Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Operando deconvolution of photovoltaic and electrocatalytic performance in ALD TiO2 protected water splitting photocathodes†
Wei
Cui
 a,
Wenzhe
Niu
ab,
René
Wick-Joliat
a,
Wenzhe
Niu
ab,
René
Wick-Joliat
 a,
Thomas
Moehl
a,
Thomas
Moehl
 a and
S. David
Tilley
a and
S. David
Tilley
 *a
*a
aDepartment of Chemistry, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland. E-mail: david.tilley@chem.uzh.ch
bState Key Laboratory of Silicon Materials, School of Materials Science and Engineering, Zhejiang University, Hangzhou, Zhejiang, China
First published on 5th June 2018
Abstract
In this work, we demonstrate that buried junction photocathodes featuring an ALD TiO2 protective overlayer can be readily characterized using a variation of the dual working electrode (DWE) technique, where the second working electrode (WE2) is spatially isolated from the hydrogen-evolving active area. The measurement of the surface potential during operation enables the operando deconvolution of the photovoltaic and electrocatalytic performance of these photocathodes, by reconstructing J–ΔV curves (reminiscent of photovoltaic J–V curves) from the 3-electrode water splitting data. Our method provides a clearer understanding of the photocathode degradation mechanism during stability tests, including loss of the catalyst from the surface, which is only possible in our isolated WE2 configuration. A pn+Si/TiO2 photocathode was first investigated as a well behaved model system, and then the technique was applied to an emerging material system based on Cu2O/Ga2O3, where we uncovered an intrinsic instability of the Cu2O/Ga2O3 junction (loss of photovoltage) during long term stability measurements.
Introduction
Photoelectrochemical (PEC) water splitting has been recognized as a promising avenue for harvesting renewable hydrogen fuel from inexhaustible solar energy and water.1–4 Large photovoltages are required for efficient and therefore cost-effective water splitting, and one approach to achieving larger open-circuit voltages (Voc) is using so-called “buried junctions”. These buried junction photoelectrodes can be modeled as a series combination of a p–n junction photoabsorber, a protective layer and surface catalyst (pn/cat),5,6 where the Voc is decoupled from the semiconductor–electrolyte interface, and the increased band bending of the p–n junction can significantly enhance electron–hole pair separation.7The efficiency of a pn/cat photocathode is largely determined by the intrinsic properties of the buried p–n junction.8 However, the semiconductor-catalyst and catalyst–electrolyte interfaces also play a critical role in the overall performance of the system. Issues such as charge transport in the protective layer, the nature of the semiconductor-catalyst contact (ohmic or Schottky-type), as well as the electrocatalytic activity at the catalyst–electrolyte interface are typically obscured within the standard current–voltage measurement data.9,10 Therefore, we sought to develop an experimental technique that could not only evaluate the PEC performance but also simultaneously provide an understanding of these different interfaces during PEC operation.
The dual working electrode (DWE) technique was first reported in the 1970s. Nakato, Pinson and Wilson reported that n-GaP and n-TiO2 photoanodes coated with thin gold films showed a photovoltaic effect, representing early examples of in situ measurements of the surface potential.11–13 Recently, the Boettcher group has used the DWE technique to study a photoanode-catalyst interface.14 It is of note that the second working electrode in all of the previous works has either been a transparent conducting oxide (TCO) or a thin metal film that covers the entire active area.15,16 For systems that do not employ TCOs as part of the buried junction structure, it has thus far not been possible to carry out DWE studies without introducing a metallic film, which influences the measurement through partial light absorption and by affecting the catalyst binding to the photoelectrode surface. We have therefore developed a new architecture of the DWE technique that is compatible with standard buried junction photocathodes featuring a protective layer, which does not introduce extraneous materials at the semiconductor–electrolyte interface. With this method, one can diagnose a problem of the stability of the catalyst on the surface versus the stability of the photovoltaic output of the p–n junction. As will be shown in this manuscript, the latter case does indeed require consideration. The diagnosis of the point of failure in unmodified PEC devices under operation is critical for identification of targets to improve the system.
