Structure-directing role of immobilized polyoxometalates in the synthesis of porphyrinic Zr-based metal–organic frameworks†
Mathis
Duguet
 ab,
Alex
Lemarchand
b,
Youven
Benseghir
ab,
Pierre
Mialane
a,
Maria
Gomez-Mingot
b,
Catherine
Roch-Marchal
a,
Mohamed
Haouas
a,
Marc
Fontecave
b,
Caroline
Mellot-Draznieks
ab,
Alex
Lemarchand
b,
Youven
Benseghir
ab,
Pierre
Mialane
a,
Maria
Gomez-Mingot
b,
Catherine
Roch-Marchal
a,
Mohamed
Haouas
a,
Marc
Fontecave
b,
Caroline
Mellot-Draznieks
 *b,
Capucine
Sassoye
*b,
Capucine
Sassoye
 *c and
Anne
Dolbecq
*c and
Anne
Dolbecq
 *a
*a
aUniversité Paris-Saclay, UVSQ, CNRS, UMR 8180, Institut Lavoisier de Versailles, 78035 Versailles Cedex, France. E-mail: anne.dolbecq@uvsq.fr
bLaboratoire de Chimie des Processus Biologiques, UMR CNRS 8229, Collège de France, Sorbonne Université, PSL Research University, 11 Place Marcelin Berthelot, 75231 Paris Cedex 05, France. E-mail: caroline.mellot-draznieks@college-de-france.fr
cSorbonne Université, UMR 7574, Collège de France, Laboratoire de Chimie de la Matière Condensée de Paris, 4 Place Jussieu, 75252 Paris cedex 05, France. E-mail: capucine.sassoye@upmc.fr
First published on 27th July 2020
Abstract
We evidence the structure-directing role of the PW12O403− polyoxometalate in porphyrinic MOF synthesis whereby it promotes the formation of the kinetic topology. Its immobilization into the MOF is successfully achieved at a high temperature yielding the kinetic MOF-525/PCN-224 phases, while prohibiting the formation of the thermodynamic MOF-545 product. A combined experimental/theoretical approach uses differential PDF and DFT calculations along with solid-state NMR to show the structural integrity of the POM and its location next to the Zr-based nodes.
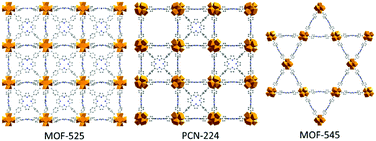
Over the years, Metal–Organic Frameworks (MOFs) have been extensively studied for a variety of applications such as gas storage, drug delivery or catalysis.1–3 In the strategic field of solar energy conversion, the use of porphyrinic Zr6-TCCP-based MOFs constructed from Zr6O4(OH)4 inorganic nodes and tetrakis(4-carboxyphenyl)porphyrin (TCPP) linkers for photo- or electrocatalysis purposes has attracted ever-growing interest, knowing that the porphyrin can act both as a photosensitizer and/or catalyst when metallated.4,5 Amongst this subfamily, three topologies have attracted particular attention, namely MOF-525,6PCN-2247 and MOF-5456 (this last being also known as PCN-222) (Fig. 1). Due to the large size of the TCPP linker, these three Zr6-TCCP-based MOFs exhibit sufficiently wide pores and high surface areas to allow the heterogenization of molecular catalysts to host energy conversion-related reactions, an emerging subfield.8 We thus recently showed that the immobilization of a cobalt sandwich-type polyoxometalate (POM) in MOF-545 leads to a noble metal-free heterogeneous photosystem with a high photocatalytic activity for water oxidation reaction, whereby MOF-545 acts as both a photosensitizer and an heterogenization matrix.9 POMs can also be co-immobilized with complexes in the pores of MOFs in order to boost their photocatalytic activity. Recently, we evidenced that the incorporation of the PW12O403− (PW12) Keggin-type POM inside the Cp*Rh@UiO-67 MOF increased its photocatalytic activity for the CO2 reduction reaction (CO2RR) when compared to that of the POM-free catalyst.10 Amongst other MOFs,11MOF-525 was shown to display significant catalytic activity for the CO2RR. While Hod et al. first showed the electrocatalytic properties of MOF-525(Fe),12 Zhang et al. demonstrated, a year later, the photocatalytic activity for CO2RR of its free-base, Co- and Zn-metallated forms under visible light.13 Based on these studies, we decided to attempt the immobilization of PW12 inside the Fe-metallated Zr6-TCPP-based MOFs in view of modifying their catalytic properties at a later stage. It must be recalled that the targeted synthesis of these phases is still a subject of intense research.12–18 For example, the influence of the synthetic temperature14 or that of the modulator16 was explored, whereby MOF-525 and PCN-224 synthesized at room temperature (RT) were shown to be kinetic outcomes while MOF-545 was identified as the thermodynamic phase obtained at a higher temperature. The consequence of the kinetic nature of MOF-525 and PCN-224 and of their structural similarity is that they are often obtained as a mixture, yielding very similar X-ray diffraction patterns. This led us to investigate the synthetic parameters which make it possible to get the desired kinetic Fe-metallated MOF-525/PCN-224 phases amongst the three possible Zr6-TCCP-based MOF products (Fig. 1), the non-metallated products also being synthesized for reference purposes.
