Coordination distortion induced water adsorption in hydrophobic flexible metal–organic frameworks†
Yoshinobu
Kamakura
a,
Arata
Hikawa
a,
Hirofumi
Yoshikawa
b,
Wataru
Kosaka
 cd,
Hitoshi
Miyasaka
cd,
Hitoshi
Miyasaka
 cd and
Daisuke
Tanaka
cd and
Daisuke
Tanaka
 *ae
*ae
aDepartment of Chemistry, School of Science and Technology, Kwansei Gakuin University, 2-1 Gakuen, Sanda, Hyogo 669-1337, Japan. E-mail: dtanaka@kwansei.ac.jp
bDepartment of Nanotechnology for Sustainable Energy, School of Science and Technology, Kwansei Gakuin University, Sanda, Hyogo 669-1337, Japan
cInstitute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
dDepartment of Chemistry, Graduate School of Science, Tohoku University, 6-3 Aramaki-Aza-Aoba, Aoba-ku, Sendai 980-8578, Japan
eJST PRESTO, 2-1 Gakuen, Sanda, Hyogo 669-1337, Japan
First published on 17th July 2020
Abstract
Herein, we synthesized four isostructural flexible interdigitated hydrophobic metal–organic frameworks that differ only in their central metal ion. The water-adsorption properties are dependent on the metal species, which was revealed to be caused by coordination distortion around the metal ions.
Recently, metal–organic frameworks (MOFs) have attracted significant attention for use as water adsorbents.1–5 MOFs exhibit several attractive properties, which make them suitable for use in several applications, including fluorescence,6–8 phosphorescence,9,10 enhanced CO2 capture,11,12 ion conduction,13,14 and dehumidification.15,16 The ability to adsorb and desorb water molecules at high relative pressures can help in reducing the amount of energy consumed during the release of water. Therefore, flexible17–19 and mesoporous20–22 MOFs have been developed. Flexible MOFs are particularly desirable as they facilitate fine control of the onset pressure; they exhibit abrupt uptake at a specific pressure, which is referred to as the gate-opening pressure.23 This onset pressure is significantly affected by the flexibility of the linker,24 structural dimensionality,25 and type of metal ion used in the nodes.26
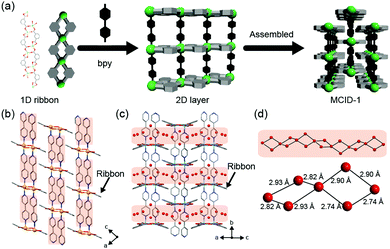
To control the onset pressure for the adsorption of water molecules by a flexible MOF, the relationship between the framework dynamics and behavior of the water molecules in the micropores of the MOF must be known. In particular, flexible MOFs with low structural dimensionality and coordination distortion within their nodes are interesting because their onset pressure is affected by various structural parameters. In this work, we chose MCID-1 ([M(ip)(bpy)]n, where M = metal ion, ip = isophthalate, and bpy = 4,4′-bipyridine) as the model compound. The metal ions in MCID-1 are connected by ip units to form one-dimensional (1D) double-chain ribbon-like structures of {[M(ip)]}n. Then, these ribbons are linked by bpy to yield a two-dimensional (2D) sheet-like motif. Finally, these 2D layers are mutually interdigitated, resulting in zero-dimensional (0D) hydrophobic pores between the layers (Fig. 1a).27 MCID-1 adsorbs guest molecules, which is accompanied by the expansion of the 2D layer gap and rearrangement of the coordination modes of the metal ions. In addition, MCID-1 forms isostructures with various metal ions.28–33 Although these characteristics are desirable for evaluating the effects of node-derived flexibility and dimensionality, apart from ZnCID-1, the water-adsorption properties of MCID-1 compounds have not yet been reported. Therefore, the effect of the type of metal ion on the water-adsorption behavior of these compounds remains unknown. Herein, we report the effects of various central metal species on the water-adsorption behavior of MCID-1 and highlight the differences in these behaviors for four MCID-1 compounds (M = Co, Ni, Cu, and Zn). Moreover, we synthesized solid-solution-type M1M2CID-1 and determined the correlation between the distribution of metal ions within the framework and its water-adsorption behavior.
