Open Access Article
Open Access ArticleA critical assessment of the environmental fate of linear and cyclic volatile methylsiloxanes using multimedia fugacity models
Dimitri
Panagopoulos
 *ab and
Matthew
MacLeod
*ab and
Matthew
MacLeod
 a
a
aDepartment of Environmental Science and Analytical Chemistry, ACES, Stockholm University, Svante Arrhenius väg 8, SE-114 18 Stockholm, Sweden. E-mail: drdimitripanagopoulos@gmail.com
bEnvironmental Energy Technologies Division, Lawrence Berkeley National Laboratory, LBNL, 1 Cyclotron Road, 94720 Berkeley, California, USA
First published on 18th December 2017
Abstract
We apply multimedia models to systematically evaluate the fate profile of cyclic volatile methyl siloxanes (VMS) D4, D5 and D6, and the linear VMS L4 and L5 using recently reported measurements of their partition ratios between organic carbon and water (KOC), their salting out constants (Ks), and their enthalpy of sorption to organic carbon (ΔHOC). Our assessment follows a multi-stage strategy where the environmental fate of the chemicals is explored in generic regional models with increasing fidelity to the real system and in a region-specific model. Modeled emissions of VMS to air remained in air and were degraded or advected out of the system with overall residence times ranging from 2.4 to 2.5 days, while emissions to water resulted in accumulation in sediment and longer residence times ranging from 29.5 to 1120 days. When emitted to water the modeled residence times of VMS in the sediment exceeded the REACH criterion for persistence in freshwater sediments. Reported KOC measurements for D5 differ by 1 log unit, which results in a 500-day difference in the overall residence times calculated in the generic regional modeling. In the specific-region modeling assessment for Adventfjorden, Svalbard in Norway, the different KOC measurements of D5 resulted in a 200-day difference in overall residence times. Model scenarios that examined combinations of previously published ΔHOC or enthalpy of phase change between octanol and water (ΔHOW) for D5 in combination with the range of the KOC measurements resulted in 1100-days difference in overall residence times. Our results demonstrate that residence times of VMS in aquatic systems are highly sensitive to their degree of sorption to organic carbon, and that residence times of VMS likely exceed several persistence criteria and therefore they cannot be considered as non-persistent.
Environmental significanceVolatile methylsiloxanes (VMS) are a group of organosilicon chemicals that are used in personal care products and in the production of silicone polymers. VMS have been found at considerable levels in the air, in sediments and in aquatic organisms. We examine the fate of VMS using multimedia models in aquatic environments and we study their residence times in generic and specific environmental scenarios. Our calculations suggest that the residence times of VMS exceed several persistence criteria in aquatic environments and therefore they cannot be regarded as non-persistent chemicals. |
Introduction
Volatile methylsiloxanes are a group of organosilicon chemicals that consist of –Si(CH3)2–O– chains in cyclic or linear form.1–7 Cyclic volatile methylsiloxanes (cVMS) are primarily used as carriers in personal care products such as deodorants, skin creams and lotions.1–7 On a smaller scale cVMS are also used as solvents and building blocks in the production of silicon polymers.1–6 The most commonly used cVMS are octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5) and dodecamethylcyclohexasiloxane (D6).1–6 Linear volatile methylsiloxanes (lVMS) are mainly used as intermediates in the production of silicon polymers and on a smaller scale as carriers in personal care products.7 Usually the concentrations of cVMS in personal care products are higher than those of lVMS. However, Lu et al.8 reported that some personal care products from the Chinese market have concentrations of lVMS that exceed those of cVMS.Cyclic and linear VMS have been found at considerable levels in air,9,10 sediments11 and aquatic organisms.12 In the air, VMS degrade within days because of their reaction with hydroxyl radicals,13 but estimated lifetimes in sediment are substantially longer.14–17 Whelan18 explored the fate of cVMS in two contrasting North American lakes using multimedia models and underlined the importance of obtaining accurate measurements of KOC, as this was the parameter that was shown to be the most sensitive in the model calculations.
Environmental risk assessments for D5 have been conducted by Environment Canada and Health Canada, the United Kingdom Environment Agency, and the European Chemicals Agency.1–7,20,21 In the initial report of Environment Canada and Health Canada the authors concluded that D5 was a toxic substance as defined under the Canadian Environmental Protection Act and should be added to the Toxic Substances List in Schedule 1.19,22,23 Shortly after this report was published, it was challenged by industry groups, who suggested that the assessment was not conducted using the best available scientific evidence of that time.19,22,23 The Canadian Minister of Environment responded by forming an independent Board of Review to reexamine and assess D5 taking into consideration physicochemical properties presented by the industry representatives.19,22,23 The Board of Review overruled the initial assessment and concluded that “D5 does not pose a danger to the environment” and that “its projected future uses will not pose a danger to the environment”.19,22,23
In the risk assessment report of the United Kingdom Environment Agency the authors concluded that although D5 meets the P and vP criteria set by REACH24 for sediment, D5 will not persist in the aquatic environment because of its plausible loss through volatilization.1–3 That conclusion was consistent with recommendations articulated by Webster et al.,25 who used model calculations to demonstrate that using compartment-specific persistence criteria to evaluate a chemical would lead to misclassification when a chemical fails the half-life criterion in an environmental compartment where it does not considerably partition. To avoid misclassification, Webster et al.,25 recommended evaluating chemicals based on overall residence times instead of single compartment criteria, and proposed an overall environmental persistence criterion of 100 days.
The European Chemicals Agency is currently considering a potential European-wide restriction of D4 and D5 in wash-off personal care products. As a part of this process, the agency published a member state committee opinion on the persistence and bioaccumulation of D4 and D5. The decision of the committee is pending.20,21
Mackay et al.19 presented an evaluation of the fate of D5 using the equilibrium criterion (EQC) level III fugacity model. In a model scenario where 100% of the emissions are released to water, 94% of D5 partitioned to the sediment, <6% to water and <1% to the air. The overall residence time was 140 days, which exceeds the P criterion24 for freshwater sediment by only 20 days. Mackay et al.19 in their modeling calculations used a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOC value of 5.17, which was measured by Kozerski et al.26
KOC value of 5.17, which was measured by Kozerski et al.26
In a recent study, we measured the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOC for D5 to be 6.12.16 This KOC value is of one order of magnitude higher than the measurements of Kozerski et al.,26 which would substantially increase the modeled residence time of D5 in aquatic environments, and potentially indicate a more marked exceedance of the P criterion for sediment set by REACH.24
KOC for D5 to be 6.12.16 This KOC value is of one order of magnitude higher than the measurements of Kozerski et al.,26 which would substantially increase the modeled residence time of D5 in aquatic environments, and potentially indicate a more marked exceedance of the P criterion for sediment set by REACH.24
A parameter that could have great influence on KOC and thus on the residence times of VMS in aquatic environments is the enthalpy of sorption to OC from water (ΔHOC). Due to the very limited literature data for ΔHOC, it is common practice in modeling calculations to assume ΔHOC is equal to the enthalpy of phase change between octanol and water (ΔHOW) in order to adjust KOC to different temperatures.27–30 Xu and Kropscott31 studied the effect of temperature on the partition ratios of VMS between octanol and water (KOW) and observed that the KOW of VMS decreased with decreasing temperature. In another recent study,32 we measured the effect of temperature on the KOC of VMS and observed that KOC increased with decreasing temperatures. This difference could result in substantial differences in the modeled environmental fate and the residence times of VMS in aquatic environments at temperatures lower than the reference temperature of KOC measurements.