In the present work, we thus investigate the immobilization of the PW12 POM into Zr6-TCCP-based MOFs and its impact on the obtained mixture of phases. A structuring effect of the POM is reported towards the targeted kinetic MOF-525/PCN-224 phases. A complete structural characterization of the product is provided by using Pair Distribution Function (PDF) analysis along with DFT calculations.
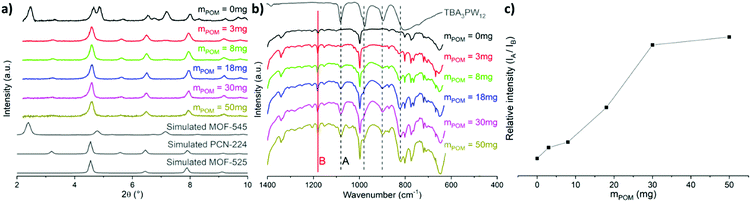
The synthesis of the mixed phases MOF-525/PCN-224, adapted from reported procedures,14,19–21 consists of the preparation of Zr6 clusters in solution at a high temperature followed by the addition of porphyrin and stirring at RT for a week (Fig. 2). We adopted the notation suggested by Gong et al.,14Zr6-TCPP-T and Zr6-TCPP-Fe-T, where T refers to the synthesis temperature. The powder X-ray diffraction (PXRD) diagrams of Zr6-TCPP-25 and Zr6-TCPP-Fe-25 correspond expectedly to the simulated diagrams of the kinetic phases, MOF-525 and/or PCN-224 (Fig. S1a, ESI†). Regarding the immobilization of PW12 into the MOFs, the post-synthetic impregnation strategy was discarded, knowing that both PCN-224 and MOF-525 exhibit small windows which preclude the diffusion of the POM into the MOF. We thus rather adopted an in situ strategy by adding (TBA)3PW12O40 into the synthetic medium. However, RT synthesis failed to incorporate PW12 into the MOF. Increasing the synthesis temperature was found to be essential to allow its successful incorporation and obtain the targeted PW12@Zr6-TCPP-120 and PW12@Zr6-TCPP-Fe-120 after 1 h at 120 °C. For the same experiment at 100 °C and 65 °C, less crystalline phases are obtained (Fig. S2, ESI†). We focused on the impact of a stepwise increase in the amount of PW12 introduced into the reaction medium of PW12@Zr6-TCPP-Fe-120 on the resulting MOFs’ topologies while keeping all the other synthetic parameters constant (Fig. 3). Notably, in the absence of PW12 in the reaction medium, the high temperature synthesis of Zr6-TCPP-Fe-120 leads to a mixture of the thermodynamic phase, MOF-545(Fe), and of the kinetic ones, MOF-525(Fe)/PCN-224(Fe). This latter result falls in line with the temperature-topology relationship recently reported.14 Strikingly, the formation of the thermodynamic phase MOF-545(Fe) is, however, suppressed upon the addition of a very low amount of PW12 (Fig. 3a, red), pointing towards a remarkable structure-directing effect of PW12 in the high temperature synthesis. All the PXRD diagrams of the PW12@Zr6-TCPP-Fe-120 composites obtained for higher PW12 amounts are similar, corresponding to PCN-224(Fe) or to the MOF-525(Fe)/PCN-224(Fe) mixture.
