In the case of SbPO4/BCx, the value of ID/IG ratio is 1.11, nearly equal to that of Sb/BCx, suggesting that the phase conversion of Sb to SbPO4 almost does not affect the BCx structure. FT-IR characterization was also carried out on these samples to elaborate the chemical bonds (Fig. 3f). In all the samples, the broad peak located at ∼3445 cm−1 was assigned to the O–H stretching vibration, and the peak centered at 1612 cm−1 was ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations. In the SbPO4/BCx sample, a prominent feature assigned to P–O stretching vibrations at ∼1054 and ∼987 cm−1 is visible, probably assigned to SbPO4.40 Except for the Sb/C sample, two extra peaks detected at 1190 and 1410 cm−1 in the SbPO4/BCx and Sb/BCx samples were attributed to B–C and B–O stretching vibrations,43,44 respectively, indicating the successful formation of the BCx structure by the substitution of boron into the carbon lattice.45
C stretching vibrations. In the SbPO4/BCx sample, a prominent feature assigned to P–O stretching vibrations at ∼1054 and ∼987 cm−1 is visible, probably assigned to SbPO4.40 Except for the Sb/C sample, two extra peaks detected at 1190 and 1410 cm−1 in the SbPO4/BCx and Sb/BCx samples were attributed to B–C and B–O stretching vibrations,43,44 respectively, indicating the successful formation of the BCx structure by the substitution of boron into the carbon lattice.45
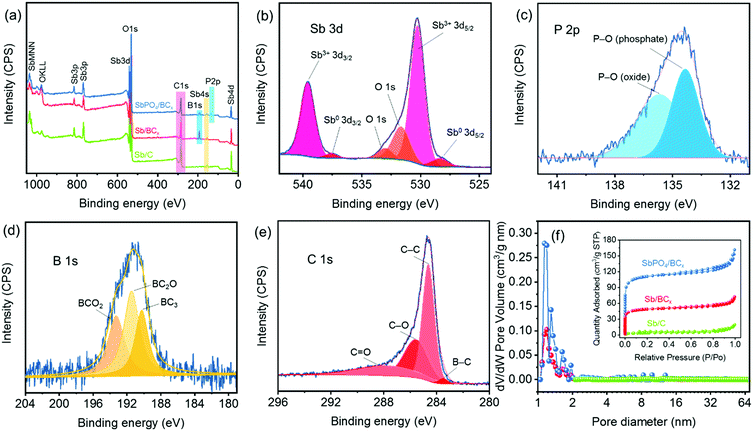
X-ray photoelectron spectroscopy (XPS) was conducted to reveal the composition and the chemical bonding states in the SbPO4/BCx composite. The signals of Sb, P, O, C and B could be easily detected in the survey spectrum (Fig. 4a). The high-resolution spectra of these elements confirm the existence of the SbPO4 and BCx structure (Fig. 4b–e). The high-resolution Sb 3d peaks for SbPO4/BCx are fitted into four peaks (Fig. 4b), which are assigned to Sb 3d5/2 (530.3 eV) and Sb 3d3/2 (539.7 eV) of Sb3+, and Sb 3d5/2 (528.4 eV) and Sb 3d3/2 (537.6 eV) of Sb0, respectively.6,7 Note that there are two O 1 s peaks located at 532.9 eV and 531.7 eV, which are assigned to the P–O bonding in P2O5 and PO43−, respectively.14 For comparison, the high-resolution Sb 3d peaks of Sb/BCx and Sb/C are also shown (Fig. S2, ESI†). The high-resolution P 2p peaks centered at 134.6 eV for SbPO4/BCx are fitted into two peaks (Fig. 4c), which are assigned to P–O (phosphate, 134.3 eV) and P–O (phosphorus oxide, 135.7 eV), respectively.46,47 These XPS results of Sb 3d and P 2p further confirm that the SbPO4/BCx composite consists of SbPO4 and Sb phases, in good accord with the XRD results (Fig. 3d). The high-resolution B 1s peaks for the BCx matrix are fitted into three peaks (Fig. 4d and Fig. S2, ESI†), corresponding to three B/C structures36,43 of BC3 (190.2 eV), BC2O (191.5 eV), and BCO2 (193.2 eV), and the other boron species is invisible, suggesting that boron atoms are completely incorporated into the carbon structure. The boron dopant concentrations in the BCx matrix are obtained through the full XPS spectrum fitting (Table S1, ESI†). The SbPO4/BCx composite has a higher boron concentration of 12.93 at%. The ratio of the three B/C structures can thus be determined, and the atomic percentage of BC3, BC2O, and BCO2 is 3.56, 5.28, and 4.10 at%, respectively. These results indicate that more boron atoms replace the carbon atoms at the edge or defect sites of the carbon structure.43 In all the samples, the C 1s peaks at ∼284.6, ∼285.6, ∼287.8 eV are assigned to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively36,45 (Fig. 3e and Fig. S2, ESI†). The presence of C–O and C