A TiO2-protected pn+-Si junction photocathode was chosen as a platform to develop this method, as the Si p–n junction is robust and stable. Atomic layer deposition (ALD) TiO2 is a common protective layer for water splitting photocathodes due to its favorable conduction band position for the hydrogen evolution reaction, optical transmittance for visible light, high stability over a wide range of electrolyte solutions and pH, and good conductivity. Moreover, the high doping density of ALD TiO2 enables an ohmic (tunnel) contact to the contacting metal of the second working electrode (WE2), no matter the work function.17,18 The top contact was made via a thin Au layer covered by epoxy, which was able to sense the surface potential of the photocathode under operando conditions, without directly contacting the electrolyte or HER catalyst. The hidden J–V curve of the buried p–n junction can then be extracted by measuring the difference in voltage between the backside and the surface of the photocathode (ΔV) and plotting versus the water splitting current. By monitoring the evolution of the hidden J–V curve in the 3-electrode water splitting measurements, one can immediately diagnose whether the degradation in the performance of the photocathode derives from a problem with the catalyst or with the photovoltaic output of the p–n junction. In this work, we evaluate both a well understood system (pn+-Si) and a promising emerging system (p–n Cu2O/Ga2O3). The results from the Si system demonstrate that the failure of the surface catalyst is easily identified with our DWE technique. The results from the Cu2O-based system reveal an intrinsic instability of the p–n junction, with reduced photovoltaic output following a stability measurement.
Results and discussion
Interface energetics of the pn+Si/TiO2/Pt(ed) photocathode
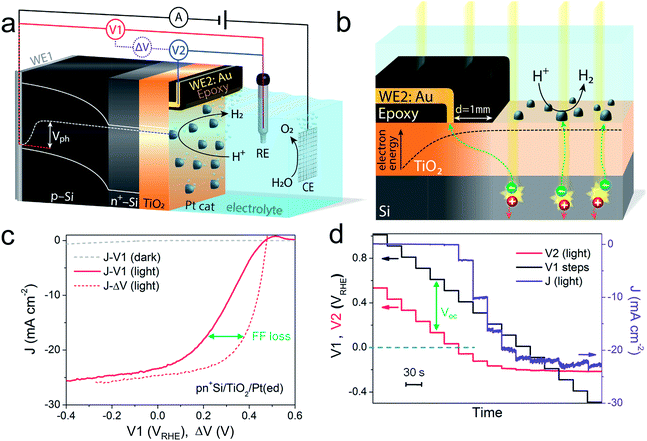
A pn+-Si photocathode with 100 nm-thick ALD-TiO2 protective layer was chosen as a model system for the development of the technique. A schematic diagram of the DWE setup is depicted in Fig. 1a. WE1 is used for controlling the back contact potential (V1) of the photocathode. WE2 is connected to the photocathode surface and kept at open circuit during PEC measurements to directly probe the surface potential (or in other words to probe the energy level of surface-accumulated electrons) in relation to the reference electrode (V2). To avoid direct contact of WE2 and the active area, WE2 was contacted a small distance away from the illuminated area (∼1 mm) and separated by a thin coating of opaque epoxy, as shown in Fig. 1b. When illuminated, electron–hole pairs are generated and separated by the built-in electric field across the p–n junction, bringing photoelectrons to the surface while sweeping holes to the back contact. Consequently, a photovoltage is created across the pn+-Si homojunction. Considering that both the n-type Si layer and TiO2 are highly doped,19 the width of the space charge region of both n+-Si and TiO2 is very narrow and ensures an Ohmic contact. The Pt catalyst also forms an Ohmic contact with the TiO2 layer.17 As a result, the measured potentials of V1 and V2 directly give the energetic positions of the quasi-Fermi level of holes and electrons, respectively. The difference between V1 and V2, denoted ΔV, is the output voltage of the pn+-Si junction. It is worth noting that as the photoelectrons diffuse away from the illuminated area (as shown in Fig. 1b), the electrons cannot enter the electrolyte and will ultimately recombine. This leads to a lower electron density than in the illuminated area and a slightly reduced Voc compared to the photovoltaic output of the p–n junction is recorded. The small drop in the measured surface potential is robust and reproducible, and does not complicate the analysis herein.After the Pt catalyst was electrodeposited onto the TiO2 surface, the conventional current density-back contact potential (J–V1) curve of the pn+Si/TiO2/Pt(ed) photocathode was obtained in 0.5 M H2SO4 with a linear sweep from positive to negative potential (Fig. 1c). Under one sun illumination, the pn+Si/TiO2/Pt(ed) photocathode exhibits an onset potential for water reduction of ∼0.5 VRHE. As V1 becomes more negative, the photocurrent density increases and eventually saturates at 25 mA cm−2 at V1 = −0.2 VRHE. WE2 enables the in situ measurement of surface potential V2 during the sweep of V1. ESI Fig. S3† presents V2 and ΔV values as a function of V1 with and without illumination.
