Rh(III)-catalyzed C–H oxidative ortho-olefination of arenes using 7-azaindole as a directing group and utilization in the construction of new tetracyclic heterocycles containing a 7-azaindole skeleton†
Bin
Liu
,
Ridong
Li
,
Wei
Zhan
,
Xin
Wang
,
Zemei
Ge
* and
Runtao
Li
*
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, China. E-mail: lirt@bjmu.edu.cn
First published on 12th May 2016
Abstract
A Rh(III)-catalyzed C–H ortho-mono-olefination of aryls directed by 7-azaindoles was reported. This method opens up a novel pathway to synthesize complex 7-azaindole derivatives and interestingly, we observed an intramolecular cascade annulation via unexpected double directing groups (7-azaindole and carbonyl).
Introduction
The 7-azaindole skeleton, widely used as key scaffolds, has been found in various pharmaceutical molecules and drug candidates (Fig. 1).1 More important, the two nitrogen atoms in 7-azaindole scaffolds could serve as both hydrogen-bond donors and acceptors to form bidentate hydrogen bonds leading to the enhanced activity in the binding site.1b However, up to now, direct modifications of 7-azaindoles are still rare.2 In 2009, Fagnou reported a Pd(II)-catalyzed arylation of 7-azaindoles via N-oxide activation.3a Later, a direct C–H arylation of N-methyl-7-azaindole at the C-2 position by diverse arylboronic acids was also realized by Das and co-workers.3b Very recently, Das et al. developed a Pd-catalyzed oxidative C-3 alkenylation of 7-azaindoles.4 Moreover, 7-azaindole also has an inherent property in C–H activation. Recently, Xu and Dong independently reported the Rh(III)-catalyzed 7-azaindole directed C–H activation of arenes forming novel complex derivatives.5 Despite this progress, the 7-azaindole directed Csp2–Csp2 coupling/addition reaction has not been studied yet.Since 1967, the report of the pioneering work by Fujiwara and Moritani, oxidative olefination of C–H bonds has attracted increasing attention.6 This strategy has been applied to the synthesis of many useful complex structures owing to its step-economic and waste-reducing process. Indeed, although various directing groups (e.g., pyrazole, pyridine, imine, amide, carboxylic acid and N-nitroso) have been identified as effective tools for selective C–C couplings,7 further development is still in strong demand regarding high ortho-monoselectivity and wide substrate scope. Thus based on the previous reports and as well as our own interest in developing novel complex 7-azaindole derivatives,1b,5c herein we reported an efficient method for the 7-azaindole directed C–H oxidative ortho-olefination of arenes catalyzed by rhodium and its utilization in the construction of tetracyclic heterocycles containing 7-azaindole skeleton. Though the Dong group reported a novel Rh(III)-catalyzed C–H activation of 7-azaindoles with electron-rich alkenyl esters while our paper was in preparation,8 there is obvious difference between their work and our work.
Results and discussion
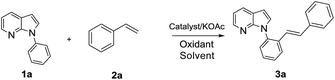
At the outset, the alkenylation of 1-phenyl-7-azaindole (1a) with styrene (2a) was used as model to optimize the reaction conditions (Table 1). First, four kinds of commonly used metal catalysts, Pd(II), Ru(II), Rh(III) and Ir(III), were examined in the in the presence of KOAc and AgOAc in toluene at 120 °C for 14 h (entries 1–5). The best result is the [RhCp*Cl2]2 as catalyst to afford the desired product 3a in 18% yield (entry 4). Then, different oxidants were screened using [RhCp*Cl2]2 as catalyst. To our delight, the use of Cu(OAc)2 proved to be a superior oxidant and product 3a was isolated in 66% yield (entries 6–8). Subsequently, the effect of solvents were examined and it was found that 1,4-dioxane as solvent could improve the yield from 66% to 76% (entries 8–13). Finally, different additives were also screened and KOAc was still the most effective (entries 13–16). Interestingly, when excess styrene was added, bis-olefinated product was not observed, suggesting a high ortho-mono-selectivity (entry 17).| Entry | Cat. | Oxidant | Solvent | Yield 3a (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), catalyst (5 mol%), additive (0.2 mmol) and oxidant (0.4 mmol) in solvent (3.0 mL), stirred in a sealed tube at 120 °C for 14 h. b Isolated yields. c NaOAc as the additives. d CsOAc as the additives. e No additives were added. f 2a in large excess (1.2 mmol). | ||||
| 1 | — | AgOAc | Toluene | n.r. |
| 2 | Pd(OAc)2 | AgOAc | Toluene | n.r. |
