Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
γ-Al2O3 nanorods with tuneable dimensions – a mechanistic understanding of their hydrothermal synthesis†
T. E. Bella,
J. M. González-Carballob,
R. P. Toozeb and
L. Torrente-Murciano *a
*a
aDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Philippa Fawcett Drive, Cambridge CB3 0AS, UK. E-mail: lt416@cam.ac.uk
bSasol Technology UK, Purdie Building, North Haugh, St Andrews, KY16 9ST, UK
First published on 24th April 2017
Abstract
This paper reports for the first time the size control of well-defined and morphologically pure alumina (γ-Al2O3) nanorods, presenting an economic and reproducible route for the manufacture of these materials with tuneable sizes for useful applications, for example serving as adsorbents, catalysts and catalyst supports. A detailed understanding of the different steps taking place during the hydrothermal synthesis has been deduced herein. Understanding the effect of temperature on the relative rates of these steps is essential for achieving size and morphology selectivity, but has often been overlooked in the literature. This systematic study identifies six distinct steps taking place during the synthesis: (1) formation of Al(OH)3, (2) dissolution of Al(OH)3 into hexameric based fragments (3) thermolysis at temperatures ≥ 170 °C into soluble AlOOH (boehmite) building blocks (4) formation of lamellar AlOOH sheets (5) scrolling into nanorod crystallites and subsequent oriented attachment into high aspect nanorods and (6) growth by Ostwald ripening to low aspect nanorods. The obtained AlOOH nanorods are converted into γ-Al2O3 with conservation of morphology by calcination at 500 °C. Nanorod formation (step 5) can only be achieved at temperatures ≥ 180 °C (after 20 hours). At 180 °C, growth of the rods (step 6) takes place simultaneously with their slow formation (step 5) leading to two distinct nanorod products with different aspect ratios. At higher temperatures (200 °C), the rate of formation (step 5) is fast, quickly reaching completion, allowing for substantial growth of the nanorods and resulting in a monomodal size distribution. Thus, we have identified that γ-Al2O3 rods with high aspect ratio can be selectively synthesised at 180 °C for ≥20 hours, while low aspect ratios are produced at 200 °C for ≥10 hours. In all cases, the average size of the nanorods increases linearly with prolonged reaction time due to their continuous growth.
1. Introduction
Ceramic materials such as, alumina (Al2O3), are inexpensive materials commonly used as catalyst supports in numerous industrial processes.1 The widespread use of Al2O3 in heterogeneous catalysis has arisen from its high chemical and thermal stability as well as its desirable textural properties such as surface area, porosity and surface acidity/basicity.2 The gamma polymorph is most commonly used for catalyst supports due to its high specific surface area.3 Despite the widespread use of bulk γ-Al2O3, in recent years, the focus has shifted towards nanostructured materials since altering the morphology at the nanoscale can potentially lead to materials with distinct physical and chemical properties. In addition, morphology control enables the selective exposure of different crystal surfaces, which may have distinct interactions with metal active sites and reactants. These specific interactions can be exploited to offer additional stability to metal nanoparticles when implementing nanostructured γ-Al2O3 as a catalyst support.Several routes have been reported for the synthesis of nanostructured materials including hydrothermal,3–13 sol–gel,14–17 anodisation,18–20 atomic layer deposition21 and wet etching.22 Amongst these, hydrothermal methods are popular as they are cheap, reproducible, simple and can be easily scaled up. There are numerous published reports of hydrothermal syntheses of γ-Al2O3 using structure directing agents (SDAs) such as surfactants7–9 and polymers,1,23 which function as a template. However, these additives can affect the product crystallinity and SDAs can be hard to remove completely from the final materials. As such, hydrothermal procedures capable of maintaining high product quality without SDAs are more desirable and are the focus of the work herein. An alternative technique to direct product morphology is the use of anions such as sulphate, chloride and nitrate, which can adsorb preferentially to particular crystal surfaces to inhibit growth in certain directions.24 Since anions can be easily removed by washing, they do not compromise the quality of the product.
The hydrothermal conditions used in the literature to synthesise 1D AlOOH and γ-Al2O3 without SDAs are summarised in Table 1. It is clear from our previous work3 that an acidic medium is required to hydrothermally produce 1D nanostructures and the literature suggests at least 12 hours at temperatures exceeding 100 °C are needed. It is important to note that Table 1 includes data for both AlOOH and γ-Al2O3 materials. While AlOOH can be easily converted to γ-Al2O3 by calcination, the crystal lattice contraction resulting from its dehydration has an effect on its size, which should be taken into consideration when comparing sizes.
| Precipitant agent or base | pH | Temperature (°C) | Time (hours) | Morphology | Size (nm) | SABET (m2 g−1) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Length | Diameter | |||||||
| a — indicates information not provided. | ||||||||
| NaOH | 3.5 | 200 | 20 | Nanorod (γ-AlOOH) | 260 | 27 | 46 | 3 |
| NaOH | 4 | 200 | 24 | Nanorod (γ-AlOOH) | 200–300 | 10–30 | 85 | 4 |
| 5 | 200 | 24 | Nanorod (γ-AlOOH) | 200–300 | 10–30 | 24 | ||
| C6H5NH2 | — | 200 | 24 | Nanorod (γ-AlOOH) | 300–700 | 25–40 | — | 25 |
| NaNH2 | — | 200 | 4–6 | Lamellar (γ-AlOOH) | 20–40 | 20–25 | — | 5 |
| — | 200 | 12 | Nanotube (γ-AlOOH) | 100 | 2–5 | — | ||
| — | 200 | 20 | Nanorod (γ-AlOOH) | <180 | 15–20 | — | ||
| NH4OH | 5 | 200 | 48 | Nanorod (γ-AlOOH) | 100–400 | 20–30 | — | 26 |
| NH3 | 5 | 200 | 48 | Nanorod (γ-AlOOH) | 140–320 | — | — | 27 |
| (NH4)2CO3 | — | 100 | 24 | Nanorod (γ-Al2O3) | 90–140 | 35–45 | 165 | 28 |
| — | 100 | 36 | Nanorod (γ-Al2O3) | 90–140 | 35–45 | 152 | ||
| HCO–N(CH3)2 | 2.5 | 150 | 24 | Nanorod (γ-Al2O3) | <50 | ∼5 | 457 | 29 |
| (C2H5)4N(OH) | — | 170 | 72 | Nanofibre (γ-Al2O3) | 300–1000 | 8–15 | 210 | 30 |
Despite the work in the area, the ability to synthesise nanostructured alumina with simultaneous control over the morphology and size has not yet been reported. For example, syntheses at 200 °C for 24 hours can produce γ-AlOOH nanorods of either 200–300 nm or 300–700 nm length as reported by Yang4 and Ma et al.25 respectively. We believe that the key to developing control of the 1D nanostructure size lies within the understanding of the mechanism.
