Phthalocyanine in perovskite solar cells: a review
Ehsan
Rezaee
ab,
Danish
Khan
 a,
Siyuan
Cai
a,
Lei
Dong
a,
Hui
Xiao
a,
S. Ravi P.
Silva
a,
Siyuan
Cai
a,
Lei
Dong
a,
Hui
Xiao
a,
S. Ravi P.
Silva
 b,
Xiaoyuan
Liu
*a and
Zong-Xiang
Xu
b,
Xiaoyuan
Liu
*a and
Zong-Xiang
Xu
 *a
*a
aDepartment of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China. E-mail: liuxy2022@sustech.edu.cn; xu.zx@sustech.edu.cn
bAdvanced Technology Institute, Department of Electrical and Electronic Engineering, University of Surrey, Guildford, Surrey GU2 7XH, UK
First published on 21st February 2023
Abstract
Presently, due to the rapid development of perovskite solar cells (PSCs), their commercialization is not only attractive but perhaps the only choice to replace both thin film photovoltaics (PV) and conventional silicon PV. However, although the power conversion efficiency (PCE) of PSCs is has reached above 25%, which is suitable for their commercialization, their poor long-term stability with scalability remains a challenge due to the sensitivity of perovskites under ambient conditions. Therefore, the top and bottom layers sandwiching the perovskite layer are critical. In this case, the charge transport layers (CTLs) not only enable charge extraction but also play the role of encrusting, passivating, and supporting the perovskite layer. Therefore, the perovskite interface has a dominant influence on the performances of PSCs. In this case, phthalocyanines (Pcs) have a large π-conjugated framework, outstanding thermal stability, excellent physical/chemical stability, low cost, and can be endowed with a tunable energy band, good carrier mobility, solubility in common solvents, and facile synthesis through their appropriate design, making them a robust charge selective layer, passivation layer or both in PSCs. In this review, we summarize the studies on the application of Pc compounds in PSCs, including dopant-free macrocycle molecules, from the perspectives of molecular engineering, doped hole transporting material (HTM), and employment of Pcs as additives in the perovskite layer or HTMs. This review provides helpful insight for further enhancing the efficiency of PSC devices, while improving their long-term operational life by utilizing Pc materials.
1. Introduction
Perovskite solar cells (PSCs) are being intensively researched given that their power conversion efficiency (PCE) has reached 25.7%, exhibiting potential for commercialization in the near future.1 However, although this remarkable PCE is the highest in the history of thin film photovoltaics, the commercialization of PSCs can only be realized by achieving long-term stability and scaling up their fabrication methods. Specifically, the performance of PSCs in two aspects, i.e., stability and PCE, degrades when PSCs are scaled up.2 The stability issues generally originate from the main absorber, i.e., the perovskite layer, when it is exposed to ambient conditions.3 At the beginning of the development of PSCs, many efforts were devoted to lengthening the lifetime of PSCs by adjusting the perovskite compositions and crystal structures. Subsequently, the charge transport layers (CTLs) were endowed with defect passivation and charge transportation properties given that the CTLs have direct interfacial contact with the perovskite layer. Accordingly, the interfaces of perovskite have become crucial, and in this case extra passivation layers have been incorporated to alleviate the built-in defects in perovskite and protect it from the external environment.4 In general, PSCs can be divided into three basic architectures including positive–intrinsic–negative (p–i–n), negative–intrinsic–positive (n–i–p) and mesoporous n–i–p. Regardless of the architectures, the perovskite/CTL interface is an opportunity to counter the built-in defects in the bulk perovskite, at the surface, or in the grain boundaries. In addition, the interfacial contact between the adjacent layers directly influences the carrier extraction and recombination.5 Therefore, numerous techniques such as compositional,6 interfacial,7 and passivation engineering8 have been applied mainly through the interactions between the perovskite/CTLs. Nevertheless, another big concern is replacing the conventional hole transport materials (HTMs) ((2,2′,7,7′-tetrakis(N,N′-di-p-methoxyphenyl-amine)-9,9′-spirobifluorene (spiro-OMeTAD), poly[bis(4-phenyl)(2,5,6-trimethylphenyl)-amine] (PTAA), and poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate)(PEDOT:PSS)) with new materials to enhance the cost-effectiveness and stability of the device, where small molecules can play a vital role.9 Small molecules enable facile design modifications to achieve the required properties, such as dopant-free HTMs, interlayers, organic–inorganic hybrid HTMs or electron transport materials (ETMs), or as additives to improve the formation of perovskite crystals.9,10 Conclusively, small molecules are beneficial to enhance the properties of the bottom, top, and perovskite layers. However, before the application of small molecules in PSCs, several factors need to be considered, such as their reproducibility, cost-effectiveness, solubility, stability, hydrophobicity, energy levels, and light absorption.11 Generally, most of these properties are acquired through the selection of an appropriate molecular core, and then further design modifications are applied with regard to the specific device.9 Besides these properties, the formation of a thin film with the desired structural and morphological characteristics is one of the main priorities, where conjugated π systems play a significant role.12Phthalocyanines (Pcs), which are aromatic heterocyclic compounds derived from porphyrins, are among the most comprehensive π-conjugated system-based molecules.13 They possess four isoindole units bridged by nitrogen atoms, forming a two-dimensional extended π-conjugated system with 18 delocalized electrons, which offers high thermal, chemical, and photostability for Pc materials.14 This extended π-conjugated system also assists in the absorption of Pcs in the red/near-infrared (NIR) region of the solar spectrum, which exhibit two characteristic bands, i.e., a Soret or B-band with a generally weaker absorption and intense Q-band with a molar extinction coefficient as high as 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1.15,16 Subsequently, the ability to design and synthesize Pcs with different properties make this interesting class of macrocyclic organic compounds suitable for a variety of applications, including dyes and pigments, photocatalysts, imaging and therapy, chemical sensors, single-molecule magnets, and as active materials in electronic devices, such as organic photovoltaics (OPVs), thin-film transistors (OTFTs), light-emitting diodes (OLEDs), dye-sensitized solar cells (DSSCs) and PSCs.17–21
000 M−1 cm−1.15,16 Subsequently, the ability to design and synthesize Pcs with different properties make this interesting class of macrocyclic organic compounds suitable for a variety of applications, including dyes and pigments, photocatalysts, imaging and therapy, chemical sensors, single-molecule magnets, and as active materials in electronic devices, such as organic photovoltaics (OPVs), thin-film transistors (OTFTs), light-emitting diodes (OLEDs), dye-sensitized solar cells (DSSCs) and PSCs.17–21
In addition to introducing various functional groups in Pcs as substituents, strategies based on the π–π interactions of Pc molecules and their orientations, electron–donor–acceptor (D–A) systems including Pcs and other electron active subunits, and covalent organic frameworks (COFs) were also developed in last two decades for the design and synthesis of novel Pc-based materials in the form of thin films, nanoparticles and other ordered condensed matters, which can be employed for the fabrication of devices with practical and potential applications.22–28 The first report on the synthesis of a Pc was published in 1907 by Braun and Tchemiac.29 They obtained a small amount of blue material as a by-product during the preparation of 2-cyanobenzamide, which was H2Pc. However, at that time, they did not know the structure of the blue material, and therefore no name was given to it. Subsequently, a copper(II) Pc compound was synthesized in 1927 during the preparation of phthalonitrile.30 Similarly, the structure of this dark blue material was not characterized. The structure of Pc compounds was first published in 1934, and further characterized by Robertson using X-ray diffraction analysis.31–38 In the decade after the characterization of the first Pc compounds, a variety of strategies has been developed for the design and synthesis of many types of Pc compounds, dividing this class of interesting organic materials into several categories, including metal-free or metal-coordinated Pcs, unsubstituted and symmetrical or unsymmetrical substituted Pc compounds, binuclear, trinuclear, tetranuclear, pentanuclear and octanuclear Pcs, fused-Pcs and ball-type Pcs.39–44
Pcs, specifically in PSCs, are used as HTMs, interlayers or additives in the absorber films or HTMs. Pc-based HTMs with both structures (n–i–p or p–i–n) play a crucial role in enhancing the performance of PSCs. In n–i–p devices, they act as a protective layer on the perovskite, while in p–i–n, they act as a support for the deposition of the perovskite. The general trend is the use of PEDOT:PSS, PTAA or spiro-OMeTAD as HTMs in efficient PSCs. However, these conventional HTMs have serious drawbacks such as high cost, low reproducibility, hygroscopicity and dopant requirement.45 These problems can be overcome through the use of Pcs derivatives owing to their high thermal stability, hydrophobicity and high hole mobility and conductivity. In addition, Pcs can be decorated with Lewis acid–base passivation capability and deposited on the perovskite as an interlayer or HTM. Lastly, as additives in the perovskite layer, Pcs enhance the film crystallinity and coverage and reduce the chance of defect generation in perovskites. At each position in the PSC device structure, the required properties of Pc compounds can be tuned by judiciously tailoring their structures. Pcs often strongly chelate to metals or metalloids of all groups of elements through two covalent and two coordination bonds, forming stable metal Pcs (MPcs).46,47 Additionally, regarding the designed target for a Pc compound, different groups with various electronic and spatial structures can be introduced in either the Pc ring as substituents or the central ion as axial ligands, endowing it with new chemical and physical features. The substituted groups may also occupy the peripheral or non-peripheral positions on the Pc ring, resulting in different properties in the final compound (Fig. 1a and b).48 For example, while applied as a dopant-free HTM, an improvement in the hole mobility can be achieved by enhancing the charge flow in the molecule in the form of electron–donor–acceptor (D–A) systems, intermolecular interactions via π-conjugation extensions or enhancement in the molecule planarity.49–51 Another approach is perovskite defection passivation in the interlayer through the incorporation of Lewis bases at the appropriate position in the structure.52 Furthermore, the approach of carrying two faces under one hood, i.e., defect passivation and dopant-free HTM in a single layer, can be applied.53
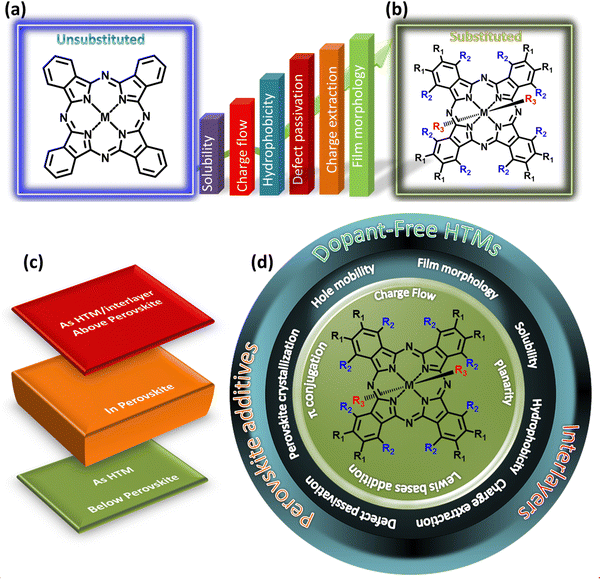 | ||
| Fig. 1 (a) Unsubstituted Pc, (b) substituted Pc, (c) Pc applications in different layers of PSCs and (d) design strategies linking the important parameters of PSCs. | ||
Initially, when unsubstituted Pcs were demonstrated as HTMs or interlayers, they faced the issues of low solubility, low film coverage, and low hole extraction. These issues result in a low open circuit voltage (VOC) and fill factor (FF), hindering the PCE. Accordingly, to overcome the problems of insolubility and low film coverage of Pcs, numerous fabrication techniques have been developed to coat thin film layers of Pcs. Consequently, the PCEs improved to a certain level. Furthermore, the decoration of tetra-substituted or octa-substituted Pcs with alkyl chains, diphenylamine (DPA), triphenylamine (TPA), thiophene, etc. resulted in many new properties in PSCs such as D–A properties, energy level optimization, defect passivation, dopant-free HTMs, solubility in different solvents, enhanced film morphology, etc. (Fig. 1a and b). Pcs are mostly incorporated as HTMs (below the perovskite layer in p–i–n devices and above the perovskite layer in n–i–p devices) and interlayers (above the perovskite layer) (Fig. 1c). All these positions are critical due to their direct contact with the sensitive perovskite. Hence, Pc compounds have been designed using different strategies such as enhancement of π-conjugation, inter or intra-charge flow by changing their center or arms, decoration with Lewis bases at the appropriate positions in their structure, or enhancement of planarity by changing the linkage positions or twisting angles of their arms. These designs govern the properties of Pcs such as solubility in organic solvents, film morphologies (perovskite and Pc-based layer), hydrophobicity, charge extraction, defect passivation, perovskite crystallization and hole mobility (Fig. 1d), which are essential for PSCs and can be optimized by substituting the necessary blocks around the Pc ring.
Due to their unique characteristics, Pc compounds have recently attract great attention in perovskite-related studies, but there is a lack of reviews summarizing all the recent developments in the literature and highlighting the unique role Pc materials can play in the future commercialization of PSC devices. Therefore, this review aims to present the basis of the functionality of Pcs as certain layers in PSCs. In Section 2, we discuss the applications of Pc compounds in five categories, including molecular-engineered dopant-free HTMs in accordance with the quantity of substituent, doped Pc, interfacial modification layer, and Pc acting as an additive in the perovskite film and HTMs. Finally, we present some remarks and conclusions. The aim of this study is to provide readers with comprehensive information about the applications of Pc materials and the most notable achievements to date.
2. Application of phthalocyanines in PSCs
2.1. Dopant-free phthalocyanine-based HTMs for PSCs
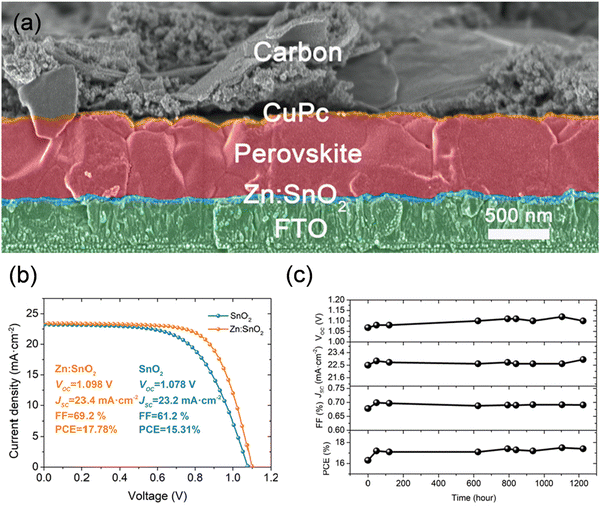 | ||
| Fig. 2 (a) High-resolution cross-sectional SEM image of the carbon-based planar-structured PSC, (b) J–V curves of the best-performing PSC with SnO2 or Zn-doped SnO2 as the ETM, (c) and evolution of the J–V parameters of the Zn-doped SnO2-based devices stored in the ambient air with a relative humidity of ∼20%. Reproduced with permission.64 Copyright 2019, Elsevier. | ||
| Device architecture | HTM | Hole mobility of HTM (cm2 V−1 s−1) | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Mesoscopic | CuPc | — | 0.75 | 16.3 | 40 | 5.0 | 54 |
| Planar, n–i–p | CuPc | ∼10−2 | 1.04 | 19.13 | 77.74 | 15.42 | 55 |
| Mesoscopic | Nanorod-like CuPc | — | 1.05 | 20.8 | 74.0 | 16.1 | 56 |
| Mesoscopic | Needle-like CuPc | — | 1.04 | 20.14 | 71.0 | 14.89 | 57 |
| Inverted planar | CuPc | — | 1.03 | 19.4 | 77 | 15.4 | 58 |
| Inverted planar | CuPc | — | 1.075 | 20.63 | 75.4 | 16.72 | 62 |
| Planar n–i–p | CuPc | — | 0.98 | 23.28 | 67 | 15.39 | 60 |
| Planar n–i–p | CuPc | — | 1.025 | 22.26 | 68.9 | 15.73 | 59 |
| Planar n–i–p | CuPc | — | 1.073 | 22.41 | 72.6 | 17.46 | 61 |
| Inverted planar | CuPc/VOx | — | 1.015 | 22.15 | 74.8 | 16.85 | 63 |
| Planar n–i–p | CuPc | — | 1.098 | 23.4 | 69.2 | 17.78 | 64 |
| Inverted planar | CuPc | — | 1.108 | 23.34 | 78.5 | 20.30 | 65 |
| Planar n–i–p | PMMA/CuPc | — | 1.08 | 24.87 | 79.29 | 21.25 | 66 |
| Mesoscopic | ZnPc | 1.9 × 10−3 | 0.83 | 22.8 | 43.4 | 8.13 | 67 |
| Inverted planar | ZnPc | — | 0.96 | 17.26 | 70 | 11.6 | 68 |
| Mesoscopic | TiOPc | — | 0.737 | 12.86 | 54 | 5.05 | 69 |
| Planar n–i–p | NiPc | — | 0.94 | 17.64 | 73 | 12.1 | 70 |
| Inverted planar | NiPc | — | 1.0 | 19.45 | 73.6 | 14.31 | 71 |
| Inverted planar | CuI/PbPc | — | 0.80 | 20.49 | 66 | 10.79 | 72 |
Changing the metal center in Pc materials can alter their electronic and structural properties dramatically. Unsubstituted Pcs with metal centers, such as Zn, Pb, Ni, Sn, VO, and TiO2, have been employed as the HTM in PSCs. In 2015, Di Carlo and coworkers employed ZnPc as dopant-free HTM in PSCs and reported an efficiency as high as 8.13% for a small area device with a mesoporous TiO2 ETM.67 They suggested that the orientation and stacking direction of the ZnPc molecules could significantly affect the electrical and optical properties. The vacuum-deposited α-phase ZnPc HTM with a thickness of 100 nm offered a hole mobility of around 1.9 × 10−3 cm2 V−1 s−1. The comparison of the incident photo-to-current efficiency (IPCE) spectra of the devices using ZnPc and spiro-OMeTAD as the HTM layers confirmed the contribution of ZnPc to the free charge generation process inside the PSC device. The ZnPc layer could also act as a shield layer for the perovskite against both light radiation and ambient moisture. A year later, Fostiropoulos et al. improved the PCE of the ZnPc-based PSC to 11.6% by using an ultrathin (5 nm) vacuum-deposited ZnPc HTM in an inverted perovskite device.68 In their study, a two-step vacuum deposition process was used for the fabrication of the perovskite layer and the film quality was improved by optimizing the deposition rate. Li and coworkers used titanylphthalocyaine (TiOPc) as an HTM for PSCs with a mesoscopic configuration. Specifically, β-form and α-form TiOPc HTMs were fabricated via thermal vapor evaporation under vacuum and solvent annealing, respectively. The β-form TiOPc as the HTM achieved a higher PCE of 5.05% than the α-form, which can be attributed to the better thin film morphology and matching energy level of the HOMO.69 Haider et al. employed unsubstituted NiPc as a dopant-free HTM in a planar n–i–p-structured PSC for the first time and achieved PCEs of 12.1% and 6.2% for small and large area devices, respectively.70 NiPc showed excellent hole extraction ability from the perovskite active layer, with a relatively high hole mobility of 1.0 × 10−1 cm2 V−1 s−1. The use of thermal evaporation deposition allowed the small-size NiPc to permeate into the perovskite grain boundaries and improve the efficiency of hole extraction due to the enhanced interface contact. The thickness of the NiPc layer was also optimized, with a 120 nm film achieving the highest performance. The photoluminescence (PL) and impedance spectroscopy studies indicated that the NiPc layer could offer superior hole extraction and transfer ability compared with the CuPc thin film. In another study by the same group in 2019, pristine NiPc was employed as an HTM in an inverted PSC and a PCE of 14.3% was reported, indicating the potential of NiPc materials for the fabrication of different PSC architectures, which can compete with CuPc as the typical representative of MPc-based HTMs.71 To achieve the best performance for the inverted perovskite devices, the NiPc HTM thickness was optimized to 30 nm, where the undesirable direct contact between the perovskite layer and the transparent electrode, the reduced incident light transmittance, and the increased series resistance were all avoided. Hou et al. used unsubstituted PbPc in a p–i–n-structured PSC fabricated under high humidity conditions.72 In their work, PbPc on a thin CuI layer was used as the HTM in PSCs with inverted structures. The hydrophobicity of the PbPc was beneficial for the formation of a porous PbI2 film and the fast preparation of a compact and homogeneous perovskite film with a large grain size. Consequently, reduced absorption in the visible area was achieved for the CuI–PbPc layer, which was critical in maintaining the light absorption level of the perovskite layer, especially in an inverted PSC device. The prepared devices showed a PCE of 10.79%, which is comparable with PEDOT:PSS-based PSCs, with good long-term stability.