| 3 | [RuCl2(p-cymene)]2 | AgOAc | Toluene | n.r. |
| 4 | [RhCp*Cl2]2 | AgOAc | Toluene | 18 |
| 5 | [IrCp*Cl2]2 | AgOAc | Toluene | n.r. |
| 6 | [RhCp*Cl2]2 | AgO | Toluene | 14 |
| 7 | [RhCp*Cl2]2 | Ag2CO3 | Toluene | 12 |
| 8 | [RhCp*Cl2]2 | Cu(OAc)2 | Toluene | 66 |
| 9 | [RhCp*Cl2]2 | Cu(OAc)2 | p-Xylene | 16 |
| 10 | [RhCp*Cl2]2 | Cu(OAc)2 | CH3CN | 59 |
| 11 | [RhCp*Cl2]2 | Cu(OAc)2 | DMF | 32 |
| 12 | [RhCp*Cl2]2 | Cu(OAc)2 | t AmOH | n.r. |
| 13 | [RhCp*Cl 2 ] 2 | Cu(OAc) 2 | 1,4-Dioxane | 76 |
| 14c | [RhCp*Cl2]2 | Cu(OAc)2 | 1,4-Dioxane | 74 |
| 15d | [RhCp*Cl2]2 | Cu(OAc)2 | 1,4-Dioxane | 54 |
| 16e | [RhCp*Cl2]2 | Cu(OAc)2 | 1,4-Dioxane | 12 |
| 17f | [RhCp*Cl2]2 | Cu(OAc)2 | 1,4-Dioxane | 75 |
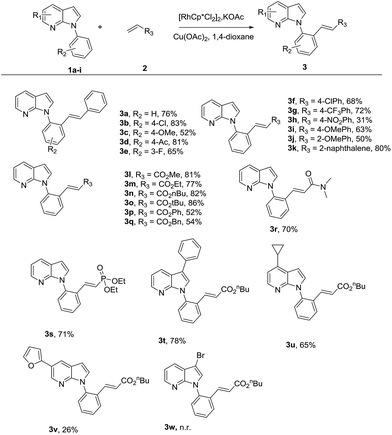
With the optimized reaction conditions in hand, the scope of the current method was investigated. We tested various substituted 7-azaindoles as well as styrenes and acrylates under the optimized reaction conditions (Table 2). Gratifyingly, the reaction condition was compatible with a variety of common functional groups substituted on 1-phenyl ring of 7-azaindole and aryl ring of styrene to afford the corresponding products in good to excellent yields (3a–k). For the 7-azaindoles, both the electron-deficient and electron-rich 1-aryls showed good reactivity (3b–e). For the coupling partners, even instead of styrene with 2-vinylnaphthalene, the product 3k was still obtained in 80% yield. When treating 1a with acrylates, the oxidative coupling reaction also proceeded well to afford the desired products in high yields (3l–o, 77–86%). However, phenyl-acrylate and benzyl-acrylate gave lower yields, presumably due to steric hindrance (3p, q). Varying the coupling partner with acrylamide and vinyl phosphonate, it was found that the reaction could be readily transformed without loss of reactivity (3r, s). Moreover, aryl, heteroaryl and alkyl substituents on the 7-azaindole core were all well tolerated to give the desired products in moderate to good yields (3t–v). Unfortunately, 3-halo substituted 7-azaindoles did not afford the expected products which need further explanation (3w).
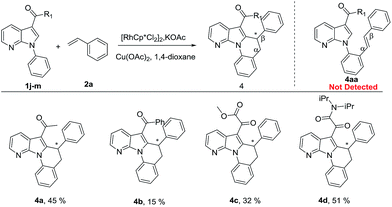
Interestingly, when 3-acetyl-1-phenyl-7-azaindole 1j was subjected to the reaction with styrene 2a under standard conditions, an unexpected intramolecular cascade annulation took place to afford the tetracyclic compound 4a in 45% yield (see ESI for characterization†). According to the literature,9 the 3-acetyl group of 1j should be a directing group, which could trigger the intramolecular annulation (Table 3). It has to be pointed out, unfortunately, the intermediates 4aa could not be detected in the process of the reaction. Gladly, when replacing the 3-acetyl group with other similar directing groups, the corresponding tetracycles 4b–d were also smoothly obtained, further demonstrating the directing function of the 3-acyl moiety and oxalyl amide/DG (4d, 51%) proved to be the most efficient. To the best of our knowledge, we are the first to synthesize this new kind of azaindole fused heterocyclic derivatives via Rh(III)-catalyzed cascade C–H alkenylation/intramolecular carbonyl directed annulation. Despite the modest yield, while in these cases, the majority of the starting materials were recovered.
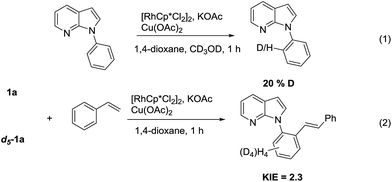
To explore the reaction pathway, the deuterium-labeling experiments were also carried out (Scheme 1). With the excess introduction of CD3OD, the H/D exchange was clearly observed (20% D incorporation), which illustrates that the C–H cleavage step occurs and is reversible. A mixture of 1a and d5-1a was also allowed to react with 2a, which demonstrated an intermolecular kinetic isotope effect (KIE) of 2.3, thus suggesting that the C–H bond cleavage of the arenes might be involved in the rate-limiting step.
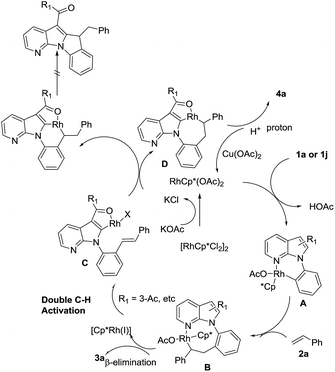
On the basis of the above results and recent achievements in Rh(III)-catalyzed oxidative C–H olefination,7,10 a plausible mechanism is proposed (Scheme 2). First, monomeric RhCp*(OAc)2 reacted with 7-azaindole to form rhodacycle species A. Subsequently, olefin 2a inserted into the C–Rh bond to give an intermediate B which undergo β-H elimination to afford products 3a. When 3-acetyl existed,9 rhodacycle species C appeared from the second C–H activation of the ortho C–H bond, followed by C–Rh addition giving the cyclometalated complex D, which undergo protonation giving the alkylation product 4a.
