Open Access Article
Open Access ArticleCharacterization of commercial eco-glitters derived from modified regenerated cellulose after laboratory exposure in two aquatic media†
Marta
Doval-Miñarro
 *,
Antonio
López-Vivancos
,
Joaquín
López-Castellanos
and
Javier
Bayo
*,
Antonio
López-Vivancos
,
Joaquín
López-Castellanos
and
Javier
Bayo
Technical University of Cartagena (UPCT), Department of Chemical and Environmental Engineering, Paseo Alfonso XIII, 52; E-30203 Cartagena, Murcia, Spain. E-mail: marta.doval@upct.es; Tel: +34 968325552
First published on 21st July 2025
Abstract
Due to their size and composition, glitter particles can be classified as primary microplastics. Their widespread use in crafts and textiles often leads to uncontrolled release into the environment, with most particles eventually reaching seawater. The European Union recently banned certain microplastics, including glitter for specific uses. Recent developments in eco-friendly glitters, primarily based on modified regenerated cellulose, claim biodegradability in aquatic environments. In this study, we assessed the degradation of commercial biodegradable glitter in purified water and seawater under laboratory conditions over 96 days, as well as the acute toxicity of their leachates on Aliivibrio fischeri. Although no toxicological effects were observed, the glitter particles retained their shape, and their chemical changes were minimal, evidencing that complete biodegradation was far from occurring, thereby posing a potential risk to higher species.
Environmental significanceGlitter particles—classified as primary microplastics—pose an environmental risk due to their widespread use and uncontrolled release. The European Union has banned certain microplastics, including conventional glitter, because of their harmful effects on organisms. In response, eco-glitters, typically made from modified regenerated cellulose and certified as biodegradable in an aqueous medium inoculated from activated sludge, have emerged. However, their biodegradability under natural seawater conditions remains uncertain since their microbial abundance in activated sludge differs significantly. In this paper, we assess the behavior of two commercial eco-glitters exposed to purified water and seawater for 96 days. Despite surface changes in color and gloss, the glitters retained their shape and size, suggesting that they may continue to adversely affect the environment. |
1. Introduction
Conventional glitters are typically composed of petroleum-based monomers, with polyethylene terephthalate (PET) being the most prevalent.1 The core material is then coated with a layer of aluminum, iron, titanium, or bismuth to achieve their final sparkly appearance, which is further covered by a transparent layer of plastic material, commonly polyethylene (PE), polymethyl methacrylate (PMMA), or PET.2 Since glitter particle sizes are usually <5 mm, they can be classified as primary microplastics.3 The effects of microplastics on health are an area of ongoing research; however, it is widely recognized that microplastics can be ingested by organisms and can accumulate, leading to inflammation and immune reactions, DNA damage, oxidative stress, and neurotoxic and metabolic effects, among others.4Microplastics have been detected in aquatic, terrestrial, and atmospheric environments and they are considered ubiquitous pollutants, as they have been found even in remote areas far from human activity.5,6 In the scientific literature, microplastics are typically classified as films, fibers, or fragments, resulting in limited specific detection of glitter in the environment, as it is often grouped with films. It is striking that only a few studies describe hexagonal fragments found in the environment, suggesting the presence of glitter,7–9 considering its extensive use in the textile, cosmetic, and arts and crafts industries.10 Indeed, several authors have argued that glitter has been overlooked as a source of microplastics in the environment, despite its potentially significant role in shaping the dynamics of microplastic pollution from source to sink.11,12 Lusher et al. (2017) expanded the classification of microplastics based on their shapes, establishing glitter as a new category.13 Recent studies have followed this classification14–16 and have begun to pay specific attention to glitter particles. These shiny particles have been found in estuaries,15 river flows,17 riverbeds,18 wastewater treatment plant sludge,13,16 and street dust.7,8 Recently, several authors have highlighted the environmental relevance of glitter, emphasizing that, as primary microplastics, they may pose a greater risk than microbeads.19 Due to their high mobility and durability, glitter particles can reach virtually any environmental compartment. Moreover, their large surface-to-volume ratio facilitates biofilm formation, which increases the likelihood of trophic transfer into food webs.20
Research on the ecotoxicity of glitter particles is limited, and further investigation into this topic is necessary. The effects of different types and concentrations of conventional glitter particles on Mytilus galloprovincialis after 7 days of exposure were assessed.21 The authors found that the digestive tract of Mytilus galloprovincialis preferably retains smaller particles, inducing an increase in the antioxidant defense response. The toxicity of glitter leachates on Aliivibrio fischeri, two algae (Raphidocelis subcapitata and Phaeodactylum tricornutum), Daphnia magna, and Paracentrotus lividus has also been studied.2 The algae were the species most susceptible to glitter leachates. These authors also concluded that the associated toxicity depends not only on the composition of the outer layer of the glitter [PMMA, PE, or polyamide (PA)], the contact area, or the colors but also on the susceptibility of the species, the exposure time, and the medium (freshwater or seawater). The toxic effects of PET glitter on Artemia salina in terms of mortality, feeding behavior, hatching efficiencies, and swimming competencies were recently studied.22 Significant levels of glitter-induced toxicity in the studied aquatic ecosystem were observed. A negative influence of non-biodegradable glitter particles on two cyanobacterial strains was also detected. The effects were dose-dependent, altering cell biovolume and growth rates.23 The toxicity of PET glitters on the microalga Desmodesmus sp as a function of glitter color24 was also assessed. Photosynthesis turned out to be partially affected due to the shading effect of the glitter. Also, the leachates of red and green glitters negatively impacted microalgal growth. These authors concluded that there is a higher toxicological risk associated with additives such as chromium, isobutanol, or hydroxy (dimethyl)silane. The metallic coating of glitter particles has been found to reduce light penetration and increase water reflectance, thereby interfering with underwater radiation and limiting light availability for photosynthetic processes.25 The effects of varying concentrations of commercial glitter on Eisenia fetida have also been investigated, highlighting its potential to persist and accumulate in the environment.26 More recently, significant ingestion of glitter particles by Culicidae and Chironomidae has been reported, accompanied by fragmentation and preferential accumulation in specific body regions, which may increase the risk of bioaccumulation and trophic transfer within aquatic food webs.27
To reduce the discharge of plastic debris in the marine environment, the European Union recently imposed new restrictions on synthetic polymer microplastics, which encompass conventional glitter, for certain applications such as arts and crafts, toys, and clothing.28 As a result, biodegradable glitter has gained increased attention. Typical alternatives to replace conventional glitters can include plant-based options, such as modified regenerated cellulose (MRC) or naturally sourced cellulose nanocrystals (CNCs);29,30 or they can have an inorganic origin, such as natural mica or synthetic mica.