O, respectively36,45 (Fig. 3e and Fig. S2, ESI†). The presence of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O provides numerous anchoring sites for electrochemically active materials and prevents shedding from the electrodes.7 Except for Sb/C, the small peak at ∼283.5 eV in SbPO4/BCx and Sb/BCx is ascribed to the B–C bonding,45 revealing that boron atoms are incorporated into the carbon lattice and the formation of the BC3 structure43 (Fig. 1). When the graphite-like BC3 structure was used as the anode material for sodium ion batteries, the sodium ions could effectively intercalate this structure with the maximum concentration, and this unique structure has a much lower potential barrier, which would boost the sodium transportation and create much faster ion diffusion kinetics in the electrode.35,39
O provides numerous anchoring sites for electrochemically active materials and prevents shedding from the electrodes.7 Except for Sb/C, the small peak at ∼283.5 eV in SbPO4/BCx and Sb/BCx is ascribed to the B–C bonding,45 revealing that boron atoms are incorporated into the carbon lattice and the formation of the BC3 structure43 (Fig. 1). When the graphite-like BC3 structure was used as the anode material for sodium ion batteries, the sodium ions could effectively intercalate this structure with the maximum concentration, and this unique structure has a much lower potential barrier, which would boost the sodium transportation and create much faster ion diffusion kinetics in the electrode.35,39
The microporous characteristics of the SbPO4/BCx composite were evaluated through the N2 adsorption/desorption isotherm analysis (the inset of Fig. 4f). The isotherm curves and the NLDFT model calculation reveal that the SbPO4/BCx composite has a highly microporous structure (type-I),48 centered at approximately 1.2 nm (Fig. 4f), and the microporous structure can also be observed in the HRTEM image of the BCx matrix (Fig. 3b), which would enable an intimate contact between the electrolyte and electrode upon cycling.7 These micropores in both SbPO4/BCx and Sb/BCx samples are ascribed to the volatilization of small molecules (the thermal decomposition of NH4B5O8) during two-step annealing processes. The surface area was estimated by the BET method and the results are shown in Table S1 (ESI†). Both boron-doping and phase conversion of Sb to SbPO4 greatly increase the BET surface area of the Sb/C composite. The BET surface area of the SbPO4/BCx and Sb/BCx samples sharply increased to 428.24 and 183.92 m2 g−1, respectively, which is probably due to the structural rearrangement in the porous carbon matrix induced by boron and the phase conversion.36 This porous structure inhibits the volume expansion of the electrode upon cycling by providing sufficient vacant sites and enables better ion transport through the electrolyte to the active material.25
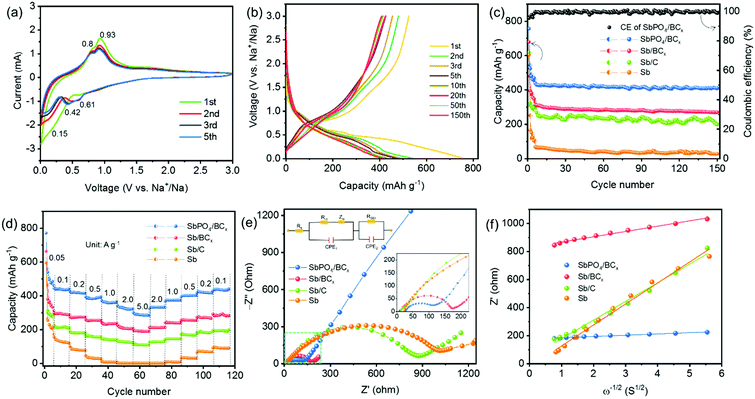