MCID-1 was synthesized by the solvothermal method through the reaction of M(NO3)2·xH2O (M = Co, Ni, and Zn, x = 6 or M = Cu, x = 3) with Na2ip and bpy (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2ip
Na2ip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bpy molar ratio = 1
bpy molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in H2O with heating at 150 °C for 48 h followed by cooling to 30 °C over 12 h. We obtained MCID-1 in powdered form under various synthesis conditions. The X-ray powder diffraction (XRPD) patterns of the synthesized samples are identical to those simulated based on the reported crystal structure of MCID-1 (Fig. S1, ESI†). The XRPD patterns of the as-synthesized CoCID-1 and NiCID-1 are identical to those of the guest-free phase, which indicates that both compounds are devoid of guest water molecules in their 0D hydrophobic cavities (Fig. 1b and Fig. S2a; see note S5 in the ESI†).30,31 However, the XRPD patterns of CuCID-1 and ZnCID-1 indicate the presence of water molecules within the pores (MCID-1⊃H2O; Fig. 1c and Fig. S2e; see note S5 in the ESI†), although the areas surrounding the pores in MCID-1 are hydrophobic (Fig. 1c and Fig. S2d, ESI†).32,33 The XRPD patterns of MCID-1⊃H2O (M = Cu or Zn) indicate ribbon-like structures, i.e., structures in which the aromatic rings in the coordination polymer of metal ions and ip are twisted alternately. The twisted ribbon-like structures result in the formation of 1D channels. These 1D channels are filled with water molecules, which form 1D chain structures within the hydrophobic space due to hydrogen bonding.34 The formation of water nanoclusters plays a key role in stabilizing these 1D chain structures (Fig. 1c and d).
1) in H2O with heating at 150 °C for 48 h followed by cooling to 30 °C over 12 h. We obtained MCID-1 in powdered form under various synthesis conditions. The X-ray powder diffraction (XRPD) patterns of the synthesized samples are identical to those simulated based on the reported crystal structure of MCID-1 (Fig. S1, ESI†). The XRPD patterns of the as-synthesized CoCID-1 and NiCID-1 are identical to those of the guest-free phase, which indicates that both compounds are devoid of guest water molecules in their 0D hydrophobic cavities (Fig. 1b and Fig. S2a; see note S5 in the ESI†).30,31 However, the XRPD patterns of CuCID-1 and ZnCID-1 indicate the presence of water molecules within the pores (MCID-1⊃H2O; Fig. 1c and Fig. S2e; see note S5 in the ESI†), although the areas surrounding the pores in MCID-1 are hydrophobic (Fig. 1c and Fig. S2d, ESI†).32,33 The XRPD patterns of MCID-1⊃H2O (M = Cu or Zn) indicate ribbon-like structures, i.e., structures in which the aromatic rings in the coordination polymer of metal ions and ip are twisted alternately. The twisted ribbon-like structures result in the formation of 1D channels. These 1D channels are filled with water molecules, which form 1D chain structures within the hydrophobic space due to hydrogen bonding.34 The formation of water nanoclusters plays a key role in stabilizing these 1D chain structures (Fig. 1c and d).