Finally, salinity has an impact on KOC. In a previous study we observed that the KOC of VMS increased with increasing salinity.17 This observation indicates that the residence times of VMS in marine environments are likely to be longer than in freshwater systems.
In this study we use a multi-stage process suggested by Mackay et al.33 as a framework to compare the environmental fate profile of VMS using property data from the Environment Canada and UK risk assessments to the fate profile using property data from our recent measurements. The stages are: (1) chemical classification, (2) evaluative assessment of chemical fate, (3) regional or far-field evaluation and (4) local or near-field evaluation. One of the focuses of this study is to assess the differences in the residence times of VMS in aquatic environments under a range of scenarios that reflect the variability in reported KOC, ΔHOC and ΔHOW. For stages 3 and 4, we model the fjord at Longyearbyen (Adventfjorden) in the Norwegian Arctic because it is a cold system, where the KOC values are expected to be substantially different from those at 21 °C and where the data on (ΔHOW and ΔHOC) will impact the model assessment. In stage 2, we used the KOC values at 21 °C. All modeling was done according to the good modeling practice guidelines as introduced by Buser et al.34
Stage 1: chemical classification
The chemicals evaluated in this study partition to all environmental media and therefore are classified as Type 1 chemicals according to the classification system suggested by Mackay et al.33 The physicochemical properties of the chemicals used in the modeling calculations together with references are presented in Table 1. The KOC of VMS was corrected for temperature changes using the values for ΔHOC but we also explored a scenario where ΔHOC were assumed to be equal to reported values of ΔHOW. Except for KOC, ΔHOC and ΔHOW all other parameters were the same in all the modeling scenarios.| D4 | D5 | D6 | L4 | L5 | |
|---|---|---|---|---|---|
a The vapor pressures for all chemicals except for L4 were measured experimentally in the study of Lei et al.,35 whereas the vapor pressure for L4 was estimated based on their regression.35
b The values for KOW and KAW of D4, D5, D6 and L4 were measured experimentally in the studies of Xu and Kropscott.31 The KOW and KAW of L5 were estimated from PP-LFER regressions, which were constructed as suggested by Goss36 combining the data of Abraham et al.37 with the measurements of Xu and Kropscott31 in their training sets. The values for KOA were calculated by subtracting the values for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW from those for log KAW from those for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW.
c The values for the salting-out constants (Ks) of VMS were measured by Panagopoulos et al.16,17
d The values for ΔHOW, ΔHAW and ΔHOA of D4, D5 and L4 were measured in the study of Xu and Kropscott.31 The values for ΔHOW, ΔHAW and ΔHOA of D6 and L5 were calculated by linearly extrapolating from the measured values based on the chemicals' molecular weight.
e The values for ΔHOC were measured by Panagopoulos et al.32
f The activation energies (Eact) for degradation in the air of D4 and D5 were measured in the study of Xiao et al.13 The value of D6 was calculated by extrapolating from the data for D4 and D5. The value for L4 was measured by Zammit38 and the value of L5 was calculated by extrapolating from the data of Zammit38 for L2, L3 and L4.
g The activation energies for hydrolysis of D4, D5 and D6 were measured by Xu and Kozerski.39 Since no clear trend was observed between these values and the size of the molecules, the values of L4 and L5 were assumed to be the same as the measured ones.
h The half-lives of D4 and D5 in air were measured by Xiao et al.,13 the half-life of D6 was calculated by extrapolating from the measurements for D4 and D5. The half-life of L4 was measured by Zammit38 and the half-life of L5 was estimated by extrapolating from the measurements for L2, L3 and L4.
i The half-lives of D4 and D5 in water at 25 °C and pH 7 and 8 were calculated by Environment Canada4–6 based on hydrolysis data from Dow Corning. The half-lives of D6, L4, and L5 were extrapolated from the data for D4 and D5.
j The half-lives of D4, D5 and D6 in soil were calculated by Xu.40 The half-lives for L4, and L5 were assumed to be the same as those of D4 and D5.
k The half-lives of D4 and D5 in sediment were measured by Xu and Miller41–43 and the half-lives of D6, L4, and L5 were estimated by extrapolating from the data for D4 and D5. Since these half-lives were measured in experiments with bulk sediments we chose not to adjust them based on concentrations in pore water. KOW.
c The values for the salting-out constants (Ks) of VMS were measured by Panagopoulos et al.16,17
d The values for ΔHOW, ΔHAW and ΔHOA of D4, D5 and L4 were measured in the study of Xu and Kropscott.31 The values for ΔHOW, ΔHAW and ΔHOA of D6 and L5 were calculated by linearly extrapolating from the measured values based on the chemicals' molecular weight.
e The values for ΔHOC were measured by Panagopoulos et al.32
f The activation energies (Eact) for degradation in the air of D4 and D5 were measured in the study of Xiao et al.13 The value of D6 was calculated by extrapolating from the data for D4 and D5. The value for L4 was measured by Zammit38 and the value of L5 was calculated by extrapolating from the data of Zammit38 for L2, L3 and L4.
g The activation energies for hydrolysis of D4, D5 and D6 were measured by Xu and Kozerski.39 Since no clear trend was observed between these values and the size of the molecules, the values of L4 and L5 were assumed to be the same as the measured ones.
h The half-lives of D4 and D5 in air were measured by Xiao et al.,13 the half-life of D6 was calculated by extrapolating from the measurements for D4 and D5. The half-life of L4 was measured by Zammit38 and the half-life of L5 was estimated by extrapolating from the measurements for L2, L3 and L4.
i The half-lives of D4 and D5 in water at 25 °C and pH 7 and 8 were calculated by Environment Canada4–6 based on hydrolysis data from Dow Corning. The half-lives of D6, L4, and L5 were extrapolated from the data for D4 and D5.
j The half-lives of D4, D5 and D6 in soil were calculated by Xu.40 The half-lives for L4, and L5 were assumed to be the same as those of D4 and D5.
k The half-lives of D4 and D5 in sediment were measured by Xu and Miller41–43 and the half-lives of D6, L4, and L5 were estimated by extrapolating from the data for D4 and D5. Since these half-lives were measured in experiments with bulk sediments we chose not to adjust them based on concentrations in pore water.
