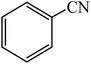
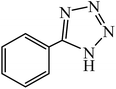
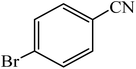
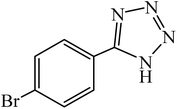
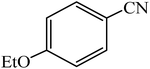
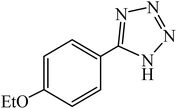
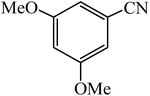
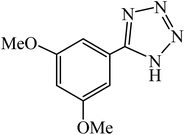
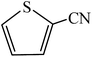
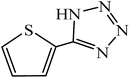
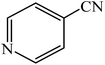
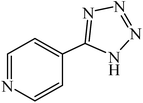
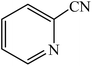
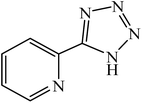
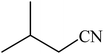
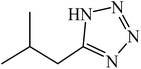
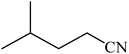
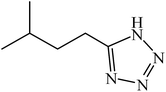
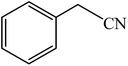
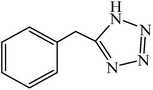
A rapid metal free synthesis of 5-substituted-1H-tetrazoles using cuttlebone as a natural high effective and low cost heterogeneous catalyst†
Sara S. E. Ghodsinia and
Batool Akhlaghinia*
Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad 9177948974, Iran. E-mail: akhlaghinia@um.ac.ir; Fax: +98-51-3879-5457; Tel: +98-51-3880-5527
First published on 29th May 2015
Abstract
A convenient, rapid and metal free synthesis of 5-substituted-1H-tetrazoles is described by [3 + 2] cycloaddition reaction of nitriles with sodium azide. The reaction was catalyzed by cuttlebone in DMSO at 110 °C. Cycloaddition reaction of nitriles with sodium azide happened in the presence of mesoporous cuttlebone by “electrophilic activation” of nitriles through hydrogen bond formation between the cuttlebone and nitrile. Cuttlebone as a natural low cost heterogeneous catalyst with high porosity, high flexural stiffness, high compressive strength and high thermal stability affords 5-substituted-1H-tetrazoles rapidly with high efficiency.
Introduction
Tetrazoles have been known for over a hundred years. They are an important and useful class of heterocycles with a wide range of applications in medicinal chemistry,1–8 coordination chemistry9 and material science.10 Tetrazoles as precursors of different nitrogen containing heterocycles (triazoles, oxazolidones and thiazoles)9a,11 have been studied as lipophilic spacers,12 ion and peptide chelating agents,13 cis-peptide bond mimics14 and catalysts in asymmetric synthesis.15 Because of their usefulness, research on the catalytic preparation of tetrazoles has attracted great attention. The first method involves the [3 + 2] cycloaddition of an azide with a nitrile which was reported by Hantzsch and Vagt in 1901.16 So far, most of the reported methods contain the cycloaddition of nitrile with an azide moiety, under the influence of several efficient catalysts. For a long time proton acid-catalyzed cycloaddition was the main routes to 5-substituted-1H-tetrazoles which was suffered from explosion of large amounts of harmful hydrazoic acid.17–27 Some of different homogenous and heterogeneous catalyst systems were also applied in 5-substituted-1H-tetrazoles synthesis via cycloaddition of nitrile with azide. Numbers of these new catalysts are Pd(OAc)2/ZnBr2,28 ZnO,29 ZnBr2,30 ZnCl2/tungstates,31 Zn/Al hydrotalcite,32 Zn(OTf)2,33 Zn hydroxyapatite,34 ZnS,35 Cu2O,36 nano ZnO/Co3O4,37 FeCl3–SiO2,38 Fe(OAc)2,39 Fe(HSO4)3,40 nano CuFe2O4,41 CdCl2,42 BF3·OEt2,43 InCl3,44 I2,45 (CH3)2SnO,46 TBAFza,47 AgNO3,48 copper triflates,49 β-cyclodextrin,50 COY zeolites,51 Pd(PPh3)4,52 AgNPs,53 WAlPO-5 microspheres,54 Fe3O4@SiO2/salen of Cu(II),55 CuFe2O4 nano particles,41 B(C6F5)3,56 AlCl3,57 Zn–Cu alloy,58 CAES,59 CuSO4·5H2O,60 In(OTf)3.61 But, in this ways, there are problems including the use of expensive and toxic metals, high-cost reagents, long reaction times (several hours to days) in combination with high reaction temperatures, strong Lewis acids, low yield, harsh reaction conditions, boring work-ups and difficulty in separation and recovery of the catalyst.Today, as employing the metal catalysts are not always eco-friendly and because of this, serious environmental pollution often results,62 and also because of the strict environmental legislation, there is a huge demand for metal-free, green and safe synthetic methods that not only reduce the use of toxic and corrosive reagents and stop the formation of inorganic wastes but also leading to high yield of the desired product.63 In continuation of our studies and to promote the methods of tetrazoles preparation,59,60 herein we report an efficient rapid metal free synthesis of 5-substituted-1H-tetrazoles in the presence of mesoporous cuttlebone as a new green heterogeneous catalyst with super catalytic activity.