Guest-free MCID-1 was obtained by washing MCID-1 with MeOH and heating it at 120 °C overnight under reduced pressure. The XRPD patterns of CoCID-1 and NiCID-1 did not change during degassing/activation (Fig. S4 and S5, ESI†). In contrast, the XRPD patterns of CuCID-1⊃H2O and ZnCID-1⊃H2O changed upon removal of water. The XRPD pattern of ZnCID-1⊃H2O after degassing is identical to the simulated pattern of degassed ZnCID-1 (Fig. S6, ESI†), whose space group is similar to that of CoCID-1 and NiCID-1 (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ).33 In contrast, the XRPD pattern of CuCID-1 acquired in an Ar atmosphere is dramatically different from that of MCID-1⊃H2O and corresponds to an unknown phase (Fig. S7 and S8, ESI†). We successfully determined the crystal structure of the guest-free CuCID-1 phase through single-crystal structural analysis (Fig. 1c and Table S1, ESI†). While the other MCID-1 samples (M = Co, Ni, and Zn) had a space group of P
).33 In contrast, the XRPD pattern of CuCID-1 acquired in an Ar atmosphere is dramatically different from that of MCID-1⊃H2O and corresponds to an unknown phase (Fig. S7 and S8, ESI†). We successfully determined the crystal structure of the guest-free CuCID-1 phase through single-crystal structural analysis (Fig. 1c and Table S1, ESI†). While the other MCID-1 samples (M = Co, Ni, and Zn) had a space group of P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , that of CuCID-1 was determined to be P21/c (Table S2, ESI†). The simulated XRPD pattern of the degassed CuCID-1 phase is identical to this unknown XRPD pattern. CuCID-1 adsorbed water in air, and its crystal structure transformed to that of CuCID-1⊃H2O within 20 min under ambient conditions (Fig. S8, ESI†).
, that of CuCID-1 was determined to be P21/c (Table S2, ESI†). The simulated XRPD pattern of the degassed CuCID-1 phase is identical to this unknown XRPD pattern. CuCID-1 adsorbed water in air, and its crystal structure transformed to that of CuCID-1⊃H2O within 20 min under ambient conditions (Fig. S8, ESI†).
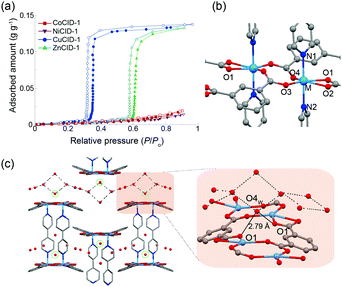
To evaluate the affinity of MCID-1 for water, the water-adsorption isotherms of MCID-1 were acquired, which showed that the onset pressure (P/P0) for ZnCID-1 was approximately 0.60 and similar to that reported previously,27 while CuCID-1 started to adsorb water at approximately P/P0 = 0.33 (Fig. 2a). In contrast, CoCID-1 and NiCID-1 did not adsorb water below P/P0 = 0.9. These results indicate that the adsorption behavior of MCID-1 changed considerably, although the hydrophobic cavity environment remained similar (see note S10 in the ESI†) and the metal ions and water molecules do not directly interact.
To understand the differences in these water-adsorption properties, we focused on the coordination environment of MCID-1 (Table S2, ESI†). The nodes of MCID-1 are dinuclear complexes, wherein the two metal ions are crystallographically equivalent and exhibit six-fold coordination structures (Fig. 2b and Fig. S10a, ESI†). The lengths of the six coordination bonds in CoCID-1 and NiCID-1 are almost the same (2.0–2.2 Å). Furthermore, the coordination geometries of CoCID-1 and NiCID-1 are similar to that of a symmetric ideal octahedron. However, for CuCID-1⊃H2O and ZnCID-1⊃H2O, the length of the bond between the metal atom and one of the oxygen atoms (M–O2) is approximately 2.6 Å, which is approximately 0.5 Å longer than that of the other bonds. In other words, the metal ion in MCID-1⊃H2O exhibits a distorted octahedral geometry. In the case of degassed ZnCID-1, the coordination distortion relaxes once the water molecules are removed. The space group of ZnCID-1 changes from C2/c to P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , which is similar to that of CoCID-1 and NiCID-1. However, the Cu–O2 bond length does not change after the removal of the guest, and CuCID-1 still shows coordination distortion, which is attributable to the Jahn–Teller effect of CuII. In addition, the space group of CuCID-1 is P21/c, which is different from that of the other degassed MCID-1 phases. These results suggest that the adsorption of water induces distortion of the coordination structure and the stability of the distorted coordination structure depends on the type of metal ion used.
, which is similar to that of CoCID-1 and NiCID-1. However, the Cu–O2 bond length does not change after the removal of the guest, and CuCID-1 still shows coordination distortion, which is attributable to the Jahn–Teller effect of CuII. In addition, the space group of CuCID-1 is P21/c, which is different from that of the other degassed MCID-1 phases. These results suggest that the adsorption of water induces distortion of the coordination structure and the stability of the distorted coordination structure depends on the type of metal ion used.
