Flexible and wearable strain sensors based on tough and self-adhesive ion conducting hydrogels†
Zhenwu
Wang
ab,
Jing
Chen
 b,
Liufang
Wang
b,
Guorong
Gao
b,
Yang
Zhou
b,
Rong
Wang
b,
Ting
Xu
b,
Jingbo
Yin
b,
Liufang
Wang
b,
Guorong
Gao
b,
Yang
Zhou
b,
Rong
Wang
b,
Ting
Xu
b,
Jingbo
Yin
 *a and
Jun
Fu
*a and
Jun
Fu
 *b
*b
aSchool of Materials Science and Engineering, Shanghai University, 99 Shangda Road, Shanghai 200444, People's Republic of China. E-mail: jbyin@oa.shu.edu.cn
bCixi Institute of Biomedical Engineering & Polymers and Composites Division, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Zhongguan West Road 1219, Zhenhai District, Ningbo, 315201, People's Republic of China. E-mail: fujun@nimte.ac.cn
First published on 19th November 2018
Abstract
Inspired by biosystem, ionic hydrogels have been extensively studied as promising materials for wearable or implantable devices. Herein, we report novel ionic hydrogels that comprise dynamically crosslinked polyzwitterion and physically crosslinked polyvinyl alcohol, which demonstrate excellent mechanical properties, repeatable self-adhesion, and high and linear strain sensitivity. The obtained hydrogels can be directly attached to human skin as sensors to detect or monitor physiological signals.
Introduction
Tremendous efforts have been devoted to develop soft electronic devices to meet the growing demands of high-performance and multi-functionalities of flexible electronics.1–4 Flexible and wearable strain sensors have been successfully developed for electronic skin,5,6 human–machine interfaces,7 biomedical implants,8,9 and human activity monitors.10–12 Currently, most strain sensors comprising elastomer matrix with metal particles/liquid,13–16 carbon materials,17 or conductive polymers18–21 have been successfully fabricated. However, most of such strain sensors usually present low fracture strain (<200%), whereas there is a need to develop sensors with much larger extensibility. Moreover, self-adhesive and adaptive strain sensors are desired for intimate contact to various object surfaces, particularly to skin, to ensure stable signal detection under repeated deformations.17,22Inspired by biosystems, where signal transmission depends on long-distance ion transport, ionic hydrogels have been extensively researched as promising materials for wearable or implantable devices due to their excellent biocompatibility and intrinsic flexibility.6,23 Wan et al. combined covalently cross-linked poly(vinyl alcohol) (PVA) and poly(vinyl pyrrolidone) with Fe3+ cross-linked cellulose nanocrystal networks to form ionic hydrogels to monitor human physiological signals with a high strain sensitivity (0.478).24 Yang et al. blended tannic acid-coated cellulose nanocrystals, poly(acrylic acid) chains and metal ions in a covalent polymer network to produce ionic hydrogels with multiple coordination, which showed strain sensitivity of 7.8 at 2000% strain.22 However, only a few ionic hydrogels with remarkable sensitivity to strain have been reported,25,26 where the gel deformation has negligible influence on ion transport.24–26 In addition, most reported wearable sensors based on ionic hydrogels present nonlinear sensitivity; thus, their high sensitivity is usually limited to a narrow sensing range.22 Moreover, the addition of polyzwitterionic chains into the ionic conducting system significantly enhances the conductivity27 since the zwitterionic moieties help the separation of ions during ion migration and thus improve the ionic conductivity.28 Therefore, zwitterionic hydrogels have been recognized as promising candidates for lithium metal batteries,27 supercapacitors,28 capacitive sensors,25,26etc.