The electrochemical performance of the SbPO4/BCx hybrid anode for SIBs was evaluated by cyclic voltammetry (CV), galvanostatic discharge–charge cycling, and electrochemical impedance spectroscopy (EIS). Fig. 3a shows the 1st, 2nd, 3rd and 5th CV curves of the SbPO4/BCx electrode at a scan rate of 0.1 mV s−1 over 3.0–0.01 V (vs. Na+/Na). The involved electrochemical reaction process upon cycling can be described as eqn (1) and (2):7
| SbPO4 + 3Na+ + 3e− → Sb + Na3PO4 | (1) |
| Sb + 3e− + 3Na+ ↔ Na3Sb | (2) |
In the first sweep, two cathodic peaks at 0.62 and 0.15 V are assigned to the reduction of SbPO4 to metallic Sb and the alloying reaction between Sb and Na, respectively.40 The anodic peaks at 0.81 and 0.93 V are attributed to the phase transition from the NaxSb alloy to Sb.12 In the second sweep, no obvious peaks vanish, implying that the irreversible reactions are greatly inhibited. The cathodic peak at 0.62 V still exists and another peak at 0.42 V appears, which also originates from the generation of a new NaxSb alloy.15 The cathodic peak at 0.15 V probably comes from the residual Sb unreacted completely during the first discharge process.13 Compared to the variations of the cathodic peaks, those of the anodic peaks are invisible. The difference in the CV curves between the first and subsequent cycles is mainly attributed to the formation of the solid electrolyte interphase (SEI) film. The CV curves of the following cycles almost overlap, indicating the good cyclability of the SbPO4/BCx electrode. It is noted that correlative plateau regions are observed in the initial five discharge/charge profiles of the SbPO4/BCx electrode (Fig. 3b). The first discharge/charge capacity is 756/524 mA h g−1, exhibiting an initial Coulombic efficiency (CE) of 69% at 0.2 A g−1. The low CE value is attributed to the irreversible reactions during the first discharge process, including the generation of the SEI films resulting from the decomposition of the electrolyte, the irreversible sodium-ion insertion into smaller pores, and the irreversible conversion from SbPO4 to metallic Sb.7 After the initial cycling, the CE value reached 98% at the 3rd cycle and thereafter maintained above 99% after the 5th cycle. Fig. 3c presents the capacity and cycling performance of the SbPO4/BCx electrode at 0.2 A g−1 in the voltage range of 0.01–3 V (vs. Na+/Na). The SbPO4/BCx electrode exhibits much better cycling stability than Sb/BCx, Sb/C and Sb. After 150 cycles at 0.2 A g−1, the SbPO4/BCx electrode shows 78.2% capacity retention and exhibits a reversible capacity of 410 mA h g−1, much higher than 64.5% (270 mA h g−1) of Sb/BCx, 56.2% (200 mA h g−1) of Sb/C and 4.7% (28 mA h g−1) of Sb, which indicates that the conversion of Sb to SbPO4 over Sb/BCx induced a sharp increase in sodium storage capacity and enhanced cycling stability, originating from the stable PO43− anions as buffers to alleviate the volume change upon cycling. The sodium storage capacity of SbPO4/BCx is higher than those of Sb-based anodes reported previously (Table S2, ESI†). These results confirm that the excellent cycling performance of the SbPO4/BCx electrode is ascribed to the prominent advantages of the highly microporous structure, the buffer of volume change (stable PO43− anions), the lower potential barrier of BC3, and abundant edge defects49 (BC2O, BCO2).