|
|||||
| Vapor pressure (Pa)a | 126 | 20.4 | 2.26 | 40.2 | 6.0 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOWb KOWb |
6.98 | 8.07 | 8.87 | 8.14 | 8.70 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAWb KAWb |
2.74 | 3.16 | 3.01 | 3.45 | 3.13 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOAb KOAb |
4.24 | 4.91 | 5.86 | 4.71 | 5.57 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOC Panagopoulos et al.16,17 KOC Panagopoulos et al.16,17 |
5.13 | 6.30 | 7.13 | 6.24 | 7.26 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOC Kozerski et al.26 KOC Kozerski et al.26 |
4.44 | 5.17 | — | 5.16 | — |
| Salting-out constant (Ks)c | 0.42 | 0.34 | 0.37 | 0.25 | 0.37 |
| ΔHOW (kJ mol−1)d | 31.9 | 68.8 | 105.7 | 11.3 | 14.0 |
| ΔHOC (kJ mol−1)e | −79.2 | −48.0 | −48.3 | −67.6 | −45.8 |
| ΔHAW (kJ mol−1)d | 73.9 | 123.9 | 173.9 | 65.5 | 81.0 |
| ΔHOA (kJ mol−1)d | −43.7 | −47.3 | −50.9 | −46.9 | −58.0 |
| E act for reaction with ˙OH (kJ mol−1)f | −0.71 | 3.31 | 6.85 | 5 | 4.8 |
| E act for hydrolysis (kJ mol−1)g | 87.6 | 87.2 | 93.5 | 87.6 | 87.2 |
| Half-life in air at 25 °C (h)h | 108 | 101 | 79 | 55 | 13 |
| Half-life in water at pH 7 and 25 °C (h)i | 89 | 1776 | 3463 | 409 | 2096 |
| Half-life in water at pH 8 and 25 °C (h)i | 70 | 216 | 362 | 98 | 244 |
| Half-life in soil at 25 °C (h)j | 127 | 302 | 9624 | 127 | 302 |
| Half-life in sediment at 25 °C (h)k | 8760 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 055 055 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 228 228 |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867 867 |
Discharge scenarios
Since this is primarily an evaluative study and the scope of the study is to evaluate the differences in the chemicals' residence times that may occur due to differences in the reported KOC and ΔH values, we have not estimated site-specific emission rates for Adventfjorden. Instead, in the Adventfjorden scenarios we assumed that all chemicals were emitted to water and all emission rates were set at a constant value. The same approach is also used in the study of Mackay et al.19Stage 2: evaluative assessment of chemical fate
As in the study by Mackay et al.,19 our evaluative assessment was conducted using the EQC model distributed by Trent University, Canada. A description of the model can be found in the studies of Mackay et al.19,33,44 The model was run for all three levels of fugacity calculations (Level I, II and III). Level I refers to a model at steady-state and equilibrium, Level 2 refers to steady-state and equilibrium but it also includes processes of advection and reaction, Level III to steady-state non-equilibrium, and Level IV refers to non-steady-state non-equilibrium.45 The area of the environment in the EQC model is 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2, and it has environmental properties similar to the U.S. state of Pennsylvania or of South Korea. Our modeling scenarios are all direct applications of the standard scenario found in EQC.
000 km2, and it has environmental properties similar to the U.S. state of Pennsylvania or of South Korea. Our modeling scenarios are all direct applications of the standard scenario found in EQC.
Results from the Level I EQC calculations indicate that under equilibrium and steady state conditions the bulk of all the VMS chemicals considered here will mainly partition to the air, and that this result is not sensitive to which KOC values are used as input to the model. A summary of the results of the Level 1 EQC modeling assessment is presented in Table 3.
| Parameter | Value for January | Value for July | Source |
|---|---|---|---|
| a We assumed a residence time of water in the fjord of 6 months for winter and 5 months for summer. This assumption is based on the observations of Basedow et al.52 for Kongsfjorden in Svalbard. No measurements were found for Adventfjorden. Basedow et al.52 measured a residence time of water in Kongsfjorden of about 6 days. Kongsfjorden is directly exposed to the Atlantic Ocean, while Adventfjorden is a small fjord inside a larger fjord (Isfjorden) and the water exchange there is expected to be substantially slower. For that reason, we chose the value of 6 months. The difference between winter and summer is due to additional water flowing into the fjord from the rivers and due to the ice cover melting.48 The residence times of water are primarily controlled by the inflow of ocean water into the fjord and by the outflow of fjord water into the ocean. b The values for MTCw and MTCa in winter were assumed to be extremely low because the fjord is covered with ice and there is no volatilization. | |||
| Air temperature | −7 | 8 | Weslawski48 |
| Water temperature | 0 | 5 | Weslawski48 |
| Fjord area (m2) | 2.8 × 107 | 2.8 × 107 | Weslawski48 |
| Fjord mean depth (m) | 75 | 75 | Weslawski48 |
| Fjord volume (m3) | 2.10 × 109 | 2.10 × 109 | Weslawski48 |
| Precipitation (m h−1) | 2.28 × 10−5 | 2.28 × 10−5 | Hanssen-Bauer51 |
| Residence time of water (months) | 6a | 5a | Weslawski48 and Basedow et al.52 |
| Concentration of suspended particles (mg L−1) | 35.3 | 223.5 | Zajaczkowski and Wlodarska-Kowalczuk49 |
| OC fraction of suspended particles (%) | 2 | 2 | Warner et al.50 |
| OC fraction of sediment particles (%) | 2 | 2 | Warner et al.50 |
| Sediment deposition rate (g m−2 d−1) | 4.2 | 464.1 | Zajaczkowski and Wlodarska-Kowalczuk49 |
| Sediment resuspension rate (g m−2 d−1) | 0.634 | 0.634 | Mackay45 |
| Sediment burial rate (g m−2 d−1) | 1.96 | 1.96 | Mackay45 |
| MTC at the water side of the air–water interphase (m h−1) | 3 × 10−7b | 0.05 | Mackay45 |
| MTC at the air side of the air–water interphase (m h−1) | 3 × 10−5b | 5 | Mackay45 |
| Sediment layer depth (m) | 0.05 | 0.05 | Mackay45 |
| MTC at the water side of the water-sediment interphase (m h−1) | 0.01 | 0.01 | Mackay45 |
| Level I substance | Amount (kg) | |||
|---|---|---|---|---|
| In air | In water | In sediment | In soil | |
| Panagopoulos et al.16,17 K OC | ||||
| D4 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 891 891 |
0.364 | 2.35 | 106 |
| D5 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
0.148 | 9.34 | 420 |
| D6 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 495 495 |
0.185 | 120 | 5381 |
| L4 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
0.0708 | 5.9 | 266 |
| L5 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 374 |
0.14 | 122 | 5500 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Kozerski et al. 26 K OC | ||||
| D4 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 986 |
0.364 | 0.297 | 13.4 |
| D5 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 951 951 |
0.148 | 1.05 | 47.3 |
| L4 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 979 979 |
0.0709 | 0.459 | 20.7 |
In Level II and III we focus our assessment on the persistence of the VMS modeled as residence times in individual compartments (water and sediment), and as the overall residence time in the modeled regions. The residence times are defined as follows.
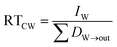 | (1) |
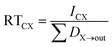 | (2) |
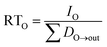 | (3) |
The Level II EQC modeling assessment shows that the overall persistence of all VMS are similar, and dominated by processes in the air compartment. Advection and reaction in the air are the main removal mechanisms (Table 4). Again, there are no notable differences between calculations using the KOC measurements from Panagopoulos et al.16,17 and those of Kozerski et al.26
| Level II substance | Amount | ||||||
|---|---|---|---|---|---|---|---|
| In air (kg) | In water | In sediment | In soil | Lost by advection | Lost by reaction | Overall residence time (h) | |
| Panagopoulos et al.16,17KOC | |||||||
| D4 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 892 |
0.222 | 1.44 | 64.6 | 609 | 391 | 61 |
| D5 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 273 273 |
0.0879 | 5.56 | 250 | 593 | 407 | 60 |
| D6 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 259 259 |
0.104 | 67.4 | 3033 | 533 | 467 | 56 |
| L4 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721 721 |
0.0438 | 3.65 | 164 | 617 | 382 | 62 |
| L5 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 123 |
0.0847 | 74 | 3329 | 571 | 421 | 61 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Kozerski et al. 26 K OC | |||||||
| D4 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911 |
0.222 | 0.181 | 8.14 | 609 | 391 | 61 |
| D5 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303 303 |
0.0879 | 0.624 | 28.1 | 593 | 407 | 59 |
| L4 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772 772 |
0.0438 | 0.284 | 12.8 | 618 | 382 | 62 |
The Level III EQC modeling assessment shows that for all VMS the medium of release strongly affects the distribution of the chemicals between air, water, soil and sediment. When released in the air all VMS tend to remain in air and they are removed from the environment through advection and reaction. When the VMS are released in water they tend to partition to the sediment, which substantially prolongs their overall residence times compared to the release to air scenario due to lack of advection and slower degradation rates. When emissions occur to soil the VMS with lower KOC tend to mainly partition to air while those with higher KOC mainly reside in soil. Out of all three emission scenarios, emissions to water showed the longest overall residence times (Table 5), and residence times increased with increasing hydrophobicity. When cVMS are emitted to water the overall residence times range from 8 to 1123 days and those of lVMS range from 97 to 1194 days. Emissions to water result in the longest residence times, and will occur through wastewater treatment plants, so we focused our comparisons on that scenario.
