Enhancing selectivity and efficiency in the electrochemical synthesis of adiponitrile†
Daniela E.
Blanco
,
Aaliyah Z.
Dookhith
and
Miguel A.
Modestino
 *
*
NYU Tandon School of Engineering, 6 Metrotech Center, Brooklyn, NY 11201, USA. E-mail: modestino@nyu.edu
First published on 15th November 2018
Abstract
Adiponitrile is a large scale chemical intermediate used in the production of Nylon 6,6. It is primarily produced via two methods: the thermal hydrocyanation of butadiene and the electrochemical hydrodimerization of acrylonitrile. The thermochemical method is an energy intensive process that involves acutely toxic reactants such as hydrogen cyanide. On the other hand, the electrosynthesis of adiponitrile is a green chemical process that uses water-based electrolytes and can be directly coupled with renewable electricity sources such as wind or sunlight. Although this process is the largest organic electrochemical process in industry, it still faces many challenges owing to its low energy conversion efficiency and selectivity. Using a systematic approach, this study provides insights into mass transport and kinetic factors that influence the reaction performance, and demonstrates that by careful control of the composition of the electrolyte, concentration of reactants, operating current densities, and temperature, selectivities as high as 83% can be achieved. Our results provide electrochemical engineering guidelines to significantly improve the efficiency of the electrochemical production of adiponitrile and open up opportunities to the direct implementation of renewable-energy sources in chemical manufacturing.
Introduction
The past decade has seen an immense increase in the deployment of renewable energy sources such as solar and wind energy, tripling its global electricity production capacity.1 This increase has been driven by a combination of world-wide initiatives to reduce the emission of greenhouse gases,2–4 and a precipitous yearly decrease of over 20% in prices of silicon photovoltaics and wind turbines.1,5,6 This paradigm shift in energy generation has led to an increased interest towards the electrification of the chemical industry.7–10 Such a transition from thermochemical to electrochemical processes would enable the direct coupling of green electricity sources with chemical manufacturing and result in a vast reduction of CO2 emissions. Currently, electrochemical processes account for only a small fraction of the chemical industry, and are dominated by chloro-alkali and Aluminum production. Organic electrochemical processes are significantly smaller in volume, but their potential impact is much larger, as their implementation could result in sustainable production processes for more than 35% of chemical products.11 To date, the electrochemical hydrodimerization of acrylonitrile (AN) to adiponitrile (ADN) is the largest and most successful organic electrochemical process implemented in industry.12ADN is a large volume precursor (>1.5 million tons per year) of hexamethylenediamine,13 which is used as a monomer in the manufacture of Nylon 6,6.14–16 In addition to the electrochemical route to ADN, two alternative thermochemical production methods exist: the hydrocyanation of 1,3 butadiene, and the dehydration of adipic acid in the presence of ammonia.17
The hydrocyanation of 1,3 butadiene is the most extensively used method for adiponitrile production, and consists of two stages carried out at temperatures from 30–150 °C (ref. 18–20) and pressures of 1 to 5 MPa.21,22 The main drawback of this method is that it requires large amounts of energy and the use of hydrogen cyanide, a highly toxic reactant. The ammoniation of adipic acid to adiponitrile is carried out in liquid (200–300 °C)23 or gas phase (300–350 °C),24 and suffers low selectivity due to self-cyclization side reactions that occur at high temperature.25
In the electrosynthesis of ADN, the reductive dimerization of AN takes place at the cathode surface (E0= −0.56 V, calculated as shown in the ESI†), while the oxygen evolution reaction occurs at the anode (Fig. 1).26–32 These reactions are carried in an aqueous electrolyte at mild temperatures and atmospheric pressure.33
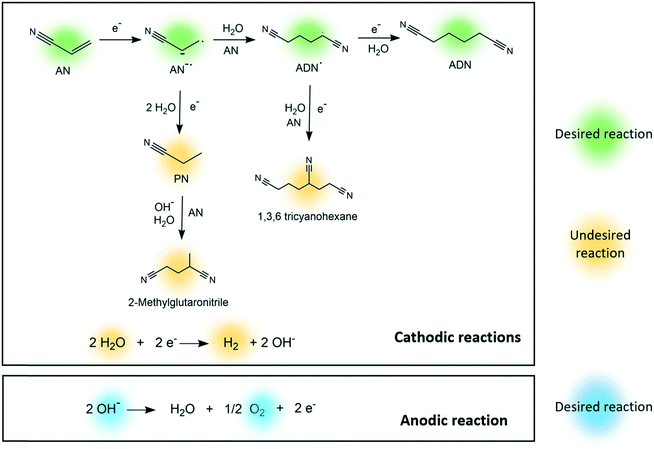 | ||
| Fig. 1 Proposed reaction pathways for the electrosynthesis of adiponitrile, cathodic side reactions, and anodic side reactions. | ||
Several competing side reactions can occur at the cathode surface, which include hydrogen evolution and the formation of organic by-products such as propionitrile (PN), 2-methylglutaronitrile (MGN), and 1,3,6 tricyanohexane, as presented in Fig. 1.34–38 The interest on improving this green production route is currently growing given that the reaction could be completed in one single step with high yield and energy efficiency, and can be easily integrated with renewable energy sources.29,39