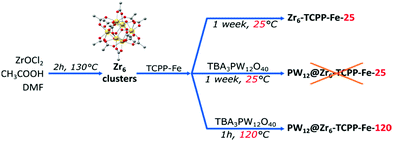 | ||
| Fig. 2 Synthetic routes adopted for Zr6-TCPP-Fe and PW12@Zr6-TCPP-Fe. The non-metallated compounds are isolated using similar procedures (see ESI†). | ||
The IR spectra of this series show the unambiguous presence of the POM within all PW12@Zr6-TCPP-Fe-120 materials, exhibiting the characteristic P–O (1077 cm−1), W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (972 cm−1) and W–O–W (892 and 797 cm−1) vibration bands of PW12 (Fig. 3b and Fig. S1b, ESI†). The absence of the aliphatic C–H vibrations in the IR spectra of the composites indicates that no TBA was inserted in the matrix during the synthetic process. A semi-quantitative analysis of the IR spectra was undertaken by following the relative intensities of the bands at 1077 cm−1 (noted A), typical of PW12, and at 1180 cm−1 attributed to the νC–C + νC–N vibrations (noted B), typical of the studied MOFs. The evolution of this ratio reveals that a maximum amount of POM immobilized into the MOF is reached for ca. 30 mg of (TBA)3PW12O40 in the reaction medium, corresponding to a PW12/Zr molar ratio of 0.05 (Fig. 3c). PW12@Zr6-TCPP-120 was also synthesized for further characterization purposes (see Experimental section). The POM loading was evaluated using EDS (Table S1, ESI†), suggesting an average of 1 POM every 2 Zr6 units in the free-base PW12@Zr6-TCPP-120 and 1 POM every 4 Zr6 units in the Fe-metallated PW12@Zr6-TCPP-Fe-120. C, H, and N analyses indicate missing linkers in both cases, a consequence of the immobilization of the negatively charged POMs and of the presence of the PCN-224 phase.
O (972 cm−1) and W–O–W (892 and 797 cm−1) vibration bands of PW12 (Fig. 3b and Fig. S1b, ESI†). The absence of the aliphatic C–H vibrations in the IR spectra of the composites indicates that no TBA was inserted in the matrix during the synthetic process. A semi-quantitative analysis of the IR spectra was undertaken by following the relative intensities of the bands at 1077 cm−1 (noted A), typical of PW12, and at 1180 cm−1 attributed to the νC–C + νC–N vibrations (noted B), typical of the studied MOFs. The evolution of this ratio reveals that a maximum amount of POM immobilized into the MOF is reached for ca. 30 mg of (TBA)3PW12O40 in the reaction medium, corresponding to a PW12/Zr molar ratio of 0.05 (Fig. 3c). PW12@Zr6-TCPP-120 was also synthesized for further characterization purposes (see Experimental section). The POM loading was evaluated using EDS (Table S1, ESI†), suggesting an average of 1 POM every 2 Zr6 units in the free-base PW12@Zr6-TCPP-120 and 1 POM every 4 Zr6 units in the Fe-metallated PW12@Zr6-TCPP-Fe-120. C, H, and N analyses indicate missing linkers in both cases, a consequence of the immobilization of the negatively charged POMs and of the presence of the PCN-224 phase.
In addition, N2 sorption isotherms (Fig. S3, ESI†) evidence a decrease of the surface area of the POM@MOF compounds when compared to that of the POM-free Zr6-TCPP-Fe-25 material, as expected from the incorporation of PW12 into the MOF's pores. More importantly, the solid-state NMR 31P{1H} CPMAS spectra of PW12@Zr6-TCPP-120 and PW12@Zr6-TCPP-Fe-120 (Fig. S4, ESI†) clearly show a characteristic single peak at −15.9 ppm and −15.5 ppm, respectively, in the expected chemical shift range for PW12,22 thus suggesting the integrity of the POM. Moreover, the line broadening and appearance of a spinning sideband in the spectrum of PW12@Zr6-TCPP-Fe-120 are due to chemical shift anisotropy and anisotropic bulk magnetic susceptibility broadening of 31P nuclei in interaction with the FeIII paramagnetic centre.