Results and discussion
Cuttlebone signifies a special class of ultra-light weight, high stiffness and high permeability cellular biomaterials, which was provided from cuttlefish with an efficient means of maintaining neutral buoyancy at considerable habitation depths.64,65 Cuttlebone as a natural material possessing the multifunctional properties with high porosity, high flexural stiffness, high compressive strength and high thermal stability functions as a rigid buoyant tank in animal. Cuttlebone with an inorganic–organic composite framework composed of aragonite, protein and β-chitin.66,67 Following our interest in the synthesis of 5-substituted-1H-tetrazoles, we speculated on the possibility of using cuttlebone to achieve 5-substituted-1H-tetrazoles by [3 + 2] cycloaddition reaction of nitriles with sodium azide (Scheme 1).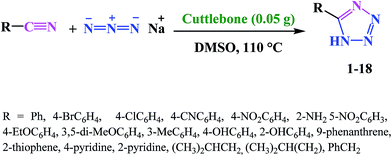 | ||
| Scheme 1 Synthesis of different structurally 5-substituted-1H-tetrazoles in the presence of cuttlebone in DMSO. | ||
Cuttlebone was taken out from cuttlefish (Sepia esculenta),64,65,68,69 which is commonly found in saltwater beaches like Persian Gulf in Iran. This sample was in a fairly good condition with minimal external damage. In order to remove pollution on the surface of cuttlebone, the catalyst was powdered, washed with distilled water and dried at 100 °C for 2 h.
The model reaction was carried out by taking the mixture of benzonitrile with sodium azide in the presence of cuttlebone. Table 1 gives the details about the amount of cuttlebone, molar ratios of benzonitrile/NaN3, solvent and temperature which were used to optimize the reaction conditions. Only 30% addition product is obtained when the reaction is carried out without catalyst in DMSO at 140 °C (Table 1, entry 1), despite of prolonged reaction time. At the same reaction condition, 100% conversion was obtained after 20 min in the presence of cuttlebone (Table 1, entry 2). This result highlights the specific role of cuttlebone in [3 + 2] cycloaddition reaction of benzonitrile with sodium azide. In an effort to develop better reaction conditions, different solvents were screened for cycloaddition reaction in the presence of cuttlebone (Table 1, entries 3–9). Other solvents, such as DMF, toluene, H2O, CH3CN gave the desired product in low yield (Table 1, entries 4–6) but no product was obtained when the reaction was performed in 1,4-dioxane, CH3NO2 and CHCl3 (Table 1, entries 7–9). As shown in Table 1 among the different solvents tested DMSO was found to be the solvent of choice for [3 + 2] cycloaddition reaction in the presence of cuttlebone. At lower reaction temperature (130 °C and 110 °C) the reaction was progressed as the same as at 140 °C (Table 1, entries 10–12). To investigate the effect of catalyst loading, the formation of 5-phenyl-1H-tetrazole was carried out in DMSO at 110 °C in the presence of 0.07 g and 0.03 g of catalyst. According to this study, increasing the catalyst loading did not lead to higher conversion (Table 1, entry 13), while lower catalyst loading has lessened the reaction yield even after longer reaction time (Table 1, entry 14). To improve [3 + 2] cycloaddition reaction, the effect of different molar ratio of benzonitrile/sodium azide was also examined in DMSO at 110 °C. It is noteworthy that additional amount of sodium azide cannot improve the reaction rate (Table 1, entry 15). By considering the cuttlebone structure66,67 and to understand which segment of cuttlebone catalyzed [3 + 2] cycloaddition reaction, the optimized reaction conditions were applied on the cyclization of benzonitrile with sodium azide in the presence of CaCO3 and freshly extracted β-chitin from cuttlebone70 respectively (Table 1, entries 16 and 17). In comparison, a trace amount of product was produced in the presence of CaCO3 while in the presence of β-chitin the reaction was completed after longer reaction time.
| Entry | Molar ratio (benzontrile/NaN3) | Catalyst (g) | Solvent | Temperature (°C) | Time (h) | Isolated yield (%) |
|---|---|---|---|---|---|---|
| a The reaction was performed in the presence of CaCO3.b The reaction was performed in the presence of freshly extracted β-chitin from cuttlebone. | ||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | DMSO | 140 | 10 | 30 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMSO | 140 | 20 min | 98 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMF | 140 | 2 | 65 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | CH3CN | Reflux | 2 | 10 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | Toluene | Reflux | 2 | 5 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | H2O | Reflux | 2 | 10 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | CH3NO2 | Reflux | 2 | 0 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | 1,4-Dioxane | Reflux | 2 | 0 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | CHCl3 | Reflux | 2 | 0 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMSO | 130 | 20 min | 98 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMSO | 110 | 20 min | 98 |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMSO | 100 | 20 min | 95 |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.07 | DMSO | 110 | 20 min | 98 |
| 14 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.03 | DMSO | 110 | 2 | 65 |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/5 1/5 |
0.05 | DMSO | 110 | 20 min | 98 |
| 16a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMSO | 110 | 20 min | Trace |
| 17b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMSO | 110 | 20/40 min | 85/98 |
It can be seen from Table 2 that, admirable yields of the desired 5-substituted-1H-tetrazoles were obtained rapidly by using 0.05 g of cuttlebone in solution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of organic nitriles/NaN3 in DMSO at 110 °C. The reaction times and product yields of aromatic nitriles bearing electron withdrawing and electron donating groups have a significant difference, because of the obvious difference in electrophilic character of nitrile. The aromatic nitriles containing electron withdrawing substituents such as –Cl, –Br, –CN, –NO2 at para position took less time (15–30 min) for complete conversion of starting material (Table 2, entries 2–5). 2-Amino-5-nitrobenzonitrile results the desired product in longer reaction time due to steric effect and electron donation of –NH2 substitution at ortho position (Table 2, entry 6). Moreover, aromatic rings with electron donating groups (–NH2, –OCH3, CH3, –OCH2CH3 and –OH) gave the corresponding tetrazoles in high yields, although longer reaction times (40 min to 2 h) were required (Table 2, entries 7–11). Interestingly, aryl nitrile containing a free hydroxy group at ortho or para positions gave desired product in high yield after 1 h or 40 min respectively (Table 2, entries 10 and 11). Hydroxy group at ortho position render inductive effect which accelerates the cycloaddition reaction of 2-hydroxybenzonitrile. Phenanthrene-9-carbonitrile with steric hindrance at 9 position produced the corresponding tetrazole after 2 h (Table 2, entry 12). Similarly, heteroaryl nitriles were found to be extremely reactive substrate affording the relative tetrazole rapidly in the presence of cuttlebone (Table 2, entries 13–15). We next examined the cycloaddition of alkyl nitriles such as 3-methylbutanenitrile, 4-methylpentanenitrile and 2-phenylacetonitrile with azide ion in the presence of cuttlebone. It was found that alkyl nitriles in the presence of cuttlebone reacted smoothly to give the corresponding 1H-tetrazoles in excellent yields after 1–1.5 h (Table 2, entries 16–18). The results obtained from Table 2 indicate that [3 + 2] cycloaddition reaction of nitriles with sodium azide in the presence of cuttlebone proceeds rapidly irrespective of the electronic nature of nitrile compound.