For industrial applications, the most popular device configuration is an undivided electrochemical cell40–49 (Fig. S1 in the ESI†). This system is composed of a multiphase electrolyte with organic microdroplets of AN, which enhance mass transport of ADN into the electrolyte. Electrolyte composition has evolved from that first studied by Baizer and Danly27,28,41 which included large concentration of tetraalkylammonium (TAA) salts as supporting electrolyte to enhance the solubility of organic reagents. More recent ADN electrohydromerization reactors use aqueous electrolytes with phosphate ions for enhanced conductivity and pH buffering capacity. Ethylenediaminetetraacetic acid (EDTA) is added as a chelating agent for dissolved metal ions resulting from corrosion of the anode, and thus avoids their electroreduction at the cathode surface. TAA ions are used in small concentrations and play an important role in controlling the selectivity towards organic products and stabilizing the organic droplets in the aqueous electrolyte.50,51 Larger substituting alkyl groups on the ammonium ions are expected to exclude water molecules and protons from the electrical double layer (EDL), hindering the generation of PN and hydrogen, thus promoting the formation of ADN at the cathode.52 Lastly, lead and cadmium are commonly used as cathode materials due to their high overpotential for hydrogen evolution, which limits this competing reaction.52
Despite the advances achieved over the years, and the importance of the electrosynthesis of ADN in industry,12 the development of this route has been dominated by empirical studies attempting to optimize the performance of the ADN production process – most of these dating back several decades and based on earlier electrolyte compositions.27,28,41 It is generally accepted that the utilization of TAA ions and the control of AN concentration, pH, and temperature are critical elements to promote high selectivity towards ADN, but a thorough understanding of their effects on the mass transport, ionic resistance, and reaction kinetics is lacking. This study fills this knowledge gap by systematically identifying the effects of reactant concentration, reaction operation conditions, and the molecular size, concentration, and affinity of TAA ions towards AN, on the selectivity and efficiency of ADN production. This thorough and up-to-date assessment of the key performance drivers in the electrochemical production of ADN is essential to improve the competitiveness of the electrochemical route, which represents a greener alternative for the production of ADN that implements non-toxic solvents (water), more benign starting materials (i.e. acrylonitrile instead of hydrogen cyanide) which can be manufactured from biomass sources such as glycerol,53,54 is less energy intensive, and offers a clear opportunity for the direct incorporation of renewable energy sources such as wind and solar electricity into chemical manufacturing.
Experimental
Materials
All chemicals used for solution preparation were acquired from Sigma-Aldrich, including sodium phosphate, tetramethylammonium hydroxide, tetraethylammonium hydroxide, tetrapropylammonium hydroxide, tetrabutylammonium hydroxide, tetrahexylammonium hydroxide, EDTA disodium salt, and acrylonitrile.A fresh aqueous solution with 0.5 M (8 wt%) sodium phosphate, 0.03 M (1 wt%) EDTA, and each type of TAA ion at 0.004–0.05 M (0.1–1.2 wt%) was prepared before adding 0.2–3.8 M (1–20 wt%) AN for each experiment. The described aqueous solution was used as catholyte, and 1 M sulfuric acid was used as anolyte. Since the cathodic pH remained constant throughout the experiments, negligible permeation of acid through the membrane was assumed.
A 1 cm2 cadmium foil (American Elements) was used as working electrode, and a platinum mesh (Alfa Aesar) as counter electrode.
Electrochemical characterization
The effects of the TAA ions, AN concentration and reaction parameters on the cathodic half-cell reactions were studied using a 3-electrode setup with a continuously stirred electrolyte. Cyclic voltammetry (CV) experiments, electrochemical impedance spectroscopy (EIS), and chronoamperometry (CA) techniques were performed using a BioLogic VSP-300 potentiostat. CV experiments were carried out from 0 to −3.2 V vs. SHE at 50 mV s−1.All experiments were carried out in an H-cell (Fig. 2) fabricated using VeroClear Polyjet resin in a Stratasys Object30 Pro additive manufacturing tool. A Nafion N117 membrane was used to separate the two chambers, avoid the deposition of metal ions from the anode on the cathode surface, and study the limitations and side reactions taking place at the cathodic surface at compensated electrode potentials. Viton o-rings were used to seal the membrane between the two chambers, and a Ag/AgCl electrode in 4 M KCl solution was used as reference electrode. The cadmium electrode surface was operated at 40 mA cm−2 with catholyte solution for 10–12 hours prior to use in the experiments.
