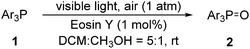Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Eosin Y-catalyzed photooxidation of triarylphosphines under visible light irradiation and aerobic conditions†
Yanbin
Zhang
 b,
Cong
Ye
b,
Cong
Ye
 b,
Shijie
Li
b,
Shijie
Li
 b,
Aishun
Ding
b,
Aishun
Ding
 b,
Guangxin
Gu
b,
Guangxin
Gu
 *a and
Hao
Guo
*a and
Hao
Guo
 *b
*b
aDepartment of Materials Science, Fudan University, 220 Handan Road, Shanghai, 200433, P. R. China. E-mail: guangxingu@fudan.edu.cn; Fax: +86-21-65648837; Tel: +86-21-65648837
bDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433, P. R. China. E-mail: Hao_Guo@fudan.edu.cn; Fax: +86-21-55664361; Tel: +86-21-55664361
First published on 27th February 2017
Abstract
We report herein a novel method for Eosin Y-catalyzed photooxidation of triarylphosphines under visible light irradiation and aerobic conditions. This new approach employed visible light as the energy source and air as the oxidant, showing great advantages in environmental benignness and operational easiness with a wide functional group tolerance.
Photochemistry is an important branch in modern chemistry.1 Distinct from traditional thermal reactions, the key intermediates in photo-reactions are the electronically excited state of organic molecules. Photochemical sensitization of triplet oxygen to singlet oxygen is one of the appealing aspects of photochemistry.2 Compared with chemical decomposition, electro-chemical, bio-chemical and other methods,3 photochemical sensitization is cheap, easy to handle, efficient and environment-friendly. More importantly, organic photocatalysts are easy to functionalize, which means the catalyst could be attached to advanced materials4 and bio-materials.5 Several photocatalysts were used in singlet oxygen generation.6 Among them, the structure of Eosin Y showed good features, such as aqueous solubility and easiness to modify (Scheme 1). Thus, much attention has been paid to Eosin Y-catalyzed singlet oxygen generation.7
Phosphine oxide is an important fragment in medicine,8 and functional supramoleculars.9 It also plays an important role in organic synthesis.10 It has been used as a catalyst for the Wittig reaction,11 a ligand for transitional metals,12 and as a gentle oxidant.13 Numerous methods have been developed for the preparation of phosphine oxides from phosphines via direct oxidations,14,15 among which some photocatalysts, such as 9,10-dicyanoanthracen,15b 9-mesityl-10-methylacridinium perchlorate,15c and sensitizers generating singlet oxygen15d showed nice catalytic activity.15 However, in many cases, external environmentally harmful oxidants,14a expensive additives,14b or harsh conditions14c were required. Moreover, stoichiometric oxidants will generate stoichiometric wastes, which makes the purification process much more complicated.14b An efficient, environmentally friendly, and convenient method for the oxidation of phosphines is desirable as part of an overall effort to develop “Green Chemistry”. With our continuous interest in this field,16 we turned to study the Eosin Y-catalyzed photo oxidation of triarylphosphines based on the following considerations. Firstly, Eosin Y is cheap and the loading of the catalyst is generally low. Secondly, harsh irradiation conditions can be avoided, since Eosin Y absorbs visible light. Thirdly, Eosin Y is stable in air and absorbs visible light, thus, special glassware and protective gas is no more required. This will provide operational convenience. Here in, we wish to report our recent results in the photooxidation of triarylphosphines using Eosin Y as the catalyst under visible light irradiation and aerobic conditions.