Conclusions
In summary, we have developed a useful method for the synthesis of a wide range of ortho-olefinated 7-azaindole derivatives. Moreover, we are the first to realize the cascade reaction of Rh(III)-catalyzed C–H alkenylation/intramolecular carbonyl directed C–C annulation for 1-phenyl-3-acyl-7-azaindoles, which lead to new azaindole fused heterocyclic derivatives. Further applications of these synthesized molecules as well as their biological activities are currently under investigation in our laboratory.Experimental section
Unless otherwise noted, starting materials were purchased from J&K, TCI, Sigma, Arcos, Alfa and used without purification. Catalysts and additives were purchased from TCI, solvents were used without pre-purification. All of the reactions were monitored by thin layer chromatography (TLC) on GF254 silica gel plates. 1H NMR spectra and 13C NMR spectra were recorded on a Bruker AVANCE III 400 (400 MHz) spectrometer in needful d-reagents (CDCl3) with tetramethylsilane (TMS) as an internal reference. HRMS of additional products were carried out on Bruker Apex IV FTMS. Melting points were determined on X5A made by Beijing Fukai Company as uncorrected values. All manipulations were conducted with a standard sealed tube.General procedure for Rh(III)-catalyzed oxidative olefination and annulation via double DGs
To a 15 mL sealed tube was added 1 (0.2 mmol), styrene (0.3 mmol, 31 mg), [RhCp*Cl2]2 (5%, 6.1 mg), KOAc (0.2 mmol, 20 mg), Cu(OAc)2 (0.4 mmol, 72 mg) and 1,4-dioxane (3 mL). The reaction mixture was vigorously stirred at 120 °C under air for 14–20 h. And then the solvent was evaporated under reduced pressure and the residue was purified by flash column chromatography on silica gel to afford the desired products 3 or 4 (PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 8
EtOAc = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
(E)-1-(2-Styrylphenyl)-1H-pyrrolo[2,3-b]pyridine (3a)
Light yellow oil, (yield, 76%), 1H NMR (400 MHz, CDCl3) δ 8.35 (dd, J = 4.7, 1.5 Hz, 1H), 8.01 (dd, J = 7.8, 1.5 Hz, 1H), 7.86 (d, J = 7.7 Hz, 1H), 7.54–7.38 (m, 3H), 7.33 (d, J = 3.5 Hz, 1H), 7.24 (d, J = 4.4 Hz, 4H), 7.22–7.17 (m, 1H), 7.13 (dd, J = 7.8, 4.7 Hz, 1H), 7.06 (d, J = 16.3 Hz, 1H), 6.71 (d, J = 16.3 Hz, 1H), 6.65 (d, J = 3.5 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 148.6, 144.0, 137.1, 136.1, 134.8, 130.9, 130.2, 129.1, 128.6, 128.4, 128.3, 127.8, 126.6, 126.5, 124.0, 120.7, 116.5, 101.0. HRMS m/z (ESI) calcd for C21H17N2 (M + H)+: 297.1386, found 297.1382.(E)-1-(4-Chloro-2-styrylphenyl)-1H-pyrrolo[2,3-b]pyridine (3b)
White solid, (yield, 83%), mp 96.3–98.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 4.7, 1.3 Hz, 1H), 8.01 (dd, J = 7.8, 1.4 Hz, 1H), 7.83 (d, J = 2.0 Hz, 1H), 7.39 (dt, J = 8.4, 5.2 Hz, 3H), 7.30 (d, J = 3.6 Hz, 1H), 7.27–7.21 (m, 4H), 7.14 (dd, J = 7.8, 4.7 Hz, 1H), 7.06 (d, J = 16.3 Hz, 1H), 6.64 (dd, J = 12.6, 9.9 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 148.6, 144.1, 136.6, 136.4, 134.5, 134.3, 132.2, 129.9, 129.8, 129.2, 128.7, 128.2, 128.2, 126.8, 126.3, 122.8, 120.7, 116.7, 101.4. HRMS m/z (ESI) calcd for C21H16ClN2 (M + H)+: 331.0997, found 331.0993.(E)-1-(4-Methoxy-2-styrylphenyl)-1H-pyrrolo[2,3-b]pyridine (3c)
Light yellow solid, (yield, 54%), mp 34.4–36.8 °C. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 4.7, 1.3 Hz, 1H), 8.04–7.97 (m, 1H), 7.35 (dd, J = 8.4, 5.7 Hz, 2H), 7.29 (d, J = 3.5 Hz, 1H), 7.27–7.19 (m, 5H), 7.11 (dd, J = 7.8, 4.7 Hz, 1H), 7.02 (d, J = 16.3 Hz, 1H), 6.97 (dd, J = 8.6, 2.8 Hz, 1H), 6.66–6.57 (m, 2H), 3.92 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.5, 148.9, 144.0, 137.0, 136.1, 131.1, 130.4, 129.8, 129.4, 129.0, 128.6, 127.9, 126.7, 123.9, 120.5, 116.3, 114.2, 111.0, 100.7, 55.7. HRMS m/z (ESI) calcd for C22H19N2O (M + H)+: 327.1492, found 327.1488.(E)-1-(4-(1H-Pyrrolo[2,3-b]pyridin-1-yl)-3-styrylphenyl)ethan-1-one (3d)