Nevertheless, zwitterionic hydrogels have been rarely utilized as strain sensors. The capability of polyzwitterionic chains to assist ion transportation may be sensitive to the strain. Wu et al. have demonstrated non-covalent crosslinking of polyzwitterionic chains to form gels through supramolecular interactions between zwitterionic units;25 however, the gels are usually weak. Although such polyzwitterionic gels have been fabricated in supercapacitors for sensing,26 few reports on polyzwitterionic hydrogels as resistance strain sensors can be found in literature. A major obstacle may be the poor mechanical properties of such gels. Chemical crosslinking and compositing may improve the mechanical properties of the hydrogels but can largely constrain the mobility of the polyzwitterionic chains, which is unfavourable for the sensitivity of the hydrogel sensors.22
Here, we demonstrate novel tough semi-interpenetrating hydrogels comprising linear zwitterionic polymers within the physically crosslinked PVA network. The zwitterionic (poly[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl), PSBMA) chains are employed to provide ionic conductivity, whereas the freeze-thawed PVA matrix serves as a strong support. The electrostatic dipole–dipole interactions25,26,29 between the SBMA moieties and intermolecular hydrogen bonding between SBMA and PVA chains reinforce hydrogels. Besides, the zwitterionic groups on the PSBMA chains serve as a continuous ionic conductive network. The ion conductive hydrogels are sensitive to strains, showing high and linear sensitivity to stretching over a wide strain range. Moreover, the zwitterionic chains on the surface of hydrogels provide electrostatic or dipole–dipole interactions with various substrates, which can enable repeated adhesion to substrates. The hydrogels are used to monitor human motions by direct contact with the skin. The results suggest the promising applications of the gels as wearable strain sensors for human motion detection.
Results and discussion
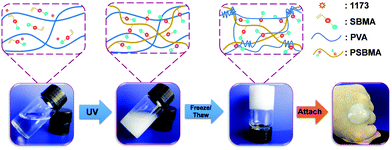
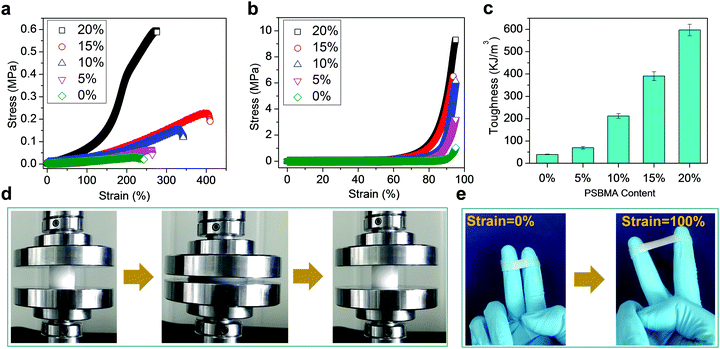
Fig. 1 schematically illustrates the synthesis of conductive hydrogels. First, PSBMA was synthesized in the PVA solution through free radical polymerization of SBMA monomers initiated by UV (Experimental section in ESI†). The transparent mixture became turbid and viscous with the formation of micrometre-sized aggregates of PSBMA (Fig. S1a, ESI†). The aggregation is due to the formation of intra- and interchain ionic contacts, resulting in an ionically cross-linked network.30 Subsequently, the PVA and PSBMA mixture solution was frozen and thawed to obtain physically crosslinked hydrogels with a semi-interpenetrating structure (Fig. S2, ESI†). Therein, the PSBMA aggregates are encapsulated in the PVA network (Fig. S1b, ESI†). The obtained hydrogel could be intimately attached onto the metacarpal joint without any gap, showing very good adhesion and adaptation. It is noted that hydrogels frozen and thawed for more time became rigid and lost the adhesion and adaptation properties. A relatively moderate cross-linking network is important for fabricating self-adhesive and adaptive strain sensors.Fig. 2a shows representative tensile stress–strain curves of hydrogels with various PSBMA contents (from 0% to 20%). The ultimate tensile strength (UTS) increases from 0.032 MPa (0%) to 0.066 MPa (5%), 0.153 MPa (10%), 0.228 MPa (15%) and 0.596 MPa (20%), whereas the fracture strain (FS) increases from 227% to 260% (5%), 330% (10%), 400% (15%) and 275 (20%). Interestingly, UTS and FS are both enhanced with increasing content of PSBA in 10% PVA solution presumably due to the electrostatic interactions and hydrogen bonds provided by PSBMA chains25 (Fig. S3, ESI†). Thus, the corresponding toughness of PVA/PSBMA hydrogels or the area under the stress–strain curves is highly enhanced with increasing PSBMA content (Fig. 2b). The highest toughness (596 kJ m−3) of composite hydrogels is 18 times higher than that of pure PVA hydrogel. Besides, the corresponding compression strength is remarkably improved from 1.039 MPa (0%) to 3.21 MPa (5%), 6.14 MPa (10%), 6.50 MPa (15%) and 9.30 MPa (20%) (Fig. 2c). The hydrogels compressed to 80% strain can fully recover after unloading (Fig. 2d).
