Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boronic acid–DMAPO cooperative catalysis for dehydrative condensation between carboxylic acids and amines†
Kazuaki
Ishihara
*ab and
Yanhui
Lu
a
aGraduate School of Engineering, Nagoya University, B2-3(611), Furo-cho, Chikusa, Nagoya 464-8603, Japan. E-mail: ishihara@cc.nagoya-u.ac.jp
bJST, CREST, B2-3(611), Furo-cho, Chikusa, Nagoya 464-8603, Japan
First published on 6th November 2015
Abstract
Arylboronic acid and 4-(N,N-dimethylamino)pyridine N-oxide (DMAPO) cooperatively catalyse the dehydrative condensation reaction between carboxylic acids and amines to give the corresponding amides under azeotropic reflux conditions. This cooperative use is much more effective than their individual use as catalysts, and chemoselectively promotes the amide condensation of (poly)conjugated carboxylic acids. The present method is practical and scalable, and has been applied to the synthesis of sitagliptin and a drug candidate.
Introduction
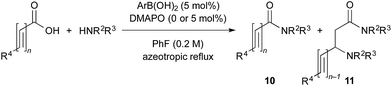
The catalytic dehydrative condensation reaction between carboxylic acids and amines is one of the most ideal methods for synthesizing the corresponding amides.1 In 1996, Yamamoto et al. reported the first example of the dehydrative amide condensation reaction catalysed by meta- or para-electron-deficient group-substituted phenylboronic acids such as 3,4,5-trifluorophenylboronic acid (1) (pKa = 6.8)2a and 3,5-bis(trifluoromethyl)phenylboronic acid (2) (pKa = 7.2)2b under azeotropic reflux conditions (Scheme 1).3 These boronic acids are more acidic than phenylboronic acid (pKa = 8.8, 8.9).2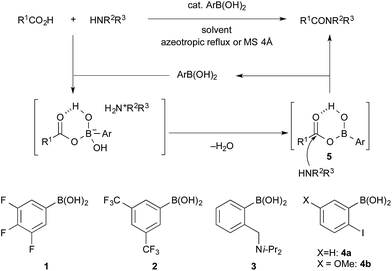 | ||
| Scheme 1 Dehydrative condensation of carboxylic acids with amines catalysed by arylboronic acids, and representative examples of catalysts. | ||
In 2006, Whiting et al. reported that ortho-Brønsted base-substituted phenylboronic acids such as 2-(N,N-diisopropylaminomethyl)phenylboronic acid (3) were effective catalysts for the amide condensation of aromatic carboxylic acids under the same conditions as above.1a,4 In 2008 and 2012, Hall et al. reported that 2-iodophenylboronic acid (4a) and 2-iodo-5-methoxyphenylboronic acid (4b) were also effective catalysts for the amide condensation in the presence of drying agents (activated 4 Å molecular sieves) at lower temperature.5 The o-iodo group of 4a and 4b assists the catalysis of amide condensation as a weak base.6 In addition to these boronic acids, boric acid,7a,c benzo[1,3,2]dioxaborol-2-ol,7b methylboronic acid,7d and some o-Brønsted base-substituted boronic acids8 have been reported to be useful as amidation catalysts. However, the substrate scope is still quite limited. For example, harsh conditions (higher temperature, prolonged reaction time, excess amounts of substrates, increased amounts of catalysts, etc.) are required for sterically hindered α-branched carboxylic acids and conjugated carboxylic acids. In 2013, Whiting et al. discovered an interesting synergistic catalytic effect between o-tolylboronic acid (50 mol%) and o-nitrophenylboronic acid (50 mol%) in dipeptide synthesis.3f To the best of our knowledge, this was the first example of two cooperative promoters for direct amidation.3f,9
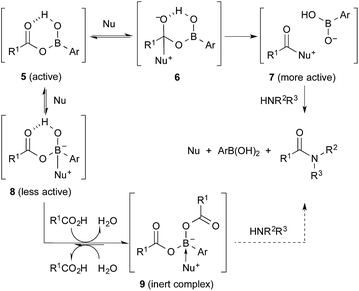
In the process catalysed by arylboronic acid, a mixed anhydride intermediate 5 is generated from the carboxylic acid and arylboronic acid under azeotropic reflux conditions or in the presence of drying agents in the first stage (Schemes 1 and 2). This is the first activation of the carboxylic acid. If a nucleophilic additive (Nu) reacts with 5 to generate a more active cationic intermediate 7 (ref. 10) (second activation) via a tetrahedral intermediate 6, the amide condensation may proceed more rapidly. However, if Nu preferentially coordinates as a Lewis base to the boron atom of 5, a less active species 8 is generated and the amide condensation may be suppressed. Here we report that arylboronic acids and N,N-dimethylaminopyridine N-oxide (DMAPO) cooperatively promote the dehydrative condensation between various carboxylic acids and amines.