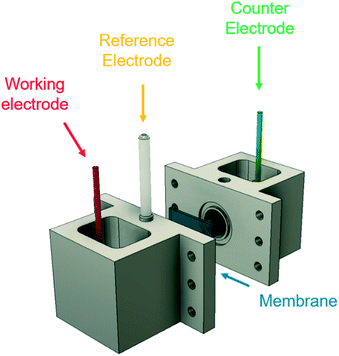 | ||
| Fig. 2 Experimental 3-electrode setup, showing the working electrode (cadmium), counter electrode (platinum), and Ag/AgCl reference electrode. A Nafion 117 membrane separates the two chambers. | ||
A constant electrolyte volume of 7 mL was used in all experiments, temperature was controlled using a hot plate and a sand bath, and the electrolyte pH was measured with a B30PCI pH meter from VWR. Vigorous stirring (700 rpm and 1.2 cm long stirring bar) was used in all experimental runs. EIS measurements were performed to determine the ohmic drop between the working and reference electrodes, and compensate the measured working electrode potential. Details on the ohmic drop measurements and calculation are provided in the (ESI†) and all reported electrode potentials correspond to the cathode potential after compensating for ohmic losses.
Chemical analysis
Liquid–liquid extraction with toluene was used to separate the organic molecules from the aqueous electrolyte after CA techniques of 40 minutes that yielded AN conversions under 30%. The organic phase was then analyzed in a GCMS-QP2010 Shimadzu gas chromatographer equipped with a mass spectrometer. Component identification and quantification were performed using calibration curves for AN, PN, and ADN at different electrolyte compositions (see Fig. S7 in the ESI†). Faraday's law was used to correlate the current measured in each experiment with the consumption/production of each species. In the results below, the selectivity towards each product is equivalent to their Faradaic efficiency. Details on the calculations for selectivity and ADN production rate are shown in the ESI.†Results and discussion
The composition of the electrolyte and reaction parameters can directly affect the kinetic, mass transport, and ohmic limitations of the electrochemical conversion. In the following results, it is shown how the length of the alkyl chains of the TAA ions, the concentration of TAA ions and AN, the temperature, and the pH affect the performance of the electrochemical conversion.Effect of the TAA ion size
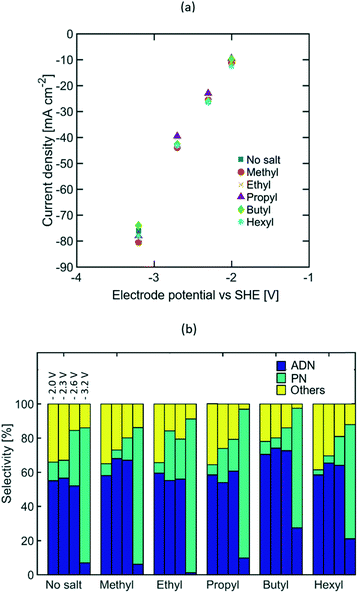
Varying lengths of the TAA ion alkyl chain (e.g. methyl, ethyl, propyl, butyl, and hexyl) were studied to assess the effect of the molecular size and intermolecular interactions between the TAA ions and AN on the selectivity of the electrochemical transformation. Longer alkyl chains are expected to lower electrolyte conductivity and increase the TAA ion's affinity towards AN, thus leading to higher AN local concentration near the EDL. EIS measurements (see Fig. S5 in the ESI†) and the steady state polarization curves shown in Fig. 3(a) indicate that the alkyl chain length does not affect electrolyte conductivity significantly, which suggests that most of the current is carried by the phosphate and sodium ions acting as supporting electrolyte. Furthermore, the similar polarization curves obtained for different TAA ion sizes and concentrations (see Fig. S3 in the ESI†) imply that the TAA ions do not affect the rate of electron transfer, but serve only to modulate the relative concentration of species in the double layer and thus the selectivity of the reaction.Fig. 3(b) summarizes the effect of the alkyl chain length on selectivity towards ADN, PN, and other by-products, such as hydrogen, MGN, 1,3,6 tricyanohexane, and other non-volatile oligomers, at −2, −2.3, −2.6, and −3.2 V vs. SHE. The observed increase in selectivity towards ADN suggests that the presence of TAA ions increases the AN concentration in the EDL, lowering the relative rates of the hydrogenation (i.e. production of PN) and hydrogen evolution reactions and enhancing the electrohydrodimerization reaction (i.e. production of ADN), which involves two AN molecules.