In contrast, the crystal structure of MCID-1⊃H2O suggests that the guest water molecules form 1D chains through hydrogen-bonding interactions within the pores. In addition, one of the water molecules (O4w) in the 1D chain forms a hydrogen bond with the oxygen atom of the carboxylate of ip (O1) (Fig. 2c and Fig. S10b, ESI†). It is likely that this host–guest interaction leads to coordination distortion, resulting in the structural transition. These results suggest that the coordination structure around the metal ion requires this distortion to interact with O4w, after which the coordination bond length of M–O2 increases. In other words, the distortion of the coordination bond is important for the formation of the hydrogen bond between O1 and O4w. Furthermore, it is likely that the differences in stabilities of the distorted structures corresponding to the various metal ions determine the water-adsorption properties of MCID-1.
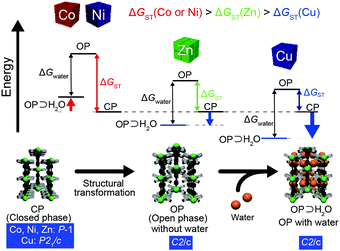
We propose an energy diagram to explain the metal-ion dependency of the water-adsorption behavior of MCID-1 (Fig. 3). We hypothesize that MCID-1 exhibits the following three phases: a closed phase (CP), imaginary open phase without water molecules (OP), and open phase with water (OP⊃H2O). In addition, two types of variations in energy are defined: one is the change in the Gibbs energy for structural transformation (ΔGST), which destabilizes OP⊃H2O, whereas the other is the change in the Gibbs energy for water-cluster formation (ΔGwater), which stabilizes OP⊃H2O. Initially, we assumed that the CP structure of the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) group of MCID-1 (M = Co, Ni, and Zn) and the CP structure of the P21/c group of CuCID-1 have the same Gibbs energies. Focusing on the value of ΔGST, for this assumption to be true, the coordination bonds must lengthen and coordination structure must distort. We believe that the ΔGST value of CuCID-1 is the smallest because the distorted coordination structure of CuII is stable even in the absence of guest molecules, owing to the Jahn–Teller effect. In contrast, since the symmetric coordination structures of NiCID-1 and CoCID-1 do not contain water molecules, their ΔGST values are expected to be very large, which is mostly due to the absence of the Jahn–Teller effect. Furthermore, the ΔGST value of ZnCID-1 is expected to be moderately high and the distorted coordination structure is somewhat stable because of the isotropic d10 electron structure of ZnII ion. In addition, we assume that similar water chain structures are formed in all MCID-1 samples. Therefore, their ΔGwater values are also the same. As shown in Fig. 3, CuCID-1 and ZnCID-1 are stabilized by the water chain, and the as-synthesized compounds are MCID-1⊃H2O (M = Cu, and Zn). Furthermore, this model suggests that CuCID-1⊃H2O is more stable than ZnCID-1⊃H2O, which is consistent with the lower onset pressure observed in the CuCID-1 water-adsorption isotherm. However, CoCID-1 and NiCID-1 are destabilized after water adsorption, as their ΔGST values are high. Thus, they cannot form OP⊃H2O due to this type of destabilization. The proposed energy diagram strongly suggests that coordination distortion is essential for the adsorption of water into the pores. Furthermore, the onset pressure depends on the stability of the distorted coordination structure, and distortion is accompanied by a structural transformation. Therefore, the adsorption behavior of MCID-1 is strongly affected by the type of central metal ion present.