A hidden J–ΔV curve, analogous to the current–voltage characteristic of a PV cell, can then be extracted and is plotted in Fig. 1c. The Voc and Jsc are 475 mV and 24.6 mA cm−2, respectively (the characteristics are also listed in ESI Table S1†). A significant loss of fill factor is observed when comparing the J–V1 and J–ΔV curves, which derives from the additional series resistances in a PEC cell versus a PV cell, namely the TiO2/catalyst junction resistance, the overpotential of the catalyst required for driving a chemical reaction, and the solution resistance.20 In essence, the J–ΔV curve shows the best possible fill factor that can be achieved by the J–V1 curve. In practice, a real PEC J–V1 curve will always have a smaller fill factor due to the catalyst overpotential as well as the series resistances mentioned above.
In order to more clearly visualize the effect of the surface potential on the current, a stepwise chronoamperometry measurement was carried out whereby the potential of V1 was stepped every 30 s and both V2 and the photocurrent were recorded (Fig. 1d). When V1 is positive of ∼0.5 VRHE (and the photocurrent is still nearly 0), V2 remains at a constant distance (constant ΔV, see also ESI Fig. S3†). Substantial cathodic photocurrents appear at V1 = 0.4 VRHE, where V2 is (due to the photovoltage) more negative than 0 VRHE (with ΔV now starting to shrink). After the onset potential, although V2 continues to move negatively with each step, ΔV shrinks further as the photocurrent increases. Ultimately, both V2 and the photocurrent become constant, even as V1 becomes more negative, eventually entering a reverse bias-type regime. ESI Fig. S4† depicts band energy diagrams under several conditions of applied bias, and ESI Fig. S5† gives a detailed discussion of the relationship between photovoltage and the onset potential.
Stability of the pn+Si/TiO2/Pt(ed) photocathode
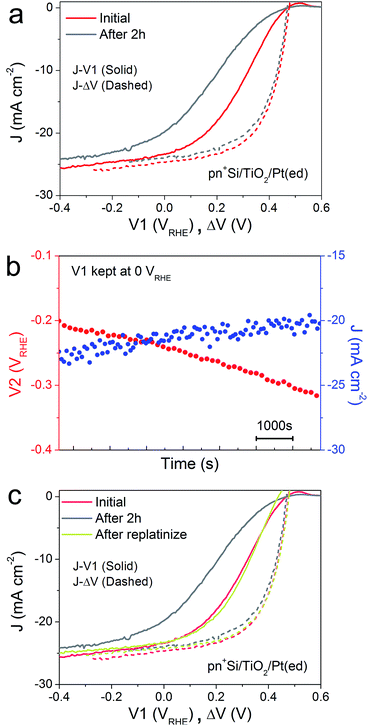
A critical issue for photoelectrodes is the long-term stability. A standard procedure for assessing the stability is to carry out a chronoamperometry experiment under a static back contact potential and to compare the J–V1 behavior before and after the stability test. This type of analysis, however, is relatively limited because the underlying degradation mechanisms are inaccessible. The decrease in PEC performance can be due to several factors. Firstly, the H2-evolving catalyst may be deactivated, poisoned or dislodged from the electrode surface. Secondly, the p–n junction may produce a reduced output Voc, due to partial photocorrosion and increased recombination. These changes in the semiconductor material also result in lower photocurrent densities and fill factor.In order to characterize the degradation mechanism in the Si photocathode, we performed a 2 h stability test by holding V1 at 0 VRHE, a typical value for these types of test in the literature.8Fig. 2a shows the J–V1 and J–ΔV curves before and after the 2 h stability test. Compared with the initial J–V1 scan, the scan after the 2 h shows similar onset potential and slightly decreased saturation photocurrent, but a remarkably poorer fill factor. As the J–ΔV curves remain the same, it is immediately apparent that the problem relates to the catalyst and not to the photovoltaic performance of the buried junction. Fig. 2b depicts how the surface potential V2 and photocurrent density change over time under a static back contact potential of 0 VRHE. Over 2 h, the photocurrent density drops from ∼23 to ∼20 