| Level III substance | Emission medium | Amount (kg) | ||||
|---|---|---|---|---|---|---|
| In air | In water | In sediment | In soil | Overall residence time (h) | ||
| Panagopoulos et al.16,17KOC | ||||||
| D4 | Air | 6090 | 0.00488 | 0.0344 | 5.01 | 61 |
| Water | 1094 | 8649 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 955 955 |
0.9 | 707 | |
| Soil | 4566 | 0.0716 | 0.505 | 4590 | 92 | |
| All three | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
8649 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 956 956 |
4596 | 287 | |
| Air | 5928 | 0.021 | 1.41 | 16.4 | 60 | |
| D5 | Water | 1183 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 871 871 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.28 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 236 236 |
| Soil | 3839 | 0.406 | 27.2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389 |
193 | |
| All three | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 951 951 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872 872 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409 409 |
5496 | |
| Air | 5326 | 0.0131 | 1.38 | 247 | 56 | |
| D6 | Water | 158 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 307 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.33 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 975 |
| Soil | 4333 | 6.6 | 697 | 259![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2638 | |
| All three | 9817 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 314 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
259![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
9889 | |
| Air | 6175 | 0.00199 | 0.0988 | 9.15 | 62 | |
| L4 | Water | 711 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691 |
826![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.05 | 8438 |
| Soil | 3389 | 0.145 | 7.2 | 8271 | 117 | |
| All three | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691 |
826![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8281 | 2872 | |
| Air | 5751 | 0.00606 | 0.608 | 39.6 | 58 | |
| L5 | Water | 120 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664 664 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.827 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980 980 |
| Soil | 680 | 0.915 | 91.8 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 433 |
392 | |
| All three | 6561 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665 665 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473 |
8143 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Kozerski et al. 26 K OC | ||||||
| D4 | Air | 6091 | 0.00496 | 0.0051 | 0.983 | 61 |
| Water | 1310 | 8831 | 9095 | 0.211 | 192 | |
| Soil | 5792 | 0.0493 | 0.0508 | 901 | 67 | |
| All three | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 193 193 |
8831 | 9095 | 902 | 107 | |
| Air | 5930 | 0.022 | 0.397 | 2.85 | 59 | |
| D5 | Water | 3044 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
457![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.46 | 4859 |
| Soil | 5568 | 0.128 | 2.31 | 2672 | 82 | |
| All three | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
457![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2676 | 1667 | |
| Air | 6177 | 0.0021 | 0.0239 | 1.4 | 62 | |
| L4 | Water | 2372 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 704 704 |
213![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.536 | 2338 |
| Soil | 5752 | 0.0376 | 0.427 | 1262 | 70 | |
| All three | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302 302 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 704 704 |
213![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1264 | 823 | |
When emitted to water in the generic EQC model, all VMS except D4 exceed the 100-day persistence criterion suggested by Webster et al.25 (Fig. 1). Large differences were observed between the simulations based on the KOC measurements of Panagopoulos et al.16,17 and those of Kozerski et al.26 The largest difference observed was for D5 emitted to water. Using the KOC measurements of Kozerski et al.26 the overall residence time for D5 is 203 d while using the KOC measurements of Panagopoulos et al.16,17 the overall residence time is 676 d. The difference of one log unit in the KOC of D5 resulted in almost 500-days difference in the modeled overall residence times.
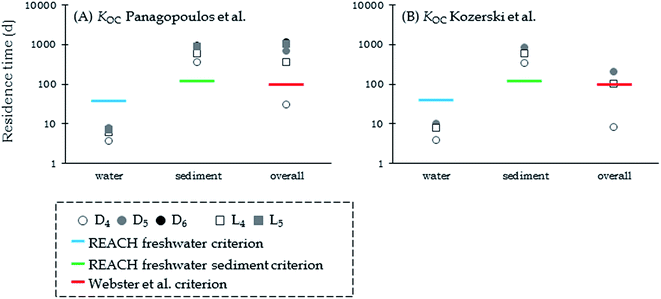 | ||
| Fig. 1 Compartment-specific and overall residence times for VMS calculated in the EQC model using the KOC measurements of (A) Panagopoulos et al.16,17 and (B) those of Kozerski et al.26 The blue line shows the REACH criterion for freshwater and the green line shows that for freshwater sediment.24 The red line shows the 100-day criterion for overall persistence suggested by Webster et al.25 | ||
The modeled residence times in the water compartment of all chemicals are below the REACH criterion for freshwater.24 However, the modeled residence times of all chemicals in the sediment compartment exceed the REACH criterion for freshwater sediment, regardless of which KOC values are used.24 Compartment specific residence times in water and sediment are almost the same for the two different KOC values, however the overall residence times are substantially longer using the KOC measured by Panagopoulos et al.16,17 (Fig. 1). The explanation lies in the distribution of cVMS among the different compartments and the total inventory. The larger inventory of cVMS in the sediment when using the KOC value of Panagopoulos et al.16,17 results in much longer overall residence times but it does not strongly affect the compartment specific residence times for sediment.
This evaluative assessment confirms the modeling results presented by Whelan et al.14,15 in that it underlines the importance of KOC and the importance of the emission medium, especially when emissions are to water, in the chemical fate and persistence of VMS in the environment. These results also agree with the studies of Hughes et al.46 and Xu and Wania.47
Stage 3 and 4: regional/local or near-field evaluation
The fate of cVMS and lVMS in Adventfjorden, Svalbard is particularly interesting because it is a coastal system with low water temperatures all year round, seasonal variability in particle deposition, and ice coverage during winter.48,49 Since 2006–2007 the ice thickness and coverage of the fjord has been declining. However, we kept that parameter in our modeling because we wanted to see how ice coverage may affect the behavior of VMS. Adventfjorden receives wastewater from Longyearbyen without any mechanical, chemical or biological treatment. Warner et al.50 showed a decrease in the concentrations of D5 in the sediment with increasing distance from the wastewater outlet indicating that wastewater is the main source of VMS. The effluent is released to the fjord at 62 m depth about 1.5 km away from the coast.Our Adventfjorden model is a non-equilibrium Level III and Level IV model, which was specifically parameterized to describe the environmental fate of chemicals in coastal environments. The model is similar to the EQC model in structure, and describes chemical behavior using the fugacity concept by Mackay.45 A diagram of the model environment is shown in Fig. 2.