In our previous work,3 we demonstrated morphological control of γ-Al2O3 materials by varying the NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio, producing morphologically pure 2D nanoplates and 1D nanorods in basic and acidic conditions respectively.3 Herein, we focus on the size control of γ-Al2O3 nanorods by developing an understanding of the synthetic mechanism using a combination of 27Al NMR and electron microscopy techniques. This work has led to the identification of distinct steps leading up to nanorod formation and growth, where variations in synthesis temperature and time leads to controlled γ-Al2O3 sizes. Subsequently, the mechanistic understanding presented herein can be applied to tailor the γ-Al2O3 nanorod size by controlling synthesis temperature and time, which was not previously possible with hydrothermal methods.
Al ratio, producing morphologically pure 2D nanoplates and 1D nanorods in basic and acidic conditions respectively.3 Herein, we focus on the size control of γ-Al2O3 nanorods by developing an understanding of the synthetic mechanism using a combination of 27Al NMR and electron microscopy techniques. This work has led to the identification of distinct steps leading up to nanorod formation and growth, where variations in synthesis temperature and time leads to controlled γ-Al2O3 sizes. Subsequently, the mechanistic understanding presented herein can be applied to tailor the γ-Al2O3 nanorod size by controlling synthesis temperature and time, which was not previously possible with hydrothermal methods.
2. Experimental
2.1 Synthesis of γ-alumina nanorods
The hydrothermal synthesis of γ-Al2O3 has been previously reported.3 In a typical synthesis, 20 mL of 1 M NaOH solution is added to an aqueous solution containing 9.6 g of Al(NO3)3·9H2O in a PTFE lined stainless steel autoclave. The reactant quantities correspond to a NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio of 0.77
Al molar ratio of 0.77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which is known from our previous work3 to yield morphologically pure γ-Al2O3 nanorods after hydrothermal synthesis at 200 °C for 20 hours. The initial pH of this mixture is 3.5. The precursors are treated hydrothermally in an air-circulating oven for 10 to 80 hours at 150 to 200 °C. After this time, the autoclave vessel is naturally cooled down to room temperature and the resulting white γ-AlOOH precipitate and solution are separated by centrifugation (4000 rpm, 3 minutes). The solid product is dried overnight in a vacuum oven at 80 °C, crushed into a fine powder and calcined at 500 °C for 3 hours (3 °C min−1 rate). The final γ-Al2O3 product is washed with distilled water and dried.
1 which is known from our previous work3 to yield morphologically pure γ-Al2O3 nanorods after hydrothermal synthesis at 200 °C for 20 hours. The initial pH of this mixture is 3.5. The precursors are treated hydrothermally in an air-circulating oven for 10 to 80 hours at 150 to 200 °C. After this time, the autoclave vessel is naturally cooled down to room temperature and the resulting white γ-AlOOH precipitate and solution are separated by centrifugation (4000 rpm, 3 minutes). The solid product is dried overnight in a vacuum oven at 80 °C, crushed into a fine powder and calcined at 500 °C for 3 hours (3 °C min−1 rate). The final γ-Al2O3 product is washed with distilled water and dried.
2.2 Characterisation
Transmission electron microscopy (TEM) was carried out using a JEOL JEM1200EXII instrument. Scanning electron microscopy (SEM) was carried out using a FEI Nova NanoSEM 450 using 5 kV accelerating voltage and 2.5 a.u. spot size at a working distance of 4.8 mm. Samples were sputter coated with approximately 6 nm layer of platinum to minimise charging. Exact platinum coating thickness was not determined and thus SEM is used for comparison and qualitative data of size and morphology rather than quantitative analysis. N2 sorption measurements were carried out at −196 °C using a Micromeritics ASAP 2020 apparatus. All samples were degassed at 150 °C prior to analysis. Specific surface area was calculated using the BET method. Powder X-ray diffraction (pXRD) patterns were obtained using a Bruker D8 Advance diffractometer and a position sensitive VANTEC-1 detector with Cu Kα radiation, operated at 40 kV and 40 mA. 27Al NMR experiments were run on a 500 MHz Bruker AV500 with 130.32 MHz resonance frequency, using a ca. 90 degree pulse, no proton decoupling (pulse sequence “zg”), spectral width of 500 ppm, acquisition time of 0.12 second, delay time of 1 second and 20 to 1200 scans for each experiment. All solutions were diluted in D2O.3. Results and discussion
3.1 Aluminium species involved in AlOOH formation
We have recently reported3 that prior to hydrothermal synthesis of nanostructured alumina, addition of an aqueous solution of aluminium nitrate (Al(NO3)3·9H2O) to aqueous NaOH solutions leads to the formation of aluminium hydroxide solid (Al(OH)3), according to reaction (1). We have also demonstrated that Al(OH)3 is the precursor to the boehmite (γ-AlOOH) hydrothermal product (reaction (2)), which is converted into γ-Al2O3 by calcination at 400–700 °C.| Al(NO3)3·9H2O (aq) + 3OH− (aq) → Al(OH)3 (s) + 9H2O (l) + 3NO3− (aq) | (1) |
| Al(OH)3 (s) → γ-AlOOH (s) + H2O (l) | (2) |
The morphology is conserved during this calcination step and consequently, the capacity to control the nanostructured γ-Al2O3 morphology and size is during the formation of γ-AlOOH step (reaction (2)).3,6 Herein, we now develop upon this understanding, recognising intermediate steps taking place during these processes, providing useful insights on the overall mechanism of formation of γ-AlOOH nanorods, ultimately leading to nanorod size control.