1 molar ratio of organic nitriles/NaN3 in DMSO at 110 °C. The reaction times and product yields of aromatic nitriles bearing electron withdrawing and electron donating groups have a significant difference, because of the obvious difference in electrophilic character of nitrile. The aromatic nitriles containing electron withdrawing substituents such as –Cl, –Br, –CN, –NO2 at para position took less time (15–30 min) for complete conversion of starting material (Table 2, entries 2–5). 2-Amino-5-nitrobenzonitrile results the desired product in longer reaction time due to steric effect and electron donation of –NH2 substitution at ortho position (Table 2, entry 6). Moreover, aromatic rings with electron donating groups (–NH2, –OCH3, CH3, –OCH2CH3 and –OH) gave the corresponding tetrazoles in high yields, although longer reaction times (40 min to 2 h) were required (Table 2, entries 7–11). Interestingly, aryl nitrile containing a free hydroxy group at ortho or para positions gave desired product in high yield after 1 h or 40 min respectively (Table 2, entries 10 and 11). Hydroxy group at ortho position render inductive effect which accelerates the cycloaddition reaction of 2-hydroxybenzonitrile. Phenanthrene-9-carbonitrile with steric hindrance at 9 position produced the corresponding tetrazole after 2 h (Table 2, entry 12). Similarly, heteroaryl nitriles were found to be extremely reactive substrate affording the relative tetrazole rapidly in the presence of cuttlebone (Table 2, entries 13–15). We next examined the cycloaddition of alkyl nitriles such as 3-methylbutanenitrile, 4-methylpentanenitrile and 2-phenylacetonitrile with azide ion in the presence of cuttlebone. It was found that alkyl nitriles in the presence of cuttlebone reacted smoothly to give the corresponding 1H-tetrazoles in excellent yields after 1–1.5 h (Table 2, entries 16–18). The results obtained from Table 2 indicate that [3 + 2] cycloaddition reaction of nitriles with sodium azide in the presence of cuttlebone proceeds rapidly irrespective of the electronic nature of nitrile compound.
FT-IR spectroscopy of all purified products revealed an absorption band at 3485–3329 cm−1 (N–H) and group of bands at 1469–1430 cm−1 (scissoring bending C–H), 1293–1233 cm−1 (N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1189–1110 and 1106–1041 cm−1 (tetrazole ring) and absence of band at 2200 cm−1 due to CN group. In 1H NMR and 13C NMR spectra, a signal at 16.9 and 161–155 ppm are assigned to the NH and quaternary carbon NH–C
N–), 1189–1110 and 1106–1041 cm−1 (tetrazole ring) and absence of band at 2200 cm−1 due to CN group. In 1H NMR and 13C NMR spectra, a signal at 16.9 and 161–155 ppm are assigned to the NH and quaternary carbon NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N respectively.
N respectively.
All of the products were known compounds and characterized by the FT-IR spectroscopy, mass spectrometry and comparison of their melting points with known compounds. The structure of selected products was further confirmed by 1H NMR and 13C NMR spectroscopy.
A plausible mechanism for the reaction methodology under current development is shown in Scheme 2. The fact that after a long reaction time, lower yield of product was observed in the absence of cuttlebone (Table 1, entry 1) established the catalytic activity of cuttlebone in [3 + 2] cycloaddition reactions of various organic nitriles with sodium azide. Although the role of cuttlebone in [3 + 2] cycloaddition reaction is not clear definitively, it is speculated that a ternary complex A among nitrile compound, azide ion and cuttlebone (β-chitin as organic segment of catalyst) would be generated in the transition state immediately as shown in Scheme 2. In accordance with Scheme 2 the following steps are postulated to occur: the hydrogen bonding of β-chitin with nitrogen atom of nitrile compound accelerates the cyclization step via increasing the electrophilicity of nitrile group. The [3 + 2] cycloaddition between the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of nitrile compound and azide ion takes place readily to form the intermediate B. After removing the catalyst by simple filtration, C was produced as sodium salt which upon acidic work-up, leads to formation of D and E. Finally the more stable tautomer E (5-substituted-1H-tetrazole) was obtained as major product. Further investigation on the elucidation of the mechanism and scope of this reaction are currently underway in our laboratory.
N bond of nitrile compound and azide ion takes place readily to form the intermediate B. After removing the catalyst by simple filtration, C was produced as sodium salt which upon acidic work-up, leads to formation of D and E. Finally the more stable tautomer E (5-substituted-1H-tetrazole) was obtained as major product. Further investigation on the elucidation of the mechanism and scope of this reaction are currently underway in our laboratory.