mA cm−2, while V2 steadily shifts to more negative values, which indicates that higher overpotential is needed in order to achieve a similar current density. A poor contact between the surface and the catalyst (TiO2/catalyst) as well as worsening kinetics at the catalyst/electrolyte interface (e.g. surface poisoning) will result in a higher overpotential for the catalytic interface.21 Pt was then re-deposited onto the electrode surface (Fig. 2c). Due to the fact that the fill factor is completely restored upon re-platinization, we can confirm that neither a degradation in the p–n junction of the silicon nor resistive losses through e.g. formation of a silicon oxide layer are responsible for the change in the J–V1 curve. The degradation likely results from desorption of the Pt nanoparticles, as has been previously observed for electrodeposited platinum on ALD TiO2.22 When the ALD TiO2 was replaced by a thin metallic Ti film, the Pt catalyst binding was much more robust over a 2 h stability measurement (ESI Fig. S7†)
Investigation of the TiO2/Pt junction
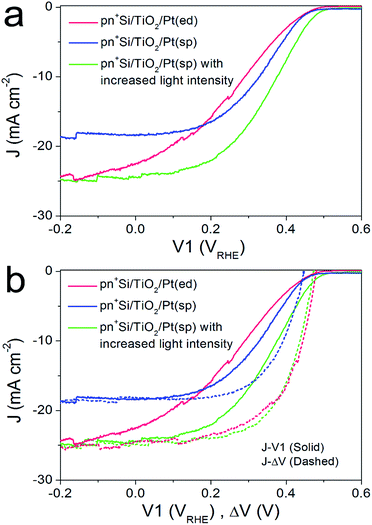
For the pn+-Si photocathodes described above, the Pt catalyst was electrodeposited onto either the TiO2 or Ti metal surface as nanoparticles with a size range of ∼10–30 nm (ESI Fig. S8a†). This non-continuous catalyst morphology may be unfavorable for efficient extraction of the surface electrons, resulting in a poor fill factor of the J–V1 curve.23 Therefore, we investigated a nominally 2 nm-thick Pt film with nearly full coverage on the TiO2 layer by sputter coating (denoted pn+Si/TiO2/Pt(sp)). The Pt deposited in this way makes the surface slightly rough (ESI Fig. S8b†). Fig. 3a compares the J–V1 curves of pn+Si/TiO2/Pt(sp) and pn+Si/TiO2/Pt(ed) photocathodes under one sun illumination. Sputtered Pt exhibits a similar onset potential and improved fill factor, but much reduced photocurrent densities due to the optical transmission loss through the 2 nm-thick Pt film (ESI Fig S9†). For better comparison between the sputtered and electrodeposited samples, we also measured the J–V1 curve of the pn+Si/TiO2/Pt(sp) at an increased light intensity to achieve a similar photocurrent density, plotted in green. The green curve exhibits an earlier onset potential despite having the same Voc as the Pt(ed) curve, suggesting a better catalytic activity of sputtered Pt over electrodeposited Pt. Support for this hypothesis is shown by comparing their individual catalytic activities towards H2 generation when deposited on FTO slides (Fig S10†). Additionally, pn+Si/TiO2/Pt(sp) always shows an enhancement in the fill factor the in J–V1 curves, reflecting the smaller TiO2/Pt/electrolyte interfacial resistance for the TiO2/Pt(sp) as compared to the TiO2/Pt(ed). In the case of a conformal Pt film, electron transfer is more probable as the catalyst surface area is increased, which is also indicated by the much more positive V2 value in pn+Si/TiO2/Pt(sp), shown in ESI Fig. S11.† For example, to reach the same saturation photocurrent, a ∼130 mV overpotential is required for pn+Si/TiO2/Pt(sp) but ∼200 mV for pn+Si/TiO2/Pt(ed).What we have already hypothesized by the performance of the different Pt on FTO is confirmed by the determination of the J–ΔV curves. At similar saturation photocurrents the J–ΔV (PV mode) of pn+Si/TiO2/Pt(sp) is essentially identical with the pn+Si/TiO2/Pt(ed) (see the green and red dashed curves) while the J–V1 curve (PEC mode) shows a clearly higher FF for the device with sputtered Pt.