Fig. 3d shows the superior rate performance of the SbPO4/BCx electrode. As the current densities progressively increase from 0.1 to 0.2, 0.5, 1.0, 2.0, and 5.0 A g−1, the electrode exhibits good capacity retention, changing from 440 to 419, 393, 369, 325, and 296 mA h g−1, respectively. When the current density returns stepwise to 2.0, 1.0, 0.5, 0.2 and 0.1 A g−1, capacities of 322, 363, 392, 415 and 430 mA h g−1 are obtained, respectively. To give insight into the excellent rate performance of the SbPO4/BCx electrode, EIS spectra were collected after 10 cycles and fitted by a Randle-type equivalent circuit model (the inset of Fig. 3e). Fig. 3e presents the Nyquist plots of the SbPO4/BCx, Sb/BCx, Sb/C and Sb electrodes; all the spectra consist of semicircles in the medium–high frequency region and sloping straight lines in the low-frequency region, which are related to the charge-transfer resistance (Rct) and solid-state diffusion of sodium in the electrode material, respectively.7,20 A shown in Table S3 (ESI†), the SbPO4/BCx electrode exhibits the lowest charge-transfer resistance (Rct = 44.43 Ω), which indicates its high electrical conductivity and enhanced reaction kinetics upon cycling. The results obtained from the 4-point probe method further confirm that the electronic conductivity of SbPO4/BCx is much larger than those of Sb/BCx and Sb/C (Table S3, ESI†). The relationship between Zre and ω−1/2 in Fig. 3f represents the Warburg impedance related to sodium-ion diffusion. The SbPO4/BCx electrode displays the lowest slope (σ = 9.18) among the three electrodes (Table S3, ESI†), implying facile sodium-ion transportation. The EIS result is consistent with its excellent cycling and rate performance, hinting the reinforced electron and ion transport. These results thus suggest that the robust B/C structure and stable PO43− anions are favorable for sodium-ion transport and play an important role in enhancing the rate performance (Fig. 5).
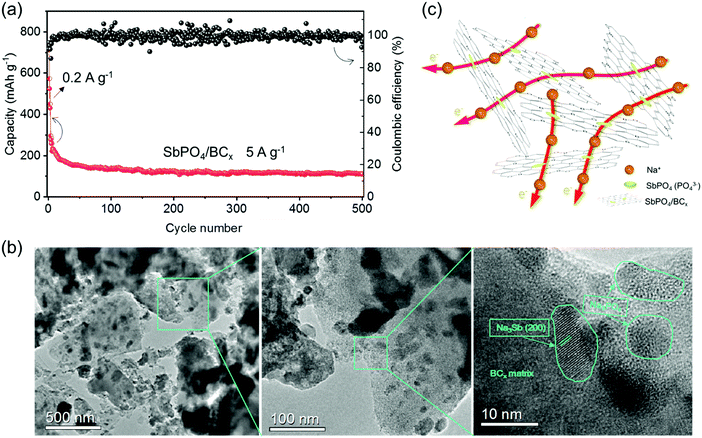
A long-term cycling at a high current rate of 5 A g−1 is achieved for the SbPO4/BCx electrode (Fig. 6a). After a few activation cycles under 0.2 A g−1, the capacity remains at 300 mA h g−1. In the following cycles, the capacity decreases to 127 mA h g−1 after 150 cycles, 118 mA h g−1 after 300 cycles, and 112 mA h g−1 after 500 cycles. Thus, the SbPO4/BCx electrode at a high current rate exhibits the low capacity retention of 37.3% as well as good cycling stability. The large initial capacity fading of SbPO4/BCx is probably attributed to the restricted buffering ability to adjust the volume expansion, which can be proved by observing the morphologies of electrodes before and after cycling (Fig. S3, ESI†). After 500 cycles, a small number of cracks are observed on the surface of the SbPO4/BCx electrode, which make it hard to retain the integrity of the electrode upon cycling. The TEM images in Fig. 6b show that the BCx matrix still retains its primary structure after 500 cycles, accounting for the good cycling stability of the SbPO4/BCx electrode at a high current rate.