At high current density, a significant increase in selectivity towards PN was observed with all TAA ion sizes. This is likely due to mass transport limitations which result in a decreased concentration of AN in the EDL that favours the formation of PN. At lower current densities (10 to 42 mA cm−2), these mass transport limitations are less pronounced given the lower depletion rate of AN. The selectivity increase observed with longer alkyl chains, from ethyl to butyl, suggests that the increased affinity towards AN of longer alkyl chains promotes higher concentrations of this molecule in the EDL. An exception to this trend is observed for electrolytes containing tetramethylammonium ions, which exhibit high ADN selectivity despite their short alkyl chains. This is consistent with the ability of small ions to effectively solvate and stabilize intermediate carbanions in the ADN formation process (see Fig. 1), preventing their early protonation and lowering the activation energy for the electrohydromerization pathway. The selectivity drop observed for electrolytes containing tetrahexylammominum (THA) hydroxide was caused by the low solubility of this salt in water. While single-phase solutions were obtained with all other TAA ion sizes, the addition of THA ions to the electrolyte resulted in the formation of a second phase. This new organic-rich phase lowers the AN and TAA ion concentration in the aqueous electrolyte and thus the selectivity towards ADN. Overall, the highest selectivity was observed for electrolytes containing tetrabutylammonium (TBA) ions, with an increase of 20% in ADN selectivity with respect to the case when no TAA ions were present. This optimum TAA ion size differs from that reported in earlier studies by Danly,55 where electrolytes contained large amounts of TAA ions as supporting electrolytes (up to 2.5 M). In the state-of-the-art electrolyte formulations studied in this work, the optimal TAA selection criteria is no longer based on conductivity enhancement – given that the current is primarily carried by phosphate and sodium ions – but instead on the ability of the TAA ions to regulate reactant concentration in the EDL and stabilize intermediate anions.
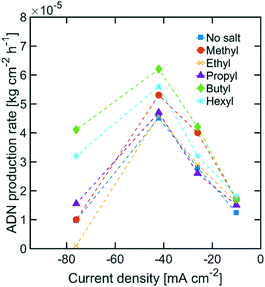
While the size of TAA ions and the applied current density play a significant role in the selectivity of the reaction, other performance metrics, such as the ADN production rate and the energy productivity, are important when defining the most desirable operating conditions. Fig. 4 displays the ADN production rate achieved with the different TAA ion sizes, where TBA ions yielded the highest production rate among the sizes tested. Furthermore, the trends obtained for all sizes showed a maximum in production rate for ADN at −42 mA cm−2, where there is an optimal trade-off between the AN consumption rate and ADN selectivity. Fig. 5 provides a measure of the energy productivity of the system in terms of ADN production per unit of energy input, where TBA ions also exhibit the best performance. As expected, the highest energy productivity was found at the lowest applied current densities, where kinetic losses are at a minimum. However, at low current density, the ADN production rate was severely affected, resulting in less favorable operating conditions for practical implementation.
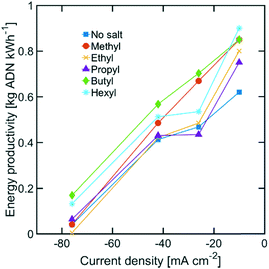 | ||
| Fig. 5 Energy productivity for various TAA ion sizes. The electrolyte contained 0.6 M of AN, 0.5 M Na3PO4, 0.03 M EDTA, and 0.02 M TAA ion. The temperature was maintained at 25 °C and the pH at 11. | ||
Effect of the TAA ion concentration
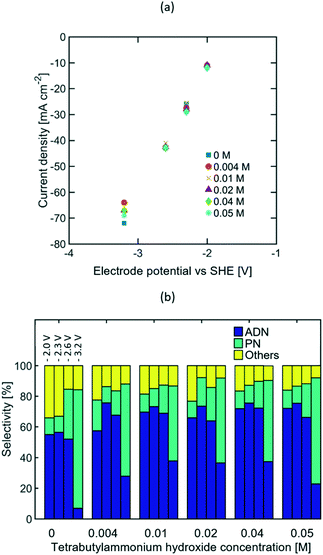
Given that the optimum selectivity towards ADN was achieved with TBA ions, studies on the TAA ion concentration effects on selectivity were carried out using this alkyl chain length. It is expected that higher bulk TAA ion concentration would increase AN solubility in the aqueous phase,27,41,56 thus resulting in a higher AN concentration near the electrode surface.Due to the low solubility of TBA salts at ambient temperature, the study was limited to concentrations ranging between 0 to 0.05 M. The concentrations of other components in the electrolyte were kept constant at 0.6 M AN, 0.5 M Na3PO4, and 0.03 M EDTA, based on the composition reported in recent patents.57–60 EIS studies (see Fig. S5 in the ESI†) and the steady state polarization curves shown in Fig. 6(a) suggest that the TAA ion concentration range tested was not large enough to significantly affect electrolyte conductivity, as most of the current is expected to be carried by phosphate and sodium ions. Fig. 6(b) summarizes the effect of the TAA ion concentration on selectivity at four different compensated working electrode potentials from −2 to −3.2 V vs. SHE. At lower electrode potentials, there was no meaningful difference on the selectivity achieved for the TAA ion concentration range studied. This suggests that the bulk concentration of TAA ions does not affect the system's performance significantly, and low bulk concentrations (0.004 to 0.05 M) are enough to ensure the TAA ion's presence at the EDL. At higher electrode potentials, the strong increase in ADN selectivity once the TAA ion was added suggests that the TAA ion's ability to increase AN concentration in the EDL becomes more critical when AN depletion is stronger and the system is mass transport limited.