In addition, PW12@Zr6-TCPP-Fe-120 and PW12@Zr6-TCPP-120 were studied using cyclic voltammetry (CV) by the deposition of a thin film of the composites on a glassy carbon electrode (Fig. S5, ESI†). The electrochemical signatures of PW1224 and TCPP-Fe12 in solution have been compared to those of the POM@MOF composites, showing that not only were they in full agreement with those reported in the literature but they also allowed the characteristic redox peaks of these two components on the composite materials to be unambiguously attributed. To further investigate the structural features of these porphyrin-based MOFs, a Pair Distribution Function (PDF) analysis was undertaken. Reflecting the probability of finding two atoms separated by a distance r, PDF data provide quantitative information about the local atomic structure, while giving a global overview of the sample. They have proved to be particularly powerful in the field of MOFs recently.25 In a first step, considering that we may have a mixture of structurally related MOFs, MOF-525 and/or PCN-224, which are difficult to distinguish using PXRD only, we investigated the use of PDF intending to identify the distinctive features related to each phase. This was undertaken using Zr6-TCPP-Fe-25 as a case study. As references, the PDF profiles of MOF-525 and PCN-224 were simulated using their DFT-optimized crystal structures. They exhibit common features due to their common Zr-oxocluster but also distinctive particularities (see ESI† for details, Fig. S6–S8). The PDF profile of Zr6-TCPP-Fe-25 exhibits specific features from both MOFs (Fig. S9, ESI†). On one hand, it has strong similarities with that of MOF-525, with a distinct peak at 3.1 Å and a relatively intense peak at 5.1 Å, which are both a signature of Zr-connected TCCP linkers. On the other hand, secondary features suggest the possible presence of PCN-224 in Zr6-TCPP-Fe-25, such as a rather intense peak at 4.0 Å (associated to –OH and H2O molecules in place of missing linkers) and a similar PDF profile to that of PCN-224 in the [5.5–7.5 Å] radial distance range. Overall, this analysis indicates that Zr6-TCPP-Fe-25 is unambiguously a mixture of MOF-525 and PCN-224.
In a second step, PDF data were further exploited to investigate the structural integrity of the immobilized POM within PW12@Zr6-TCPP-Fe-120. Using the differential PDF method (d-PDF)26 recently applied to characterize a POM@UiO-67 composite,10 the d-PDF of the immobilized PW12 was obtained by subtracting directly the PDF of Zr6-TCPP-Fe-25 from that of PW12@Zr6-TCPP-Fe-120 (Fig. 4a and Fig. S10, see ESI† for details). The POM's d-PDF profile is compared in the 1–10 Å range to that of an isolated PW12O403− calculated from reported crystallographic data23 (Fig. 4b). The two PDFs are indeed very similar allowing a precise assignment of all PDF peaks to characteristic W–O and W–W distances and a refinement of the PW12 structure as illustrated in Fig. 4c (see ESI† for details, Fig S11 and Table S2). The relatively good quality of the refinement (Rw = 29.3%) and the refined structure of PW12 indicate that the POM is indeed fully preserved upon its immobilization in Zr6-TCPP-Fe-120.