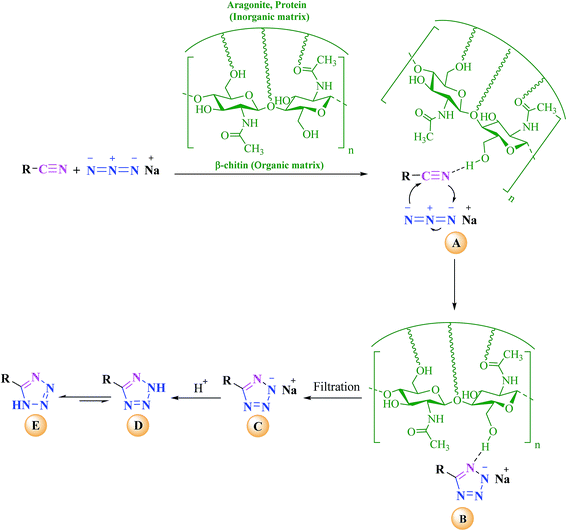 | ||
| Scheme 2 Proposed mechanism for the synthesis of 5-substituted-1H-tetrazoles in the presence of cuttlebone. | ||
[3 + 2] Cycloaddition reaction of organic nitriles with sodium azide in the presence of cuttlebone was vigorously dependent on physical form of the catalyst. Excellent yield of product was obtained in the presence of fine powdered cuttlebone, while 5-phenyl-1H-tetrazole was obtained in 20% yield in the presence of non-powdered cuttlebone. On the basis of the results obtained from Table 1 (entries 16 and 17), organic segment of cuttlebone (β-chitin) has an essential role in catalytic activity of cuttlebone. So, in the presence of β-chitin (which was extracted from cuttlebone according the method reported previously)70 the reaction was completed after longer reaction time, and in the presence of CaCO3 (as inorganic segment of cuttlebone) a trace amount of product was produced. Performing the reaction in the presence of β-chitin suffered from long reaction time and tedious work-up procedure, because of gelation of β-chitin in the reaction mixture. Also, β-chitin is not reusable catalyst in [3 + 2] cycloaddition reaction. Interestingly, cuttlebone matrix improved the catalytic activity of β-chitin significantly with providing easy and simple removing of catalyst which could be reusable several times (Fig. 1).
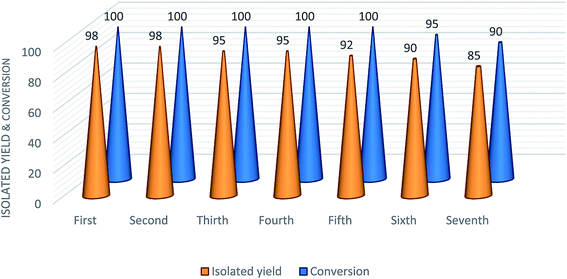 | ||
| Fig. 1 [3 + 2] Cycloaddition reactions of benzonitrile with sodium azide in the presence of reused cuttlebone. | ||
Recyclability is important for industrial application of a catalyst. Therefore, the reuse performance of cuttlebone was investigated. After completion of [3 + 2] cycloaddition reaction of benzonitrile with sodium azide, cuttlebone was separated by simple filtration from the reaction mixture, washed with distilled water and ethanol several times to remove the organic products. The catalyst was dried at 100 °C for 1 h. The heterogeneous catalyst was used for 6 successive times in the new experiments without dramatic yield loss and generate product with purity similar to that obtained in the first run (Fig. 1).
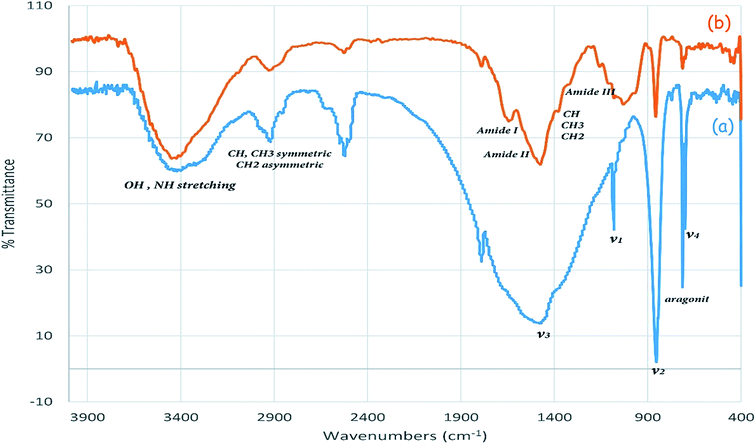
The FT-IR spectrum of cuttlebone and the 6th recovered cuttlebone was shown in Fig. 2. As can be seen, the shape, position and relative intensity of all characteristic peaks are well preserved. The results indicate no considerable changes were observed on the chemical structure of functional groups and the hydrogen bonding network (Fig. 2).
In comparison, [3 + 2] cycloaddition reaction of nitriles with sodium azide in the presence of cuttlebone affords 5-sustituted-1H-tetrazole rapidly in high yield than the earlier reported catalysts (Table 3). As can be seen from Table 3, all of the previously reported methods suffer from long reaction times to achieve suitable yields as well as use of strong Lewis acids, expensive and toxic metals and tedious work-up procedure.