group of MCID-1 (M = Co, Ni, and Zn) and the CP structure of the P21/c group of CuCID-1 have the same Gibbs energies. Focusing on the value of ΔGST, for this assumption to be true, the coordination bonds must lengthen and coordination structure must distort. We believe that the ΔGST value of CuCID-1 is the smallest because the distorted coordination structure of CuII is stable even in the absence of guest molecules, owing to the Jahn–Teller effect. In contrast, since the symmetric coordination structures of NiCID-1 and CoCID-1 do not contain water molecules, their ΔGST values are expected to be very large, which is mostly due to the absence of the Jahn–Teller effect. Furthermore, the ΔGST value of ZnCID-1 is expected to be moderately high and the distorted coordination structure is somewhat stable because of the isotropic d10 electron structure of ZnII ion. In addition, we assume that similar water chain structures are formed in all MCID-1 samples. Therefore, their ΔGwater values are also the same. As shown in Fig. 3, CuCID-1 and ZnCID-1 are stabilized by the water chain, and the as-synthesized compounds are MCID-1⊃H2O (M = Cu, and Zn). Furthermore, this model suggests that CuCID-1⊃H2O is more stable than ZnCID-1⊃H2O, which is consistent with the lower onset pressure observed in the CuCID-1 water-adsorption isotherm. However, CoCID-1 and NiCID-1 are destabilized after water adsorption, as their ΔGST values are high. Thus, they cannot form OP⊃H2O due to this type of destabilization. The proposed energy diagram strongly suggests that coordination distortion is essential for the adsorption of water into the pores. Furthermore, the onset pressure depends on the stability of the distorted coordination structure, and distortion is accompanied by a structural transformation. Therefore, the adsorption behavior of MCID-1 is strongly affected by the type of central metal ion present.
The metal-based solid solution approach is a recently developed method for precisely controlling the flexibility of a MOF.35 With the aim of controlling the adsorption behavior of MCID-1, we evaluated the adsorption behavior of solid-solution-type CID-1 (M1xM21−xCID-1). We synthesized solid-solution-type M1xM21−xCID-1 with various metal ratios under the same synthesis conditions as those used to prepare MCID-1, with the only difference being the ratio of M1 and M2 (M1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M2
M2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2ip
Na2ip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bpy = x
bpy = x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 − x
1 − x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
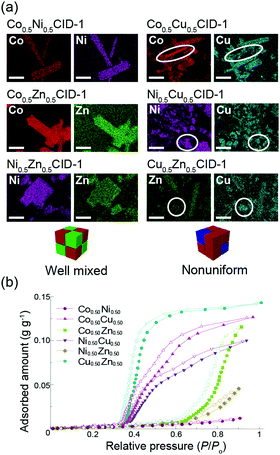
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; x = 0.15, 0.33, 0.50, 0.67, and 0.85). The XRPD patterns and scanning electron microscopy-energy-dispersive X-ray spectroscopy (SEM-EDX) maps show that Cu-containing CID-1 (CuxM1−xCID-1) does not form a uniform solid-solution-type CID-1 at any metal ratio. However, other metal combinations yielded well-mixed M1xM21−xCID-1 (Fig. 4a; also see notes S12–S15 in the ESI†). To evaluate the distribution structures, we measured their magnetic susceptibilities.36 The results suggest that M0.5Zn0.5CID-1 (M = Co and Ni) forms homo-metal dinuclear structures. However, Co0.5Ni0.5CID-1 exhibited a randomly mixed hetero- and homo-metal dinuclear structure (see note S16 in the ESI†).
1; x = 0.15, 0.33, 0.50, 0.67, and 0.85). The XRPD patterns and scanning electron microscopy-energy-dispersive X-ray spectroscopy (SEM-EDX) maps show that Cu-containing CID-1 (CuxM1−xCID-1) does not form a uniform solid-solution-type CID-1 at any metal ratio. However, other metal combinations yielded well-mixed M1xM21−xCID-1 (Fig. 4a; also see notes S12–S15 in the ESI†). To evaluate the distribution structures, we measured their magnetic susceptibilities.36 The results suggest that M0.5Zn0.5CID-1 (M = Co and Ni) forms homo-metal dinuclear structures. However, Co0.5Ni0.5CID-1 exhibited a randomly mixed hetero- and homo-metal dinuclear structure (see note S16 in the ESI†).