Yellow solid, (yield, 81%), mp 40.0–41.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.48–8.41 (m, 1H), 8.38–8.31 (m, 1H), 8.08–7.95 (m, 2H), 7.68–7.57 (m, 1H), 7.38–7.33 (m, 1H), 7.31–7.13 (m, 7H), 6.84–6.75 (m, 1H), 6.72–6.63 (m, 1H), 2.70 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 197.4, 148.5, 144.1, 139.9, 136.7, 136.5, 134.7, 132.1, 129.6, 129.3, 128.7, 128.6, 128.2, 128.0, 126.8, 126.8, 123.4, 120.9, 117.0, 101.9, 26.9. HRMS m/z (ESI) calcd for C23H19N2O (M + H)+: 339.1492, found 339.1484.(E)-1-(5-Fluoro-2-styrylphenyl)-1H-pyrrolo[2,3-b]pyridine (3e)
Colorless oil, (yield, 65%), 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 4.7, 1.5 Hz, 1H), 8.01 (dd, J = 7.8, 1.6 Hz, 1H), 7.38–7.28 (m, 3H), 7.25–7.15 (m, 6H), 7.15–7.11 (m, 1H), 7.04 (d, J = 16.7 Hz, 1H), 6.66 (d, J = 3.6 Hz, 1H), 6.53 (d, J = 16.7 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 162.6, 148.5, 144.1, 137.6, 137.4, 135.4 (d, JC–F = 11 Hz), 129.8, 129.2, 128.5, 128.1, 128.0, 126.5, 124.6 (d, JC–F = 4 Hz), 123.6 (d, JC–F = 13 Hz), 120.65, 118.35, 116.71, 116.0 (d, JC–F = 24 Hz), 101.47. HRMS m/z (ESI) calcd for C21H16FN2 (M + H)+: 315.1292, found 315.1290.(E)-1-(2-(4-Chlorostyryl)phenyl)-1H-pyrrolo[2,3-b]pyridine (3f)
Yellow solid, (yield, 68%), mp 39.9–41.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 4.7, 1.5 Hz, 1H), 8.01 (dd, J = 7.8, 1.5 Hz, 1H), 7.90–7.77 (m, 1H), 7.52–7.42 (m, 3H), 7.32 (d, J = 3.5 Hz, 1H), 7.22–7.17 (m, 2H), 7.13 (dd, J = 7.9, 4.6 Hz, 3H), 6.99 (d, J = 16.3 Hz, 1H), 6.66 (dd, J = 9.9, 6.4 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 148.6, 144.0, 136.2, 135.6, 134.4, 133.4, 130.1, 129.5, 129.1, 128.8, 128.6, 128.6, 128.5, 127.8, 126.5, 124.7, 120.6, 116.6, 101.2. HRMS m/z (ESI) calcd for C21H16ClN2 (M + H)+: 331.0997, found 331.0990.(E)-1-(2-(4-(Trifluoromethyl)styryl)phenyl)-1H-pyrrolo[2,3-b]pyridine (3g)
Light red oil, (yield, 72%), 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 4.6 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 7.85 (d, J = 7.3 Hz, 1H), 7.56–7.38 (m, 5H), 7.30 (dd, J = 11.4, 5.8 Hz, 3H), 7.13 (dd, J = 7.8, 4.7 Hz, 1H), 7.05 (d, J = 16.3 Hz, 1H), 6.78 (d, J = 16.3 Hz, 1H), 6.66 (d, J = 3.5 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 148.7, 144.1, 140.6, 136.5, 134.1, 130.0, 129.6, 129.5, 129.2, 129.1, 129.0, 128.7, 128.5, 126.7, 126.6, 125.5 (CF3, q, JC–F = 11 Hz), 122.8, 120.7, 116.6, 101.3. HRMS m/z (ESI) calcd for C22H16F3N2 (M + H)+: 365.1260, found 365.1268.(E)-1-(2-(4-Nitrostyryl)phenyl)-1H-pyrrolo[2,3-b]pyridine (3h)
Yellow oil, (yield, 31%), 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 4.7, 1.3 Hz, 1H), 8.09 (d, J = 8.8 Hz, 2H), 8.04 (dd, J = 7.9, 1.4 Hz, 1H), 7.92–7.84 (m, 1H), 7.56–7.46 (m, 3H), 7.37–7.30 (m, 3H), 7.16 (dd, J = 7.8, 4.7 Hz, 1H), 7.09 (d, J = 16.3 Hz, 1H), 6.86 (d, J = 16.3 Hz, 1H), 6.69 (d, J = 3.5 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 148.6, 146.9, 144.1, 143.6, 136.8, 133.6, 129.9, 129.5, 129.3, 129.2, 128.8, 128.6, 128.3, 127.0, 126.7, 124.0, 120.7, 116.7, 101.5. HRMS m/z (ESI) calcd for C21H16N3O2 (M + H)+: 342.1237, found 342.1238.(E)-1-(2-(4-Methoxystyryl)phenyl)-1H-pyrrolo[2,3-b]pyridine (3i)
Light yellow solid, (yield, 63%), mp 36.5–37.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.34 (dd, J = 4.7, 1.3 Hz, 1H), 8.01 (dd, J = 7.8, 1.4 Hz, 1H), 7.84 (d, J = 7.4 Hz, 1H), 7.50–7.37 (m, 3H), 7.33 (d, J = 3.5 Hz, 1H), 7.18 (d, J = 8.7 Hz, 2H), 7.13 (dd, J = 7.8, 4.7 Hz, 1H), 7.01 (d, J = 16.3 Hz, 1H), 6.78 (d, J = 8.7 Hz, 2H), 6.64 (d, J = 3.5 Hz, 1H), 6.56 (d, J = 16.2 Hz, 1H), 3.76 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.4, 148.6, 143.9, 135.9, 135.1, 130.4, 130.2, 130.0, 129.0, 128.6, 128.4, 127.9, 127.9, 126.2, 121.8, 120.6, 116.5, 114.0, 100.9, 55.3. HRMS m/z (ESI) calcd for C22H19N2O (M + H)+: 327.1492, found 327.1486.(E)-1-(2-(2-Methoxystyryl)phenyl)-1H-pyrrolo[2,3-b]pyridine (3j)
Colorless oil, (yield, 50%), 1H NMR (400 MHz, CDCl3) δ 8.35 (dd, J = 4.7, 1.5 Hz, 1H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 7.93–7.87 (m, 1H), 7.51–7.36 (m, 4H), 7.34 (d, J = 3.5 Hz, 1H), 7.20–7.08 (m, 3H), 6.81 (dd, J = 12.1, 4.3 Hz, 2H), 6.73 (d, J = 16.4 Hz, 1H), 6.63 (d, J = 3.5 Hz, 1H), 3.76 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 157.0, 148.7, 143.9, 136.1, 135.4, 130.3, 129.0, 128.8, 128.49, 128.4, 128.0, 126.8, 126.5, 126.2, 125.8, 124.4, 120.6, 116.4, 110.9, 100.8, 55.4. HRMS m/z (ESI) calcd for C22H19N2O (M + H)+: 327.1492, found: 327.1497.(E)-1-(2-(2-(Naphthalen-2-yl)vinyl)phenyl)-1H-pyrrolo[2,3-b]pyridine (3k)