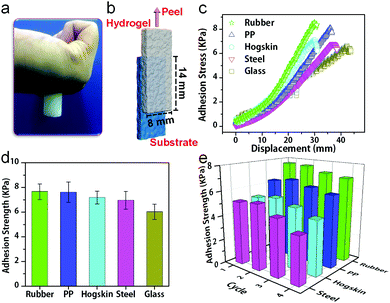
Moreover, the hydrogels can stick onto many substrates and sustain cyclic stretching with large strains. For example, the hydrogels with 15 wt% PSBMA formed a stable adhesion to gloves, and no detachment occurred during cyclic stretching at 100% strain (Fig. 2e). The recoverability is mainly ascribed to dynamic electrostatic interactions within the networks, whereas the electrostatic and/or dipole–dipole interactions at the interface may provide good adhesion. In fact, PVA/PSBMA hydrogels are adhesive to many substrates including skin, polypropylene, glass, varnish, polyethylene, rubbers and steel (Fig. 3a and Fig. S4a–f, ESI†). The adhesive and soft hydrogels formed conformal adhesion to the substrate, which is extremely important for accurate acquisition of signals. Such adhesion is stable and sustains vigorous shaking (Movie S1, ESI†).
The adhesion strength was measured by the lap shear test. The hydrogel sheet was placed on the substrate with an 8 mm × 14 mm contacting area. The substrate was fixed, whereas the hydrogel sheet was stretched at 100 mm min−1 (Fig. 3b). The hydrogel with 15 wt% PSBMA showed the strongest adhesion to rubber (Fig. S5, ESI†) and was used for subsequent studies unless otherwise specified. Fig. 3c shows representative shear load–displacement curves of hydrogels with various substrates. The adhesion strengths of the hydrogels with rubber, PP, hogskin, steel and glass were measured (Fig. 3c), and a maximum value was obtained with rubber (7.66 kPa). This could be due to the roughness of rubber, which provides the largest contact area with hydrogels. Besides, the synergy of electrostatic interaction and hydrogen bonding at the gel and substrate interface may play a critical role for adhesion. Moreover, hydrophobic interaction is probably another reason for the adhesion of hydrogels on PP (7.61 kPa).
Furthermore, cyclic lap shear tests demonstrate repeatable adhesion with substrates, where the adhesion strength remains almost constant for at least four cycles (Fig. 3e). For example, the adhesion strength with the hogskin only decreases from 5.22 kPa for the first test to 4.87 kPa for the fourth test or 10%, which is in sharp contrast to the 30% decline for the adhesive strain sensors after three peel-off/adhesion cycles reported previously.22 The current stable and repeated adhesion may be due to the interactions between the charged groups on skin and the zwitterionic groups in the gels. These results suggest the feasibility of the hydrogels as wearable sensors for repeated use. The highly repeatable adhesion with various substrates is quite desirable in practical applications such as correction during assembly, adjustment in the course of use, recovery and reuse after service.31,32
The conductivity of PVA/PSBMA hydrogels increases with the PSBMA content from 10.1 ± 0.2 mS m−1 (5%) to 33.7 ± 1.1 mS m−1 (10%), 42.9 ± 2.0 mS m−1 (15%) and 51.0 ± 1.8 mS m−1 (20%) (Fig. S6, ESI†), which is close to the conductivity of lithium copolymer polyzwitterion systems (0.056 S m−1).27 The polyzwitterionic chains in the network serve as ion transport carrier networks to achieve ion conductivity.