| Entry | Catalyst | Solvent | Temp. °C | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Mesoporous ZnS35 | DMF | 120 | 36 | 96 |
| 2 | Silica sulfuric acid71 | DMF | Reflux | 5 | 88 |
| 3 | Imidazole-based zwitterionic-type molten salts72 | — | 120 | 12 | 84 |
| 4 | Chitosan derived magnetic ionic liquid73 | — | 70 | 7 | 87 |
| 5 | AgNO3 (ref. 48) | DMF | 120 | 5 | 83 |
| 6 | CoY zeolite74 | DMF | 120 | 14 | 90 |
| 7 | Fe3O4@SiO2/Salen Cu(II)55 | DMF | 120 | 7 | 90 |
| 8 | Zn/Al hydrocalcite32 | DMF | 120–130 | 12 | 84 |
| 9 | Zn hydroxyapatite34 | DMF | 120 | 12 | 78 |
| 10 | AgNPs53 | DMF | 120 | 8 | 92 |
| 11 | B(C6F5)3 (ref. 56) | DMF | 120 | 8 | 94 |
| 12 | CAES59 | DMSO | 130 | 1 | 95 |
| 13 | CuSO4·5H2O60 | DMSO | 140 | 1 | 98 |
| 14 | Cuttlebone | DMSO | 110 | 20 min | 98 |
Experimental
General
The purity determinations of the products were accomplished by TLC on silica gel polygram STL G/UV 254 plates. The melting points of products were determined with an Electrothermal Type 9100 melting point apparatus. The FT-IR spectra were recorded on an Avatar 370 FT-IR Therma Nicolet spectrometer. The NMR spectra were provided on Brucker Avance 100 and 400 MHz instruments in acetone-d6, DMSO-d6 and CD3CN. Elemental analyses were performed using a Thermo Finnegan Flash EA 1112 Series instrument. Mass spectra were recorded with Agilent Technologies (HP) 5973 Network Mass Selective Detector, Shimadzu GC-MS-QP5050 instruments and a CH7A Varianmat Bremem instrument (Germany) at 70 eV. Elemental compositions were determined with a Leo 1450 VP scanning electron microscope equipped with an SC7620 energy dispersive spectrometer (SEM-EDS) presenting a 133 eV resolution at 20 kV. All of the products were known compounds and characterized by the FT-IR spectroscopy and comparison of their melting points with known compounds. The structure of selected products was further confirmed by 1H NMR, 13C NMR spectroscopy, and mass spectrometry. Cuttlebone was taken out from cuttlefish (Sepia esculenta),64,65,68,69 which is commonly found in saltwater beaches like Persian Gulf in Iran.Pre-preparation of cuttlebone
In order to remove pollution on the surface of cuttlebone, the catalyst was powdered, washed with distilled water and dried at 100 °C for 2 h.Characterization of cuttlebone
Cuttlebone was identified by FT-IR spectroscopy, scanning electron microscope and energy dispersive spectrum (SEM-EDS). The FT-IR spectrum of the cuttlebone (Sepia esculenta) shows absorption bands between 3440–699 cm−1 (Fig. 3). The observed absorptions bands for aragonite phases (calcium carbonate) in cuttlebone structure are due to the planar CO3−2 ion. There are four vibrational modes in the free CO3−2 ion:75 the symmetric stretching vibration of the carbonate ion at about 1082 cm−1 (ν1); the out-of-plane bending absorption at about 857 cm−1 (ν2); the asymmetric stretching vibrations at about 1400–1500 cm−1 (ν3); the split in-plane bending vibrations at about 700 cm−1 (ν4). Also, as shown in the FT-IR spectrum of cuttlebone (Fig. 3), the bands at 3448 and 3318 cm−1 are attributed to the OH and NH stretching vibrations respectively, which are related to absorption bands of β-chitin segment of cuttlebone. The hydrogen bonding network of β-chitin in cuttlebone would change the shape and intensity of the above mentioned absorption bands. The absorption bands ranging from 2977 to 2855 cm−1 were related to symmetric stretching of CH, CH3 and asymmetric stretching vibrations of CH2. The CH bending, CH3 symmetric deformation and CH2 wagging bands were appeared at 1384 to 1312 cm−1. The absorption bands at 1662 and 1644 cm−1 are assigned to amide I band (two types of hydrogen bonds in a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group with the NH group of the adjacent chain and the OH group of the inter-chain). Amide II band (in-plane N–H bending and C–N stretching mode) at about 1550 and 1360 cm−1 and amide III band (in-plane mode of the CONH group) are observed at 1315 cm−1. The absorption bands ranging from 1160 to 1030 cm−1 are attributed to the asymmetric bridge oxygen and C–O stretching vibrations.76,77
O group with the NH group of the adjacent chain and the OH group of the inter-chain). Amide II band (in-plane N–H bending and C–N stretching mode) at about 1550 and 1360 cm−1 and amide III band (in-plane mode of the CONH group) are observed at 1315 cm−1. The absorption bands ranging from 1160 to 1030 cm−1 are attributed to the asymmetric bridge oxygen and C–O stretching vibrations.76,77
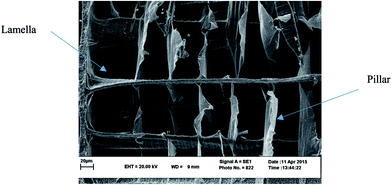
Scanning electronic microscopy (SEM) of transverse section of cuttlebone commonly reveals an approximately periodic microstructure. The most common base cell (or smallest representative volume element – RVE) has a bridge-like configuration (Fig. 4). This base cell geometry can be used to approximate the mechanical properties for cuttlebone.66
Also, as was shown in Fig. 5 scanning electronic microscopy (SEM) of finely powdered cuttlebone shows an amorphous morphology.
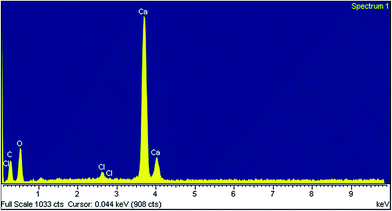
The energy dispersive spectrum (EDS) indicates the presence of Ca, C, O and Cl elements in cuttlebone structure (Fig. 6).
