Formal synthesis of (−)-platensimycin†
Zhi-Wei
Jiao
a,
Yong-Qiang
Tu
ac,
Qing
Zhang
a,
Wen-Xing
Liu
a,
Shao-Hua
Wang
a and
Min
Wang
*b
aSchool of Pharmacy & State Key Laboratory of Applied Organic Chemistry Lanzhou University, Lanzhou, 730000, P. R. China
bCollege of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou 310036, P. R. China. E-mail: mwang@hznu.edu.cn
cCollaborative Innovation Center of Chemical Science and Engineering, Tianjin, P. R. China
First published on 14th May 2015
Abstract
A formal synthesis of (−)-platensimycin has been successfully carried out using a tandem C–H oxidation/C–C coupling (cyclization)/rearrangement as the key step.
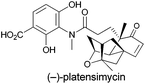
Exploring new antibiotics is an evergreen topic in the field of drug discovery because of the continuous emergence of multi-resistant bacteria.1 Among the antibiotics developed/discovered in the past several years, (−)-platensimycin (Fig. 1), as a potential antibiotic drug candidate, was firstly isolated from a strain of Streptomyces platensis by Merck scientists in 2006.2 Subsequent biological activity test of such a polycyclic natural product showed that it could selectively inhibit FabF, a key enzyme in the fatty acid synthetic pathway in bacteria, in the 48–160 nM range.
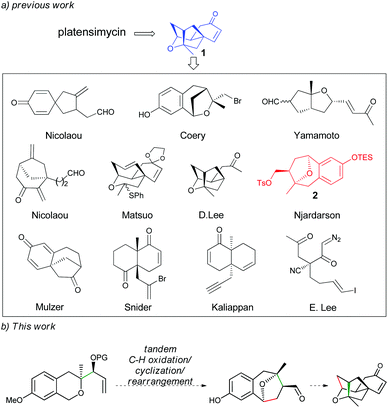
Due to its special bioactivity and structural molecular skeleton, this molecule has been an ideal target for total synthesis. Right after its isolation, Nicolaou's group reported the first total synthesis of platensimycin in its racemic form.3a Since then, a number of research groups have also attempted the synthesis of the molecule. Most of them take compound 1 as the key intermediate to develop the corresponding synthetic strategies (Scheme 1).3,4 Among them, the strategy reported by Njardarson and co-workers, who used compound 2 with a benzoxa[3.2.1]octane skeleton to afford precursor 1,3o has drawn our attention as such type of an intermediate might be readily obtained through a tandem C–H oxidation/C–C coupling (cyclization)/rearrangement of isochroman-derived allylic silylether, which has been recently developed by our group and has been applied to the asymmetric total syntheses of (−)-brussonol and (−)-przewalskine E.5,6 Based on the above information and combined with our long interest in α-C–H bond functionalization of oxygen atoms7 and synthesis of bioactive natural products,8 we proposed a strategy toward the synthesis of (−)-platensimycin (Scheme 1). In this study, we present our results of the formal synthesis of (−)-platensimycin using the above-mentioned tandem reaction as the key step.