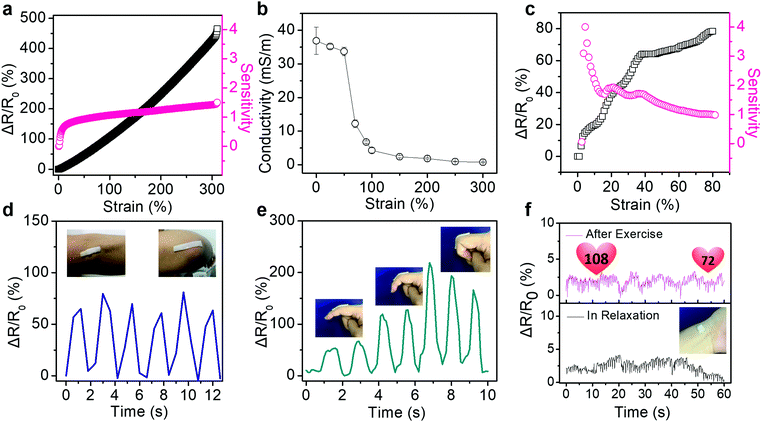
Such PVA/PSBMA hydrogels with reliable and repeatable adhesion behaviour, favourable conductivity and excellent mechanical properties are sensitive to strains. The resistance change ratio upon loading is defined as ΔR/R0 = |R0 − R|/R0, where R0 and R are the initial resistance and that under strain. Besides, the strain sensitivity or gauge factor (GF) is defined as GF = (ΔR/R0)/ε, where ε is the strain. Fig. 4a shows an unprecedented linear increase in the resistance change ratio during tensile strain (0–300%) for hydrogels with 10% PSBMA. GF of the sensor increased from 0.8 to 1.5 within 0–50% strain. However, over 50% strain, GF increased to 1.5 and remained constant, and it was higher than that of ionic gels reported previously (1.0 at 0–80% strain).22 Importantly, in the strain range of 0–300%, the hydrogel sensor shows linear sensitivity to the strain, which provides an excellent working range for sensors in practical applications.
On the other hand, the conductivity of hydrogels is sensitive to the tensile strain. Fig. 4b shows that the conductivity of hydrogels decreased slightly from 36.8 ± 4.1 mS m−1 to 33.7 ± 1.3 mS m−1 at strain of 0–50% and declined sharply to 12.2 ± 1.2 mS m−1 at 70% strain. This small drop at low strain may be caused by the disentanglement of PSBMA chains under low tensile strain. Moreover, the dependence of resistance change ratio and sensitivity of hydrogels with various contents of PSBMA on the tensile strain are also shown in Fig. S7 (ESI†). GF of hydrogels with 5 wt% PSBMA showed a sharp rise from 0.07 to 0.8 at 0–15% strain. However, the hydrogels with 15% PSBMA required larger strain to obtain a comparatively high sensitivity (0.3 at strain of 139%, Fig. S7b, ESI†). Furthermore, hydrogels with 20 wt% PSBMA showed quite low sensitivity to the tensile strain (<0.4, Fig. S7c, ESI†). Below the strain of 120%, the resistance of hydrogels remained almost unchanged. When the strain was over 120%, the resistance change rate of hydrogel increased significantly (GF = 0.1). Hydrogels with more PSBMA required a larger strain to attain high sensitivity, which may confirm the influence of disentanglement of PSBMA chains on the resistance change ratio. Additionally, the relative resistance changes and sensitivity upon compression were measured (Fig. 4c). The PVA/PSBMA hydrogels show the largest sensitivity of 4 at 3% strain, which is adequate for potential applications in human-activity monitoring.33 Furthermore, these hydrogel strain sensors exhibit outstanding insensitivity to other mechanical disturbance. For example, as the hydrogels are bent or twisted, the resistance change ratios are very low (less than 5%, Fig. S8, ESI†). The insensitivity to bending and twisting is favourable for strain sensors in practical applications, which can minimize the influence from external disturbance.