The excellent rate capability and cyclability with high capacity could be illustrated by the robust BCx structure and stable PO43− anions (Fig. 6c). The amorphous BCx structure with a large d-spacing of ∼0.41 nm can intercalate more sodium ions. The abundant micropores serve as reservoirs for storing the sodium ions and shorten the diffusion distance. The high SBET value leads to the large contact area between the electrode and electrolyte, thus exhibiting the rapid charge-transfer reaction. Additionally, since valence band holes are created by boron doping, more sodium ions would be captured easily in the BCx structure, which motivates more sodium ions to intercalate electrochemically. Both the robust BCx matrix and stable PO43− anions as buffers could alleviate the volume change upon cycling, optimizing the cycling performance. The strong attachment between SbPO4 and BCx matrix would benefit mutual charge transfer between them, and keep the integrity of the electrode during the sodiation/desodiation processes. All of these merits make the SbPO4/BCx composite promising as an anode material for SIBs.
4. Conclusions
In summary, we propose a SbPO4/BCx hybrid anode for sodium-ion batteries by a facile conversion of Sb to SbPO4 through one-step annealing of a solid mixed powder of Sb/BCx and NH4H2PO4, where the Sb/BCx composites are prepared by pyrolyzing antimony acetate impregnated ammonium pentaborate/starch xerogels under an Ar atmosphere at 800 °C. When used as the anode of SIBs, the SbPO4/BCx composite exhibits a high initial reversible capacity of 871 mA h g−1 at 50 mA g−1, a good rate capability of about 300 mA h g−1 even at 5 A g−1 and an excellent cycling stability of 500 cycles. These results confirm that the excellent cycling performance of the SbPO4/BCx electrode is ascribed to the prominent advantages of a highly microporous structure, the buffer of volume change (stable PO43− anions), the lower potential barrier of BC3, and abundant edge defects (BC2O, BCO2). The novel B/C structure and stable PO43− anions are favorable for sodium-ion transport and play an important role in enhancing the rate performance. It is believed that this easy method of combining SbPO4 with a BCx matrix might be extended to other anode materials for reinforced electrochemical performance of SIBs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21703209, 51502270, U1510125, 51272301), the Key Research and Development (R&D) Projects of Shanxi Province (No. 201803D121037), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, the Specialized Research Fund for Sanjin Scholars Program of Shanxi Province, and the North University of China Fund for Scientific Innovation Team.Notes and references
- W. T. Jing, C. C. Yang and Q. Jiang, J. Mater. Chem. A, 2020, 8, 2913–2933 RSC.
- C. Kudakwashe, M. Grietus, D. L. Danilov and P. H. L. Notten, Adv. Energy Mater., 2018, 8, 1800079 CrossRef.
- Y. Fang, L. Xiao, Z. Chen, X. Ai, Y. Cao and H. Yang, Electrochem. Energy Rev., 2018, 1, 294–323 CrossRef CAS.
- C. Delmas, Adv. Energy Mater., 2018, 8, 1703137 CrossRef.
- Y. Y. Zhou, Y. L. Yang, M. G. Jiao and Z. Zhou, Sci. Bull., 2018, 63, 146–148 CrossRef CAS.
- J. Pan, Y. Zhang, L. Li, Z. Cheng, Y. Li, X. Yang, J. Yang and Y. Qian, Small Methods, 2019, 3, 1900231 CrossRef CAS.
- J. Pan, S. Chen, Q. Fu, Y. Sun, Y. Zhang, N. Lin, P. Gao, J. Yang and Y. Qian, ACS Nano, 2018, 12, 12869–12878 CrossRef CAS PubMed.
- L. Liang, Y. Xu, C. Wang, L. Wen, Y. Fang, Y. Mi, M. Zhou, H. Zhao and Y. Lei, Energy Environ. Sci., 2015, 8, 2954–2962 RSC.