Although TAA ions have been reported to act as surfactants in the electrolyte, their effects in the bulk concentration of AN were not significant in the study, given that AN is soluble in water at the concentration range tested and there was no evidence of the formation of a dispersed phase in the electrolyte. Considering that the TAA ion concentration did not impact selectivity significantly, the highest selectivity towards ADN was observed at approximately −2.3 V vs. SHE and −27 mA cm−2.
Effect of AN concentration
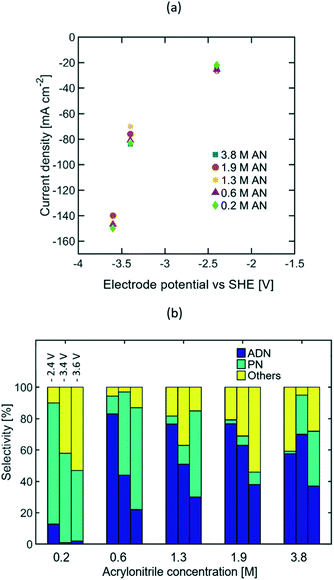
The results reported above reveal that current density is the reaction parameter with strongest influence on selectivity, production rate, and energy productivity. This is attributed to the mass transport limitations observed at high AN consumption rates. Increasing the bulk concentration of AN can mitigate these limitations, and thus allow for optimal operation at higher current densities. The effects that larger AN bulk concentrations have in the reaction system include: (i) the increase of AN concentration in the EDL, (ii) the possible formation of a second phase in the electrolyte, (iii) the decrease in electrolyte conductivity, and (iv) the promotion of AN self-polymerization reactions.In order to better understand the extent of each of these effects, reactions were studied in the presence of AN bulk concentrations from 0.2 to 3.8 M at three compensated working electrode potentials from −2.4 to −3.6 V vs. SHE. These electrode potentials were used given that higher steady state current densities, depicted on Fig. 7(a), were essential to clearly elucidate the effect of AN concentration. Fig. 7(b) summarizes the selectivity values found for the concentration range mentioned above. The results show that as the current density increased, higher bulk concentrations of AN were required to maximize ADN selectivity. These results confirm that higher bulk AN concentrations help mitigate mass transport limitations and reduce the production rate of PN.
The formation of a second phase in the electrolyte was observed when the AN concentration exceeded 1.3 M (see Fig. S1 in the ESI†), which led to the presence of small organic droplets under vigorous stirring for experimental runs. The presence of a secondary phase can have an impact on the electrolyte conductivity, but as demonstrated from both EIS and cyclic voltammetry measurements (see Fig. S3 and S5 in the ESI†), these effects were negligible in our experiments.
Additionally, the PN selectivity decreased at high AN concentrations, while the selectivity towards other by-products increased. Observation of an increased AN self-polymerization rate in the bulk electrolyte suggests that these by-products are likely to be dominated by AN-derived oligomers (Fig. S9 in the ESI†). Electrochemical radicals generated in the cathode could further trigger self-polymerization reactions in the EDL thus leading to these oligomers.
The highest ADN selectivity (>80%) was achieved with 0.6 M (3 wt%) AN at −26 mA cm−2. This indicates that AN-derived electrochemical by-products are hard to control under basic electrolytes and at high AN concentration. However, as observed in Fig. 8, there was a significant increase in adiponitrile production rate at the highest AN concentration (3.8 M AN) and current density (−82 mA cm−2). These operating conditions may be preferable for practical implementation, despite the reduced energy productivity (Fig. 9) and selectivity.
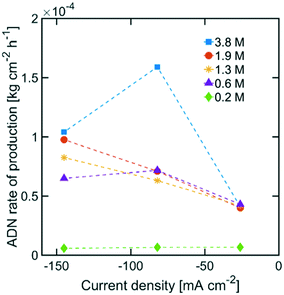 | ||
| Fig. 8 ADN production rate with various AN concentrations. The electrolyte contained 0.5 M Na3PO4, 0.03 M EDTA, and 0.02 M TBA hydroxide. The temperature was maintained at 25 °C and the pH at 11. | ||
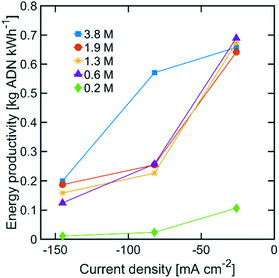 | ||
| Fig. 9 Energy productivity with various AN concentrations. The electrolyte contained 0.5 M Na3PO4, 0.03 M EDTA, and 0.02 M TBA hydroxide. The temperature was maintained at 25 °C and the pH at 11. | ||
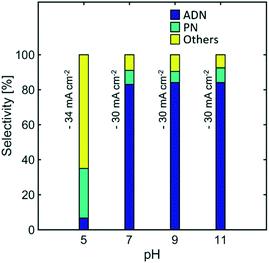
Effect of pH
Electrolyte pH can have significant effects in selectivity, as higher concentration of protons would facilitate the hydrogen evolution reaction, and the early protonation of AN radical anions, leading to the formation of PN. Furthermore, EDTA has low solubility at pH < 7 while the TAA hydroxide precipitates at pH > 11. This limits the operation of the reaction to intermediate pH values.Fig. 10 shows the effect of pH on the selectivity of the reaction. No significant effect is observed when the electrolyte was maintained at pH values between 11 and 7. Nevertheless, for pH lower than 7, where no EDTA was added in the electrolyte, the selectivity towards ADN dropped significantly, while the formation of PN and hydrogen was favoured. As expected, this directly affected and significantly lowered ADN production and the energy productivity of the reaction (See Fig. S4 in the ESI†). These results suggest that the higher water content used in state-of-the-art electrolyte compositions favours hydrogen evolution and PN formation at even moderately acidic conditions (pH = 5). This limitation was not observed in earlier electrolytes reported by Danly,55 which contained lower amounts of water and exhibited high selectivities for pH values as low as 4.