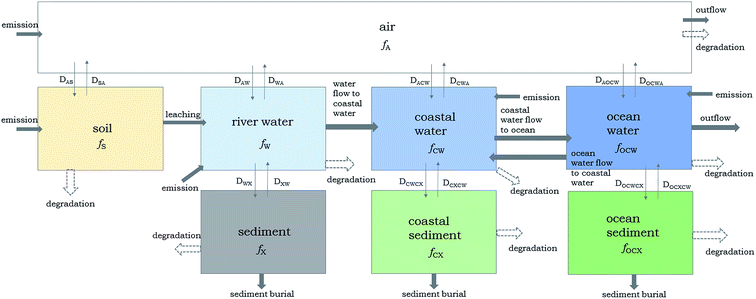 | ||
| Fig. 2 Diagram of the Adventfjorden model based on the fugacity approach as introduced by Mackay.45f refers to the fugacity (Pa) of each individual compartment and D is the fugacity rate descriptor (mol h−1 Pa−1) between compartments. For example, the fugacity rate descriptor from air to water is shown as DAW. | ||
The physical characteristics of Adventfjorden are summarized in Table 2. Adventfjorden is a small fjord located close to Longyearbyen in Svalbard, Norway, with a total area of about 28 km2.48 It has an average depth of 75 m and is rather steep, with a 50 m km−1 depth increase for the first km from the coast.48 The main source of water into the fjord is exchange of seawater from the ocean. Adventfjorden receives freshwater primarily from the rivers Adventelva and Longyearelva with average water flows of 3 m3 s−1 for each river and from sea ice and snow cover melting.48 The residence time of the water in the fjord is controlled by the inflow of ocean water into the fjord and by the outflow of fjord water into the ocean. The freshwater from the rivers and the ice cover melting are minor contributions to the overall residence time.48 Zajaczkowski and Wlodarska-Kowalczuk49 measured the concentrations of suspended particles and the sedimentation rates at different sites across the fjord. Based on their observations an average concentration of suspended particles of 223.5 mg L−1 was used in our model scenarios for July and 35.3 mg L−1 was used for model scenarios for January. For the months in between we calculated the concentration of suspended particles assuming that it increases logarithmically. A value of 464.1 g m−2 per day sedimentation rate was used in scenarios for July and a value of 4.2 g m−2 per day was used for January. For the months between, the sedimentation rates were calculated as described above for the concentration of suspended particles. The fraction of total OC in the sediment of Adventfjorden is around 2% (Warner et al.50). The same OC content was assumed for the suspended particles.
In winter the mass transfer coefficients at the water side (MTCw) and at the air side (MTCa) of the air–water interface were assumed to be extremely low in January and December to simulate ice cover, and to logarithmically increase in the months in between (Table 2). For all other parameters, we used data from the literature for each month.
In Fig. 3 and 4 we present steady-state calculations of the residence times of VMS in Adventfjorden for each month over a period of one year. In all scenarios, the emissions of the chemicals were directed 100% into water. In cases where residence times exceed 1 month, the modeled steady-state conditions will not be approached in the real system, and unsteady-state (Level IV) model results are presented below. However, we chose to model hypothetical steady-state conditions for each month in order to explore bounding scenario for variability in the system that illustrate the effects of temperature, ice coverage and varying sedimentation rates on the residence times of the chemicals in the fjord. Results in Fig. 3 are for KOC corrected for temperature using the ΔHOW measurements of Xu and Kropscott31 and results in Fig. 4 are for a scenario in which KOC was corrected for temperature changes using the ΔHOC measurements of Panagopoulos et al.32 We present both compartment-specific and overall residence times.
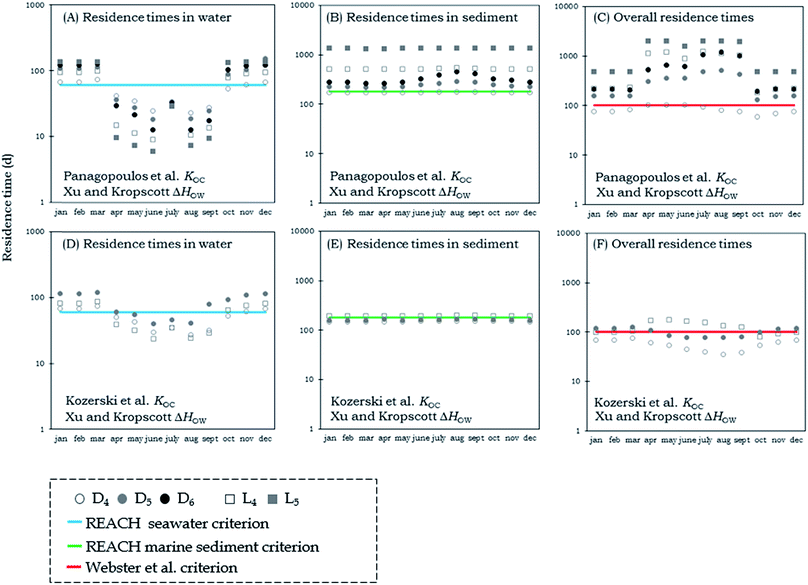 | ||
| Fig. 3 Water, sediment and overall residence times for cVMS (circles) and lVMS (squares) using both the KOC measurements of Kozerski et al.26 (lower panels) and those of Panagopoulos et al.16,17 (upper panels). The KOC was corrected for temperature changes using the ΔHOW measurements of Xu and Kropscott.31 The blue line shows the REACH criterion for persistence in marine waters and the green line shows the REACH criterion for persistence in marine sediments.24 The red line shows the 100-day overall persistence criterion suggested by Webster et al.25 | ||
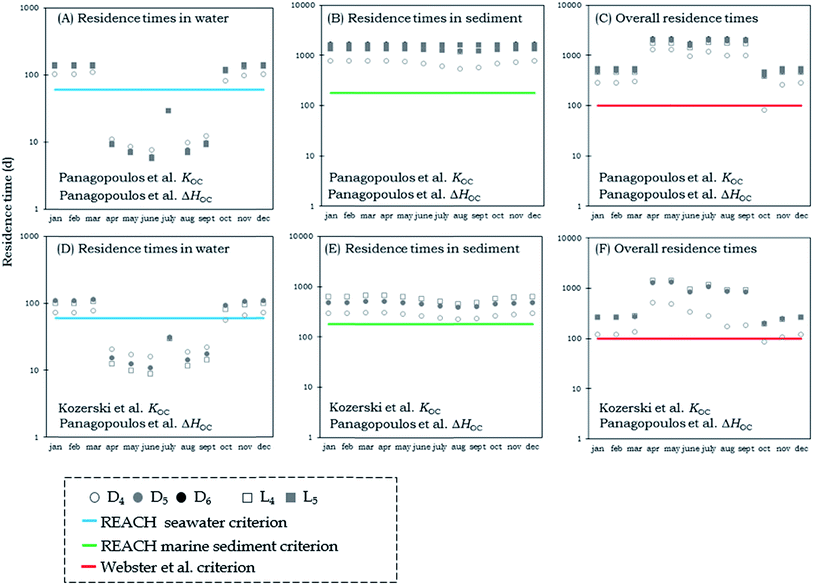 | ||
| Fig. 4 Water, sediment and overall residence times for cVMS (circles) and lVMS (squares) using both the KOC measurements of Kozerski et al.26 (lower panels) and those of Panagopoulos et al.16,17 (upper panels). The KOC was corrected for temperature changes using the ΔHOC measurements of Panagopoulos et al.32 The blue line shows the REACH criterion for persistence in marine waters and the green line shows the REACH criterion for persistence in marine sediments.24 The red line shows the 100-day overall persistence criterion suggested by Webster et al.25 | ||
The residence times of the chemicals in water were found to vary considerably in the steady state scenarios for different months (Fig. 3 & 4). The variation depends on the ice formation and melting in the fjord. In the winter months when the fjord is covered with ice the residence times of the chemicals in water exceed the REACH criterion for marine waters.24 In the summer months when the ice has melted and volatilization is not restricted the residence times are below the REACH criterion for marine waters.24 In July the concentrations of VMS increase substantially compared to the values for June and August. The reason behind that difference is that in July we have the highest concentration of suspended particles. The larger amount of organic carbon in water in July increases the residence times in water.