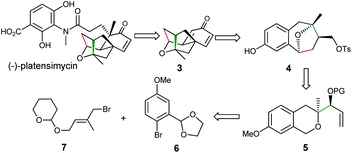
Our retrosynthetic analysis is illustrated in Scheme 2 and mainly focused on the synthesis of the key intermediate 3, which could be obtained through the corresponding dearomatization/cyclization reaction of compound 4.9 Compound 4 with a benzoxa[3.2.1]octane skeleton could be prepared through our newly developed methodology from precursor 5, which could be assembled via a simple construction of the isochroman moiety from readily available bromide 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 7.11
10 and 7.11
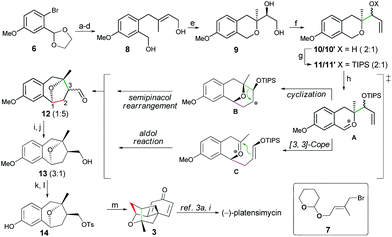
As shown in Scheme 3, our synthesis started with the bromide 6, which was coupled with segment 7 followed by sequential transformations including acetal-deprotection/reduction/THP-deprotection to provide smoothly the allylic alcohol 8. Next, we investigated the tandem Sharpless asymmetric epoxidation/epoxy opening reaction of 8, and careful condition screening showed that the use of the classical Sharpless catalyst (1.5 equiv.) at −50 °C could give the best result (90% yield, 91% ee) of the expected diol 9. However, when the catalyst loading was less than 1.0 equiv., the reaction gave product 9 with both low yield and poor enantioselectivity. For example, only 33% ee was achieved while a 0.3 equiv. Sharpless catalyst was employed. Cleavage of the diol 9 with NaIO4 followed by the Grignard reaction with excess vinyl magnesium bromide afforded the allylic alcohol 10 as two diastereoisomers (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which were protected as TIPS ethers 11 (2 isomers, dr = 2
1), which were protected as TIPS ethers 11 (2 isomers, dr = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). To our delight, when 11 (2 isomers) was subjected to the standard conditions of 0.1 eq. InCl3 and 2.0 eq. DDQ., the expected aldehyde 12 with a benzoxa[3.2.1]octane skeleton was obtained as two diastereoisomers (dr = 1
1). To our delight, when 11 (2 isomers) was subjected to the standard conditions of 0.1 eq. InCl3 and 2.0 eq. DDQ., the expected aldehyde 12 with a benzoxa[3.2.1]octane skeleton was obtained as two diastereoisomers (dr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) in moderate 52% yield. As mentioned in our previous report, there are two possible ways to go through after the formation of intermediate A with a benzylic oxacarbenium cation under the oxidative conditions. One is a tandem cyclization/semipinacol rearrangement to give B and the other is a tandem [3,3]-cope rearrangement/aldol reaction.12
5) in moderate 52% yield. As mentioned in our previous report, there are two possible ways to go through after the formation of intermediate A with a benzylic oxacarbenium cation under the oxidative conditions. One is a tandem cyclization/semipinacol rearrangement to give B and the other is a tandem [3,3]-cope rearrangement/aldol reaction.12
Although the major isomer of 12 did not possess the desired C-3 configuration, fortunately, a final basification of the reaction system with Na2CO3/MeOH could reverse the stereochemistry of aldehyde 12, which was directly reduced with NaBH4 without purification to give alcohol 13 in 43% yield (dr = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).13 Tosylation of the major isomer of 13 followed by demethylation with AlCl3/EtSH14 gave precursor 14. Finally, treatment of 14 with anhydrous TBAF in xylene could initiate the dearomatization/intramolecular cyclization to afford the tetracyclic core 3 in 85% yield. Intermediate 3 showed the identical spectroscopic data (1H and 13C NMR, IR, HRMS, and optical rotation) with the previously reported compound,3o and could be easily converted to (−)-platensimycin by using the methods described by Corey3i and Nicolaou.3a
1).13 Tosylation of the major isomer of 13 followed by demethylation with AlCl3/EtSH14 gave precursor 14. Finally, treatment of 14 with anhydrous TBAF in xylene could initiate the dearomatization/intramolecular cyclization to afford the tetracyclic core 3 in 85% yield. Intermediate 3 showed the identical spectroscopic data (1H and 13C NMR, IR, HRMS, and optical rotation) with the previously reported compound,3o and could be easily converted to (−)-platensimycin by using the methods described by Corey3i and Nicolaou.3a
Conclusions
In conclusion, the formal synthesis of (−)-platensimycin has been finished through the use of a new strategy, in which a newly developed tandem C–H oxidation/C–C coupling (cyclization)/rearrangement has been used as the key step. These results not only further demonstrate the utility of such a tandem reaction, but also provide additional choice for the construction of molecules with a benzoxa[3.2.1]octane skeleton.Acknowledgements
This work was supported by the NSFC (no.: 21202073, 21290180, 21272097, 21372056, 21372104 and 21472077), the “111” Program of MOE, the Project of MOST (2012ZX 09201101–003) and the fundamental research funds for the central universities (lzujbky-2013-236, lzujbky-2014-k20 and lzujbky-2015-k12).Notes and references
- (a) S. B. Singh and J. F. Barrett, Biochem. Pharmacol., 2006, 71, 10062 CrossRef PubMed; (b) M. S. Butler and A. D. Buss, Biochem. Pharmacol., 2006, 71, 919 CrossRef CAS PubMed.