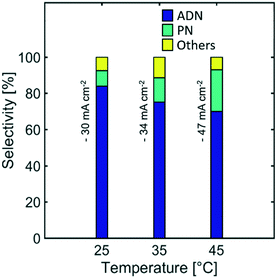
Effect of temperature
Temperature is a reaction parameter that can have strong influence in the reaction kinetics and mass transport. Higher temperatures increase the diffusion coefficient of species, electrolyte conductivity, and electrode reaction rates. These effects lead to the observed higher current densities (Fig. S3 in the ESI†) for a given cathodic potential.Furthermore, the selectivity towards ADN decreased with increasing temperature, while the formation of PN was favoured, as shown on Fig. 11. This suggests that the reactant electroreduction rate had a stronger temperature dependence than the diffusivity of AN, leading to mass transport limitations and a decrease in selectivity at higher temperatures.
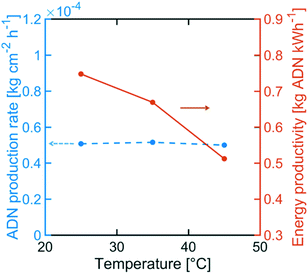
Although the selectivity towards ADN decreased with increasing temperature, Fig. 12 indicates that the ADN production rate can be maintained. On the other hand, the energy productivity decreased with increasing temperature. These results suggest that despite the fact that temperature does not strongly affect the production rate for the desired product, it can significantly lower ADN selectivity, together with the energy productivity of the system.
Conclusions
This work provides a thorough understanding of the factors affecting the electrochemical hydrodimerization of AN to ADN, and demonstrates that this reaction is strongly limited by mass transport of organic reactants at high current densities. TAA ions have the ability to increase AN concentration in the EDL and thus increase selectivity towards ADN. Different TAA ion sizes and concentrations do not affect the electrolyte conductivity significantly when highly conductive supporting ions are implemented. There is, however, a compromise between alkyl chain length of TAA ions and ADN selectivity. Ions with smaller alkyl chains are likely to solvate and stabilize intermediate carbanions more effectively, preventing their early protonation, while larger sizes have increased affinity towards AN and increase AN concentration in the EDL. Alkyl chains with more than four carbons provide lower improvements on selectivity due to their phase separation from the aqueous electrolyte. Tetrabutylammonium ions showed the best performance in terms of selectivity, ADN production, and energy productivity. The concentration of the TAA ions must be kept below 0.05 M to avoid their precipitation at the cathode surface. Furthermore, there was not a significant effect of TAA ion concentration on selectivity.Given that the reaction becomes mass transport limited at high current density, increasing the reactant concentrations in the bulk results in improved reaction performance, as measured by the ADN production rates and energy productivity. A maximum of 83% on ADN selectivity was found with 0.6 M AN and −26 mA cm−2. Nevertheless, the use of AN concentrations above its solubility limit leads to increased formation of oligomers derived from bulk self-polymerization reactions, lowering ADN yields. The production rate of ADN also increases with higher reactant concentration, while the energy productivity of the system decreases.
Intermediate regimes of pH (between 7 and 11) are favorable for the production of ADN, and performance metrics do not change significantly within this pH window. Nevertheless, at pH < 7, hydrogen evolution and PN production are dominant, lowering notably the selectivity, ADN production rate, and energy productivity. Lastly, temperature strongly affects the current density at a given potential, which can lead to mass transport limitations at high temperatures, and thus, reduced energy productivity.