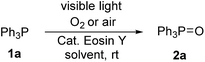
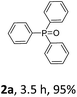
Our initial attempts were carried out using triphenylphosphine 1a as the model substrate, 15 mol% of Eosin Y as the catalyst, a 23 W household lamp as the light source, and oxygen as the oxidant. A quantitative yield of the corresponding triphenylphosphine oxide 2a was obtained in all tested solvents (entries 1–6, Table 1). Given that the reaction in methanol showed the highest reaction speed (entry 6, Table 1), methanol was chosen as the standard solvent. However, when we tried to explore the substrate scope, some reactants were not dissolved in methanol. Thus, a mixture of dichloromethane (DCM)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (MeOH) = 5
methanol (MeOH) = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used as the optimal solvent (entry 7, Table 1). Further studies showed that the loading of catalyst could be dramatically reduced (entries 7–10, Table 1). We were delight to see that only 1 mol% of Eosin Y was enough to efficiently catalyze this transformation (entry 10, Table 1). Air was then used as oxidant instead of pure oxygen, the same excellent yield was observed (entry 11, Table 1) with high quantum yield (Φ = 0.72). Eosin Y disodium salt was then used instead of Eosin Y but showed slightly decreased yield (entry 12, Table 1). Non-halogenated Eosin Y derivative, fluorescein, was also tested but showed far more less reactivity (entry 13, Table 1). Finally, control experiments without Eosin Y (entry 12, Table 1) or light (entry 13, Table 1) were carried out. The results indicated that both Eosin Y and light played a key role in this transformation. Thus, condition A (Eosin Y (1 mol%), DCM
1 was used as the optimal solvent (entry 7, Table 1). Further studies showed that the loading of catalyst could be dramatically reduced (entries 7–10, Table 1). We were delight to see that only 1 mol% of Eosin Y was enough to efficiently catalyze this transformation (entry 10, Table 1). Air was then used as oxidant instead of pure oxygen, the same excellent yield was observed (entry 11, Table 1) with high quantum yield (Φ = 0.72). Eosin Y disodium salt was then used instead of Eosin Y but showed slightly decreased yield (entry 12, Table 1). Non-halogenated Eosin Y derivative, fluorescein, was also tested but showed far more less reactivity (entry 13, Table 1). Finally, control experiments without Eosin Y (entry 12, Table 1) or light (entry 13, Table 1) were carried out. The results indicated that both Eosin Y and light played a key role in this transformation. Thus, condition A (Eosin Y (1 mol%), DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5
MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, air (1 atm), 23 W household lamp, and rt) was applied for further studies.
1, air (1 atm), 23 W household lamp, and rt) was applied for further studies.
| Entry | Solvent | Eosin Y (mol%) | Time (h) | NMR yieldb (%) |
|---|---|---|---|---|
| a A solution of 1a (0.2 mmol) in the tested solvent (3 mL in total) was irradiated by a 23 W household lamp at rt under O2 atmosphere (1 atm). b Yield determined by 31P NMR spectroscopy of the crude reaction mixture using tricyclohexylphosphin as internal standard. c The reactions were carried out under air atmosphere (1 atm). d Isolated yield. e Eosin Y disodium salt was used instead of Eosin Y. f Recovered yield of 1a. g Fluorescein was used instead of Eosin Y. h The reaction was carried out without light. | ||||
| 1 | THF | 15 | 7 | >99 |
| 2 | CCl4 | 15 | 6 | >99 |
| 3 | DCM | 15 | 5 | >99 |
| 4 | Ethyl acetate | 15 | 4 | >99 |
| 5 | CH3CN | 15 | 4 | >99 |
| 6 | MeOH | 15 | 1.5 | >99 |
| 7 | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 3.5 | >99 |
| 8 | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 3.5 | >99 |
| 9 | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 3.5 | >99 |
| 10 | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 3.5 | >99 |
| 11c | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 3.5 | >99 (95)d |
| 12e | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 3.5 | 91 (5)f |
| 13g | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 3.5 | 40 (55)f |
| 14c | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 3.5 | 30 (66)f |
| 15c,h | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5 MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 10 | NR |
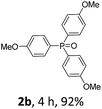
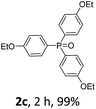
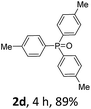
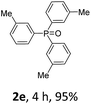
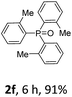
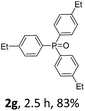
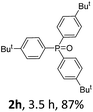
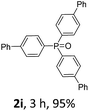
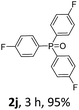
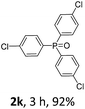
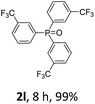
With the optimal conditions in hand, we next examined the substrate scope of this photooxidation with a series of triarylphosphines (Table 2). Reactants with a strong electron donating group, like methoxy (2b) or ethoxy (2c), were firstly examined, which gave the desired products in excellent yields. Then some weak electron donating groups, such as methyl (2d, 2e, and 2f), ethyl (2g), tert-butyl (2h), and phenyl (2i), were induced into the aryl ring of the triarylphosphines and their reactivities were tested. Good to excellent yields were generated. Electron deficient substrates with weak electron withdrawing groups (2j and 2k) or strong electron withdrawing groups (2l, 2m, and 2n) were then applied under the standard conditions. The corresponding yields were quite satisfying. Notably, trinaphthylphosphine (1o) and triphenylphosphine (1p) were also tolerant and showed nice reactivities in this reaction. These results demonstrated that tolerance for a wide range of arylphosphines was achieved in this photooxidation methodology.