Light yellow solid, (yield, 80%), mp 156.2–156.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.36 (dd, J = 4.7, 1.3 Hz, 1H), 8.03 (dd, J = 7.8, 1.5 Hz, 1H), 7.91 (d, J = 7.4 Hz, 1H), 7.80–7.70 (m, 2H), 7.66 (d, J = 5.8 Hz, 2H), 7.55–7.30 (m, 7H), 7.24 (d, J = 2.7 Hz, 1H), 7.14 (dd, J = 7.8, 4.7 Hz, 1H), 6.82 (d, J = 16.3 Hz, 1H), 6.67 (d, J = 3.5 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 148.7, 144.0, 136.2, 134.8, 134.7, 133.5, 133.1, 131.0, 130.2, 129.1, 128.6, 128.4, 128.4, 128.2, 128.0, 127.6, 126.9, 126.5, 126.3, 126.0, 124.4, 123.4, 120.7, 116.5, 101.1. HRMS m/z (ESI) calcd for C25H19N2 (M + H)+: 347.1543, found 347.1539.Methyl (E)-3-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3l)
Light yellow oil, (yield, 81%), 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 4.7, 1.5 Hz, 1H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.59–7.43 (m, 3H), 7.38 (d, J = 16.0 Hz, 1H), 7.27 (d, J = 3.6 Hz, 1H), 7.18–7.09 (m, 1H), 6.67 (d, J = 3.6 Hz, 1H), 6.37 (d, J = 16.0 Hz, 1H), 3.68 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 167.0, 148.6, 144.0, 140.3, 137.6, 131.8, 130.9, 129.7, 129.3, 128.9, 128.5, 127.6, 120.8, 120.0, 116.8, 101.8, 51.7. HRMS m/z (ESI) calcd for C17H15N2O2 (M + H)+: 279.1128, found 279.1132.Ethyl (E)-3-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3m)
Light yellow oil, (yield, 77%), 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 4.7, 1.4 Hz, 1H), 8.00 (dd, J = 7.8, 1.4 Hz, 1H), 7.81 (d, J = 7.9 Hz, 1H), 7.57–7.42 (m, 3H), 7.37 (d, J = 16.0 Hz, 1H), 7.31–7.24 (m, 1H), 7.13 (dd, J = 7.8, 4.7 Hz, 1H), 6.67 (d, J = 3.6 Hz, 1H), 6.38 (d, J = 16.0 Hz, 1H), 4.14 (q, J = 7.1 Hz, 2H), 1.22 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 166.5, 148.6, 144.0, 140.0, 137.6, 131.9, 130.8, 129.8, 129.2, 128.8, 128.4, 127.5, 120.8, 120.4, 116.8, 101.8, 60.5, 14.2. HRMS m/z (ESI) calcd for C18H17N2O2 (M + H)+: 293.1285, found 293.1287.n Butyl (E)-3-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3n)
Light yellow oil, (yield, 82%), 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 4.7, 1.5 Hz, 1H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 7.81 (d, J = 7.9 Hz, 1H), 7.57–7.43 (m, 3H), 7.34 (d, J = 16.0 Hz, 1H), 7.28 (d, J = 3.6 Hz, 1H), 7.13 (dd, J = 7.8, 4.7 Hz, 1H), 6.67 (d, J = 3.6 Hz, 1H), 6.38 (d, J = 16.0 Hz, 1H), 4.07 (t, J = 6.6 Hz, 2H), 1.55 (dt, J = 14.5, 6.6 Hz, 2H), 1.33–1.26 (m, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 166.6, 148.6, 144.0, 139.9, 137.6, 131.9, 130.8, 129.8, 129.2, 128.8, 128.4, 127.4, 120.8, 120.4, 116.7, 101.7, 64.3, 30.6, 19.1, 13.7. HRMS m/z (ESI) calcd for C20H21N2O2 (M + H)+: 321.1598, found 321.1596.t Butyl (E)-3-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3o)
Light yellow oil, (yield, 86%), 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 4.7, 1.4 Hz, 1H), 7.99 (dd, J = 7.8, 1.5 Hz, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.62–7.43 (m, 3H), 7.26 (dd, J = 12.8, 7.0 Hz, 2H), 7.13 (dd, J = 7.8, 4.7 Hz, 1H), 6.66 (d, J = 3.6 Hz, 1H), 6.33 (d, J = 15.9 Hz, 1H), 1.40 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 165.8, 148.7, 143.9, 138.8, 137.5, 132.0, 130.6, 129.8, 129.2, 128.8, 128.4, 127.3, 122.2, 120.8, 116.7, 101.6, 80.5, 28.1. HRMS m/z (ESI) calcd for C20H21N2O2 (M + H)+: 321.1598, found 321.1601.Phenyl (E)-3-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3p)
Yellow solid, (yield, 52%), mp 102.5–104.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 4.7, 1.4 Hz, 1H), 7.99 (dd, J = 7.8, 1.5 Hz, 1H), 7.95–7.85 (m, 1H), 7.64–7.48 (m, 4H), 7.40–7.29 (m, 3H), 7.19 (t, J = 7.4 Hz, 1H), 7.13 (dd, J = 7.9, 4.7 Hz, 1H), 7.05 (d, J = 7.6 Hz, 2H), 6.68 (d, J = 3.6 Hz, 1H), 6.57 (d, J = 16.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 164.9, 150.7, 148.7, 144.1, 142.0, 137.9, 131.6, 131.3, 129.7, 129.4, 129.3, 128.9, 128.5, 127.7, 125.7, 121.5, 120.8, 119.3, 116.9, 102.0. HRMS m/z (ESI) calcd for C22H17N2O2 (M + H)+: 341.1285, found 341.1282.Benzyl (E)-3-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3q)