 | ||
| Scheme 2 Our proposal: the second activation of a mixed anhydride 5 with a nucleophilic additive (Nu) to generate a more active cationic intermediate 7. | ||
Results and discussion
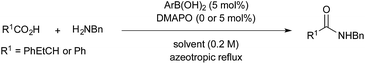
First, the amide condensation reaction between 2-phenylbutyric acid and benzylamine was examined in the presence of 5 mol% each of boronic acid 2 and a nucleophilic additive under azeotropic reflux conditions in fluorobenzene (bp. 85 °C)3f for 17 h (Table 1). Boronic acid 2 did not promote the reaction in the absence of additive under these conditions (entry 1). Tertiary amines such as N,N-diisopropylethylamine and 4-(N,N-dimethylamino)pyridine (DMAP)11 were not effective as additives (entries 2 and 3). 4-Methoxypyridine N-oxide (MPO) was also less active (entry 4). In contrast, a more nucleophilic but weak base, DMAPO,12 was quite effective for the amide condensation (entry 5). However, a more nucleophilic additive such as 4-(pyrrolidin-1-yl)pyridine N-oxide (PPYO) was less effective than DMAPO (entry 6), perhaps because the strong nucleophilicity of PPYO might reduce the activity of 7.| Entry | Additive | Yieldb (%) | Entry | Additive | Yieldb (%) |
|---|---|---|---|---|---|
| a A solution of 2-phenylbutyric acid (0.5 mmol) and benzylamine (0.5 mmol) in fluorobenzene was heated in the presence of 2 (5 mol%) and additive (0 or 5 mol%) under azeotropic reflux conditions. b Isolated yield. | |||||
| 1 | None | <5 | 4 | MPO | <5 |
| 2 | i-Pr2EtN | <5 | 5 | DMAPO | 99 |
| 3 | DMAP | <5 | 6 | PPYO | 27 |
Next, the cooperative effects of several boronic acids (5 mol%) were compared in the condensation reaction between 2-phenylbutyric acid or benzoic acid and benzylamine in the presence of DMAPO (5 mol%) (Table 2). These less reactive carboxylic acids were not activated by the individual use of boronic acids under the same conditions. As expected, 2–DMAPO and 4b–DMAPO efficiently activated 2-phenylbutyric acid (entries 1 and 3). Phenylboronic acid and 3 were almost inert, even in the presence of DMAPO (entries 2 and 4). Interestingly, 2–DMAPO was more effective than 4b–DMAPO for the amide condensation of benzoic acid (entries 1 and 3). While Whiting's catalyst 3 was quite effective for the amide condensation of benzoic acid, the catalytic activity of 3 was suppressed in the presence of DMAPO (entry 4).13
| Entry | ArB(OH)2 | Yieldb,c (%) of PhEtCHCONHBn | Yieldb,d (%) of PhCONHBn |
|---|---|---|---|
| a 0.5 mmol of carboxylic acid and 0.5 mmol of benzylamine were used in the presence of 5 mol% of ArB(OH)2 and 0 or 5 mol% of DMAPO. b The results when both catalysts were used are shown. For comparison, the results without DMAPO are shown in brackets. c Conditions: fluorobenzene (bp. 85 °C), 17 h. d Conditions: toluene (bp. 110 °C), 4 h. | |||