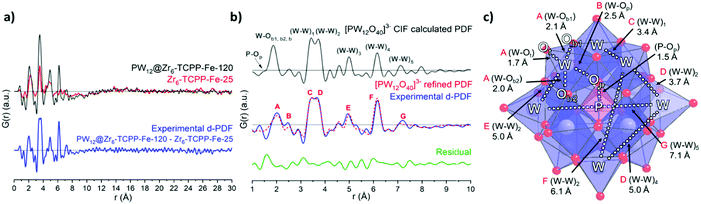 | ||
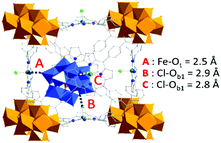
| Fig. 4 (a) PDF profiles for PW12@Zr6-TCPP-Fe-120, Zr6-TCPP-Fe-25 and the resulting d-PDF associated with the immobilized PW12. (b) Comparison of the calculated PDF of an isolated PW12 from reported crystallographic data23 and the experimental d-PDF associated with PW12 in PW12@Zr6-TCPP-Fe-120, superimposed with the refined d-PDF and residual profile. A–H labels of peaks correspond to the indicated refined distances in the POM structure as illustrated in (c). WO6, blue octahedra; PO4, pink tetrahedron; O, red sphere; W, grey spheres; P, pink sphere. | ||
To get an in-depth insight into the host–guest interactions at play between the PW12 and the MOF, Density Functional Theory (DFT) calculations were performed using MOF-525(Fe) phase as the main host. In a first step, simulated annealing (SA) calculations followed by dispersion-corrected DFT-D3 geometry optimizations were done to identify the most likely position of the POM in MOF-525(Fe) and qualify its host–guest interactions within the MOF. They reveal a non-centered positioning of the POM within the MOF's cubic cage showing PW12 cornered close to a Zr-oxocluster inorganic node (Fig. 5). The interaction energy between PW12 and MOF-525(Fe) was estimated to be −422 kJ mol−1, which corresponds to coordination-like interactions between the OPOM atoms and Fe ions of the TCCP linkers and dispersive interactions with Cl atoms, in addition to POM-TCCP hydrogen bonds (Fig. S12a, ESI†). These results correlate well with the 2D 31P–1H correlation MAS NMR experiment (Fig. S12b, ESI†) demonstrating the spatial proximity of the POMs to the inorganic nodes. Overall, DFT calculations coupled with solid-state NMR added remarkable insights on the POM-MOF interactions to PDF analysis.
In conclusion, with the aim of targeting porphyrinic POM@MOF systems, the addition of the PW12 polyoxometalate in the synthetic medium of porphyrin MOFs was explored using a high temperature one-pot synthetic protocol. In the absence of POM, a mixture of the kinetic MOF-525/PCN-224 and thermodynamic MOF-545 topologies is obtained. Remarkably, the addition of PW12 in the synthetic medium prevents the formation of the expected thermodynamic MOF-545 phase while favoring the formation of the kinetic ones even at a high temperature, pointing towards the structure-directing role of the POM. Simultaneously we developed in-depth characterization methods for PW12@MOF by utilizing the differential Pair Distribution Function as a direct structural signature of the POM, solid-state NMR and DFT calculations. They unequivocally showed the structural integrity of the POM inside the porphyrinic MOF along with an insight into the host–guest interactions between the different components, circumventing the difficulties inherent to the Rietveld refinement of powder X-ray diffraction data of such long-range disordered materials. These results open the way for more complete research on the templating effect of POMs on the topology of porphyrinic MOFs and their effect on their catalytic properties.
This work was supported by CNRS, UVSQ, the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation, the French ANR as part of the ‘Investissements d’Avenir’ program no. ANR-11-IDEX-0003-02 and CHARMMMAT ANR-11-LABX-0039. This work has been sponsored by the Ile-de-France Region in the framework of Respore, the Île-de-France network of Excellence in Porous Solids. The IMAP laboratory is gratefully acknowledged for N2 porosimetry measurements. The calculations have been performed using the HPC resources from GENCI (CINES) through Grant A0050907343.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev. DOI:10.1021/acs.chemrev.9b00685
.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed
.
- A. Kirchon, L. Feng, H. F. Drake, E. A. Joseph and H.-C. Zhou, Chem. Soc. Rev., 2018, 47, 8611–8638 RSC
.
- Z.-Y. Gu, J. Park, A. Raiff, Z. Wei and H.-C. Zhou, ChemCatChem, 2014, 6, 67–75 CrossRef CAS
.
- W.-Y. Gao, M. Chrzanowski and S. Ma, Chem. Soc. Rev., 2014, 43, 5841–5866 RSC
.
- W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443–6445 CrossRef CAS PubMed
.
- D. Feng, W.-C. Chung, Z. Wei, Z.-Y. Gu, H.-L. Jiang, Y.-P. Chen, D. J. Darensbourg and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 17105–17110 CrossRef CAS PubMed
.