Colorless oil, (yield, 54%), 1H NMR (400 MHz, CDCl3) δ 8.32 (dd, J = 4.7, 1.5 Hz, 1H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.57–7.44 (m, 3H), 7.41 (d, J = 16.0 Hz, 1H), 7.37–7.29 (m, 3H), 7.29–7.23 (m, 3H), 7.14 (dd, J = 7.8, 4.7 Hz, 1H), 6.67 (d, J = 3.6 Hz, 1H), 6.43 (d, J = 16.0 Hz, 1H), 5.12 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 166.3, 148.7, 144.0, 140.6, 137.7, 136.0, 131.8, 131.0, 129.7, 129.3, 128.8, 128.5, 128.5, 128.1, 127.9, 127.5, 120.8, 119.9, 116.8, 101.8, 66.2. HRMS m/z (ESI) calcd for C23H19N2O2 (M + H)+: 355.1441, found 355.1436.(E)-3-(2-(1H-Pyrrolo[2,3-b]pyridin-1-yl)phenyl)-N,N-dimethylacrylamide (3r)
Red solid, (yield, 70%), mp 91.7–93.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.30 (dd, J = 4.7, 1.4 Hz, 1H), 7.97 (dd, J = 7.8, 1.5 Hz, 1H), 7.82–7.70 (m, 1H), 7.55–7.42 (m, 3H), 7.37–7.24 (m, 2H), 7.11 (dd, J = 7.8, 4.7 Hz, 1H), 6.65 (d, J = 3.5 Hz, 1H), 6.54 (d, J = 15.6 Hz, 1H), 2.93 (s, 3H), 2.91 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 166.3, 148.6, 144.0, 137.4, 137.1, 133.1, 130.1, 129.6, 129.2, 129.1, 128.5, 128.3, 120.8, 120.2, 116.6, 101.6, 37.2, 35.7. HRMS m/z (ESI) calcd for C18H18N3O (M + H)+: 292.1444, found 292.1441.Diethyl (E)-(2-(1H-pyrrolo[2,3-b]pyridin-1-yl)styryl)phosphonate (3s)
Colorless oil, (yield, 71%), 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 3.9 Hz, 1H), 8.00 (d, J = 7.7 Hz, 1H), 7.77 (d, J = 7.3 Hz, 1H), 7.49 (ddd, J = 25.0, 15.8, 7.4 Hz, 3H), 7.30 (d, J = 3.5 Hz, 1H), 7.13 (dd, J = 7.7, 4.8 Hz, 1H), 7.01 (dd, J = 22.2, 17.5 Hz, 1H), 6.68 (d, J = 3.4 Hz, 1H), 6.14 (t, J = 17.6 Hz, 1H), 4.06–3.83 (m, 4H), 1.18 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 148.5, 144.0, 137.0, 132.6, 132.4, 130.8, 129.6, 129.5, 128.7, 128.6, 127.4, 121.0, 116.8, 101.9, 61.9, 61.9, 16.3, 16.2. HRMS m/z (ESI) calcd for C19H22N2O3P (M + H)+: 357.1363, found: 357.1350.n Butyl (E)-3-(2-(3-phenyl-1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3t)
White solid, (yield, 78%), mp 79.2–80.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.37 (dd, J = 4.7, 1.4 Hz, 1H), 8.30 (dd, J = 8.0, 1.5 Hz, 1H), 7.84 (d, J = 7.4 Hz, 1H), 7.71–7.63 (m, 2H), 7.60–7.39 (m, 7H), 7.34 (t, J = 7.4 Hz, 1H), 7.21 (dd, J = 8.0, 4.7 Hz, 1H), 6.42 (d, J = 16.0 Hz, 1H), 4.07 (t, J = 6.6 Hz, 2H), 1.53 (dt, J = 14.5, 6.6 Hz, 2H), 1.30–1.23 (m, 2H), 0.83 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 166.5, 149.1, 144.4, 139.8, 137.3, 134.3, 131.9, 130.9, 129.0, 128.8, 128.6, 128.6, 127.5, 127.3, 126.7, 126.6, 120.6, 119.1, 117.3, 117.2, 64.4, 30.6, 19.1, 13.7. HRMS m/z (ESI) calcd for C26H25N2O2 (M + H)+: 397.1911, found 397.1911.n Butyl (E)-3-(2-(4-cyclopropyl-1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3u)
Colorless oil, (yield, 65%), 1H NMR (400 MHz, CDCl3) δ 8.18 (d, J = 5.0 Hz, 1H), 7.80 (d, J = 7.9 Hz, 1H), 7.58–7.41 (m, 3H), 7.37 (d, J = 16.0 Hz, 1H), 7.23 (d, J = 3.6 Hz, 1H), 6.78 (d, J = 3.6 Hz, 1H), 6.63 (d, J = 5.1 Hz, 1H), 6.39 (d, J = 16.0 Hz, 1H), 4.08 (t, J = 6.6 Hz, 2H), 2.36–2.22 (m, 1H), 1.56 (dt, J = 14.5, 6.6 Hz, 2H), 1.30 (dd, J = 15.0, 7.5 Hz, 2H), 1.22–1.15 (m, 2H), 1.02 (dt, J = 6.2, 4.3 Hz, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 166.6, 148.4, 146.8, 144.3, 140.1, 137.8, 131.9, 130.8, 128.8, 128.7, 128.3, 127.4, 120.4, 120.3, 111.3, 100.1, 64.3, 30.6, 19.1, 13.7, 13.0, 9.7. HRMS m/z (ESI) calcd for C23H25N2O2 (M + H)+: 361.1911, found 361.1914.n Butyl (E)-3-(2-(5-(furan-2-yl)-1H-pyrrolo[2,3-b]pyridin-1-yl)phenyl)acrylate (3v)
Yellow oil, (yield 26%), 1H NMR (400 MHz, CDCl3) δ 8.66 (d, J = 2.0 Hz, 1H), 8.27 (d, J = 2.0 Hz, 1H), 7.82 (d, J = 7.9 Hz, 1H), 7.60–7.47 (m, 4H), 7.36 (d, J = 16.0 Hz, 1H), 7.29 (d, J = 3.6 Hz, 1H), 6.70 (d, J = 3.6 Hz, 1H), 6.66 (d, J = 3.2 Hz, 1H), 6.50 (dd, J = 3.3, 1.8 Hz, 1H), 6.39 (d, J = 16.0 Hz, 1H), 4.07 (t, J = 6.6 Hz, 2H), 1.59–1.52 (m, 2H), 1.29 (d, J = 7.6 Hz, 2H), 0.88–0.84 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 166.5, 152.8, 147.9, 142.0, 140.9, 139.8, 137.4, 131.9, 130.9, 130.6, 128.7, 128.6, 127.5, 124.4, 121.0, 120.5, 120.5, 111.6, 104.3, 102.1, 64.4, 30.6, 19.1, 13.6. HRMS m/z (ESI) calcd for C24H23N2O3 (M + H)+: 387.1703, found 387.1706.1-(6-Phenyl-5,6-dihydro-6l3-pyrido[3′,2′:4,5]pyrrolo[1,2-a]quinolin-7-yl)ethan-1-one (4a)