Cu2O/Ga2O3 junction photocathode
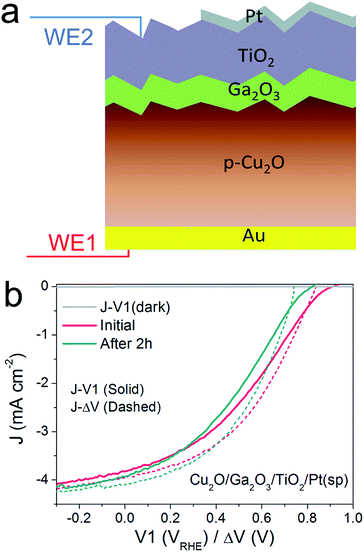
Thus far, we have developed the DWE technique with a model pn+Si/TiO2/Pt(ed) photocathode, with which we can gain a deeper insight into the PEC process and the photocathode stability. Next, we applied this technique to the emerging material ALD TiO2-protected Cu2O to demonstrate the generality of the technique and to probe a potential instability of the photovoltaic output of these materials.22,24 An n-type Ga2O3 was used as a buffer layer between the Cu2O and TiO2 overlayer because this interlayer ensures a positively shifted onset potential, compared to that of n-Al:ZnO (AZO) (ESI Fig. S12†).25,26Fig. 4a schematically depicts the multilayer structure of the Cu2O/Ga2O3/TiO2 photocathode. In a similar fashion as for the silicon photocathodes described previously, a second working electrode was introduced to probe the surface potential V2, prior to deposition of the Pt catalyst by sputtering.PEC measurements were performed in a pH 5 phosphate/sulfate solution. Fig. 4b displays the J–V1 and J–ΔV curves. A positive onset potential of ∼0.9 VRHE is observed in the J–V1 curve, and at V1 = 0 VRHE, the photocurrent density is 3.9 mA cm−2. The onset potential is much more positive than that from both Cu2O/ZnO and Cu2O/AZO photocathodes, reflecting the larger photovoltage generated by the Cu2O/Ga2O3 junction. In the case of the J–ΔV curve, the Voc, Jsc and fill factor are 836 mV, 4.0 mA cm−2 and 36.1%, respectively. Compared with reported Cu2O/Ga2O3 solar cells in the literature, the Voc and fill factor values are comparable, but the Jsc is lower due to light absorption by the Pt catalyst.26,27 Still, resistance at the TiO2/Pt/electrolyte interfaces contribute to the fill factor loss between the J–V1 and J–ΔV curves. The fill factor loss is not very significant. As the J–ΔV curve mirrors the J–V1 curve, it is clear that the photovoltaic output of the buried Cu2O/Ga2O3 junction is responsible for the shape of the J–V1 curve of the photocathode, and not the catalytic activity of the Pt catalyst. When using an electrodeposited Pt catalyst the PEC system exhibits a lower fill factor, indicating that the TiO2/Pt/electrolyte resistance indeed can also limit J–V1 performance, as shown in ESI Fig. S15.† We further carried out a stepwise test on the photocathode under illumination. Results and discussion are provided in ESI Fig. S16 and S17† and confirm our statements above.