| 1 | 2 | 99 [<5] | 97 [<5] |
| 2 | PhB(OH)2 | <5 [<5] | <5 [<5] |
| 3 | 4b | 92 [<5] | 20 [8] |
| 4 | 3 | 7 [15] | 80 [95] |
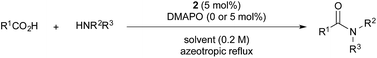
To explore the substrate scope using the cooperative catalysts, 2–DMAPO, the amide condensation reactions of several less reactive α-branched carboxylic acids and arenecarboxylic acids were examined under azeotropic reflux conditions in fluorobenzene (bp. 85 °C) or toluene (bp. 110 °C). As shown in Table 3, in each example, the cooperative catalysts were much more effective than 2 alone, the results for which are shown in brackets. Notably, not only aliphatic primary amines but also sterically hindered aliphatic secondary amines, less nucleophilic anilines and alkoxyamines reacted with these carboxylic acids. In particular, 2–DMAPO was effective in the amidation of arenecarboxylic acids with sterically hindered amines, in comparison with 3 and 4b (entries 9–14). This cooperative method is scalable to practical volumes: the catalytic loading of 2–DMAPO could be reduced to 2.5 mol% for the dehydrative condensation on an 80 mmol scale (entry 4).
| Entry | Amide | Solvent, time | Yieldb (%) |
|---|---|---|---|
| a Unless noted otherwise, 0.5 mmol of carboxylic acid and 0.5 mmol of amine were used in the presence of 5 mol% of 2 and 0 or 5 mol% of DMAPO. b The results when both catalysts were used are shown. For comparison, the results without DMAPO are shown in brackets. c 10 mol% of each of the catalysts was used. d 2.5 mol% of each of 2 and DMAPO was used on an 80 mmol scale in 70 mL of toluene. e 15 mol% of each of the catalysts was used. f 99% ee. g 3 was used. h 4b was used. | |||
| 1c |
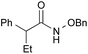
|
PhF, 25 h | 93 [<5] |
| 2c |
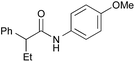
|
PhF, 17 h | 90 [<5] |
| 3 |
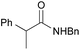
|
PhH, 8 h | 95 [<5] |
| 4d | PhCH3, 11 h | 98 | |
| 5 |
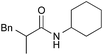
|
PhCH3, 8 h | 97 [<5] |
| 6c |
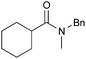
|
PhCH3, 18 h | 92 [<5] |
| 7c |
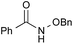
|
PhCH3, 12 h | 70 [23] |
| 8 |
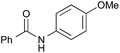
|
PhCH3, 23 h | 91 [19] |
| 9e |
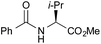
|
PhF, 23 h | 85f [30] |
| 10e,g | PhF, 23 h | [2] | |
| 11e,h | PhF, 23 h | [17] | |
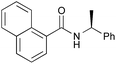
| 12 |
|
PhCH3, 9 h | 95 [<5] |
| 13g | PhCH3, 9 h | [39] | |
| 14h | PhCH3, 9 h | [<5] | |
| 15 |
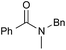
|
PhCH3, 8 h | 92 [32] |
The boronic acid-catalysed condensation of relatively more reactive α-nonbranched carboxylic acids with sterically hindered secondary amines and less nucleophilic anilines proceeded even in the absence of DMAPO, as shown in brackets in Table 4.