The precise nature of the species resulting from hydrolysis of aluminium salts depends on a delicate balance of Al3+ concentration, base concentration, pH, degree of stirring, temperature, rate of base addition and aging time.31 Further insights into the transformation of Al(NO3)3·9H2O to Al(OH)3 (reaction (1)), reveals that the conversion comprises of several intermediate steps. The process starts with the initial formation of [Al(H2O)6]3+ cations (reaction (3)), easily susceptible to deprotonation as the small, highly charged Al3+ cation polarises and weakens the O–H bonds, resulting in sequential deprotonation to form aluminium monomers shown in reaction (4) and (5). It is worth noting that in an aqueous solution of aluminium nitrate, without the addition of base, the equilibrium position of reaction (4) and (5) is far to the left. However, the addition of base, such as NaOH, shifts the equilibria in reactions (4)–(6) towards the precipitation of solid Al(OH)3.
| Al(NO3)3·9H2O (aq) → [Al(H2O)6]3+ (aq) + 3NO3− (aq) + 3H2O (l) | (3) |
| [Al(H2O)6]3+ (aq) + OH− (aq) ⇆ [Al(OH)(H2O)5]2+ (aq) + H2O (l) | (4) |
| [Al(OH)(H2O)5]2+ (aq) + OH− (aq) ⇆ [Al(OH)2(H2O)4]+ (aq) + H2O (l) | (5) |
| [Al(OH)2(H2O)4]+ (aq) + OH− (aq) ⇆ Al(OH)3 (s) + 4H2O (l) | (6) |
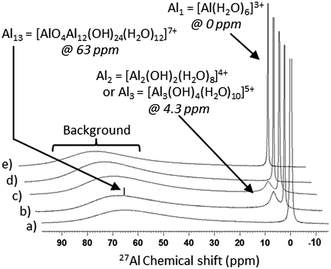
The use of 27Al NMR provides information about the species present in the reaction solution before and after hydrothermal treatment, facilitating unequivocal identification of any symmetrical aluminium species including monomers, dimers/trimers and tridecamers.32 Due to the quadrupolar nature of 27Al, only symmetrical species are detected, resulting in a vast number of unidentifiable aluminium monomers, oligomers and polymers potentially present in solution.33
The formation of monomeric aluminium species by aqueous dissolution of Al(NO3)3·9H2O is evidenced by 27Al NMR spectra, shown in Fig. 1a. The peak at 0 ppm corresponds to all symmetrical, monomeric aluminium species in an octahedral arrangement including [Al(H2O)6]3+, [Al(OH)(H2O)5]2+ and [Al(OH)2(H2O)4]+.31
The mechanism of Al(OH)3 formation by polymerisation of [Al(OH)2(H2O)4]+ cations (reaction (6)) is described in detail by reactions (7)–(9). In this process, the deprotonated monomers can dimerise, as shown for [Al(OH)2(H2O)4]+ in reaction (7) and subsequently, monomers and dimers can join to form trimers as in reaction (8). The dimers can aggregate into Al6 hexamer rings ([Al6(OH)12(H2O)12]6+) as shown in reaction (9). Further aggregation of the hexameric rings results in the precipitation of Al(OH)3 which will be discussed in further detail later in this section but first the solution composition is examined.
| 2[Al(OH)2(H2O)4]+ (aq) → [Al2(OH)2(H2O)8]4+ (aq) + 2OH− | (7) |
| [Al(OH)2(H2O)4]+ (aq) + [Al2(OH)2(H2O)8]4+ (aq) → [Al3(OH)4(H2O)10]5+ (aq) + 2H2O (l) | (8) |
| 3[Al2(OH)2(H2O)8]4+ (aq) → [Al6(OH)12(H2O)12]6+ (aq) + 6H2O (l) + 6H+ (aq) | (9) |
Upon the addition of NaOH to the aqueous solution of Al3+, the formation of dimers/trimers (reactions (7) and (8)) and tridecamers ([Al13O4(OH)24(H2O)12]7+) in solution is evidenced by the 27Al NMR peaks at 4.3 and 63 ppm respectively in Fig. 1b.31,34 The formation of these species does not proceed in the absence of base under the studied conditions as shown by 27Al NMR (Fig. 1a), despite the fact that nitrates are believed to facilitate dimerisation.35
At a low NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 0.77, corresponding to pH values <4, the aluminium species in solution tend to be monomeric and dimeric by nature.36 Its basic hydrolysis results in the formation of Al13, provided that the OH
Al ratio of 0.77, corresponding to pH values <4, the aluminium species in solution tend to be monomeric and dimeric by nature.36 Its basic hydrolysis results in the formation of Al13, provided that the OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio is between 0.5 and 2.5.33,37 Al13 corresponds to a 3D tridecamer, made up of a central aluminium tetrahedron (AlO4) surrounded by four planar trimers ([Al3(OH)4(H2O)10]5+), with the chemical formula of [Al13O4(OH)24(H2O)12]7+. It is based on the structure of a Keggin cluster,38 initially elucidated to describe the structure of heteropoly acids.37 This structure forms because the high concentration of OH− in the local environment during base addition yields the tetrahedral centre of [Al(OH)4]−, which is subsequently surrounded by four planar trimers ([Al3(OH)4(H2O)10]5+), individually formed as per reaction (8).39 The concentration of Al13 increases with slow addition of base, whereas fast base injection favours the precipitation of Al(OH)3.39 The Al13 species is detectable by 27Al NMR (peak at 63 ppm) due to the symmetrical, tetrahedral environment of the central AlO4. It is worth noting that the relative concentration of aluminium atoms within the Keggin cluster is 13 times higher than that shown by the area of its corresponding 27Al NMR peak, as only the central tetrahedral aluminium atom is detectable by 27Al NMR.34
Al ratio is between 0.5 and 2.5.33,37 Al13 corresponds to a 3D tridecamer, made up of a central aluminium tetrahedron (AlO4) surrounded by four planar trimers ([Al3(OH)4(H2O)10]5+), with the chemical formula of [Al13O4(OH)24(H2O)12]7+. It is based on the structure of a Keggin cluster,38 initially elucidated to describe the structure of heteropoly acids.37 This structure forms because the high concentration of OH− in the local environment during base addition yields the tetrahedral centre of [Al(OH)4]−, which is subsequently surrounded by four planar trimers ([Al3(OH)4(H2O)10]5+), individually formed as per reaction (8).39 The concentration of Al13 increases with slow addition of base, whereas fast base injection favours the precipitation of Al(OH)3.39 The Al13 species is detectable by 27Al NMR (peak at 63 ppm) due to the symmetrical, tetrahedral environment of the central AlO4. It is worth noting that the relative concentration of aluminium atoms within the Keggin cluster is 13 times higher than that shown by the area of its corresponding 27Al NMR peak, as only the central tetrahedral aluminium atom is detectable by 27Al NMR.34
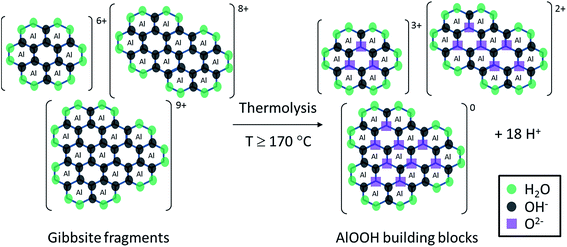