- J.-S. Qin, S. Yuan, C. Lollar, J. Pang, A. Alsalme and H.-C. Zhou, Chem. Commun., 2018, 54, 4231–4249 RSC
.
- G. Paille, M. Gomez-Mingot, C. Roch-Marchal, B. Lassalle-Kaiser, P. Mialane, M. Fontecave, C. Mellot-Draznieks and A. Dolbecq, J. Am. Chem. Soc., 2018, 140, 3613–3618 CrossRef CAS PubMed
.
- Y. Benseghir, A. Lemarchand, M. Duguet, P. Mialane, M. Gomez-Mingot, C. Roch-Marchal, T. Pino, M.-H. Ha-Thi, M. Haouas, M. Fontecave, A. Dolbecq, C. Sassoye and C. Mellot-Draznieks, J. Am. Chem. Soc., 2020, 142, 9428–9438 CrossRef CAS PubMed
.
- D. Li, M. Kassymova, X. Cai, S.-Q. Zang and H.-L. Jiang, Coord. Chem. Rev., 2020, 412, 213262 CrossRef CAS
.
- I. Hod, M. D. Sampson, P. Deria, C. P. Kubiak, O. K. Farha and J. T. Hupp, ACS Catal., 2015, 5, 6302–6309 CrossRef CAS
.
- H. Zhang, J. Wei, J. Dong, G. Liu, L. Shi, P. An, G. Zhao, J. Kong, X. Wang, X. Meng, J. Zhang and J. Ye, Angew. Chem., Int. Ed., 2016, 55, 14310–14314 CrossRef CAS PubMed
.
- X. Gong, H. Noh, N. C. Gianneschi and O. K. Farha, J. Am. Chem. Soc., 2019, 141, 6146–6151 CrossRef CAS PubMed
.
- M. L. Kelty, W. Morris, A. T. Gallagher, J. S. Anderson, K. A. Brown, C. A. Mirkin and T. D. Harris, Chem. Commun., 2016, 52, 7854–7857 RSC
.
- S. M. Shaikh, P. M. Usov, J. Zhu, M. Cai, J. Alatis and A. J. Morris, Inorg. Chem., 2019, 58, 5145–5153 CrossRef CAS PubMed
.
- P. Deria, J. Yu, R. P. Balaraman, J. Mashni and S. N. White, Chem. Commun., 2016, 52, 13031–13034 RSC
.
- P. Deria, D. A. Gómez-Gualdrón, I. Hod, R. Q. Snurr, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2016, 138, 14449–14457 CrossRef CAS PubMed
.
- M. R. DeStefano, T. Islamoglu, S. J. Garibay, J. T. Hupp and O. K. Farha, Chem. Mater., 2018, 30, 2193–2197 CrossRef
.
- H. Noh, C.-W. Kung, T. Islamoglu, A. W. Peters, Y. Liao, P. Li, S. J. Garibay, X. Zhang, M. R. DeStefano, J. T. Hupp and O. K. Farha, Chem. Mater., 2018, 30, 2193–2197 CrossRef CAS
.
- G. Kickelbick, P. Wiede and U. Schubert, Inorganica Chim. Acta, 1999, 284, 1–7 CrossRef CAS
.
- W. Salomon, C. Roch-Marchal, P. Mialane, P. Rouschmeyer, C. Serre, M. Haouas, F. Taulelle, S. Yang, L. Ruhlmann and A. Dolbecq, Chem. Commun., 2015, 51, 2972–2975 RSC
.
- A. Kremenović, A. Spasojević-de Biré, R. Dimitrijević, P. Sciau, U. B. Mioč and P. Colomban, Solid State Ion., 2000, 132, 39–53 CrossRef
.
- K. Eda and T. Osakai, Inorg. Chem., 2015, 54, 2793–2801 CrossRef CAS PubMed
.
- C. Castillo-Blas, J. M. Moreno, I. Romero-Muñiz and A. E. Platero-Prats, Nanoscale 10.1039/D0NR01673J
.
- K. W. Chapman, P. J. Chupas and C. J. Kepert, J. Am. Chem. Soc., 2005, 127, 11232–11233 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis, characterizations and computations. See DOI: 10.1039/d0cc04283h |
| This journal is © The Royal Society of Chemistry 2020 |