The residence times of VMS in sediment are less variable in the range of scenarios. Ice formation and melting does not affect the sediment residence times substantially. In the scenarios using the KOC measurements of Kozerski et al.,26 the residence times were all shorter than in scenarios using the measurements of Panagopoulos et al.16,17 and in the case where we used the ΔHOW measurements of Xu and Kropscott,31 the residence times were very close to the criterion values for persistence in marine sediments. On the other hand, scenarios using the ΔHOC measurements of Panagopoulos et al.32 had residence times of VMS that exceeded the REACH criterion for marine sediments in all cases except for D4, where residence times were almost equal to the residence time of the REACH criterion.24
Similar results were observed for the overall residence times. In the majority of scenarios, the overall residence times exceed the 100-day criterion of Webster et al.25 but the magnitude of that exceedance varies considerably among the different scenarios. In the scenario using KOC measurements of Kozerski et al.26 and ΔHOW measurements of Xu and Kropscott31 the overall residence times were in all cases between 50 and 200 days, while in the scenario using the KOC measurements and ΔHOC of Panagopoulos et al.16,17,32 the majority of calculated overall residence times were between 100 and 1000 days. Our calculations using scenarios based on measurements of Panagopoulos et al.16,17,32 suggest that, in contrast to findings in the study of Mackay et al.19 and the assessments of UK Environment Agency1–3 and Environment Canada,4–6 VMS cannot be categorized as non-persistent since their residence times exceed by far the REACH criterion for marine sediments24 and the 100-day criterion of Webster et al.25 One could expect to see longer residence times in the winter than in the summer due to the ice-melting and warmer water temperatures. However, the effect of higher sediment deposition rates in the summer outweighs the effect of ice-melting and higher water temperatures.
In Fig. 5 we present the modeled elimination of VMS from Adventfjorden in a Level IV unsteady-state model scenario over the course of one year if the system starts at steady-state conditions for average values of environmental parameters and emissions are stopped at time 0. Large differences are observed for the different scenarios. In the scenario using KOC measurements of Kozerski et al.26 and ΔHOW measurements of Xu and Kropscott,31 1 year after the end of emissions the modeled amount of D5 in Adventfjorden decreases to almost 0% of the initial amount. However, in the scenarios using KOC measurements and ΔHOC of Panagopoulos et al.16,17,32 the modeled amount of D5 declines to only about 65% of the initial amount.
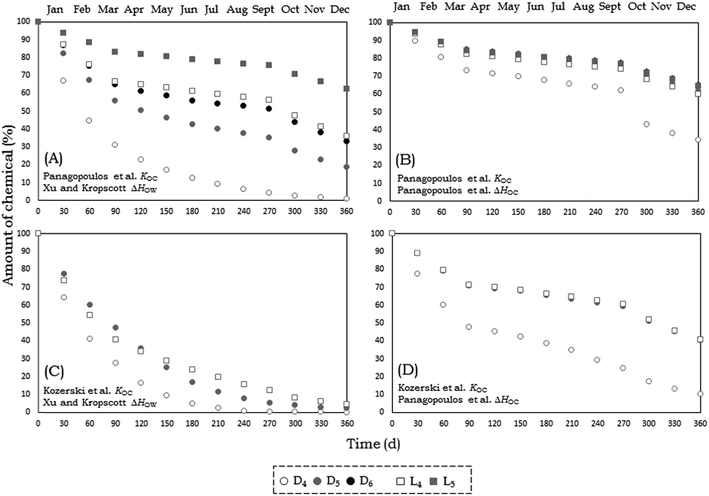 | ||
| Fig. 5 Elimination of cVMS (circles) and lVMS (squares) from Adventfjorden over the course of one year if emissions stopped at time 0. Panels A and B show the elimination data for cVMS and lVMS using the KOC measurements of Panagopoulos et al.16,17 and panels C and D show the elimination data for cVMS and lVMS using the KOC measurements of Kozerski et al.26 The panels on the left (A and C) show the elimination data for cVMS and lVMS using the ΔHOW measurements of Xu and Kropscott31 to correct for temperature and the panels on the right show the elimination data for cVMS and lVMS using the ΔHOW measurements of Panagopoulos et al.32 | ||
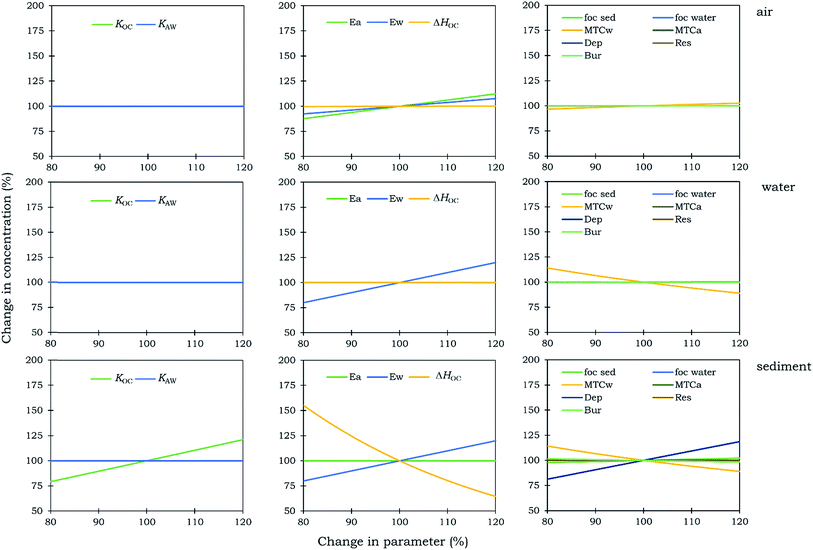
Fig. 6 shows results of a sensitivity analysis of the steady-state (Level III) version of the Adventfjorden model. The parameters that were included in the sensitivity analysis were selected based on whether they have an influence on the residence times of the chemicals in the sediment and the overall residence times. Parameters that influence only the residence times of the chemicals in water, such as the residence time of water in the fjord were not included in the sensitivity analysis. The parameters that were found to be most sensitive are ΔHOC, KOC, the fraction of organic carbon in the water (fOC) and the deposition rate of sediment particles (Dep). This observation is also supported by the findings of Krogseth et al.53 Out of all compartments, these parameters were found to be especially sensitive for the concentrations of VMS in the sediment. One could expect to see large differences in the concentrations of VMS in water too but the amount of siloxane in the water compartment is small under all scenarios and thus appears insensitive to changes in ΔHOC, KOC, fOC and Dep. The results of the sensitivity analysis underline the importance of accurately determining the KOC and ΔHOC of VMS in order to study their environmental fate using multimedia models. Our findings from the sensitivity analysis are in good agreement with those of Whelan.14,15 Both studies agree that KOC and other parameters directly related to KOC, such as sediment deposition and resuspension rates are the most sensitive parameters of the models.