One of the few studies involving biodegradable glitter31 examined the effects of conventional glitter (PET-based), MRC-based glitter, and natural and synthetic mica on the biodiversity and ecosystem functioning of a lotic, sedimentary habitat (Lemna minor and Potamopyrgus antipodarum). The authors concluded that both conventional and alternative glitters can have negative impacts on this environment. These authors also expressed concern regarding certifications required to obtain the biodegradable stamp, as tests were conducted on glitters not in their final form but rather on the core material. Recently, it was concluded that MRC-based glitters decreased the root length, biomass, and chlorophyll content of L. minor;32 in contrast to other authors who observed a reduction in the reproduction rate of Folsomia candida when exposed to PET glitters and no detrimental effects when exposed to cellulose nanocrystalline glitter.33
In this paper, we assess the degradation of two commercial biodegradable glitters (pink and golden) in purified and sea water under laboratory conditions after 96 days of exposure. We study the degradation of the glitter particles and the potential acute toxicity of the leachates on Aliivibrio fischeri. This study contributes to a better understanding of the degradation and the effects of biodegradable glitter on the environment.
2. Materials and methods
2.1. Glitter characterization
Commercial biodegradable chunky glitters (Moon™ Creations, UK) were purchased in three colors (pink, golden and blue). According to the manufacturer, the composition of the glitters includes rayon (a type of modified regenerated cellulose), glycerin, styrene/acrylate copolymer, water, and urea. Additionally, it may contain varying proportions of aluminum powder (CI77000), red 7 (CI 15850 1), yellow 5 lake (CI19140 1), and blue 1 lake (CI42090 1), depending on the glitter color. The shape of the glitters was hexagonal, and they came as a mix of two different sizes (0.5 mm and 1 mm apothem). It should be noted that the glitter samples tested in this study were commercially sourced, and no information was provided by the manufacturer regarding batch consistency or potential formulation variability across different production lots. Furthermore, the detailed composition of the glitters—including the relative proportions of cellulose, styrene/acrylate copolymer, pigments, and other additives—was not disclosed. As a result, our findings should be interpreted with caution, as they may not be fully representative of all glitters marketed as biodegradable or eco-friendly. Future studies should consider testing a broader range of samples with known and controlled compositions to improve comparability and generalizability.The glitter morphology was studied with a ZEISS Crossbeam 350 Field Emission Scanning Electron Microscope (FESEM) (Carl Zeiss Microscopy, Oberkochen, Germany), and the elemental composition of the glitter surface was characterized by energy-dispersive spectrometry (EDS) with an Xplore 30 detector (Oxford Instruments Nanotechnology Tools Ltd, UK). SEM images were used to measure the dimensions of the glitter particles, using their longest diagonal. For this purpose, the Ridge Detection plugin in the open-source software ImageJ (National Institutes of Health, USA) was employed. The chemical composition of the outer layer was further investigated by Fourier transform infrared spectroscopy (FTIR) with a Thermo Nicolet 5700 spectrometer (ThermoFisher Scientific, Waltham, Massachusetts, USA). The thermal behavior of the glitters was studied by Differential Scanning Calorimetry (DSC) with a DSC 822E (Mettler Toledo, Columbus, Ohio, USA). Glitter samples were analyzed by FESEM, FTIR and DSC at the beginning and end of the experiment (after 96 days).
Glitter color was measured with a colorimeter (Minolta CR-300 series, Ramsey, NJ, USA) in the CIELAB color space. Differences in color at the beginning and end of the experiment were determined from the values of L (lightness), a and b (chromatic coordinates) measured, according to eqn (1):2
 | (1) |
2.2. Aquatic media
Experiments were conducted in type II water, from now on, purified water, obtained from a Purelab Chorus 2 RO/DI system (ELGA Labwater, High Wycombe, UK), and sea water collected from the Mediterranean Sea on the southeast coast of Spain (37° 38′ 04.0′′ N, 0° 41′ 37.7′′ W). Characterization of this seawater is provided in Table S1 in the ESI.† The seawater was vacuum-filtered in the laboratory using quantitative analysis paper filters to remove any potential particles (pore size 7–9 μm).Experiments conducted in purified water provide insight into the physicochemical degradation of materials under abiotic aqueous conditions. In contrast, experiments using seawater offer a more environmentally realistic scenario, where both abiotic and potentially biotic degradation processes may occur.
2.3. Degradation and toxicological tests
0.5037 ± 0.0026 g of glitter (pink or golden) were placed in a flask containing either purified water or seawater (500 mL) (Fig. S1†). Experiments were conducted in duplicate, and blank controls for each type of water were also prepared. The flasks were connected to an air pump (EHEIM air200, Deizisau, Germany) to maintain aerobic conditions. After 96 days, the content of each flask was filtered, and the glitter was carefully collected and rinsed with purified water. and subsequently dried for 4 h at 40 °C. Afterwards, it was weighed to study the mass loss and color was measured again. Glitters were again characterized by FESEM, FTIR and DSC.Regarding the leachates, MICROTOX® tests with Aliivibrio fischeri were carried out to determine acute toxicity.34 For this purpose, the bacteria were exposed for 15 min to different dilutions of the leachates (dilution factors: 0.9, 0.45, 0.225, and 0.1125) and a blank. The bioluminescence was measured afterwards (I15) and compared to the initial one (I0) with a photometer (Microtox® M500, Modern Water plc, York, UK). Lyophilized bacteria and solutions (reconstitution solution, osmotic adjusting solution and the diluent) were purchased from Modern Water plc (York, UK). For the determination of the EC50, the parameter gamma (γ) was calculated through eqn (2):
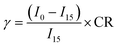 | (2) |
To further characterize the leachates, metal content was determined by using an Agilent 7900 ICP-MS (Agilent Technologies, Inc., California, USA). Sample codification is shown in Table 1.
| Sample | Color | Aquatic medium |
|---|---|---|
| AR1 | Pink | Purified water |
| AR2 | Pink | Purified water |
| MR1 | Pink | Seawater |
| MR2 | Pink | Seawater |
| AD1 | Golden | Purified water |
| AD2 | Golden | Purified water |
| MD1 | Golden | Seawater |
| MD2 | Golden | Seawater |
Additionally, to study the loss of mass and color with time, tests with blue glitter were carried out in 8 flasks, 4 of them containing 250 mL of purified water, and the other 4 flasks containing 250 mL of seawater. 0.3067 ± 0.0025 g of glitter were added to each flask. Air was pumped into the flasks to ensure aerobic conditions. Every 10 days, one of the flasks with purified water and one of the flasks with seawater were removed. The content was filtered, and the collected glitter was dried at 40 °C for 4 h. Subsequently, the glitter was weighed, and the color was measured.