The thorough electrochemical engineering guidelines provided in this work can help optimize operation parameters in ADN electrosynthesis and maximize the performance of the reaction as measured by selectivity, ADN production rate, and energy productivity. Furthermore, the challenges faced in the electrochemical ADN production are common to many organic electrosynthetic processes. These challenges include the low solubility of reactants in aqueous electrolytes, the narrow electrochemical stability window of water, and the generation of multiple side products in electrode and bulk reactions. The insights provided by this study can help mitigate these obstacles and result in improvements for other organic electrosynthesis processes where careful control of the concentration of reactants and intermediates in the EDL is required to achieve high selectivity and efficiency. Finally, although the understanding provided by this study can aid at designing high-efficiency and scalable ADN electrohydrodimerization reactors, complex challenges associated with controlling side-reactions, mass transport, and hydrodynamics of the electrolyte at scale will need to be resolved before the findings of this work can be implemented in industrial settings.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge the support and work of Alexandra Ryan, as well as the support and insightful comments of Dr. Minfeng Fang, Prof. Yoshiyuki Okamoto, Prof. Mark Green, Prof. Andre Taylor, and Prof. Ryan Hartman. They also acknowledge the financial support provided by H&M Foundation through the Global Change Award and from New York University, Tandon School of Engineering, through the MAM startup fund.References
- I. E. Agency, Renewables 2017,Report, 978-92-64-28187-5, International Energy Agency, 2017 Search PubMed.
- Paris Agreement, Paris, 2015 Search PubMed.
- Sustainable development goals United Nations Development Programme, 2015 Search PubMed.
- United Nations Framework Convention on Climate Change, United Nations, 1992 Search PubMed.
- Renewables 2017, Global Status Report Renewable Energy Policy Network for the 21st Century, 2017 Search PubMed.
- K. Van Kranenburg, E. Schols, H. Gelevert, R. De Kler, Y. Van Delft and M. Weeda, Opportunities for electrification. Empowering the chemical industry, TNO, 2016 Search PubMed.
- Z. J. Schiffer and K. Manthiram, Joule, 2017, 1, 10–14 CrossRef.
- C. A. Rodriguez, M. A. Modestino, D. Psaltis and C. Moser, Energy Environ. Sci., 2014, 7, 3828–3835 RSC.
- S. Ardo, D. Fernandez Rivas, M. A. Modestino, V. Schulze Greiving, F. Abdi, E. Alarcon-Llado, V. Artero, K. E. Ayers, C. Battaglia, J.-P. Becker, D. Bederak, A. Berger, F. Buda, E. Chinello, B. Dam, V. Di Palma, T. Edvinsson, K. Fujii, H. J. G. E. Gardeniers, H. Geerlings, S. M. Hosseini Hashemi, S. Haussener, F. A. Houle, J. Huskens, B. James, K. Konrad, A. Kudo, P. P. Kunturu, D. Lohse, B. Mei, E. Miller, G. Moere, J. Muller, K. L. Orchard, T. Rosser, F. H. Saadi, J.-W. Schüttauf, B. J. Seger, S. W. Sheehan, W. A. Smith, J. Spurgeon, M. Tang, R. van de Krol, P. C. K. Vesborg and P. Westerik, Energy Environ. Sci., 2018, 11, 2768–2783 RSC.
- J.-W. Schüttauf, M. A. Modestino, E. Chinello, D. Lambelet, A. Delfino, D. Dominé, A. Faes, M. Despeisse, J. Bailat, D. Psaltis, C. Moser and C. Ballif, J. Electrochem. Soc., 2016, 163, F1177–F1181 CrossRef.
- D. L. Vely, CEP Magazine, 2015, pp. 10–12 Search PubMed.
- G. G. Botte, Electrochem. Soc. Interface, 2014, 23, 49–55 CrossRef.
- J. J. Dai, Y. B. Huang, C. Fang, Q. X. Guo and Y. Fu, ChemSusChem, 2012, 5, 617–620 CrossRef CAS PubMed.
- T. Fuchigami and S. Inagi, Fundamentals and Applications of Organic Electrochemistry, John Wiley & Sons, Japan, 2015 Search PubMed.
- K. Scott, I. F. McConvey and J. Henderson, J. Appl. Electrochem., 1987, 17, 329–339 CrossRef CAS.
- M. Watson, D. Pletcher and D. W. Sopher, J. Electrochem. Soc., 2000, 147, 3751–3758 CrossRef CAS.
- G. Bellussi and C. Perego, Industrial Catalytic Aspects of the Synthesis of Monomers for Nylon Production, Catalysis and Process Technology Research Centre, Italy, 1999 Search PubMed.
- M. Rapoport, US Pat., US4330483A, 1981 Search PubMed.
- B. Zhu, Y. Shi, J. Dong and W. Huang, China Pat., CN101985431A, 2011 Search PubMed.
- W. Yulin, China Pat., CN101676261A, 2010 Search PubMed.
- T. Jungkamp, H.-M. Polka, R. Baumann, M. Bartsch, G. Haderlein, H. Luyken and J. Scheidel, US Pat., US7504529B2, 2009 Search PubMed.
- A. Breikss, US Pat., US5523453A, 1996 Search PubMed.
- J. Shi, X. Zhang, F. Chen, L. Fan, C. Shu, G. Dang, X. Zhan, S. Feng, H. Wang, Z. Cao, Y. Zhang, D. Dong and J. Zhao, China Pat., CN106146345B, 2018 Search PubMed.
- M. Decker, J. Schmidt, H. Hoffmann and H. J. Pistor, US Pat., US3671566A, 1969 Search PubMed.