To study the stability of the Cu2O/Ga2O3/TiO2/Pt(sp) photocathode, a 2 h chronoamperometric measurement was performed under illumination at V1 = 0 VRHE. Fig. 4b shows the comparison of the J–V1 curves before and after a 2 h stability test. The onset potential shows a negative shift of nearly 120 mV, although the photocurrent density remains similar. Fig. 4b also shows the initial J–ΔV curve and the one after the 2 h stability test. An obvious decrease in Voc is evident, from 836 mV to 743 mV, while the Jsc shows a slight increase from 4.0 to 4.1 mA cm−2 (ESI Table S1†). In contrast to the pn+-Si photocathode, the Cu2O/Ga2O3 photocathode shows a degradation of the photovoltaic output of the underlying buried junction. For the silicon system, J–ΔV remained constant while the J–V1 changed (Fig. 2). For the Cu2O/Ga2O3 case, J–ΔV has changed while J–V1 remains similar (retains a similar photocurrent and fill factor). In order to determine the origin of the degraded photovoltage, a solid-state measurement was carried out using the DWE photocathode in a 2-electrode configuration, directly obtaining photovoltaic J–V curves. Fig. S18† shows the J–V characteristics of the Cu2O/Ga2O3/TiO2 DWE device before and after a stability measurement that was carried out at short circuit for 2 h, where an obvious Voc decrease in a range of 100 mV was observed. Since corrosion through any pinholes in the ALD protective layer can be ruled out in the solid state measurement, we attribute the loss of the PEC performance in this system to an intrinsic problem with the Cu2O/Ga2O3 junction. Further studies are underway to more clearly identify the underlying reason for the instability of this junction.
Conclusions
We have developed a new configuration of the DWE technique that is able to probe the surface potential of a water splitting photocathode under operation without interfering in the charge transfer processes at the different interfaces in actual water splitting PEC devices. Although we have focused on ALD TiO2 in this work due to its widespread use, we expect that our DWE architecture is applicable to other overlayer materials as well, provided they are sufficiently conductive, which is a requirement for PEC electrodes in any case.28 This technique has been demonstrated as a universal method to systematically investigate independently the photovoltaic and electrocatalytic properties of catalyst-modified buried junction photocathodes. A pn+Si/TiO2/Pt photocathode was first fabricated as a platform to model the DWE system. By means of surface potential measurements, the intrinsic properties of the buried p–n junction were studied, and the hidden J–V curve of a photovoltaic cell was extracted. Additionally, the fill factor loss between J–V1 and J–ΔV curves was identified as a parameter that characterizes the TiO2/Pt/electrolyte interface, where the morphology of the catalyst plays an important role. Furthermore, the PEC performance degradation mechanism was investigated and discussed. We have demonstrated that the stability of underlying p–n junctions in buried junction photocathodes can be characterized under operando conditions. Finally, we applied the DWE technique to a promising emerging system based on Cu2O/Ga2O3, where it was found that the large photovoltage decreases over time. The degradation in the photovoltage with time was also observed in the solid state, ruling out any potential corrosion by the electrolyte. As new material combinations are synthesized for PEC measurements, the DWE electrode technique enables a rapid diagnosis of the cause of degradation in these systems, while also obtaining the PV characteristics of these newly developed junctions without the need to construct separate PV cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The University of Zurich, the University Research Priority Program (URPP) LightChEC, and the Swiss National Science foundation (AP Energy Grant # PYAPP2 160586) are gratefully acknowledged for financial support. Wenzhe Niu thanks Zhejiang University for an international study and communication fellowship. The authors acknowledge the assistance and support of the Center for Microscopy and Image Analysis at the University of Zurich for the SEM characterization. The authors would also like to thank Dr Olivier Blacque (Department of Chemistry, University of Zurich) for his assistance in the XRD characterization.References
- A. Heller, Science, 1984, 223, 1141–1148 Search PubMed.
- M. Gratzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- E. L. Miller, Energy Environ. Sci., 2015, 8, 1809 Search PubMed.
- M. S. Prévot and K. Sivula, J. Phys. Chem. C, 2013, 117, 17879–17893 Search PubMed.
- Y. Nakato, Y. Egi, M. Hiramoto and H. Tsubomura, J. Phys. Chem., 1984, 88, 4218–4222 CrossRef.
