Controlled self-aggregation of perylene bisimide and its application in thick photoconductive interlayers for high performance polymer solar cells†
Hengtao
Zhao
,
Yinqi
Luo
,
Linlin
Liu
,
Zengqi
Xie
* and
Yuguang
Ma
Institute of Polymer Optoelectronic Materials and Devices, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: msxiez@scut.edu.cn
First published on 14th December 2016
Abstract
Highly conductive cathode interlayers that can work efficiently when the film is thick, are essentially important for polymer solar cells since this would facilitate their mass production in the future. Herein, an asymmetric organic dye molecule, perylene bisimide (PBI) 3, is synthesized as a photosensitizer for zinc oxide (ZnO) to achieve a photoconductive hybrid material. The self-aggregation of PBI3 was efficiently restricted by the introduction of an alkyl group at one of the imide positions in the structure of the molecule, whereas the formation of Zn–N chemical bonding between ZnO and PBI3 ensures the formation of a robust hybrid thin film. The photoconductive hybrid thin film shows highly enhanced conductivity under white light irradiation. Inverted polymer solar cells (PSCs) based on the photoconductive cathode interlayers (ZnO:PBI3 (3 wt%)) show a very high power conversion efficiency (PCE) of 8.79% when the thickness of the interlayer is 100 nm, which is three times higher than that of the ZnO cathode interlayer-based device.
1. Introduction
Polymer solar cells (PSCs) have shown great promise as one of the renewable and sustainable photovoltaic techniques for solar energy conversion in the past few decades, with the benefits of low-cost and the possibility of large scale solution processing.1–6 Rapid progress has been made on the power conversion efficiency (PCE) in recent years, promoted by the development of both materials and device engineering.7–10 Both cathode and anode interlayers, located between the active layer and electrodes in the device, play essential roles in the electron and hole extraction efficiency to reduce the interfacial recombination. In the champion devices, reported to date, the thickness of the interlayers is very small (∼2–30 nm) due to the relatively low charge mobility and conductivity of the interfacial materials.11–13 It is still problematic for high-throughput processing methods, such as the “role-to-role” approach, to produce these thin films without any pin-holes. To be compatible with the current production techniques, devices that can efficiently work under the thick film conditions are highly expected.Highly conductive interlayers are particularly essential for the production of large scale modules. For this purpose, self-doped fullerene derivatives and conjugated polymers have been developed by Jen et al. and Huang et al., respectively.14,15 Although the doped conjugated systems usually show strong absorption in the visible light region, this limits their applications in the inverted PSCs as cathode interlayers, especially when the film is thick. Zinc oxide (ZnO), which is usually fabricated through a so-called “sol–gel” method, is a widely used cathode interlayer material in the inverted PSCs with advantages such as high transparency, low-cost, easy solution processing, and proper energy levels.16–18 Amorphous thin film derived by this method shows rather low electron mobility on the level of 10−3–10−4 cm2 V−1 s−1 due to plenty of defects as electron trapping sites and shows rather low electron conductivity.17 Various methods, including elements doping, such as aluminum-doped ZnO (AZO),19 gallium-doped ZnO (GZO),20 indium-doped ZnO (IZO),21 and tin-doped ZnO (ZTO), have been applied to increase the conductivity of the ZnO thin film.22 Most recently, organic dye molecules, such as perylene bisimide molecules (PBI), were doped into the ZnO thin films to achieve the photoconductive cathode interlayers, which possess rather high conductivity under sunlight irradiation and the working condition of PSCs due to the photoinduced electron transfer from PBI molecules to ZnO.23,24 Organic dye molecules possess large extinction coefficients over a wide wavelength range, whereas ZnO shows fast electron transport efficiency. The organic–inorganic hybridized photoconductive materials simultaneously avail the advantage of the inherent features of both the organic and inorganic materials, but it still remains challenging to achieve a molecularly dispersed system due to the easy phase separation. In our previous studies, chemical bonding between ZnO and nitrogen atoms of PBI facilitated the formation of uniform composition;23 however, the very strong self-assembly ability of PBI molecules still makes the fabrication of the photoconductive interlayers difficult.
In this study, we report an asymmetric perylene bisimide (PBI3) bearing one N–H group at one tip of the molecule and one N-alkyl group at the another (Scheme 1). The N–H unit helps to form the chemical bond between ZnO and PBI, generating a robust thin film, whereas the N-alkyl group restricts the self-aggregation of PBI, producing a molecularly dispersed uniform composition. The hybrid thin films with different doping concentrations showed very high photoconductive behavior under sunlight irradiation and were used as cathode interlayers in the inverted PSCs. The PCE of the best PSCs based on the photoconductive interlayers was up to three times higher than that of the control device using ZnO as the interlayer, where the thickness of all the interlayers was 100 nm.
2. Results and discussion
2.1 Synthesis
A newly designed target compound PBI3 was synthesized according to the route outlined in Scheme 1. The PBI1 was synthesized from tetrachloro-substituted perylene bisanhydride according to the procedures reported in literature.25 The partial saponification of PBI1 with potassium hydroxide in t-BuOH at 80 °C provided PBI2, which was converted to the target asymmetric PBI3 by reaction with an excess amount of ammonium acetate in propionic acid at 150 °C. All these compounds were fully characterized by 1H NMR, 13C NMR, and MALDI-TOF.2.2 Controlled self-aggregation
Previously, an analogue of PBI3 with two N–H units at each end of the molecule (herein, designated as PBI-H) was reported as a supergelator in the nonpolar organic solvents such as toluene and chlorobenzene.26 This super strong self-aggregation ability of the symmetric PBI-H was attributed to the synchronous intermolecular multiple hydrogen bonds and π–π interactions, which induced the formation of fiber-like aggregates. In the molecular structure of PBI3, the introduction of an N-alkyl unit at one tip of the molecule hinders the consecutive molecular alignment but limits the formation of molecular dimers through either hydrogen bonding or π–π interactions. The aggregation behavior of PBI3 was then studied in methylcyclohexane (MCH), which is a nonpolar poor solvent.Fig. 1 displays the temperature-dependent absorption spectra of PBI3 in MCH. PBI3 was well dissolved in MCH at 70 °C, according to the typical fine structured absorption spectrum of tetraphenoxy-substituted PBIs with an absorption maximum at 560 nm.25,26 With a decrease in temperature, an absorption band emerged and increased at around 605 nm, which indicates the formation of aggregates, and the band is assigned to the J-band of PBI3 aggregates. Three well-defined isosbestic points were observed at 575, 476, and 442 nm. The red-shift of the absorption spectra of the J-aggregates of PBI3 is 45 nm relative to the molecule solution, which is much smaller than that of the J-aggregates of the PBI-H with two N–H units (85 nm).26 The smaller red-shifts clearly indicate that fewer molecules are involved in the J-aggregates of PBI3, which means that the self-aggregation of the PBI3 molecule is effectively hindered by the introduction of one N-alkyl unit in the molecular structure. Indeed, the solubility of PBI3 is about 3–5 times higher than that of PBI-H, and no gelation behavior was observed in any solvents.
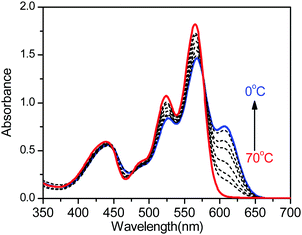 | ||
| Fig. 1 Temperature-dependent absorption spectra of PBI3 in MCH at a concentration of 0.5 × 10−3 M in a 1 mm cell. The arrow indicates the spectral change with the decreasing temperature. | ||
To gain an insight into the morphology of PBI3 aggregates, a thin film was fabricated on a quartz substrate from its chloroform solution by the spin-coating method. The film was subjected to scanning electron microscopy (SEM). As shown in Fig. S2c (ESI†), a smooth surface without any aggregated domain was observed for PBI3, which is totally different as compared to that of the fiber-like aggregates of PBI-H (Fig. S2b, ESI†). The reduced aggregation behavior of PBI3 may help form a well dispersed uniform thin film when doped in a host matrix.
2.3 Hybrid materials based on ZnO and PBI3
Attempts to dope ZnO with the unconjugated polymers, such as PVP,27 PEO,28 PEG,29 and conjugated polymer, such as PFEP, have been reported;30 however, the electron mobility of these doping systems was either unchanged or even decreased. Conjugated small molecules were also used as dopants to ZnO, whereas an enhanced electron mobility was observed in various systems.14 Regardless of method, a uniform doping system at the molecular level is important. To obtain a molecularly dispersed organic–inorganic hybrid material, the interaction between the organic species and inorganic materials must suppress that between the organic molecules. Herein, zinc acetate and PBI3 were dissolved in the co-solvent of 2-methoxyethanol and 2-aminoethanol to form a precursor solution, and the film of hybridized ZnO and PBI3 was fabricated by the spin-coating method followed by thermal treatment at 200 °C for 60 min. The solubility of PBI3 was good enough that it facilitated the variation of the doping concentration in a large region. To confirm the interaction between ZnO and PBI3, a sample with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio between these was prepared and scraped from the glass substrate for the FT-IR measurement. As shown in Fig. 2, a peak located at 675 cm−1 with medial intensity was observed for the hybrid material, which is totally different from that of PBI3. The absorption peak at 675 cm−1 was assigned as that of the Zn–N bond according to the literature.31 The FT-IR results herein confirmed the coordination of the zinc ion and the ligand nitrogen atom, which is in agreement with our previous conclusion from the results of X-ray photoelectron spectroscopy (XPS).23 Both the reduced self-aggregation of PBI3 as discussed above and the formation of the Zn–N chemical bond facilitate the formation of a uniform doping system.
1 weight ratio between these was prepared and scraped from the glass substrate for the FT-IR measurement. As shown in Fig. 2, a peak located at 675 cm−1 with medial intensity was observed for the hybrid material, which is totally different from that of PBI3. The absorption peak at 675 cm−1 was assigned as that of the Zn–N bond according to the literature.31 The FT-IR results herein confirmed the coordination of the zinc ion and the ligand nitrogen atom, which is in agreement with our previous conclusion from the results of X-ray photoelectron spectroscopy (XPS).23 Both the reduced self-aggregation of PBI3 as discussed above and the formation of the Zn–N chemical bond facilitate the formation of a uniform doping system.
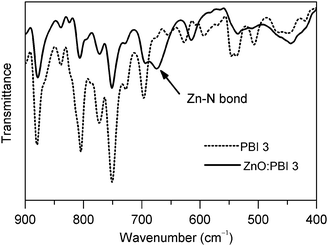 | ||
Fig. 2 FT-IR spectra of PBI3 (dotted line) and the hybrid of ZnO and PBI3 with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (solid line). 1 (solid line). | ||
2.4 Photoconductive behavior of the hybrid material
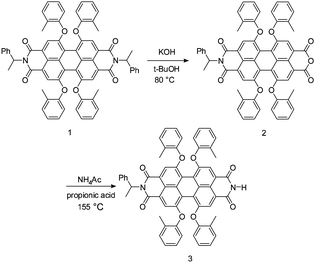
To evaluate the photoconductive properties of the hybrid material of ZnO:PBI3, devices with a configuration of ITO/ZnO:PBI3/Al were fabricated and the doping concentration of PBI3 was optimized. As a reference, the photoconductive behavior of ZnO thin film was also determined under the same conditions. As we previously reported, the ZnO thin film showed diode behavior with low current density both in the dark and under the illumination of visible light because it is transparent in the visible light region. Fig. 3 shows I–V curves of these thin films under AM 1.5G 1000 W m−2 illumination. As can be seen, all the doping films showed ten to twenty times higher current when compared with that of the ZnO film, and the I–V curves became linear. This means that the doping films showed conductor behavior under illumination. The highly increased conductivity was attributed to the photo-induced electron transfer from PBI3 to the conduction band of ZnO. When the doping concentration was low, the current through the film under the light was increased with the increasing concentration of PBI3, and the maximum photoconductivity was observed when the concentration was 3 wt%. If the doping concentration was further increased, the photoconductivity decreased, which means the aggregation morphology of the hybrid thin film might be changed under a high doping concentration. However, the ZnO thin film showed diode behavior even under illumination.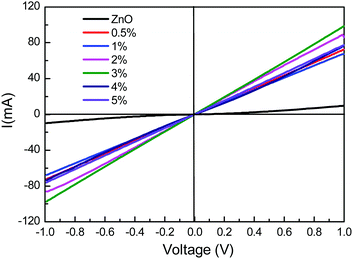 | ||
| Fig. 3 I–V curves of the devices for ITO/ZnO:PBI3 (100 nm) or ZnO (100 nm)/Al under AM 1.5G 1000 W m−2 illumination. | ||
2.5 High performance PSCs based on the photoconductive cathode interlayer
The hybrid thin films of ZnO:PBI3 with different doping concentrations from 0.5 wt% to 5 wt% were used as cathode interlayers to fabricate the inverted PSCs. Herein, the thickness of the ZnO:PBI3 interlayer was 100 nm, which is much thicker than the typical thickness of ZnO (∼30 nm) reported in the studies. The device configuration of the inverted PSCs was ITO/ZnO:PBI3 (100 nm)/PTB7:PC71BM (100 nm)/MoO3 (10 nm)/Al, where PTB7 was the electron donating polymer and PC71BM was the electron accepting material and the ratio between them was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
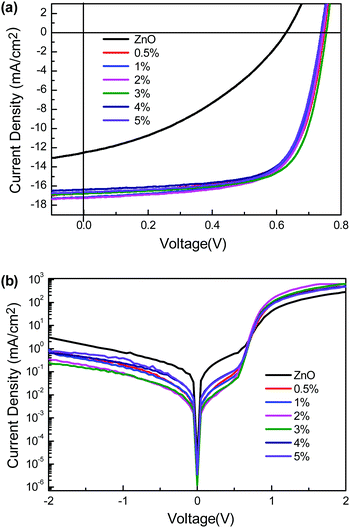
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, whereas MoO3 was used as the anode interlayer. As a reference, the device with ZnO as the cathode interlayer was also fabricated. The current density–voltage (J–V) characteristics of the inverted PSCs under AM 1.5G irradiation at 1000 W m−2 and in the dark are shown in Fig. 4, and the device performance metrics are summarized in Table 1. Note that for all the devices, performance using the photoconductive interlayers (ZnO:PBI3) was obviously improved relative to that of the ZnO interlayer-based device, even the doping concentration of PBI3 varied from 0.5 wt% to 5 wt%, which clearly indicates that the aggregation of PBI3 was well restricted. Specifically, a highly efficient i-OPV was achieved with an average PCE of 8.65% based on the hybrid interlayer of ZnO:PBI3 (3 wt%), which is about three times higher than that of a ZnO based device (2.84%). All the factors of open circuit voltage (Voc), short circuit current (Jsc), and fill factor (FF) are simultaneously enhanced for the ZnO:PBI3 interlayers-based devices. When comparing the factors of the ZnO interlayer and ZnO:PBI3 (3 wt%) interlayer-based devices, the Voc improved from 0.62 V to 0.745 V, the Jsc improved from 12.23 mA cm−2 to 16.66 mA cm−2, and the FF increased from 38.53% to 69.56%. The increased Voc of the photoconductive interlayer-based device can be attributed to the reduced work function of the modified electrode under the working conditions.23 The higher Jsc and FF indicated an improved charge selectivity and reduced recombination at the cathode interface and increased charge transport ability of the cathode interlayer. As shown in Fig. 4b, the dark current under reversed bias was significantly reduced and the dark current under the forward bias was enhanced in the dark J–V curves of the ZnO:PBI3 (3 wt%) based device, which corresponds well to the increased Jsc and FF. Indeed, the ZnO:PBI-H based device showed about 30-fold improvement in the rectification ratio relative to the pure ZnO based device at ±2 V.
1.5, whereas MoO3 was used as the anode interlayer. As a reference, the device with ZnO as the cathode interlayer was also fabricated. The current density–voltage (J–V) characteristics of the inverted PSCs under AM 1.5G irradiation at 1000 W m−2 and in the dark are shown in Fig. 4, and the device performance metrics are summarized in Table 1. Note that for all the devices, performance using the photoconductive interlayers (ZnO:PBI3) was obviously improved relative to that of the ZnO interlayer-based device, even the doping concentration of PBI3 varied from 0.5 wt% to 5 wt%, which clearly indicates that the aggregation of PBI3 was well restricted. Specifically, a highly efficient i-OPV was achieved with an average PCE of 8.65% based on the hybrid interlayer of ZnO:PBI3 (3 wt%), which is about three times higher than that of a ZnO based device (2.84%). All the factors of open circuit voltage (Voc), short circuit current (Jsc), and fill factor (FF) are simultaneously enhanced for the ZnO:PBI3 interlayers-based devices. When comparing the factors of the ZnO interlayer and ZnO:PBI3 (3 wt%) interlayer-based devices, the Voc improved from 0.62 V to 0.745 V, the Jsc improved from 12.23 mA cm−2 to 16.66 mA cm−2, and the FF increased from 38.53% to 69.56%. The increased Voc of the photoconductive interlayer-based device can be attributed to the reduced work function of the modified electrode under the working conditions.23 The higher Jsc and FF indicated an improved charge selectivity and reduced recombination at the cathode interface and increased charge transport ability of the cathode interlayer. As shown in Fig. 4b, the dark current under reversed bias was significantly reduced and the dark current under the forward bias was enhanced in the dark J–V curves of the ZnO:PBI3 (3 wt%) based device, which corresponds well to the increased Jsc and FF. Indeed, the ZnO:PBI-H based device showed about 30-fold improvement in the rectification ratio relative to the pure ZnO based device at ±2 V.
| Cathode interlayer | V oc (V) | J sc (mA cm−1) | FF (%) | PCEa (%) |
|---|---|---|---|---|
| a The best PCEs are given in the brackets. | ||||
| ZnO | 0.62 ± 0.01 | 12.23 ± 0.29 | 38.53 ± 2.34 | 2.84 ± 0.10 (2.95) |
| ZnO:PBI3 (0.5 wt%) | 0.745 ± 0.005 | 16.85 ± 0.07 | 65.45 ± 1.34 | 8.19 ± 0.18 (8.40) |
| ZnO:PBI3 (1 wt%) | 0.745 ± 0.005 | 17.06 ± 0.08 | 66.36 ± 0.51 | 8.38 ± 0.11 (8.48) |
| ZnO:PBI3 (2 wt%) | 0.745 ± 0.005 | 16.98 ± 0.15 | 66.81 ± 0.81 | 8.46 ± 0.21 (8.63) |
| ZnO:PBI3 (3 wt%) | 0.745 ± 0.005 | 16.66 ± 0.09 | 69.56 ± 0.46 | 8.63 ± 0.13 (8.79) |
| ZnO:PBI3 (4 wt%) | 0.745 ± 0.005 | 16.21 ± 0.13 | 68.15 ± 0.09 | 8.18 ± 0.16 (8.35) |
| ZnO:PBI3 (5 wt%) | 0.745 ± 0.005 | 16.40 ± 0.19 | 68.82 ± 0.23 | 8.39 ± 0.04 (8.42) |
3. Conclusion
In conclusion, we designed and synthesized a novel asymmetric PBI3 molecule. The self-aggregation of this PBI3 was restricted by introducing one N-alkyl unit in the molecular structure that hinders the consecutive molecular alignment. A robust thin film of ZnO:PBI3 was obtained by forming the Zn–N chemical bonds, which was confirmed by the FT-IR characterization. The hybrid of ZnO:PBI3 showed high photoconductivity under visible light irradiation and the thin films were applied as photoconductive cathode interlayers in the inverted PSCs to achieve high performance devices even though the thickness of the interlayer was 100 nm.4. Experiments
4.1 Materials
All reagents, unless otherwise specified, were purchased from Alfa Aesar or Sigma-Aldrich and used without further purification.4.2 Synthesis of PBI2
A portion of 1.5 g (1.5 mmol) of PBI3 and 98 mg (1.76 mmol) KOH solid were suspended in a 40 mL t-BuOH and stirred at 80 °C for 1.5 h. After being cooled down to room temperature, the reaction mixture was added to 50 mL glacial acetic acid under stirring. Then, 200 mL water was added to dilute the mixture. The solid was separated by filtration and washed with water (3 × 30 mL). The crude product was purified by column chromatography on silica gel using CH2Cl2/n-hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent. The pure product (550 mg, yield 40%) was dried in vacuum (10−3 mbar, SiO2) and was used for the next step. 1H NMR (600 MHz, CDCl3, ppm): δ = 8.02 (d, 4H), 7.42–7.44 (d, 2H), 7.27–7.30 (m, 2H), 7.17–7.21 (m, 5H), 7.01–7.06 (m, 8H), 6.89–6.90 (d, 4H), 6.44–6.45 (m, 1H), 2.05–2.07 (d, 12H), 1.90–1.91 (d, 3H). 13C NMR (100 MHz, CDCl3, ppm): δ = 162.26, 159.02, 155.01, 151.95, 139.40, 132.41, 132.41, 130.67, 128.87, 128.83, 127.15, 126.60, 126.55, 126.05, 125.98, 124.40, 124.30, 122.42, 120.65, 120.49, 119.47, 119.29, 118.15, 117.80, 117.07, 49.24, 28.68, 15.00. MALDI-TOF: calculated for C60H41NO9 919.28 m/z, found 919.31.
1) as the eluent. The pure product (550 mg, yield 40%) was dried in vacuum (10−3 mbar, SiO2) and was used for the next step. 1H NMR (600 MHz, CDCl3, ppm): δ = 8.02 (d, 4H), 7.42–7.44 (d, 2H), 7.27–7.30 (m, 2H), 7.17–7.21 (m, 5H), 7.01–7.06 (m, 8H), 6.89–6.90 (d, 4H), 6.44–6.45 (m, 1H), 2.05–2.07 (d, 12H), 1.90–1.91 (d, 3H). 13C NMR (100 MHz, CDCl3, ppm): δ = 162.26, 159.02, 155.01, 151.95, 139.40, 132.41, 132.41, 130.67, 128.87, 128.83, 127.15, 126.60, 126.55, 126.05, 125.98, 124.40, 124.30, 122.42, 120.65, 120.49, 119.47, 119.29, 118.15, 117.80, 117.07, 49.24, 28.68, 15.00. MALDI-TOF: calculated for C60H41NO9 919.28 m/z, found 919.31.
4.3 Synthesis of PBI3
A solution of 500 mg (0.55 mmol) PBI2 and 450 mg ammonium acetate in a 30 mL propionic acid was stirred at 140 °C for 12 h. After being cooled down to room temperature, the mixture was added to 300 mL water under stirring. The precipitated solid was separated by filtration. After being dried under vacuum (70 °C, 10−3 mbar, SiO2), 480 mg (yield: 95%) pure product was obtained. 1H NMR (600 MHz, CDCl3, ppm): δ = 8.37 (s, 1H), 8.02–8.03 (d, 4H), 7.42–7.44 (d, 2H), 7.27–7.30 (t, 2H), 7.15–7.20 (m, 5H), 7.0–7.03 (m, 8H), 6.89–6.91 (m, 4H), 6.44–6.66 (m, 1H), 2.05–2.07 (d, 12H), 1.90–1.91 (d, 3H). 13C NMR (100 MHz, CDCl3, ppm): δ = 162.00, 155.49, 155.01, 152.18, 139.56, 132.55, 132.03, 130.54, 130.51, 128.87, 128.81, 127.13, 126.48, 125.99, 125.98, 124.12, 121.99, 121.15, 119.69, 119.48, 119.44, 119.30, 118.83, 118.18, 117.86, 117.62, 49.16, 28.68, 15.04. MALDI-TOF: calculated for C60H42N2O8 918.29 m/z, found 918.27. UV/vis (CH2Cl2): λmax = 575 nm. Fluorescence (CH2Cl2): λmax = 595 nm.4.4 Device fabrication
Patterned ITO-glass substrates were used as cathode in the polymer solar cells. The ITO coated glass substrates were cleaned by sonication in detergent, deionized water, acetone, and isopropyl alcohol and dried in a nitrogen stream. The photoconductive cathode interlayer ZnO:PBI-H (100 nm) and ZnO (100 nm) were prepared according to the method reported in literature.23 The thickness of the films was determined by the surface profiler (Alfa Step-500, Tencor). The substrates were then transferred into a nitrogen-filled glove box. Active layer solutions (D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) with donor concentrations of 10 mg mL−1 were prepared in chlorobenzene (CB) with 3% (volume fraction) of DIO. The solutions were then spin-coated onto the substrates. The thicknesses of the active layers were approximately 100 nm. The resulting photoactive layer was dried in vacuum for 3 h before the electrode deposition. A 10 nm MoO3 layer and a 100 nm Al layer were subsequently evaporated through a shadow mask to define the active area of the devices (16 mm2) and form the top anode. All device fabrication processes were carried out in a N2-filled glove box (Braun GmbH). The PCE was determined from the J–V curve measurements (using a Keithley 2400 sourcemeter) under a 1 sun, AM 1.5G spectrum from a solar simulator (Oriel model 91192; 1000 W m−2). All the tests exhibited consistent results with relative errors within 5%. The solar simulator illumination intensity was determined using a monocrystal silicon reference cell (Hamamatsu S1133, with KG-5 visible colour filter) calibrated by the National Renewable Energy Laboratory (NREL).
1.5) with donor concentrations of 10 mg mL−1 were prepared in chlorobenzene (CB) with 3% (volume fraction) of DIO. The solutions were then spin-coated onto the substrates. The thicknesses of the active layers were approximately 100 nm. The resulting photoactive layer was dried in vacuum for 3 h before the electrode deposition. A 10 nm MoO3 layer and a 100 nm Al layer were subsequently evaporated through a shadow mask to define the active area of the devices (16 mm2) and form the top anode. All device fabrication processes were carried out in a N2-filled glove box (Braun GmbH). The PCE was determined from the J–V curve measurements (using a Keithley 2400 sourcemeter) under a 1 sun, AM 1.5G spectrum from a solar simulator (Oriel model 91192; 1000 W m−2). All the tests exhibited consistent results with relative errors within 5%. The solar simulator illumination intensity was determined using a monocrystal silicon reference cell (Hamamatsu S1133, with KG-5 visible colour filter) calibrated by the National Renewable Energy Laboratory (NREL).
4.5 Measurements
Author contributions
H. Zhao and Y. Luo equally contributed to this work.Acknowledgements
We are thankful for the financial support received from the Natural Science Foundation of China (51373054, 51573055), the National Basic Research Program of China (973 Program) (2014CB643504), and the Fundamental Research Funds for the Central Universities.References
- G. Li, R. Zhu and Y. Yang, Polymer solar cells, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Bulk heterojunction solar cells with internal quantum efficiency approaching 100%, Nat. Photonics, 2009, 3, 297–303 CrossRef CAS.
- S. Günes, H. Neugebauer and N. S. Sariciftci, Conjugated polymer-based organic solar cells, Chem. Rev., 2007, 107, 1324–1338 CrossRef PubMed.
- F. C. Krebs, Fabrication and processing of polymer solar cells: a review of printing and coating techniques, Sol. Energy Mater. Sol. Cells, 2009, 93, 394–412 CrossRef CAS.
- B. C. Thompson and J. M. J. Fréchet, Polymer–fullerene composite solar cells, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- Z. C. He, C. M. Zhong, X. Huang, W. Y. Wong, H. B. Wu, L. W. Chen, S. J. Su and Y. Cao, Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells, Adv. Mater., 2011, 23, 4636–4643 CrossRef CAS PubMed.
- L. M. Chen, Z. Xu, Z. Hong and Y. Yang, Interface investigation and engineering – achieving high performance polymer photovoltaic devices, J. Mater. Chem., 2010, 20, 2575–2598 RSC.
- Y. F. Li, Molecular design of photovoltaic materials for polymer solar cells: toward suitable electronic energy levels and broad absorption, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- Y. Lin, J. Y. Wang, Z. G. Zhang, H. T. Bai, Y. F. Li, D. B. Zhu and X. W. Zhan, An electron acceptor challenging fullerenes for efficient polymer solar cells, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS PubMed.
- L. Nian, J. D. Zhou, K. Zeng, X. Y. Wu, L. L. Liu, Z. Q. Xie, F. Huang and Y. G. Ma, The effect of interfacial diffusion on device performance of polymer solar cells: a quantitative view by active-layer doping, Sci. China: Chem., 2015, 58, 317–322 CrossRef CAS.
- Z. C. He, C. M. Zhong, S. J. Su, M. Xu, H. B. Wu and Y. Cao, Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure, Nat. Photonics, 2012, 190, 1–5 Search PubMed.
- Y. H. Liu, J. B. Zhao, Z. K. Li, C. Mu, W. Ma, H. W. Hu, K. Jiang, H. R. Lin, H. Ade and H. Yan, Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells, Nat. Commun., 2014, 10, 1–8 Search PubMed.
- S. S. Li, L. Ye, W. C. Zhao, S. Q. Zhang, S. Mukherjee, H. Ade and J. H. Hou, Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells, Adv. Mater., 2016, 28, 9423–9429 CrossRef CAS PubMed.
- K. M. O’Malley, C. Z. Li, H. L. Yip and A. K. Y. Jen, Enhanced open-circuit voltage in high performance polymer/fullerene bulk-heterojunction solar cells by cathode modification with a C60 surfactant, Adv. Energy Mater., 2012, 2, 82–86 CrossRef.
- F. Huang, K. S. Chen, H. L. Yip, S. K. Hau, O. Acton, Y. Zhang, J. D. Luo and A. K. Y. Jen, Development of new conjugated polymers with donor-π-bridge-acceptor side chains for high performance solar cells, J. Am. Chem. Soc., 2009, 131, 13886–13887 CrossRef CAS PubMed.
- Z. G. Yin, J. J. Wei and Q. D. Zheng, Interfacial materials for organic solar cells: recent advances and perspectives, Adv. Sci., 2016, 3, 1–37 Search PubMed.
- M. S. White, D. C. Olson, S. E. Shaheen, N. Kopidakis and D. S. Ginley, Inverted bulk-heterojunction organic photovoltaic device using a solution-derived ZnO underlayer, Appl. Phys. Lett., 2006, 89, 143517 CrossRef.
- Y. M. Sun, J. H. Seo, C. J. Takacs, J. Seifter and A. J. Heeger, Inverted polymer solar cells integrated with a low temperature-annealed sol–gel-derived ZnO film as an electron transport layer, Adv. Mater., 2011, 23, 1679–1683 CrossRef CAS PubMed.
- X. H. Liu, X. D. Li, Y. R. Li, C. J. Song, L. P. Zhu, W. J. Zhang, H. Q. Wang and J. F. Fang, High-performance polymer solar cells with PCE of 10.42% via Al-doped ZnO cathode interlayer, Adv. Mater., 2016, 28, 7405–7412 CrossRef CAS PubMed.
- K. S. Shin, K. H. Lee, H. H. Lee, D. Choi and S. W. Kim, Enhanced power conversion efficiency of inverted organic solar cells with a Ga-doped ZnO nanostructured thin film prepared using aqueous solution, J. Phys. Chem. C, 2010, 114, 15782–15785 CAS.
- A. Puetz, T. Stubhan, M. Reinhard, O. Loesch, E. Hammarberg, S. Wolf, C. Feldmann, H. Kalt, A. Colsmann and U. Lemmer, Organic solar cells incorporating buffer layers from indium doped zinc oxide nanoparticles, Sol. Energy Mater. Sol. Cells, 2011, 95, 579–585 CrossRef CAS.
- T. Z. Oo, R. D. Chandra, N. Yantara, R. R. Prabhakar, L. H. Wong, N. Mathews and S. G. Mhaisalkar, Zinc tin oxide (ZTO) electron transporting buffer layer in inverted organic solar cell, Org. Electron., 2012, 13, 870–874 CrossRef CAS.
- L. Nian, W. Q. Zhang, N. Zhu, L. L. Liu, Z. Q. Xie, H. B. Wu, F. Würthner and Y. G. Ma, Photoconductive cathode interlayer for highly efficient inverted polymer solar cells, J. Am. Chem. Soc., 2015, 137, 6995–6998 CrossRef CAS PubMed.
- L. Nian, Z. H. Chen, S. Herbst, Q. Y. Li, C. Z. Yu, X. F. Jiang, H. L. Dong, F. H. Li, L. L. Liu, F. Würthner, J. W. Chen, Z. Q. Xie and Y. G. Ma, Aqueous solution processed photoconductive cathode interlayer for high performance polymer solar cells with thick interlayer and thick active layer, Adv. Mater., 2016, 28, 7521–7526 CrossRef CAS PubMed.
- T. E. Kaiser, H. Wang, V. Stepanenk and F. Würthner, Supramolecular construction of fluorescent J-aggregates based on hydrogen-bonded perylene dyes, Angew. Chem., Int. Ed., 2007, 46, 5541–5544 CrossRef CAS PubMed.
- Z. Q. Xie, V. Stepanenko, B. Fimmel and F. Würthner, An organogelator design without solubilizing side chains by backbone contortion of a perylene bisimide pigment, Mater. Horiz., 2014, 1, 355–359 RSC.
- C. E. Small, S. Chen, J. Subbiah, C. M. Amb, S. W. Tsang, T. H. Lai, J. R. Reynolds and F. So, High-efficiency inverted dithienogermole–thienopyrrolodione-based polymer solar cells, Nat. Photonics, 2012, 6, 115–120 CrossRef CAS.
- H. M. Xiong, X. Zhao and J. S. Chen, New polymer-inorganic nanocomposites: PEO–ZnO and PEO–ZnO–LiClO4 films, J. Phys. Chem. B, 2001, 105, 10169–10174 CrossRef CAS.
- S. B. Jo, J. H. Lee, M. Sim, M. Kim, J. H. Park, Y. S. Choi, Y. Kim, S. G. Ihn and K. Cho, High performance organic photovoltaic cells using polymer-hybridized ZnO nanocrystals as a cathode interlayer, Adv. Energy Mater., 2011, 1, 690–698 CrossRef CAS.
- J. Liu, J. Wu, S. Y. Shao, Y. F. Deng, B. Meng, Z. Y. Xie, Y. Y. Geng, L. X. Wang and F. L. Zhang, Printable highly conductive conjugated polymer sensitized ZnO NCs as cathode interfacial layer for efficient polymer solar cells, ACS Appl. Mater. Interfaces, 2014, 6, 8237–8245 CAS.
- C. Liu, L. L. Zhang, D. L. Wang, J. J. Yang and X. H. Liu, Synthesis, crystal structure and catalytic properties of novel Zn–N metal complexes based on ethylenediamine derivatives, J. Synth. Cryst., 2015, 44, 287–293 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra, MALDI-TOF spectra, CV curves, SEM images, UV-vis absorption and photoluminescence spectra. See DOI: 10.1039/c6qm00286b |
| This journal is © the Partner Organisations 2017 |