Typical procedure for the preparation of 5-phenyl-1H-tetrazole in the presence of cuttlebone
Finely powdered cuttlebone (0.050) was added to a mixture of benzonitrile (0.1031 g, 1 mmol) and sodium azide (0.0650 g, 1 mmol) in DMSO (2 mL) with stirring at room temperature. The reaction temperature was raised up to 110 °C for 20 min. The progress of the reaction was monitored by TLC. After completion of reaction (monitored by thin-layer chromatography, TLC), the reaction mixture was cooled and the catalyst was filtered. The filtrate was treated with HCl (4 N, 10 mL) and then ethyl acetate (2 × 10 mL). The resultant organic layer was washed with distilled water, dried over anhydrous sodium sulfate, and concentrated to give the crude solid crystalline 5-phenyl-1H-tetrazole. The crude product was recrystallized from n-hexane/ethylacetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) obtaining 0.1430 g of colourless crystals (98% isolated yield).
1) obtaining 0.1430 g of colourless crystals (98% isolated yield).
Conclusion
In conclusion, a convenient and highly efficient metal free synthesis of 5-substituted-1H-tetrazoles has been developed by reacting nitriles with sodium azide in the presence of cuttlebone as a green and effective catalyst. This new type of low cost and reusable catalyst plays a crucial role by “electrophilic activation” of nitrile through hydrogen bond formation between cuttlebone and nitrile. Excellent yields of product, short reaction time, elimination of dangerous and harmful hydrazoic acid, avoidance of toxic metal derivatives, a simple work-up procedure, simple pre-preparation, and easy handling of the catalyst are the notable and valuable features of this metal free methodology. This procedure can be used to convert aryl, heteroaryl and alkyl nitriles, sterically hindered ortho-substituted aryl nitriles, organic nitriles containing bulky aryl groups and halo aryl nitriles to corresponding 5-substituted-1H-tetrazoles. Thus, it provides a better and more practical alternative to the existing methodologies.Acknowledgements
The authors gratefully acknowledge the partial support of this study by Ferdowsi University of Mashhad Research Council.Notes and references
- R. J. Herr, Bioorg. Med. Chem., 2002, 10, 3379 CrossRef CAS.
- R. E. Ford, P. Knowles, E. Lunt, S. M. Marshal, A. J. Penrose, C. A. Ramsden, A. J. H. Summers, J. L. Walker and D. E. Wrigth, J. Med. Chem., 1986, 29, 538 CrossRef CAS.
- E. A. Hallinan, S. Tsymbalov, C. R. Dorn, B. S. Pitzele and D. W. Hansen Jr, J. Med. Chem., 2002, 45, 1686 CrossRef CAS PubMed.
- J. H. Toney, P. Fitzgerald, N. Grover-Sharma, S. H. Olson, W. J. May, J. G. Sundelof, D. E. Vanderwall, K. A. Cleary, S. K. Grant and J. K. Wu, Chem. Biol., 1998, 5, 185 CrossRef CAS.
- Y. Inada, T. Wada, Y. Shibouta, M. Ojima, T. Sanada, K. Ohtsuki, K. Itoh, K. Kubo, Y. Kohara and T. Naka, J. Pharmacol. Exp. Ther., 1994, 268, 1540 CAS.
- C. N. S. S. P. Kumar, D. K. Parida, A. Santhoshi, A. K. Kota, B. Sridhar and V. J. Rao, Med. Chem. Commun., 2011, 2, 486 RSC.
- (a) E. Dolusic, P. Larrieu, L. Moineaux, V. Stroobant, L. Pilotte, D. Colau, L. Pochet, B. T. Van den Eynde, B. Masereel and J. Wouters, J. Med. Chem., 2011, 54, 5320 CrossRef CAS PubMed; (b) A. R. Modarresi-Alam, F. Khamooshi, M. Rostamizadeh, H. Keykha, M. Nasrollahzadeh, H. R. Bijanzadeh and E. Kleinpeter, J. Mol. Struct., 2007, 841, 61 CrossRef CAS PubMed.
- E. Vieira, S. Huwyler, S. Jolidon, F. Knoflach, V. Mutel and J. Wichmann, Bioorg. Med. Chem. Lett., 2005, 15, 4628 CrossRef CAS PubMed.
- (a) R. Huisgen, J. Sauer, H. J. Sturn and J. H. Markgraf, Chem. Ber., 1960, 93, 2106 CrossRef CAS PubMed; (b) X. S. Wang, Y. Z. Tang, X. F. Huang, Z. R. Qu, C. M. Che, P. W. H. Chan and R. G. Xiong, Inorg. Chem., 2005, 44, 5278 CrossRef CAS PubMed.
- (a) M. Kiskey, D. E. Chavez, D. C. Noud, S. F. Son, H. L. Berghout and C. A. Bome, Proc. Int. Pyrotech. Semin., 2000, 27, 3 Search PubMed; (b) V. A. Ostrovskii, M. S. Pevzner, T. P. Kofmna, M. B. Shcherbinin and I. V. Tselinskii, Targets Heterocycl. Syst., 1999, 3, 467 CAS; (c) G. I. Koldobskii and V. A. Ostrovskii, Usp. Khim., 1994, 63, 847 CrossRef CAS PubMed.
- D. J. Moderhack, J. Prakt. Chem./Chem.-Ztg., 1988, 340, 687 CrossRef PubMed.
- F. Himo, Z. P. Demko, L. Noodleman and K. B. Sharpless, J. Am. Chem. Soc., 2003, 125, 9983 CrossRef CAS PubMed.
- E. Lodyga-Chruscinska, D. Sanna, G. Micera, L. Chruscinski, J. Olejnik, R. J. Nachman and J. Zabrocki, Acta Biochim. Pol., 2006, 53, 65 CAS.