- Y. Zhu, L. Gao, L. Wen and B. Zong, Chin. Sci. Bull., 2015, 60, 1488 CrossRef.
- M. Baizer, C. Campbell, R. Fariss and J. Robert, US Pat., US3193480A, 1963 Search PubMed.
- M. M. Baizer, J. Electrochem. Soc., 1964, 111, 215–222 CrossRef CAS.
- M. M. Baizer and J. D. Anderson, J. Org. Chem., 1965, 30, 1351–1356 CrossRef CAS.
- P. Suwanvaipattana, S. Limtrakul, T. Vatanatham and P. A. Ramachandran, J. Cleaner Prod., 2017, 142, 1296–1308 CrossRef CAS.
- G. Hills, Elecrochemistry, Burlington House, London, 1973 Search PubMed.
- H. Putter, Industrial Electroorganic Chemistry, Marcel Dekker, New York, 4th edn, 2001 Search PubMed.
- P. Barlett, Bioelectrochemistry, Fundamentals, Experimental Techniques and Applications, John Wiley & Sons Ltd, England, 2008 Search PubMed.
- D. Pletcher, Z. Tian and D. Williams, Developments in Electrochemistry, John Wiley & Sons Ltd, United Kingdom, 2014 Search PubMed.
- K. Scott and B. Hayati, Chem. Eng. Process., 1993, 32, 253–260 CrossRef CAS.
- K. Scott, B. Hayati, A. N. Haines and I. F. McConvey, Chem. Eng. Technol., 1990, 13, 376–383 CrossRef CAS.
- M. M. Baizer, J. D. Anderson, J. H. Wagenknecht, M. R. Ort and J. P. Petrovich, Electrochim. Acta, 1967, 12, 1377–1381 CrossRef CAS.
- K. Scott and B. Hayati, Chem. Eng. Sci., 1990, 45, 2341–2347 CrossRef CAS.
- M. Baizer and D. Danly, CHEMTECH, 1980, 10, 161 CAS.
- B. Frontana-Uribe, R. Daniel Little, J. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099–2119 RSC.
- C. A. C. Sequeira and D. M. F. Santos, J. Braz. Chem. Soc., 2009, 20, 387–406 CrossRef CAS.
- D. E. Danly, J. Electrochem. Soc., 1984, 131, 435C–442C CrossRef.
- K. Nakagawa and Y. Nagamori, US Pat., US4789442A, 1988 Search PubMed.
- D. Pletcher and F. Walsh, Industrial Electrochemistry , Chapman and Hall, Cambridge, 1990 Search PubMed.
- K. Mihara, M. Seko, S. Ogawa, S. Kumazaki, R. Komori and M. Yoshida, US Pat., US3497429A, 1970 Search PubMed.
- C. Campbell, D. Danly and W. Mueller, US Pat., 3830712A, 1974 Search PubMed.
- D. Darrell, US Pat., 3481846A, 1969 Search PubMed.
- O. Onsager, US Pat., 3644476, 1972 Search PubMed.
- F. Beck and H. Leitner, US Pat., 3616320A, 1969 Search PubMed.
- M. Seko, K. Mihara, S. Kumazaki, R. Komori and M. Yoshida, US Pat., 3595764A, 1971 Search PubMed.
- F. Beck, J. Appl. Electrochem., 1972, 2, 59–69 CrossRef CAS.
- P. F. Karimi, S. N. Ashrafizadeh and F. Mohammadi, Chem. Eng. J., 2012, 183, 402–407 CrossRef.
- F. Karimi, F. Mohammadi and S. N. Ashrafizadeh, J. Electrochem. Soc., 2011, 158, E129–E135 CrossRef CAS.
- R. K. Grasselli and F. Trifirò, Top. Catal., 2016, 59, 1651–1658 CrossRef CAS.
- M. O. Guerrero-Pérez and M. A. Bañares, ChemSusChem, 2008, 1, 511–513 CrossRef PubMed.
- D. Danly and C. Campbell, Techniques of Electroorganic Synthesis, Part III, John Wiley & Sons, 1982 Search PubMed.
- F. Karimi, S. N. Ashrafizadeh and F. Mohammadi, Chem. Eng. J., 2012, 183, 402–407 CrossRef CAS.
- B. Liu, Y. Li, H. Wang and Y. Han, China Pat., CN202359209U, 2011 Search PubMed.
- Y. Ding, B. Feng, G. Li, G. Wang, X. Guan and S. Zhao, China Pat., CN102002726A, 2011 Search PubMed.
- F. Wang, Y. Han, L. Xiao, R. Yao, D. Zheng, L. Wang, G. Jiang and S. Zhang, China Pat., CN105543888A, 2016 Search PubMed.
- B. Feng, H. Zhang, G. Wang, J. Wang, W. Xian and X. Ma, China Pat., CN102061482A, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8re00262b |
| This journal is © The Royal Society of Chemistry 2019 |