| a A solution of 1 (0.2 mmol), Eosin Y (1 mol%), DCM (2.5 mL), and MeOH (0.5 mL) was irradiated by a 23 W household lamp at rt under air atmosphere (1 atm). Isolated yield was reported. | ||
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
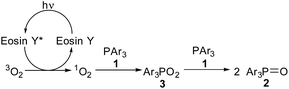
To gain insight to the mechanism, singlet oxygen quenching experiments were carried out using 9,10-dimethylanthracene as singlet oxygen quencher.2b The results were shown in Fig. 1. The whole reaction process was inhibited by 1 equivalent of 9,10-dimethylanthracene (triangle symbol). The reaction rate was even lower with the addition of 2 equivalents of 9,10-dimethylanthracene (square symbol). Since 9,10-dimethylanthracene was a typical singlet oxygen quencher,2b we reasoned that singlet oxygen might be the key oxidative species in the triarylphosphine oxidation process. The trapping product, 9,10-dimethyl-9,10-dihydro-9,10-epidioxyanthracene, was also observed in crude 1H NMR analysis.17 Styrene and cyclohexene was also added to the reaction system to trap phosphadioxirane, but no trapping species was detected.18a
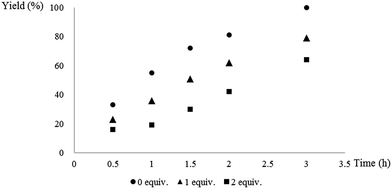 | ||
| Fig. 1 Singlet oxygen quenching experiments. (Reactions were carried out under optimized conditions with 0, 1 or 2 equivalents of 9,10-dimethylanthracene.) | ||
On the basis of the above results and literature precedence,18 a possible mechanism was proposed in Scheme 2. Upon the irradiation of visible light, Eosin Y reaches its excited state.2c Then energy transfer between Eosin Y* and oxygen (3O2) gave singlet oxygen (1O2), and Eosin Y went back to ground state.2a Triarylphosphine 1 and singlet oxygen (1O2) combined to afford triarylphosphadioxane 3.18a3 and another molecule of 1 finally furnished 2 as the product.18b
Conclusions
In conclusion, a visible light-induced metal-free photo oxidation of triarylphosphines was developed. Eosin Y was employed as the catalyst and air was used as the oxidant. Considering that this protocol is cheap, environmental friendly, easy to operate, and the substrate scope is broad, this method may be widely used in the oxidation of phosphine derivatives.Acknowledgements
We greatly acknowledge the financial support from International Science & Technology Cooperation Program of China (2014DFE40130).Notes and references
- (a) D. P. Hari and B. König, Angew. Chem., Int. Ed., 2013, 52, 4734–4743 CrossRef CAS; (b) R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872–3890 CrossRef CAS; (c) S. Poplata, A. Tröster, Y. Zou and T. Bach, Chem. Rev., 2016, 116, 9748–9815 CrossRef CAS.
- (a) M. C. Derosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233/234, 351–371 CrossRef; (b) A. A. Gorman and M. A. J. Rodgers, Chem. Soc. Rev., 1981, 10, 205 RSC; (c) F. Amat-Guerri, M. M. C. López-González, R. Martínez-Utrilla and R. Sastre, J. Photochem. Photobiol., A, 1990, 53, 199–210 CrossRef CAS.
- D. R. Kearns, Chem. Rev., 1971, 71, 395–427 CrossRef CAS.
- F. Xue, M. Shi, Y. Yan, H. Yang, Z. Zhou and S. Yang, RSC Adv., 2016, 6, 15509–15512 RSC.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181 RSC.
- (a) A. Pace and E. L. Clennan, J. Am. Chem. Soc., 2002, 124, 11236–11237 CrossRef CAS; (b) D. Kalaitzakis, T. Montagnon, I. Alexopoulou and G. Vassilikogiannakis, Angew. Chem., Int. Ed., 2012, 51, 8868–8871 CrossRef CAS; (c) R. Gao, D. G. Ho, B. Hernandez, M. Selke, D. Murphy, P. I. Djurovich and M. E. Thompson, J. Am. Chem. Soc., 2002, 124, 14828–14829 CrossRef CAS; (d) D. B. Ushakov, K. Gilmore, D. Kopetzki, D. T. McQuade and P. H. Seeberger, Angew. Chem., Int. Ed., 2014, 53, 557–561 CrossRef CAS; (e) T. Taniguchi, D. Hirose and H. Ishibashi, ACS Catal., 2011, 1, 1469–1474 CrossRef CAS.
- (a) B. M. Estevão, D. S. Pellosi, C. F. de Freitas, D. Vanzin, D. S. Franciscato, W. Caetano and N. Hioka, J. Photochem. Photobiol., A, 2014, 287, 30–39 CrossRef; (b) L. S. Herculano, L. C. Malacarne, V. S. Zanuto, G. V. B. Lukasievicz, O. A. Capeloto and N. G. C. Astrath, J. Phys. Chem. B, 2013, 117, 1932–1937 CrossRef CAS; (c) Y. C. Teo, Y. Pan and C. H. Tan, ChemCatChem, 2013, 5, 235–240 CrossRef CAS; (d) Z. Barbieriková, M. Mihalíková and V. Brezová, Photochem. Photobiol., 2012, 88, 1442–1454 CrossRef; (e) D. S. Pellosi, B. M. Estevão, J. Semensato, D. Severino, M. S. Baptista, M. J. Politi, N. Hioka and W. Caetano, J. Photochem. Photobiol., A, 2012, 247, 8–15 CrossRef CAS; (f) M. Scholz, R. Dědic, T. Breitenbach and J. Hála, Photochem. Photobiol. Sci., 2013, 12, 1873–1884 RSC.