- D. D. Beusen, J. Zabrocki, U. Slomczynska, R. D. Head, J. L. F. Kao and G. R. Marshall, Biopolymers, 1995, 36, 181 CrossRef CAS PubMed.
- K. R. Knudsen, C. E. T. Mitchell and S. V. Ley, Chem. Commun., 2006, 66 RSC.
- A. Hantzsch and A. Vagt, Justus Liebigs Ann. Chem., 1901, 314, 339 CrossRef CAS PubMed.
- D. M. Zimmerman and R. A. Olofson, Tetrahedron Lett., 1969, 10, 5081 CrossRef.
- A. K. Gupta and C. H. Oh, Tetrahedron Lett., 2004, 45, 4113 CrossRef CAS PubMed.
- D. Habibi, H. Nabavi and M. Nasrollahzadeh, J. Chem., 2012, 1 Search PubMed.
- D. Kundu, A. Majee and A. Hajra, Tetrahedron Lett., 2009, 50, 2668 CrossRef CAS PubMed.
- K. Koguro, T. Oga, S. Mitsui and R. Orita, Synthesis, 1998, 910 CrossRef CAS PubMed.
- S. Furmeier and J. O. Metzger, Eur. J. Org. Chem., 2003, 885 CrossRef CAS PubMed.
- B. S. Jursic and B. W. LeBlanc, J. Heterocycl. Chem., 1998, 35, 405 CrossRef CAS PubMed.
- J. Roh, T. V. Artamonova, K. Vavrova, G. I. Koldobskii and A. Hrabalek, Synthesis, 2009, 2175 CrossRef CAS PubMed.
- M. Alterman and A. Hallberg, J. Org. Chem., 2000, 65, 7984 CrossRef CAS PubMed.
- (a) K. A. Hofmann and H. Hock, Ber. Dtsch. Chem. Ges., 1910, 43, 1866 CrossRef CAS PubMed; (b) J. S. Mihina and R. M. Herbst, J. Org. Chem., 1950, 15, 1082 CrossRef CAS; (c) R. M. Herbst and K. R. Wilson, J. Org. Chem., 1957, 22, 1142 CrossRef CAS.
- W. G. Finnegan, R. A. Henry and R. Lofquist, J. Am. Chem. Soc., 1958, 80, 3908 CrossRef CAS.
- Z. Yizhong, R. Yiming and C. Chun, Helv. Chim. Acta, 2009, 92, 171 CrossRef PubMed.
- M. L. Kantam, K. B. Shiva Kumar and C. Sridhar, Adv. Synth. Catal., 2005, 347, 1212 CrossRef CAS PubMed.
- Z. P. Demko and K. B. Sharpless, J. Org. Chem., 2001, 66, 7945 CrossRef CAS PubMed.
- S. Rostamizadeh, H. Ghaieni, R. Aryan and A. Amani, Chin. Chem. Lett., 2009, 20, 1311 CrossRef CAS PubMed.
- M. L. Kantam, K. B. Shiva Kumar and K. J. Phani Raja, J. Mol. Catal. A: Chem., 2006, 247, 186 CrossRef CAS PubMed.
- S. Hajra, D. Sinha and M. Bhowmick, J. Org. Chem., 2007, 72, 1852 CrossRef CAS PubMed.
- M. L. Kantam, V. Balasubrahmanyam and K. B. Shiva Kumar, Synth. Commun., 2006, 1809 CrossRef CAS PubMed.
- (a) L. Lang, B. Li, W. Liu, L. Jiang, Z. Xu and G. Yin, Chem. Commun., 2010, 46, 448 RSC; (b) L. Lang, H. Zhou, M. Xue, X. Wang and Z. Xu, Mater. Lett., 2013, 106, 443 CrossRef CAS PubMed.
- T. Jin, F. Kitahara, S. Kamijo and Y. Yamamoto, Tetrahedron Lett., 2008, 49, 2824 CrossRef CAS PubMed.
- S. M. Agawane and J. M. Nagarkar, Catal. Sci. Technol., 2012, 2, 1324 CAS.
- M. Nasrollahzadeh, Y. Bayat, D. Habibi and S. Moshaee, Tetrahedron Lett., 2009, 50, 4435 CrossRef CAS PubMed.
- J. Bonnamour and C. Bolm, Chem.–Eur. J., 2009, 15, 4543 CrossRef CAS PubMed.
- H. Eshghi, S. M. Seyedi and E. Rahimi Zarei, ISRN Org. Chem., 2011, 1 CrossRef PubMed.
- B. Sreedhar, A. S. Kumar and D. Yadav, Tetrahedron Lett., 2011, 52, 3565 CrossRef CAS PubMed.
- G. Venkateshwarlu, A. Premalatha, K. C. Rajanna and P. K. Saiprakash, Synth. Commun., 2009, 39, 4479 CrossRef CAS PubMed.
- A. Kumar, R. Narayanan and H. Shechter, J. Org. Chem., 1996, 61, 4462 CrossRef CAS.
- V. S. Patil, K. P. Nandre, A. U. Borse and S. V. Bhosale, E-J. Chem., 2012, 9, 1145 CrossRef CAS PubMed.
- B. Das, C. R. Reddy, D. N. Kumar, M. Krishnaiah and R. Narender, Synlett, 2010, 391 CrossRef CAS PubMed.
- S. J. Wittenberger and B. G. Donner, J. Org. Chem., 1993, 58, 4139 CrossRef CAS.
- D. Amantini, R. Beleggia, F. Fringuelli, F. Pizzo and L. Vaccaro, J. Org. Chem., 2004, 69, 2896 CrossRef CAS PubMed.