MPc materials offer a large and fully conjugated aromatic framework and high thermal and chemical stability. They can be thermally evaporated over large areas and are suitable for the fabrication of both small and large area perovskite devices. Altering the metal center in MPc compounds can adjust their characteristics, such as electronic and optical properties, charge transfer ability, carrier diffusion length, and solid-state molecular alignment, which make them suitable choices for various tasks. Studies have established the compatibility of these materials for various device structures and their unique potential to act as either the underlying smooth thin film or the capping shield layer for the light absorbing perovskite film in PSCs. However, it is worth noting that in a conventional n–i–p PSC, Pc materials can also help PSC devices to absorb light in the wavelength range of 550 to 800 nm as a result of their wide Q band absorption. Therefore, the low energy photon penetrating perovskite layer can be absorbed by the Pc-based HTM layer and the photogenerated electrons in the Pc thin film can be injected into the perovskite layer to enhance the PCE of the device.
| Chemical structure no. | Device architecture | HTM | Hole mobility of HTM (cm2 V−1 s−1) | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Mesoscopic | CuMePc | — | 0.70 | 16.9 | 40 | 5.2 | 73 |
| 1 | Planar n–i–p | CuMePc | 1.95 × 10−3 | 0.968 | 23.11 | 52 | 11.7 | 74 |
| 2 | Planar n–i–p | CuEtPc | 3.75 × 10−4 | 1.01 | 22.81 | 49.9 | 11.5 | 74 |
| 3 | Planar n–i–p | CuPrPc | 2.16 × 10−3 | 1.01 | 23.2 | 76.0 | 17.8 | 75 |
| 4 | Planar n–i–p | ZnPrPc | 1.948 × 10−3 | 1.09 | 23.98 | 63.33 | 16.15 | 76 |
| 5 | Planar n–i–p | PdPrPc | 1.057 × 10−3 | 1.07 | 23.81 | 71.04 | 18.09 | 76 |
| 6 | Mesoscopic | n-BuCuPc | (5.0 ± 0.6) × 10−5 | 0.89 | 17.3 | 56 | 8.5 | 77 |
| 6 | Mesoscopic | n-BuCuPc | — | 1.04 | 20.9 | 66 | 14.4 | 78 |
| 6 | Mesoscopic | n-BuCuPc | — | 1.07 | 21.0 | 71 | 15.9 | 79 |
| 7 | Mesoscopic | t-BuCuPc | (1.3 ± 0.2) × 10−6 | 0.94 | 13.8 | 43 | 5.6 | 77 |
| 7 | Mesoscopic | t-BuCuPc | — | 1.07 | 22.6 | 77.5 | 18.8 | 80 |
| 7 | Mesoscopic | t-BuCuPc | — | 1.13 | 23.19 | 71.3 | 18.68 | 81 |
| 2 | Planar n–i–p | CuEtPc | 20.4 × 10−4 | 0.95 | 20.4 | 66.6 | 12.9 | 82 |
| 6 | Planar n–i–p | CuBuPc | 12.6 × 10−4 | 1.02 | 21.0 | 50 | 15.0 | 82 |
| 8 | Planar n–i–p | CuHePc | 8.77 × 10−4 | 0.97 | 17.4 | 68.1 | 11.5 | 82 |
| 9 | Mesoscopic | CuPc–TIPS | — | 1.01 | 21.4 | 65 | 14.0 | 83 |
| 10 | Mesoscopic | CuPc–OBu | 1.23 × 10−4 | 1.06 | 22.8 | 73 | 17.6 | 84 |
| 11 | Mesoscopic | CuPc–Bu | 4.30 × 10−4 | 1.03 | 21.0 | 66 | 14.3 | 84 |
| 12 | Planar n–i–p | OMe–DPA–CuPc | 1.55 × 10−3 | 1.054 | 22.44 | 70.74 | 16.73 | 85 |
| 13 | Planar n–i–p | OMe–TPA–CuPc | 6.39 × 10−3 | 1.096 | 23.41 | 76.67 | 19.67 | 85 |
| 14 | Planar n–i–p | SMe–TPA–CuPc | — | 1.132 | 24.10 | 84.31 | 23.00 | 53 |
| 15 | Mesoscopic | ZnPcNO2–OPh | 2.80 × 10−5 | 1.071 | 20.832 | 64.4 | 14.35 | 86 |
| 16 | Mesoscopic | CuPcNO2–OPh | 1.86 × 10−5 | 1.064 | 20.461 | 58.4 | 12.72 | 86 |
| 17 | Mesoscopic | CoPcNO2–OPh | — | 1.04 | 11.79 | 64 | 8.24 | 87 |
| 18 | Mesoscopic | CuPcNO2–OMFPh | 5.02 × 10−5 | 1.02 | 20.01 | 60 | 12.52 | 88 |
| 19 | Mesoscopic | CuPcNO2–OBFPh | 8.21 × 10−5 | 1.04 | 20.62 | 64 | 13.66 | 88 |
| 20 | Mesoscopic | ZnPcNO2–OBFPh | 11.4 × 10−5 | 1.10 | 21.00 | 68 | 15.74 | 88 |
| 21 | Mesoscopic | ZnPc(t-Bu)4 | — | 0.879 | 17.19 | 34.1 | 5.16 | 89 |
| 22 | Mesoscopic | (n-Bu)4ZnPc | — | 0.928 | 14.5 | 61.8 | 9.00 | 90 |
| 23 | Mesoscopic | ZnPcAE | 66.10 × 10−4 | 1.064 | 21.19 | 63.2 | 14.25 | 94 |
| 24 | Planar n–i–p | RE-ZnBu4Pc | 7.59 × 10−4 | 0.94 | 21.83 | 63.10 | 12.94 | 91 |
| 25 | Planar n–i–p | ZnBu4Pc | 4.78 × 10−4 | 0.95 | 20.74 | 69.64 | 12.06 | 91 |
| 26 | Mesoscopic | H2-(t-Bu)4Pc | — | 1.10 | 22.70 | 80.70 | 20.10 | 95 |
| 27 | Mesoscopic | OTPA–ZnPc | 1.08 × 10−5 | 1.02 | 22.36 | 71.43 | 16.23 | 92 |
| 28 | Mesoscopic | ZnPH22 | 2.8 × 10−4 | 1.06 | 22.5 | 77.0 | 18.3 | 96 |
| 29 | Mesoscopic | Methoxyethoxy-NiPc | 1.1 × 10−6 | 1.13 | 23.92 | 78.66 | 21.23 | 50 |
| 30 | Mesoscopic | NiPc–Cou | 4.78 × 10−6 | 1.061 | 19.50 | 49.4 | 10.23 | 93 |
| 31 | Mesoscopic | FePc–Cou | 3.65 × 10−6 | 1.51 | 18.59 | 48.0 | 9.40 | 93 |
| 32 | Planar n–i–p | CuMe2Pc | 4.79 × 10−2 | 1.085 | 21.32 | 68 | 15.73 | 97 |
| 33 | Planar n–i–p | PdMe2Pc | 3.42 × 10−2 | 1.06 | 21.08 | 73 | 16.28 | 98 |
| 34 | Planar n–i–p | HMe2Pc | 2.72 × 10−2 | 1.035 | 21.28 | 70.8 | 15.59 | 99 |
| 35 | Planar n–i–p | ZnMe2Pc | 1.80 × 10−2 | 1.005 | 21.03 | 70.8 | 15.59 | 99 |
| 36 | Planar n–i–p | Me6Bu–ZnPc | 6.85 × 10−4 | 1.09 | 23.09 | 69.18 | 17.41 | 100 |
| 37 | Planar n–i–p | NiEt2Pc | 1.33 × 10−5 | 0.96 | 18.65 | 48.32 | 8.63 | 101 |
| 38 | Planar n–i–p | NiPr2Pc | 3.64 × 10−4 | 1.04 | 22.83 | 59.40 | 14.07 | 101 |
| 39 | Planar n–i–p | CuPc-(OMe)8 | 1.1 × 10−3 | 1.05 | 22.1 | 79 | 18.3 | 102 |
| 40 | Planar n–i–p | P-SC6-TiOPc | 1.16 × 10−5 | 0.95 | 19.92 | 36.04 | 6.82 | 103 |
| 41 | Planar n–i–p | NP-SC6-TiOPc | 1.17 × 10−4 | 1.06 | 22.31 | 71.34 | 16.87 | 103 |
Lianos and Xu's group added longer alkyl chains, such as n- and tert-butyl groups to the Pc ring (6 and 7) and investigated their performance as HTMs in PSCs, respectively.77 The long alkyl groups increased the molecular ordering and crystallinity of the Pc materials in the thin film samples. The higher crystallinity of the n-butyl-substituted CuPc (n-BuCuPc) was the origin of its higher hole mobility, which was almost 40 times higher than that of the tert-butyl-substituted CuPc material. Consequently, a better performance was observed for the perovskite devices based on tetra-n-butyl CuPc, with a PCE as high as 8.5%, compared with that of 5.6% for the PSCs employing tert-butyl-substituted CuPc as the dopant-free HTM. In another work, Lianos’ group introduced a buffer layer of graphene oxide (GO) between n-BuCuPc as the HTM and the perovskite layer, which significantly improved the performance of their devices.78 The presence of the buffer layer helped to reduce the shunt paths towards the electrode, offered higher stability to the perovskite layer, facilitated hole transfer, and reduced the charge recombination due to the improved HTM layer. Due to the mesoporous structure of GO, the extended aggregation and presence of voids in the Pc-based thin film were limited. Therefore, compared with the devices without a buffer layer, the PSCs using n-BuCuPc as the HTM and GO as the buffer layer in their structure showed enhanced FF and VOC values and a dramatic increase in PCE from 7.3% to 14.4%. A further increase in the cell efficiency was achieved when TiO2/reduced GO was used as the ETM in a mesoscopic PSC instead of TiO2, and a PCE of 15.9% was obtained.79
Kim and coworkers fabricated mesoscopic PSCs with high efficiency and thermal stability, utilizing tetra-tert-butyl-substituted CuPc (t-BuCuPc) as the HTM and a mixed perovskite of (FAPbI3)0.85(MAPbBr3)0.15 as the light absorbing material (Fig. 4a).80 A virtually face-on orientation of t-BuCuPc was expected at the interface of the perovskite crystal facets, which was considered the main origin of the increase in both the charge transfer efficiency and long-term stability of the devices. The best-performing PSC achieved a PCE of 18.8%, with VOC, JSC, and FF values of 1.07 V, 22.6 mA cm−2 and 77.5%, respectively. To investigate the long-term thermal stability of the devices, the t-BuCuPc-based PSC was kept on a hotplate at 85 °C for 1100 h inside a glovebox under an inert atmosphere. The device could maintain 97% of its initial efficiency for more than 1000 h of thermal annealing (Fig. 4b), which is particularly important, given that the operating temperature of the solar cells can easily become high under direct sunlight. Thus, the use of Pc materials in the device structure can be advantageous for PSCs working under high temperature. In 2018, Duong et al. used the soluble t-BuCuPc as the HTM in mesoscopic PSC devices and achieved PCEs as high as 20% through interfacial engineering.81 It was observed that the solution-processed t-BuCuPc layer on top of the perovskite film possessed abundant cracks on its surface, which caused direct contact between the perovskite layer and the top electrode. Subsequently, heat treatment at 85 °C was applied to the devices, which resulted in negligible shunts and interface recombination (Fig. 4c). Interestingly, the cracks persisted on the CuPc film, even with the post heat treatment. However, it was verified that the Au particles on the contact rearranged after the heat treatment and migrated away from the cracks on the surface of the HTM layer. It is worth noting that only by using highly thermally stable Pc materials as the HTM in PSC devices structure it is possible to develop heating treatments for healing the device structure and enhancing its performance and operating lifetime. The J–V characteristics, together with the performance parameters of the champion device with the post heat treatment are presented in Fig. 4d, where negligible hysteresis can be observed for the device. In a recent study, our research group focused on the impact of the length of the alkyl group in peripherally tetra-substituted CuPc compounds when used as dopant-free HTMs on the PSC photovoltaic performance.82 The results confirmed the role of the alkyl chain in the molecular orientation and intermolecular π–π stacking of Pc materials, and subsequently the hole mobility of their thin films. With an extended carbon chain length, improved crystallization was observed. Matching experimental data with calculations showed that alkyl-substituted CuPc materials have a tilted orientation on the perovskite layer with the angles of 46.56°, 39.58° and 34.00° for CuEtPc (2), CuBuPc (6), and CuHePc (8), respectively. Although the lower tilt angle of CuHePc was more beneficial for hole transport in the PSC, its greater π–π stacking distance suggested a weaker interaction, leading to lower hole mobility. The hole mobilities of CuEtPc, CuBuPc and CuHePc were measured by the SCLC method to be 20.4 × 10−4, 12.6 × 10−4 and 8.77 × 10−4 cm2 V−1 s−1, respectively. The CuBuPc-based PSCs exhibited a higher efficiency (15%) compared with the devices using CuEtPc (12.9%) and CuHePc HTMs (11.5%), which was attributed to the better morphology of the CuBuPc films on perovskite and their high hole mobility and more balanced charge transport.
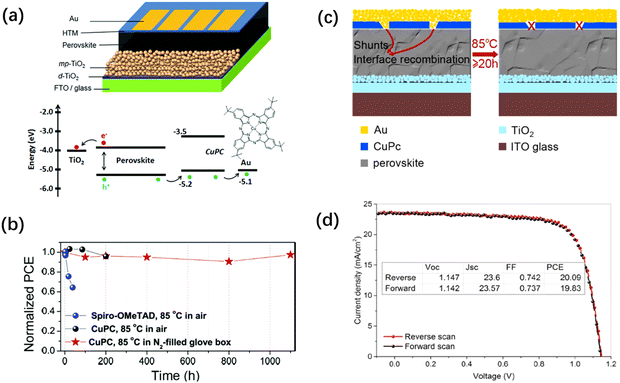 | ||
| Fig. 4 (a) Schematic architecture of CuPc-applied perovskite solar cells (PSCs) and the energy-level diagram of the components used in these devices. (b) Long-term stability of the devices stressed at 85 °C in air (25–30% humidity), employing FAPbI3/CuPc (black color) and FAPbI3/spiro-OMeTAD (blue color). 1100 h stability of the device utilizing FAPbI3/CuPc at 85 °C in a nitrogen-filled glove box (red color). Reproduced with permission.80 Copyright 2017, RSC. (c) Schematic illustration of the impact of the post-heat treatment on the ion migration paths in the device structure. (d) J−V scans of the champion perovskite cell in both reverse and forward direction at a scan rate of 50 mV s−1. The inset shows the photovoltaic parameters extracted from the J–V curves. Reproduced with permission.81 Copyright 2018, ACS. | ||
Sun and coworkers introduced triisopropylsilylethynyl (TIPS) substituents in the periphery of the Pc ring to improve its solubility and hydrophobicity. CuPc–TIPS (9) was explored as an HTM in mesoscopic perovskite devices, in combination with mixed-ion perovskite materials and vacuum-free carbon counter electrode, resulting in a PCE of 14.0% under 1 Sun illumination intensity.83 CuPc–TIPS showed significantly higher conductivity (5 × 10−6 S cm−1) than pristine spiro-OMeTAD (10−7–10−8 S cm−1).83 In another work in 2019, the same team developed two dopant-free HTMs based on CuPc derivatives with non-peripheral substituents.84 Their study confirmed that even small structural changes in the substituents could have a significant influence on the molecular ordering in thin films and the hole transport ability. The PSCs based on CuPc–OBu (10), with butoxy chains attached to phenoxy substituents, exhibited the best PCE of 17.6%, which was substantially higher than that of the devices employing the CuPc–Bu (11) HTM (14.3%), bearing butyl chains on the phenoxy groups. It was demonstrated that CuPc–OBu could offer higher hole mobility and more effective charge collection than CuPc–Bu due to the presence of extra oxygen molecules and higher flexibility of the C–O bond, and consequently a closer packing of the molecules. Recently, our research group achieved remarkable PCEs for PSCs using dopant-free HTMs based on arylamine-substituted CuPcs.85 Methoxydiphenylamine-substituted copper phthalocyanine (OMe–DPA–CuPc, 12) and methoxytriphenylamine-substituted copper phthalocyanine (OMe–TPA–CuPc, 13) were employed as HTMs in planar n–i–p devices using Cs0.05(MA0.13FA0.87)0.95Pb(I0.87Br0.13)3 as the light absorber and SnO2 thin layer as the ETM, reaching efficiencies as high as 16.7% and 19.7%, respectively. According to the molecular dynamics (MD) simulation results, a higher density was calculated for OMe–DPA–CuPc (1.26 g cm−3) with respect to OMe–TPA–CuPc (1.23 g cm−3) in the bulk amorphous aggregates (Fig. 5a). Focusing on the intermolecular radial distribution function between the molecular centers of mass, short-range aggregation was obtained for OMe–TPA–CuPc compared to OMe–DPA–CuPc (Fig. 5b). In addition, the distribution of the intermolecular alignment of the nearest neighboring molecules revealed an orientation between about 80° to 85° for OMe–DPA–CuPc compared with the orientation values approaching 0° for the OMe–TPA–CuPc molecules, leading to more efficient π–π interactions for the latter (Fig. 5c). Due to the enhanced tendency of OMe–TPA–CuPc to form partially ordered aggregates, a larger mobility for OMe–TPA–CuPc can be expected with respect to OMe–DPA–CuPc. Accordingly, a higher hole mobility was measured by the SCLC method for OMe–TPA–CuPc (6.39 × 10−3 cm2 V−1 s−1) than OMe–DPA–CuPc (1.55 × 10−3 cm2 V−1 s−1). Moreover, the synthesis cost of OMe–TPA–CuPc was estimated to be 28.19 $ per g, which is much lower than that of spiro-OMeTAD. Moreover, in the aging test, the OMe–TPA–CuPc-based devices showed good stability, retaining 92% of their initial efficiency over 960 h (without encapsulation, in air, at 25 °C and under 75% humidity).