Yellow solid, (yield 45%), racemic, 1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 8.2 Hz, 1H), 8.58–8.41 (m, 2H), 7.46–7.37 (m, 1H), 7.31 (dd, J = 7.9, 4.8 Hz, 1H), 7.20–7.07 (m, 5H), 6.98 (dd, J = 7.5, 1.6 Hz, 2H), 5.51 (dd, J = 5.9, 1.5 Hz, 1H), 3.46 (dd, J = 15.4, 6.0 Hz, 1H), 3.18 (dd, J = 15.5, 1.7 Hz, 1H), 2.60 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 193.7, 147.3, 145.9, 143.7, 140.0, 135.1, 129.7, 129.2, 128.5, 127.8, 127.4, 126.9, 126.3, 125.7, 120.4, 120.2, 119.0, 112.0, 37.9, 33.9, 31.5. HRMS m/z (ESI) calcd for C23H19N2O (M + H)+: 339.1492, found 339.1493.Phenyl(6-phenyl-5,6-dihydro-6l3-pyrido[3′,2′:4,5]pyrrolo[1,2-a]quinolin-7-yl)methanone (4b)
Light yellow solid, (yield 15%), racemic, 1H NMR (400 MHz, CDCl3) δ 9.13 (d, J = 8.1 Hz, 1H), 8.46 (dd, J = 4.7, 1.6 Hz, 1H), 7.74 (dd, J = 8.0, 1.6 Hz, 1H), 7.70–7.61 (m, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.48–7.41 (m, 1H), 7.42–7.32 (m, 2H), 7.21–7.05 (m, 6H), 6.91 (dd, J = 7.0, 2.3 Hz, 2H), 5.12 (dd, J = 5.7, 2.5 Hz, 1H), 3.45 (dd, J = 15.5, 5.8 Hz, 1H), 3.15 (dd, J = 15.5, 2.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 192.0, 147.2, 145.7, 143.7, 140.9, 140.5, 135.3, 131.9, 129.6, 129.2, 128.9, 128.4, 128.3, 127.8, 127.3, 126.7, 126.6, 125.6, 121.0, 120.1, 118.5, 111.4, 37.6, 34.2. HRMS m/z (ESI) calcd for C28H21N2O (M + H)+: 401.1648, found 401.1655.Methyl 2-oxo-2-(6-phenyl-5,6-dihydro-6l3-pyrido[3′,2′:4,5]pyrrolo[1,2-a]quinolin-7-yl)acetate (4c)
Yellow solid, (yield 32%), racemic, 1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 8.2 Hz, 1H), 8.52 (dd, J = 4.7, 1.6 Hz, 1H), 8.38 (dd, J = 8.0, 1.6 Hz, 1H), 7.43 (dt, J = 8.4, 4.2 Hz, 1H), 7.34 (dd, J = 8.0, 4.7 Hz, 1H), 7.21–7.07 (m, 5H), 6.97 (dd, J = 7.4, 1.8 Hz, 2H), 5.35 (dd, J = 5.9, 1.9 Hz, 1H), 3.75 (s, 3H), 3.48 (dd, J = 15.5, 6.0 Hz, 1H), 3.19 (dd, J = 15.6, 2.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 181.0, 165.6, 148.8, 147.6, 144.5, 139.5, 134.7, 129.6, 129.2, 128.6, 127.9, 127.3, 127.0, 126.4, 126.3, 120.4, 119.9, 119.7, 107.5, 52.6, 37.4, 33.8. HRMS m/z (ESI) calcd for C24H19N2O3 (M + H)+: 383.1390, found 383.1394.N,N-Diisopropyl-2-oxo-2-(6-phenyl-5,6-dihydro-6l3-pyrido[3′,2′:4,5]pyrrolo[1,2-a]quinolin-7-yl)acetamide (4d)
Yellow solid, (yield 51%), racemic, 1H NMR (400 MHz, CDCl3) δ 9.12 (d, J = 8.2 Hz, 1H), 8.50 (dd, J = 6.5, 5.0 Hz, 2H), 7.44–7.36 (m, 1H), 7.32 (dd, J = 7.9, 4.8 Hz, 1H), 7.18–7.04 (m, 5H), 6.99–6.87 (m, 2H), 5.62 (d, J = 4.8 Hz, 1H), 3.62–3.49 (m, 2H), 3.44 (dd, J = 13.6, 6.8 Hz, 1H), 3.19 (dd, J = 15.6, 1.5 Hz, 1H), 1.53 (dd, J = 13.9, 6.8 Hz, 6H), 1.05 (d, J = 6.6 Hz, 3H), 0.66 (d, J = 5.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 186.3, 167.8, 147.6, 144.2, 139.9, 134.9, 129.9, 129.2, 128.5, 127.8, 127.3, 126.8, 126.4, 126.0, 120.3, 120.2, 119.4, 108.0, 50.4, 45.9, 36.8, 33.8, 20.6, 20.2, 20.2, 19.8. HRMS m/z (ESI) calcd for C29H30N3O2 (M + H)+: 452.2332, found 452.2328.Acknowledgements
We gratefully acknowledge the financial support from National Science Foundation of China (No. 21172011).Notes and references
- (a) A. Carbone, M. Pennati, B. Parrino, A. Lopergolo, P. Barraja, A. Montalbano, V. Spano, S. Sbarra, V. Doldi, M. D. Cesare, G. Cirrincione, P. Diana and N. Zaffaroni, J. Med. Chem., 2013, 56, 7060 CrossRef CAS; (b) Y. Zhang, B. Liu, X. Y. Wu, R. D. Li, X. L. Ning, Y. Liu, Z. M. Liu, Z. M. Ge, R. T. Li and Y. X. Yin, Bioorg. Med. Chem., 2015, 23, 4815 CrossRef CAS; (c) D. A. Sandham, C. Adcock, K. Bala, L. Barker, Z. Brown, G. Dubois, D. Budd, B. Cox, R. A. Fairhurst, M. Furegati, C. Leblanc, J. Manini, R. Profit, J. Reilly, R. Stringer, A. Schmidt, K. L. Turner, S. J. Watson, J. Willis, G. Williams and C. Wilson, Bioorg. Med. Chem., 2009, 19, 4794 CrossRef CAS; (d) A. Paczal, B. Bálint, C. Weber, Z. B. Szabo, L. Ondi, I. Theret, F. D. Ceuninck, C. Bernard, A. Ktorza, F. Perron-Sierra and A. Kotschy, J. Med. Chem., 2016, 59, 687 CrossRef CAS.