- Z. Liu, T. Song and U. Paik, J. Mater. Chem. A, 2018, 6, 8159–8193 RSC.
- B. Wang, Z. Deng, Y. Xia, J. Hu, H. Li, H. Wu, Q. Zhang, Y. Zhang, H. Liu and S. Dou, Adv. Energy Mater., 2020, 10, 1903119 CrossRef CAS.
- H. Hou, Y. Yang, Y. Zhu, M. Jing, C. Pan, L. Fang, W. Song, X. Yang and X. Ji, Electrochim. Acta, 2014, 146, 328–334 CrossRef CAS.
- X. Zhou, Y. Zhong, M. Yang, M. Hu, J. Wei and Z. Zhou, Chem. Commun., 2014, 50, 12888–12891 RSC.
- M. Wang, Z. Yang, J. Wang, W. Li, L. Gu and Y. Yu, Small, 2015, 11, 5381–5387 CrossRef CAS PubMed.
- T. Ramireddy, N. Sharma, T. Xing, Y. Chen, J. Leforestier and A. M. Glushenkov, ACS Appl. Mater. Interfaces, 2016, 8, 30152–30164 CrossRef CAS PubMed.
- J. Duan, W. Zhang, C. Wu, Q. Fan, W. Zhang, X. Hu and Y. Huang, Nano Energy, 2015, 16, 479–487 CrossRef CAS.
- P. Li, L. Yu, S. Ji, X. Xu, Z. Liu, J. Liu and J. Liu, Chem. Eng. J., 2019, 374, 502–510 CrossRef CAS.
- S. Qiu, X. Wu, L. Xiao, X. Ai, H. Yang and Y. Cao, ACS Appl. Mater. Interfaces, 2016, 8, 1337–1343 CrossRef CAS PubMed.
- T. Wu, H. Hou, C. Zhang, P. Ge, Z. Huang, M. Jing, X. Qiu and X. Ji, ACS Appl. Mater. Interfaces, 2017, 9, 26118–26125 CrossRef CAS PubMed.
- H. Hou, M. Jing, Y. Yang, Y. Zhang, W. Song, X. Yang, J. Chen, Q. Chen and X. Ji, J. Power Sources, 2015, 284, 227–235 CrossRef CAS.
- X. Zhou, Z. Dai, J. Bao and Y.-G. Guo, J. Mater. Chem. A, 2013, 1, 13727–13731 RSC.
- X. Liu, M. Gao, H. Yang, X. Zhong and Y. Yu, Nano Res., 2017, 10, 4360–4367 CrossRef CAS.
- L. Li, K. H. Seng, D. Li, Y. Xia, H. K. Liu and Z. Guo, Nano Res., 2014, 7, 1466–1476 CrossRef CAS.
- K. Wang, Y. Xu, Y. Li, V. Dravid, J. Wu and Y. Huang, J. Mater. Chem. A, 2019, 7, 3327–3335 RSC.
- Y.-L. Ding, C. Wu, P. Kopold, P. A. van Aken, J. Maier and Y. Yu, Small, 2015, 11, 6026–6035 CrossRef CAS PubMed.
- X.-M. Pham, D. T. Ngo, H. T. T. Le, P. N. Didwal, R. Verma, C.-W. Min, C.-N. Park and C.-J. Park, Nanoscale, 2018, 10, 19399–19408 RSC.
- X. Zhou and Y.-G. Guo, ChemElectroChem, 2014, 1, 83–86 CrossRef.
- N. Sun, Z. Guan, Y. Liu, Y. Cao, Q. Zhu, H. Liu, Z. Wang, P. Zhang and B. Xu, Adv. Energy Mater., 2019, 9, 1901351 CrossRef.
- X. Dou, I. Hasa, D. Saurel, C. Vaalma, L. Wu, D. Buchholz, D. Bresser, S. Komaba and S. Passerini, Mater. Today, 2019, 23, 87–104 CrossRef CAS.