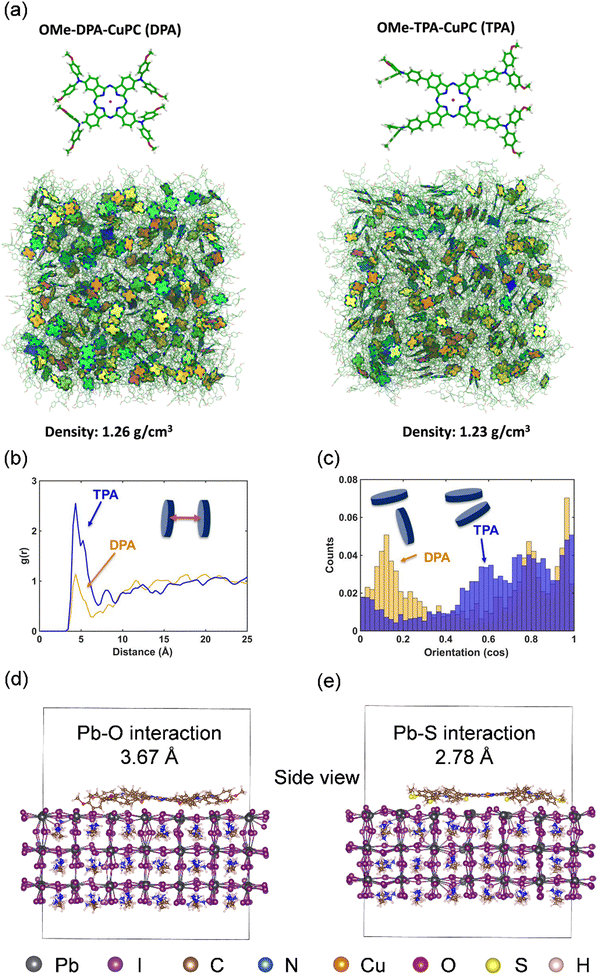 | ||
| Fig. 5 (a) Molecular structure and simulated bulk aggregates (periodic cubic box, about 8 × 8 × 8 nm) of OMe–DPA–CuPc and OMe–TPA–CuPc. (b) Centers of mass and (c) distribution of the intermolecular alignment of nearest neighboring molecules (center of mass distance of <6.5 Å), defined as the angle between the normal to the planes of the two Pc cores. Adsorption structures equilibrated by AIMD simulation: side views of (d) OMe–TPA–CuPc/MAPbI3 and (e) SMe–TPA–CuPc/MAPbI3. Reproduced with permission.85 Copyright 2019, Wiley. | ||
In a recent publication, the same research group explored the performance of methylthiotriphenylamine-substituted copper phthalocyanine (SMe–TPA–CuPc) (14) as a dopant-free HTM in PSCs with the n–i–p structure.53 It was demonstrated that the SMe–TPA–CuPc molecules could diffuse into the perovskite film, effectively passivating the defects in the grain boundaries, as well as the surface of the film through the stronger interactions between the under-coordinated lead sites in the film and the methylthio moiety than the methoxy moiety of the Pc-based HTM (Fig. 5d and e). This holistic approach for passivating the perovskite layer resulted in an excellent performance and stability for the devices, delivering an incredible efficiency of 23.0%. This is among the highest efficiencies reported to date for PSC devices using dopant-free HTMs. After 3624 h of aging at 85 °C, the optimized PSCs could maintain 96% of their initial PCE. Guo et al. used asymmetric MPc materials as dopant-free HTMs in PSCs and reported PCEs of 12.72%, 14.35%, and 8.24% for the devices employing Cu-, Zn-, and Co-based HTMs, respectively.86,87 With three phenoxy and a nitro substituent at the peripheral positions around the Pc ring, acting as “push” and “pull” groups, the developed Pc-based HTMs offered a donor–π–acceptor (D–π–A) structure, favoring their hole transport ability. Moreover, the flexible phenoxy groups enhanced the solubility of the MPc materials, as well as their molecular packing order. The improved performance of the ZnPcNO2–OPh (15)-based PSCs was attributed to the higher hole mobility of ZnPcNO2–OPh and its lower series resistance compared with CuPcNO2–OPh (16) and CoPcNO2–OPh (17). Guo and coworkers extended their research on NO2-substituted asymmetric MPcs by introducing either (4-methylformate)phenoxy or (4-butylformate)phenoxy groups in the Pc macrocycle, using Cu and Zn as metal centers.88 Three novel dopant-free HTMs were developed and denoted as CuPcNO2–OMFPh (18), CuPcNO2–OBFPh (19), and ZnPcNO2–OBFPh (20) and incorporated in mesoscopic PSCs. The higher hole mobility of ZnPcNO2–OBFPh resulted in a higher JSC value and better performance for the cell in comparison to the PSCs utilizing the CuPc HTMs. The best-performing devices based on ZnPcNO2–OBFPh presented a PCE of 15.74%, with VOC, JSC, and FF of 1.10 V, 21.00 mA cm−2 and 68%, respectively. This study demonstrated the great potential of tetra-substituted ZnPc materials as HTMs in PSCs. In 2016, Wu et al. reported an efficiency of 5.16% and 7.98% under forward and reverse scans, respectively, for a mesoscopic perovskite device employing peripherally tetra-tert-butyl substituted ZnPc (21) as a dopant-free HTM.89 Their device suffered from a very low FF and severe hysteresis, which is mainly due to the poor coverage of the perovskite layer on the mesoporous TiO2 film. Similarly, Katz and coworkers observed considerable hysteresis in their PSC devices based on a tetra-n-butoxy-substituted ZnPc ((n-BuO)4ZnPc, 22) HTM and achieved PCEs of 6.06% and 9.00% under forward and reverse scans, respectively.90 Undesirable interfacial contact was considered the main origin of the observed hysteresis behavior in the devices. Consequently, by replacing one of the tert-butyl groups in tetra-substituted Zn and CuPc compounds, six asymmetric MPc materials were developed and used as dopant-free HTMs in PSC devices.94 MPcs often possess a narrow band gap of 1.4 to 2.1 eV, which cause poor electron blocking and increased charge recombination at their interface with the perovskite film.13 Thus, to overcome this problem, substituents are introduced in various positions around the Pc ring to tune the band gap of MPc materials. Additionally, through the appropriate selection of the substituents, the carrier diffusion length and the carrier mobility can be improved in the Pc thin film. Perturbing the electron conjugated system in the Pc ring by asymmetrically introducing different substituents will deliver a unique set of photophysical and electrochemical properties in the Pc compound. By investigating the charge carrier dynamics of the thin films of the developed MPcs via transient absorption spectroscopy (TAS), it was found that the charge carrier lifetime was synergically controlled by both the core metal and the different substitutes around the ring. Asymmetrically attached substituents around the Pc ring can impact the triplet state properties and deliver a longer lifetime for the generated charges. Eventually, the evaluation of the photovoltaic performance of the PSCs based on asymmetrical substituted ZnPcs indicated a jump in the efficiency of the asymmetric device (ZnPcAE (23)) with a PCE of 14.25% compared with that of the tetra-tert-Bu-substituted ZnPc (PCE = 10.52%).
Recently, we compared the performance of PSCs using either a pure isomer or isomer mixture of tetra-n-butyl-substituted ZnPc as a dopant-free HTM in their structure.91 The use of pure isomer materials in the device structure as the active layers has been confirmed as an efficient approach to achieve a higher performance and lifetime in the devices.104,105 It has been verified that various isomers of a Pc compound can offer different values of charge carrier mobility.106 Accordingly, the use of a pure isomer Pc compound with the highest charge mobility and conductivity may endow the PSC device with a higher performance and stability. However, the common chromatography is not a suitable technique for separating and purifying various isomers of a Pc compound with four similar groups attached non-peripherally to the Pc ring. Therefore, a ring expansion method was used to synthesize the pure isomer ZnPc material (RE-ZnBu4Pc, 24). A considerably higher X-ray diffraction peak intensity was observed for RE-ZnBu4Pc compared to ZnPc with the isomer mixture (ZnBu4Pc, 25), proving the greater tendency of the pure isomer compound to form films with higher crystallinity, which is advantageous for its charge carrier transport ability. The devices based on RE-ZnBu4Pc showed a higher average performance with better reproducibility in the fabrication process, as well as better stability compared with the PSCs employing the isomer mixture Pc-based HTM (ZnBu4Pc). The highest PCEs of 12.94% and 12.06% were achieved for the planar devices based on RE-ZnBu4Pc and ZnBu4Pc, respectively. The better film quality with higher crystallinity and higher hole mobility of RE-ZnBu4Pc than ZnBu4Pc were considered as the main reason for the better performance observed for the devices using this HTM material. The PSCs based on both pure isomer and mixture ZnPc materials showed good long-term stability after storage in an atmosphere with high relative humidity. In a study in 2021, the performance of tetra-tert-butyl-substituted metal-free Pc (26) as an HTM for n–i–p devices was explored, reporting efficiencies of over 20% for n–i–p devices, and compared to that of metal-Pc HTMs.95 It is well-known that the pyrrolic nitrogen groups in the inner ring of the Pc molecule are highly reactive towards metal ions, and therefore can strongly interact with the under-coordinated Pb2+ sites on the surface of the perovskite film and passivate its defects, further enhancing the VOC of the devices compared to their metal-coordinated counterparts. Cui and coworkers modified the performance of TPA-substituted ZnPc HTM by adding methoxy groups to the TPA moiety.92 The methoxy groups endowed the ZnPc material with enhanced solubility in various organic solvents, leading to a more controlled thin film fabrication process using solution-based deposition techniques. The methoxy functional groups could also improve the interface between the perovskite and HTM layers and passivate the defects in the perovskite layer, leading to a better performance for the devices. OTPA–ZnPc (27) was used as a dopant-free HTM in PSC devices with a mesoporous TiO2 ETM layer and mixed perovskite ((FAPbI3)0.85(MAPbBr3)0.15) absorber. OTPA–ZnPc showed a HOMO level of −5.58 eV, which is slightly higher than that of the mixed perovskite material (−5.65 eV), and its SCLC hole mobility was calculated to be 1.08 × 10−5 cm2 V−1 s−1. A smooth film of the HTM was uniformly formed on the top of the perovskite layer, inhibiting the direct contact between the perovskite layer and the electrode. The best-performing device exhibited a PCE as high as 16.23% under the reverse scan. In 2021, a series of ZnPc derivatives was synthesized bearing four phenyl-based substituents around the Pc scaffold and employed as dopant-free HTMs in n–i–p PSCs.96 The device using the best-performing HTM, ZnPH22 (28), containing benzo[d][1,3]dioxole peripheral substituents, could deliver a competitive PCE of 18.3%. It was suggested that the cyclic/planar conformation of ZnPH22, due to the unique structure of its peripheral groups, led to superior charge extraction and transport capability for the developed Pc-based HTM.
A series of Pc materials (CoPc, NiPc, ZnPc and H2Pc) was designed using four methoxyethoxy units and employed in PSC devices as HTMs.50 A record efficiency of 21.23% was achieved for a conventional n–i–p-structured PSC device based on the dopant-free tetra methoxyethoxy-substituted NiPc (29) (Fig. 6). It was demonstrated that among the used metal ions, Ni as the central atom could contribute more electrons to the Pc ring and construct a larger intramolecular electric field, which facilitated the interfacial charge extraction, improving the performance of the PSCs. In addition, the presence of two oxygen atoms in the methoxyethoxy substituents not only enhanced the molecular dipole, but also modified the dipole direction. The electron–hole pair density of four NiPc units can form a single connected flow manifold by intermolecular π orbitals, delivering efficient intermolecular charge transport (Fig. 6b). The high hole mobility of 1.1 ± 0.2 × 10−4 cm2 V−1 s−1 was reported for the NiPc compound, which was considerably higher than that of CoPc (8.8 ± 0.3 × 10−6 cm2 V−1 s−1), ZnPc (1.5 ± 0.4 × 10−5 cm2 V−1 s−1), CuPc (7.3 ± 0.2 × 10−5 cm2 V−1 s−1) and H2Pc (6.1 ± 0.3 × 10−5 cm2 V−1 s−1).
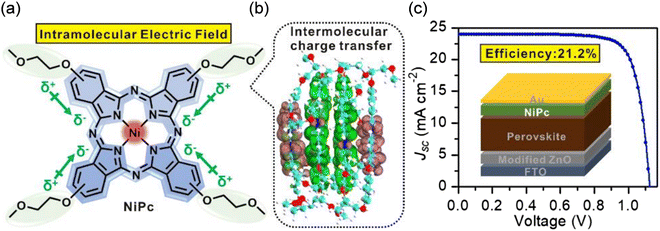 | ||
| Fig. 6 (a) Structure of NiPc and direction of intramolecular electric field. (b) Hole–electron pair density isosurface (isovalue = 0.005 a.u.) in the simulated 4-periods NiPc structure unit. The pink and green isosurface represent electron and hole density, respectively. (c) J–V curve for the best-performing PSC with NiPc as HTM (Inset: Schematic illustration of the PSC with NiPc as HTM). Reproduced with permission.85 Copyright 2019, Wiley. | ||
By introducing four 7-coumarinoxy-4-methyl groups in the periphery of NiPc and FePc, Qi et al. developed new HTMs, which were denoted as NiPc–Cou (30) and FePc–Cou (31), respectively.93 The coumarin groups facilitated a reduction in the aggregation of the Pc molecules and enhanced their solubility in organic solvents. PSCs with relatively good stability and PCEs of 10.23% and 9.40% were fabricated based on NiPc–Cou and FePc–Cou, respectively. NiPc–Cou showed a higher hole mobility (4.78 × 10−6 cm2 V−1 s−1) compared with FePc–Cou (3.65 × 10−6 cm2 V−1 s−1). With its HOMO level matched with that of perovskite layer and LUMO level higher than FePc–Cou, NiPc–Cou could offer greater hole extraction and conduction, as well as better electron blocking ability.
It was evidently established that the electronic nature and size of the substituents around the Pc macrocycle play a crucial role in defining the function of the Pc compound employed in PSC devices and its efficiency. A suitable substituent can adjust the solubility of the Pc materials in the appropriate solvents, and subsequently improve the quality of the deposited thin film by altering its morphology, crystallinity and thickness. By changing the electronic structure of the Pc compound, a substituent can help to match the energy levels of the frontier molecular orbitals in optoelectronic devices. Substituents can modify the intermolecular π–π stacking of the Pc core in the thin film, as well as the electrochemical properties of the Pc compounds, and consequently their performance as the active charge selective layer in the perovskite device. The bulkiness of the substituents can directly impact the aggregation of the Pc derivatives and their molecular arrangement and orientation inside the thin film, which successively influence their charge transport ability. Various substituents can also regulate the hydrophobicity of Pc compounds as an HTM layer in PSCs and improve the long-term stability of the devices.