2.4. Statistical analysis
The statistical analyses were conducted using SPSS Statistics version 26.0 (IBM, Co. Ltd, USA). The Kolmogorov–Smirnov test was applied to evaluate whether the data followed a normal distribution. The results were presented as mean values accompanied by their standard error (SE). To determine significant differences among group means, a one-way analysis of variance (ANOVA) was performed, based on the assumptions of normality and homogeneity of variances. Statistical significance was considered at p-values <0.05. When the ANOVA F-test indicated significant differences, Fisher's Least Significant Difference (LSD) test was subsequently used as a post hoc method to identify which specific group pairs differed from each other.3. Results and discussion
3.1. Glitter degradation
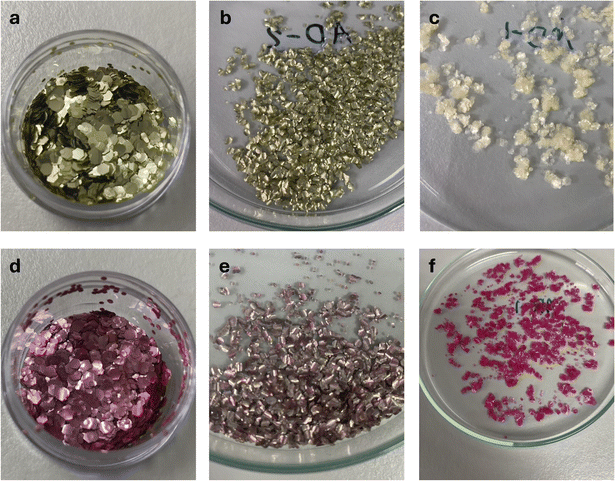
Due to the nature of our experimental setup, the tests performed provide information on the degradation of the glitters, understood according to ISO 23832:2021 (ref. 35) as any irreversible process leading to a significant change in the structure of a material, typically characterized by a change in properties and/or fragmentation, affected by environmental conditions, occurring over time, and potentially involving multiple steps. We do not refer to biodegradation, which is a specific type of degradation caused by biological activity, as neither oxygen consumption nor CO2 evolution has been measured. Since disintegration—defined as the physical breakdown of a material into smaller fragments—can sometimes result from biodegradation processes,35 we use the broader term degradation to encompass all these potential mechanisms that may occur simultaneously.3.1.1.1. Color change of golden and pink glitters. Fig. 1 allows comparison of the change in appearance of the glitters. In seawater, the glitters lost their characteristic shine, whereas in purified water, the glitters retained some of their sheen, though the color slightly faded.
The color of each sample was measured in the CIELAB color space. Table 2 shows the values of L, a and b for the original glitters and for each sample after 96 days of exposure in the aquatic medium. As can be seen, the highest differences in color are manifested in the L value, that is, the luminosity, which, in every case, is reduced. The overall color variation can be estimated using ΔE (eqn (1)). The samples in purified water exhibit a slightly higher average ΔE (25.59 ± 2.34) compared to those in seawater (average ΔE = 20.78 ± 1.09), although these differences were not statistically significant (p > 0.05). As for the glitter colors, no significant differences in fading were observed either (ΔE = 23.47 ± 2.46 for the golden glitter and ΔE = 22.90 ± 2.09 for the pink glitter, p > 0.05). Interestingly, the golden glitter replicates displayed consistent behavior, while larger variations were noted among the pink glitter replicates.
| Initial | |||
|---|---|---|---|
| 30/1/24 | L | A | B |
| GOLDEN | 83.65 | −6.98 | 25.65 |
| PINK | 57.41 | 33.59 | −0.87 |
| Final | |||||||
|---|---|---|---|---|---|---|---|
| 8/5/24 | L | a | b | ΔL | Δa | Δb | ΔE |
| AD1 | 57.67 | −4.15 | 16.39 | −25.98 | 2.83 | −9.26 | 27.73 |
| AD2 | 58.03 | −4.20 | 15.42 | −25.62 | 2.78 | −10.23 | 27.73 |
| AR1 | 47.13 | 24.49 | 11.67 | −10.28 | −9.10 | 12.54 | 18.59 |
| AR2 | 52.77 | 6.29 | 4.94 | −4.64 | −27.30 | 5.81 | 28.29 |
| MD1 | 67.75 | −0.93 | 17.31 | −15.90 | 6.05 | −8.34 | 18.95 |
| MD2 | 67.51 | −0.40 | 16.95 | −16.14 | 6.58 | −8.70 | 19.48 |
| MR1 | 39.50 | 36.72 | 9.38 | −17.91 | 3.13 | 10.25 | 20.87 |
| MR2 | 36.16 | 34.79 | 9.84 | −21.25 | 1.20 | 10.71 | 23.83 |
3.1.1.2. Color change in blue glitters. Regarding the blue glitters, the change in color with exposure time is presented in Fig. 2. The trend of the global color change (ΔE), shown in Fig. 2c, was parabolic in both aquatic media, indicating that the color changed throughout the entire duration of the experiment. To better identify which color coordinate was changing, Fig. 2a and b present the differences in L, a, and b of the glitter in purified water and seawater, respectively. Once again, the L coordinate showed the most significant change in both media, indicating a variation in luminosity. This change reached a maximum and then decreased again, in contrast to a and b, which experienced changes at the beginning of the tests and then remained constant over time. The total color difference was significantly higher in seawater (ΔE = 50.83 ± 2.69) than in purified water (ΔE = 35.30 ± 4.63) (F-test = 8.409; p-value = 0.027).
3.1.2.1. Mass loss of golden and pink glitters. From the beginning of the experiments, it was evident that some mass loss had occurred, as the water in the flasks was quickly dyed after the glitter was added. However, all the glitters retained their hexagonal shape and were far from being completely degraded. The glitters were weighed before and after the experiments, and no significant differences in mass loss were found as a function of color or the aquatic medium (p > 0.05). Table 3 presents the average mass loss for each type of glitter and water. The average mass loss for the pink and golden glitters was −19.10 ± 0.73% and −16.29 ± 1.25%, respectively. The average mass loss in seawater and purified water, regardless of glitter color, was −17.54 ± 1.77% and −17.85 ± 0.52%, respectively.
| Sample | Glitter | Water | Initial mass (g) | Final mass (after 96 days) (g) | Change (%) |
|---|---|---|---|---|---|
| MR1 | Pink | Seawater | 0.5037 | 0.4092 | −18.76 |
| MR2 | Pink | Seawater | 0.5042 | 0.3981 | −21.04 |
| MD1 | Golden | Seawater | 0.5059 | 0.4165 | −17.67 |
| MD2 | Golden | Seawater | 0.5052 | 0.4412 | −12.67 |
| AR1 | Pink | Purified | 0.5035 | 0.4073 | −19.11 |
| AR2 | Pink | Purified | 0.5072 | 0.4185 | −17.49 |
| AD1 | Golden | Purified | 0.4995 | 0.4163 | −16.66 |
| AD2 | Golden | Purified | 0.5005 | 0.4096 | −18.16 |
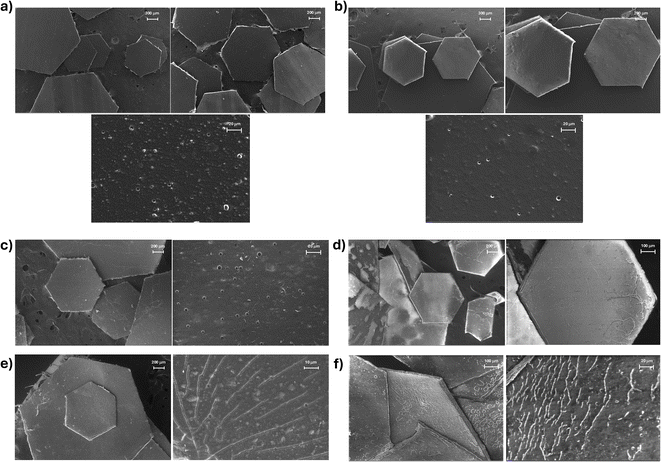
A comparison of the dimensions of the original and aged glitters was conducted to determine whether the observed mass differences were due to a reduction in size or thickness. The distance between two opposite apexes of the glitters was measured using ImageJ and FESEM images. Fisher's test for multiple comparisons revealed no significant differences in the lateral dimensions of the glitters, suggesting that the mass loss is more likely attributable to a reduction in thickness rather than in surface area (Fig. S2†).
3.1.2.2. Mass loss of blue glitters. Experiments with blue glitter were conducted to study the change in color over time (11, 20, 29, and 39 days). The mass of the glitters was also measured to investigate the potential correlation between mass loss and exposure time (Fig. 3). Interestingly, the mass loss was higher for the samples with shorter exposure times in both aquatic media, likely due to an initial loss of soluble pigments and additives at the beginning of the experiments followed by some water absorption. In fact, rayon and other natural and manmade cellulosic materials are well-known for their high-water absorption.36 Also, glycerin, an ingredient of glitters, is a hygroscopic substance.37 The mass loss was slightly higher in seawater (20.05 ± 2.56%) than in purified water (18.09 ± 1.91%), though not significant (p > 0.05). The mass loss trend was similar in both media.
The analysis by EDS showed the composition (as mass percentage) of the external layer of the glitter before and after the experiments (Table 4). Measurements were conducted in different regions and the results were averaged. The original glitters were mainly composed of C and O, as expected due to the organic nature of the materials. As minor elements, the glitters contained Na, Al, Si, S, and Cl. The pink glitter also contained some Ca, and the amount of Cl was significantly higher in the golden than in the pink glitter. The glitters exposed to seawater showed some amounts of Mg and Ca, which were likely due to residues of these elements from the seawater. There was also a slight increase in the proportion of Al, likely due to the loss of part of the external layer, which exposed more Al to the aquatic media. There was also a migration of part of the exposed Al to the waters, as confirmed by the metal analysis of the lixiviates (Table S2†). Some superficial chemical changes in the glitters were also observed in the FTIR spectra of the original and aged glitters, which support a partial loss of the outermost layer. These findings are discussed in detail in Subsection 3.1.4.
| Element | Golden | Pink | ||||
|---|---|---|---|---|---|---|
| Original | Seawater | Purified water | Original | Seawater | Purified water | |
| C | 77.01 (0.14) | 73.32 (0.35) | 78.20 (0.50) | 79.45 (1.74) | 77.71 (2.12) | 81.99 (2.99) |
| O | 16.48 (0.31) | 15.52 (2.27) | 13.27 (1.68) | 17.74 (1.90) | 14.30 (0.37) | 13.09 (2.09) |
| Na | 0.35 (0.02) | 0.31 (0.08) | 0.34 (0.00) | 0.62 (0.14) | ||
| Mg | 1.06 (0.28) | 0.03 (0.03) | 1.19 (0.11) | |||
| Al | 1.78 (0.02) | 3.38 (0.80) | 4.32 (1.37) | 1.27 (0.07) | 2.65 (0.50) | 3.98 (4.67) |
| Si | 0.31 (0.01) | 0.57 (0.13) | 0.42 (0.14) | 0.28 (0.02) | 0.57 (0.17) | 0.46 (0.19) |
| S | 0.51 (0.01) | 0.28 (0.07) | 0.31 (0.04) | 0.57 (0.04) | 0.55 (0.35) | 0.32 (0.23) |
| Cl | 3.58 (0.42) | 4.64 (0.18) | 2.35 (0.88) | 0.08 (0.01) | 1.00 (0.44) | |
| Ca | 0.96 (0.36) | 0.28 (0.02) | 1.42 (0.80) | 0.05 (0.11) | ||
The glitters soaked in purified water showed less variety of elements on their surfaces, confirming that the presence of Mg and Ca in the glitters in contact with seawater came indeed from the salts dissolved in the seawater. Significant differences in element concentrations between aged glitters and the original ones were found by EDS for C and S (p < 0.05), and for O and Al (p < 0.1) in golden glitters; as well as for O in pink glitters (Fig. S3†). The change in the proportion of C and O in the aged glitters may also indicate a partial loss of the organic acrylic layer of the glitters. The presence of S can be related to the fact that carbon disulfide is used in the production of rayon38 and trace amounts of sulfur may remain in the final product.39
Overall, the most remarkable changes occurred in the Al content of the aged glitters, compared to the original ones. The composition of C and O remained relatively stable in the aged glitters, indicating that no major structural changes took place.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), and a band at 700 cm−1 (corresponding to monosubstituted aromatic rings).40 This confirmed that the external layer of the glitters was composed of the styrene/acrylate copolymer.
O stretching), and a band at 700 cm−1 (corresponding to monosubstituted aromatic rings).40 This confirmed that the external layer of the glitters was composed of the styrene/acrylate copolymer.
After exposure to aquatic media, both types of glitters showed an increased absorption band in the 3300–3500 cm−1 region (*), which was indicative of hydroxyl groups and hydrogen bonds, as shown in Fig. 5. This increase could result either from water absorption or from greater exposure of other components -such as glycerin or rayon-that are rich in hydroxyl groups. In the pink glitters, other characteristic rayon bands did not appear in the spectra, suggesting that the rayon remained covered by the other layers. For instance, the rayon broad band at 1060 cm−1, shown in Fig. S4 in the ESI file,† indicative of C–O stretching,41 was absent, which challenges the hypothesis of rayon exposure. Although the aged golden glitters exhibited a new broad band at approximately 1029 cm−1 (**), which is also indicative of C–O stretching, the absence of other characteristic absorption bands of rayon precludes the confirmation of partial exposure of the rayon core. For instance, the diagnostic band at 895–900 cm−1 associated with β-(1→4)-glycosidic linkages42 is present in the rayon spectrum but absent in the aged glitter samples.
 | ||
| Fig. 5 FTIR spectra of the original glitters and after 96 days of exposure in purified water and seawater for (a) golden glitter and (b) pink glitter. | ||
Additionally, three notable spectral differences appeared at around 1700 cm−1, 1450 cm−1, and 1225 cm−1 (the latter only in the pink glitter in seawater). The band at 1700 cm−1 (***) increased in the exposed glitters, which could be attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of urea present in the glitter formulation. Another typical urea peak at 1625 cm−1 (****) (due to N–H vibrations) was small in the original glitters but became more pronounced after exposure; this peak shifted slightly to 1556 cm−1 in the pink glitter soaked in seawater (MR1). Furthermore, a characteristic urea peak at 1453 cm−1 (*****), due to C–N stretching, was notably enhanced in the exposed pink glitters (in both media) and only slightly in the golden glitter exposed to seawater (MD1). The band at 1225 cm−1 (******) observed in MR1 is difficult to interpret since it falls in the fingerprint region; it may result from C–O stretching in the styrene/acrylate copolymer or rayon, or from C–N stretching of amine groups from urea. Meanwhile, many of the original peaks attributed to the styrene/acrylate copolymer remain in the spectra of the exposed glitters.
O stretching of urea present in the glitter formulation. Another typical urea peak at 1625 cm−1 (****) (due to N–H vibrations) was small in the original glitters but became more pronounced after exposure; this peak shifted slightly to 1556 cm−1 in the pink glitter soaked in seawater (MR1). Furthermore, a characteristic urea peak at 1453 cm−1 (*****), due to C–N stretching, was notably enhanced in the exposed pink glitters (in both media) and only slightly in the golden glitter exposed to seawater (MD1). The band at 1225 cm−1 (******) observed in MR1 is difficult to interpret since it falls in the fingerprint region; it may result from C–O stretching in the styrene/acrylate copolymer or rayon, or from C–N stretching of amine groups from urea. Meanwhile, many of the original peaks attributed to the styrene/acrylate copolymer remain in the spectra of the exposed glitters.
In summary, although the FTIR spectra indicated some superficial changes, part of the outermost layer of the glitters (styrene/acrylate copolymer) appeared to remain in the aged samples, with no evident exposure of the rayon, which is the core material. Some increased bands in the aged glitters may be due to a higher exposure of urea.
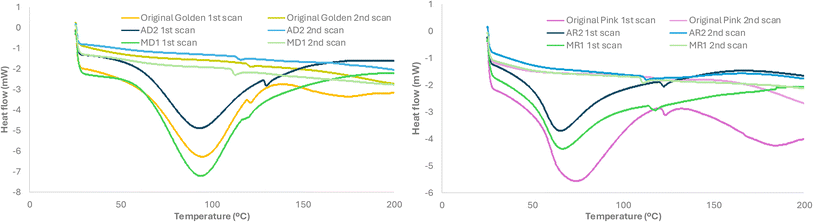 | ||
| Fig. 6 DSC heating curves of the original glitters and after 96 days of exposure, (a) golden glitter and (b) pink glitter. | ||
This decrease is typically associated with weakened intermolecular interactions and increased polymer chain mobility, potentially resulting from surface oxidation, hydrolysis, or the leaching of plasticizers and pigments. The partial loss of aluminum and the outermost surface layers—rich in aromatic groups that contribute to stiffness—may have also accounted for these changes. These thermal effects are consistent with morphological alterations observed via FESEM, which revealed surface roughening and microcracking, particularly in the samples exposed to seawater. Although mechanical properties were not directly assessed, the combined evidence suggests that environmental exposure not only altered the chemical composition but may have also compromised the structural integrity and mechanical stability of the glitters. Further investigations incorporating mechanical testing could help to better quantify these degradation effects.
3.2. Analysis of leachates
Sodium (Na) concentrations also increased significantly in the leachates from purified water. This increase may have originated from the glitter itself, which is consistent with the EDS analysis: Na was not detected on the surface of the glitters after exposure to purified water, although it was present in the original, unexposed samples. Na can also be present in ambient air as a component of marine aerosols. It is worth noting that during the experiments, the samples were continuously aerated with ambient air to maintain aerobic conditions, and some Na may have been absorbed into the water.
Molybdenum (Mo), copper (Cu), and bromine (Br) also showed increases in the experiments conducted with purified water, particularly in the case of the pink glitters. While Br may be derived from marine aerosols and be absorbed into the water from air, the other elements are more likely to originate from the glitters, as they are commonly found in metallic salts, pigments, and stabilizers. The low concentrations of Mo and Cu in the leachates may indicate only trace amounts in the glitters, even though they were not detected in the original material by EDS. Significant differences between the concentration of these elements in the leachates of the aged glitters (p < 0.05) were found for Mo in the lixiviates of golden glitter in sea water and for Mn in the lixiviates of pink glitter in purified water.
In contrast, the concentrations of other elements decreased. For example, antimony (Sb) showed a slight decline in both media, while silicon (Si) and barium (Ba) decreased notably in distilled water but remain stable in seawater. These findings suggest that glitter particles can act as both sources and sinks of different elements.
Given that the leachates originated from 0.5 g of glitter soaked in 500 mL of water, the concentration of potentially harmful substances in the test solutions is presumed to be significantly higher than what would be expected under typical environmental conditions in the event of uncontrolled glitter release. Thus, the observed absence of acute toxicity in this model organism suggests a limited short-term ecotoxicological risk under the specific conditions tested. This result is in line with Piccardo et al. (2022),2 who found no ecotoxicological effects of eight conventional glitters on Aliivibrio fischeri, although one glitter type showed moderate toxicity. However, that study—and others—reported greater effects on different organisms (e.g., algae), not evaluated in the present work. Moreover, the MICROTOX® bioassay fails to detect sublethal, genotoxic, endocrine-disrupting, or chronic effects that may impact organisms at other trophic levels. Therefore, the results obtained should be interpreted strictly within the context of acute bacterial toxicity. Future studies should aim to incorporate complementary bioassays involving multiple trophic levels and more sensitive endpoints to provide a more comprehensive ecotoxicological assessment.
It is also important to note that only inorganic elements in the leachates were analyzed (via ICP-MS), and no chemical characterization of potential organic additives (e.g., pigments, plasticizers, and surfactants) was performed. Consequently, while the tested leachates did not exhibit toxicity for Aliivibrio fischeri, a complete assessment of chemical safety would require further analysis. Ecotoxicological conclusions should thus be considered preliminary.
Our experiments, which lasted a little over three months, revealed that the so-called bioglitters did not undergo rapid degradation in seawater. While glitters progressively lost their color and shine, the leachates remained non-toxic to Aliivibrio fischeri under the tested conditions. Nevertheless, other studies have documented adverse effects of MRC glitter leachates on different species, such as reduced root length in Lemna minor after 14 and 36 days of exposure.31,32
Previous studies have also shown that materials marketed as sustainable can still release harmful substances or degrade slowly under environmental conditions.45 In line with these findings, Zanini et al. (2024) highlighted that biodegradable glitter—often presented as an eco-friendly alternative—may exhibit similar or even greater toxic effects compared to conventional plastic-based glitter, due in part to the leaching of coatings, dyes, and additives during degradation.46 The authors emphasize the current lack of standardized testing protocols to accurately assess biodegradability and ecotoxicity, particularly in aquatic environments where glitter pollution is most prevalent. Thus, to improve the robustness of biodegradability assessments and environmental safety evaluations, regulatory frameworks should incorporate multi-trophic ecotoxicity testing involving bacteria, primary producers, and invertebrates, alongside more environmentally realistic conditions such as long-term exposure in natural waters or sediments. Implementing these comprehensive and ecologically relevant approaches would help align testing protocols with actual environmental dynamics, thereby enhancing the reliability of eco-labels and supporting truly sustainable product development.
4. Conclusions
Exposure of commercial biodegradable glitter to purified water and seawater for over three months revealed fading colors (especially a decrease in luminosity) and loss of shine, particularly in glitters exposed to seawater. The average mass loss of the glitters was close to 18%, potentially due to the dissolution of part of the outermost coating (styrene/acrylate copolymer) and the loss of aluminum and pigments from the next layer. Tests with blue glitters showed that, after a relatively quick initial mass loss, a progressive gain in mass occurred over time, possibly due to water absorption.FESEM images indicated that degradation was more pronounced in seawater than in purified water, and that the mass loss was associated with a reduction in thickness rather than in lateral dimensions. EDS analysis of the glitter surface composition showed that the mass percentage of aluminum in aged glitters was higher than in the original glitters, likely due to increased exposure of the aluminum layer after the partial loss of the styrene/acrylate coating.
FTIR and DSC analyses also showed some changes in the glitters. FTIR indicated that the styrene/acrylate copolymer is still present in aged glitters but may have locally disappeared exposing other components of the glitters such as the metallic layer, urea, or glycerin. DSC revealed a slight decrease in the glass transition temperature of the aged glitters. Finally, the leachates did not show acute toxicity for Aliivibrio fischeri, although further tests on other species are necessary to confirm the safety of both the glitters and their leachates.
Despite the observed superficial changes, the glitters retained their hexagonal shape and were far from being completely degraded by the end of our tests (96 days), suggesting that these materials may pose long-term physical and/or chemical risks to aquatic organisms that ingest them. Many eco-friendly glitters are certified as biodegradable according to standard ISO 14851,47 which involves inoculating an aqueous medium with activated sludge. These conditions differ significantly from a real marine environment, where microorganism concentrations are much lower. Given that most glitter waste ends up in our seas and oceans, it is crucial to develop and certify new glitter formulations under conditions that simulate the marine environment. Future research should aim to characterize degradation products—particularly organic compounds—and to assess their potential toxicity to a broader range of marine species, thereby enabling a more comprehensive evaluation of the environmental impact of eco-friendly glitters.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
MDM – conceptualization/data curation/writing – original draft. ALV – investigation/formal analysis/writing – review & editing. JLC – investigation/writing – review & editing. JB – methodology/resources/supervision/writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has been funded by the Cátedra de Medio Ambiente Autoridad Portuaria de Cartagena Campus Mare Nostrum (CMA_APC_CMN) (ref. 8761/25IQA).References
- M. Yurtsever, Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics, J. Agric. Environ. Ethics, 2019, 32(3), 459–478 CrossRef.
- M. Piccardo, F. Provenza, S. Anselmi and M. Renzi, Ecotoxicological Assessment of “Glitter” Leachates in Aquatic Ecosystems: An Integrated Approach, Toxics, 2022, 10, 677 CrossRef CAS PubMed.
- M. Yurtsever, Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics, J. Agric. Environ. Ethics, 2019, 32(3), 459–478 CrossRef.
- C. Q. Y. Yong, S. Valiyaveettil and B. L. Tang, Toxicity of Microplastics and Nanoplastics in Mammalian Systems, Int. J. Environ. Res. Public Health, 2020, 17(5), 1509 CrossRef CAS PubMed . Available from: https://www.mdpi.com/1660-4601/17/5/1509.
- M. González-Pleiter, C. Edo, Á. Aguilera, D. Viúdez-Moreiras, G. Pulido-Reyes and E. González-Toril, et al., Occurrence and transport of microplastics sampled within and above the planetary boundary layer, Sci. Total Environ., 2021, 761, 143213 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0048969720367449.
- M. González-Pleiter, D. Velázquez, C. Edo, O. Carretero, J. Gago and Á. Barón-Sola, et al., Fibers spreading worldwide: Microplastics and other anthropogenic litter in an Arctic freshwater lake, Sci. Total Environ., 2020, 722, 137904 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0048969720314170.
- S. Abbasi, B. Keshavarzi, F. Moore, H. Delshab, N. Soltani and A. Sorooshian, Investigation of microrubbers, microplastics and heavy metals in street dust: a study in Bushehr city, Iran, Environ. Earth Sci., 2017, 76, 798 CrossRef.
- S. Dehghani, F. Moore and R. Akhbarizadeh, Microplastic pollution in deposited urban dust, Tehran metropolis, Iran, Environ. Sci. Pollut. Res., 2017, 24(25), 20360–20371 CrossRef CAS PubMed.
- A. Ballent, P. L. Corcoran, O. Madden, P. A. Helm and F. J. Longstaffe, Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments, Mar. Pollut. Bull., 2016, 110(1), 383–395 CrossRef CAS PubMed.
- M. Perosa, C. Guerranti, M. Renzi and S. Bevilacqua, Taking the sparkle off the sparkling time, Mar. Pollut. Bull., 2021, 170, 112660 CrossRef CAS PubMed.
- A. S. Tagg and J. A. Ivar do Sul, Is this your glitter? An overlooked but potentially environmentally-valuable microplastic, Mar. Pollut. Bull., 2019, 146, 50–53 CrossRef CAS PubMed.
- N. Bikiaris, N. F. Nikolaidis and P. Barmpalexis, Microplastics (MPs) in Cosmetics: A Review on Their Presence in Personal-Care, Cosmetic, and Cleaning Products (PCCPs) and Sustainable Alternatives from Biobased and Biodegradable Polymers, Cosmetics, 2024, 11(5), 145 CrossRef CAS.
- A. Lusher, R. Hurley, C. Vogelsang, L. Nizzeto and M. Olsen, Mapping microplastics in sludge, 2017. Available from: https://www.researchgate.net/publication/324220835 Search PubMed.
- S. Gündoğdu, B. Kutlu, T. Özcan, F. Büyükdeveci and M. C. M. Blettler, Microplastic pollution in two remote rivers of Türkiye, Environ. Monit. Assess., 2023, 195(6), 791 CrossRef PubMed.
- A. Nithin, A. Sundaramanickam and M. Sathish, Seasonal distribution of microplastics in the surface water and sediments of the Vellar estuary, Parangipettai, southeast coast of India, Mar. Pollut. Bull., 2022, 174, 113248 CrossRef CAS PubMed.
- S. Raju, M. Carbery, A. Kuttykattil, K. Senthirajah, A. Lundmark and Z. Rogers, et al., Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant, Water Res., 2020, 15, 173 Search PubMed.
- I. D. da Costa, E. de Freitas Queiroz, N. N. dos S. Nunes, L. L. Costa and I. R. Zalmon, The revelry of plastic! Quali-quantitative variation of microplastics in freshwater before and after Carnival in south-eastern Brazil, Mar. Freshwater Res., 2024, 75(12), MF24092 CrossRef.
- R. Hurley, J. Woodward and J. J. Rothwell, Microplastic contamination of river beds significantly reduced by catchment-wide flooding, Nat. Geosci., 2018, 11(4), 251–257, DOI:10.1038/s41561-018-0080-1.
- S. B. Kurniawan, N. S. M. Said, M. F. Imron and S. R. S. Abdullah, Microplastic pollution in the environment: Insights into emerging sources and potential threats, Environ. Technol. Innovation, 2021, 23, 101790 CrossRef CAS.
- A. Dąbrowska, M. Stachowicz and M. Szymiczek, Glitters in fishing ground baits – A direct source of primary microplastics in soil and freshwater ecosystems, Chemosphere, 2024, 369, 143842 CrossRef PubMed.
- F. Provenza, S. Anselmi, A. Specchiulli, M. Piccardo, D. Barceló and M. Prearo, et al., Sparkling plastic: Effects of exposure to glitter on the Mediterranean mussel Mytilus galloprovincialis, Environ. Toxicol. Pharmacol., 2022, 96, 103994 CrossRef CAS PubMed.
- D. Das Pramanik, S. Lei, P. Kay and F. M. Goycoolea, Investigating on the toxicity and bio-magnification potential of synthetic glitters on Artemia salina, Mar. Pollut. Bull., 2023, 190, 114828 CrossRef CAS PubMed.
- M. J. Machado, R. B. Dextro, R. B. Cruz, S. R. Cotta and M. F. Fiore, Response of two cyanobacterial strains to non-biodegradable glitter particles, Aquat. Toxicol., 2023, 260, 106590 CrossRef CAS PubMed.
- C. Wang, L. Jiang, Y. Zhang, C. Wang and M. He, Stealth microplastics pollutants: Toxicological evaluation of polyethylene terephthalate-based glitters on the microalga Desmodesmus sp. and its color effect, Environ. Sci. Pollut. Res., 2023, 30(42), 95975–95987 CrossRef CAS PubMed.