- Y. Gao, G. Lu, P. Zhang, L. Zhang, G. Tang and Y. Zhao, Org. Lett., 2016, 18, 1242–1245 CrossRef CAS.
- J. Li, D. Ding, Y. Tao, Y. Wei, R. Chen, L. Xie, W. Huang and H. Xu, Adv. Mater., 2016, 28, 3122–3130 CrossRef CAS.
- (a) T. Yu, Y. Wang, X. Hu and P. Xu, Chem. Commun., 2014, 50, 7817–7820 RSC; (b) X. Zeng, Z. Cao, X. Wang, L. Chen, F. Zhou, F. Zhu, C. Wang and J. Zhou, J. Am. Chem. Soc., 2016, 138, 416–425 CrossRef CAS; (c) C. J. A. Warner, A. T. Reeder and S. Jones, Tetrahedron: Asymmetry, 2016, 27, 136–141 CrossRef CAS; (d) H. M. Al-Saidi, J. Saudi Chem. Soc., 2016, 20, 615–624 CrossRef CAS.
- (a) L. Wang, Y. Wang, M. Chen and M. Ding, Adv. Synth. Catal., 2014, 356, 1098–1104 CrossRef CAS; (b) M. Schirmer, S. Adomeit, A. Spannenberg and T. Werner, Chem.–Eur. J., 2016, 22, 2458–2465 CrossRef CAS.
- H. Pan, H. Huang, W. Liu, H. Tian and Y. Shi, Org. Lett., 2016, 18, 896–899 CrossRef CAS.
- N. Oka, R. Kajino, K. Takeuchi, H. Nagakawa and K. Ando, J. Org. Chem., 2014, 79, 7656–7664 CrossRef CAS.
- (a) K. A. Prokop and D. P. Goldberg, J. Am. Chem. Soc., 2012, 134, 8014–8017 CrossRef CAS; (b) E. K. Beloglazkina, A. G. Majouga, A. A. Moiseeva, N. V. Zyk and N. S. Zefirov, Mendeleev Commun., 2009, 19, 69–71 CrossRef CAS; (c) K. E. Elson, I. D. Jenkins and W. A. Loughlin, Tetrahedron Lett., 2004, 45, 2491–2493 CrossRef CAS.
- (a) K. Ohkubo, T. Nanjo and S. Fukuzumi, Bull. Chem. Soc. Jpn., 2006, 79, 1489–1500 CrossRef CAS; (b) S. Yasui, S. Tojo and T. Majima, J. Org. Chem., 2005, 70, 1276–1280 CrossRef CAS; (c) K. Ohkubo, H. Kotani and S. Fukuzumi, Chem. Commun., 2005, 4520–4522 RSC; (d) R. Gao, D. G. Ho, T. Dong, D. Khuu, N. Franco, O. Sezer and M. Selke, Org. Lett., 2001, 3, 3719–3722 CrossRef CAS.
- A. Ding, Y. Wang, R. Rios, J. Sun, H. Li and H. Guo, J. Saudi Chem. Soc., 2015, 19, 706–709 CrossRef.
- (a) J. M. Carney, R. J. Hammer, M. Hulce, C. M. Lomas and D. Miyashiro, Tetrahedron Lett., 2011, 52, 352–355 CrossRef CAS; (b) J. M. Carney, R. J. Hammer, M. Hulce, C. M. Lomas and D. Miyashiro, Synthesis, 2012, 44, 2560–2566 CrossRef CAS.
- (a) D. G. Ho, R. Gao, J. Celaje, H. Y. Chung and M. Selke, Science, 2003, 302, 259–262 CrossRef CAS; (b) S. Tsuji, M. Kondo, K. Ishiguro and Y. Sawaki, J. Org. Chem., 1993, 58, 5055–5059 CrossRef CAS; (c) C. P. Mane, S. V. Mahamuni, A. P. Gaikwad, R. V. Shejwal, S. S. Kolekar and M. A. Anuse, J. Saudi Chem. Soc., 2015, 19, 46–53 CrossRef; (d) B. Stewart, A. Harriman and L. J. Higham, Organometallics, 2011, 30, 5338–5343 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and characterization data for all compounds. See DOI: 10.1039/c6ra25469a |
| This journal is © The Royal Society of Chemistry 2017 |