As well as the formation of a complex combination of soluble aluminium species discussed so far, the addition of NaOH to aqueous solutions of aluminium nitrate also results in the formation of solid Al(OH)3 via polymerisation. Prior to hydrothermal treatment, the initial formation of solid Al(OH)3 by the addition of NaOH is limited by the OH− concentration in the solution (reactions (3)–(6)). Thus, NaOH is the limiting reagent for the formation of AlOOH product (reaction (2))3 after hydrothermal synthesis, despite the presence of other aluminium species in solution. Interestingly, due to the low synthesis pH, the formed Al(OH)3 undergoes hydrolysis to reform soluble gibbsite fragments, whose size depends on the OH/Al ratio.31 Indeed, we found that at pH 3.5 (OH/Al = 0.77), solid Al(OH)3 fully dissolves in aqueous solution at room temperature after 48 hours. Furthermore, when the initial solution is heated to 50 °C, the solid Al(OH)3 rapidly (<30 minutes) dissolves, demonstrating that, as expected, the dissolution rate increases with temperature. Consequently, under acidic hydrothermal conditions (OH/Al = 0.77), solid Al(OH)3 dissolves rapidly into gibbsite fragments (pictured as reactants in Fig. 2). If the hydrothermal temperature is high enough (above 170 °C), deprotonation or hydrolysis of the gibbsite fragments by heating (thermolysis) takes place, associated with an observed decreased in pH due to the liberation of protons as depicted simplistically in Fig. 2. As deprotonation advances, the formation of relatively stable, uncharged species leads eventually to the precipitation of solid AlOOH by crystallisation.
We previously reported3 that the amount of Al(OH)3 formed determines the amount of AlOOH produced and this can now be explained as it governs the amount of gibbsite fragments which can reform in solution upon dissolution, which cannot form without base addition.
The final amount of AlOOH product formed after hydrothermal treatment during 20 hours increases as the reaction temperature is increased, as shown in Table 2. Under the studied NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio conditions of 0.77, the maximum theoretically achievable Al2O3 yield is approximately 30 wt% as conversion to essential precursor Al(OH)3 (reaction (3)–(6)) and subsequent gibbsite fragments, is incomplete due to the non-stoichiometric NaOH
Al molar ratio conditions of 0.77, the maximum theoretically achievable Al2O3 yield is approximately 30 wt% as conversion to essential precursor Al(OH)3 (reaction (3)–(6)) and subsequent gibbsite fragments, is incomplete due to the non-stoichiometric NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio.3 Such maximum conversion is only achieved after 20 hour synthesis at temperatures ≥ 200 °C, below which, the rate of conversion of gibbsite fragments into AlOOH building blocks is kinetically limited to yield less than the maximum theoretical product yield at ≤180 °C.
Al ratio.3 Such maximum conversion is only achieved after 20 hour synthesis at temperatures ≥ 200 °C, below which, the rate of conversion of gibbsite fragments into AlOOH building blocks is kinetically limited to yield less than the maximum theoretical product yield at ≤180 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio) for 20 hours at different reaction temperatures
Al molar ratio) for 20 hours at different reaction temperatures
| Synthesis temperature (°C) | γ-Al2O3 yield (wt%) |
|---|---|
| 150 | 0 |
| 165 | 0 |
| 170 | 7 |
| 180 | 21 |
| 200 | 30 |
On the contrary, at hydrothermal temperatures ≤ 165 °C thermolysis of the gibbsite fragments (Fig. 2) is not achieved and consequently no solid product is formed as Al(OH)3 is irreversibly dissolved into gibbsite fragments due to the solution acidity. Indeed, there is no change in the solution pH, confirming the absence of thermolysis reaction. The same is observed even if the synthesis is carried out for longer times (40 hours), confirming that at temperatures ≤ 165 °C, the process is not simply kinetically limited but rather it is not feasible. In addition, if this remaining solution is subsequently treated at 200 °C over 20 hours, a boehmite product is obtained with the same physical properties as boehmite synthesised by a single treatment at 200 °C for 20 hours. It is important to mention that the literature contains several studies where the synthesis of boehmite is reported at temperatures as low as 120 °C.34,40 However, this is likely due to the different nature of aluminium and base precursors employed in these cases which undoubtedly affects the equilibria and activation energies of the intermediate synthetic steps.
The 27Al NMR spectra of the supernatant solutions after hydrothermal treatment at different temperatures (Fig. 1) also show that when boehmite product is formed (≥170 °C), the dimer/trimer peak at 4.3 ppm disappears whereas it remains when no solid product is formed at ≤165 °C. This observation confirms that the dimer/trimer plays an important role in forming the final AlOOH solid product. Monomers are present in the post reaction solution, as observed by 27Al NMR, after hydrothermal treatment in the studied temperature range (Fig. 1c–e) due to the non-stoichiometric NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 0.77. In addition, 27Al NMR spectra in Fig. 1c–e show that after hydrothermal treatment at all temperatures within the studied range, the Al13 Keggin cluster is no longer present regardless of the formation of solid boehmite product. This suggests that the Al13 Keggin cluster is not directly significant for the formation of AlOOH. Indeed, the concentration of Al13 Keggin cluster is reported to decrease overtime in acidic conditions due to its clustering into species undetectable by 27Al NMR.34
Al ratio of 0.77. In addition, 27Al NMR spectra in Fig. 1c–e show that after hydrothermal treatment at all temperatures within the studied range, the Al13 Keggin cluster is no longer present regardless of the formation of solid boehmite product. This suggests that the Al13 Keggin cluster is not directly significant for the formation of AlOOH. Indeed, the concentration of Al13 Keggin cluster is reported to decrease overtime in acidic conditions due to its clustering into species undetectable by 27Al NMR.34
3.2 Mechanism of formation of 1D AlOOH nanorods
Comparison of the alumina rods produced over 20 hours with a NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio 0.77 at different temperatures provides further insights of the formation mechanism of the boehmite structures. At temperatures ≥ 180 °C, pure nanorods are formed however, at lower temperatures (e.g. 170 °C), a variety of morphologies, including 2D rectangular nanoplates and nanoparticles, are observed (Fig. 3a). This is due to the lower rate of conversion of these intermediate products into the more thermodynamically stable nanorods via scrolling followed by oriented attachment (Fig. 8). This scrolling step forming nanorods from lamellar sheets does not take place at 170 °C, while it is kinetically limited at 180 °C, as shown by the low aluminium yield obtained (Table 2). In all cases, the pXRD pattern (Fig. S1†) of the calcined boehmite materials (500 °C) can be indexed to the γ phase of Al2O3 (JCPDS card no. 10-0425), without the presence of other crystalline phases. Additionally, the materials produced at temperatures ≥ 180 °C present similar BET surface areas (∼145 m2 g−1), irrespective of the distinct nanorod dimensions shown by TEM (Fig. 3b and c). By contrast, the alumina material produced under the same conditions at 170 °C has a lower BET surface area (122 m2 g−1), likely due to the difference in morphology (Fig. 3a) and porosity (Fig. S5†).
Al molar ratio 0.77 at different temperatures provides further insights of the formation mechanism of the boehmite structures. At temperatures ≥ 180 °C, pure nanorods are formed however, at lower temperatures (e.g. 170 °C), a variety of morphologies, including 2D rectangular nanoplates and nanoparticles, are observed (Fig. 3a). This is due to the lower rate of conversion of these intermediate products into the more thermodynamically stable nanorods via scrolling followed by oriented attachment (Fig. 8). This scrolling step forming nanorods from lamellar sheets does not take place at 170 °C, while it is kinetically limited at 180 °C, as shown by the low aluminium yield obtained (Table 2). In all cases, the pXRD pattern (Fig. S1†) of the calcined boehmite materials (500 °C) can be indexed to the γ phase of Al2O3 (JCPDS card no. 10-0425), without the presence of other crystalline phases. Additionally, the materials produced at temperatures ≥ 180 °C present similar BET surface areas (∼145 m2 g−1), irrespective of the distinct nanorod dimensions shown by TEM (Fig. 3b and c). By contrast, the alumina material produced under the same conditions at 170 °C has a lower BET surface area (122 m2 g−1), likely due to the difference in morphology (Fig. 3a) and porosity (Fig. S5†).
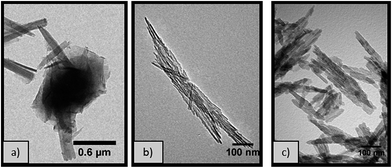 | ||
Fig. 3 TEM micrographs of γ-Al2O3 obtained from hydrothermal treatment for 20 hours with 1 M NaOH (0.77 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio) at (a) 170 °C (b) 180 °C and (c) 200 °C. Al molar ratio) at (a) 170 °C (b) 180 °C and (c) 200 °C. | ||
Table 3 shows that as the synthesis temperature increases, the crystallite size increases. Interestingly, a 10 °C increase from 170 to 180 °C results in doubling of the crystallite size in both (440) and (400) directions whereas a 20 °C increase from 180 °C to 200 °C only results in a <1 nm increase in size due to higher rate of crystallite formation as temperature increases. Additionally, rod formation is not observed at 170 °C, while it is the principal morphology obtained at higher temperatures.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio)
Al molar ratio)
| Synthesis temperature (°C) | Morphology | SABETa (m2 g−1) | Average crystallite sizeb (nm) | |
|---|---|---|---|---|
| (440) | (400) | |||
| a SABET: specific surface area calculated using the BET approximation using N2 sorption data at −196 °C.b Calculated from line broadening at half maximum intensity of (440) and (400) XRD peaks using the Scherrer equation. | ||||
| 170 | Rectangular sheets and nanoparticles | 122 | 3.1 | 2.9 |
| 180 | Rods | 144 | 6.4 | 6.1 |
| 200 | Rods | 146 | 7.1 | 6.8 |
For the samples synthesised at 180 °C and 200 °C, the crystallite size in both the (400) and the (440) direction is of comparable size of 6 to 7 nm. However, SEM micrographs in Fig. 4 show that the nanorods synthesised at 180 °C are longer and thinner than those synthesised under comparable conditions at 200 °C. It is important to mention that the samples were coated with a thin layer of platinum to reduce charging and consequently quantitative analysis of these micrographs is not applicable. High resolution TEM analysis of the material synthesised for 20 hours at 200 °C (Fig. 5) confirms that the rods consists of attached crystallites, in agreement with the small crystallite size calculated by pXRD (Table 3). The individual crystallites aggregate by the oriented attachment mechanism.
 | ||
Fig. 4 SEM micrographs of γ-Al2O3 nanorods synthesised for 20 hours with 1 M NaOH (0.77 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio) at (a) 180 °C and (b) 200 °C. Al molar ratio) at (a) 180 °C and (b) 200 °C. | ||
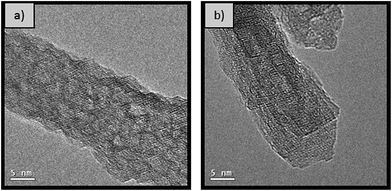 | ||
Fig. 5 High resolution TEM micrograph of nanorods synthesised with 1 M NaOH (0.77 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio) at 200 °C for 20 hours (a) before and (b) after calcination at 500 °C. Al molar ratio) at 200 °C for 20 hours (a) before and (b) after calcination at 500 °C. | ||
The mechanism of oriented attachment is driven by the presence of NO3− anions in the solution which stabilise specific AlOOH surfaces, inhibiting the growth in that direction. Specifically, NO3− anions bind to the AlOOH surface through the surface hydroxyl groups which are protonated in acidic conditions to –OH2+, making it capable of attracting a negative ion.24 This effect can be tuned by controlling the adsorption strength of the anion and their complexing ability with Al3+, following the order SO42− > Cl− > NO3−, whereby SO42− presents the strongest growth promotion in the direction where no adsorption interaction is possible.24 Interestingly, poor crystallinity and the formation of short nanorods (aspect ratio 2–4) have been reported elsewhere with NO3−, which may due to the morphology directing polymer employed.1 This is contrary to our observations, likely due to the fact that our synthesis does not use a structure directing agent.
3.3 Nanorod growth
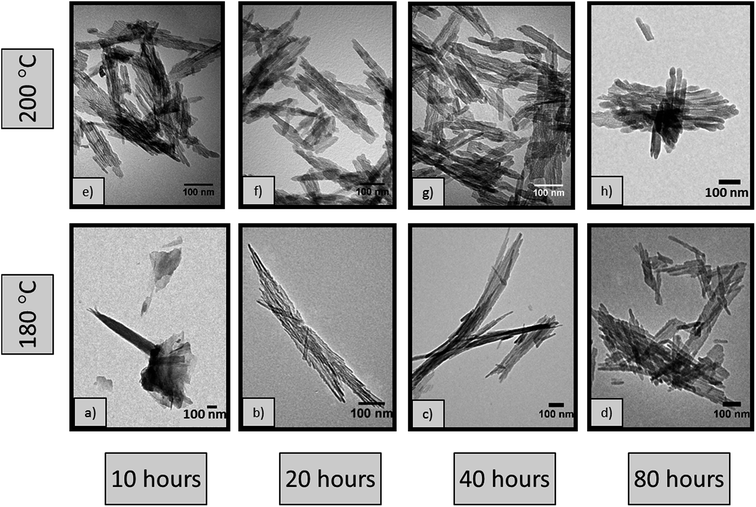
In order to fully understand the reasons behind the different nanorod dimensions observed in Fig. 3b and c and consequently the effect of temperature on the size of the rods, a series of hydrothermal syntheses were carried out at 180 °C and 200 °C for different durations from 10 to 80 hours. Fig. 6 shows representative TEM micrographs of the materials. All of the samples show high morphological purity, except the sample synthesised at 180 °C for 10 hours (Fig. 6a) where nanorods are not formed but rather a mixture of morphologies, including sheets and nanoparticles, are observed, resembling the sample synthesised at 170 °C for 20 hours (Fig. 3a). This observation confirms that the scrolling of the AlOOH sheets, taking place under acidic conditions3 herein, is kinetically limited at such low temperatures.Careful characterisation of the samples also reveals the presence of two distinct products formed at 180 °C, including rods with a high aspect ratio (>40), mixed with considerably lower aspect ratio (<17) rods. This is clearly illustrated by the bimodal length and diameter size distribution (Fig. S2†). Both products are separated by post-synthesis sedimentation; the high aspect ratio rods have high surface energy and aggregate, falling to the bottom of the autoclave while the low aspect ratio rods remain in suspension and are collected using centrifugation. Histograms of the lengths and diameters are shown in the ESI (Fig. S2†), with average values summarised in Table 4. The pXRD patterns (Fig. S3 and S4†) of the materials in Fig. 6 show the presence of a single crystalline phase corresponding to γ-Al2O3 (JCPDS card no. 10-0425).
| Temperature (°C) | Synthesis time (hours) | Average length (nm) | Average diameter (nm) | Aspect ratiob | SABETc (m2 g−1) | Average crystallite sized (nm) | Yield (wt%) | |
|---|---|---|---|---|---|---|---|---|
| (440) | (400) | |||||||
a γ-Al2O3 synthesised at 180 °C and 200 °C with 1 M NaOH (0.77 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio). Data for high aspect ratio rods shown in italics.b Length Al molar ratio). Data for high aspect ratio rods shown in italics.b Length![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diameter ratio.c SABET: specific surface area calculated using the BET approximation using N2 sorption data at −196 °C.d Calculated with Scherrer equation using line broadening at half maximum intensity of (440) and (400) XRD peaks. diameter ratio.c SABET: specific surface area calculated using the BET approximation using N2 sorption data at −196 °C.d Calculated with Scherrer equation using line broadening at half maximum intensity of (440) and (400) XRD peaks. |
||||||||
| 180 °C | 10 | N/A | N/A | N/A | 117 | 2.6 | 50.1 | 11 |
| 20 | 115 | 8 | 14 | 144 | 6.4 | 6.1 | 21 | |
| 318 | 5 | 64 | ||||||
| 40 | 133 | 11 | 12 | 147 | 5.1 | 4.8 | 28 | |
| 287 | 7 | 41 | ||||||
| 80 | 213 | 18 | 12 | 123 | 5.9 | 5.8 | 23 | |
| 518 | 13 | 40 | ||||||
| 200 °C | 10 | 80 | 8 | 10 | 161 | 6.5 | 5.9 | 20 |
| 20 | 128 | 13 | 10 | 146 | 7.1 | 6.8 | 30 | |
| 40 | 182 | 20 | 9 | 86 | 6.9 | 6.4 | 25 | |
| 80 | 290 | 26 | 11 | 106 | 7.3 | 6.4 | 15 | |
As shown in Table 4, similar surface areas are observed for γ-Al2O3 nanorods synthesised for 20 hours at 180 °C and 200 °C despite the difference in size of the low aspect ratio nanorods. This is likely due to the sample produced at 180 °C containing both low and high aspect ratio nanorods, the later has a higher surface area, thus increasing the average surface area of the sample. Despite the variability of nanorod dimensions as the hydrothermal duration changes, all samples produced at 200 °C present a similar aspect ratio (∼10), suggesting a multi-directional nature of the dissolution–recrystallisation mechanism.
All the samples summarised in Table 4 are mesoporous with a characteristic type IV N2 sorption isotherm with hysteresis (Fig. S5†). Additionally, all nanorods produced, independently of the synthesis time, present a similar crystallite size (∼5–7 nm), calculated using the Scherrer equation with the line broadening at half maximum intensity of the (440) and (400) diffraction peaks. The high resolution TEM micrographs shown in Fig. 5 further confirms that the rods are indeed formed by agglomeration of crystals, via the oriented attachment of AlOOH nanorod crystallites formed by scrolling of AlOOH sheets.
Rods with high aspect ratio (long and thin) are formed by oriented attachment of the scrolled crystallites formed under acidic conditions. Once the 1D high aspect boehmite nanorods have been formed, they are susceptible to growth via Ostwald ripening41 following a dissolution/recrystallisation mechanism, which has been observed in other 1D ceramic structures.42 This growth step takes place simultaneously with further rod formation, leading to the bimodal size distribution observed for the samples synthesised at 180 °C. In addition, due to the formation and growth steps taking place simultaneously, the narrow diameter and length size distribution achieved after 10 hours of synthesis, gets broader as the hydrothermal time is increased to 80 hours. At 200 °C, the rate of both steps is increased, resulting not only in a monomodal length and diameter size distribution, but also in rods with smaller aspect ratios. The increased rate of scrolling and oriented attachment of AlOOH crystallites forming rods at 200 °C compared to 180 °C is evidenced by the higher aluminium yields achieved in the former case for a given synthesis time (Table 4). Furthermore, the ease of growth by dissolution–recrystallisation depends on the degree of solubility of the crystallites,27 which increases as the temperature increases, leading to a higher growth rate at 200 °C compared to 180 °C. This is evidenced in Table 4 and Fig. 7 as the diameter for the low aspect rods synthesised at 200 °C are larger than the equivalent material produced for the same time but at 180 °C. This is in agreement with previous observations by Xu et al.40 who reported an increase in the diameter and length of the rods with increasing reaction temperature from 120 to 180 °C.
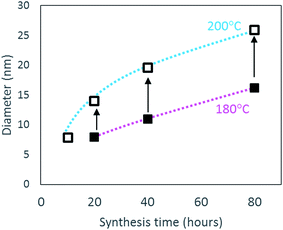 | ||
| Fig. 7 Variation in diameter of low aspect ratio nanorods following increased synthesis time (10 to 80 hours) at 180 °C (closed square) and at 200 °C (open square). | ||
In addition, the length and diameter of the rods is increased by extending the synthesis time as shown in Fig. 7 for the low aspect nanorods. One should notice that the average diameter of the low aspect ratio rods produced after 20 hours at 180 °C is similar to the diameter of the nanorods produced after only 10 hours at 200 °C. Since nanorods are not formed after 10 hours at 180 °C, no data point is included in Fig. 7. These observations demonstrate that low aspect ratio nanorods with similar dimensions can be produced at 200 °C for shorter synthesis time (10 hours) than at 180 °C with double synthesis time (20 hours). In the latter case, the rods will be simultaneously produced with high aspect ratio rods, which can be separated by sedimentation as mentioned previously.
Based on the systematic study presented herein, the overall process for the hydrothermal synthesis of nanorods in acidic conditions has been elucidated, revealing a six step mechanism, summarised in Fig. 8. Initially, NaOH addition to an aqueous solution of Al(NO3)3·9H2O forms Al(OH)3 (step 1), which undergoes hydrolysis into fragments of gibbsite due to the acidic conditions (step 2). At a hydrothermal temperature ≥ 170 °C, thermolysis (step 3) takes place to yield AlOOH building blocks that subsequently crystallise into lamellar sheets of AlOOH, (step 4), evidenced by TEM analysis of the product obtained after hydrothermal treatment at 170 °C. The formation of nanorods (step 5) proceeds only at ≥180 °C for 20 hours, involving scrolling to form nanorods crystallites which can attach in an oriented way to form high aspect ratio nanorods. At higher temperatures (200 °C), step 5 can be achieved after a shorter hydrothermal treatment time of 10 hours. These nanorods can grow (step 6) in a non-selective, multi-directional manner by Ostwald ripening, resulting in low aspect ratio (<20) nanorods. At 200 °C, step 5 is sufficiently fast that only low aspect ratio nanorods are observed, whereas 180 °C results in a mixture of low and high aspect ratio nanorods.
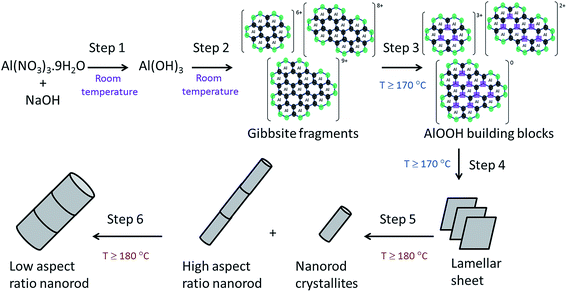 | ||
| Fig. 8 Summary of the elucidated six step mechanism for nanorod formation by hydrothermal synthesis under acidic conditions. | ||
4. Conclusion
In conclusion, a systematic study of the effect of temperature and time on the hydrothermal synthesis of γ-Al2O3 nanorods is presented here, revealing an in-depth understanding of the formation mechanism. Herein, we have presented and discussed an evidence based six-step mechanism, taking into account all aspects of the process. The formation of Al(OH)3 (step 1) and its consequent dissolution into hexameric gibbsite fragments (step 2) takes place before hydrothermal treatment. This is followed by thermolysis into AlOOH blocks (step 3) followed by lamellar AlOOH formation (step 4), their scrolling and oriented attachment to form AlOOH rods (step 5) and their simultaneous growth (step 6). Electron microscopy, 27Al NMR studies and experimental observations demonstrate that the reaction temperature plays a key role in the formation of nanostructured AlOOH. A minimum hydrothermal temperature of 170 °C is needed to form AlOOH (step 4) but a higher temperature of 180 °C is needed to form AlOOH nanorods (step 5). Understanding the relative rate of steps 4–6 at different temperatures allows for control of the nanorod dimensions. We, thus, demonstrate that γ-Al2O3 nanorods of selective diameter and length can be synthesised by varying the hydrothermal treatment time and temperature. The nanorod diameter can be increased by raising the hydrothermal temperature from 180 °C to 200 °C or by prolonging the synthesis time up to 80 hours via growth by Ostwald ripening (step 6). However after 80 hours synthesis, the nanorod size distribution is considerably broader, losing size control.Acknowledgements
The authors would like to acknowledge the UK Engineering and Physical Science Research Council (grant number EP/L020432/2) for funding, the Department of Chemical Engineering and Biotechnology at the University of Cambridge and SASOL UK for TEB's studentship. We would like to thank Dr Lowe (University of Bath) for help in obtaining the 27Al NMR spectra.References
- W. Cai, Y. Hu, J. Chen, G. Zhang and T. Xia, CrystEngComm, 2012, 14, 972–977 RSC.
- M. Trueba and S. P. Trasatti, Eur. J. Inorg. Chem., 2005, 2005, 3393–3403 CrossRef.
- T. E. Bell, J. M. Gonzalez-Carballo, R. P. Tooze and L. Torrente-Murciano, J. Mater. Chem. A, 2015, 3, 6196–6201 CAS.
- Q. Yang, Inorg. Mater., 2010, 46, 953–958 CrossRef CAS.
- Y. X. H. Hou, Q. Yang, Q. Guo and C. Tan, Nanotechnology, 2005, 16, 741–745 CrossRef.
- M.-G. Ma, Y.-J. Zhu and Z.-L. Xu, Mater. Lett., 2007, 61, 1812–1815 CrossRef CAS.
- H. C. Lee, H. J. Kim, S. H. Chung, K. H. Lee, H. C. Lee and J. S. Lee, J. Am. Chem. Soc., 2003, 125, 2882–2883 CrossRef CAS PubMed.
- Z. Zhang and T. J. Pinnavaia, Langmuir, 2010, 26, 10063–10067 CrossRef CAS PubMed.
- L. Qu, C. He, Y. Yang, Y. He and Z. Liu, Mater. Lett., 2005, 59, 4034–4037 CrossRef CAS.
- H. A. Dabbagh, K. Taban and M. Zamani, J. Mol. Catal. A: Chem., 2010, 326, 55–68 CrossRef CAS.
- T. Kim, J. Lian, J. Ma, X. Duan and W. Zheng, Cryst. Growth Des., 2010, 10, 2928–2933 CAS.
- B. Xu, T. Xiao, Z. Yan, X. Sun, J. Sloan, S. L. González-Cortés, F. Alshahrani and M. L. H. Green, Microporous Mesoporous Mater., 2006, 91, 293–295 CrossRef CAS.
- Y. Zhang, J. Liu, R. He, Q. Zhang, X. Zhang and J. Zhu, Chem. Phys. Lett., 2002, 360, 579–584 CrossRef CAS.
- M. Sasani Ghamsari, M. A. Abdul-Hamid, S. Radiman, A. H. Sasani Ghamsari and S. Rahmani, Mater. Lett., 2013, 106, 439–442 CrossRef CAS.
- S. C. Kuiry, E. Megen, S. D. Patil, S. A. Deshpande and S. Seal, J. Phys. Chem. B, 2005, 109, 3868–3872 CrossRef CAS PubMed.
- F. Rashidi, A. N. Kharat, A. M. Rashidi, E. Lima, V. Lara and J. S. Valente, Eur. J. Inorg. Chem., 2010, 2010, 1544–1551 CrossRef.
- J. N. D. de Leon, V. Petranovskii, J. A. de los Reyes, G. Alonso-Nunez, T. A. Zepeda, S. Fuentes and J. L. Garcia-Fierro, Appl. Catal., A, 2014, 472, 1–10 CrossRef.
- L. Pu, X. Bao, J. Zou and D. Feng, Angew. Chem., Int. Ed., 2001, 40, 1490–1493 CrossRef CAS PubMed.
- J. Zou, L. Pu, X. Bao and D. Feng, Appl. Phys. Lett., 2002, 80, 1079–1081 CrossRef CAS.
- L. Yi, L. Zhiyuan, H. Xing, L. Yisen and C. Yi, Chem. Commun., 2011, 47, 2173–2175 RSC.
- J. S. Lee, B. Min, K. Cho, S. Kim, J. Park, Y. T. Lee, N. S. Kim, M. S. Lee, S. O. Park and J. T. Moon, J. Cryst. Growth, 2003, 254, 443–448 CrossRef CAS.
- J. Hwang, B. Min, J. S. Lee, K. Keem, K. Cho, M. Y. Sung, M. S. Lee and S. Kim, Adv. Mater., 2004, 16, 422–425 CrossRef CAS.
- Z. Zhu, H. Sun, H. Liu and D. Yang, J. Mater. Sci., 2010, 45, 46–50 CrossRef CAS.
- T. He, L. Xiang and S. Zhu, CrystEngComm, 2009, 11, 1338–1342 RSC.
- Y. Z. M. Ma, G. Cheng and Y. Huang, J. Mater. Sci. Technol., 2008, 24, 637–640 Search PubMed.
- S. C. Shen, Q. Chen, P. S. Chow, G. H. Tan, X. T. Zeng, Z. Wang and R. B. H. Tan, J. Phys. Chem. C, 2006, 111, 700–707 CrossRef.
- P. Pardo, N. Montoya and J. Alarcon, CrystEngComm, 2015, 17, 2091–2100 RSC.
- J. Wang, K. Shang, Y. Guo and W.-C. Li, Microporous Mesoporous Mater., 2013, 181, 141–145 CrossRef CAS.
- S. Ghosh, R. Das and M. K. Naskar, J. Am. Ceram. Soc., 2016, 99, 2273–2282 CrossRef CAS.
- Y.-F. Han, Z. Zhong, K. Ramesh, F. Chen and L. Chen, J. Phys. Chem. C, 2007, 111, 3163–3170 CAS.
- S. Bi, C. Wang, Q. Cao and C. Zhang, Coord. Chem. Rev., 2004, 248, 441–455 CrossRef CAS.
- G. Fu, L. F. Nazar and A. D. Bain, Chem. Mater., 1991, 3, 602–610 CrossRef CAS.
- S. M. Bradley, R. A. Kydd and R. F. Howe, J. Colloid Interface Sci., 1993, 159, 405–412 CrossRef CAS.
- R. J. M. J. Vogels, J. T. Kloprogge, P. A. Buining, D. Seykens, J. B. H. Jansen and J. W. Geus, J. Non-Cryst. Solids, 1995, 191, 38–44 CrossRef CAS.
- C. V. Ruiz Madroñero and J. E. Rodríguez Paéz, Ing. Invest., 2010, 30, 16–24 Search PubMed.
- H. Zhao, H. Liu and J. Qu, J. Colloid Interface Sci., 2009, 330, 105–112 CrossRef CAS PubMed.
- G. Furrer, C. Ludwig and P. W. Schindler, J. Colloid Interface Sci., 1992, 149, 56–67 CrossRef CAS.
- J. F. Keggin, Nature, 1933, 131, 908–909 CrossRef CAS.
- J. T. Kloprogge, D. Seykens, J. B. H. Jansen and J. W. Geus, J. Non-Cryst. Solids, 1992, 142, 94–102 CrossRef CAS.
- X. Xu, Y. Liu, Z. Li, Z. Lv, J. Song, M. He, Q. Wang, L. Yan and Z. Li, J. Therm. Anal. Calorim., 2014, 115, 1111–1117 CrossRef CAS.
- L. Zhang, X. Jiao, D. Chen and M. Jiao, Eur. J. Inorg. Chem., 2011, 2011, 5258–5264 CrossRef CAS.
- L. Torrente-Murciano, A. A. Lapkin and D. Chadwick, J. Mater. Chem., 2010, 20, 6484–6489 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02590d |
| This journal is © The Royal Society of Chemistry 2017 |