- (a) Y. Chen, S. Guo, K. Li, J. Qu, H. Yuan, Q. Hua and B. Chen, Adv. Synth. Catal., 2013, 355, 711 CrossRef CAS; (b) A. Plas, C. Martin, N. Joubert and M. C. Viaud-Massuard, Org. Lett., 2015, 17, 4710 CrossRef CAS; (c) Y. Kim and S. Hong, Chem. Commun., 2015, 51, 11202 RSC.
- (a) M. P. Huestis and K. Fagnou, Org. Lett., 2009, 11, 1357 CrossRef CAS; (b) P. Kannaboina, K. Anilkumar, S. Aravinda, R. A. Vishwakarma and P. Das, Org. Lett., 2013, 15, 5718 CrossRef CAS.
- P. Kannaboina, K. A. Kumar and P. Das, Org. Lett., 2016, 18, 900 CrossRef CAS.
- (a) G. Y. Qian, X. H. Hong, B. X. Liu, H. Mao and B. Xu, Org. Lett., 2014, 16, 5294 CrossRef CAS; (b) S. S. Li, C. Q. Wang, H. Lin, X. M. Zhang and L. Dong, Org. Lett., 2015, 17, 3018 CrossRef CAS PubMed; (c) B. Liu, X. Wang, Z. M. Ge and R. T. Li, Org. Biomol. Chem., 2016, 14, 2944 RSC.
- (a) I. Moritani and Y. Fujiwara, Tetrahedron Lett., 1967, 8, 1119 CrossRef; (b) Y. Fujiwara, I. Moritani and M. Matsuda, Tetrahedron, 1968, 24, 4819 CrossRef CAS; (c) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633 CrossRef CAS.
- For selected examples of Rh-catalyzed C–C coupling using various DGs, see: (a) N. Umeda, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 7094 CrossRef CAS; (b) H. Li, Y. Li, X. S. Zhang, K. Chen, X. Wang and Z. J. Shi, J. Am. Chem. Soc., 2011, 133, 15244 CrossRef CAS; (c) Z. S. Qi and X. W. Li, Angew. Chem., Int. Ed., 2013, 52, 8995 CrossRef CAS; (d) A. S. Tsai, M. Brasse, R. G. Bergman and J. A. Ellman, Org. Lett., 2011, 13, 540 CrossRef CAS; (e) P. Zhao, F. Wang, K. Han and X. W. Li, Org. Lett., 2012, 14, 3400 CrossRef CAS PubMed; (f) F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9982 CrossRef CAS; (g) F. Wang, G. Y. Song and X. W. Li, Org. Lett., 2010, 12, 5430 CrossRef CAS; (h) K. Ueura, T. Satoh and M. Miura, Org. Lett., 2007, 9, 1407 CrossRef CAS PubMed; (i) S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 6295 CrossRef CAS; (j) B. Q. Liu, Y. Fan, Y. Gao, C. Sun, C. Xu and J. Zhu, J. Am. Chem. Soc., 2013, 135, 468 CrossRef CAS; (k) N. J. Wang, S. T. Mei, L. Shuai, Y. Yuan and Y. Wei, Org. Lett., 2014, 16, 3040 CrossRef CAS; (l) S. T. Mei, N. J. Wang, Q. Ouyang and Y. Wei, Chem. Commun., 2015, 51, 2980 RSC; (m) L. T. Xu, C. Zhang, Y. P. He, L. S. Tan and D. W. Ma, Angew. Chem., Int. Ed., 2016, 55, 321 CrossRef CAS.
- S. S. Li, C. Q. Wang, H. Lin, X. M. Zhang and L. Dong, Org. Biomol. Chem., 2016, 14, 229 RSC.
- For selected examples of carbonyl directed C–C addition reactions, see: (a) S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 366, 529 CrossRef CAS; (b) S. G. Lim, J. A. Ahn and C. H. Jun, Org. Lett., 2004, 6, 4687 CrossRef CAS; (c) X. Y. Shi and C. J. Li, Org. Lett., 2013, 15, 1476 CrossRef CAS PubMed; (d) Y. Kommagalla, K. Srinivas and C. V. Ramana, Chem.–Eur. J., 2014, 20, 7884 CrossRef CAS.
- For selected reviews, see: (a) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053 CrossRef CAS; (b) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS; (c) K. M. Engle, T. S. Mei, M. Wasa and J. Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS; (d) G. Y. Song, F. Wang and X. W. Li, Chem. Soc. Rev., 2012, 41, 3651 RSC; (e) G. Y. Song and X. W. Li, Acc. Chem. Res., 2015, 48, 1007 CrossRef CAS; (f) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS; (g) L. Yang and H. M. Huang, Chem. Rev., 2015, 115, 3468 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07478b |
| This journal is © The Royal Society of Chemistry 2016 |