Conclusions
A major challenge in modeling the environmental fate of VMS in aquatic environments using multimedia models has been obtaining reliable data for KOC and ΔHOC. Our work demonstrates that these two parameters are the most sensitive in the region-specific modeling assessment. The difference of one log unit between the KOC measurements of Kozerski et al.26 and those of Panagopoulos et al.16,17 in combination with differences in reported ΔHOW and ΔHOC resulted in substantial differences in the environmental fate and residence times of VMS. Our results suggest that residence times of VMS may be substantially longer when using ΔHOC instead of ΔHOW in the modeling calculations of VMS. Also because of the difference in the sign (+ or −) of ΔHOW and ΔHOC of VMS, modeling calculations of the effect of temperature on the residence times of VMS may show contradictory results. Calculations using ΔHOW would indicate that the residence times of VMS are shorter in cold waters,54 while calculations using ΔHOC would indicate that they are longer in cold waters. In the light of these new results we suggest that VMS are monitored in aquatic environments in order to assess their persistence and the potential environmental threat they may pose in the future.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the Swedish Research Council FORMAS for funding this project (project number 2011-484) and Mick Whelan for useful comments and suggestions.References
- D. N. Brooke, M. J. Crookes, D. Gray and S. Robertson, Risk Assessment Report: Octamethylcyclotetrasiloxane, Environment Agency of Great Britain, 2009 Search PubMed.
- D. N. Brooke, M. J. Crookes, D. Gray and S. Robertson, Risk Assessment Report: Decamethylcyclopentasiloxane, Environment Agency of Great Britain, 2009 Search PubMed.
- D. N. Brooke, M. J. Crookes, D. Gray and S. Robertson, Risk Assessment Report: Dodecamethylcyclohexasiloxane, Environment Agency of Great Britain, 2009 Search PubMed.
- Environment Canada, Screening assessment for the challenge octamethylcyclotetrasiloxane, Chemical abstracts service registry number 556-67-2, Environment Canada, 2008 Search PubMed.
- Environment Canada, Screening assessment for the challenge decamethylcyclopentasiloxane, Chemical abstracts service registry number 541-02-6, Environment Canada, 2008 Search PubMed.
- Environment Canada, Screening assessment for the challenge dodecamethylcyclohexasiloxane, Chemical abstracts service registry number 540-97-6, Environment Canada, 2008 Search PubMed.
- Environment Canada, Screening assessment for the challenge siloxanes and silicones, di-Me, hydrogen-terminated, Chemical abstracts service registry number 70900-21-9, Environment Canada, 2011 Search PubMed.
- Y. Lu, T. Yuan, W. Wang and K. Kannan, Concentrations and assessment of exposure to siloxanes and synthetic musks in personal care products from China, Environ. Pollut., 2011, 159, 3522–3528 CrossRef CAS PubMed.
- I. S. Krogseth, A. Kierkegaard, M. S. McLachlan, K. Breivik, K. M. Hansen and M. Schlabach, Occurrence and seasonality of cyclic volatile methyl siloxanes in Arctic air, Environ. Sci. Technol., 2013, 47, 502–509 CrossRef CAS PubMed.
- M. McLachlan, A. Kierkegaard, K. M. Hansen, R. van Egmond, J. H. Christensen and C. A. Skjøth, Concentrations and fate of decamethylcyclopentasiloxane (D5) in the atmosphere, Environ. Sci. Technol., 2010, 44, 5365–5370 CrossRef CAS PubMed.
- C. Sparham, R. van Egmond, C. Hastie, S. O'Connor, D. Gore and N. Chowdhury, Determination of decamethylcyclopentasiloxane in river and estuarine sediments in the UK, J. Chromatogr. A, 2011, 1218(6), 817–823 CrossRef CAS PubMed.
- K. Borgå, E. Fjeld, A. Kierkegaard and M. S. McLachlan, Consistency in trophic magnification factors of cyclic methyl siloxanes in pelagic freshwater food webs leading to brown trout, Environ. Sci. Technol., 2013, 47, 14394–14402 CrossRef PubMed.
- R. Xiao, I. Zammit, Z. Wei, W.-P. Hu, M. MacLeod and R. Spinney, Kinetics and mechanism of the oxidation of cyclic methylsiloxanes by hydroxyl radicals in the gas phase: An experimental and theoretical Study, Environ. Sci. Technol., 2015, 49, 13322–13330 CrossRef CAS PubMed.
- M. J. Whelan, D. Sanders and R. van Egmond, Effect of Aldrich humic acid on water-atmosphere transfer of decamethylcyclopentasiloxane, Chemosphere, 2009, 74, 1111–1116 CrossRef CAS PubMed.
- M. J. Whelan, R. van Egmond, D. Gore and D. Sanders, Dynamic multi-phase partitioning of decamethylcyclopentasiloxane (D5) in river water, Water Res., 2010, 44, 3679–3686 CrossRef CAS PubMed.
- D. Panagopoulos, A. Jahnke, A. Kierkegaard and M. MacLeod, Organic carbon/water and dissolved organic carbon/water partitioning of cyclic volatile methylsiloxanes: measurements and polyparameter linear free energy relationships, Environ. Sci. Technol., 2015, 49, 12161–12168 CrossRef CAS PubMed.
- D. Panagopoulos, A. Kierkegaard, A. Jahnke and M. MacLeod, Evaluating the salting-out effect on the organic carbon/water partition ratios (KOC and KDOC) of linear and cyclic volatile methylsiloxanes: Measurements and polyparameter free energy relationships, J. Chem. Eng. Data, 2016, 61, 3098–3108 CrossRef CAS.
- M. Whelan, Evaluating the fate and behaviour of cyclic volatile methyl siloxanes in two contrasting North American lakes using a multi-media model, Chemosphere, 2013, 91, 1566–1576 CrossRef CAS PubMed.
- D. Mackay, C. E. Cowan-Ellsberry, D. E. Powell, K. B. Woodburn, S. Xu, G. E. Kozerski and J. Kim, Decamethylcyclopentasiloxane (D5) environmental sources, fate, transport, routes of exposure, Environ. Toxicol. Chem., 2015, 34, 1–14 CrossRef.
- European Chemicals Agency, Previous Consultations on Restriction Proposals for Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5), 2015, https://echa.europa.eu/previous-consultations-on-restriction-proposals/-/substance-rev/9444/term, latest access: December 11, 2017 Search PubMed.
- European Chemicals Agency, 2015, Member State Committee Opinion on Persistence and Bioaccumulation of Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5), https://echa.europa.eu/documents/10162/13641/art77-3c_msc_opinion_on_d4_and_d5_20150422_en.pdf, latest access December 11, 2017 Search PubMed.
- Government of Canada, Canadian Environmental Protection Act, 1999. Canada Gazette Part III, 1999, vol 22, http://www.ec.gc.ca/lcpe-cepa/default.asp?lang1/4En%26n1/4CC0DE5E2–1%26toc1/4hide, latest access: 10/11/16 Search PubMed.
- Siloxane D5 Board of Review, Report of the Board of Review for decamethylpentacyclosiloxane (D5), Government of Canada, Ottawa, ON, Canada, 2011, http://www.ec.gc.ca/lcpe-cepa/default.asp?lang1/4En%26n1/4515887B7–1%26offset1/41%26toc1/4show#archived, latest access: 10/11/16 Search PubMed.