The ionic conductive hydrogels have been demonstrated as sensors to detect or monitor various human motions in real time. The hydrogels were attached onto the skin directly, and the resistance change ratios upon movements were recorded. For example, the hydrogel bar adhered on the elbow generated cyclically changing ΔR/R0 values as the elbow was cyclically bent and stretched (Fig. 4d). The output signals show a strong correlation with the elbow movements. The data captured by the strain sensor could help the basketball player to improve the shooting posture. Fig. 4e shows the detection of finger bending at 30°, 60° and 90°. The ΔR/R0 values of the sensor increased with the bending angle, which suggests possible quantitative detection of the bending angle of fingers. Furthermore, the sensor could be used in the compression mode to detect very weak vibrations. Fig. 4f compares the real-time electronic signals from the sensor attached on the wrist of a volunteer before and after exercise. It clearly displays typical pulse shapes and frequency increase from 60 min−1 at rest to 108 min−1 after exercise. Moreover, the top curve (after exercise) exhibits a gradual decay of the beating rate from 108 min−1 to 72 min−1 in one minute, demonstrating the gradual calming down of the body after exercise.34,35 These results indicate the outstanding immediate, sensitive and precise response of hydrogel sensors to subtle vibrations.
The PVA/PSBMA hydrogels show remarkable potential for wearable sensors with linear and high sensitivity, repeatable adhesion and admirable mechanical properties. In contrast to the poor mechanical properties of most polyzwitterionic hydrogels with chemical crosslinking,25,26 the non-covalent interactions between the PVA and PSBMA chains are ascribed to these outstanding properties. First, the high extensibility of the PSBMA/PVA hydrogels enables high and linear strain sensitivity over a large strain range (GF = 1.5 for 0–300% of strain) and repeatable adhesion (only 10% decline of adhesion strength for 4 cycles), which were otherwise not accessible for the previously reported wearable sensors based on cellulose nanocomposite ionic hydrogels.22 To achieve robust adhesion of conductive hydrogels with substrates, strategies including mussel-inspired catechol modifications have been used,36,37 which usually involve oxidation failure and pH limitation in practical applications.32,38,39 To this end, the polyzwitterionic chains are advantageous because no chemical modifications are needed to gain self-adhesion, particularly at low modulus. Such robust adhesion can be repeated for a few times due to the non-covalent nature of interface interactions.40 With excellent linearity of the hydrogels and robust adhesion, the ion conductive hydrogels may find applications not only in strain sensors, but also as implantable sensors considering the biocompatibility of both PVA and PSBMA chains.
Conclusions
In conclusion, we have designed and prepared novel tough and adhesive ionic hydrogels comprising interpenetrating networks of PSBMA and PVA. The hydrogels demonstrate linear and high sensitivity (GF = 1.5) at large strain (0–300%) and remarkable repeatable adhesiveness (10% decline after 4 cycles) with various substrates including rubbers, PP, steel and skin. These outstanding strengths provide accurate and reliable detections of subtle physiological signals such as bending of various joints and wrist pulse before and after exercise. This hydrogel shows tremendous potential in applications such as ionic skin, human/machine interactions, biomedical implants, and human heath monitors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51873224, 51603220, 51803227), Natural Science Foundation of Zhejiang Province (LQ16E030002, LY17E030011), and Natural Science Foundation of Ningbo (2017A610057, 2016A610255).Notes and references
- X. Wang, Z. Liu and T. Zhang, Small, 2017, 13, 1602790 CrossRef PubMed.
- D. Chen and Q. Pei, Chem. Rev., 2017, 117, 11239–11268 CrossRef CAS PubMed.
- S. Zhao, J. Li, D. Cao, G. Zhang, J. Li, K. Li, Y. Yang, W. Wang, Y. Jin, R. Sun and C.-P. Wong, ACS Appl. Mater. Interfaces, 2017, 9, 12147–12164 CrossRef CAS PubMed.
- C. S. Luo, P. Wan, H. Yang, S. A. A. Shah and X. Chen, Adv. Funct. Mater., 2017, 27, 1606339 CrossRef.
- H.-H. Chou, A. Nguyen, A. Chortos, J. W. F. To, C. Lu, J. Mei, T. Kurosawa, W.-G. Bae, J. B. H. Tok and Z. Bao, Nat. Commun., 2015, 6, 8011 CrossRef CAS PubMed.
- J. Y. Sun, C. Keplinger, M. Whitesides George and Z. Suo, Adv. Mater., 2014, 26, 7608–7614 CrossRef CAS PubMed.
- G. Shi, Z. Zhao, J. H. Pai, I. Lee, L. Zhang, C. Stevenson, K. Ishara, R. Zhang, H. Zhu and J. Ma, Adv. Funct. Mater., 2016, 26, 7614–7625 CrossRef CAS.
- A. Biswas, L. R. Bornhoeft, S. Banerjee, Y.-H. You and M. J. McShane, ACS Sens., 2017, 2, 1584–1588 CrossRef CAS PubMed.
- H. Huang, S. Chauhan, J. Geng, Y. Qin, D. F. Watson and J. F. Lovell, Biomacromolecules, 2017, 18, 562–567 CrossRef CAS PubMed.
- L. Han, X. Lu, M. Wang, D. Gan, W. Deng, K. Wang, L. Fang, K. Liu, W. Chan Chun, Y. Tang, L. T. Weng and H. Yuan, Small, 2016, 13, 1601916 CrossRef PubMed.
- Y. Liu, Y. Hu, J. Zhao, G. Wu, X. Tao and W. Chen, Small, 2016, 12, 5074–5080 CrossRef CAS.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. Izadi-Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296 CrossRef CAS PubMed.
- N. Lu, C. Lu, S. Yang and J. Rogers, Adv. Funct. Mater., 2012, 22, 4044–4050 CrossRef CAS.
- J. A. Rogers, T. Someya and Y. Huang, Science, 2010, 327, 1603 CrossRef CAS PubMed.
- Z. Yu, X. Niu, Z. Liu and Q. Pei, Adv. Mater., 2011, 23, 3989–3994 CrossRef CAS PubMed.
- C. Yan, J. Wang, X. Wang, W. Kang, M. Cui, Y. Foo Ce and S. Lee Pooi, Adv. Mater., 2013, 26, 943–950 CrossRef PubMed.
- M. Liao, P. Wan, J. Wen, M. Gong, X. Wu, Y. Wang, R. Shi and L. Zhang, Adv. Funct. Mater., 2017, 27, 1703852 CrossRef.
- Y. Y. Lee, H. Y. Kang, H. Gwon Seok, M. Choi Gwang, S. M. Lim, J. Y. Sun and Y. C. Joo, Adv. Mater., 2015, 28, 1636–1643 CrossRef PubMed.
- F. Zhao, Y. Shi, L. Pan and G. Yu, Acc. Chem. Res., 2017, 50, 1734–1743 CrossRef CAS PubMed.
- C. L. Choong, M. B. Shim, B. S. Lee, S. Jeon, D. S. Ko, T. H. Kang, J. Bae, H. Lee Sung, K. E. Byun, J. Im, J. Jeong Yong, E. Park Chan, J. J. Park and U. I. Chung, Adv. Mater., 2014, 26, 3451–3458 CrossRef CAS.