- P. Mani, A. K. Singh and S. K. Awasthi, Tetrahedron Lett., 2014, 55, 1879 CrossRef CAS PubMed.
- L. Bosch and J. Vilarrasa, Angew. Chem., 2007, 46, 3926 CrossRef CAS PubMed.
- D. R. Patil, Y. B. Wagh, P. G. Ingole, K. Singh and D. S. Dalal, New J. Chem., 2013, 37, 3261 RSC.
- V. Rama, K. Kanagaraj and K. Pitchumani, J. Org. Chem., 2011, 76, 9090 CrossRef CAS PubMed.
- Y. S. Gyoung, J. G. Shim and Y. Yamamoto, Tetrahedron Lett., 2000, 41, 4193 CrossRef CAS.
- P. Mani, C. Sharma, S. Kumar and S. K. Awasthi, J. Mol. Catal. A: Chem., 2014, 392, 150 CrossRef CAS PubMed.
- D. Kong, Y. Liu, J. Zhang, H. Li, X. Wang, G. Liu, B. Li and Z. Xu, New J. Chem., 2014, 38, 3078 RSC.
- F. Dehghani, A. R. Sardarian and M. Esmaeilpour, J. Organomet. Chem., 2013, 743, 87 CrossRef CAS PubMed.
- S. K. Prajapti, A. Nagarsenkar and B. N. Babu, Tetrahedron Lett., 2014, 55, 3507 CrossRef CAS PubMed.
- D. P. Matthews, J. E. Green and A. J. Shuker, J. Comb. Chem., 2000, 2, 19 CrossRef CAS PubMed.
- G. Aridoss and K. K. Laali, Eur. J. Org. Chem., 2011, 6343 CrossRef CAS PubMed.
- N. Razavi and B. Akhlaghinia, RSC Adv., 2015, 5, 12372 RSC.
- B. Akhlaghinia and S. Rezazadeh, J. Braz. Chem. Soc., 2012, 23, 2197 CrossRef CAS PubMed.
- K. Dhiman, M. Adinath and H. Alakananda, Tetrahedron Lett., 2009, 50, 2668 CrossRef PubMed.
- K. P. Nandre, J. K. Salunke, J. P. Nandre, A. U. Patil, V. S. Borse and S. V. Bhosale, Chin. Chem. Lett., 2012, 23, 161 CrossRef CAS PubMed.
- R. A. Sheldon, J. Chem. Technol. Biotechnol., 1997, 68, 381 CrossRef CAS.
- J. D. Birchall and N. L. Thomas, J. Mater. Sci., 1983, 18, 2081 CrossRef CAS.
- S. Vogel, J. Biosci., 2006, 31, 309 CrossRef CAS.
- J. Cadman, Sh. Zhou, Y. Chen, W. Li, R. Appleyard and Q. Li, Acta Mech. Sin., 2010, 26, 27 CrossRef PubMed.
- X. Jia, W. Qian, D. Wu, D. Wei, G. Xu and X. Liu, Colloids Surf., B, 2009, 68, 231 CrossRef CAS PubMed.
- J. Cadman, Y. Chen, Sh. Zhou and Q. Li, Adv. Mater. Res., 2010, 123, 295 CrossRef PubMed.
- N. Jothi and R. Kunthavai Nachiyar, Global J. Biotechnol. Biochem., 2013, 8, 33 CAS.
- W. Ogasawara, W. Shenton, S. A. Davis and S. Mann, Chem. Mater., 2000, 12, 2835 CrossRef CAS.
- Z. Du, C. Si, Y. Li, Y. Wang and J. Lu, Int. J. Mol. Sci., 2012, 13, 4696 CrossRef CAS PubMed.
- M. Rahman, A. Roy, M. Ghosh, Sh. Mitra, A. Majee and A. Hajra, RSC Adv., 2014, 4, 6116 RSC.
- A. Khalafi-Nezhad and S. Mohammadi, RSC Adv., 2013, 3, 4362 RSC.
- V. Rama, K. Kanagaraj and K. Pitchumani, J. Org. Chem., 2011, 76, 9090 CrossRef CAS PubMed.
- B. Plavsic, S. Kobe and B. Orel, KZLTET, 1999, 33, 517 CAS.
- M. Osada, C. Miura, Y. S. Nakagawa, M. Kaihara, M. Nikaido and K. Totani, Carbohydr. Polym., 2013, 92, 1573 CrossRef CAS PubMed.
- F. G. Pearson, R. H. Marchessault and C. Y. Liang, J. Polym. Sci., 1960, 43, 101 CrossRef PubMed.
- V. Aureggi and G. Sedelmeier, Angew. Chem., Int. Ed., 2007, 46, 8440 CrossRef CAS PubMed.
- S. Lossen and J. Liebigs, Ann. Chem., 1897, 298, 102 Search PubMed.
- A. Koennecke, R. Dome, E. Kleinpeter and E. Lippmann, Tetrahedron, 1997, 35, 1957 CrossRef.
- F. Ek, L. G. Wistrand and T. Frejd, J. Org. Chem., 2003, 68, 1911 CrossRef CAS PubMed.
- Y. W. Yao, Y. Zhou, B. P. Lin and Ch. Yao, Tetrahedron Lett., 2013, 54, 6779 CrossRef CAS PubMed.
- G. Satzinger and J. Liebigs, Ann. Chem., 1960, 638, 159 CAS.
- A. Najafi Chermahini, A. Teimouri and A. Moaddeli, Heteroat. Chem., 2011, 22, 168 CrossRef PubMed.
- A. R. Katritzky, B. E. M. El-Gendy, B. Hall, C. D. Draghici and P. J. Steel, J. Org. Chem., 2010, 75, 6468 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08147e |
| This journal is © The Royal Society of Chemistry 2015 |