- H. Hou, X. Qiu, W. Wei, Y. Zhang and X. Ji, Adv. Energy Mater., 2017, 7, 1602898 CrossRef.
- Y. Yu, X. Zhong, Y. Wu and S. Zen, Chem. – Asian J., 2018, 13, 1248–1265 CrossRef PubMed.
- M. Wang, Y. Yang, Z. Yang, L. Gu, Q. Chen and Y. Yu, J. Adv. Sci., 2017, 4, 1600468 CrossRef PubMed.
- S. Qiu, L. Xiao, M. L. Sushko, K. S. Han, Y. Shao, M. Yan, X. Liang, L. Mai, J. Feng, Y. Cao, X. Ai, H. Yang and J. Liu, Adv. Energy Mater., 2017, 7, 1700403 CrossRef.
- H. Chen, Y. Xiong, T. Yu, P. Zhu, X. Yan, Z. Wang and S. Guan, Carbon, 2017, 113, 266–273 CrossRef CAS.
- D. Ni, W. Sun, Z. Wang, Y. Bai, H. Lei, X. Lai and K. Sun, Adv. Energy Mater., 2019, 9, 1900036 CrossRef.
- R. P. Joshi, B. Ozdemir, V. Barone and J. E. Peralta, J. Phys. Chem. Lett., 2015, 6, 2728–2732 CrossRef CAS PubMed.
- H. Wang, Y. Li, Y. Wang, S. Hu and H. Hou, J. Mater. Chem. A, 2017, 5, 2835–2843 RSC.
- S. Gong and Q. Wang, J. Phys. Chem. C, 2017, 121, 24418–24424 CrossRef CAS.
- P. Bhauriyal, A. Mahata and B. Pathak, J. Phys. Chem. C, 2017, 121, 9748–9756 CrossRef CAS.
- A. A. Kuzubov, A. S. Fedorov, N. S. Eliseeva, F. N. Tomilin, P. V. Avramov and D. G. Fedorov, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 195415 CrossRef.
- Y. Wang, L. Li and G. Li, RSC Adv., 2012, 2, 12999–13006 RSC.
- M. Ou, Y. Ling, L. Ma, Z. Liu, D. Luo and L. Xu, Mater. Lett., 2018, 224, 100–104 CrossRef CAS.
- X.-F. Luo, C.-H. Yang, Y.-Y. Peng, N.-W. Pu, M.-D. Ger, C.-T. Hsieh and J.-K. Chang, J. Mater. Chem. A, 2015, 3, 10320–10326 RSC.
- X. Yu, P. Han, Z. Wei, L. Huang, Z. Gu, S. Peng, J. Ma and G. Zheng, Joule, 2018, 2, 1610–1622 CrossRef CAS.
- S. Wang and X.-B. Zhang, Adv. Mater., 2019, 31, 1805432 CrossRef PubMed.
- H. Wang, Q. Guo, J. Yang, Z. Liu, Y. Zhao, J. Li, Z. Feng and L. Liu, Carbon, 2013, 56, 296–308 CrossRef CAS.
- C. Zhang, X. Wang, Q. Liang, X. Liu, Q. Weng, J. Liu, Y. Yang, Z. Dai, K. Ding, Y. Bando, J. Tang and D. Golberg, Nano Lett., 2016, 16, 2054–2060 CrossRef CAS PubMed.
- K. Lu, S. Lu, T. Gu, X. Zheng, K. Ke, X. Li and R. Yang, Electrochem. Commun., 2019, 103, 22–26 CrossRef CAS.
- L. Hao, B. Luo, X. Li, M. Jin, Y. Fang, Z. Tang, Y. Jia, M. Liang, A. Thomas, J. Yang and L. Zhi, Energy Environ. Sci., 2012, 5, 9747–9751 RSC.
- H. Wang, D. An, N. Li, Y. Li, M. Wang, J. Zhang, S. Hu and Y.-B. He, J. Power Sources, 2020, 461 DOI:10.1016/j.jpowsour.2020.228110.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00024h |
| This journal is © The Royal Society of Chemistry 2020 |