- S. Bai, C. Sun, P. Wan, C. Wang, R. Luo, Y. Li, J. Liu and X. Sun, Small, 2014, 11, 306–310 CrossRef.
- C. Shao, M. Wang, L. Meng, H. Chang, B. Wang, F. Xu, J. Yang and P. Wan, Chem. Mater., 2018, 30, 3110–3121 CrossRef CAS.
- H. Chun and T. D. Chung, Annu. Rev. Anal. Chem., 2015, 8, 441–462 CrossRef CAS.
- Y.-J. Liu, W.-T. Cao, M.-G. Ma and P.-B. Wan, ACS Appl. Mater. Interfaces, 2017, 9, 25559–25570 CrossRef CAS PubMed.
- Z. Lei and P. Wu, Nat. Commun., 2018, 9, 1134 CrossRef.
- Z. Lei, Q. Wang, S. Sun, W. Zhu and P. Wu, Adv. Mater., 2017, 29, 1700321 CrossRef.
- C. Tiyapiboonchaiya, J. M. Pringle, J. Sun, N. Byrne, P. C. Howlett, D. R. MacFarlane and M. Forsyth, Nat. Mater., 2003, 3, 29 CrossRef.
- X. Peng, H. Liu, Q. Yin, J. Wu, P. Chen, G. Zhang, G. Liu, C. Wu and Y. Xie, Nat. Commun., 2016, 7, 11782 CrossRef CAS.
- C. K. Roy, H. L. Guo, T. L. Sun, A. B. Ihsan, T. Kurokawa, M. Takahata, T. Nonoyama, T. Nakajima and J. P. Gong, Adv. Mater., 2015, 27, 7344–7348 CrossRef CAS PubMed.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177–4190 CrossRef CAS PubMed.
- Y. Mengüç, S. Y. Yang, S. Kim, J. A. Rogers and M. Sitti, Adv. Funct. Mater., 2012, 22, 1246–1254 CrossRef.
- H. Yi, S. H. Lee, M. Seong, M. K. Kwak and H. E. Jeong, J. Mater. Chem. B, 2018 10.1039/C8TB02598C.
- T. Q. Trung and N.-E. Lee, Adv. Mater., 2016, 28, 4338–4372 CrossRef CAS PubMed.
- Z. Wang, H. Zhou, W. Chen, Q. Li, B. Yan, X. Jin, A. Ma, H. Liu and W. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 14045–14054 CrossRef CAS PubMed.
- Z. Wang, H. Zhou, J. Lai, B. Yan, H. Liu, X. Jin, A. Ma, G. Zhang, W. Zhao and W. Chen, J. Mater. Chem. C, 2018, 6, 9200–9207 CAS.
- W. Chen, R. Wang, T. Xu, X. Ma, Z. Yao, B. Chi and H. Xu, J. Mater. Chem. B, 2017, 5, 5668–5678 RSC.
- X. Zhao, M. Zhang, B. Guo and P. X. Ma, J. Mater. Chem. B, 2016, 4, 6644–6651 RSC.
- H. Yang, K. Liao, H. Huang, Q. Wu, L. Wan and Z. Xu, J. Mater. Chem. A, 2014, 2, 10225–10230 RSC.
- L. Han, L. Yan, K. Wang, L. Fang, H. Zhang, Y. Tang, Y. Ding, L.-T. Weng, J. Xu, J. Weng, Y. Liu, F. Ren and X. Lu, NPG Asia Mater., 2017, 9, e372 CrossRef CAS.
- W. Li, R. Feng, R. Wang, D. Li, W. Jiang, H. Liu, Z. Guo, M. J. Serpe and L. Hu, J. Mater. Chem. B, 2018, 6, 4799–4807 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/c8tb02629g |
| This journal is © The Royal Society of Chemistry 2019 |