| Entry | Amide | ArB(OH)2 | Solvent, time | Yieldb (%) |
|---|---|---|---|---|
| a Unless noted otherwise, 0.55 mmol of carboxylic acid and 0.50 mmol of amine were used in the presence of 5 mol% of ArB(OH)2 and 0 or 5 mol% of DMAPO. b The results when both catalysts were used are shown. For comparison, the results without DMAPO are shown in brackets. c 10 mol% of each of the catalysts was used. d The reaction was carried out at a 5 mmol scale. | ||||
| 1c |
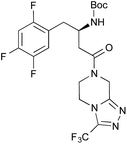
|
2 | PhF, 23 h | 81 [55] |
| 2c | PhB(OH)2 | PhF, 23 h | 92 [50] | |
| 3c | 4b | PhF, 23 h | 98 [53] | |
| 4d | 4b | PhF, 40 h | >99 | |
| 5c | — | PhF, 23 h | <5 [<5] | |
| 6 |
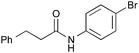
|
PhB(OH)2 | PhH, 17 h | 82 [44] |
Nevertheless, the addition of DMAPO was also quite effective for these reactions. Interestingly, 4b and phenylboronic acid were slightly more reactive than 2 in the presence of DMAPO. In particular, the utility of inexpensive phenylboronic acid is industrially significant. This catalytic method is readily scalable. 2.5 g of N-Boc protected sitagliptin,14 an anti-diabetic drug, was obtained by carrying out the condensation on a 5 mmol scale (entry 4).
The results in Tables 1–4 suggest that both the nucleophilicity of the additive and the Lewis acidity and steric effect of the boronic acid are important in the cooperative catalysis with an ArB(OH)2–nucleophilic base (Table 5). The reactivity from highest to lowest followed the order arenecarboxylic acids, α-branched carboxylic acids, α-nonbranched carboxylic acids. As a result, 2 was more effective for arenecarboxylic acids and α-branched carboxylic acids. On the other hand, 4b and phenylboronic acid were more effective for α-nonbranched carboxylic acids.
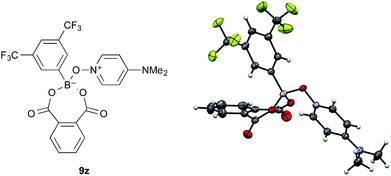
The amide condensation reaction should occur through the active intermediate 6 (Scheme 2). However, not only 6 but also the undesired complex 8 would be generated in an equilibrium mixture. Complex 8 might be converted to the more stable complex 9, which is inert to the amide condensation. In fact, the generation of inert complex 9 was ascertained by 11B and 1H NMR analysis in the amidation of less-hindered carboxylic acids.15 Also, the chemical structure of the cyclic complex prepared from 2, phthalic acid, and DMAPO was determined to be that of 9z by X-ray diffraction analysis (Fig. 1).16
For sterically hindered carboxylic acids such as arenecarboxylic acids and α-branched carboxylic acids, the desired intermediate 7 was preferentially generated. Thus, o-nonsubstituted and m- or p-electron-deficient group-substituted phenylboronic acids such as 1 and 2 were more suitable. In contrast, for less sterically hindered α-nonbranched carboxylic acids, the undesired complex 9 was generated more easily. In addition, the strong Lewis acidity of 2 helped to stabilize 9 by the tight coordination of DMAPO to the boron centre. This is why 4b and phenylboronic acid were slightly more effective than 2 for the condensation of α-nonbranched carboxylic acids. Not only Lewis acidity, but also the bulkiness of the o-substituent of the boronic acid might suppress the generation and stability of 9. It is noted that the effect of DMAPO was not striking at ambient temperature. Heating was required to accelerate the equilibrium between 6 and 8.
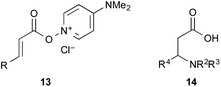
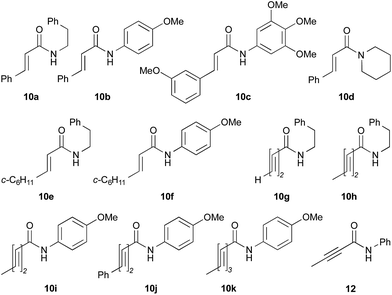
The utility of the cooperative catalysts was also demonstrated for the selective amide condensation of β-substituted acrylic acids to give the corresponding amides 10 (Table 6). The production of Michael adducts 11 was fairly minimal. In contrast, when boronic acids were used in the absence of DMAPO, the yield and selectivity of the reaction for 10 were moderate. Control experiments ascertained that 10 (n = 1) was selectively obtained from 13,17 and 11 (n = 1) was not generated from 10 (n = 1) but 14. Amide 10c is known to be a potential antimitotic agent, especially for brain cancers (entry 6).18 The cooperative catalysts were effective for the selective amide condensation of not only β-substituted acrylic acids, but also polyconjugated carboxylic acids and but-2-ynoic acid (entries 12–17).
| Entry | ArB(OH)2 | Time (h) | Product 10 or 12 | |
|---|---|---|---|---|
| Yieldb (%) | Selectivityb (%) | |||
| a Unless noted otherwise, 0.5 mmol of carboxylic acid and 0.5 mmol of amine were used in the presence of 5 mol% of ArB(OH)2 and 0 or 5 mol% of DMAPO. b The results when both catalysts were used are shown. For comparison, the results without DMAPO are shown in brackets. c β-Aminoamide 11 (n = 1) was obtained as the sole minor product. d 10 mol% of each of the catalysts was used. e Toluene was used as a solvent. f Several minor products including 11 were obtained. | ||||
| 1 | 2 | 12 | 10a, 87 [42] | 93 [74]c |
| 2 | 4b | 10 | 10a, 82 [79] | 94 [91]c |
| 3 | PhB(OH)2 | 12 | 10a, 15 [4] | 95 [78]c |
| 4 | 2 | 15 | 10b, 77 [17] | 89 [30]c |
| 5 | 4b | 16 | 10b, 65 [29] | 89 [47]c |
| 6d,e | 2 | 15 | 10c, 96 [49] | 96 [64]c |
| 7 | 2 | 38 | 10d, 90 [78] | >95 [>95]c |
| 8 | 2 | 8 | 10e, 68 [19] | 82 [37]c |
| 9 | 4b | 7 | 10e, 72 [62] | 84 [78]c |
| 10d | PhB(OH)2 | 19 | 10e, 81 [56] | 92 [76]c |
| 11d | PhB(OH)2 | 22 | 10f, 73 [32] | 96 [55]c |
| 12d | 2 | 16 | 10g, 98 [45] | >99 [45]f |
| 13d | 2 | 16 | 10h, 97 [69] | >99 [79]f |
| 14d | 2 | 16 | 10i, 73 [<5] | 97 [<5]f |
| 15d | 2 | 23 | 10j, 92 [-] | >99 [50]f |
| 16d | 2 | 24 | 10k, 99 [25] | >99 [56]f |
| 17d | 2 | 14 | 12, 98 [46] | >99 [82]f |
|
||||
Conclusions
In conclusion, this new cooperative catalytic system is quite effective for the amidation reaction of less reactive carboxylic acids, such as sterically hindered α-branched carboxylic acids and arenecarboxylic acids, and the chemoselective amidation reaction of conjugated carboxylic acids. Based on the NMR spectra and X-ray diffraction analysis of inert species 9, a preliminary mechanism was proposed. Further mechanistic studies are in progress. We believe that these findings will trigger the further development of high-performance amidation catalysts.Acknowledgements
Financial support for this project was partially provided by the Program for Leading Graduate Schools: IGER Program in Green Natural Sciences (MEXT), JSPS KAKENHI Grant Numbers 26620079, 15K13692, and 15H05755.Notes and references
- For selected reviews, see: (a) I. Georgiou, G. Ilyashenko and A. Whiting, Acc. Chem. Res., 2009, 42, 756 CrossRef CAS PubMed; (b) K. Ishihara, Tetrahedron, 2009, 65, 1085 CrossRef CAS; (c) H. Charville, D. Jackson, G. Hodges and A. Whiting, Chem. Commun., 2010, 46, 1813 RSC; (d) C. Grosjean, J. Parker, C. Thirsk and A. R. Wright, Org. Process Res. Dev., 2012, 16, 781 CrossRef CAS; (e) E. Dimitrijević and M. S. Taylor, ACS Catal., 2013, 3, 945 CrossRef; (f) H. Zheng and D. G. Hall, Aldrichimica Acta, 2014, 47, 41 CAS; (g) H. Lundberg, F. Tinnis, N. Selander and H. Adolfsson, Chem. Soc. Rev., 2014, 43, 2714 RSC; (h) K. Ishihara, in Synthesis and Application of Organoboron Compounds, Topics in Organometallic Chemistry, ed. E. Fernández and A. Whiting, Springer, Heidelberg, 2015, vol. 49, pp. 243–270 Search PubMed.
- (a) J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205 CrossRef CAS; (b) S. Kheirjou, A. Abedin and A. Fattahi, Comput. Theor. Chem., 2012, 1000, 1 CrossRef CAS.
- For the electron-deficient arylboronic acid-catalysed condensation of carboxylic acids, see: (a) K. Ishihara, S. Ohara and H. Yamamoto, J. Org. Chem., 1996, 61, 4196 CrossRef CAS PubMed; (b) K. Ishihara, S. Ohara and H. Yamamoto, Macromolecules, 2000, 33, 3511 CrossRef CAS; (c) K. Ishihara, S. Kondo and H. Yamamoto, Synlett, 2001, 1371 CrossRef CAS; (d) K. Ishihara, S. Ohara and H. Yamamoto, Org. Synth., 2002, 79, 176 CrossRef CAS; (e) T. Maki, K. Ishihara and H. Yamamoto, Synlett, 2004, 1355 CAS; (f) S. Liu, Y. Yang, X. Liu, F. K. Ferdousi, A. S. Batsanov and A. Whiting, Eur. J. Org. Chem., 2013, 5692 CrossRef CAS.
- (a) K. Arnold, A. S. Batsanov, B. Davies and A. Whiting, Green Chem., 2008, 10, 124 RSC; (b) I. Georgiou, G. Ilyashenko and A. Whiting, Acc. Chem. Res., 2009, 42, 756 CrossRef CAS PubMed.
- (a) R. M. Al-Zoubi, O. Marion and D. G. Hall, Angew. Chem., Int. Ed., 2008, 47, 2876 CrossRef CAS PubMed; (b) R. M. Al-Zoubi and D. G. Hall, Org. Lett., 2010, 12, 2480 CrossRef CAS PubMed; (c) N. Gernigon, R. M. Al-Zoubi and D. G. Hall, J. Org. Chem., 2012, 77, 8386 CrossRef CAS PubMed; (d) N. Gernigon, H. Zheng and D. G. Hall, Tetrahedron Lett., 2013, 54, 4475 CrossRef CAS.
- (a) T. Marcelli, Angew. Chem., Int. Ed., 2010, 49, 6840 CrossRef CAS PubMed; (b) C. Wang, H.-Z. Yu, Y. Fu and Q.-X. Guo, Org. Biomol. Chem., 2013, 11, 2140 RSC.
- (a) P. Tang, Org. Synth., 2005, 81, 262 CrossRef CAS; (b) T. Maki, K. Ishihara and H. Yamamoto, Org. Lett., 2006, 8, 1431 CrossRef CAS PubMed; (c) R. K. Mylavarapu, K. Gcm, N. Kolla, R. Veeramalla, P. Koilkonda, A. Bhattacharya and R. Bandichhor, Org. Process Res. Dev., 2007, 11, 1065 CrossRef CAS; (d) R. Yamashita, A. Sakakura and K. Ishihara, Org. Lett., 2013, 15, 3654 CrossRef CAS PubMed.
- For other examples of the o-Brønsted base-substituted phenylboronic acid-catalysed condensation of carboxylic acids, see: (a) A. Sakakura, T. Ohkubo, R. Yamashita, M. Akakura and K. Ishihara, Org. Lett., 2011, 13, 892 CrossRef CAS PubMed; (b) A. Sakakura, R. Yamashita, M. Akakura and K. Ishihara, Aust. J. Chem., 2011, 64, 1458 CrossRef CAS; (c) T. M. El Dine, W. Erb, Y. Berhault, J. Rouden and J. Blanchet, J. Org. Chem., 2015, 80, 4532 CrossRef PubMed; (d) E. K. W. Tam, Rita, L. Y. Liu and A. Chen, Eur. J. Org. Chem., 2015, 1100 CrossRef CAS.
- For recent examples of boron catalysts for reactions involving carboxylic acids, see: (a) T. Azuma, A. Murata, Y. Kobayashi, T. Inokuma and Y. Takemoto, Org. Lett., 2014, 16, 4256 CrossRef CAS PubMed; (b) Y. Morita, T. Yamamoto, H. Nagai, Y. Shimizu and M. Kanai, J. Am. Chem. Soc., 2015, 137, 7075 CrossRef CAS PubMed.
- The unstable counter anion of 7, ArB(OH)O−, may be immediately converted to [ArBL3]− in the presence of carboxylic acids and amines.
- (a) Review: N. de Rycke, F. Couty and O. R. P. David, Chem.–Eur. J., 2011, 17, 12852 CrossRef CAS PubMed; (b) A. Sakakura, K. Kawajiri, T. Ohkubo and K. Ishihara, J. Am. Chem. Soc., 2007, 129, 14775 CrossRef CAS PubMed; (c) R. Tandon, T. Unzer, T. A. Nigst, N. de Rycke, P. Mayer, B. Wendt, O. P. R. David and H. Zipse, Chem.–Eur. J., 2013, 19, 6435 CrossRef CAS PubMed.
- I. Shina, H. Ushiyama, Y. Yamada, Y. Kawakita and K. Nakata, Chem.–Asian J., 2008, 3, 454 CrossRef PubMed.
- The bulkiness of the o-substituent of 3 may suppress the second activation from 6 to 7 with DMAPO. Alternatively, DMAPO may suppress the Brønsted base role of the o-substituent of 3.
- D. Kim, L. Wang, M. Beconi, G. J. Eiermann, M. H. Fisher, H. He, G. J. Hickey, J. E. Kowalchick, B. Leiting, K. Lyons, F. Marsilio, M. E. McCann, R. A. Patel, A. Petrov, G. Scapin, S. B. Patel, R. S. Roy, J. K. Wu, M. J. Wyvratt, B. B. Zhang, L. Zhu, N. A. Thornberry and A. E. Weber, J. Med. Chem., 2005, 48, 141 CrossRef CAS PubMed.
- The reactivity and stability of 9 were investigated. See the ESI for details.†.
- 9z was produced with N-benzylphthalimide through the dehydrative condensation reaction between phthalic acid and benzylamine in the presence of 2 (5 mol%) and DMAPO (5 mol%) in fluorobenzene under azeotropic reflux conditions. In this reaction, the cooperative use of 2 and DMAPO was less effective than the single use of 2. See the ESI (Table S2†) for details.
- Intermediate 13 was prepared from DMAPO and the corresponding acryl chloride. The reaction of 13 with amine gave 10 as the sole product. See the ESI for details.†.
- J. B. Leslie, R. C. Holaday, T. Nguyen and P. J. Hergenrother, J. Med. Chem., 2010, 53, 3964 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure and characterization data of new compounds are provided. CCDC 1429213. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc03761a |
| This journal is © The Royal Society of Chemistry 2016 |