- S. W. Boettcher, E. L. Warren, M. C. Putnam, E. A. Santori, D. Turner-Evans, M. D. Kelzenberg, M. G. Walter, J. R. McKone, B. S. Brunschwig, H. A. Atwater and N. S. Lewis, J. Am. Chem. Soc., 2011, 133, 1216–1219 CrossRef PubMed.
- Q. Huang, Z. Ye and X. Xiao, J. Mater. Chem. A, 2015, 3, 15824–15837 Search PubMed.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- Z. Zhang and J. T. Yates, Chem. Rev., 2012, 112, 5520–5551 CrossRef PubMed.
- T. A. Pham, Y. Ping and G. Galli, Nat. Mater., 2017, 1–8 Search PubMed.
- Y. Nakato, T. Ohnishi and H. Tsubomura, Chem. Lett., 1975, 4, 883–886 CrossRef.
- W. E. Pinson, Nature, 1977, 269, 316–318 CrossRef.
- R. Wilson, L. Harris and M. Gerstner, J. Electrochem. Soc., 1977, 124, 2–3 CrossRef.
- F. Lin and S. W. Boettcher, Nat. Mater., 2013, 13, 81–86 CrossRef PubMed.
- G. Hodes, L. Thompson, J. Dubow and K. Rajeshwar, J. Am. Chem. Soc., 1983, 1289, 324–330 CrossRef.
- J. R. White, F.-R. F. Fan and A. J. Bard, J. Electrochem. Soc., 1985, 132, 544–550 CrossRef.
- B. Seger, T. Pedersen, A. B. Laursen, P. C. K. Vesborg, O. Hansen and I. Chorkendorff, J. Am. Chem. Soc., 2013, 135, 1057–1064 CrossRef PubMed.
- J. Gu, Y. Yan, J. L. Young, K. X. Steirer, N. R. Neale and J. a. Turner, Nat. Mater., 2015, 15, 456 CrossRef PubMed.
- W. Septina, R. R. Prabhakar, R. Wick, T. Moehl and S. D. Tilley, Chem. Mater., 2017, 29, 1735–1743 CrossRef.
- K. T. Fountaine, H. J. Lewerenz and H. A. Atwater, Nat. Commun., 2016, 7, 13706 CrossRef PubMed.
- B. Seger, D. S. Tilley, T. Pedersen, P. C. K. Vesborg, O. Hansen, M. Gratzel and I. Chorkendorff, RSC Adv., 2013, 3, 25902–25907 RSC.
- A. Paracchino, V. Laporte, K. Sivula, M. Gratzel and E. Thimsen, Nat. Mater., 2011, 10, 456–461 CrossRef PubMed.
- E. Kemppainen, A. Bodin, B. Sebok, T. Pedersen, B. Seger, B. Mei, D. Bae, P. C. K. Vesborg, J. Halme, O. Hansen, P. D. Lund and I. Chorkendorff, Energy Environ. Sci., 2015, 8, 2991–2999 Search PubMed.
- A. Paracchino, N. Mathews, T. Hisatomi, M. Stefik, S. D. Tilley and M. Graetzel, Energy Environ. Sci., 2012, 5, 8673–8681 Search PubMed.
- C. Li, T. Hisatomi, O. Watanabe, M. Nakabayashi, N. Shibata, K. Domen and J.-J. Delaunay, Energy Environ. Sci., 2015, 8, 1493–1500 Search PubMed.
- Y. S. Lee, D. Chua, R. E. Brandt, S. C. Siah, J. V Li, J. P. Mailoa, S. W. Lee, R. G. Gordon and T. Buonassisi, Adv. Mater., 2014, 26, 4704–4710 CrossRef PubMed.
- T. Minami, Y. Nishi and T. Miyata, Appl. Phys. Express, 2013, 6, 44101 CrossRef.
- J. Azevedo, S. D. Tilley, M. Schreier, M. Stefik, C. Sousa, J. P. Araújo, A. Mendes, M. Grätzel and M. T. Mayer, Nano Energy, 2016, 24, 10–16 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images, Faradaic efficiencies, V2/ΔV–V1 curve, etc. See DOI: 10.1039/c8sc01453a |
| This journal is © The Royal Society of Chemistry 2018 |