Next, we acquired the water-sorption isotherms of M1xM21−xCID-1 at 298 K to evaluate the effects of the type of metal ion in the solid solution, which revealed that the onset pressure changes gradually with metal-ion ratio (see note S17 in the ESI†). The isotherms for ZnxM1−xCID-1 (M = Co and Ni) suggest that the amount of water adsorption increases gradually with increasing x, while the isotherms for CuxM1−xCID-1 (M = Co, Ni, and Zn) indicate stepwise adsorption, i.e., the adsorption has two components, which is consistent with the heterogeneous distribution of Cu ions observed in the SEM-EDX map in Fig. 4a. These results suggest that the onset pressure and adsorption amount can be controlled by mixing the metal ions. With respect to the amount of water adsorbed by M10.5M20.5CID-1 (Fig. 4b), the amount of adsorbed water by Co0.5Zn0.5CID-1 was larger than that of Ni0.5Zn0.5CID-1, which is attributable to the weak Jahn–Teller effect of the CoII (d7) metal ion, which aids distortion and structural transformation as a consequence.
In summary, we demonstrated that the water-adsorption behavior of MCID-1 (M = Co, Ni, Cu, and Zn) strongly depends on the type of metal ion. The formation of 1D water chain-like structures is the key factor that determines the adsorption of water within the hydrophobic pores. In addition, Jahn–Teller stabilization of the distorted coordination structure enables water adsorption at lower pressures. Further, the adsorbed amount and uptake pressure of water are determined by the composition of the M1M2CID-1 solid solution. This study provides a new strategy for controlling the water-adsorption properties of MOFs based on their design.
D. T. acknowledges the PRESTO program (Grant No. JPMJPR17NA) of the Japan Science and Technology Agency (JST) and the JSPS for KAKENHI Grant No. 20H02577. H. Y. acknowledges the JSPS for KAKENHI Grant No. 20H04680, 20H04646, and 18H04528. This work was performed under the GIMRT program of the Institute for Materials Research, Tohoku University (Proposal No. 18K0063). We thank Prof. R. Matsuda for his helpful discussion.
Conflicts of interest
There are no conflicts to declare.References
- M. J. Kalmutzki, C. S. Diercks and O. M. Yaghi, Adv. Mater., 2018, 30, e1704304 CrossRef PubMed.
- J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Chem. Soc. Rev., 2014, 43, 5594 RSC.
- Z. Chen, P. Li, X. Zhang, P. Li, M. C. Wasson, T. Islamoglu, J. F. Stoddart and O. K. Farha, J. Am. Chem. Soc., 2019, 141, 2900 CrossRef CAS PubMed.
- C. Wang, X. Liu, N. Keser Demir, J. P. Chen and K. Li, Chem. Soc. Rev., 2016, 45, 5107 RSC.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Science, 2017, 356, 430 CrossRef CAS PubMed.
- X. Yang and D. Yan, J. Mater. Chem. C, 2017, 5, 7898 RSC.
- D. Yan, Y. Tang, H. Lin and D. Wang, Sci. Rep., 2014, 4, 4337 CrossRef PubMed.
- X. Yang and D. Yan, Chem. Sci., 2016, 7, 4519 RSC.
- X. Yang and D. Yan, Adv. Opt. Mater., 2016, 4, 897 CrossRef CAS.
- Y. Yang, K.-Z. Wang and D. Yan, ACS Appl. Mater. Interfaces, 2016, 8, 15849 Search PubMed.
- E. Soubeyrand-Lenoir, C. Vagner, J. W. Yoon, P. Bazin, F. Ragon, Y. K. Hwang, C. Serre, J. S. Chang and P. L. Llewellyn, J. Am. Chem. Soc., 2012, 134, 10174 CrossRef CAS PubMed.
- A. O. Yazaydin, A. I. Benin, S. A. Faheem, P. Jakubczak, J. J. Low, R. R. Willis and R. Q. Snurr, Chem. Mater., 2009, 21, 1425 CrossRef CAS.
- S. S. Nagarkar, S. M. Unni, A. Sharma, S. Kurungot and S. K. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2638 CrossRef CAS PubMed.
- J. M. Taylor, K. W. Dawson and G. K. Shimizu, J. Am. Chem. Soc., 2013, 135, 1193 CrossRef CAS PubMed.