- L. L. Yoshida, B. Irineu and M. B. da Cunha-Santino, Interference of glitter with the photosynthetic rates of a submerged macrophyte, Egeria densa. N Z J Bot, 2023, 1–14, DOI:10.1080/0028825X.2023.2276284.
- T. Trakić, F. Popović, J. Sekulić and D. K. Hackenberger, Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae), Agriculture, 2024, 14(2), 267 CrossRef.
- A. C. de Oliveira, L. O. Drummond, S. De Grande and F. M. Nuvoloni, Glitter ingestion by bromeliad-dwelling macroinvertebrates: implications for freshwater microplastic contamination, Environ. Toxicol. Chem., 2025, vgaf111, DOI:10.1093/etojnl/vgaf111.
- European Commision, Comission regulation (UE) 2023/2055 of 25 September 2023 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards synthetic polymer microparticles [Internet], Official Journal of the European Union, L 238/67, 2023, Available from:https://echa.europa.
- B. E. Droguet, H. L. Liang, B. Frka-Petesic, R. M. Parker, M. De Volder and J. J. Baumberg, et al., Large-scale fabrication of structurally coloured cellulose nanocrystal films and effect pigments, Nat. Mater., 2022, 21, 352–358 CrossRef CAS PubMed.
- T. Wang, Y. Wang, C. Ji, Z. Xu, Q. Ding and H. Yang, All-Cellulose-Based Photonic Glitters, Adv. Funct. Mater., 2025, 30, 95975–95987 Search PubMed.
- D. S. Green, M. Jefferson, B. Boots and L. Stone, All that glitters is litter? Ecological impacts of conventional versus biodegradable glitter in a freshwater habitat, J. Hazard. Mater., 2021, 402, 124070 CrossRef CAS PubMed.
- B. Boots, D. S. Green, B. Olah-Kovacs, F. De Falco and E. Lupo, Physical and chemical effects of conventional microplastic glitter versus alternative glitter particles on a freshwater plant (Lemnaceae: Lemna minor), Ecotoxicol. Environ. Saf., 2023, 263, 115291 CrossRef CAS PubMed.
- P. H. Chen, B. E. Droguet, I. Lam, D. S. Green, S. Vignolini and Z. Gu, et al., Assessing the ecotoxicological effects of novel cellulose nanocrystalline glitter compared to conventional polyethylene terephthalate glitter: Toxicity to springtails (Folsomia candida), Chemosphere, 2024, 366, 143315 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045653524022136.
- International Organization for Standardization. ISO 11348-3:2007 Water Quality — Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test) — Part 3: Method Using Freeze-Dried Bacteria. Geneva, Switzerland: ISO; 2007 Search PubMed.
- International Organization for Standardization. ISO 23832:2021 Plastics — Test Methods for Determination of Degradation Rate and Disintegration Degree of Plastic Materials Exposed to Marine Environmental Matrices under Laboratory Conditions. Geneva, Switzerland: ISO; 2021 Search PubMed.
- I. R. Comnea-Stancu, K. Wieland, G. Ramer, A. Schwaighofer and B. Lendl, On the Identification of Rayon/Viscose as a Major Fraction of Microplastics in the Marine Environment: Discrimination between Natural and Manmade Cellulosic Fibers Using Fourier Transform Infrared Spectroscopy, Appl. Spectrosc., 2017, 71(5), 939–950 CrossRef CAS PubMed.
- X. Lin, W. Ma, L. Chen, L. Huang, H. Wu and A. Takahara, Influence of water evaporation/absorption on the stability of glycerol-water marbles, RSC Adv., 2019, 9(59), 34465–34471 RSC.
- D. Majumdar, A. Bhanarkar, C. Rao and D. Gouda, Carbon disulphide and hydrogen sulphide emissions from viscose fibre manufacturing industry: A case study in India, Atmos. Environ.:X, 2022, 13, 100157 CAS.
- A. T. Özgüney, A. E. Körlü, I. M. Bahtiyari and M. Bahar, A NOVEL APPROACH FOR SULPHUR TEST ON VISCOSE BASED MATERIALS, JTATM, 2006, 5(2), 1–8 Search PubMed.
- T. Barman, H. Chen, J. Liu, G. Yang, W. Zhao and C. Peng, et al., Synthesis and characterization of styrene-based polyfluoroacrylate film for hydrophobic/icephobic applications, Thin Solid Films, 2019, 687, 137462 CrossRef.
- I. Kaur, N. Sharma and V. Kumari, Modification of fiber properties through grafting of acrylonitrile to rayon by chemical and radiation methods, J. Adv. Res., 2013, 4(6), 547–557 CrossRef PubMed.
- K. S. Salem, N. K. Kasera, M. A. Rahman, H. Jameel, Y. Habibi and S. J. Eichhorn, et al., Comparison and assessment of methods for cellulose crystallinity determination. Chemical Society Reviews. Royal Society of Chemistry; 2023, Vol. 52. pp. 6417–46 Search PubMed.
- M. Özdemir, M. Özdemir Alp, A. Aytaç and V. Deniz. A study of the properties of paper sized with styrene-butyl acrylate copolymers. In: Acta Physica Polonica A. Polish Academy of Sciences; 2017. pp. 1098–101 Search PubMed.
- K. Khezri and Y. Fazli, Characterization of Diatomite Platelets and Its Application for In Situ Atom Transfer Radical Random Copolymerization of Styrene and Butyl Acrylate: Normal Approach, J. Inorg. Organomet. Polym. Mater., 2017, 27(1), 266–274 CrossRef CAS.
- L. Zimmermann, A. Dombrowski, C. Völker and M. Wagner, Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition, Environ. Int., 2020, 145, 106066 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0160412020320213.
- M. L. de O. Zanini, L. M. Fonseca, C. R. Piecha, C. de P. L. Corrêa, L. B. Dode and E. da R. Zavareze, et al., A Review of Conventional and Environmentally Sustainable Glitter. Environmental Quality Management. John Wiley and Sons Inc; 2024, Vol. 34 Search PubMed.
- International Organization for Standardization. ISO 14851:2019 Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium — Method by Measuring the Oxygen Demand in a Closed Respirometer. Geneva, Switzerland; 2019 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5em00105f |
| This journal is © The Royal Society of Chemistry 2025 |