- (a) J. Wang, S. M. Soisson, K. Young, W. Shoop, S. Kodali, A. Galgoci, R. Painter, G. Parthasarathy, Y. Tang, R. Cummings, S. Ha, K. Dorso, M. Motyl, H. Jayasuriya, J. Ondeyka, K. Herath, C. Zhang, L. Hernandez, J. Alloco, J. R. A'BasilioTormo, O. Genilloud, F. Vicente, F. Pelaez, L. Colwell, S. H. Lee, B. Michael, T. Felcetto, C. Gill, L. L. Silver, J. Hermes, K. Bartizal, J. Barrett, D. J. Schmatz, W. Becker, D. Cully and S. B. Singh, Nature, 2006, 441, 358 CrossRef CAS PubMed; (b) S. B. Singh, H. Jayasuriya, J. G. Ondeyka, K. B. Herath, C. Zhang, D. L. Zink, N. N. Tsou, R. G. Ball, A. Basilio, O. Genilloud, M. T. Diez, F. Vicente, F. Pelaez, K. Young and J. Wang, J. Am. Chem. Soc., 2006, 128, 11916 CrossRef CAS PubMed.
- For the total synthesis of platensimycin and related strategies, see: (a) K. C. Nicolaou, A. Li and D. J. Edmonds, Angew. Chem., Int. Ed., 2006, 45, 7086 CrossRef CAS PubMed; (b) K. C. Nicolaou, D. J. Edmonds, A. Li and G. S. Tria, Angew. Chem., Int. Ed., 2007, 46, 3942 CrossRef CAS PubMed; (c) Y. Zou, C.-H. Chen, C. D. Taylor, B. M. Foxman and B. B. Snider, Org. Lett., 2007, 9, 1825 CrossRef CAS PubMed; (d) K. P. Kaliappan and V. Ravikumar, Org. Lett., 2007, 9, 2417 CrossRef CAS PubMed; (e) P. Li, J. N. Payette and H. Yamamoto, J. Am. Chem. Soc., 2007, 129, 9534 CrossRef CAS PubMed; (f) A. K. Ghosh and X. Kai, Org. Lett., 2007, 9, 4013 CrossRef CAS PubMed; (g) A. K. Ghosh and K. Xi, J. Org. Chem., 2009, 74, 1163 CrossRef CAS PubMed; (h) K. Tiefenbacher and J. Mulzer, Angew. Chem., Int. Ed., 2007, 46, 8074 CrossRef CAS PubMed; (i) G. Lalic and E. J. Corey, Org. Lett., 2007, 9, 4921 CrossRef CAS PubMed; (j) K. C. Nicolaou, D. Pappo, K. Y. Tsang, R. Gibe and D. Y.-K. Chen, Angew. Chem., Int. Ed., 2008, 47, 944 CrossRef CAS PubMed; (k) C. H. Kim, K. Jang, P. S. Choi, Y. Y. K. Chung and E. Lee, Angew. Chem., Int. Ed., 2008, 47, 4009 CrossRef CAS PubMed; (l) J. Matsuo, K. Takeuchi and H. Ishibashi, Org. Lett., 2008, 10, 4049 CrossRef CAS PubMed; (m) S. Y. Yun, J.-C. Zheng and D. Lee, J. Am. Chem. Soc., 2009, 131, 8413 CrossRef CAS PubMed; (n) K. C. Nicolaou, A. Li, S. P. Ellery and D. J. Edmonds, Angew. Chem., Int. Ed., 2009, 48, 6293 CrossRef CAS PubMed; (o) N. A. McGrath, E. S. Bartlett, S. Sittihan and J. T. Njardarson, Angew. Chem., Int. Ed., 2009, 48, 8543 CrossRef CAS PubMed; (p) S. T.-C. Eey and M. J. Lear, Org. Lett., 2010, 12, 5510 CrossRef CAS PubMed; (q) P. Magnus, H. Rivera and V. Lynch, Org. Lett., 2010, 12, 5677 CrossRef CAS PubMed; (r) E. Z. Oblak and D. L. Wright, Org. Lett., 2011, 13, 2263 CrossRef CAS PubMed; (s) J. Wang and H. O. Sintim, Chem. – Eur. J., 2011, 17, 3352 CrossRef CAS PubMed; (t) L. Zhu, Y. Han, G. Du and C.-S. Lee, Org. Lett., 2013, 15, 524 CrossRef CAS PubMed; (u) S. Hirai and M. Nakada, Tetrahedron, 2011, 67, 518 CrossRef CAS PubMed; (v) E. Z. Oblak and D. L. Wright, Org. Lett., 2011, 13, 2263 CrossRef CAS PubMed; (w) Y. Ueda, K. Iwahashi, K. Iguchi and H. Ito, Synthesis, 2011, 1532 CAS; (x) M.-A. Beaulieu, K. C. Guérard, G. Maertens, C. Sabot and S. Canesi, J. Org. Chem., 2011, 76, 9460 CrossRef CAS PubMed; (y) S. Horii, M. Torihata, T. Nagasawa and S. Kuwahara, J. Org. Chem., 2013, 78, 2798 CrossRef CAS PubMed; (z) L. Zhu, C. Zhou, W. Yang, S. He, G. Cheng, X. Zhang and C. Lee, J. Org. Chem., 2013, 78, 7912 CrossRef CAS PubMed; (a a) S. Xing, W. Pan, C. Liu, J. Ren and Z. Wang, Angew. Chem., Int. Ed., 2010, 49, 3215 CrossRef CAS PubMed.
- recent reviews of synthesis of platensimycin, see: (a) K. Tiefenbacher and J. Mulzer, Angew. Chem., Int. Ed., 2008, 47, 2548 CrossRef CAS PubMed; (b) D. T. Manallack, I. T. Crosby, Y. Khakham and B. Capuano, Curr. Med. Chem., 2008, 15, 705 CrossRef CAS; (c) Y.-S. Yao and Z.-J. Yao, Chin. J. Org. Chem., 2008, 28, 1553 CAS; (d) P. Harsh and G. A. O'Doherty, Chemtracts, 2009, 22, 31 CAS; (e) K. C. Nicolaou, J. S. D. Chen, J. Edmonds and A. A. Estrada, Angew. Chem., Int. Ed., 2009, 48, 660 CrossRef CAS PubMed; (f) K. C. Nicolaou, J. S. Chen and S. M. Dalby, Bioorg. Med. Chem., 2009, 17, 2290 CrossRef CAS PubMed; (g) X. Lu and Q. You, Curr. Med. Chem., 2010, 17, 1139 CrossRef CAS; (h) K. Palanichamy and K. P. Kaliappan, Chem. – Asian J., 2010, 5, 668 CrossRef CAS PubMed; (i) M. Saleem, H. Hussain, I. Ahmed, T. van Ree and K. Krohn, Nat. Prod. Rep., 2011, 28, 1534 RSC.
- Z.-W. Jiao, Y.-Q. Tu, Q. Zhang, W.-X. Liu, S.-Y. Zhang, S.-H. Wang and F.-M. Zhang and S. Jiang, Nat. Commun., 2015, DOI:10.1038/ncomms8332.
- For references on other DDQ mediated C–H oxidative coupling reactions, see reviews: (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) C. J. Scheuermann, Chem. – Asian J., 2010, 5, 436 CrossRef CAS PubMed; (c) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed. For selected recent examples: (d) Y. Zhang and C.-J. Li, Angew. Chem., Int. Ed., 2006, 45, 1949 CrossRef CAS PubMed; (e) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2006, 128, 56 CrossRef CAS PubMed; (f) Y. Zhang and C.-J. Li, J. Am. Chem. Soc., 2006, 128, 4242 CrossRef CAS PubMed; (g) L. Zhao and C.-J. Li, Angew. Chem., Int. Ed., 2008, 47, 7075 CrossRef CAS PubMed; (h) W. Tu, L. Liu and P. E. Floreancig, Angew. Chem., Int. Ed., 2008, 47, 4184 CrossRef CAS PubMed; (i) W. Tu and P. E. Floreancig, Angew. Chem., Int. Ed., 2009, 48, 4567 CrossRef CAS PubMed; (j) L. Liu and P. E. Floreancig, Org. Lett., 2009, 11, 3132 CrossRef PubMed; (k) L. Liu and P. E. Floreancig, Angew. Chem., Int. Ed., 2010, 49, 3069 CrossRef CAS PubMed; (l) L. Liu and P. E. Floreancig, Angew. Chem., Int. Ed., 2010, 49, 5894 CrossRef CAS PubMed; (m) Y.-Z. Li, B.-J. Li, X.-Y. Lu, S. Lin and Z.-J. Shi, Angew. Chem., Int. Ed., 2009, 48, 3817 CrossRef CAS PubMed; (n) B. Yu, T. Jiang, J. Li, Y. Sun, X. Pan and X. She, Org. Lett., 2009, 11, 3442 CrossRef CAS PubMed; (o) A. K. Ghosh and X. Cheng, Tetrahedron Lett., 2012, 53, 2568 CrossRef CAS PubMed; (p) Z. Meng, S. Sun, H. Yuan, H. Lou and L. Liu, Angew. Chem., Int. Ed., 2014, 53, 543 CrossRef CAS PubMed; (q) Z. Lu, M. Yang, P. Chen, X. Xiong and A. Li, Angew. Chem., Int. Ed., 2014, 53, 13840 CrossRef CAS PubMed.
- For reviews, see: (a) S.-Y. Zhang, F.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2011, 40, 1937 RSC; For recent examples, see: (b) L. Shi, Y.-Q. Tu, M. Wang, F.-M. Zhang, C.-A. Fan, Y.-M. Zhao and W.-J. Xia, J. Am. Chem. Soc., 2005, 127, 10836 CrossRef CAS PubMed; (c) K. Cao, Y.-J. Jiang, S.-Y. Zhang, C.-A. Fan, Y.-Q. Tu and Y.-J. Pan, Tetrahedron Lett., 2008, 49, 4652 CrossRef CAS PubMed; (d) S.-Y. Zhang, Y.-Q. Tu, C.-A. Fan, F.-M. Zhang and L. Shi, Angew. Chem., Int. Ed., 2009, 48, 8761 CrossRef CAS PubMed; (e) Z.-W. Jiao, S.-Y. Zhang, C. He, Y.-Q. Tu, S.-H. Wang, F.-M. Zhang, Y.-Q. Zhang and H. Li, Angew. Chem., Int. Ed., 2012, 51, 8811 CrossRef CAS PubMed.
- For reviews, see: (a) Z.-L. Song, C.-A. Fan and Y.-Q. Tu, Chem. Rev., 2011, 111, 7523 CrossRef CAS PubMed; (b) B. Wang and Y.-Q. Tu, Acc. Chem. Res., 2011, 44, 1207 CrossRef CAS PubMed; (c) S. H. Wang, B. S. Li and Y.-Q. Tu, Chem. Commun., 2014, 50, 2393 RSC; For recent examples, see: (d) X.-M. Zhang, Y.-Q. Tu, F.-M. Zhang, H. Shao and X. Meng, Angew. Chem., Int. Ed., 2011, 50, 3916 CrossRef CAS PubMed; (e) J. Peng, X. Hou, S. Zhang and Y. Tu, Acta Chim. Sin., 2012, 70, 2232 CrossRef CAS; (f) J.-M. Tian, X. Zhao, Y.-Q. Tu, W. Gong, F.-M. Zhang, S.-Y. Zhang and S.-H. Wang, Chem. – Asian J., 2014, 9, 724 CrossRef CAS PubMed.
- (a) W. Winstein and R. Baird, J. Am. Chem. Soc., 1957, 79, 756 CrossRef; (b) S. Masamune, J. Am. Chem. Soc., 1961, 83, 1009 CrossRef CAS.
- M.-Y. Lin, A. Das and R.-L. Liu, J. Am. Chem. Soc., 2006, 128, 9340 CrossRef CAS PubMed.
- J. Kuk, B. S. Kim, H. Jung, S. Choi, J.-Y. Park and S. Koo, J. Org. Chem., 2008, 73, 1991 CrossRef CAS PubMed.
- M. H. Hopkins, L. E. Overman and G. M. Rishton, J. Am. Chem. Soc., 1991, 113, 5354 CrossRef CAS.
- The relative configuration of 13 was determined by comparing it with a known compound reported by Njardarson and co-workers: ref. 3o.
- M. Node, K. Nishide, K. Fuji and E. Fujita, J. Org. Chem., 1980, 45, 4275 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00109a |
| This journal is © the Partner Organisations 2015 |