- REACH Online, Annex XIII: Criteria for the identification of persistent, bioaccumulative and toxic substances, and very persistent and very bioaccumulative substances, http://www.reachonline.eu/REACH/EN/REACH_EN/articleXIII.html, latest access: September 6, 2016.
- E. Webster, D. Mackay and F. Wania, Evaluating environmental persistence, Environ. Toxicol. Chem., 1998, 17, 2148–2158 CrossRef CAS.
- G. E. Kozerski, S. Xu, J. Miller and J. Durham, Determination of soil-water partition coefficients of volatile methylsiloxanes, Environ. Toxicol. Chem., 2014, 33(9), 1937–1945 CrossRef CAS PubMed.
- T. Gouin and T. Harner, Modelling the environmental fate of the polybrominated diphenyl ethers, Environ. Int., 2003, 29, 717–724 CrossRef CAS PubMed.
- A. Palm, I. T. Cousins, D. Mackay, M. Tysklind, C. Metcalfe and M. Alaee, Assessing the environmental fate of chemicals of emerging concern: a case study of the polybrominated diphenyl ethers, Environ. Pollut., 2002, 117, 195–213 CrossRef CAS PubMed.
- T. N. Brown and F. Wania, Development and exploration of an organic contaminant fate model using poly-parameter linear free energy relationships, Environ. Sci. Technol., 2009, 43, 6676–6683 CrossRef CAS PubMed.
- U. Schenker, M. MacLeod, M. Scheringer and K. Hungerbühler, Improving data quality for environmental fate models: A least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds, Environ. Sci. Technol., 2005, 39, 8434–8441 CrossRef CAS PubMed.
- S. Xu and B. Kropscott, Evaluation of the three-phase equilibrium method for measuring temperature dependence of internally consistent partition coefficients (KOW, KOA and KAW) for volatile methylsiloxanes and trimethylsilanol, Environ. Toxicol. Chem., 2014, 33, 2702–2710 CrossRef CAS PubMed.
- D. Panagopoulos, A. Jahnke, A. Kierkegaard and M. MacLeod, Temperature dependence of the organic carbon/water partition ratios (KOC) of volatile methylsiloxanes, Environ. Sci. Technol. Lett., 2017, 4, 240–245 CrossRef CAS.
- D. Mackay, A. Di Guardo, S. Paterson, G. Kicsi and C. Cowan, Assessing the fate of new and existing chemicals: A five-stage process, Environ. Toxicol. Chem., 1996, 15, 1618–1626 CrossRef CAS.
- A. M. Buser, M. MacLeod, M. Scheringer, D. Mackay, M. Bonnell, M. H. Russel, J. V. DePinto and K. Hungerbuhler, Good modeling practice guidelines for applying multimedia models in chemicals assessments, Integr. Environ. Assess. Manage., 2012, 8, 703–708 CrossRef CAS PubMed.
- Y. D. Lei, F. Wania and D. Mathers, Temperature-dependent vapor pressure of selected cyclic and linear polydimethylsiloxane oligomers, J. Chem. Eng. Data, 2010, 55, 5865–5873 Search PubMed.
- K.-U. Goss, Predicting the equilibrium partitioning of organic compounds using just one linear solvation energy relationship (LSER), Fluid Phase Equilib., 2005, 233, 19–22 CrossRef CAS.
- M. H. Abraham, H. S. Chadha, G. S. Whiting and R. C. Mitchell, Hydrogen bonding. 32. An analysis of water-octanol and water-alkane partitioning and the log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P parameter of seiler, J. Pharm. Sci., 1994, 83, 1085–1100 CrossRef CAS PubMed.
P parameter of seiler, J. Pharm. Sci., 1994, 83, 1085–1100 CrossRef CAS PubMed. - I. Zammit, Gaseous phase reaction kinetics of cyclic and linear siloxanes with hydroxyl radicals, Master thesis, Department of Environmental Science and Analytical Chemistry, Stockholm University, June 2015.
- S. Xu and G. E. Kozerski, Assessment of the fundamental partitioning properties of permethylated cyclosiloxanes, Poster presented at SETAC Europe, Porto, Portugal, May 2007 Search PubMed.
- S. Xu, Fate of cyclic methylsiloxanes in soils. 1. The degradation pathway, Environ. Sci. Technol., 1999, 33, 603–608 CrossRef CAS.
- S. Xu and J. Miller, Aerobic transformation of octamethylcyclotetrasiloxane in aquatic sediment systems, Final Report To CES. Dow Corning TIS Report No. 2009-I0000-61317. 2009a Search PubMed.
- S. Xu and J. Miller, Anaerobic transformation of octamethylcyclotetrasiloxane in aquatic sediment systems, Final Report To CES. Dow Corning TIS Report No. 2009-I0000-61734, 2009b Search PubMed.
- S. Xu and J. Miller, Aerobic and anaerobic transformation of decamethylcyclopentasiloxane in aquatic sediment systems, Final Report To CES. Dow Corning TIS Report No. 2010-I0000-62003, 2010 Search PubMed.
- D. Mackay, S. Paterson, A. Di Guardo and C. E. Cowan, Evaluating the environmental fate of a variety of types of chemicals using the EQC model, Environ. Toxicol. Chem., 1996, 15, 1627–1637 CrossRef CAS.
- D. Mackay, Multimedia environmental models. The fugacity approach, CRC Press, Taylor & Francis Group, 2nd edn, 2001 Search PubMed.
- L. Hughes, D. Mackay, D. E. Powell and J. Kim, An updated state of the science EQC model for evaluating chemical fate in the environment: Application to D5 (decamethylcyclopentasiloxane), Chemosphere, 2012, 87, 118–124 CrossRef CAS PubMed.
- S. Xu and F. Wania, Chemical fate, latitudinal distribution and long-range transport of cyclic volatile methylsiloxanes in the global environment: A modeling assessment, Chemosphere, 2013, 93, 835–843 CrossRef CAS PubMed.
- J. D. Weslawski, Adventfjorden. Arctic sea in the backyard, Institute of Oceanography PAS, Sopot, Poland, 2011 Search PubMed.
- M. Zajaczkowski and M. Wlodarska-Kowalczuk, Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). I. Flux, deposition, and sediment dynamics, Estuarine, Coastal Shelf Sci., 2007, 74, 285–296 CrossRef.
- N. A. Warner, A. Evenset, G. Christensen, G. W. Gabrielsen, K. Borgå and H. Leknes, Volatile siloxanes in the European Arctic: Assessment of sources and spatial distribution, Environ. Sci. Technol., 2010, 44, 7705–7710 CrossRef CAS PubMed.
- I. Hanssen-Bauer, Temperature and precipitation in Svalbard 1912-2050: Measurements and scenarios, Polar Rec., 2002, 38, 225–232 CrossRef.
- S. L. Basedow, K. Eiane, V. Tverberg and M. Spindler, Advection of zooplankton in an Arctic fjord (Kongsfjorden, Svalbard), Estuarine, Coastal Shelf Sci., 2004, 60, 113–124 CrossRef.
- I. S. Krogseth, M. J. Whelan, G. N. Christensen, K. Breivik, A. Evenset and N. A. Warner, Understanding of cyclic volatile methyl siloxane fate in a high latitude lake is constrained by uncertainty in organic carbon–water partitioning, Environ. Sci. Technol., 2017, 51, 401–409 CrossRef CAS PubMed.
- J. Kim, D. Mackay and M. J. Whelan, Predicted persistence and response times of linear and cyclic volatile methylsiloxanes in global and local environments, Chemosphere, 2018, 195, 325–335 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |