Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sulfoxide-directed metal-free cross-couplings in the expedient synthesis of benzothiophene-based components of materials†
Andrew J.
Eberhart
,
Harry
Shrives
,
Yuntong
Zhang
,
Amandine
Carrër
,
Adam V. S.
Parry
,
Daniel J.
Tate
,
Michael L.
Turner
and
David J.
Procter
*
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL, UK. E-mail: david.j.procter@manchester.ac.uk
First published on 9th November 2015
Abstract
A metal-free approach combining sulfoxide-directed metal-free C–H cross-couplings with tuneable electrophile-mediated heterocyclizations and carbocyclative dimerizations, allows expedient access to benzothiophene-based systems that are components of important materials or are proven organic materials in their own right. As benzothiophene-based materials are typically prepared using Pd-catalyzed cross-coupling processes, our approach allows potential issues of metal cost and supply, and metal-contamination of products, to be avoided.
Introduction
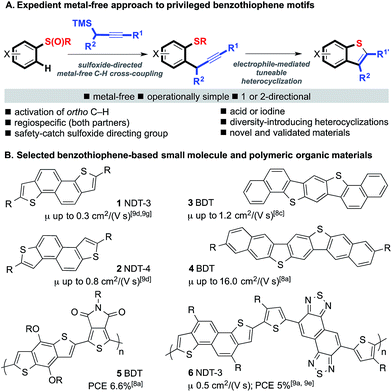
The selective formation of carbon–carbon bonds to aromatic rings is a critical goal in science.1 Although the use of first row metals is an important and growing field (e.g. Fe, Co, Ni, Cu), modern cross-coupling technology still typically involves the use of platinum group metals (e.g. Pd, Ru, Rh, Pt),2 and the development of cross-coupling reactions that do not involve the use of an expensive and/or supply-risk metal is highly desirable.3 Such processes have additional benefits as trace metal contamination in products arising from metal-catalyzed processes is a concern in industry, particularly the pharmaceutical and organic electronic sectors.4 In the latter field, even ‘undetectable’ levels of palladium contaminant can have a detrimental effect on the electrical properties of materials and thin film device performance.4dHere we describe an expedient metal-free approach to important benzothiophene-based architectures, including motifs that are crucial components in valuable organic small molecule and polymeric materials (Scheme 1A).5 Organic materials are increasingly prepared using C–H activation methods mediated by platinum group metals6 in addition to cross-couplings using classical Pd-catalyzed methods.7 Benzodithiophenes (BDTs)8 and napthodithiophenes (NDTs),9 in particular, have been exploited in high-performance small-molecule/oligomeric semi-conducting materials (e.g.1–4), polymeric semiconductors,8,9 and in co-polymers for solar cells8,9 (e.g.5 and 6) (Scheme 1B).
The crucial benzothiophene-based motifs are typically prepared using Pd-catalyzed cross-coupling processes in one or more key steps. Our approach utilizes sulfoxide-directed metal-free C–H cross-coupling processes,10 allowing established Pd-catalyzed couplings and associated metal-contamination to be avoided, and new diversity-introducing hetero- and carbocyclizations mediated by electrophiles. The novel approach has been used in the metal-free synthesis of a range of benzothiophenes including components of materials, and both validated organic materials and previously unknown organic materials for evaluation.
Results and discussion
A two-directional, metal-free route to NDT materials
We began by exploring the conversion of the products of our sulfoxide-directed, metal-free, propargylative C–H cross-coupling10 to benzothiophenes. Pleasingly, straight-forward variation of iodine or acid-mediated conditions (A–C) allowed access to a wide range of decorated benzothiophene motifs bearing different levels of oxidation at the carbon adjacent to the benzothiophene ring (Scheme 2).11 In addition, a wide range of substituents on the aromatic ring are tolerated thus allowing the electronic properties of target materials to be tuned (vide infra).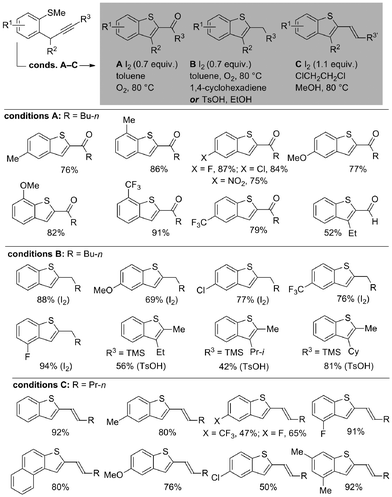 | ||
| Scheme 2 Tuneable electrophile-mediated, heterocyclization to give decorated benzothiophenes (isolated yields after purification). | ||
A proposed mechanism for the formation of benzothiophenes under iodine-mediated conditions A–C is set out in Scheme 3. Upon treatment with iodine, cyclization gives sulfonium salts I that undergo demethylation and tautomerization to form common iodide intermediates II. Upon heating, homolysis gives radicals III that either undergo quenching with O2 (conditions A) to give ketone products, possibly via alkylhydroperoxide intermediates IV, or with 1,4-cyclohexadiene (conditions B) to give alkyl substituted benzothiophene products. Under conditions C, elimination of iodides II gives alkenyl benzothiophene products. Acid-mediated cyclization (alternative conditions B) gives alkyl substituted benzothiophene products directly after demethylation of sulfonium salt intermediates.
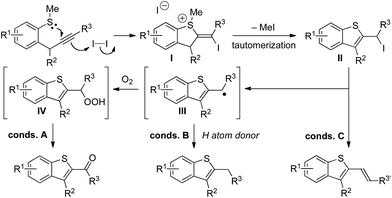 | ||
| Scheme 3 Proposed mechanism for the tuneable iodine-mediated, heterocyclization to give decorated benzothiophenes. | ||
We next investigated the feasibility of combining sulfoxide-directed cross-coupling with electrophile-mediated heterocyclizations in metal-free approaches to benzothiophene-based components of organic materials. We first examined a metal-free, two-directional synthesis of naphthodithiophene (NDT) motifs, typically prepared using Pd-catalyzed Sonogashira couplings,9d,g and found in high performance small molecule and polymeric organic materials.9 Bis-sulfoxide starter units 7 and 8, readily-prepared from 2,6-dihydroxynapthalene (82%, 2 steps) and 1,5-dihydroxynapthalene (66%, 2 steps) (n-C6H13SH, toluene, Dean–Stark; mCPBA, CH2Cl2),12 underwent two-directional metal-free C–H propargylation, under our previously reported conditions,10 to deliver adducts 9 and 10 in high yield (Scheme 4). In the cross-coupling to give 9, complete regiocontrol is observed. The process proved scalable and the cross-coupling of 8 gave 10b in 76% on a 1.5 g scale.
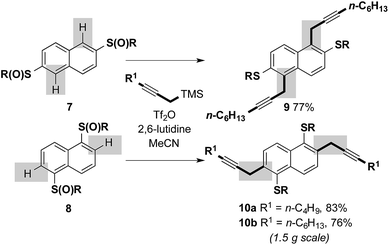 | ||
| Scheme 4 Two-directional, sulfoxide-directed, metal-free cross-couplings involving substrates derived from dihydroxynaphthalenes; R = n-C6H13 (isolated yields after purification). | ||
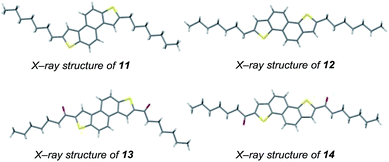
Further diversity can be introduced by tuning the conditions of the subsequent two-directional heterocyclization. For example, while acid-mediated cyclization of 9 and 10b (variant of conditions B; the use of NaI facilitates demethylation of sulfur in an intermediate sulfonium salt; Schemes 2 and 3) delivers alkyl-substituted materials 11 and 12, respectively, iodine-mediated cyclization delivers acyl-substituted materials 13 and 14 (all 2 steps from sulfoxide). Furthermore, iodine-mediated cyclization of 9 and 10b under eliminative conditions gave alkenylated products 15 and 16 (Scheme 5). NDT products 11–16 are potential small molecule organic materials in their own right (vide infra)9d,g and 11–14 were characterized by X-ray crystallographic analysis (Fig. 1).13 Thus, sulfoxide-directed cross-couplings allow palladium-mediated couplings to be avoided in the four-step synthesis of validated (11 and 12) and unexplored (13–16) types of organic material.
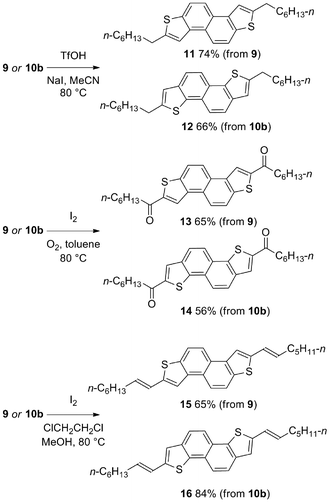 | ||
| Scheme 5 ‘Tuneable’ 2-directional heterocyclizations for the metal-free synthesis of NDT materials (isolated yields after purification). | ||
A metal-free route to BDT materials
Attractively, the sulfoxide moiety in our approach can be viewed as a ‘safety-catch’ directing group: only upon oxidation to the sulfoxide is the substrate receptive to cross-coupling. Furthermore, the directing group is reduced during the coupling, the directing effect is ‘switched-off’, and over-alkylation is impossible.10b Selective coupling in bis-sulfide substrates bearing latent directing groups is therefore possible provided selective oxidation can be achieved. For example, symmetrical bis-sulfide starter unit 17 and intermediate 19 can be selectively activated and thus undergo selective metal-free cross-coupling. This allows the controlled metal-free construction of unsymmetrical targets such as BDT 21 using iterative sulfoxide-directed cross-coupling (Scheme 6). The ability to prepare unsymmetrical small-molecule materials is crucial for several applications including the immobilization of materials on surfaces in the exploitation of self-assembled monolayers (SAM) in organic electronic devices.14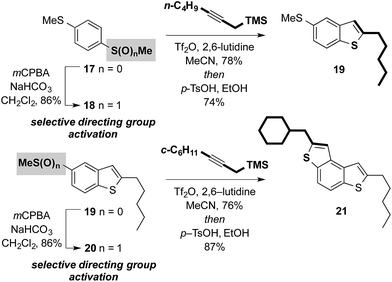 | ||
| Scheme 6 ‘Safety-catch’ sulfoxide directing groups in metal-free cross-couplings: potential for the selective synthesis of unsymmetrical benzothiophene-based components. | ||
We next addressed the challenge of developing a short, metal-free synthesis of extended BDT motifs. Such motifs are typically prepared by Pd-catalyzed Sonogashira, Suzuki, or Negishi couplings.8 Tuning the heterocyclization of the products from metal-free cross-coupling delivers benzothiophenes bearing conjugated alkenes (Scheme 2, conditions C). Subsequent, unprecedented iodine-mediated, carbocyclative dimerization conveniently delivers novel, substituted benzodithiophenes 22–28 in moderate to excellent yield (3 steps overall from sulfoxide). The structure of 22 was confirmed by X-ray crystallography.13 Crucially, the alkyl substituents on the central benzene ring, and the electronic properties of the flanking benzene rings, can be readily varied through judicial choice of propargylsilane and sulfoxide coupling partners in the metal-free approach (Scheme 7A). A proposed mechanism for the novel dimerization is shown in Scheme 7B: homocoupling of the activated alkene 29 followed by acid-mediated cyclization gives intermediate 30en route to dimers 22–28.
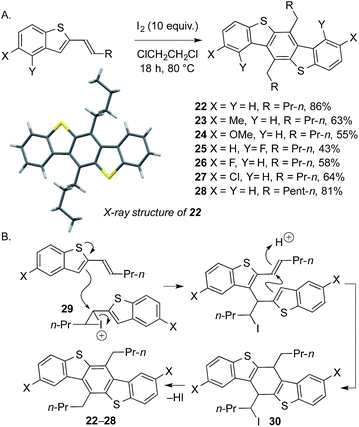 | ||
| Scheme 7 (A) Carbocyclative dimerization in a metal-free approach to novel dialkyl-substituted benzodithiophenes (BDTs). (B) Proposed mechanism for the dimerization. | ||
We have carried out preliminary evaluation of the materials prepared. Cyclic voltammetry and UV/Vis spectroscopy were used to determine the HOMO/LUMO levels and energy gaps for compounds 11–16 and 22–28. In particular, compounds 11–14 showed a low lying HOMO of <−5.5 eV and energy band gap greater than 3 eV, consistent with previous reports suggesting the orientation of the thiophene ring has little effect on the electronic structure of the core.9h As expected, a lowering of the HOMO/LUMO was observed for the acyl-substituted derivatives.
Thin films of compounds 11–14 were characterised using X-ray diffraction and atomic force microscopy, and organic field effect transistors (OFETs) were prepared and analysed using the conventional thin film transistor techniques.15 Consistent with previous observations,9d,g,h novel materials 11 and 13 formed smooth thin films and demonstrated p-type (hole transporting) behaviour in a field effect transistor. In particular, compound 11 demonstrated a mobility of 0.2 cm2 V−1 s−1, high current on/off ratio of 107 and a threshold voltage of −22 V over an average of 9 devices. This performance is consistent with those reported for fused benzothiophene semiconductors.9h
Conclusions
In conclusion, a metal-free approach allows expedient access to benzothiophene-based systems that are components of important materials or are proven organic materials in their own right. The approach combines sulfoxide-directed metal-free C–H cross-couplings with novel tuneable electrophile-mediated heterocyclizations and carbocyclative dimerizations. As benzothiophene-based materials are typically prepared using Pd-catalyzed cross-coupling processes, our approach allows potential issues of metal cost and supply, and the metal-contamination of products, to be avoided.Acknowledgements
We thank the EPSRC (Established Career Fellowship to D. J. P. and Doctoral Prize to A. J. E), The Leverhulme Trust (Research Fellowship to D. J. P), and Novartis (Studentship to H. S.).Notes and references
-
(a) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451 CrossRef CAS PubMed
; (b) J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177 CrossRef CAS PubMed
; (c) Transition Metal-Catalyzed Couplings in Process Chemistry: Case Studies From the Pharmaceutical Industry, ed. J. Magano and J. R. Dunetz, Wiley, 2013 Search PubMed
; (d) C. Toberg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027 CrossRef
.
- For recent reviews, see:
(a) P. B. Arockiam, C. Bruneau and P. H. Dixneauf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed
; (b) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC
; (c) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed
; (d) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef PubMed
; (e) X. Chen, K. M. Engle, D. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed
.
- For recent metal-free cross-coupling approaches based on Pummerer-type processes, see:
(a) T. Kobatake, D. Fujino, S. Yoshida, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2010, 132, 11838 CrossRef CAS PubMed
; (b) T. Kobatake, S. Yoshida, H. Yorimitsu and K. Oshima, Angew. Chem., Int. Ed., 2010, 49, 2340 CrossRef CAS PubMed
; (c) A. J. Eberhart, J. E. Imbriglio and D. J. Procter, Org. Lett., 2011, 13, 5882 CrossRef CAS PubMed
; (d) Y. Ookubo, A. Wakamiya, H. Yorimitsu and A. Osuka, Chem.–Eur. J., 2012, 18, 12690 CrossRef CAS PubMed
; (e) A. J. Eberhart, C. Cicoira and D. J. Procter, Org. Lett., 2013, 15, 3994 CrossRef CAS PubMed
; (f) K. Murakami, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2014, 53, 7510 CrossRef CAS PubMed
; (g) X. Huang and N. Maulide, J. Am. Chem. Soc., 2011, 133, 8510 CrossRef CAS PubMed
; (h) X. Huang, M. Patil, C. Fares, W. Thiel and N. Maulide, J. Am. Chem. Soc., 2013, 135, 7312 CrossRef CAS PubMed
; (i) B. Peng, D. Geerdink, C. Farés and N. Maulide, Angew. Chem., Int. Ed., 2014, 53, 5462 CrossRef PubMed
; (j) B. Peng, X. Huang, L.-G. Xie and N. Maulide, Angew. Chem., Int. Ed., 2014, 53, 8718 CrossRef CAS PubMed
. For selected reviews of the Pummerer reaction, see: (k) L. H. S. Smith, S. C. Coote, H. F. Sneddon and D. J. Procter, Angew. Chem., Int. Ed., 2010, 49, 5832 CrossRef CAS PubMed
; (l) S. Akai and Y. Kita, Top. Curr. Chem., 2007, 274, 35 CrossRef CAS
; (m) K. S. Feldman, Tetrahedron, 2006, 62, 5003 CrossRef CAS
; (n) S. K. Bur and A. Padwa, Chem. Rev., 2004, 104, 2401 CrossRef CAS PubMed
.
- For reviews on palladium removal, see:
(a) C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889 CrossRef CAS
. See also ref. 1c. (b) K. Königsberger, G.-P. Chen, R. R. Wu, M. J. Girgis, K. Prasad, O. Repič and T. J. Blacklock, Org. Process Res. Dev., 2003, 7, 733 CrossRef
. For a recent example of the negative impact of metal impurities, see: (c) J. C. Hermann, Y. Chen, C. Wartchow, J. Menke, L. Gao, S. K. Gleason, N.-E. Haynes, N. Scott, A. Petersen, S. Gabriel, B. Vu, K. M. George, A. Narayanan, S. H. Li, H. Qian, N. Beatini, L. Niu and Q.-F. Gan, ACS Med. Chem. Lett., 2013, 4, 197 CrossRef CAS PubMed
. For an example of the palladium contamination of large organic molecules and polymers, see: (d) K. T. Nielsen, K. Bechgaard and F. C. Krebs, Macromolecules, 2005, 38, 658 CrossRef CAS
. See also ref. 7d and e.
- For recent reviews on thiophene-based organic materials, see:
(a) K. Takimiya and I. Osaka, Chem. Rec., 2015, 15, 175 CrossRef CAS PubMed
; (b) K. Takimiya, I. Osaka, T. Mori and M. Nakano, Acc. Chem. Res., 2014, 47, 1493 CrossRef CAS PubMed
; (c) K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 4347 CrossRef CAS PubMed
.
- For recent reviews, see:
(a) Y. Segawa, T. Maekawa and K. Itami, Angew. Chem., Int. Ed., 2015, 54, 66 CrossRef CAS PubMed
; (b) A. Facchetti, L. Vaccaro and A. Marrocchi, Angew. Chem., Int. Ed., 2012, 51, 3520 CrossRef CAS PubMed
.
-
(a)
Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Developments, ed. A. Molnar, Wiley, 2013 Search PubMed
; (b) J. Tsuji, Palladium Reagents and Catalysts: New Perspectives for the 21st Century, Wiley, Sussex, England, 2004 Search PubMed
; (c) E. Negishi, and A. de Meijere, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, New York, 2002 Search PubMed
. For specific application in organic materials synthesis, see for example; (d) G. Marzano, C. V. Ciasca, F. Babudri, G. Bianchi, A. Pellegrino, R. Po and G. M. Farinol, Eur. J. Org. Chem., 2014, 6583 CrossRef CAS
; (e) B. Carsten, F. He, H. J. Son and T. X. L. Yu, Chem. Rev., 2011, 111, 1493 CrossRef CAS PubMed
.
- For selected references on BDTs, see:
(a) C. Mitsui, T. Okamoto, M. Yamagishi, J. Tsurumi, K. Yoshimoto, K. Nakahara, J. Soeda, Y. Hirose, H. Sato, A. Yamano, T. Ueura and J. Takeya, Adv. Mater., 2014, 26, 4546 CrossRef CAS PubMed
; (b) C. Cabanetos, A. El Labban, J. A. Bartelt, J. D. Douglas, W. R. Mateker, J. M. J. Fréchet, M. D. McGhee and P. M. Beaujuge, J. Am. Chem. Soc., 2013, 135, 4656 CrossRef CAS PubMed
; (c) Y. Chen, H. Chang, H. Tian, C. Bao, W. Li, D. Yan, Y. Geng and F. Wang, Org. Electron., 2012, 13, 3268 CrossRef CAS
; (d) T. Kashiki, S. Shinamura, M. Kohara, E. Miyazaki, K. Takimiya, M. Ikeda and H. Kuwabara, Org. Lett., 2009, 11, 2473 CrossRef CAS PubMed
; (e) P. Gao, D. Beckmann, H. N. Tsao, X. Feng, V. Enkelmann, W. Pisula and K. Müllen, Chem. Commun., 2008, 1548 RSC
; (f) H. Ebata, E. Miyazaki, T. Yamamoto and K. Takimiya, Org. Lett., 2007, 9, 4499 CrossRef CAS PubMed
; (g) K. Takimiya, Y. Honda, H. Ebata, N. Niihara and T. Otsubo, J. Org. Chem., 2005, 70, 10569 CrossRef CAS PubMed
. See also ref. 5.
- For selected references on NDTs, see:
(a) I. Osaka, T. Kakara, N. Takemura, T. Koganezawa and K. Takimiya, J. Am. Chem. Soc., 2013, 135, 8834 CrossRef CAS PubMed
; (b) S. Shi, X. Xie, P. Jang, S. Chen, L. Wang, M. Wang, H. Wang, X. Li, G. Yu and Y. Li, Macromolecules, 2013, 46, 3358 CrossRef CAS
; (c) C. Viglianisi, L. Becucci, C. Faggi, S. Piantini, P. Procacci and S. Menichetti, J. Org. Chem., 2013, 78, 3496 CrossRef CAS PubMed
; (d) I. Osaka, S. Shinamura, T. Abe and K. Takimiya, J. Mater. Chem. C, 2013, 1, 1297 RSC
; (e) I. Osaka, T. Abe, M. Shimawaki, T. Koganezawa and K. Takimiya, ACS Macro Lett., 2012, 1, 437 CrossRef CAS
; (f) S. Loser, H. Miyauchi, J. W. Hennek, J. Smith, C. Huang, A. Facchetti and T. J. Marks, Chem. Commun., 2012, 48, 8511 RSC
; (g) S. Shinamura, E. Miyazaki and K. Takimiya, J. Org. Chem., 2010, 75, 1228 CrossRef CAS PubMed
; (h) S. Shinamura, I. Osaka, E. Miyazaki, A. Nakao, M. Yamagishi, J. Takeya and K. Takimiya, J. Am. Chem. Soc., 2011, 133, 5024 CrossRef CAS PubMed
. See also ref. 5.
-
(a) A. J. Eberhart and D. J. Procter, Angew. Chem., Int. Ed., 2013, 52, 4008 CrossRef CAS PubMed
; (b) A. J. Eberhart, H. J. Shrives, E. Álvarez, A. Carrër, Y. Zhang and D. J. Procter, Chem.–Eur. J., 2015, 21, 7428 CrossRef CAS PubMed
. For an application of the metal-free coupling, see: (c) T. Sugahara, K. Murakami, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2014, 53, 9329 CrossRef CAS PubMed
.
- For reviews of I2-mediated cyclizations, see:
(a) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Chem.–Eur. J., 2012, 18, 5460 CrossRef CAS PubMed
; (b) A. Palisse and S. F. Kirsch, Org. Biomol. Chem., 2012, 10, 8041 RSC
; (c) B. Godoi, R. F. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937 CrossRef CAS PubMed
.
- ESI†.
- ESI†.
-
(a) For a review, see: M. Halik and A. Hirsch, Adv. Mater., 2011, 23, 2689 CrossRef CAS PubMed
; (b) For a recent example, see: A. V. S. Parry, K. Lu, D. J. Tate, B. Urasinska-Wojcik, D. Caras-Quintero, L. A. Majewski and M. L. Turner, Adv. Funct. Mater., 2014, 24, 6677 CrossRef CAS
.
- ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, NMR spectra, CCDC numbers for X-ray structures, and details of preliminary materials evaluation and device preparation. CCDC 1415330–1415334. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc03823e |
| This journal is © The Royal Society of Chemistry 2016 |