- R. G. AbdulHalim, P. M. Bhatt, Y. Belmabkhout, A. Shkurenko, K. Adil, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2017, 139, 10715 CrossRef CAS PubMed.
- P. Guo, A. G. Wong-Foy and A. J. Matzger, Langmuir, 2014, 30, 1921 CrossRef CAS PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062 RSC.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695 CrossRef CAS PubMed.
- G. Ferey and C. Serre, Chem. Soc. Rev., 2009, 38, 1380 RSC.
- A. Samokhvalov, Chem. – Eur. J., 2015, 21, 16726 CrossRef CAS PubMed.
- W. Xuan, C. Zhu, Y. Liu and Y. Cui, Chem. Soc. Rev., 2012, 41, 1677 RSC.
- P. Silva, S. M. Vilela, J. P. Tome and F. A. Almeida Paz, Chem. Soc. Rev., 2015, 44, 6774 RSC.
- D. Tanaka, K. Nakagawa, M. Higuchi, S. Horike, Y. Kubota, T. C. Kobayashi, M. Takata and S. Kitagawa, Angew. Chem., Int. Ed., 2008, 47, 3914 CrossRef CAS PubMed.
- A. X. Zhu, Q. Y. Yang, S. Mukherjee, A. Kumar, C. H. Deng, A. A. Bezrukov, M. Shivanna and M. J. Zaworotko, Angew. Chem. Int. Ed., 2019, 58, 18212 CrossRef CAS PubMed.
- A. Kondo, H. Kajiro, H. Noguchi, L. Carlucci, D. M. Proserpio, G. Ciani, K. Kato, M. Takata, H. Seki, M. Sakamoto, Y. Hattori, F. Okino, K. Maeda, T. Ohba, K. Kaneko and H. Kanoh, J. Am. Chem. Soc., 2011, 133, 10512 CrossRef CAS PubMed.
- J. Canivet, J. Bonnefoy, C. Daniel, A. Legrand, B. Coasne and D. Farrusseng, New J. Chem., 2014, 38, 3102 RSC.
- S. Horike, D. Tanaka, K. Nakagawa and S. Kitagawa, Chem. Commun., 2007, 3395 RSC.
- S. Horike, M. Sugimoto, K. Kongpatpanich, Y. Hijikata, M. Inukai, D. Umeyama, S. Kitao, M. Seto and S. Kitagawa, J. Mater. Chem. A, 2013, 1, 3675 RSC.
- C. Ma, C. Chen, Q. Liu, D. Liao, L. Li and L. Sun, New J. Chem., 2003, 27, 890 RSC.
- L.-F. Song, C.-H. Jiang, C.-L. Jiao, J. Zhang, L.-X. Sun, F. Xu, W.-S. You, Z.-G. Wang and J.-J. Zhao, Cryst. Growth Des., 2010, 10, 5020 CrossRef CAS.
- J. Tao, X.-M. Chen, R.-B. Huang and L.-S. Zheng, J. Solid State Chem., 2003, 170, 130 CrossRef CAS.
- Y.-H. Wen, J.-K. Cheng, Y.-L. Feng, J. Zhang, Z.-J. Li and Y.-G. Yao, Inorg. Chim. Acta, 2005, 358, 3347 CrossRef CAS.
- Y. Kamakura, N. Hosono, A. Terashima, S. Kitagawa, H. Yoshikawa and D. Tanaka, ChemPhysChem, 2018, 19, 2134 CrossRef CAS PubMed.
- T. Ohba, H. Kanoh and K. Kaneko, J. Am. Chem. Soc., 2004, 126, 1560 CrossRef CAS PubMed.
- J. Zhang, W. Kosaka and H. Miyasaka, Chem. Lett., 2019, 48, 1308 CrossRef CAS.
- G. Mali, M. Mazaj, I. Arcon, D. Hanzel, D. Arcon and Z. Jaglicic, J. Phys. Chem. Lett., 2019, 10, 1464 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental information, structural figures and tables, additional data. CCDC 1978140. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc03772a |
| This journal is © The Royal Society of Chemistry 2020 |