 | ||
| Fig. 7 GIXRD patterns of Me6Bu–ZnPc deposited on (a) SiO2 and (b) perovskite (thin film thickness of ∼60 nm). Molecular orientation model of Me6Bu–ZnPc deposited on (c) SiO2 and (d) perovskite. Reproduced with permission.100 Copyright 2019, Wiley. | ||
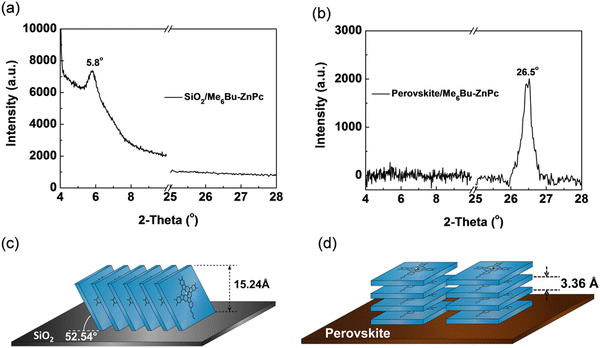
Continuing our research on octaalkyl-substituted MPc materials, we have recently reported the performance of PSCs employing dopant-free HTMs based on NiEt2Pc (37) and NiPr2Pc (38), with the alkyl groups attached to the non-peripheral positions around the Pc ring.101 It was found that the length of the alkyl chain could play an important role in defining the energy level of the thin film. Due to the better alignment between the energy levels at the perovskite/HTL interface, NiPr2Pc with a longer alkyl chain could offer reduced charge recombination, and therefore higher VOC and JSC values for the device. The PSC based on NiPr2Pc could achieve the PCE of 14.07%. Recently, non-peripheral methoxy-substituted copper(II) phthalocyanine, denoted as CuPc-(OMe)8, 39, was investigated by the Cheng group.102 The solution-processed CuPc-(OMe)8 film exhibited comparable conductivity and hole mobility with the reference doped spiro-OMeTAD. Moreover, the as-prepared CuPc-(OMe)8 showed better energy alignment with (FAPbI3)0.85(MAPbBr3)0.15 as a comparison of reference HTMs. The deposited uniform and compact CuPc-(OMe)8 thin film could hinder the direct contact between the perovskite and top electrode layers. Consequently, the dopant-free CuPc-(OMe)8-based PSCs showed a champion PCE of 18.3% accompanied with the VOC, JSC, and FF of 1.05 V, 22.1 mA cm−2 and 79%, respectively. Recently, we developed two TiOPc derivatives, possessing eight n-hexylthio groups attached to either the peripheral (P-SC6-TiOPc, 40) or non-peripheral positions (NP-SC6-TiOPc, 41) of the Pc ring and applied them as dopant-free HTMs in planar n–i–p PSCs.103 According to density functional theory (DFT) calculation results, a larger rotation angle for the substituents on the peripheral, compared with non-peripheral positions, led to an increased height for the P-SC6-TiOPc compound, and consequently a greater distance and weaker π–π interactions between the molecules in the P-SC6-TiOPc thin film (Fig. 8a). Therefore, significantly higher hole extraction and mobility were obtained for NP-SC6-TiOPc than the P-SC6-TiOPc thin film. Furthermore, the different sizes and intermolecular interactions of the P-SC6-TiOPc and NP-SC6-TiOPc compounds resulted in some alterations in their thin film morphology. A thin film with an improved morphology, lower surface roughness, and better coverage on the perovskite layer was achieved for NP-SC6-TiOPc compared with P-SC6-TiOPc (Fig. 8b). The PSCs using NP-SC6-TiOPc achieved the highest PCE of 16.87%, which was remarkably higher than that of P-SC6-TiOPc-based devices (6.82%). It was demonstrated that tuning the position of the attached substituents can be considered as an effective strategy to enhance the performance of Pc-based PSC devices. Significantly higher reproducibility was achieved for the devices based on NP-SC6-TiOPc as a dopant-free HTM due to its higher ordering and higher-quality thin film (Fig. 8c). The photovoltaic properties of some selected perovskite devices using octa-substituted Pc compounds in their structure are summarized in Table 2.50,93,97–103
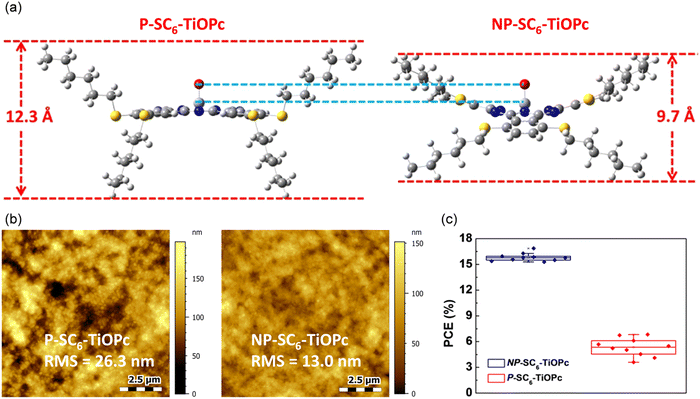 | ||
| Fig. 8 (a) DFT results of the side view of the P-SC6-TiOPc- and NP-SC6-TiOPc-optimized ground state geometry (Gaussian 09 optimization with DFT B3LYP/6-31g(d)). (b) AFM images of P-SC6-TiOPc deposited and NP-SC6-TiOPc deposited on perovskite. (c) PCE statistics of devices fabricated from the same batch. Reproduced with permission.103 Copyright 2019, ACS. | ||
2.2. Doped phthalocyanines for PSCs
The introduction of a bulky group at the peripheral sites of the Pc core may suppress the aggregation and reduce the π–π interaction.14 However, it also results in low hole mobility and conductivity, which are unfavorable for efficient hole transport in devices. Accordingly, a common method to overcome this issue is the doping strategy. As a successful approach in the case of spiro-OMeTAD, doping with bis(trifluoromethylsulphonyl)imide (Li-TFSI), 4-tert-butylpyridine (tBP) and tri(bis(trifluoromethane)sulfonimide) (FK209) led to a reduction in the spiro-OMeTAD molecule LUMO level. Accordingly, the energy gap between the pristine spiro-OMeTAD and the radical cation species provides the driving force for charge hopping between these two materials, significantly improving the conductivity and mobility in the doped form.107 Considering this, doping was utilized in Pc HTMs to improve the performance of PSCs. The molecular structures of the Pc materials used as doped HTMs in PSCs are shown in Fig. 9 (42–65). | ||
| Fig. 9 Molecular structures of Pc as doped HTMs (42–65) and additive in HTM or perovskite layer in PSCs (66–75). | ||
In 2015, Nazeeruddin's group explored the performance of Zn(II)octa(2,6-diphenylphenoxy)phthalocyanine (TT80, 42) as a doped HTM in PSCs and reported a PCE of 6.7% under AM1.5G standard conditions.108 The introduction of eight bulky substituents in the peripheral sites around the Pc ring led to the suppression of aggregation and a significant increase in the solubility of the ZnPc material, allowing the use of solution-processed deposition techniques. The same research group further introduced different secondary amine substituents in the periphery of the ZnPc ring to develop new HTMs for perovskite devices.109 The PSCs using the newly synthesized HTMs, denoted as BI25 (43), BL07 (44), and BL08 (45), achieved the PCEs of 11.75%, 6.65%, and 11.44%, respectively. In 2019, they developed a series of octa-substituted MPcs with eight secondary aromatic amines bound to the peripheral positions of the macrocycle by C–N bonds, which were denoted as BL25 (46), BL38 (47), BL40 (48), BL50 (49), BL51 (50) and BL52 (51).110 The results showed that the MPcs featuring bis(p-alkoxyphenyl)amino substituents delivered a better photovoltaic performance than their 9H-carbazol-9-yl and diphenylamino analogs, and the core metal in the Pc derivatives with the same structure had little effect on their overall performances. A superior PCE of up to 18.10% was achieved by the HTM of ZnPc bearing eight bis(p-butoxyphenyl)amino substituents (BL40), which is comparable to that of spiro-OMeTAD with dopants (19.25%). The explanation for the huge gap in the PCE between BL25 and BL38 with the remaining ones was investigated by PL decay kinetics, electric field dependent time-resolved photoluminescence experiments, and photocurrent kinetics upon photoexcitation of the device with short nanosecond laser pulses, indicating that the former two types of HTMs have large nonradiative surface recombination and an injection barrier for holes. In a recent study by the same group, efficiencies of around 20% were reported by the same team, using a newly synthesized HTM from the same family named Zn-BL54 (52) with tetra-substituted Pc ring, and double or triple cation perovskite materials as the light-absorbing film in the device.111 The achieved efficiencies were comparable with that of the reference devices using spiro-OMeTAD, while offering considerably higher long-term stability for the non-encapsulated devices. In 2017, Calio and coworkers altered the diphenyl groups attached to the phenoxy by tert-octyl, and developed two new Pc materials substituted by 4-tert-octylphenol groups, with Cu(II) and Zn(II) as the metal center, which were denoted as (tOctPhO)8CuPc (53) and (tOctPhO)8ZnPc (54), respectively.112 The MPc-based HTMs were doped by adding lithium LiTFSI and (tBP) and tested in PSCs with mixed perovskites ((FAPbBr3)0.85(MAPbI3)0.15), and the thickness of their thin layers was optimized. It was demonstrated that a thinner layer of either HTM could offer a lower series resistance, and consequently an improved performance for the devices. The best-performing devices based on (tOctPhO)8CuPc and (tOctPhO)8ZnPc exhibited PCEs of 8.33% and 7.25%, respectively. In their study, the Lianos’ group employed tetra-phenylamine-substituted ZnPc (TPA-ZnPc, 55) as an HTM doped with tBP and LiTFSI in PSCs, reaching an efficiency of 9.0%, which was further enhanced to 13.65% in the presence of an Al2O3 buffer layer between the perovskite and HTM layers.113 The presence of the buffer layer offered a higher shunt resistance in the device, as well as higher stability for the perovskite layer, protecting it against the corrosive additives used in the HTM. Moreover, the porous framework of Al2O3 facilitated the formation of a thin film as the upper HTM layer, leading to better hole transfer and conduction and limited charge recombination. According to the cross-sectional FESEM image, a thicker hole transporting layer was achieved on the top of the Al2O3 buffer layer compared with neat TPA-ZnPc. The performance of the device was enhanced with the optimum thickness of 200 nm for the buffer and HTM layers combined.
Sun's group introduced eight butoxy groups in the non-peripheral sites around the NiPc macrocycle (NiPc–(OBu)8, 56), and in combination with vanadium(V) oxide (V2O5), developed an organic–inorganic integrated hole transport layer for incorporation in mesoscopic PSC devices.114 The PSCs based on pristine NiPc–(OBu)8 exhibited efficiencies of up to 10.6%, while doping NiPc–(OBu)8 with LiTFSI and tBP, together with an increase in its thin film thickness could significantly enhance the PCEs of the devices to 17.9%. Although the pristine NiPc–(OBu)8 showed lower conductivity (1.18 × 10−4 S cm−1) than the doped spiro-OMeTAD (1.91 × 10−4 S cm−1), the doping strategy could considerably enhance its conductivity to a value of 1.97 × 10−4 S cm−1. The use of a V2O5 buffer layer on top of the dopant-free NiPc–(OBu)8 film led to the highest efficiency of 18.3% for the devices. A PCE of 10.8% was achieved for the PSCs with only a V2O5 HTM, confirming the critical role of the organic–inorganic HTM in achieving high efficiencies. The integrated HTM could fully cover the perovskite crystals and lower the recombination losses between the perovskite and Au electrode. Nazeeruddin's group introduced thiophene-based functional groups in the ZnPc ring and studied their performance as an HTM in perovskite devices.115 Using a hybrid interfacial hole transport layer, consisting of a tetra 5-hexyl-2-thiophene-substituted ZnPc (Sym-HTPcH, 57) HTM and meso-Al2O3 buffer layer, devices with efficiencies of up to 12.8% were achieved.115 The device performance has been considerably improved by changing the thiophene-based functional groups around the ZnPc ring.116 Tetra-5-hexyl-2,2′-bisthiophene-substituted ZnPc (HBT-ZnPc, 58) and tetra-5-hexylthiophene-substituted ZnPc (HT-ZnPc, 59) were employed in mesoscopic PSCs with (FAPbI3)0.85(MAPbBr3)0.15 perovskite absorber, showing the remarkable efficiencies of 15.5% and 17.5%, respectively. The higher efficiency of the HT-ZnPc-based PSCs was attributed to the enhanced molecular packing order of HT-ZnPc. Sun's group studied non-peripherally substituted CuPc materials by incorporating four 2,4-dimethyl-3-pentoxy groups in the CuPc ring and used as them as the HTM in mesoscopic PSCs based on (FAPbI3)0.85(MAPbBr3)0.15 light absorber and CuPc-DMP (60) HTM doped with LiTFSI and tBP, achieving PCEs of up to 17.1%.117
Tetrafluorotetracyanoquinodimethane (F4-TCNQ) as a p-type doping material has attracted significant attention because of its strong electron affinity.118–121 The prerequisite for molecular doping is that the electron affinity of the dopant is in the range of the ionization energy of the HTM. The extra mobile holes induced by the p-type dopant boost the charge carrier density in the HTM, leading to higher conductivity.45 Therefore, the selection of a Pc with a suitable HOMO value matching the LUMO of F4-TCNQ is feasible to realize a charge–transfer reaction between Pc and the dopant. Liao's group employed TS-CuPc doped with F4-TCNQ in planar PSCs with normal and inverted architectures.122 The addition of 2.5 wt% of F4-TCNQ to the Pc material considerably enhanced the hole mobility of the thin film from 2.3 × 10−4 to 9.2 × 10−3 cm2 V−1 s−1. In addition, an aqueous solution of TS-CuPc (61) with F4-TCNQ showed a pH value of 7.4, which favored the long-term stability of the devices. The use of the doped TS-CuPc as the HTM in an inverted PSC led to a PCE of 16.14% compared to the reference device based on PEDOT:PSS, with the highest efficiency of 13.22%. TS-CuPc:F4-TCNQ was also used as a hole modification layer between spiro-OMeTAD as the HTM and Ag electrode in n–i–p devices, which increased the efficiency of the PSCs from 16.23% to 20.16%. F4-TCNQ was also employed by Sun's research group as a dopant for non-peripherally substituted tetra-4-(bis(4-tert-butyl)phenyl)amino)phenoxy copper phthalocyanine (CuPc-OTPAtBu, 62) HTM in PSCs.123 As a well-known electron donor, four triphenylamine (TPA) groups were introduced in the CuPc ring via oxygen atoms. The attached tert-butyl chains also enhanced the solubility of the molecularly engineered HTM in common organic solvents and improved the hydrophobicity of its thin film. When 6 wt% of dopant was added to the HTM, the bulk conductivity of the HTM significantly increased, leading to an efficiency of 15.0% for the mesoscopic devices, which is comparable to the reference devices based on spiro-OMeTAD doped with LiTFSI and tBP(highest PCE of 15.3%).
Linking two or more Pc compounds can expand their π delocalization system and enhance their charge mobility. Although an expanded 2D planar structure for Pc materials can increase the risk of achieving rough and thick films with low coverage due to the formation of extensive crystalline domains, well-designed 3D structures can deliver more uniform films given that they have less intense intermolecular interactions. In 2022, three ZnPc dimers were developed as HTMs in perovskite devices.124 In their structures, three different linkers, i.e., diketopyrrolopyrrole (DPP) (ZnPc 1 (63)), two contiguous triple bonds (ZnPc 2 (64)), and spirobifluorene (ZnPc 3 (65)), were used to endow the designed HTMs with a wider range of electronic and structural properties. The PC dimers were doped with tBP and LiTFSI and employed in n–i–p-structured PSCs. The PCE of the devices using ZnPc 1, ZnPc 2 and ZnPc 3 were 16.49%, 12.82% and 18.32%, respectively, compared to the reference device based on doped spiro-OMeTAD with the PCE of 17.42%. The lower performance of ZnPc 1 and ZnPc 2 was attributed to their lower solubility or higher molecular aggregation. The photovoltaic properties of some selected perovskite devices using doped Pc compounds as the HTM layer are summarized in Table 3.108–117,122–124
| Chemical structure no. | Device architecture | HTM | Hole mobility of HTM (cm2 V−1 s−1) | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Refers to the device with double cation perovskite and. b Refers to the device with triple cation perovskite. | ||||||||
| 42 | Mesoscopic | TT80 | 0.80 | 16.35 | 50.3 | 6.7 | 108 | |
| 43 | Mesoscopic | BI25 | 1.014 | 16.67 | 68.1 | 11.75 | 109 | |
| 44 | Mesoscopic | BL07 | 1.001 | 10.69 | 59.8 | 6.65 | 109 | |
| 45 | Mesoscopic | BL08 | 1.034 | 17.43 | 61.0 | 11.44 | 109 | |
| 46 | Mesoscopic | BL25 | 0.69 | 20.07 | 35.7 | 4.93 | 110 | |
| 47 | Mesoscopic | BL38 | 0.89 | 21.46 | 56.8 | 10.89 | 110 | |
| 48 | Mesoscopic | BL40 | 1.07 | 22.92 | 73.5 | 18.10 | 110 | |
| 49 | Mesoscopic | BL50 | 1.06 | 22.52 | 74.2 | 17.81 | 110 | |
| 50 | Mesoscopic | BL51 | 1.04 | 22.58 | 69.4 | 16.25 | 110 | |
| 51 | Mesoscopic | BL52 | 1.04 | 22.54 | 70.3 | 16.46 | 110 | |
| 52 | Mesoscopic | Zn-BL54 | 1.07 | 23.87 | 79.0 | 20.18a | 111 | |
| 52 | Mesoscopic | Zn-BL54 | 1.081 | 23.73 | 78.0 | 20.00b | 111 | |
| 53 | Mesoscopic | (tOctPhO)8CuPc | 0.87 | 19.01 | 50.56 | 8.33 | 112 | |
| 54 | Mesoscopic | (tOctPhO)8ZnPc | 0.89 | 17.52 | 46.52 | 7.25 | 112 | |
| 55 | Mesoscopic | TPA-ZnPc | 0.95 | 20 | 72 | 13.65 | 113 | |
| 56 | Mesoscopic | NiPc–(OBu)8 | 1.070 | 23.0 | 72.8 | 17.9 | 114 | |
| 56 | Mesoscopic | NiPc–(OBu)8 | 1.90 × 10−4 | 0.895 | 18.5 | 63.8 | 10.6 | 114 |
| 56 | Mesoscopic | NiPc–(OBu)8/V2O5 | 1.080 | 23.1 | 73.4 | 18.3 | 114 | |
| 57 | Mesoscopic | Sym-HTPcH | 0.988 | 17.2 | 74 | 12.8 | 115 | |
| 58 | Mesoscopic | HBT-ZnPc | 1.10 | 20.16 | 69.4 | 15.5 | 116 | |
| 59 | Mesoscopic | HT-ZnPc | 1.05 | 20.28 | 80.3 | 17.1 | 116 | |
| 60 | Mesoscopic | CuPc-DMP | 1.04 | 23.2 | 71 | 17.1 | 117 | |
| 61 | Planar n–i–p | spiro-OMeTAD/ TS-CuPc:F4-TCNQ | 9.2 × 10−3 | 1.12 | 24.32 | 74 | 20.16 | 125 |
| 61 | Planar p–i–n | TS-CuPc:F4-TCNQ | 9.2 × 10−3 | 0.96 | 21.71 | 77 | 16.14 | 125 |
| 62 | Mesoscopic | CuPc-OTPAtBu:F4-TCNQ | 1.01 | 21.9 | 68 | 15.0 | 123 | |
| 63 | Mesoscopic | ZnPc 1 | 2.83 × 10−5 | 1.03 | 23.21 | 68.9 | 16.49 | 124 |
| 64 | Mesoscopic | ZnPc 2 | 1.50 × 10−5 | 1.04 | 22.25 | 55.3 | 12.82 | 124 |
| 65 | Mesoscopic | ZnPc 3 | 4.08 × 10−5 | 1.04 | 23.63 | 74.6 | 18.32 | 124 |
2.3. Phthalocyanines as hole modification layer in PSCs
Dai's group introduced the insertion of a ZnPc hydrophobic hole modification layer between the light harvesting layer and doped spiro-OMeTAD to address the instability issue of PSCs.69 The Bilayer HTM-based PSC with Nb-doped TiOx as the ETM exhibited an improved PCE of 16.8% compared to 15.1% for the unmodified HTM devices. With the introduction of the ZnPc modification layer, the series resistance (Rs) slightly increased, while the interface resistance of the HTM/absorber (RHTM) and the total resistance (Rs + RHTM) showed the opposite trend, indicating that the addition of the interfacial buffer layer reduced the charge recombination and resulted in lower resistive voltage loss, current loss and higher PCE. Because of the hydrophobic properties of the ZnPc, the modified devices retained 91% of the initial PCE after 2400 h in an ambient environment without encapsulation. In 2022, our research group employed two thiophene-functionalized metal-free Pc isomers, Pc S2 (66) and Pc S3 (67), as the passivating agent for the MAPbI3 perovskite in an n–i–p-structured device.52 The Pc materials were added to the perovskite layer by dissolving in an anti-solvent, allowing them to impact the perovskite crystal growth mechanism. Using DFT calculations, the interaction between the S atoms in the structure of the developed Pc compounds and the under-coordinated Pb2+ was predicted. The Pc molecules not only passivate the bulk defect, but also form a rigid thin film layer on the perovskite surface for surface defect passivation. The introduction of the Pc materials in the perovskite layer helped improve the grain size by slowing down the crystallization step during the formation of the thin film and drying process. However, the S atoms were located at different positions in the two Pc materials, resulting in different affinities toward the perovskite material. This led to a higher quality for the Pc S3-added perovskite film and an efficiency as high as 18% for the fabricated PSC based on the same material. The maximum PCE obtained for the PSCs based on MAPbI3 without Pc and Pc S2-added perovskite was 16.88% and 16.64%, respectively. Additionally, the passivated devices showed a significant improvement in their moisture and thermal stability.2.4. Phthalocyanines as additives in HTMs
The introduction of a p-type dopant in the HTM is an effective method to strengthen the properties of the composites, for instance, enhancement in conductivity and reduction in ohmic losses in the hole-selective layers and injection barriers at the interface with the electrodes, which can be achieved by controlling the energy levels of the dopants or HTMs.126 The composite HTM, accompanied by increased charge carrier density and higher conductivity, facilitated the achievement of a high PCE.Sang Il Seok and coworkers reported the use of tert-butyl-CuPC (CuPC) used as an additive in the HTM layer of po-spiro-OMeTAD (po-spiro) in a PSC based on an (FAPbI3)0.85(MAPbBr3)0.15 absorbing layer.127 This optimized “bilayer” platform achieved a high FF of 0.747, VOC of 1.11 V and high PCE of 18.5%, whereas the single HTM-based po-spiro and CuPC showed a modest FF of 0.718 and 0.663, VOC of 1.09 V and 1.045 V, and PCE of 17.5% and 15.2%, respectively. The improvement in the VOC and FF values was attributed to the incorporation of CuPC in po-spiro, preventing the unwanted electrons from crossing the energy barrier of po-spiro. Transient photovoltage decay (TPD) measurements showed that the recombination lifetime of the device with CuPC-doped po-spiro was higher than that of the device with single po-spiro HTM, suggesting that the introduction of CuPC as a dopant in the po-spiro HTM layer could retard charge recombination, and then result in an enhancement in FF and VOC.
Lianos’ group and our group used n-butyl tetra-substituted CuPc (CuBuPc, 6) as an additive in spiro-OMeTAD in a mesoscopic perovskite device and achieved an impressive improvement in the PCE values of the PSCs.128 This was associated with the creation of deep traps in the hole transportation and better blocking of the back-travelling electrons due to the low-lying LUMO level of CuBuPc compared to that of spiro-OMeTAD. It was also demonstrated that the presence of the Pc materials could enhance the thin film quality of spiro-OMeTAD by decreasing the size and number of pinhole defects. Accordingly, with the decreased shunt paths, the FF and VOC of the cells increased, leading to a PSC with PCE of 15.4% for the devices based on spiro-OMeTAD with 10% CuBuPc as the HTM. The reference device showed an efficiency of 10.4%. Liao's group developed a solution-processed composite HTM consisting of PEDOT:PSS and copper phthalocyanine-3,4′,4′′,4′′′-tetrasulfonated acid tetrasodium salt (TS−CuPc, 61) and investigated its performance in PSCs with an inverted p–i–n structure.129 The results suggested an improved cell performance and stability due to the incorporation of TS-CuPc in PEDOT:PSS. The optimized composite film of TS-CuPc-doped PEDOT:PSS (50 wt%) showed better film uniformity and lower roughness compared with the pristine PEDOT:PSS and TS-CuPc thin films. The composite HTM also offered better energy alignment for efficient hole transport and extraction. In addition, the perovskite film fabricated on the composite HTM layer offered higher crystallinity with a larger grain size, together with better film coverage and enhanced carrier mobility. Finally, PSCs with a PCE of 17.29% were achieved, while the control device using pristine PEDOT:PSS exhibited an efficiency of 13.29%. In 2018, our group studied composites based on non-peripheral octamethyl-substituted CuPc nanowire (N-CuMe2Pc, 68) and P3HT and employed them as HTMs in PSCs, reaching PCEs of up to 16.61% for the optimized weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.130 The composite thin films with this optimized ratio showed both the best film quality and the highest hole mobility, resulting in a good performance. Achieving a high charge carrier mobility for P3HT requires precise control of the morphology and crystallinity of its thin film. The hole mobility of P3HT can be enhanced with optimized π–π stacking of the polymer side chain. Peng and coworkers produced a highly crystalline P3HT thin film by blending it with CuPc and employing a post-deposition solvent-annealing step with chlorobenzene.131 According to the XRD results, a significant improvement in crystallinity and conductivity of the thin film occurred when the solvent annealing treatment was applied, and a further enhancement was observed by blending P3HT with CuPc. The devices based on the developed solution-based composite HTM achieved a high efficiency of 21.6% for an active area of 1 cm.2 The use of the hydrophobic and thermally stable P3HT:CuPc composite HTM could also increase the stability of the PSC devices against light, moisture and heat. The encapsulated devices could retain 91.7% of their initial performance after 1000 h of exposure to damp heat and high humidity (Fig. 10).
1.130 The composite thin films with this optimized ratio showed both the best film quality and the highest hole mobility, resulting in a good performance. Achieving a high charge carrier mobility for P3HT requires precise control of the morphology and crystallinity of its thin film. The hole mobility of P3HT can be enhanced with optimized π–π stacking of the polymer side chain. Peng and coworkers produced a highly crystalline P3HT thin film by blending it with CuPc and employing a post-deposition solvent-annealing step with chlorobenzene.131 According to the XRD results, a significant improvement in crystallinity and conductivity of the thin film occurred when the solvent annealing treatment was applied, and a further enhancement was observed by blending P3HT with CuPc. The devices based on the developed solution-based composite HTM achieved a high efficiency of 21.6% for an active area of 1 cm.2 The use of the hydrophobic and thermally stable P3HT:CuPc composite HTM could also increase the stability of the PSC devices against light, moisture and heat. The encapsulated devices could retain 91.7% of their initial performance after 1000 h of exposure to damp heat and high humidity (Fig. 10).
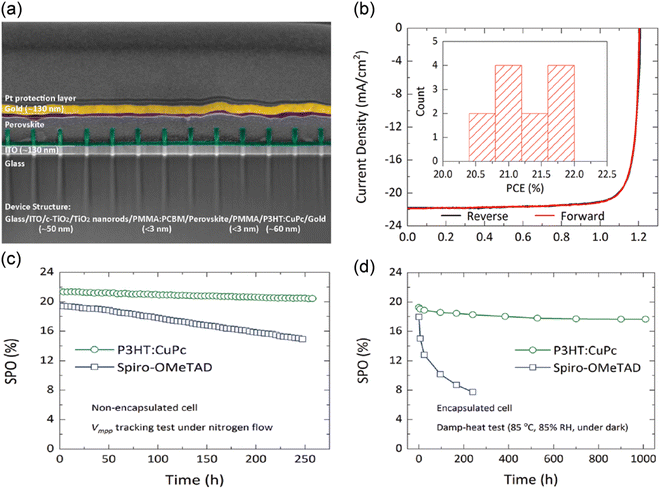 | ||
| Fig. 10 (a) Cross-sectional SEM image, (b) J–V performance and (c) SPO of nonencapsulated cells based on P3HT:CuPc and spiro-OMeTAD HTL measured by Vmpp tracking under continuous 1 Sun illumination intensity. (d) SPO of encapsulated cells based on P3HT:CuPc and Spiro-OMeTAD HTL. Reproduced with permission.131 Copyright 2021, Science. | ||
NiPcS4 (69), containing four sulphonate sodium salt as peripherally attached substituents, has been used as either a dopant-free HTM or a dopant in PEDOT:PSS, and applied in inverted PSCs.132 The addition of 10 wt% of NiPcS4 to PEDOT:PSS led to PSCs with a PCE of up to 18.90%, which was significantly higher than that for the PSCs based on other components, i.e., 16.47% and 9.29% for PEDOT:PSS and NiPcS4, respectively. The observed improvement in the performance of PSCs was attributed to factors such as enhanced photon absorption, improved film quality of perovskite, enhanced work function of HTM and its hole transporting ability, and increased cell stability. The photovoltaic properties of some selected perovskite devices using Pc compounds as additives in the HTM layer are summarized in Table 4.52,127–130,132 The molecular structures of the Pc materials used as additives in HTMs or perovskite layers in PSCs are shown in Fig. 9.
| Chemical structures no. | Device architecture | HTM | Additive | Hole mobility of HTM (cm2 V−1 s−1) | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 66 | Planar n–i–p | spiro-OMeTAD | Zn S2 | 1.012 | 22.57 | 71.45 | 16.64 | 52 | |
| 67 | Planar n–i–p | spiro-OMeTAD | Zn S3 | 1.033 | 22.75 | 76.55 | 18.01 | 52 | |
| 6 | Mesoscopic | spiro-OMeTAD | n-BuPc | 0.97 | 24.9 | 64 | 15.4 | 128 | |
| 71 | Planar p–i–n | PEDOT:PSS | TS-CuPc | 1.01 | 22.23 | 77 | 17.29 | 129 | |
| 68 | Planar n–i–p | P3HT | N-CuMe2Pc | 3.84 × 10−3 | 1.008 | 22.44 | 73.43 | 16.61 | 130 |
| 69 | Planar p–i–n | PEDOT:PSS | NiPcS4 | 1.08 | 23.01 | 77 | 18.90 | 132 | |
| 5 | Mesoscopic | po-spiro-OMeTAD | t-BuPc | 1.11 | 22.3 | 74.7 | 18.5 | 127 | |
| Mesoscopic | spiro-OMeTAD | CuPc | 0.88 | 15.30 | 61.7 | 8.4 | 139 | ||
| 5 | Planar n–i–p | spiro-OMeTAD | t-BuCuPc | 1.02 | 22.6 | 75 | 17.3 | 141 | |
| Planar n–i–p | spiro-OMeTAD | NiPc | 1.10 | 23.29 | 74.78 | 19.18 | 140 | ||
| 70 | Planar p–i–n | PTAA | HCuPc | 1.032 | 25.57 | 81.05 | 21.39 | 142 | |
| 71 | Mesoscopic | cobalt porphyrin | tetra-NH2-ZnPc | 1.11 | 23.55 | 77.28 | 20.3 | 144 | |
| Mesoscopic | spiro-OMeTAD | CuPc | 1.112 | 22.7 | 78 | 19.7 | 143 | ||
| 72 | Mesoscopic | spiro-OMeTAD | F16CuPc | 1.145 | 22.9 | 77 | 20.2 | 143 | |
| 73 | Mesoscopic | spiro-OMeTAD | FeTAP | 1.11 | 23.78 | 73.26 | 18.81 | 146 | |
| 74 | Planar n–i–p | spiro-OMeTAD | 8TPAEPC | 1.123 | 24.0 | 82.00 | 22.10 | 147 | |
| 75 | Planar n–i–p | spiro-OMeTAD | NP-SC6-ZnPc | 1.09 | 23.18 | 71.4 | 18.04 | 145 | |
| 41 | Planar n–i–p | spiro-OMeTAD | NP-SC6-TiOPc | 1.12 | 23.27 | 74.4 | 19.39 | 145 |
2.5. Pc complexes using as additives in perovskite layer
In the process of fabricating polycrystalline organometal halide perovskite thin films or operation of PSCs, defects are inevitably generated in the films. These defects can be categorized as 1D point defects (vacancies, interstitial and anti-site atoms), 2D defects consisting of grain boundaries and abundant surface defects and 3D bulk crystal defects such as lead clusters.133 These intrinsic defects, such as halide and cation vacancies, under-coordinated halide anions, lead cations, lead clusters and lead–halide antisite defects exist at the grain boundaries and surfaces, resulting in trap states, which cause non-radiative recombination of the photo-induced carriers and undesirably lower the photovoltaic parameters, and thus PCE. The hysteresis effect in the J–V curves of PSCs can also be attributed to these defects. Moreover, the instability issue of PSCs is associated with the defective grain boundaries because heat and moisture degrade the film from initiating at the surface of the film. Then, the grain boundaries filled with defects finally diffuse into the bulk perovskite.134 The charged defects also migrate through the grain boundaries under an electrical bias.As one of the main approaches to passivate defects in perovskite, diverse additives ranging from organic molecules to salts and polymers have achieved an inspiring improvement in the modification of PSCs. The method for the incorporation of additives in perovskite films can be classified as two types, i.e., the widely adopted introduction of additives in the perovskite precursor solution and dissolving the additive in an anti-solvent such as chlorobenzene, via a one-step dripping process. In the former, the morphology of the perovskite film is affected by the additives given that the dopant is scattered in the precursor solution and the colloidal clusters serve as nucleation centers for the growth and crystallization of the perovskite film.135 In the latter, the additives, in most cases, can remain in the surface of the film, influencing the surface or grain boundaries of perovskite films.136 Therefore, the additive engineering strategy can be utilized for the modulation of the morphology of perovskite films, the dimensionality of the perovskite, the band alignment, the durability of the harvesting layer and passivation of the defects, or realization of the combination of all of them.137,138 Many compounds as additives employed in PSC have been reported. However, the introduction of Pc as an additive in perovskite is rare to date and its application is in its infancy.
Unsubstituted CuPc has been used as an additive in perovskite or an interlayer to modify the surface and tailor the interfacial structure in PSCs. Behjat et al. added different amounts of CuPc to the perovskite material in PSCs and achieved higher JSC values for the devices using 0.05 wt% of CuPc in CH3NH3PbI3.139 It has been suggested that CuPc can improve the quality of the perovskite thin film and enhance its light absorption ability. The addition of CuPc to the perovskite increased the PCE of the device by 2% compared to the reference device with no CuPc in the perovskite layer. Also, spiro-OMeTAD was used as the HTM in this study, and the highest efficiency of 8.4% was achieved for the PSCs. Jin et al. improved the PCE of their spiro-OMeTAD-based PSCs from 17.4% to 19.18% by introducing NiPc as an additive in the perovskite layer.140 To achieve this goal, NiPc was added to the anti-solvent chlorobenzene, and subsequently infiltrated in the perovskite surface, which improved the crystal quality of the perovskite film and slightly reduce the defects on its surface, enhancing its interface with the HTM layer. The developed method effectively reduced the recombination rate and enhanced the charge extraction and transfer efficiency, leading to a higher performance for the PSCs. Peng et al. used a very low concentration (4.4 × 10−3 mM) of t-BuCuPc as an additive in perovskite materials in a planar PSC with a TiO2 thin layer as the ETM and spiro-OMeTAD as the HTM, improving the performance of the solar cell from 15.3% to 17.3%.141 It was found that t-BuCuPc could enhance the quality of the perovskite layer with higher crystallinity and surface coverage. Dong et al. synthesized a hyperbranched CuPc (HCuPc) (70) as an additive to FAPbI3 perovskite.142 The developed Pc molecules could encapsulate the grain boundaries at the molecular level through their twisted Pc units and improve the phase stability of the FAPbI3 perovskite against humidity, as well as the charge extraction and transport. The p–i–n devices using HCuPc additive could achieve the remarkable efficiency of 21.39%. The photovoltaic properties of some selected perovskite devices using Pc compounds as additives in the perovskite layer are summarized in Table 4.139–147
Tang and coworkers explored the impact of post-treating the MAPbI3 perovskite layer using tetra-ammonium-substituted ZnPc on the device performance.144 The two-dimensional (ZnPc)0.5MAn−1PbnI3n+1 was constructed in the grain boundaries of the MAPbI3 film by immersing the as-fabricated perovskite layer in an isopropanol solution of the ZnPc derivative (71), resulting in the successful passivation of defects and reduced trap-assisted recombination. The modified PSCs with doped spiro-OMeTAD HTM showed improved PCEs of up to 20.3% compared with 19.01% for the reference devices, and long-term stability against humidity and heating, with self-repairing capability under mild heating. The use of the spin-coating method for the deposition of the two-dimensional (ZnPc)0.5MAn−1PbnI3n+1 layer led to an efficiency of 19.6% for the modified PSC.148
Unsubstituted CuPc and its fluorinated derivative F16CuPc (72) were utilized as additives in the perovskite films reported by Ma and coworkers in 2018.143 The introduction of a dipole layer not only aligned the interfacial energy levels but also filled the grain boundaries and passivated the perovskite surface, which led to significantly suppressed charge recombination. The Pc-treated PSCs showed an improved PCE, where the best PCE was 20.2%, VOC was 1.145 V, JSC was 22.9 mA cm−2, and FF was 0.77 for F16CuPc, and a PCE of 19.7% coupled with VOC of 1.112 V, JSC of 22.7 mA cm−2 and FF of 0.78 for CuPc. In comparison with the pristine device, the improvement in the PCE of the modified devices is mainly attributed to the increase in the VOC, stemming from the reduced interfacial recombination by the introduction of CuPc and F16CuPc in the perovskite. Furthermore, treatment with F16CuPc and CuPc could also preserve the perovskite layer from moisture penetration to some extent. Hence, the PCE of the modified devices sacrificed 5% (F16CuPc) and ∼10% (CuPc) after 1000 h storage under atmosphere conditions without any sealing, while the control devices retained around 80% of their initial PCE. In a recent study, CuPc and F16CuPc were studied as hole and electron transport layers in an MAPI3-based PSC device, respectively, through DFT calculations and compared to the state-of-art charge transport materials TiO2 and spiro-OMeTAD, suggesting that better efficiency can be achieved for the Pc-based device.149 The device simulation study showed that an FF of 79.01% and PCE of 22.30% can be expected for this PSC device. In 2022, iron(II)-2,9,16,23-tetraamino-phthalocyanine (FeTAP) (73) was used as an additive inside a mixed-halide perovskite layer to passivate the defects at the surface and grain boundaries through the formation of strong bonds between the unsaturated Pb2+ ions and N–H moieties.146 This allowed the adjacent crystal grains to be crosslinked in the perovskite film, resulting in a high-quality thin film with improved charge extraction in the device. This could additionally enhance the device stability by inhibiting the perovskite film degradation process. The best-performing FeTAP-modified device achieved the PCE of 18.81%. 8TPAEPC (74), a CuPc compound with eight triphenylamine groups grafted into the peripheral side of Pc, was used as the dopant for a mixed-ion perovskite film and enhanced the PCE of the perovskite devices from 20% to 22.10%.147 As a narrow band gap additive, 8TPAEPC could extend the NIR photo-response of the perovskite film from 760 to 850 nm, which consequently enhanced the JSC value of the device from 22.8 to 24 mA cm−2. It was indicated that there are electrostatic interactions between 8TPAEPC and perovskite molecules, which assisted the crystal growth of the perovskite, leading to more packed crystal grains and smoother surface in the final film. Interestingly, using the p-type 8TPAEPC as a dopant-free HTM, PSCs with a PCE of 20.42% were achieved.
In a study conducted by our research group, two types of Pc derivatives, NP-SC6-ZnPc (75) and NP-SC6-TiOPc (41), could successfully passivate the defects in the perovskite layer (Fig. 11).150 The lone electron pairs on the S, N and O atoms in the Pc molecular structures provide the chance to form a coordination bond with under-coordinated Pb2+ in CH3NH3PbI3 together with passivating Pb–I antisite defects, which was verified by theoretical calculation and X-ray photoelectron spectroscopy (XPS) analysis. Using DFT calculations, the absorption behaviors of the Pc molecules (NP-SC6-ZnPc and NP-SC6-TiOPc) were investigated on the MAPbI3 (001) surface. The computational results confirmed that both Pc molecules prefer to absorb on the Pb–I2-terminated (001) surface, with absorption energies of −3.36 and −3.61 eV for NP-SC6-ZnPc and NP-SC6-TiOPc, respectively, which indicates the efficient absorption of the developed Pc molecules on the surface of MAPbI3. Moreover, an increase in the absorption energies was observed when the NP-SC6-ZnPc and NP-SC6-TiOPc molecules point to the I atoms in MAPbI3. It was also demonstrated that the formation of the strong O-Pb bond with a bond length of 2.34 Å between NP-SC6-TiOPc and MAPbI3 molecules may play a crucial role in the absorption of this molecule on the perovskite surface. The relaxed structures of MAPbI3/NP-SC6-ZnPc and MAPbI3/NP-SC6-TiOPc are displayed in Fig. 11. The SEM study showed that Pc passivation affected the perovskite growth and assisted the formation of more condensed, continuous and uniform perovskite films with larger grain sizes. The PSCs fabricated with Pc-decorated CH3NH3PbI3 as the light harvesting layer showed a champion PCE of up to 19.39% and 18.04% for the NP-SC6-TiOPc- and NP-SC6-ZnPc-based devices, respectively, in comparison with the additive-free modified devices (17.67%). The presence of an oxygen atom coordinated to the axial position of the metal center in NP-SC6-TiOPc enabled the easier formation of coordination bonds with the under-coordinated Pb2+ and better passivation effect compared with the NP-SC6-ZnPc counterpart. Furthermore, the resultant high-quality perovskite film with reduced traps and hydrophobic Pc displayed good durability both in the moisture and thermal test. Employing a thin passivation layer of the hydrophobic Pc materials on the top of the perovskite film can offer effective protection against humidity. Exposing the bare perovskite film to a high moisture level led to a color change from black to yellow after 60 s. Simultaneously, the MAPbI3/NP-SC6-TiOPc film showed significantly higher resistance to high humidity. Furthermore, both passivated devices showed high stability against elevated temperature. Although the control PSC lost 67% of its initial performance after increasing the temperature to 200 °C, the champion PSC with the perovskite film passivated by NP-SC6-TiOPc could maintain over 80% of its initial efficiency.
 | ||
| Fig. 11 (a) Top and (b) side views of MAPbI3/NP-SC6-ZnPc. (c) Top and (d) side views of MAPbI3/NP-SC6-TiOPc for relaxed structures by DFT calculation. Reproduced with permission.150 Copyright 2021, Wiley. | ||
3. Conclusion and prospects
Perovskite solar cells have experienced astounding progress in their efficiency in the last 10 years, almost approaching the Shockley–Queisser theoretical efficiency. However, their instability and the adaption of industrial-scale fabrication techniques are the two main factors hindering their commercialization. Several factors, such as thermal treatment, light illumination, and humidity, affect the stability of the devices, among which moisture is verified to be one of the leading causes of the degradation of perovskites. Li-salt-doped spiro-OMeTAD as the HTM in PSCs has achieved great success. However, the hydrophilic property of the additive induces moisture to invade the perovskite films, leading to their decomposition. Thus, to address the instability problem, numerous novel semiconducting materials have been explored to improve the stability of PSCs, while keeping the PCEs of the devices high. Among them, phthalocyanine compounds are the most promising candidates, owing to their excellent carrier mobility, chemical/physical stability, low cost, suitable energy levels, etc. However, to match the industrial-scale film-coating techniques, the fabrication of thin and dense organic layers, especially small molecule-based layers, is rarely reported, which can be an upcoming research direction.In this review, the applications of Pc materials were summarized in categories including Pcs employed in perovskite layer, functioning as passivate agents or pinhole/crack filler, and HTM layers, interfacial films and dopants in HTMs. The ultimate challenge for Pc-based PSCs is to achieve a high PCE comparable to the widely used spiro-OMeTAD, as well as a long operation time in the ambient environment. Thus, to achieve this goal, novel Pcs with the following properties (Fig. 12) should be considered to minimize the defects in perovskites or on their surface, enhance their interfacial contact with the active layer and inhibit the incursion of moisture.
(1) Low cost: newly developed materials should be cheaper with regard to both material price and production cost than the commonly used spiro-OMeTAD for their potential mass industrialization.
(2) Introducing heteroatoms or groups in the periphery of the Pc ring, as well as the axial ligands, which can act as Lewis acids/bases and passivate the perovskite defects; peripheral groups containing heteroatoms of N, O and S as well as some Lewis-base groups such as carbonyl can favor efficient hole transport and interface passivation; besides, axial ligands connected with the central metal in the Pc core can also provide the site for perovskite passivation.
(3) Good hydrophobicity: as an HTM in the device with n–i–p structure, Pc should possess both high mobility and good hydrophobicity given that it functions as not only a hole extraction and transport layer but also a capping layer to protect the perovskite film from moisture ingress. Alternatively, as an additive in perovskite for the passivation of the perovskite film or filling the cracks, moderate hydrophobicity is desirable because high hydrophobicity may cause the wetting issue in upper HTMs.
(4) Amorphous morphology: Pc films with high crystallization generally offer higher mobility but at the expense of the thin film quality. Consequently, the low film quality can have a negative effect on the charge transfer and device performance. Therefore, an amorphous film with smooth and pinhole-free morphology is a prerequisite for realizing a high performance in PSCs. Besides, a dense film also inhibits moisture flow from the ambient conditions. Regarding the solution-based deposition techniques for the fabrication of Pc films, the size and nature, and attached position of the substituents around the Pc ring can notably manipulate the solid-state interactions between Pc molecules, and therefore, should be selected wisely to balance the π–π interaction level and the spatial hinderance to form a thin film with sufficient crystallinity and good film quality with smooth surface morphology.
(5) High mobility and conductivity: the modification of peripheral substituents can alter optoelectronic and electrochemical properties of Pc, while this modulation may weaken the degree of aggregation of the Pc, which is not beneficial to achieve a high PCE in PSCs given that high conductivity and hole mobility are required in the device. In this regard, some structural engineering in Pcs should be conducted under the guidance of computational simulations, realizing π–π stacking interactions as well as high conductivities.
(6) Finally, a suitable energy level and resistance to thermal, optical, chemical, and environmental stresses.
In summary, newly designed Pcs should be closely coordinated with perovskite passivation and improved hydrophobicity for elevating the PCE and durability of PSCs. This review presented a summary of the recent progress and insights into the future development of Pc-based HTMs, and we anticipate that it can offer new insight for developing novel preeminent Pc-based semiconducting materials to further enhance the efficiency and stability of PSCs.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
E. R., and D. K. contributed equally to this work. The work at the Southern University of Science and Technology was supported by the National Science Foundation of China (No. 21975116) and General Program of Basic Research in Shenzhen (JCYJ20220530112801004).References
- X. Li, W. Zhang, X. Guo, C. Lu, J. Wei and J. Fang, Constructing heterojunctions by surface sulfidation for efficient inverted perovskite solar cells, Science, 2022, 375, 434–437 CrossRef CAS PubMed
.
- N.-G. Park and K. Zhu, Scalable fabrication and coating methods for perovskite solar cells and solar modules, Nat. Rev. Mater., 2020, 5, 333–350 CrossRef CAS
.
- L. Duan and A. Uddin, Defects and stability of perovskite solar cells: a critical analysis, Mater. Chem. Front., 2022, 6, 400–417 RSC
.
- P. Zhao, B. J. Kim and H. S. Jung, Passivation in perovskite solar cells: a review, Mater. Today Energy, 2018, 7, 267–286 CrossRef
.
- V. M. Le Corre, M. Stolterfoht, L. Perdigón Toro, M. Feuerstein, C. Wolff, L. Gil-Escrig, H. J. Bolink, D. Neher and L. J. A. Koster, Charge Transport Layers Limiting the Efficiency of Perovskite Solar Cells: How To Optimize Conductivity, Doping, and Thickness, ACS Appl. Energy Mater., 2019, 2, 6280–6287 CrossRef CAS
.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Compositional engineering of perovskite materials for high-performance solar cells, Nature, 2015, 517, 476–480 CrossRef CAS PubMed
.
- Z. Zhou, S. Pang, Z. Liu, H. Xu and G. Cui, Interface engineering for high-performance perovskite hybrid solar cells, J. Mater. Chem. A, 2015, 3, 19205–19217 RSC
.
- F. Wang, S. Bai, W. Tress, A. Hagfeldt and F. Gao, Defects engineering for high-performance perovskite solar cells, npj Flexible Electron., 2018, 2, 22 CrossRef
.
- D. Khan, X. Liu, G. Qu, A. R. Nath, P. Xie and Z.-X. Xu, Nexuses Between the Chemical Design and Performance of Small Molecule Dopant-Free Hole Transporting Materials in Perovskite Solar Cells, Small, 2023, 2205926 Search PubMed
.
- A. A. Said, J. Xie and Q. Zhang, Recent Progress in Organic Electron Transport Materials in Inverted Perovskite Solar Cells, Small, 2019, 15, 1900854 CrossRef PubMed
.
- P. Murugan, T. Hu, X. Hu and Y. Chen, Advancements in organic small molecule hole-transporting materials for perovskite solar cells: past and future, J. Mater. Chem. A, 2022, 10, 5044–5081 RSC
.
-
K. Akaike and K. Kanai, in Physics and Chemistry of Carbon-Based Materials: Basics and Applications, ed. Y. Kubozono, Springer Singapore, Singapore, 2019 DOI:10.1007/978-981-13-3417-7_10, pp. 293–332
.
- H. Lu and N. Kobayashi, Optically active porphyrin and phthalocyanine systems, Chem. Rev., 2016, 116, 6184–6261 CrossRef CAS PubMed
.
- O. A. Melville, B. H. Lessard and T. P. Bender, Phthalocyanine-based organic thin-film transistors: a review of recent advances, ACS Appl. Mater. Interfaces, 2015, 7, 13105–13118 CrossRef CAS PubMed
.
-
R. Guilard, K. M. Kadish, K. M. Smith and R. Guilard, The porphyrin handbook, Academic Press, New York, 2003 Search PubMed
.
-
A. B. P. Lever and C. C. Leznoff, Phthalocyanines: properties and applications, VCH, New York, 1996, pp. 1989–c1996 Search PubMed
.
- O. Inganas, Organic Photovoltaics over Three Decades, Adv. Mater., 2018, 30, 26 CrossRef PubMed
.
- H. T. Chandran, T. Liu, D. Shen, Z. Guan, M. Li, J. Antonio Zapien, S.-W. Tsang, M.-F. Lo and C.-S. Lee, A Family of Small Molecular Materials Enabling Consistently Lower Recombination Losses in Organic Photovoltaic Devices, Sol. RRL, 2020, 4, 202000245 Search PubMed
.
- M. Urbani, M. E. Ragoussi, M. K. Nazeeruddin and T. Torres, Phthalocyanines for dye-sensitized solar cells, Coord. Chem. Rev., 2019, 381, 1–64 CrossRef CAS
.
- I. Mfouo-Tynga and H. Abrahamse, Cell Death Pathways and Phthalocyanine as an Efficient Agent for Photodynamic Cancer Therapy, Int. J. Mol. Sci., 2015, 16, 10228–10241 CrossRef CAS PubMed
.
- C. Rodriguez-Seco, L. Cabau, A. Vidal-Ferran and E. Palomares, Advances in the Synthesis of Small Molecules as Hole Transport Materials for Lead Halide Perovskite Solar Cells, Acc. Chem. Res., 2018, 51, 869–880 CrossRef CAS PubMed
.
- G. de la Torre, C. G. Claessens and T. Torres, Phthalocyanines: old dyes, new materials. Putting color in nanotechnology, Chem. Commun., 2007, 2000–2015, 10.1039/B614234F
.
- G. Bottari, G. D. La Torre, D. M. Guldi and T. Torres, Covalent and noncovalent phthalocyanine-carbon nanostructure systems: synthesis, photoinduced electron transfer, and application to molecular photovoltaics, Chem. Rev., 2010, 110, 6768–6816 CrossRef CAS PubMed
.
- M. Urdampilleta, S. Klyatskaya, J. Cleuziou, M. Ruben and W. Wernsdorfer, Supramolecular spin valves, Nat. Mater., 2011, 10, 502–506 CrossRef CAS PubMed
.
- M. Abel, S. Clair, O. Ourdjini, M. Mossoyan and L. Porte, Single Layer of Polymeric Fe–Phthalocyanine: An Organometallic Sheet on Metal and Thin Insulating Film, J. Am. Chem. Soc., 2011, 133, 1203–1205 CrossRef CAS PubMed
.
- O. Matsushita, V. M. Derkacheva, A. Muranaka, S. Shimizu, M. Uchiyama, E. A. Lukyanets and N. Kobayashi, Rectangular-Shaped Expanded Phthalocyanines with Two Central Metal Atoms, J. Am. Chem. Soc., 2012, 134, 3411–3418 CrossRef CAS PubMed
.
- H. Wang, D. Qi, Z. Xie, W. Cao, K. Wang, H. Shang and J. Jiang, A sandwich-type phthalocyaninato metal sextuple-decker complex: synthesis and NLO properties, Chem. Commun., 2013, 49, 889–891 RSC
.
- G. Bottari, G. D. La Torre and T. Torres, Phthalocyanine–Nanocarbon Ensembles: From Discrete Molecular and Supramolecular Systems to Hybrid Nanomaterials, Acc. Chem. Res., 2015, 48, 900–910 CrossRef CAS PubMed
.
- A. Braun and J. Tcherniac, The products of the action of acet-anhydride on phthalamide, Ber. Dtsch. Chem. Ges., 1907, 40, 2709–2714 CrossRef CAS
.
- H. Y. Diesbach and W. E. Dec, Quelques sel compleses des o-dinitrilcs avec le cuivreet lapyridine, Helv. Claim., 1927, 10, 886–888 CrossRef
.
- G. T. Byrne, R. P. Linstead and A. R. Lowe, 213. Phthalocyanines. Part II. The preparation of phthalocyanine and some metallic derivatives from o-cyanobenzamide and phthalimide, J. Chem. Soc., 1934, 1017–1022, 10.1039/JR9340001017
.
- C. E. Dent and R. P. Linstead, 215. Phthalocyanines. Part IV. Copper phthalocyanines, J. Chem. Soc., 1934, 1027–1031, 10.1039/JR9340001027
.
- C. E. Dent, R. P. Linstead and A. R. Lowe, 217. Phthalocyanines. Part VI. The structure of the phthalocyanines, J. Chem. Soc., 1934, 1033–1039, 10.1039/JR9340001033
.
- R. P. Linstead and A. R. Lowe, 214. Phthalocyanines. Part III. Preliminary experiments on the preparation of phthalocyanines from phthalonitrile, J. Chem. Soc., 1934, 1022–1027, 10.1039/JR9340001022
.
- R. P. Linstead and A. R. Lowe, 216. Phthalocyanines. Part V. The molecular weight of magnesium phthalocyanine, J. Chem. Soc., 1934, 1031–1033, 10.1039/JR9340001031
.
- J. M. Robertson, 136. An X-ray study of the structure of the phthalocyanines. Part I. The metal-free, nickel, copper, and platinum compounds, J. Chem. Soc., 1935, 615–621, 10.1039/JR9350000615
.
- J. M. Robertson, 255. An X-ray study of the phthalocyanines. Part II. Quantitative structure determination of the metal-free compound, J. Chem. Soc., 1936, 1195–1209, 10.1039/JR9360001195
.
- J. M. Robertson and I. Woodward, 37. An X-ray study of the phthalocyanines. Part III. Quantitative structure determination of nickel phthalocyanine, J. Chem. Soc., 1937, 219–230, 10.1039/JR9370000219
.
- N. Kobayashi, Dimers, trimers and oligomers of phthalocyanines and related compounds, Coord. Chem. Rev., 2002, 227, 129–152 CrossRef CAS
.
- J. De Saja and M. Rodriguez-Mendez, Sensors based on double-decker rare earth phthalocyanines, Adv. Colloid Interface Sci., 2005, 116, 1–11 CrossRef CAS PubMed
.
- Ş. Abdurrahmanoğlu, A. Altındal, M. Bulut and Ö. Bekaroğlu, Synthesis and electrical properties of novel supramolecular octa-phthalocyaninato-dicobalt(II)-hexazinc(II) and dicobalt(II)-dimeric-phthalocyanine with six ferrocenylimin pendant groups, Polyhedron, 2006, 25, 3639–3646 CrossRef
.
- T. Ceyhan, A. Altındal, M. K. Erbil and Ö. Bekaroğlu, Synthesis, characterization, conduction and gas sensing properties of novel multinuclear metallo phthalocyanines (Zn, Co) with alkylthio substituents, Polyhedron, 2006, 25, 737–746 CrossRef CAS
.
-
Ö. Bekaroğlu, Functional Phthalocyanine Molecular Materials, Springer, 2010, pp. 105–136 Search PubMed
.
- Z. Li, F. Gao, Z. Xiao, X. Wu, J. Zuo, Y. J. O. Song and L. Technology, Nonlinear optical properties and excited state dynamics of sandwich-type mixed (phthalocyaninato)(Schiff-base) triple-decker complexes: effect of rare earth atom, Opt. Laser Technol., 2018, 103, 42–47 CrossRef CAS
.
- W. Zhou, Z. Wen and P. Gao, Less is More: Dopant-Free Hole Transporting Materials for High-Efficiency Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 1702512 CrossRef
.
-
J. Jiang, Functional phthalocyanine molecular materials, Springer, 2010 Search PubMed
.
- D. V. Konarev, A. V. Kuzmin, Y. Nakano, M. A. Faraonov, S. S. Khasanov, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Coordination Complexes of Transition Metals (M = Mo, Fe, Rh, and Ru) with Tin(II) Phthalocyanine in Neutral, Monoanionic, and Dianionic States, Inorg. Chem., 2016, 55, 1390–1402 CrossRef CAS PubMed
.
- M.-S. Liao, T. Kar, S. M. Gorun and S. Scheiner, Effects of peripheral substituents and axial ligands on the electronic structure and properties of iron phthalocyanine, Inorg. Chem., 2004, 43, 7151–7161 CrossRef CAS PubMed
.
- Y. Feng, Q. Hu, E. Rezaee, M. Li, Z.-X. Xu, A. Lorenzoni, F. Mercuri and M. Muccini, High-Performance and Stable Perovskite Solar Cells Based on Dopant-Free Arylamine-Substituted Copper(II) Phthalocyanine Hole-Transporting Materials, Adv. Energy Mater., 2019, 9, 1901019 CrossRef
.
- Z. Yu, L. Wang, X. Mu, C.-C. Chen, Y. Wu, J. Cao and Y. Tang, Intramolecular Electric Field Construction in Metal Phthalocyanine as Dopant-Free Hole Transporting Material for Stable Perovskite Solar Cells with >21% Efficiency, Angew. Chem., Int. Ed., 2021, 60, 6294–6299 CrossRef CAS PubMed
.
- W. Chen, H. Zhang, J. Sun, R. Ghadari, Z. Zhang, F. Pan, K. Lv, X. Sun, F. Guo and C. Shi, Molecular tailor-making of zinc phthalocyanines as dopant-free hole-transporting materials for efficient and stable perovskite solar cells, J. Power Sources, 2021, 505, 230095 CrossRef CAS
.
- G. Qu, D. Khan, F. Yan, A. Atsay, H. Xiao, Q. Chen, H. Xu, I. Nar and Z.-X. Xu, Reformation of thiophene-functionalized phthalocyanine isomers for defect passivation to achieve stable and efficient perovskite solar cells, J. Energy Chem., 2022, 67, 263–275 CrossRef CAS
.
- G. Qu, L. Dong, Y. Qiao, D. Khan, Q. Chen, P. Xie, X. Yu, X. Liu, Y. Wang, J. Chen, X. Chen and Z.-X. Xu, Dopant-Free Phthalocyanine Hole Conductor with Thermal-Induced Holistic Passivation for Stable Perovskite Solar Cells with 23% Efficiency, Adv. Funct. Mater., 2022, 32, 2206585 CrossRef CAS
.
- C. V. Kumar, G. Sfyri, D. Raptis, E. Stathatos and P. Lianos, Perovskite solar cell with low cost Cu-phthalocyanine as hole transporting material, RSC Adv., 2014, 5, 3786–3791 RSC
.
- W. Ke, D. Zhao, C. R. Grice, A. J. Cimaroli, G. Fang and Y. Yan, Efficient fully-vacuum-processed perovskite solar cells using copper phthalocyanine as hole selective layers, J. Mater. Chem. A, 2015, 3, 23888–23894 RSC
.
- F. G. Zhang, X. C. Yang, M. Cheng, W. H. Wang and L. C. Sun, Boosting the efficiency and the stability of low cost perovskite solar cells by using CuPc nanorods as hole transport material and carbon as counter electrode, Nano Energy, 2016, 20, 108–116 CrossRef CAS
.
- N. Torabi, A. Rahnamanic, H. Amrollahi, F. Mirjalili, M. A. Sadeghzade and A. Behjat, Performance enhancement of perovskite solar cell by controlling deposition temperature of copper phthalocyanine as a dopant-free hole transporting layer, Org. Electron., 2017, 48, 211–216 CrossRef CAS
.
- J. H. Han, Y. X. Tu, Z. Y. Liu, X. Y. Liu, H. B. Ye, Z. R. Tang, T. L. Shi and G. L. Liao, Efficient and stable inverted planar perovskite solar cells using dopant-free CuPc as hole transport layer, Electrochim. Acta, 2018, 273, 273–281 CrossRef CAS
.
- X. Y. Liu, Z. Y. Liu, B. Sun, X. H. Tan, H. B. Ye, Y. X. Tu, T. L. Shi, Z. R. Tang and G. L. Liao, All low-temperature processed carbon-based planar heterojunction perovskite solar cells employing Mg-doped rutile TiO2 as electron transport layer, Electrochim. Acta, 2018, 283, 1115–1124 CrossRef CAS
.
- Z. Y. Liu, B. Sun, X. Y. Liu, J. H. Han, H. B. Ye, Y. X. Tu, C. Chen, T. L. Shi, Z. R. Tang and G. L. Liao, 15% efficient carbon based planar-heterojunction perovskite solar cells using a TiO2/SnO2 bilayer as the electron transport layer, J. Mater. Chem. A, 2018, 6, 7409–7419 RSC
.
- X. Y. Liu, Z. Y. Liu, B. Sun, X. H. Tan, H. B. Ye, Y. X. Tu, T. L. Shi, Z. R. Tang and G. L. Liao, 17.46% efficient and highly stable carbon-based planar perovskite solar cells employing Ni-doped rutile TiO2 as electron transport layer, Nano Energy, 2018, 50, 201–211 CrossRef CAS
.
- X. Y. Liu, Z. Y. Liu, H. B. Ye, Y. X. Tu, B. Sun, X. H. Tan, T. L. Shi, Z. R. Tang and G. L. Liao, Novel efficient C-60-based inverted perovskite solar cells with negligible hysteresis, Electrochim. Acta, 2018, 288, 115–125 CrossRef CAS
.
- T. Lei, H. Dong, J. Xi, Y. Niu, J. Xu, F. Yuan, B. Jiao, W. W. Zhang, X. Hou and Z. X. Wu, Highly-efficient and low-temperature perovskite solar cells by employing a Bi-hole transport layer consisting of vanadium oxide and copper phthalocyanine, Chem. Commun., 2018, 54, 6177–6180 RSC
.
- H. B. Ye, Z. Y. Liu, X. Y. Liu, B. Sun, X. H. Tan, Y. X. Tu, T. L. Shi, Z. R. Tang and G. L. Liao, 17.78% efficient low-temperature carbon-based planar perovskite solar cells using Zn-doped SnO2 electron transport layer, Appl. Surf. Sci., 2019, 478, 417–425 CrossRef CAS
.
- M. M. Tavakoli, P. Yadav, D. Prochowicz and R. Tavakoli, Efficient, Hysteresis-Free, and Flexible Inverted Perovskite Solar Cells Using All-Vacuum Processing, Sol. RRL, 2021, 5, 2000552 CrossRef CAS
.
- H. Kim, K. S. Lee, M. J. Paik, D. Y. Lee, S.-U. Lee, E. Choi, J. S. Yun and S. I. Seok, Polymethyl Methacrylate as an Interlayer Between the Halide Perovskite and Copper Phthalocyanine Layers for Stable and Efficient Perovskite Solar Cells, Adv. Funct. Mater., 2022, 32, 2110473 CrossRef CAS
.
- A. Agresti, S. Pescetelli, S. Casaluci, A. D. Carlo, R. Lettieri and M. V. Ieee, High Efficient Perovskite Solar Cells by Employing
Zinc-Phthalocyanine as Hole Transporting Layer, IEEE Int. Conf. Nanotechnol., 2015, 732–735 Search PubMed
.
- A. Ioakeimidis, C. Christodoulou, M. Lux-Steiner and K. Fostiropoulos, Effect of PbI2 deposition rate on two-step PVD/CVD all-vacuum prepared perovskite, J. Solid State Chem., 2016, 244, 20–24 CrossRef CAS
.
- C. Chen, H. Li, J. Jin, Y. Cheng, D. Liu, H. Song and Q. Dai, Highly enhanced long time stability of perovskite solar cells by involving a hydrophobic hole modification layer, Nano Energy, 2017, 32, 165–173 CrossRef CAS
.
- M. Haider, C. Zhen, T. T. Wu, G. Liu and H. M. Cheng, Boosting efficiency and stability of perovskite solar cells with nickel phthalocyanine as a low-cost hole transporting layer material, J. Mater. Sci. Technol., 2018, 34, 1474–1480 CrossRef CAS
.
- M. Haider, C. Zhen, T. T. Wu, J. B. Wu, C. X. Jia, G. Liu and H. M. Cheng, Nickel phthalocyanine as an excellent hole-transport material in inverted planar perovskite solar cells, Chem. Commun., 2019, 55, 5343–5346 RSC
.
- F. Hou, F. Jin, B. Chu, Z. Su, Y. Gao, H. Zhao, P. Cheng, J. Tang and W. Li, Hydrophobic hole-transporting layer induced porous PbI2 film for stable and efficient perovskite solar cells in 50% humidity, Solar Energy Mater. Solar Cells, 2016, 157, 989–995 CrossRef CAS
.
- G. Sfyri, C. V. Kumar, Y. L. Wang, Z. X. Xu, C. A. Krontiras and P. Lianos, Tetra methyl substituted Cu(II) phthalocyanine as alternative hole transporting material for organometal halide perovskite solar cells, Appl. Surf. Sci., 2016, 360, 767–771 CrossRef CAS
.
- Y. Wang, X. Liu, H. Shan, Q. Chen, T. Liu, X. Sun, D. Ma, Z. Zhang, J. Xu and Z.-X. Xu, Tetra-alkyl-substituted copper(II) phthalocyanines as dopant-free hole-transport layers for planar perovskite solar cells with enhanced open circuit voltage and stability, Dyes Pigm., 2017, 139, 619–626 CrossRef
.
- X. Liu, Y. Wang, E. Rezaee, Q. Chen, Y. Feng, X. Sun, L. Dong, Q. Hu, C. Li and Z.-X. Xu, Tetra-Propyl-Substituted Copper(II) Phthalocyanine as Dopant-Free Hole Transporting Material for Planar Perovskite Solar Cells, Sol. RRL, 2018, 2, 1800050 CrossRef
.
- C. Li, Q. Hu, Q. Chen, W. Yu, J. Xu and Z.-X. Xu, Tetrapropyl-substituted palladium phthalocyanine used as an efficient hole transport material in perovskite solar cells, Org. Electron., 2021, 88, 106018 CrossRef CAS
.
- G. Sfyri, Q. Chen, Y. W. Lin, Y. L. Wang, E. Nouri, Z. X. Xu and P. Lianos, Soluble butyl substituted copper phthalocyanine as alternative hole-transporting material for solution processed perovskite solar cells, Electrochim. Acta, 2016, 212, 929–933 CrossRef CAS
.
- E. Nouri, Y.-L. Wang, Q. Chen, J.-J. Xu, G. Paterakis, V. Dracopoulos, Z.-X. Xu, D. Tasis, M. R. Mohammadi and P. Lianos, Introduction of Graphene Oxide as Buffer Layer in Perovskite Solar Cells and the Promotion of Soluble n-Butyl-substituted Copper Phthalocyanine as Efficient Hole Transporting Material, Electrochim. Acta, 2017, 233, 36–43 CrossRef CAS
.
- E. Nouri, M. R. Mohammadi, Z. X. Xu, V. Dracopoulos and P. Lianos, Improvement of the photovoltaic parameters of perovskite solar cells using a reduced-graphene-oxide-modified titania layer and soluble copper phthalocyanine as a hole transporter, Phys. Chem. Chem. Phys., 2018, 20, 2388–2395 RSC
.
- Y. C. Kim, T. Y. Yang, N. J. Jeon, J. Im, S. Jang, T. J. Shin, H. W. Shin, S. Kim, E. Lee, S. Kim, J. H. Noh, S. I. Seok and J. Seo, Engineering interface structures between lead halide perovskite and copper phthalocyanine for efficient and stable perovskite solar cells, Energy Environ. Sci., 2017, 10, 2109–2116 RSC
.
- T. Duong, J. Peng, D. Walter, J. Xiang, H. P. Shen, D. Chugh, M. Lockrey, D. Y. Zhong, J. T. Li, K. Weber, T. P. White and K. R. Catchpole, Perovskite Solar Cells Employing Copper Phthalocyanine Hole-Transport Material with an Efficiency over 20% and Excellent Thermal Stability, ACS Energy Lett., 2018, 3, 2441–2448 CrossRef CAS
.
- Y. M. Feng, Q. Chen, L. Dong, Z. H. Zhang, C. Li, S. H. Yang, S. Y. Cai and Z. X. Xu, Carbon-chain length substituent effects on Cu(II) phthalocyanines as dopant-free hole-transport materials for perovskite solar cells, Sol. Energy, 2019, 184, 649–656 CrossRef CAS
.
- X. Jiang, Z. Yu, H.-B. Li, Y. Zhao, J. Qu, J. Lai, W. Ma, D. Wang, X. Yang and L. Sun, A solution-processable copper(II) phthalocyanine derivative as a dopant-free hole-transporting material for efficient and stable carbon counter electrode-based perovskite solar cells, J. Mater. Chem. A, 2017, 5, 17862–17866 RSC
.
- X. Q. Jiang, D. P. Wang, Z. Yu, W. Y. Ma, H. B. Li, X. C. Yang, F. Liu, A. Hagfeldt and L. C. Sun, Molecular Engineering of Copper Phthalocyanines: A Strategy in Developing Dopant-Free Hole-Transporting Materials for Efficient and Ambient-Stable Perovskite Solar Cells, Adv. Energy Mater., 2019, 9, 9 Search PubMed
.
- Y. Feng, Q. Hu, E. Rezaee, M. Li, Z. Xu, A. Lorenzoni, F. Mercuri and M. Muccini, High-Performance and Stable Perovskite Solar Cells Based on Dopant-Free Arylamine-Substituted Copper(II) Phthalocyanine Hole-Transporting Materials, Adv. Energy Mater., 2019, 9, 1901019 CrossRef
.
- J.-J. Guo, Z.-C. Bai, X.-F. Meng, M.-M. Sun, J.-H. Song, Z.-S. Shen, N. Ma, Z.-L. Chen and F. Zhang, Novel dopant-free metallophthalocyanines based hole transporting materials for perovskite solar cells: the effect of core metal on photovoltaic performance, Solar Energy, 2017, 155, 121–129 CrossRef CAS
.
- J.-J. Guo, X.-F. Meng, J. Niu, Y. Yin, M.-M. Han, X.-H. Ma, G.-S. Song and F. Zhang, A novel asymmetric phthalocyanine-based hole transporting material for perovskite solar cells with an open-circuit voltage above 1.0 V, Synth. Metals, 2016, 220, 462–468 CrossRef CAS
.
- J. J. Guo, X. F. Meng, H. W. Zhu, M. M. Sun, Y. B. Wang, W. N. Wang, M. Y. Xing and F. Zhang, Boosting the performance and stability of perovskite solar cells with phthalocyanine-based dopant-free hole transporting materials through core metal and peripheral groups engineering, Org. Electron., 2019, 64, 71–78 CrossRef CAS
.
- S. Wu, Y. Zheng, Q. Liu, R. Li and T. Peng, Low cost and solution-processable zinc phthalocyanine as alternative hole transport material for perovskite solar cells, RSC Adv., 2016, 6, 107723–107731 RSC
.
- G. Zanotti, G. Mattioli, A. M. Paoletti, G. Pennesi, D. Caschera, N. Maman, I. Visoly-Fisher, R. K. Misra, L. Etgar and E. A. Katz, A Solution-Processed Tetra-Alkoxylated Zinc Phthalocyanine as Hole Transporting Material for Emerging Photovoltaic Technologies, Int. J. Photoenergy, 2018, 2473152 CAS
.
- L. Dong, Q. Hu, E. Rezaee, Q. Chen, S. Yang, S. Cai, B. Liu, J.-H. Pan and Z.-X. Xu, Dopant-Free Hole-Transporting Layer Based on Isomer-Pure Tetra-Butyl-Substituted Zinc(II) Phthalocyanine for Planar Perovskite Solar Cells, Sol. RRL, 2019, 3, 1900119 CrossRef
.
- Z. D. Cui, Y. Q. Wang, Y. Chen, X. Z. Chen, X. L. Deng, W. C. Chen and C. W. Shi, Soluble tetra-methoxyltriphenylamine substituted zinc phthalocyanine as dopant-free hole transporting materials for perovskite solar cells, Org. Electron., 2019, 69, 248–254 CrossRef CAS
.
- P. Qi, F. Zhang, X. Li, Y. Xiao, J. Guo and S. Wang, 2,9,16,23-Tetrakis(7-coumarinoxy-4-methyl)-metallophthalocyanines-based hole transporting material for mixed-perovskite solar cells, Synth. Met., 2017, 226, 1–6 CrossRef CAS
.
- P. Huang, A. Hernández, S. Kazim, J. Follana-Berná, J. Ortiz, L. Lezama, Á. Sastre-Santos and S. Ahmad, Asymmetrically Substituted Phthalocyanines as Dopant-Free Hole Selective Layers for Reliability in Perovskite Solar Cells, ACS Appl. Energy Mater., 2021, 4, 10124–10135 CrossRef CAS
.
- S. W. Kim, G. Kim, C. S. Moon, T. Y. Yang and J. Seo, Metal-Free Phthalocyanine as a Hole Transporting Material and a Surface Passivator for Efficient and Stable Perovskite Solar Cells, Small Methods, 2021, 5, 2001248 CrossRef CAS PubMed
.
- W. C. Chen, H. Y. Zhang, J. J. Sun, R. Ghadari, Z. G. Zhang, F. Pan, K. Lv, X. Sun, F. L. Guo and C. W. Shi, Molecular tailor-making of zinc phthalocyanines as dopant-free hole-transporting materials for efficient and stable perovskite solar cells, J. Power Sources, 2021, 505, 230095 CrossRef CAS
.
- G. Yang, Y.-L. Wang, J.-J. Xu, H.-W. Lei, C. Chen, H.-Q. Shan, X.-Y. Liu, Z.-X. Xu and G.-J. Fang, A facile molecularly engineered copper(II) phthalocyanine as hole transport material for planar perovskite solar cells with enhanced performance and stability, Nano Energy, 2017, 31, 322–330 CrossRef CAS
.
- X. Zheng, Y. Wang, J. Hu, G. Yang, Z. Guo, J. Xia, Z. Xu and G. Fang, Octamethyl-substituted Pd(II) phthalocyanine with long carrier lifetime as a dopant-free hole selective material for performance enhancement of perovskite solar cells, J. Mater. Chem. A, 2017, 5, 24416–24424 RSC
.
- Y. L. Wang, X. L. Zheng, X. Y. Liu, Y. M. Feng, H. Q. Shan, L. Dong, G. J. Fang and Z. X. Xu, A study of different central metals in octamethyl-substituted phthalocyanines as dopant-free hole-transport layers for planar perovskite solar cells, Org. Electron., 2018, 56, 276–283 CrossRef CAS
.
- Q. Hu, E. Rezaee, L. Dong, Q. Dong, H. Shan, Q. Chen, M. Li, S. Cai, L. Wang and Z.-X. Xu, Molecularly Designed Zinc(II) Phthalocyanine Derivative as Dopant-Free Hole-Transporting Material of Planar Perovskite Solar Cell with Preferential Face-on Orientation, Sol. RRL, 2019, 3, 1900182 CrossRef CAS
.
- F. Qi, B. Wu, J. Xu, Q. Chen, H. Shan, J. Xu and Z.-X. Xu, Non-peripherally octaalkyl-substituted nickel phthalocyanines used as non-dopant hole transport materials in perovskite solar cells, Chin. Phys. B, 2021, 30, 108801 CrossRef CAS
.
- X. Ding, C. Chen, L. Tao, C. Wu, M. Zheng, H. Lu, H. Xu, H. Li and M. Cheng, Dopant-free methoxy substituted copper(II) phthalocyanine for highly efficient and stable perovskite solar cells, Chem. Eng. J., 2020, 387, 124130 CrossRef CAS
.
- Q. Hu, E. Rezaee, M. Li, Q. Chen, Y. Cao, M. Mayukh, D. V. McGrath and Z.-X. Xu, Molecular Design Strategy in Developing Titanyl Phthalocyanines as Dopant-Free Hole-Transporting Materials for Perovskite Solar Cells: Peripheral or Nonperipheral Substituents?, ACS Appl. Mater. Interfaces, 2019, 11, 36535–36543 CrossRef CAS PubMed
.
- F. Zhang, D. Bi, N. Pellet, C. Xiao, Z. Li, J. J. Berry, S. M. Zakeeruddin, K. Zhu and M. Grätzel, Suppressing defects through the synergistic effect of a Lewis base and a Lewis acid for highly efficient and stable perovskite solar cells, Energy Environ. Sci., 2018, 11, 3480–3490 RSC
.
- F. Zhang, W. Shi, J. Luo, N. Pellet, C. Yi, X. Li, X. Zhao, T. J. S. Dennis, X. Li and S. Wang, Isomer-pure bis-PCBM-assisted crystal engineering of perovskite solar cells showing excellent efficiency and stability, Adv. Mater., 2017, 29, 1606806 CrossRef PubMed
.
- S. Dong, H. Tian, L. Huang, J. Zhang, D. Yan, Y. Geng and F. Wang, Non-Peripheral Tetrahexyl-Substituted Vanadyl Phthalocyanines with Intermolecular Cofacial π–π Stacking for Solution-Processed Organic Field-Effect Transistors, Adv. Mater., 2011, 23, 2850–2854 CrossRef CAS PubMed
.
- X. Yin, Z. Song, Z. Li and W. Tang, Toward ideal hole transport materials: a review on recent progress in dopant-free hole transport materials for fabricating efficient and stable perovskite solar cells, Energy Environ. Sci., 2020, 13, 4057–4086 RSC
.
- F. J. Ramos, M. Ince, M. Urbani, A. Abate, M. Graetzel, S. Ahmad, T. Torres and M. K. Nazeeruddin, Non-aggregated Zn(II)octa(2,6-diphenylphenoxy) phthalocyanine as a hole transporting material for efficient perovskite solar cells, Dalton Trans., 2015, 44, 10847–10851 RSC
.
- K. T. Cho, K. Rakstys, M. Cavazzini, S. Orlandi, G. Pozzi and M. K. Nazeeruddin, Perovskite Solar Cells Employing Molecularly Engineered Zn(II) Phthalocyanines as Hole-transporting Materials, Nano Energy, 2016, 30, 853–857 CrossRef CAS
.
- K. T. Cho, M. Cavazzini, K. Rakstys, S. Orlandi, S. Paek, M. Franckevičius, H. Kanda, R. Gegevičius, Q. V. Emmanuel, G. Pozzi and M. K. Nazeeruddin, Perovskite Solar Cells: 18% Efficiency Using Zn(II) and Cu(II) Octakis(diarylamine)phthalocyanines as Hole-Transporting Materials, ACS Appl. Energy Mater., 2019, 2, 6195–6199 CrossRef CAS
.
- N. Klipfel, J. Xia, P. Čulík, S. Orlandi, M. Cavazzini, N. Shibayama, H. Kanda, C. Igci, W. Li, Y.-B. Cheng, V. Jankauskas, K. Genevicius, A. M. Asiri, C. Momblona, K. Rakstys, G. Pozzi and M. K. Nazeeruddin, Zn(II) and Cu(II) tetrakis(diarylamine)phthalocyanines as hole-transporting materials for perovskite solar cells, Mater. Today Energy, 2022, 29, 101110 CrossRef CAS
.
- L. Caliò, J. Follana-Berná, S. Kazim, M. Madsen, H.-G. Rubahn, Á. Sastre-Santos and S. Ahmad, Cu(II) and Zn(II) based phthalocyanines as hole selective layers for perovskite solar cells, Sustainable Energy Fuels, 2017, 1, 2071–2077 RSC
.
- E. Nouri, J. V. S. Krishna, C. V. Kumar, V. Dracopoulos, L. Giribabu, M. R. Mohammadi and P. Lianos, Soluble tetratriphenylamine Zn phthalocyanine as Hole Transporting Material for Perovskite Solar Cells, Electrochim. Acta, 2016, 222, 875–880 CrossRef CAS
.
- M. Cheng, Y. Li, M. Safdari, C. Chen, P. Liu, L. Kloo and L. Sun, Efficient Perovskite Solar Cells Based on a Solution Processable Nickel(II) Phthalocyanine and Vanadium Oxide Integrated Hole Transport Layer, Adv. Energy Mater., 2017, 7, 1602556 CrossRef
.
- P. Gao, K. T. Cho, A. Abate, G. Grancini, P. Y. Reddy, M. Srivasu, M. Adachi, A. Suzuki, K. Tsuchimoto, M. Gratzel and M. K. Nazeeruddin, An efficient perovskite solar cell with symmetrical Zn(II) phthalocyanine infiltrated buffering porous Al2O3 as the hybrid interfacial hole-transporting layer, Phys. Chem. Chem. Phys., 2016, 18, 27083–27089 RSC
.
- K. T. Cho, O. Trukhina, C. Roldán-Carmona, M. Ince, P. Gratia, G. Grancini, P. Gao, T. Marszalek, W. Pisula, P. Y. Reddy, T. Torres and M. K. Nazeeruddin, Molecularly Engineered Phthalocyanines as Hole-Transporting Materials in Perovskite Solar Cells Reaching Power Conversion Efficiency of 17.5%, Adv. Energy Mater., 2017, 7, 1601733 CrossRef
.
- X. Jiang, Z. Yu, J. Lai, Y. Zhang, N. Lei, D. Wang and L. Sun, Efficient perovskite solar cells employing a solution-processable copper phthalocyanine as a hole-transporting material, Sci. China: Chem., 2017, 60, 423–430 CrossRef CAS
.
- P. Ru, E. Bi, Y. Zhang, Y. Wang, W. Kong, Y. Sha, W. Tang, P. Zhang, Y. Wu, W. Chen, X. Yang, H. Chen and L. Han, High Electron Affinity Enables Fast Hole Extraction for Efficient Flexible Inverted Perovskite Solar Cells, Adv. Energy Mater., 2020, 10, 1903487 CrossRef CAS
.
- J. Sun, N. Chandrasekaran, C. Liu, A. D. Scully, W. Yin, C. K. Ng and J. J. Jasieniak, Enhancement of 3D/2D Perovskite Solar Cells Using an F4TCNQ Molecular Additive, ACS Appl. Energy Mater., 2020, 3, 8205–8215 CrossRef CAS
.
- W. Q. Wu, Q. Wang, Y. Fang, Y. Shao, S. Tang, Y. Deng, H. Lu, Y. Liu, T. Li, Z. Yang, A. Gruverman and J. Huang, Molecular doping enabled scalable blading of efficient hole-transport-layer-free perovskite solar cells, Nat. Commun., 2018, 9, 1625 CrossRef PubMed
.
- D. Liu, Y. Li, J. Yuan, Q. Hong, G. Shi, D. Yuan, J. Wei, C. Huang, J. Tang and M.-K. Fung, Improved performance of inverted planar perovskite solar cells with F4-TCNQ doped PEDOT:PSS hole transport layers, J. Mater. Chem. A, 2017, 5, 5701–5708 RSC
.
- J. M. Wang, Z. K. Wang, M. Li, C. C. Zhang, L. L. Jiang, K. H. Hu, Q. Q. Ye and L. S. Liao, Doped Copper Phthalocyanine via an Aqueous Solution Process for Normal and Inverted Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 8 Search PubMed
.
- X. Jiang, Z. Yu, J. Lai, Y. Zhang, M. Hu, N. Lei, D. Wang, X. Yang and L. Sun, Interfacial Engineering of Perovskite Solar Cells by Employing a Hydrophobic Copper Phthalocyanine Derivative as Hole-Transporting Material with Improved Performance and Stability, ChemSusChem, 2017, 10, 1838–1845 CrossRef CAS PubMed
.
- M. Pegu, D. Molina, M. J. Álvaro-Martins, M. Castillo, L. Ferrer, P. Huang, S. Kazim, Á. Sastre-Santos and S. Ahmad, Dimers of diethynyl-conjugated zinc-phthalocyanine as hole selective layers for perovskite solar cell fabrication, J. Mater. Chem. C, 2022, 10, 11975–11982 RSC
.
- Y. Deng, X. Zheng, Y. Bai, Q. Wang, J. Zhao and J. Huang, Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules, Nat. Energy, 2018, 3, 560–566 CrossRef CAS
.
- K. Rakstys, C. Igci and M. K. Nazeeruddin, Efficiency vs. stability: dopant-free hole transporting materials towards stabilized perovskite solar cells, Chem. Sci., 2019, 10, 6748–6769 RSC
.
- J. Seo, N. J. Jeon, W. S. Yang, H.-W. Shin, T. K. Ahn, J. Lee, J. H. Noh and S. I. Seok, Effective Electron Blocking of CuPC-Doped Spiro-OMeTAD for Highly Efficient Inorganic-Organic Hybrid Perovskite Solar Cells, Adv. Energy Mater., 2015, 5, 1501320 CrossRef
.
- E. Nouri, Y.-L. Wang, Q. Chen, J.-J. Xu, V. Dracopoulos, L. Sygellou, Z.-X. Xu, M. R. Mohammadi and P. Lianos, The beneficial effects of mixing spiro-OMeTAD with n-butyl-substituted copper phthalocyanine for perovskite solar cells, Electrochim. Acta, 2016, 222, 1417–1423 CrossRef CAS
.
- J.-M. Wang, Z.-K. Wang, M. Li, K.-H. Hu, Y.-G. Yang, Y. Hu, X.-Y. Gao and L.-S. Liao, Small Molecule Polymer Composite Hole-Transporting Layer for Highly Efficient and Stable Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2017, 9, 13240–13246 CrossRef CAS PubMed
.
- Q. Hu, E. Rezaee, Q. Dong, H. Shan, Q. Chen, L. Wang, B. Liu, J.-H. Pan and Z.-X. Xu, P3HT/Phthalocyanine Nanocomposites as Efficient Hole-Transporting Materials for Perovskite Solar Cells, Sol. RRL, 2019, 3, 1800264 CrossRef
.
- J. Peng, D. Walter, Y. Ren, M. Tebyetekerwa, Y. Wu, T. Duong, Q. Lin, J. Li, T. Lu, M. A. Mahmud, O. L. C. Lem, S. Zhao, W. Liu, Y. Liu, H. Shen, L. Li, F. Kremer, H. T. Nguyen, D.-Y. Choi, K. J. Weber, K. R. Catchpole and T. P. White, Nanoscale localized contacts for high fill factors in polymer-passivated perovskite solar cells, Science, 2021, 371, 390–395 CrossRef CAS PubMed
.
- X. F. Zhang, X. Y. Zhou, L. Z. Zhang and B. M. Xu, Facile phthalocyanine doping into PEDOT leads to highly efficient and stable inverted metal halide perovskite solar cells, J. Mater. Chem. A, 2018, 6, 12515–12522 RSC
.
- J. M. Ball and A. Petrozza, Defects in perovskite-halides and their effects in solar cells, Nat. Energy, 2016, 1, 16149 CrossRef CAS
.
- Q. Wang, B. Chen, Y. Liu, Y. Deng, Y. Bai, Q. Dong and J. Huang, Scaling behavior of moisture-induced grain degradation in polycrystalline hybrid perovskite thin films, Energy Environ. Sci., 2017, 10, 516–522 RSC
.
- M. Vasilopoulou, A. Fakharuddin, A. G. Coutsolelos, P. Falaras, P. Argitis, A. Yusoff and M. K. Nazeeruddin, Molecular materials as interfacial layers and additives in perovskite solar cells, Chem. Soc. Rev., 2020, 49, 4496–4526 RSC
.
- Y. Wu, X. Yang, W. Chen, Y. Yue, M. Cai, F. Xie, E. Bi, A. Islam and L. Han, Perovskite solar cells with 18.21% efficiency and area over 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm2 fabricated by heterojunction engineering, Nat. Energy, 2016, 1, 16148 CrossRef CAS
cm2 fabricated by heterojunction engineering, Nat. Energy, 2016, 1, 16148 CrossRef CAS .
- T. Zhao, C.-C. Chueh, Q. Chen, A. Rajagopal and A. K. Y. Jen, Defect Passivation of Organic–Inorganic Hybrid Perovskites by Diammonium Iodide toward High-Performance Photovoltaic Devices, ACS Energy Lett., 2016, 1, 757–763 CrossRef CAS
.
- T. S. Sherkar, C. Momblona, L. Gil-Escrig, J. Avila, M. Sessolo, H. J. Bolink and L. J. A. Koster, Recombination in Perovskite Solar Cells: Significance of Grain Boundaries, Interface Traps, and Defect Ions, ACS Energy Lett., 2017, 2, 1214–1222 CrossRef CAS PubMed
.
- M. Mozaffari, A. Behjat and N. Torabi, Application of copper phthalocyanin for surface modification of perovskite solar cells, J. Mater. Sci.: Mater. Electron., 2018, 29, 18187–18192 CrossRef CAS
.
- S. Jin, Y. L. Wei, F. Y. Huang, X. M. Yang, D. Luo, Y. Fang, Y. Z. Zhao, Q. Y. Guo, Y. F. Huang and J. H. Wu, Enhancing the perovskite solar cell performance by the treatment with mixed anti-solvent, J. Power Sources, 2018, 404, 64–72 CrossRef CAS
.
- S. Wu, Q. Liu, Y. Zheng, R. Li and T. Peng, An efficient copper phthalocyanine additive of perovskite precursor for improving the photovoltaic performance of planar perovskite solar cells, J. Power Sources, 2017, 359, 303–310 CrossRef CAS
.
- R. Li, J. L. Ding, X. J. Mu, Y. F. Kang, A. R. Wang, W. H. Bi, Y. H. Zhang, J. Cao and Q. F. Dong, Hyperbranched phthalocyanine enabling black-phase formamidinium perovskite solar cells processing and operating in humidity open air, J. Energy Chem., 2022, 71, 141–149 CrossRef CAS
.
- F. C. Li, J. Y. Yuan, X. F. Ling, L. Z. Huang, N. Rujisamphan, Y. Y. Li, L. F. Chi and W. L. Ma, Metallophthalocyanine-Based Molecular Dipole Layer as a Universal and Versatile Approach to Realize Efficient and Stable Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10, 42397–42405 CrossRef CAS PubMed
.
- J. Cao, X. D. Lv, X. X. Feng, R. Q. Meng, Y. Y. Wu and Y. Tang, Efficient Grain Boundary Suture by Low-Cost Tetra-ammonium Zinc Phthalocyanine for Stable Perovskite Solar Cells with Expanded Photoresponse, J. Am. Chem. Soc., 2018, 140, 11577–11580 CrossRef CAS PubMed
.
- C. Li, R. He, Q. Liang, J. Cao, J. Yin and Y. Tang, 4-Tert-butylpyridine-assisted low-cost and soluble copper phthalocyanine as dopant-free hole transport layer for efficient Pb-and Sn-based perovskite solar cells, Sci. China: Chem., 2020, 63, 1053–1058 CrossRef CAS
.
- K. W. Lai, C. Hanmandlu, C. C. Chang and C. W. Chu, An amino-phthalocyanine additive enhances the efficiency of perovskite solar cells through defect passivation in mixed-halide films, Org. Electron., 2022, 108, 106568 CrossRef CAS
.
- Z. Zhang, H. Liu, S. Wang, H. Bao, F. Zhang and X. Li, Extended Near-Infrared Photovoltaic Responses of Perovskite Solar Cells by p-Type Phthalocyanine Derivative, Adv. Funct. Mater., 2022, 32, 2208539 CrossRef CAS
.
- C. P. Li, X. D. Lv, J. Cao and Y. Tang, Tetra-ammonium Zinc Phthalocyanine to Construct a Graded 2D-3D Perovskite Interface for Efficient and Stable Solar Cells, Chin. J. Chem., 2019, 37, 30–34 CrossRef CAS
.
- G. Pindolia, J. Pandya, S. Shinde and P. K. Jha, Fluorinated copper phthalocyanine as an electron transport material in perovskite solar cell, Int. J. Energy Res., 2022, 46, 15127–15142 CrossRef CAS
.
- Q. Hu, E. Rezaee, W. Xu, R. Ramachandran, Q. Chen, H. Xu, T. El-Assaad, D. V. McGrath and Z. X. Xu, Dual Defect-Passivation Using Phthalocyanine for Enhanced Efficiency and Stability of Perovskite Solar Cells, Small, 2021, 17, 2005216 CrossRef CAS PubMed
.
| This journal is © the Partner Organisations 2023 |